Abstract
The laboratory zebrafish (Danio rerio) is now an accepted model in toxicologic research. The zebrafish model fills a niche between in vitro models and mammalian biomedical models. The developmental characteristics of the small fish are strategically being used by scientists to study topics ranging from high-throughput toxicity screens to toxicity in multi- and transgenerational studies. High-throughput technology has increased the utility of zebrafish embryonic toxicity assays in screening of chemicals and drugs for toxicity or effect. Additionally, advances in behavioral characterization and experimental methodology allow for observation of recognizable phenotypic changes after xenobiotic exposure. Future directions in zebrafish research are predicted to take advantage of CRISPR-Cas9 genome editing methods in creating models of disease and interrogating mechanisms of action with fluorescent reporters or tagged proteins. Zebrafish can also model developmental origins of health and disease and multi- and transgenerational toxicity. The zebrafish has many advantages as a toxicologic model and new methodologies and areas of study continue to expand the usefulness and application of the zebrafish.
Keywords: zebrafish, toxicology, behavior, developmental origins of health and disease, epigenetics, CRISPR, transgenerational
The zebrafish (Danio rerio) is now a well-recognized biological model system for toxicology research and can be used to study and model toxicity from molecular initiating events to alterations in organismal health and behavior (Figure 1). The small, freshwater cyprinid fills a scientific niche between in vitro models and higher organisms. Zebrafish can be used for high-throughput chemical toxicity testing, allowing for quick, large scale screening similar to in vitro assays. Yet zebrafish are complex organisms with highly conserved organ systems and metabolic pathways that enable the evaluation of the toxicokinetics and toxicodynamics of xenobiotics similar to mammalian models. As a model organism, zebrafish are small, economical, and have easy husbandry; however, the strength of the model lies with certain features of zebrafish development that are exploited in toxicity testing (Bailey et al., 2013). Zebrafish embryos develop ex vivo, allowing for easy xenobiotic exposure; develop rapidly, with all major body systems formed by 72 h postfertilization (hpf); and are optically translucent in early stages of development. In addition, to the well described development (Kimmel et al., 1995), zebrafish have a sequenced genome (Howe et al., 2013) and are suitable to genetic manipulation (Varshney et al., 2015) and “–omics” level evaluations (Horzmann and Freeman, 2017).
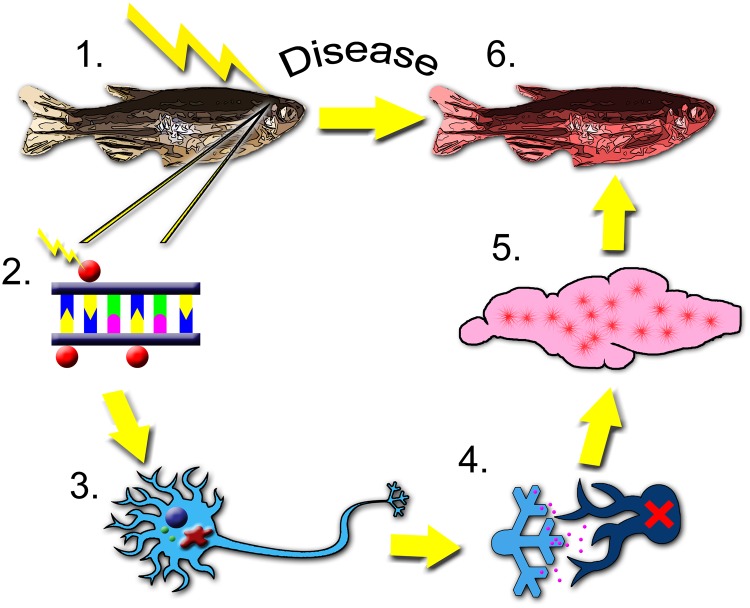
Figure 1.
