Abstract
The objective of the present study was to investigate the association of B7-H3 expression and cluster of differentiation (CD)163+ tumor-associated macrophage (TAM) infiltration with clinicopathological parameters in urothelial cell carcinoma of the bladder (UCB), and to investigate their potential conjoint effects on progression of UCB. B7-H3 expression and CD163+ TAM infiltration in tumor specimens from 134 consecutive patients that underwent radical cystectomy for UCB were tested using immunohistochemistry, followed by statistical analysis. In these 134 patients, B7-H3 expression and CD163+ TAM infiltration in the bladder carcinoma tissues were significantly associated with an increased ratio of vascular invasion (P=0.009; P=0.012) and distant metastasis (P=0.015; P=0.038); however, they were not associated with gender, age, pathologic grade, tumor stage, recurrence or lymphatic metastasis. The results of χ2 test analysis indicated that CD163+ TAM infiltration and B7-H3 expression were positively correlated (χ2=20.714; P<0.001). Overall survival (OS) and progression-free survival (PFS) rates were significantly worsened by high B7-H3 expression (P=0.002; P=0.020). However, CD163+ TAM infiltration was not associated with OS or PFS rate. Notably, the OS and PFS rates in patients with high B7-H3 expression or high CD163+ TAM infiltration were significantly poorer than the patients with low B7-H3 expression (P<0.001; P<0.001) or low CD163+ TAM infiltration (P=0.022; P=0.017) in the subgroup of 115 patients with muscle-invasive bladder cancer. The results of the present study indicate that B7-H3 expression level could be used as an independent prognostic indicator following radical cystectomy for UCB and patients with high B7-H3 expression and high CD163+ TAM infiltration experience a poorer prognosis.
Keywords: B7-H3, cluster of differentiation 163, tumor-associated macrophages, clinicopathological parameters, urothelial carcinoma of the bladder
Introduction
Bladder cancer is the second most common malignancy of the urinary system, with urothelial cell carcinoma of the bladder (UCB) being the most common type of bladder cancer (1). It is estimated that 76,960 Americans were diagnosed with bladder cancer in 2016, and 16,390 of them will succumb the disease (1). Surgical resection is the primary treatment method for patients with muscle-invasive and recurrent non-muscle-invasive UCB. However, metastasis resulted in 90% of incidences of mortality caused by UCB following gradical cystectomy or transurethral resection, the underlying molecular mechanism of which remains poorly understood (2–7).
The initiation and progression of tumors is closely associated with immune dysfunction in the tumor microenvironment. B7-H3 is a member of the B7 family of molecules, and serves an immunoregulatory function between tumor and immune cells (8,9). B7-H3 is widely expressed in urological tumors and numerous other types of human malignancy, and is associated with tumor progression, metastasis, recurrence and other adverse clinical features (10–15).
Monocytes can enter tumor tissues through vessel walls, where they are able to differentiate into macrophages, designated tumor-associated macrophages (TAMs). TAMs are associated with tumor metastasis; conflicting studies regarding the positive or negative function of TAMs in survival outcomes exist for a number of human malignancies (16–19). This phenomenon may be partly due to the heterogeneity of TAMs and the tumor microenvironment, particularly the varied nature of immunoregulatory factors. It has been reported that TAMs affect tumor angiogenesis, invasion and patient prognosis in various types of cancer, including bladder cancer (3–5). TAMs have been divided into two phenotypes: M1. the pro-inflammatory response, characterized by cells with surface markers cluster of differentiation (CD)80, CD8 or major histocompatibility complex II; and M2, the anti-inflammatory response, characterized by cells with surface markers CD20, CD30, CD16, or SLAM and responsible for tissue repair; it has been reported that the M2 phenotype affects the microvessels, pathological outcome, tumor grade and invasion of UCB (6). The cluster of differentiation 163 (CD163) antibody has been reported to be an M2-polarized-specific cell-surface receptor (6,7).
In the present study, which has 10 years of follow up data, the level of B7-H3 expression and the density of CD163+ TAM infiltration were investigated in postoperative tumor specimens from 134 patients undergoing radical cystectomy (RC) for UCB, and the associations with various clinicopathological features were analyzed. Survival analysis was performed to determine the prognostic significance of B7-H3 expression and the association of CD163+ TAMs with the postoperative survival of patients, and to evaluate the potential effects of these factors on the progression of UCB.
Materials and methods
Specimen collection
Data and tumor and adjacent non-tumor tissue samples were obtained during surgical resection from 134 consecutive patients (67.2±6.89 years old, 115 males and 19 females) who were followed-up following radical cystectomy (RC) for UCB in the Urology Department, Fourth Hospital of Hebei Medical University (Hebei, China), between March 2004 and October 2005, were analyzed. The criteria for RC were: Muscle-invasive bladder carcinoma, recurrent Ta/T1 stage non-muscle-invasive bladder carcinoma and carcinoma in situ refractory to transurethral resection with intravesical chemotherapy. Patients were classified according to the methodology described in previous studies (3,6). Patients who did not manifest signs of tumor metastasis, as evidenced by cross-sectional imaging, and had no histories of preoperative radiotherapy or chemotherapy and neo-adjuvant chemotherapy were included.
