Abstract
Introduction: Total knee arthroplasty (TKA) is associated with intense postoperative pain for which effective analgesia is essential to facilitate early postoperative recovery. Adductor canal block (ACB) and local infiltration analgesia (LIA) have become increasingly involved in postoperative pain management after TKA. We aimed to compare their efficacy and outcomes in patients undergoing TKA.
Materials and Methods: Sixty patients undergoing unilateral TKA were randomized to receive either postoperative single-injection ACB (Group A) or LIA (Group L) during the operation. All patients received spinal anaesthesia. Primary outcome was total morphine consumption over postoperative 24 hours. Visual analog pain scale, time to first and total dosage of rescue analgesia, performance-based evaluations [timed-up and go (TUG) test, quadriceps strength], side-effects, length of hospital stay and patient satisfaction were measured.
Results: Fifty-seven patients were available for analysis. Median total morphine consumption over 24 and 48 postoperative hours of Group A were significantly less than Group L (6/10 mg vs 13/25 mg, p, 0.008 and 0.001, respectively). Similarly, Group A had significantly lower VAS at postoperative 6, 12 and 18 hours, VAS at ambulation on postoperative (POD) 1-3, better TUG tests on POD 2 and during POD 3 than those of Group L. However, quadriceps strength and patient satisfaction were not different between both groups.
Conclusion: Patients undergoing TKA with single-injection ACB required less postoperative opioids than those with LIA. Furthermore, multimodal analgesia using ACB provided better postoperative analgesia, as well as performance-based activities, than those with LIA.
Keywords: adductor canal block; local infiltration analgesia; pain, functional outcome; knee arthroplasty
Introduction
Total knee arthroplasty (TKA) is usually associated with moderate to severe postoperative pain1,2. Early postoperative mobilization is critical to both reduction of immobility-related complications and achieving the optimal functional outcome following surgery. Effective postoperative analgesia, including peripheral nerve block, opioids and non-opioid medications, has been found to facilitate rehabilitation, improve patient satisfaction, and may reduce length of hospital stay3-5.
Femoral nerve block (FNB) may provide superior pain relief to patient-controlled analgesia (PCA) with opioids5,6. However, it is associated with increased risk of fall from prolonged motor blockade7,8. Adductor canal block (ACB) has been shown to be an alternative technique to FNB for postoperative pain control after TKA. Recent data suggested that ACB may contribute to adequate analgesia with a multimodal analgesic regimen9-11 and be associated with better quadriceps strength, postoperatively, in comparison with FNB12,13.
LIA has been shown to provide superior postoperative analgesia and earlier mobilization compared to placebo14,15, intrathecal morphine16, epidural analgesia17,18 and FNB19-22. Furthermore, LIA is less expensive and easier to perform than FNB, albeit with similar analgesic effects23-28. To the best of our knowledge, there has been no study comparing the head-to-head efficacy between ACB and LIA in patients undergoing TKA.
In this single-center, randomized, parallel-group, double-blinded trial, we aimed to compare the effect of ACB and LIA on established pain during postoperative period as well as ambulation ability after TKA in patients receiving spinal anaesthesia with a multimodal analgesic regimen. We hypothesized that ACB would provide similar reduction of morphine consumption during the first 24 postoperative hours (primary outcome), as well as alleviate pain during rest, movement and improve functional outcome (secondary outcomes) to those of LIA.
Materials and Methods
This study was approved by the Institutional Review Board of Chulalongkorn University, Bangkok, Thailand on March 2015 (Ref: 559/57) and registered with Clinicaltrials.in.th (TCTR20150720003). Seventy subjects scheduled to undergo elective TKA with two orthopaedic surgeons (A.T and S.N) were enrolled for this study, 60 subjects provided written consent to participate in this study and 57 subjects were available for per-protocol analysis (Fig. 1).
Fig. 1:
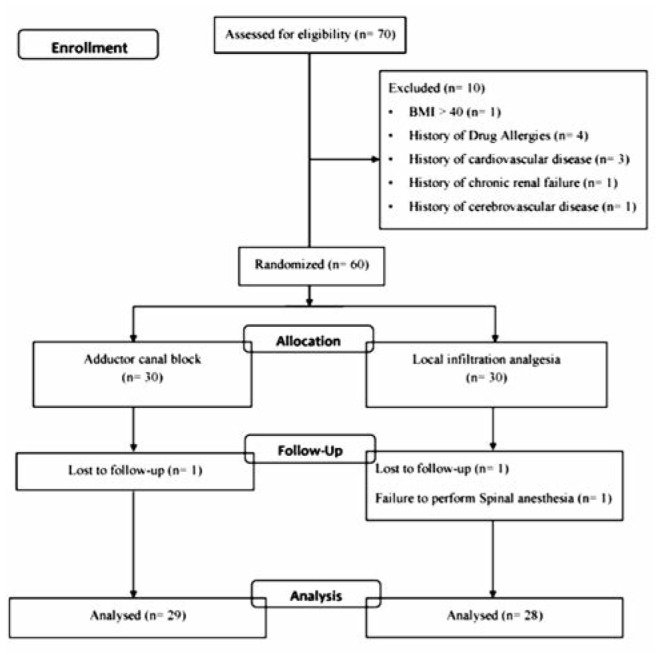
Consolidated standards of reporting trials statement flow diagram.