Zebrafish can be used to define and connect changes along the entire spectrum of toxicity research. Zebrafish can be used to model toxicity from the initial intoxication (1), through the molecular initiating event (2), and changes at the cellular (3), tissue (4), and organ (5) levels. Finally, changes in phenotype (6), including alterations in growth and development or behavior and disease outcomes can be observed. In this example, the methylation status of a gene is altered by xenobiotic exposure (2), resulting in altered gene transcription (3), altered neurotransmission (4), and brain dysfunction (5).
Although zebrafish are also used for assaying organ system toxicology (Goessling and Sadler, 2015), investigating mechanisms of action (Peterson and Macrae, 2012), and evaluating ecotoxicity and environmental toxicants (Bambino and Chu, 2017), in recent years, zebrafish have become a workhorse model in chemical toxicity screening (Rennekamp and Peterson, 2015), drug development (Gibert et al., 2013), and developmental neurotoxicity (Bailey et al., 2013; Nishimura et al., 2015). The zebrafish model performs comparably to mammalian models in developmental toxicity assays. As reviewed by Sipes et al. (2011), the concordance between zebrafish and mammalian models in evaluating chemicals for developmental toxicity is 55%–100% (Brannen et al., 2010; Padilla et al., 2011; Selderslaghs et al., 2009). However, in screens of known fetotoxic substances, assays with rats identified 79%, rabbits 75%, and mice 75% of chemicals, though the concordance between the rats, rabbits, and mice is only 56% (Hurtt et al., 2003). The percent concordance across the mammalian species suggests that the response of zebrafish is on par with mammalian models of toxicity and supports the utility of the zebrafish model in toxicology research.
One of the most highly read and cited articles published in Toxicological Sciences reviews zebrafish as model of chemical toxicity (Hill et al., 2005). In their review, Hill et al. predicted that high-throughput screening, genome editing, and the development of novel bioassays for toxicology testing would enhance the evaluation of mechanisms of chemical toxicity. Technology, molecular techniques, and their application in zebrafish have, of course, advanced in the past decade. Screening tests have increasingly become automated and automation has increased the utility of behavioral endpoints as phenotypic measures of toxicity. In addition, new molecular techniques for genome editing permit creation of specific models of human disease and interrogation of specific molecular pathways for mechanistic studies (Bambino and Chu, 2017; Phillips and Westerfield, 2014). Zebrafish are also being used to study emerging topics in toxicological research such as the developmental origins of health and disease (DOHaD) and the persistence of xenobiotic induced epigenetic alterations through generations. The aim of this review is to introduce readers to a few emerging trends in zebrafish research.
HIGH-THROUGHPUT TECHNOLOGY
The zebrafish developmental toxicity assay has become a mainstay of high-throughput analyses. Generally multiple zebrafish embryos (48–96) are exposed to a range of chemicals or concentrations. Toxicity is usually monitored by evaluating lethality, teratogenicity, or other phenotypic changes. Developmental toxicity zebrafish screens are a component of the United States Environmental Protection Agency’s Toxicology Testing in the 21st Century (Tox21) ToxCast phase I (Padilla et al., 2012) and phase II screens (Truong et al., 2014; Volz et al., 2015). ToxCast aims to use high-throughput screens and computational modeling to rank and prioritize chemicals of interest. The utility of zebrafish in chemical screening is well established, with acute toxicity assays identifying priority chemicals with greater sensitivity than predictive calculations based on chemical physicochemical properties. For example, both the octanol/water partition coefficient values of chemicals and their bioconcentration factor lack significant correlation to acute zebrafish toxicology endpoints such as the lethal concentration for 50% (LC50), but zebrafish LC50 values are significantly correlated with developmental malformations such as skeletal and swim bladder defects and yolk sac edema (Ducharme et al., 2013). Furthermore, the results of the zebrafish ToxCast screens show good correlations to mammalian toxicity assays, with zebrafish LC50 values for acute toxicity correlating with rat LC50 inhalation values, and zebrafish Lowest Observed Adverse Developmental Effect Dose values correlating to the results of rabbit dermal and rat oral exposures (Ducharme et al., 2015). In a separate screen of ToxCast chemicals, zebrafish developmental toxicity had a high concordance with rodent reproductive studies with liver and kidney pathology and a high concordance with developmental rat or rabbit maternal related toxicity (Truong et al., 2014). 75% of the chemicals identified by Truong et al. (2014) were also identified by Padilla et al.(2012) as having toxicity. It should also be noted that zebrafish are also being utilized in high-throughput screening for drug discovery (reviewed by Wiley et al. (2017)), both for the discovery of new compounds and for the identification of new, unrecognized targets (reviewed by MacRae and Peterson, 2015).