Ethics statement
The use of human samples and the research involving human participants in this study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (Hebei, China). All participants provided written informed consent prior to participation in the present study.
Immunohistochemistry and scoring
Specimens were fixed using 10% formalin at room temperature for 4 h, followed by paraffin embedding. Samples were sliced into 5 µm thick sections. The latter were dewaxed in xylene at 60°C, and hydrated with serial dilutions of ethanol (100, 95, 90, 85 and 75%), heated in 1 mmol/l EDTA (pH 8.0) to 121°C, cooled to 90°C, and incubated for 5 min. The sections were submerged in 3% H2O2, followed by deionized water for 10 min to eliminate endogenous peroxidase activity, and blocked with goat serum at 37°C for 30 min (Origene Technologies, Inc., Beijing, China) for 30 min. Sections were incubated with primary mouse anti-human B7-H3 antibody (1:500; cat. no., ab134161;Abcam, Cambridge, UK) or mouse anti-human CD163 antibody (1:100; cat. no., GTX42364, EDHu-1; Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 4°C overnight. The sections were incubated with horseradish peroxidase-conjugated goat anti-mouse Immunoglobulin G (1:500; cat. no., TA130004, Origene Technologies, Inc.) as the secondary antibody at room temperature for 30 min, and rendered with diaminobenzidine for 3–5 min. Subsequently, sections were counterstained with 5% hematoxylin at room temperature for 10 min, dehydrated in ethanol at room temperature (75 for 30 sec, 85 for 30 sec, 90 for 30 sec, 95 for 30 sec and 100% for 10 sec), cleared in 100% xylene for 30 sec, and mounted on coverslips. PBS was used to replace the primary antibody as the negative control.
A total of 5 random fields of view on each stained slide were viewed by light microscopy at magnification, ×400, with the positive rate of every 100 tumor cells counted each view, and the immunohistochemical results were analyzed according to the tumor cells positively-dyed percentage (percentage=number of positively dyed cells in each 100 cells counted) and strength evaluation. The scoring method of B7-H3 expression was based on the stained area and intensity of staining. The frequencies of positive cells were categorized into low, medium and high groups that represented <33%, 34–67% and >67% positively-stained cells, respectively (20). Sections exhibiting low B7-H3 expression were categorized as having negative/weak staining intensity, whereas sections exhibiting high B7-H3 expression were categorized as samples with moderate and strong staining intensity, as described previously (20).
The density of CD163+ TAM infiltration was based on the number of CD163+ cells per field(magnification, ×200). Quantification was performed as described previously (21), as follows: CD163+ TAM infiltration of stroma and islets was scored I–III (score I, low CD163+ TAM infiltration; score II, moderate CD163+ TAM infiltration; score III, high CD163+ TAM infiltration). Weak (score I), moderate (score II) and strong (score III) CD163+ TAM infiltrations are presented in this article. Statistical analyses were performed using the following groupings: Low and high CD163+ TAM infiltrations.
Statistical analysis
Data were analyzed with SPSS software (version 19.0; IBM Corp., Armonk, NY, USA). Data are presented as the mean ± standard error of the mean. Associations betweenB7-H3 expression or CD163+ TAM infiltration and clinicopathological features were evaluated using χ2 tests and Fisher's exact tests. The correlation between B7-H3 staining and CD163+ TAM infiltration were analyzed with χ2 test. Associations of survival and tumor progression with B7-H3 expression or CD163+ TAM infiltration were estimated using the Kaplan-Meier method, and differences were assessed using log-rank tests. All tests were two sided and P<0.05 was considered to indicate a statistically significant difference.
Results
Associations of B7-H3 expression and CD163+ TAM infiltration with clinicopathological features in UCB patients
Representative images of weak, moderate, and strong intensity of B7-H3 expression are presented in Fig. 1. High B7-H3 positive expression was identified in 93/134 (69.4%) cases and low B7-H3 expression was observed in 41/134 (30.6%) cases (Table I). Notably, high B7-H3 expression was significantly associated with increased vascular invasion (69.9 vs. 46.3%; P=0.009) and distant metastasis (49.5 vs. 26.8%; P=0.015) compared with low B7-H3 expression. No significant associations were identified between B7-H3 expression and other pathological factors including gender, age, tumor grade, tumor stage, recurrence and lymph node metastasis (Table I).
Figure 1.