Eligibility criteria were primary, unilateral TKA under spinal anaesthesia, age >18 years, body mass index 18–40 kg/m2 and American Society of Anesthesiologists (ASA) functional status I–III. Exclusion criteria included contraindication for peripheral nerve or neuraxial blockade, history of allergy to drugs implicated in this study, history of abnormal liver enzymes, hepatic failure, renal insufficiency, uncontrolled hypertension, congestive heart failure, previous heart or coronary bypass surgery, history of stroke or major neurological deficit, sensory and motor disorders in the operated limb, gastritis or gastrointestinal bleeding, organ transplantation, chronic pain requiring opioid medications, neuropathic pain, failure in preoperative Timed-Up and Go (TUG) test, and subject refusal.
Demographic characteristics, preoperative VAS, functional performance-based evaluation including TUG test and quadriceps muscle strength, were recorded by a research assistant. TUG test measures the time to rise from an armchair (seat height, 46 cm), walk 3 metres, turn, and return to sitting in the same chair29. Quadriceps muscle strength of each subject was evaluated by a digital dynamometer [MicroFET2TM, Hoggan Health Industries, Salt Lake City, USA].
All patients were assigned to receive either LIA or ACB (1:1 allocation, parallel trial design), based on a computer-generated randomization (block size= 4). Group assignment was concealed via opaque envelops that were opened after enrollment. The anaesthesiologist performing ACB (W.K) was aware of the treatment, but the two surgeons (A.T and S.N) were blinded to group randomization and were not allowed to participate in postoperative patient care. Outcome assessors and clinical personnel were blinded to the study arm. The investigators were not involved in data collection.
All patients received oral acetaminophen 650 mg, 30 minutes before surgery. All surgeries were performed under spinal with paramedian approach using a 27-gauge BDTM Quincke spinal needle at the L3/L4 or L2/L3 intervertebral space with the patient in the lateral position. Spinal anaesthesia using 0.5% hyperbaric bupivacaine 3 ml was used in all patients. If the spread of the sensory block was insufficient, the patient was excluded from the study, and general anaesthesia was later administered. All patients received intravenous dexamethasone 10 mg and ondansetron 4 mg for postoperative nausea and vomiting prophylaxis. The decision of whether to provide intravenous fluid during operation or to sedate using propofol was made at the discretion of the anaesthesiologist. The minimally invasive mini-midvastus approach was applied in all knees, with the use of tourniquet.
The LIA cocktail, prepared by an anaesthesiologist (W.K), was composed of 0.5% levobupivacaine 20 ml, morphine 5 mg, 1:1000 adrenaline 0.3 ml, and isotonic sodium chloride solution 40 ml. After implantation of the component and lavage of the surgical site were completed, 60 ml of LIA cocktail was injected around the prosthesis, fat and subcutaneous tissue before skin closure. In the ACB group, patients received only isotonic sodium chloride solution 60 ml for local infiltration.
We used the amount of LIA and most of mixture similar to previous study23 because the subjects were quite similar in weight and ethnicity to our study. Moreover, we needed to use the same dose of local anaesthetic drug to truly compare with the ACB group.
In the ACB group, ACB was performed after the surgery by a single anaesthesiologist (W.K). A high-frequency linear array ultrasound transducer [Sonosite M-Turbo, Sonosite, Bothell, Washington] was used to identify the adductor canal. The transducer was surveyed at the mid-thigh, half the distance between the inguinal crease and the patella. Next, the superficial femoral artery, the sartorius, the adductor longus and magnus muscles were identified. At this level, the adductor longus muscle could be identified underneath the sartorius muscle. The hyperechoic structure located anterolateral to the artery (saphenous nerve and nerve to vastus medialis) was identified as the target injection site. A 22-gauge, 100 mm needle [stimuplex; B Braun, Bethlehem, Pennsylvania] was introduced in-plane lateral to medial and 0.5% levobupivacine 20 ml was injected after ensuring the correct placement of the needle by using saline 2-3 ml. For the LIA group, patients received only saline 3 ml. In case of some patients wearing knee compression bandages which obscured the procedure area, temporary release at the upper part Was done.