The applications of technology in zebrafish high-throughput screening are advancing both the number and nature of endpoints and the scale of testing. Robotic systems are being used for xenobiotic dosing of the multiwell plates in developmental zebrafish toxicity assays and advances in digital dispensing of xenobiotic through ink-jet printing technology has the potential to increase the quality and repeatability of data while decreasing error (Truong et al., 2016). Limitations of high-throughput screening include the study to study variation in identification and ranking of chemical toxicity, a lack of overlapping data between species that can be used for comparison, and the fact that the methods still need refining to increase through-put and reduce embryo handing. Manual evaluation of phenotype or morphologic features can be time and labor intensive, and evaluation of earlier endpoints may allow for easier screening, as 72 hpf embryo survival predicts chemical scoring at 96 or 144 hpf (Volz et al., 2015). Alternatively, integrated systems, such as the VAST BioImager, have the potential to increase endpoints targeted for high-throughput screening by streamlining morphologic evaluation with fluorescent imaging of reporters or other fluorescently labeled proteins of interest (Pulak, 2016). Previously, multiple targets, such as different fluorescent reporters or physical measurements, were limiting on a large scale.
BEHAVIOR
Zebrafish behavior is increasingly being incorporated into toxicological testing as a measurable phenotype reflecting alterations in normal physiology. Behavioral tests for both larval and adult zebrafish take advantage of well-characterized behavioral responses (Kalueff, 2017; Kalueff et al., 2013). The assays are largely modified from rodent behavioral methods and test conserved behavioral endpoints, such as thigmotaxis (wall hugging), scototaxis (light/dark preference), geotaxis (diving preference), exploration, habituation, and stress- and anxiety-related parameters (reviewed by Ahmad et al., 2012). Deviations from normal behavior can highlight possible functional outcomes of chemical toxicity, provide information on drug efficacy, or suggest mechanisms of action or including the direct disruption of neurotransmission or other neural elements, indirection alterations in intracellular signaling, disruption of the musculoskeletal system, and altered growth and development (Reif et al., 2016).
Larval Behavior
Larval zebrafish behavior is well characterized and is often incorporated into toxicological assays. The utility of using larval zebrafish behavior for toxicity assessment was validated by exposing larvae to compounds with known mechanisms of action and known behavioral outcomes in rodents. For example, zebrafish larvae exposed to the drug valproate experienced hyperactivity similar as to what has been described in rodent models and humans (MacPhail et al., 2011). Endpoints such distance moved, velocity, time moving, angular velocity, and turn angle can be used to link xenobiotic exposure to behavior outcomes which could suggest developmental neurotoxicity or other adverse effects. Commercial zebrafish behavioral systems have allowed for easier, high-throughput analysis of larval behavior through the integration of infrared cameras with contained testing arenas, programmable stimuli control, and analysis software.