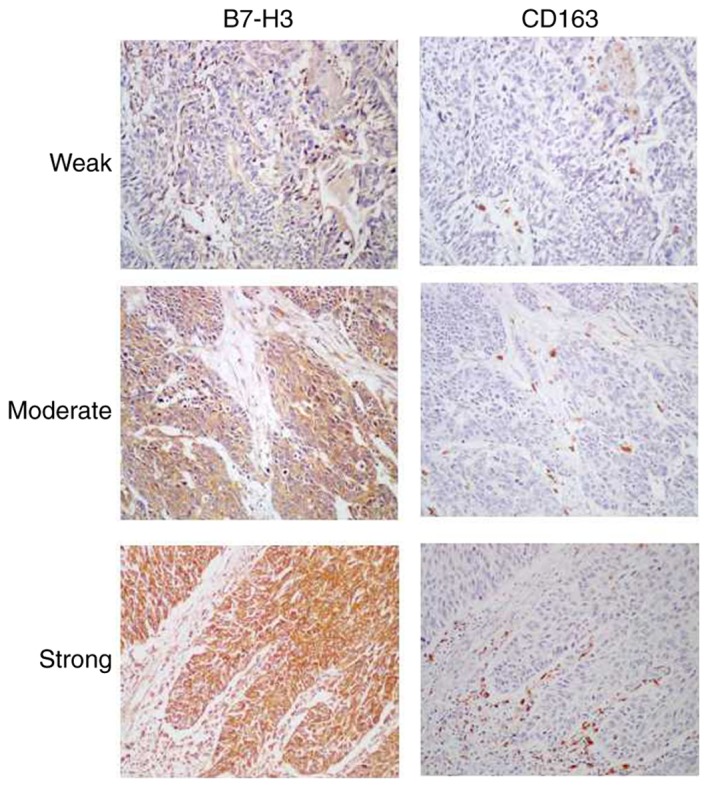
Images of B7-H3 expression and CD163+ tumor-associated macrophage infiltration in urothelial cell carcinoma of the bladder tissues. Weak, moderate, and strong intensity of B7-H3 and CD163 expression are presented (magnification, ×200). Left panels, expression levels of B7-H3 protein. Right panels, expression of CD163 protein in the same samples as the adjacent section. CD163, cluster of differentiation 163.
Table I.
Association between B7-H3 or CD163+ TAMs and clinicopathological features in 134 patients with UCB.
| B7-H3 expression | CD163+ TAM infiltration | ||||||
|---|---|---|---|---|---|---|---|
| Feature | Patients, n% | Low, n (%) | High, n (%) | P-value | Low, n (%) | High, n (%) | P-value |
| Age, years | 0.067 | 0.634 | |||||
| <65 | 53 (39.6) | 21 (51.2) | 32 (34.4) | 31 (41.3) | 22 (37.3) | ||
| ≥65 | 81 (60.4) | 20 (48.8) | 61 (65.6) | 44 (58.7) | 37 (62.7) | ||
| Gender | 0.330 | 0.855 | |||||
| Male | 115 (85.8) | 37 (90.2) | 78 (83.9) | 64 (85.3) | 51 (86.4) | ||
| Female | 19 (14.2) | 4 (9.8) | 15 (16.1) | 11 (14.7) | 8 (13.6) | ||
| Tumor stage | 0.202 | 0.220 | |||||
| Ta/CIS/T1 | 19 (14.1) | 8 (19.5) | 11 (11.8) | 12 (16.0) | 7 (11.9) | ||
| T2 | 58 (43.3) | 21 (51.2) | 37 (39.8) | 36 (48.0) | 22 (37.3) | ||
| T3 | 40 (29.9) | 9 (22.0) | 31 (33.3) | 21 (28.0) | 19 (32.2) | ||
| T4 | 17 (12.7) | 3 (7.3) | 14 (15.1) | 6 (8.0) | 11 (18.6) | ||
| Tumor grade | 0.201 | 0.177 | |||||
| Low | 27 (20.1) | 11 (26.8) | 16 (17.2) | 12 (16.0) | 15 (25.4) | ||
| High | 107 (79.9) | 30 (73.2) | 77 (82.8) | 63 (84.0) | 44 (74.6) | ||
| Lymph node metastasis | 0.185 | 0.364 | |||||
| Yes | 40 (29.9) | 9 (22.0) | 31 (33.3) | 20 (26.7) | 20 (33.9) | ||
| No | 94 (70.1) | 32 (78.0) | 62 (66.7) | 55 (73.3) | 39 (66.1) | ||
| Recurrence | 0.306 | 0.192 | |||||
| Yes | 71 (53.0) | 19 (46.3) | 52 (55.9) | 36 (48.0) | 35 (59.3) | ||
| No | 63 (47.0) | 22 (53.7) | 41 (44.1) | 39 (52.0) | 24 (40.7) | ||
| Distant metastasis | 0.015 | 0.038 | |||||
| Yes | 57 (42.5) | 11 (26.8) | 46 (49.5) | 26 (34.7) | 31 (52.5) | ||
| No | 77 (57.5) | 30 (73.2) | 47 (50.5) | 49 (65.3) | 28 (47.5) | ||
| Vascular invasion | 0.009 | 0.012 | |||||
| Yes | 84 (62.7) | 19 (46.3) | 65 (69.9) | 40 (53.3) | 44 (74.6) | ||
| No | 50 (37.3) | 22 (53.7) | 28 (30.1) | 35 (46.7) | 15 (25.4) | ||
CD, cluster of differentiation; TAM, tumor-associated macrophage.