The pain control regimen was multimodal analgesic technique. At the recovery room, all subjects were administered intravenous PCA with morphine (1mg/ml, no basal rate, 2 mg/dose, lockout interval 10 minutes, and four hours limit 30 mg) until 48 hours postoperative period. Other medications included 3 consecutive doses of intravenous parecoxib 20 mg [Dynastat, Pfizer, New York, USA] at 12-hour interval, 5 consecutive doses of oral acetaminophen 650 mg at 6-hour interval, pregabalin 75 mg [Lyrica, Pfizer, New York, USA] once daily, and celecoxib 400 mg [Celebrex, Pfizer, New York, USA], started at the last dose of parecoxib, once daily. All patients received intravenous esomeprazole 40 mg [Nexium, Astrazeneca, UK] for prevention of upper gastrointestinal bleeding, intravenous metoclopramide 5-10 mg for nausea/vomiting, intravenous chlorpheniramine 5-10 mg for itching. At discharge, home medications included half tablet of tramadol hydrochloride/acetaminophen [Ultracet, Johnson & Johnson, New Brunswick, USA] twice daily, celecoxib 200 mg, pregabalin 75 mg and esomeprazole 20 mg once a day. For severe pain, tramadol 50 mg was prescribed, orally at 6-hour interval.
Postoperative pain at rest was measured using VAS at 6, 12 and 18 hours after surgery. VAS during knee flexion and extension were measured in the morning and evening on postoperative day (POD) 1. VAS during stand-up and walking was measured on POD 2, 3. The results were recorded by research assistants who were blinded from group randomization.
Morphine consumption via PCA device was recorded at the first time requirement and 12, 24 and 48 hours, postoperatively. Quadriceps strength and TUG test on POD 2, 3 were recorded by a physiotherapist who was blinded to studied group. The incidence of nausea and vomiting (1= none, 2= queasy, 3= severe nausea, 4= vomiting), pruritus (1= none, 2= mild, 3=moderate, treatment requested, 4= severe, treatment requested), patient satisfaction (0-10), length of hospital stay, adverse events including local anesthetic toxicity and incidence of fall were recorded.
Home discharge criteria included (1) no pain on functional activities of daily living, (2) ability to get in and out of bed and a chair with little help, (3) walk along a hallway independently or with standard walker, crutches or cane, (4) ability to go up and down stairs safely. If a higher level of ongoing support was required, the patient was retained for further rehabilitation facilities.
The primary outcome was the total morphine consumption during postoperative 24 hours. Secondary outcomes included postoperative pain score, time to first and total dosage of rescue morphine in postoperative 48 hours, early and late postoperative period (from POD 0 to 3 months follow-up) performance-based test (TUG test, and quadriceps strength). Postoperative nausea and vomiting, length of hospital stay, patient satisfaction and other adverse events were also evaluated.
Sample-size calculations were done by using the morphine consumption in postoperative 24 hours as the primary endpoint. In a pilot study of 12 patients, 6 of whom received either spinal anaesthesia added to LIA or ACB. The PCA morphine consumptions were at 9± 9.2 mg and 3.7± 3.2 mg, respectively. We calculated that 27 patients would be required in each group to detect the difference with an α of 0.05 and β of 0.2. Considering the risk of dropouts, 30 patients were included in each of the two groups.
Repeated-measures analysis of variance (ANOVA) was used for the analysis of the primary outcome and secondary outcomes. Categorical data were analyzed using the Chi-square test or Fisher’s exact test. Normal distributed data were statistically tested with the independent’s t-test, and data that did not fulfill the assumptions of normal distribution were analyzed with the Mann-Whitney U-test. The results were presented as mean ± SD or median with inter-quartile range as appropriate. A p-value of <0.05 was considered statistically significant. The data was analyzed using the SPSS version 22.0 software.
Results
There were no significant differences between groups in demographic data including age, gender, body mass index, ASA, pre-operative VAS, site of surgery, duration of surgery and length of hospital stay (Table I). Although time to first request for rescue analgesia and total median morphine consumption at the first 12 postoperative hours were not significantly different between the groups, the total median morphine consumption in Group A was significantly lower than Group L in both 24 (primary outcome) and 48 postoperative hours [6 mg (range, 0-12) vs. 13 mg (range, 5-24), p=0.008, and 10 mg (range, 4-20) vs. 25 mg (range, 12-41), p=0.001, respectively] as shown in Table II.