Two of the most common assays for high-throughput analysis are an embryonic photomotor response (PMR) and a larval locomotor assay called either a visual motor response (VMR) or a larval PMR test. Zebrafish have rhythmic tail coiling movements starting at 17–21 hpf. Exposure to a light source around 24–30 hpf will cause a reflex increase in activity level, called the PMR. The reflex is mediated through nonvisual, hindbrain pathways (Kokel et al., 2013) and provides an early testable phenotype in developmental toxicity assays. In a screen of ToxCast chemicals, hypoactivity or hyperactivity during the 24 hpf PMR is associated with an increased relative risk for developmental malformations at 120 hpf (Reif et al., 2016). Interestingly in the same study, behavioral alterations could be observed for some chemicals, such as abamectin, milbemectin, and emamectin benzoate, at lower chemical concentrations than what causes morphological alterations (Reif et al., 2016). Larval zebrafish at approximately 5 days postfertilization have robust locomotor responses to changes in light based through a visually mediated reflex (VMR). Larval zebrafish tend to have increased activity in light compared with dark, but exhibit paradoxical increased behavior in dark after a sudden change from light to dark (MacPhail et al., 2009). VMR endpoints, such as duration of movement, distance moved, and velocity, if altered by chemical exposure, can provide support for toxicity and can help establish pathways effected or mechanisms of action. For example, the VMR has been used to evaluate the irritant effect of particulate matter (PM) on larval zebrafish behavior and investigate possible mechanisms. Stevens et al. (2018) evaluated the effects of acrolein and compressor-generated diesel exhaust PM on the locomotor behavior of zebrafish and found that inhibition of the transient receptor potential cation channel blocked the response to acrolein and exhaust PM. Although the PMR and VMR tests provide a phenotypic outcome of toxicity, limitations of both tests are a general lack of standardization in methods across laboratories, which is concern for reproducibility of results, and the challenge of tying behavioral data to molecular initiating events.
Adult Behavior
Adult behavioral assays are emerging as tools to evaluate changes in activity level, anxiety, learning, and other neurobehavioral outcomes after xenobiotic exposure. Adult zebrafish can be used to model complex behaviors related to stress and anxiety, learning, and social interactions, such as shoaling or aggression. Methods of monitoring adult behavior are becoming more sophisticated. Video tracking software can routinely calculate parameters such as total distance moved, velocity, turn angle, and angular velocity of single zebrafish. The basic technology has expanded to include the monitoring of multiple fish in a single area (Green et al., 2012) and 3D methods that allow for more accurate tracking and a greater range of observable endpoints from a single test (Stewart et al., 2015).
Although many behaviors can be altered by chemical or drug exposure, tests that measure parameters associated with stress and anxiety are often used in studies of neurotoxicity to determine functional outcomes of xenobiotic exposure. Three common tests are the novel tank test (NTT), the light-dark box (LDB), and the open field test (OFT) (Figure 2). Other tests modified from rodents, such as T-mazes and opioid self-administration assay (Bosse and Peterson, 2017) can provide measures of learning and cognition or addictive behavior respectively. For example, Gao et al. (2017) evaluated the effect of early life exposure to benzo[a]pyrene on adult behavioral performance on a NTT and a T-maze and was able to link a phenotype of neurodegeneration to changes in DNA methyltransferase expression, decreased neurotransmitter levels, and decreased dopaminergic neurons in brain sections. Although adult zebrafish behavior testing is well described, factors such as biological replicate, test day, and experimental protocols can have a strong effect on behavioral results (Kalueff et al., 2016), highlighting the need for reporting and standardization of information, such as acclimation period, to help improve reproducibility of results (Melvin et al., 2017).
Figure 2.