Representative images of weak, moderate and strong expression CD163+ TAM infiltrations are presented in Fig. 1. Low CD163+ TAM infiltration was identified in 75/134 cases (56.0%), and high infiltration was observed in 59/134 cases (44.0%) (Table I). High CD163+ TAM infiltration was significantly associated with increased vascular invasion (74.6 vs. 53.3%, P=0.012) and distant metastasis (52.5 vs. 34.7%, P=0.038) compared with low CD163+ TAM infiltration. No significant associations were identified between CD163+ TAM infiltration and gender, age, tumor grade, tumor stage, recurrence and lymph node metastasis (Table I).
Correlation between B7-H3 expression and CD163+ TAM infiltration in UCB patients
Among the 75 UCB patients exhibiting a low density of CD163+ TAM infiltration, 35 (46.7%) exhibited a low level of B7-H3 expression. Of the 59 patients with a high density of CD163+ TAM infiltration, 53 cases (89.8%) also exhibited a high level of B7-H3 expression. The results of Spearman's rank correlation analysis indicated that CD163+ TAM infiltration and B7-H3 expression were positively correlated (rs=0.393, P<0.001, Table II).
Table II.
Correlation between B7-H3 expression and CD163+ TAM infiltration in UCB patients.
| B7-H3 expression | |||||
|---|---|---|---|---|---|
| CD163+ TAM infiltration | Patients, n | Low, n 41 | High, n 93 | χ2 | P-value |
| Low | 75 | 35 | 40 | 20.714 | <0.001 |
| High | 59 | 6 | 53 | ||
CD, cluster of differentiation; Tam, tumor-associated macrophage.
Association between B7-H3 expression or CD163+ TAM infiltration and clinical outcomes in patients with UCB
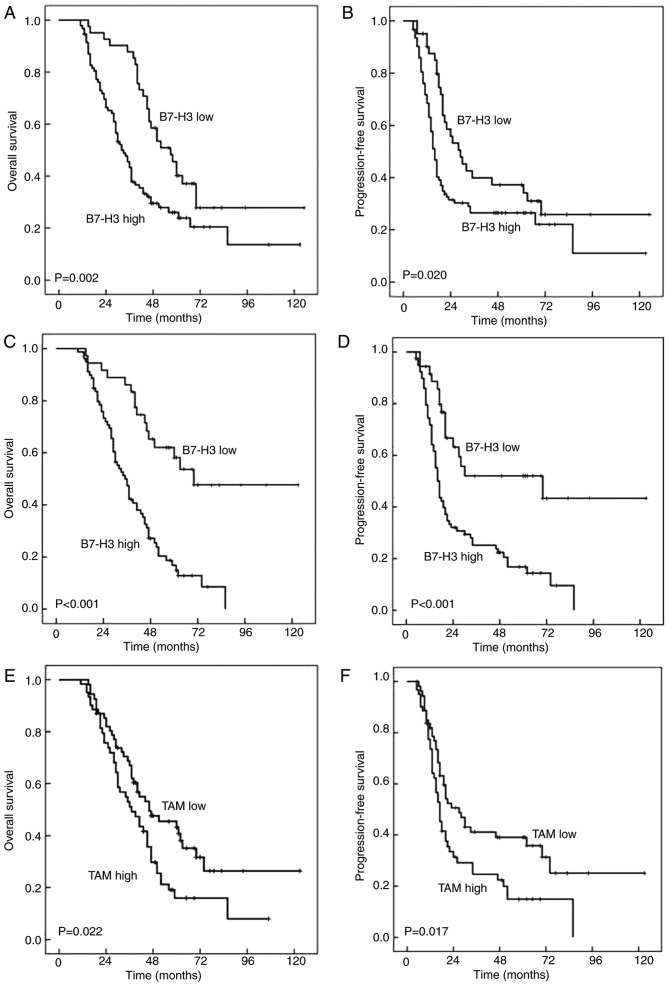
Kaplan-Meier survival analyses revealed that the estimated cancer overall survival (OS) rates at 1, 5 and 10 years following RC for patients with high B7-H3 expression were 96.8±1.8, 23.9±4.9 and 13.6±6.6%, respectively, compared with 97.6±2.4, 40.2±7.8 and 27.8±8.2% for patients with low B7-H3 expression, respectively. The estimated progression free survival (PFS) rates at 1, 5 and 10 years following RC for patients with high B7-H3 expression were 68.5±4.8, 22.1±5.6 and 11.0±8.3%, respectively, compared with 90.1±4.7, 34.1±7.8 and 25.9±.0% for patients with low B7-H3 expression respectively. Thus, high B7-H3 expression was significantly associated with decreased OS (P=0.002) and PFS (P=0.020) in 134 patients with UCB (Fig. 2A and B). However, in this cohort of 134 patients, the density of CD163+ TAM infiltration was irrelevant to the OS (P=0.074) and PFS (P=0.090) (Table III). Notably, the OS and PFS rates in patients with high B7-H3 expression or high CD163+ TAM infiltration were significantly poorer than those in patients with low B7-H3 expression (OS, P<0.001; PFS, P<0.001; Table III; Fig. 2C and D) or low CD163+ TAM infiltration (OS, P=0.022; PFS, P=0.017; Table III; Fig. 2E and F) in the subgroup of 115 UCB patients with muscle-invasive bladder cancer.