Table I:
Patient characteristics
| ACB (n=29) | LIA (n=28) | p-Value | |
|---|---|---|---|
| Age (years) | 72.14±8.06 | 68.89 5.65 | 0.083 |
| Gender | |||
| Male | 3(10.3%) | 4(14.3%) | 0.706 |
| Female | 26 (89.7%) | 24 (85.7%) | |
| Height (cm) | 153.59±6.91 | 155.52±6.72 | 0.289 |
| Weight (kg) | 60.94±10.45 | 67.38±13.95 | 0.053 |
| BMI (kg/m2) | 25.78±3.84 | 27.79±4.89 | 0.088 |
| ASA | |||
| ASA2 | 28 (96.6%) | 28 (100%) | 1 |
| ASA3 | 1(3.4%) | 0(0%) | |
| Pre-op VAS | |||
| Rest | 3.18±2.07 | 3.61±2.39 | 0.467 |
| Movement | 7.8±2.36 | 7.6±2.28 | 0.478 |
| Surgeon | |||
| A.T | 21 (72.4%) | 20 (71.4%) | 0.934 |
| S.N | 8(27.6%) | 8(28.6%) | |
| Duration of surgery (min) | 120.14±25.95 | 130.64±28.13 | 0.148 |
| Hospital stay (days) | 4±0 | 4.11±0.31 | 0.083 |
Table II:
Total morphine consumption (mg)
| ACB (n=29) | LIA (n=28) | p-Value | |
|---|---|---|---|
| Morphine 1st time (min) | 130 (105, 498) | 92 (61.5, 242.5) | 0.158 |
| Morphine at 12 hr | 4 (0, 8) | 4 (3, 13) | 0.056 |
| Morphine at 24 hr | 6 (0, 12) | 13 (5, 24) | 0.008* |
| Morphine at 48 hr | 10 (4, 20) | 25 (12, 41) | 0.001* |
*= significant at level 0.05
No difference in VAS was found during preoperative period between Group A and Group L (Table I). The mean VAS at 6, 12, and 18 postoperative hours in Group A were significantly lower than Group L with the differences of 1.21 (95% CI = -2.31 to -0.1, p=0.034), 1.51 (95% CI = -2.76 to - 0.27, p=0.018) and 1.4 (95% CI = -2.45 to -0.34, p=0.01), respectively.
At the first time to sit and knee extension in the morning of POD 1, the mean VAS were significantly lower in Group A than Group L (1.79±1.58 vs. 2.84±1.93, 95% CI = -1.99 to - 0.12, p=0.028; 2.24±1.65 vs. 3.61± 2.43, 95%CI = -2.47 to - 0.25, p=0.017, respectively). In the evening of POD 1, the mean VAS of knee extension in Group A was significantly lower than Group L (2.38±1.44 vs. 3.68±2.21, 95%CI = -2.3 to -0.3, p=0.012) (Table III).
Table III:
Visual analog scale on post-operative day 1-3
| ACB (n=29) | LIA (n=28) | p-Value | ||
|---|---|---|---|---|
| VAS Day 1 am | ||||
| Rest | 1.5 ± 1.63 | 2.28 ± 2.01 | 0.113 | |
| Sit | 1.79 ± 1.58 | 2.84 ± 1.93 | 0.028* | |
| Knee flexion | 2.79 ± 1.53 | 3.75 ± 2.25 | 0.068 | |
| Knee extension | 2.24 ± 1.65 | 3.61 ± 2.43 | 0.017* | |
| VAS Day 1 pm | ||||
| Rest | 1.41 ± 1.28 | 1.7 ± 1.55 | 0.438 | |
| Sit | 2.02 ± 1.48 | 2.51 ± 1.89 | 0.276 | |
| Knee flexion | 3.18 ± 1.77 | 4.12 ± 2.33 | 0.092 | |
| Knee extension | 2.38 ± 1.44 | 3.68 ± 2.21 | 0.012* | |
| VAS Day 2 | ||||
| Rest | 1.2 ± 1.31 | 1.55 ± 1 | 0.258 | |
| Sit | 1.79 ± 1.61 | 2.5 ± 1.29 | 0.072 | |
| Stand | 2.38 ± 1.93 | 3.64 ± 1.98 | 0.018* | |
| Walk | 2.51 ± 1.79 | 3.79 ± 2.06 | 0.015* | |
| VAS Day 3 | ||||
| Rest | 1.36 ± 1.36 | 1.24 ± 1.22 | 0.735 | |
| Sit | 1.59 ± 1.43 | 2.1 ± 1.74 | 0.236 | |
| Stand | 1.93 ± 1.38 | 2.93 ± 1.75 | 0.02* | |
| Walk | 2.21 ± 1.38 | 2.92 ± 1.89 | 0.112 |
*= significant at level 0.05
At the first time to stand and walk on POD 2, the mean VAS in Group A was significantly lower than Group L (2.38±1.93 vs. 3.64±1.98, 95%CI = -2.3 to -0.22, p= 0.018; 2.51± 1.79 vs. 3.79± 2.06, 95%CI= -2.3 to -0.26, p= 0.015, respectively) (Table III). The mean VAS on standing on POD 3 was significantly lower in Group A than Group L (1.93±1.38 vs. 2.93±1.75, 95% CI = -1.83 to -0.16, p=0.02). However, the mean VAS during walking on POD 3 was not significantly different between the groups (Table III).
At preoperative period, there were no differences in TUG test and quadriceps strength on full knee extension, 45 degrees of knee flexion and 90 degrees of knee flexion between the groups (p>0.05) (Fig. 2). TUG test on POD 2 in Group A was remarkably better than Group L (mean difference= -23.95 sec, 95%CI = -42.07 to -5.83, p=0.011) (Fig. 2). Moreover, TUG test during POD 3 of Group A was significantly better than Group L (p=0.035). However, the quadriceps strengths on POD 2 and 3 of both groups were not different (p>0.05) (Fig. 3).