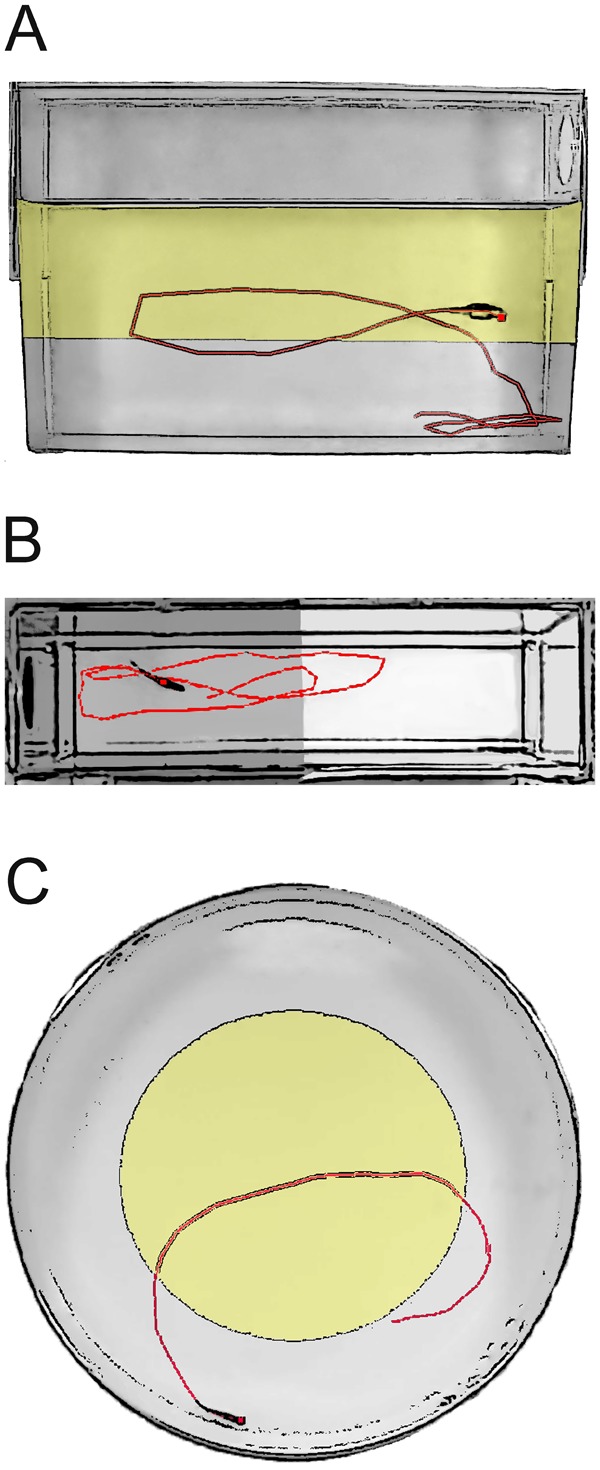
Adult zebrafish behavioral assays. Three common adult zebrafish behavioral assays are the NTT, the LDB, and the OFT. The NTT (A) introduces an adult zebrafish to a novel tank and evaluates endpoints such as time spent in upper and lower zone, latency to zone transitions, and number of zone entries, with an anxious phenotype spending more in the bottom zone. The LDB (B) introduces a zebrafish to a tank set up with dark walls on one half of the tank and white walls on the other half and evaluations similar endpoints such as time spent in light and dark zones, number of zone entries, and the latency between zone entries. The OFT (C) is similar to the rodent test and evaluates thigmotaxis (wall hugging) and exploratory behavior in a novel environment, with anxious fish. Time spent in central versus peripheral zones, latency to zone entry, and number of zone entries, as well as zebrafish startle movements, are recorded. Tracks in each example highlight the swimming pattern of the fish (dot) in the test arena.
ZEBRAFISH GENETICS AND GENOME MODULATION
Zebrafish Genetics
Zebrafish are highly amenable to genetic modification and currently over 34 000 transgenic zebrafish lines are listed with the Zebrafish International Resource Center (http://zebrafish.org; last accessed February 22, 2018). The ex vivo development of the zebrafish embryo permits easy manipulation and the transparency of the early embryos enables observation of fluorescent tagged proteins and reporters. Although 1981 marked the first publication on the genetic manipulation of zebrafish (Streisinger et al., 1981), advancing molecular technologies in recent years and a sequenced genome facilitated the ability to target mutations to specific genes within the genome (reviewed by Varshney et al., 2015). With respect to toxicological research, genome editing has the potential to create models of disease, speed screening assays through the evaluation of fluorescent reporters, and easily provide tools to interrogate mechanisms of action.