Figure 2.
Association of B7-H3 expression and CD163+ TAM infiltration with OS and PFS in patients with UCB. (A) OS rates with B7-H3 expression in cohort of 134 patients. Shorter OS times were observed in patients with high B7-H3 expression compared with low B7-H3 expression (P=0.002). (B) PFS rates with B7-H3 expression in cohort of 134 patients. Shorter PFS times were observed in patients with high B7-H3 expression than that in in low B7-H3 expression (P=0.020). (C) OS rates with B7-H3 expression in the subgroup of patients with muscle-invasive bladder cancer. Shorter OS times were observed in patients with high B7-H3 expression compare with low B7-H3 expression (P<0.001). (D) PFS rates with B7-H3 expression in the subgroup of patients with muscle-invasive bladder cancer. Shorter PFS times were observed in patients with high B7-H3 expression compared with low B7-H3 expression (P<0.001). (E) OS rates with CD163+ TAM infiltration in the subgroup of patients with muscle-invasive bladder cancer. Shorter OS times were observed in patients with high CD163+ TAM infiltration compared with low CD163+ TAM infiltration (P=0.022). (F) PFS rates with CD163+ TAM infiltration in the subgroup of patients with muscle-invasive bladder cancer patients. Shorter OS times were observed in patients with high CD163+ TAM infiltration compared with low CD163+ TAM infiltration (P=0.017). CD163, cluster of differentiation 163; OS, overall survival; PFS, progression-free survival; UCB, urothelial cell carcinoma of the bladder; TAM, tumor-associated macrophages.
Table III.
OS and PFS rates at different levels of B7-H3 expression and CD163+ TAM infiltration in patients with UCB.
| A, All patients (n=134) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| OS, % (±SE) | PFS, % (±SE) | ||||||||
| Feature | n | 1 year | 5 years | 10 years | P-value | 1 year | 5 years | 10 years | P-value |
| B7-H3 expression | 0.002 | 0.020 | |||||||
| High | 93 | 96.8 (1.8) | 23.9 (4.9) | 13.6 (6.6) | 68.5 (4.8) | 22.1 (5.6) | 11.0 (8.3) | ||
| Low | 41 | 97.6 (2.4) | 40.2 (7.8) | 27.8 (8.2) | 90.1 (4.7) | 34.1 (7.8) | 25.9 (8.0) | ||
| CD163+ TAM infiltration | 0.074 | 0.090 | |||||||
| High | 59 | 98.3 (1.7) | 22.0 (5.8) | 16.5 (6.4) | 65.5 (6.2) | 23.7 (5.6) | 15.8 (7.5) | ||
| Low | 75 | 97.3 (1.9) | 35.3 (5.8) | 22.1 (6.1) | 82.6 (4.4) | 32.2 (5.7) | 22.8 (6.2) | ||
| B, Patients with muscle-invasive bladder cancer (n=115) | |||||||||
| OS, % (±SE) | PFS, % (±SE) | ||||||||
| Feature | n | 1 year | 5 years | 10 years | P-value | 1 year | 5 years | 10 years | P-value |
| B7-H3 expression | <0.001 | <0.001 | |||||||
| High | 79 | 98.7 (1.3) | 14.9 (4.5) | 0 (0.0) | 71.8 (5.1) | 16.8 (4.6) | 0 (0.0) | ||
| Low | 36 | 100 (0.0) | 58.1 (8.7) | 47.7 (9.9) | 94.4 (3.8) | 52.0 (9.2) | 43.4 (11.0) | ||
| CD163+ TAM infiltration | 0.022 | 0.017 | |||||||
| High | 54 | 100 (0.0) | 15.9 (5.5) | 8.0 (6.3) | 73.6 (6.1) | 14.9 (5.3) | 0 (0.0) | ||
| Low | 61 | 98.4 (1.6) | 43.2 (6.7) | 26.4 (7.6) | 81.9 (4.9) | 35.8 (6.8) | 25.1 (8.1) | ||
OS, overall survival; PFS, progression-free survival; SE, standard error; CD163, cluster of differentiation; TAM, tumor-associated macrophage; UCB, urinary carcinoma of the bladder.
Discussion
In this cohort of 134 patients with UCB, a positive correlation was identified between the level of B7-H3 expression and CD163+ TAM infiltration, which were significantly associated with vascular invasion, distant metastasis and poor outcomes in the patients undergoing RC for UCB, indicating that B7-H3 may be involved in the progression of UCB with CD163+ TAM infiltration, and that B7-H3 may be a novel immunotherapeutic target for patients with UCB.