Fig. 2:
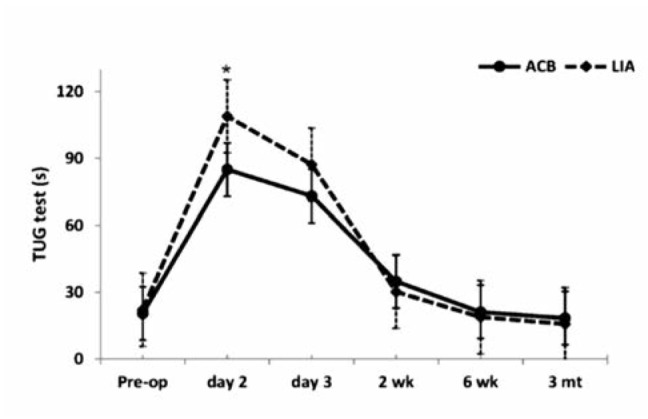
Timed-up and Go test, *= significant at level 0.05.
Fig. 3:
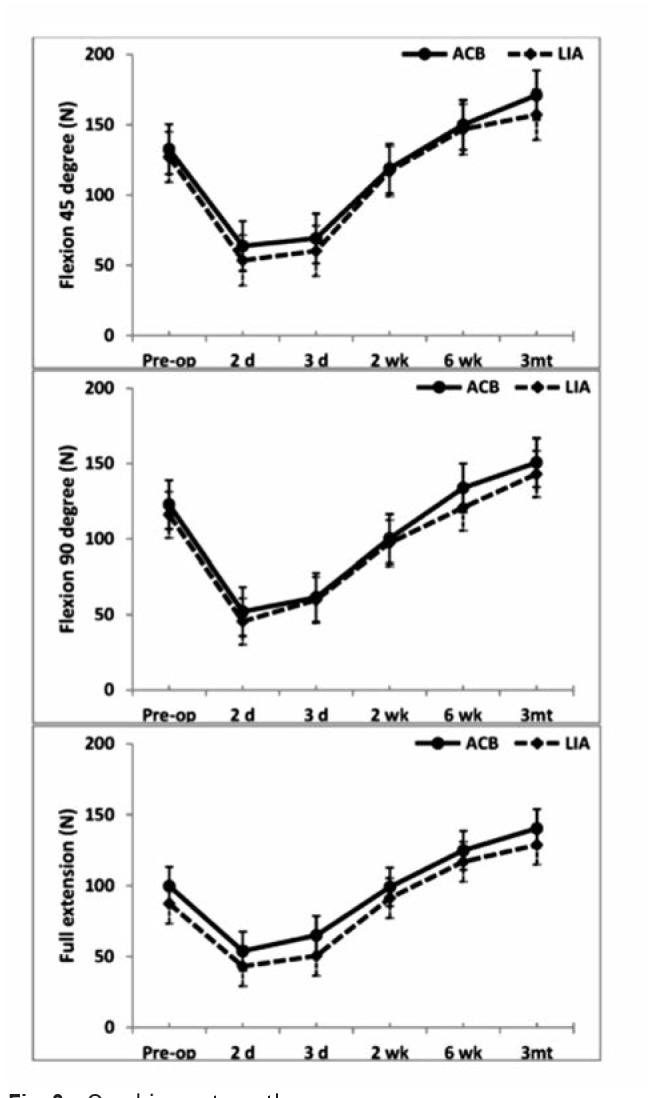
Quadriceps strength.
No differences in patient satisfaction, as well as incidence of nausea or vomiting and pruritus during postoperative period, were found between the groups (Table IV). No falls were recorded in either arm during the study period. There were no documented cases of major or minor symptoms suggestive of local anaesthetic systemic toxicity (LAST), as well as documented complications directly attributable to the nerve blocks, such as local bleeding, infection, or postoperative neuropathy.
Table IV:
Patient satisfaction and adverse events
| ACB (n=29) | LIA (n=28) | p-Value | |
|---|---|---|---|
| Satisfaction score | 8.69 ± 1.6 | 8.43 ± 1.38 | 0.503 |
| Fall | 0 | 0 | N/A |
| Nausea and vomiting | |||
| Day 1 | |||
| none/queasy/severe nausea/vomiting | 23/3/0/3 | 16/5/2/5 | 0.237 |
| Day 2 | |||
| none/queasy/severe nausea/vomiting | 24/4/0/1 | 21/6/0/1 | 0.747 |
| Day 3 | |||
| none/queasy/severe nausea/vomiting | 25/2/0/2 | 23/3/0/2 | 0.876 |
| Pruritus | |||
| none/mild/moderate/severe | 21/5/3/0 | 16/9/3/0 | 0.406 |
| Day 2 | |||
| none/mild/moderate/severe | 20/9/0/0 | 19/9/0/0 | 0.928 |
| Day 3 | |||
| none/mild/moderate/severe | 26/3/0/0 | 21/7/0/0 | 0.179 |
Discussion
In this study, under prospective, randomized, double-blind controlled trial and multimodal analgesia with comparing between ACB and LIA, we found good pain control and high satisfaction in both groups. So that both techniques can be utilised for establishing pain relief after TKA when combined with multimodal analgesic regimen especially within 12 hours postoperatively because we found no differences of morphine consumption and low pain score between the groups. However, our primary endpoint, total morphine consumption, was lower in Group A (single-shot ACB) than Group L (single-shot LIA) during both 24 and 48 hours, postoperatively. Single-shot ACB was able to provide greater pain relief than single-shot LIA during 18 hours, postoperatively. In addition, pain on movement at different times of Group A was significantly lower than Group L. In conclusion, TUG test in Group A was significantly better than Group L during 72 hours, postoperatively. These results may be considered of significant advantage since better pain relief on motion can enhance early mobilization and facilitate physiotherapy after the surgery.