B7-H3, a member of the B7 family, has been demonstrated to mediate the proliferation of CD4+ and CD8+ T cells, and to enhance interferon-γ (IFN-γ) production (8). B7-H3 may confer protection from natural killer (NK)-cell-mediated cytolysis (22). However, other studies have indicated that B7-H3 is able to inhibit the proliferation of T cells and reduce the secretion of IFN-γ and interleukin-2 (IL-2) (23,24). Although there is no consensus on the immunological or pathophysiological functions of B7-H3, aberrant B7-H3 expression has been demonstrated to be associated with tumor progression and poor prognosis in human urological neoplasms (13,14). The present study demonstrated that patients with UCB displaying high B7-H3 expression were more likely to exhibit vascular invasion and distant organ metastasis, and experience shorter OS and PFS times than patients displaying low B7-H3 expression. These findings differ from those obtained by Boorjian et al (25), who identified no association between B7-H3 expression and disease progression following cystectomy. These differences may be partly due to the higher proportion of muscle-invasive bladder cancer in the present cohort (85.8%) than in the Boorjian et al cohort (65.2%), thus the risk of postoperative tumor metastasis and mortality would be potentially increased in the present cohort of patients with UCB. Subsequently, survival analyses were conducted in the subgroup of muscle-invasive bladder cancer and it was identified that the OS and PFS rates for patients with high B7-H3 expression were significantly poorer than those for patients with low B7-H3 expression. B7-H3 may serve a pro-tumor function in patients with UCB and could be regarded as an independent indicator for malignant progression of UCB, particularly in patients with muscle-invasive bladder cancer, which, to the best of our knowledge, has not yet been reported. Additionally, B7-H3 expression was not associated with pathological features such as tumor grade, stage and lymph node metastasis in the present study, which is inconsistent with the findings of a previous study (26).
Numerous growth factors, cytokines, chemokines and enzymes released by TAMs promote tumor progression and poor survival rates (16,27,28). CD163+ TAM infiltration was correlated with high-stage and –grade disease, and associated with the formation of micro vessels, pathological outcome, tumor grade and invasiveness of UCB (3,6,21). Furthermore, the expression of CD163 was markedly associated with local expression of IL-6 and IL-10, which function as mediators to induce CD163 expression in vitro (21). The association between M2 TAM infiltration and histologically advanced UCB was confirmed in 46 patients (21). In the present study, 134 patients were treated with RC for UCB, of which 115 patients had muscle-invasive UCB. The levels of vascular invasion and distant organ metastasis were increased significantly in the subgroup exhibiting high CD163+ TAM infiltration compared with the patients with low CD163+ TAM infiltration, whereas no association was observed between the two subgroups for OS rate, PFS rate and other clinicopathological features, including gender, tumor grade, stage and lymph node metastasis. However, in the subgroup of 115 patients with muscle-invasive bladder cancer, it was identified that the OS and PFS rates in patients with high CD163+ TAM infiltration were significantly poorer than inpatients with low infiltration. These findings reveal that CD163+ TAM infiltration may be useful for prognostic prediction of survival in patients with UCB, indicating a possible pro-tumor function for CD163+ TAM infiltration in the malignant progression of UCB, which has not been accurately described in a large cohort with a high proportion of patients with muscle-invasive bladder cancer.
The rates of vascular invasion and distant metastasis were significantly elevated in the subgroup of patients with high B7-H3 expression or high CD163+ TAM infiltration. The results of χ2 test analysis revealed that there was a positive correlation between CD163+ TAM infiltration and B7-H3 expression, indicating that B7-H3 and TAMs may be involved in the initiation and malignant progression of UCB and jointly exhibit immunosuppressive effects on the tumor microenvironment. Pro-tumor TAM polarization facilitated by over-expression of B7-H3 expression maybe involved in the B7-H3/signal transducer and activation of transcription 3 signaling pathway (29).
As a novel co-regulatory molecule, the immunological function of B7-H3 remains controversial, which may reflect the potential presence of two receptors for B7-H3 with opposing co-regulatory functions on the proliferation of CD4+ T cells and induction of cytotoxic T cells (8,24). B7-H3 and TAMs in UCB may be involved in the adaptive immune response to tumors mediated by CD4+ T cells, particularly T helper cells, in addition to their potential interaction with other tumor-infiltrating immune cells, such as regulatory T cells, CTLs and NK cells. Additionally, it is hypothesized that the aberrant expression of B7-H3 or CD163+ TAM infiltration resulting from the complicated UCB tumor microenvironment may attract peripheral circulating monocytes to UCB tissues, priming them to induce the polarization of macrophages to pro-tumor TAMs (6–15,22–29). These pro-tumor TAMs are characterized by production of epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs) and high levels of IL-10, IL-17 and transforming growth factor-β (TGF-β) (18,30). These cytokines markedly promote angiogenesis, stromal invasion and metastasis, and suppress adaptive antitumor immune responses (18,30). Therefore, it is hypothesized that B7-H3 expression and CD163+ TAM infiltration maybe effective predictors of tumor progression and prognosis, particularly in patients with muscle-invasive bladder cancer.
The present study represents a valuable evaluation of the prognostic significance of B7-H3 and CD163+ TAM infiltration in UCB; however, the present study is not devoid of limitations. Although the identification of CD163+ TAM infiltration has been demonstrated to be associated with a poor prognosis in several types of cancer, including UCB (30–32), CD163 is also expressed by a significant portion of the malignant cells in tumors and lymph nodes (7,21,32,33). Additionally, TAMs were not mechanically divided into M1-type and M2-type phenotype macrophages, as was performed in previous studies (2–7,14–18). Therefore, continued study of the effects of B7-H3 and CD163+ TAM infiltration with other macrophage phenotypes in the progression of UCB are required in larger series and different cohorts.
To conclude, the expression levels of the co-stimulatory molecule B7-H3 and CD163+ TAM infiltration were investigated in tumor specimens following RC for UCB. Patients with high B7-H3 expression and high CD163+ TAM infiltration exhibited malignant progression and poor survival rates, particularly those with muscle-invasive bladder cancer. It was also identified that B7-H3 expression level maybe used as a valuable prognostic indicator following RC for UCB. B7-H3 and CD163+ TAM infiltration may function as prognostic indicators and, with additional study, may be a novel immunotherapeutic target for patients with UCB.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
ZX and BS conceived and designed the experiments. ZX, LW, JT, HM and PL performed the experiments. LW, JT, HM and PL analyzed the data. ZX, LW, JT, HM, PL and BS contributed reagents, materials and analytical tools. ZX, LW and BS wrote the paper.
Ethics approval and consent to participate
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The use of human samples and the research involving human participants in this study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (Hebei, China). All participants provided written informed consent prior to participation in the present study.
Consent for publication
The patients, or legal guardians, have provided written informed consent for the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 3.Pichler R, Fritz J, Zavadil C, Schäfer G, Culig Z, Brunner A. Tumor-infiltrating immune cell subpopulations influence the oncologic outcome after intravesical bacillus calmette-guérin therapy in bladder cancer. Oncotarget. 2016;7:39916–39930. doi: 10.18632/oncotarget.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang F, Linlin M, Ye T, Yuhai Z. Alterations of dendritic cell subsets and TH1/TH2 cytokines in the peripheral circulation of patients with superfcial transitional cell carcinoma of the bladder. J Clin Lab Anal. 2012;26:365–371. doi: 10.1002/jcla.21532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Aal AA, Emran AM, Al-Antably AS, El Saftawy EA, Bayoumy IR, Hassan NS, Badawi M. Immunohistochemical pattern of T lymphocytes population within bilharzial-associated bladder neoplasm microenvironment. Int J Immunopathol Pharmacol. 2015;28:209–217. doi: 10.1177/0394632015584733. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi H, Tanaka M, Tanaka A, Tsunemi A, Yamamoto H. Predominance of M2-polarized macrophages in bladder cancer affects angiogenesis, tumor grade and invasiveness. Oncol Lett. 2016;11:3403–3408. doi: 10.3892/ol.2016.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TR, Reedquist KA, Tak PP, Baeten DL. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2012;375:196–206. doi: 10.1016/j.jim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L. B7-H3: A costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 9.Hofmeyer KA, Ray A, Zang X. The contrasting role of B7-H3. Proc Natl Acad Sci USA. 2008;105:10277–10278. doi: 10.1073/pnas.0805458105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu KF, Zhao JM, Zhang GB, Zhang XG. Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J Gastroenterol. 2006;12:457–459. doi: 10.3748/wjg.v12.i3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamato I, Sho M, Nomi T, Akahori T, Shimada K, Hotta K, Kanehiro H, Konishi N, Yagita H, Nakajima Y. Clinical importance of B7-H3 expression in human pancreatic cancer. Br J Cancer. 2009;101:1709–1716. doi: 10.1038/sj.bjc.6605375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, Wang X. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53:143–151. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Crispen PL, Sheinin Y, Roth TJ, Lohse CM, Kuntz SM, Frigola X, Thompson RH, Boorjian SA, Dong H, Leibovich BC, et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14:5150–5157. doi: 10.1158/1078-0432.CCR-08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, Krambeck AE, McKenney ME, Karnes RJ, Blute ML, et al. B7-H3 ligand expression by prostate cancer: A novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67:7893–7900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 15.Ingebrigtsen VA, Boye K, Tekle C, Nesland JM, Flatmark K, Fodstad O. B7-H3 expression in colorectal cancer: Nuclear localization strongly predicts poor outcome in colon cancer. Int J Cancer. 2012;131:2528–2536. doi: 10.1002/ijc.27566. [DOI] [PubMed] [Google Scholar]