First, it is likely that the nerve supply of knee sensation is more complex than expected and it may be difficult to reliably block locally after the knee is exposed30 and a recent study has shown better pain relief and reduced morphine consumption when addition of the ACB to LIA31. Second, the duration of effect of LIA may be shorter than ACB. Most previous studies had demonstrated that LIA is effective for analgesia about 6-12 hours postoperatively32. For the ACB, recent study33 showed the duration of sensory blockade of about 18-22 hours. These data were consistent with the present study in which postoperative pain score and morphine consumption in Group A were found to be less than Group L after postoperative 12 hours. Third, there were no different techniques or variability in the ACB technique as only one experienced anaesthesiologist was used in our study. Therefore, the effectiveness of the ACB may be more stable. In addition, we used the same dose of local anaesthesia, NSAIDs and other multimodal drugs in both groups to avoid areas of conflict in our study.
Our result is contradictory to a study by Sawhney et al34 who demonstrated greater pain relief at rest and movement in periarticular infiltration analgesia compared with ACB. However, the doses of local anaesthesia in both groups were not equal. They used twice the dose of local anaesthesia in periarticular infiltration analgesia which may provide better effect than ACB. Moreover, they did not exclude patients who could not receive NSAIDs by rising creatinine levels. Another study showed similar result with our study but they used different type, dose and concentration of local anesthesia between the groups35.
Postoperative quadriceps strength in Group A was similar to Group L, and this might be due to the low levels of postoperative pain which were observed to be similar in both groups. The motor preservation was also suggestive that ACB did not interfere with quadriceps strength, as noted in previous studies. Kwofie et al13 and Elkassabany et al14 demonstrated significant quadriceps motor sparing in ACB compared with FNB.
However, there were some limitations in our study. Although we were able to show differences in morphine consumption and pain score, a comparatively larger sample size may have helped in further reducing bias. In our study, we injected local anaesthetic drugs in proximal adductor canal near the femoral triangle. The effect of anaesthetic spread and volume may be affected more than expectation. Bendtsen et al31 applied local anaesthetic injection to the apex of femoral triangle or proximal to the adductor canal, similar to our technique, which covered more nerves that supply the knee and would control pain after TKA more than an injection inside the adductor canal. Therefore, the difference in injection site may bring about different spread of local anaesthetic drugs and may affect the duration and effectiveness of pain relief and physical outcome. Further studies would be needed to define the optimal injection site of ACB for TKA.
Conclusion
In conclusion, a single-injection ACB with multimodal analgesia for TKA was associated with a greater reduction of morphine consumption than single-injection LIA. Furthermore, it provided superior analgesia during the postoperative 18 hours and mobilization duration. It facilitated earlier mobility after TKA than single-injection LIA. However, in clinical practice, the LIA is still easier to perform than the ACB which requires experienced anesthesiologist and may not be available in all situations.
Conflict of Interest
The authors have no conflicts of interest to disclose.
Funding
This work was supported by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University, grant number RA58/047.
Acknowledgements
We would like to thank Dr. Aekkarat Boonshua and Ms Dollapas Punpanich for their participation in the study.