- 16.Suriano F, Santini D, Perrone G, Amato M, Vincenzi B, Tonini G, Muda A, Boggia S, Buscarini M, Pantano F. Tumor associated macrophages polarization dictates the efficacy of BCG instillation in non-muscle invasive urothelial bladder cancer. J Exp Clin Cancer Res. 2013;32:87. doi: 10.1186/1756-9966-32-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukhtar RA, Nseyo O, Campbell MJ, Esserman LJ. Tumor-associated macrophages in breast cancer as potential biomarkers for new treatments and diagnostics. Expert Rev Mol Diagn. 2011;11:91–100. doi: 10.1586/erm.10.97. [DOI] [PubMed] [Google Scholar]
- 18.Ohtaki Y, Ishii G, Nagai K, Ashimine S, Kuwata T, Hishida T, Nishimura M, Yoshida J, Takeyoshi I, Ochiai A. Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J Thorac Oncol. 2010;5:1507–1515. doi: 10.1097/JTO.0b013e3181eba692. [DOI] [PubMed] [Google Scholar]
- 19.Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, Sakoda M, Ueno S, Natsugoe S, Takao S. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res. 2011;167:e211–e219. doi: 10.1016/j.jss.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Maeda N, Yoshimura K, Yamamoto S, Kuramasu A, Inoue M, Suzuki N, Watanabe Y, Maeda Y, Kamei R, Tsunedomi R, et al. Expression of B7-H3, a potential factor of tumor immune evasion in combination with the number of regulatory T cells, affects against recurrence-free survival in breast cancer patients. Ann Surg Oncol. 2014;21(Suppl 4):S546–S554. doi: 10.1245/s10434-014-3564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maniecki MB, Etzerodt A, Ulhøi BP, Steiniche T, Borre M, Dyrskjøt L, Orntoft TF, Moestrup SK, Møller HJ. Tumor-promoting macrophages induce the expression of the macrophage-specific receptor CD163 in malignant cells. Int J Cancer. 2012;131:2320–2331. doi: 10.1002/ijc.27506. [DOI] [PubMed] [Google Scholar]
- 22.Castriconi R, Dondero A, Augugliaro R, Cantoni C, Carnemolla B, Sementa AR, Negri F, Conte R, Corrias MV, Moretta L, et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci USA. 2004;101:12640–12645. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling V, Wu PW, Spaulding V, Kieleczawa J, Luxenberg D, Carreno BM, Collins M. Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: Divergent history of functional redundancy and exon loss. Genomics. 2003;82:365–377. doi: 10.1016/S0888-7543(03)00126-5. [DOI] [PubMed] [Google Scholar]
- 24.Nygren MK, Tekle C, Ingebrigtsen VA, Fodstad O. B7-H3 and its relevance in cancer, immunological and non-immunological perspectives. Front Biosci (Elite Ed) 2011;3:989–993. doi: 10.2741/e304. [DOI] [PubMed] [Google Scholar]
- 25.Boorjian SA, Sheinin Y, Crispen PL, Farmer SA, Lohse CM, Kuntz SM, Leibovich BC, Kwon ED, Frank I. T-cell coregulatory molecule expression in urothelial cell carcinoma: Clinicopathologic correlations and association with survival. Clin Cancer Res. 2008;14:4800–4808. doi: 10.1158/1078-0432.CCR-08-0731. [DOI] [PubMed] [Google Scholar]
- 26.Xylinas E, Robinson BD, Kluth LA, Volkmer BG, Hautmann R, Küfer R, Zerbib M, Kwon E, Thompson RH, Boorjian SA, et al. Association of T-cell coregula tory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Eur J Surg Oncol. 2014;40:121–127. doi: 10.1016/j.ejso.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 27.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor micro environments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 29.Kang FB, Wang L, Li D, Zhang YG, Sun DX. Hepatocellular carcinomas promote tumor-associated macrophage M2-polarization via increased B7-H3 expression. Oncol Rep. 2015;33:274–282. doi: 10.3892/or.2014.3587. [DOI] [PubMed] [Google Scholar]
- 30.Medrek C, Pontén F, Jirström K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic signifcance of tumor-associated macrophages in solid tumor: A meta-analysis of the literature. PLoS One. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sjödahl G, Lövgren K, Lauss M, Chebil G, Patschan O, Gudjonsson S, Månsson W, Fernö M, Leandersson K, Lindgren D, et al. Infiltration of CD3+ and CD68+ cells in bladder cancer is subtype specific and affects the outcome of patients with muscle-invasive tumors. Urol Oncol. 2014;32:791–797. doi: 10.1016/j.urolonc.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Lima L, Oliveira D, Tavares A, Amaro T, Cruz R, Oliveira MJ, Ferreira JA, Santos L. The predominance of M2-polarized macrophages in the stroma of low-hypoxic bladder tumors is associated with BCG immunotherapy failure. Urol Oncol. 2014;32:449–457. doi: 10.1016/j.urolonc.2013.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.