References
- 1.Strassels SA, Chen C, Carr DB. Postoperative analgesia: economics, resource use, and patient satisfaction in an urban teaching hospital. Anesth Analg. 2002;94(1):130–7. doi: 10.1097/00000539-200201000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Wu CL, Richman JM. Postoperative pain and quality of recovery. Curr Opin Anaesthesiol. 2004;17(5):455–60. doi: 10.1097/00001503-200410000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Sinatra RS, Torres J, Bustos AM. Pain management after major orthopaedic surgery: current strategies and new concepts. J Am Acad Orthop Surg. 2002;10(2):117–29. doi: 10.5435/00124635-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Paul JE, Arya A, Hurlburt L, Cheng J, Thabane L, Tidy A. Femoral nerve block improves analgesia outcomes after total knee arthroplasty: a meta-analysis of randomized controlled trials. Anesthesiology. 2010;113(5):1144–62. doi: 10.1097/ALN.0b013e3181f4b18. et al. [DOI] [PubMed] [Google Scholar]
- 5.Chan MH, Chen WH, Tung YW, Liu K, Tan PH, Chia YY. Single-injection femoral nerve block lacks preemptive effect on postoperative pain and morphine consumption in total knee arthroplasty. Acta Anaesthesiol Taiwan. 2012;50(2):54–8. doi: 10.1016/j.aat.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson HD, Hamid I, Gupte CM, Russell RC, Handy JM. Postoperative fall after the use of the 3-in-1 femoral nerve block for knee surgery: A report of four cases. J Orthop Surg (Hong Kong). 2008;16(3):381–4. doi: 10.1177/230949900801600324. [DOI] [PubMed] [Google Scholar]
- 7.Kandasami M, Kinninmonth AW, Sarungi M, Baines J, Scott NB. Femoral nerve block for total knee replacement- a word of caution. Knee. 2009;16:98–100. doi: 10.1016/j.knee.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Jaeger P, Grevstad U, Henningsen MH, Gottschau B, Mathiesen O, Dahl JB. Effect of adductor-canal-blockade on established, severe post-operative pain after total knee arthroplasty: a randomised study. Acta Anaesthesiol Scand. 2012;56(8):1013–9. doi: 10.1111/j.1399-6576.2012.02737.x. [DOI] [PubMed] [Google Scholar]
- 9.Jenstrup MT, Jæger P, Lund J, Fomsgaard JS, Bache S, Mathiesen O. Effect of adductor-canal-blockade on pain and ambulation after total knee arthroplasty: a randomized study. Acta Anaesthesiol Scand. 2012;56(3):357–64. doi: 10.1111/j.1399-6576.2011.02621.x. et al. [DOI] [PubMed] [Google Scholar]
- 10.Hanson NA, Allen CJ, Hostetter LS, Nagy R, Derby RE, Slee AE. Continuous ultrasound-guided adductor canal block for total knee arthroplasty: a randomized, double-blind trial. Anesth Analg. 2014;118(6):1370–7. doi: 10.1213/ANE.0000000000000197. et al. [DOI] [PubMed] [Google Scholar]
- 11.Andersen HL, Gyrn J, Møller L, Christensen B, Zaric D. Continuous saphenous nerve block as supplement to single-dose local infiltration analgesia for postoperative pain management after total knee arthroplasty. Reg Anesth Pain Med. 2013;38(2):106–11. doi: 10.1097/AAP.0b013e31827900a9. [DOI] [PubMed] [Google Scholar]
- 12.Nader A, Kendall MC, Manning DW, Beal M, Rahangdale R, Dekker R. Single-dose adductor canal block with local infiltrative analgesia compared with local infiltrate analgesia after total knee arthroplasty: a randomized, double-blind, placebo-controlled trial. Reg Anesth Pain Med. 2016;41(6):678–84. doi: 10.1097/AAP.0000000000000494. et al. [DOI] [PubMed] [Google Scholar]
- 13.Kwofie MK, Shastri UD, Gadsden JC, Sinha SK, Abrams JH, Xu D. The effects of ultrasound-guided adductor canal block versus femoral nerve block on quadriceps strength and fall risk: a blinded, randomized trial of volunteers. Reg Anesth Pain Med. 2013;38(4):321–5. doi: 10.1097/AAP.0b013e318295df80. et al. [DOI] [PubMed] [Google Scholar]
- 14.Elkassabany NM, Antosh S, Ahmed M, Nelson C, Israelite C, Badiola I. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block: A double-blinded randomized controlled study. Anesth Analg. 2016;122(5):1696–703. doi: 10.1213/ANE.0000000000001237. et al. [DOI] [PubMed] [Google Scholar]
- 15.Busch CA, Shore BJ, Bhandari R, Ganapathy S, MacDonald SJ, Bourne RB. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am. 2006;88(5):959–63. doi: 10.2106/JBJS.E.00344. et al. [DOI] [PubMed] [Google Scholar]
- 16.Vendittoli PA, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin MC. A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. J Bone Joint Surg Am. 2006;88(2):282–9. doi: 10.2106/JBJS.E.00173. et al. [DOI] [PubMed] [Google Scholar]
- 17.Essving P, Axelsson K, Aberg E, Spännar H, Gupta A, Lundin A. Local infiltration analgesia versus intrathecal morphine for postoperative pain management after total knee arthroplasty: a randomized controlled trial. Anesth Analg. 2011;113(4):926–33. doi: 10.1213/ANE.0b013e3182288deb. [DOI] [PubMed] [Google Scholar]
- 18.Andersen KV, Bak M, Christensen BV, Harazuk J, Pedersen NA, Soballe K. A randomized, controlled trial comparing local infiltration analgesia with epidural infusion for total knee arthroplasty. Acta Orthop. 2010;81(5):606–10. doi: 10.3109/17453674.2010.519165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorsell M, Holst P, Hyldahl HC, Weidenhielm L. Pain control after total knee arthroplasty: a prospective study comparing local infiltration anesthesia and epidural anesthesia. Orthopedics. 2010;33(2):75–80. doi: 10.3928/01477447-20100104-13. [DOI] [PubMed] [Google Scholar]
- 20.Toftdahl K, Nikolajsen L, Haraldsted V, Madsen F, Tonnesen EK, Soballe K. Comparison of peri- and intraarticular analgesia with femoral nerve block after total knee arthroplasty: a randomized clinical trial. Acta Orthop. 2007;78(2):172–9. doi: 10.1080/17453670710013645. [DOI] [PubMed] [Google Scholar]
- 21.Chaumeron A, Audy D, Drolet P, Lavigne M, Vendittoli PA. Periarticular injection in knee arthroplasty improves quadriceps function. Clin Orthop Relat Res. 2013;471(7):2284–95. doi: 10.1007/s11999-013-2928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashraf A, Raut VV, Canty SJ, McLauchlan GJ. Pain control after primary total knee replacement. A prospective randomised controlled trial of local infiltration versus single shot femoral nerve block. Knee. 2013;20(5):324–7. doi: 10.1016/j.knee.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Uesugi K, Kitano N, Kikuchi T, Sekiguchi M, Konno SI. Comparison of peripheral nerve block with periarticular injection analgesia after total knee arthroplasty: a randomized, controlled study. Knee. 2014;21(4):848–52. doi: 10.1016/j.knee.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injection: a prospective randomized study. J Arthroplasty. 2007;22(6 Suppl 2):33–8. doi: 10.1016/j.arth.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Affas F, Nygards EB, Stiller CO, Wretenberg P, Olofssen C. Pain control after total knee arthroplasty: a randomized trial comparing local infiltration anesthesia and continuous femoral nerve block. Acta Orthop. 2011;82(4):441–7. doi: 10.3109/17453674.2011.581264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spangehl MJ, Clarke HD, Hentz JG, Misra L, Blocher JL, Seamans DP. Periarticular injections and femoral & sciatic blocks provide similar pain relief after TKA: A randomized clinical trial. Clin Orthop Relat Res. 2015;473(1):45–53. doi: 10.1007/s11999-014-3603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng FY, Ng JKF, Chiu KY, Yan CH, Chan CW. Multimodal periarticular injection vs continuous femoral nerve block after total knee arthroplasty: a prospective, crossover, randomized clinical trial. J Arthroplasty. 2012;27(6):1234–8. doi: 10.1016/j.arth.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 28.Carli F, Clemente A, Asenjo JF, Kim DJ, Mistraletti G, Gomarasca M.et alAnalgesia and functional outcome after total knee arthroplasty: periarticular infiltration vs continuous femoral nerve block Br J Anaesth. 20101052185–95. [DOI] [PubMed] [Google Scholar]
- 29.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 30.Sanna M, Sanna C, Caputo F, Piu G, Salvi M. Surgical approaches in total knee arthroplasty. Joints. 2013;1(2):34–44. [PMC free article] [PubMed] [Google Scholar]
- 31.Bendtsen TF, Moriggl B, Chan V, Børglum J. The optimal analgesic block for total knee arthroplasty. Reg Anesth Pain Med. 2016;41(6):711–9. doi: 10.1097/AAP.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 32.Andersen LØ, Kehlet H. Analgesic efficacy of local infiltration analgesia in hip and knee arthroplasty: a systematic review. Br J Anaesth. 2014;113(3):360–74. doi: 10.1093/bja/aeu155. [DOI] [PubMed] [Google Scholar]
- 33.Jæger P, Grevstad U, Koscielniak-Nielsen ZJ, Sauter AR, Sørensen JK, Dahl JB. Does dexamethasone have a perineural mechanism of action? A paired, blinded, randomized, controlled study in healthy volunteers. Br J Anaesth. 2016;117(5):635–41. doi: 10.1093/bja/aew318. [DOI] [PubMed] [Google Scholar]
- 34.Sawhney M, Mehdian H, Kashin B, Ip G, Bent M, Choy J. Pain after unilateral total knee arthroplasty: a prospective randomized controlled trial examining the analgesic effectiveness of a combined adductor canal peripheral nerve block with periarticular infiltration versus adductor canal nerve block alone versus periarticular infiltration alone. Anesth Analg. 2016;122(6):2040–6. doi: 10.1213/ANE.0000000000001210. et al. [DOI] [PubMed] [Google Scholar]
- 35.Beausang DH, Pozek JP, Chen AF, Hozack WJ, Kaufmann MW, Torjman MC. A randomized controlled trial comparing adductor canal catheter and intraarticular catheter after primary total knee arthroplasty. J Arthroplasty. 2016;31(9):298–301. doi: 10.1016/j.arth.2016.01.064. et al. Suppl. [DOI] [PubMed] [Google Scholar]