CRISPR-Cas9 Genome Modification and Applications in Zebrafish
Although chemical mutation, targeted induced local lesion in genomes (TILLING), zinc finger nucleases (ZFNs), and TAL effector nucleases (TALENs) have all been used to manipulate the zebrafish genome, in 2013, clustered, regularly interspaced, short palindromic repeats (CRISPR) and the CRISPR-associated system 9 (Cas9) endonuclease were first used to selectively edit the zebrafish genome (Hwang et al., 2013a,b). The CRISPR-Cas9 system is a precise and flexible tool for genome engineering that is based on a bacterial adaptive immune response (as reviewed by Wright et al., 2016). The advantages of the CRISPR-Cas9 system include easy and flexible design of guide RNA (gRNA) to target a specific gene, low cost, high efficiency, lower off-target effects compared with other technologies, and the ability to target more than 1 gene concurrently through multiplexed systems. However, the CRISPR-Cas9 system does not totally eliminate the potential for off-target effects, and genetic mosaics and multiple mutations may be introduced through non-homologous end joining at alleles of interest.
Hwang et al. (2013b) published the first description of CRISPR-Cas9 system induced site-specific mutations in the zebrafish genome. In the initial work, the frequency of targeted indel mutations was 24.1%–59.4%, with 6%–36% of embryos having biallelic mutations. Since then, the CRISPR-Cas9 system has been used to cause heritable, biallelic mutations with 75%–99% efficiency (Jao et al., 2013), knock-in genes with an indel mutation rate of 22%–67% with 31%–50% heritability (Auer et al., 2014), induce single nucleotide polymorphisms with 46% efficiency and 10% heritability (Irion et al., 2014), knock-in reporters at >25% efficiency (Kimura et al., 2014), integrate in-frame exogenous DNA with 61% efficiency (Hisano et al., 2015), perform reverse genetic screening with 90% coverage (Shah et al., 2015), target mutations to specific tissues (Ablain et al., 2015), and for base editing with 28% site-specific efficiency (Zhang et al., 2017). Although CRISPR-Cas9 appears to be highly efficient and highly specific, with rare off-target effects, one interesting phenomenon is a variability in phenotype between morpholino knockdown and CRISPR-Cas9 knockout techniques. Kok et al. (2015) suggest that the difference in phenotypes is actually a result of off-target effects of the morpholinos, and recommend the use of mutant lines over morpholino technology. However, it is also suspected that in-frame indels result in a mosaic phenotype that may result in a weaker phenotype despite a high number of indel mutations (Jao et al., 2013; Shah et al., 2015). The use of CRISPR-Cas9 systems in zebrafish is further reviewed by Albadri et al. (2017), Gonzales and Yeh (2014), and Li et al. (2016).
CRISPR-Cas9 technology is only now being integrated into the zebrafish toxicology toolbox. Tian et al. (2017) recently described the use of CRISPR-Cas9 to create multiresistance-associated protein (mrp) 1 knock-out zebrafish for use in studying the role of Mrp1 in cadmium chloride and benzo[a]pyrene toxicity. The group was able to determine that Mrp1 has a protective role in cadmium and benzo[a]pyrene toxicity, as increased malformations and delayed hatching were observed in mrp1 depleted embryos. This study highlights the utility of CRISPR-Cas9 systems in elucidating mechanisms of action in toxicological research—namely through the linking of genotype and chemical exposure to an altered phenotype. The identification of genes associated with susceptibility or resistance to toxicants has the potential to better inform molecular mechanisms, and consequently, better translate the potential adverse health risks to humans (Garcia et al., 2016).
EMERGING AREAS IN TOXICOLOGY
Developmental Origins of Health and Disease
The DOHaD hypothesis suggests that developmental exposure to environmental stressors, including toxicants, can result in genetic, epigenetic, or functional changes in tissues that increase disease risk later in life (Heindel et al., 2015). Although the DOHaD hypothesis has expanded to recognize the importance of environmental exposures (Haugen et al., 2015; Heindel et al., 2015), the paradigm is only starting to be integrated into the field of toxicology (Schug et al., 2013).
In addition to their strength in developmental toxicity research, zebrafish are excellent models for studying aging and the life course (reviewed by Sasaki and Kishi, 2013), especially in the context of the DOHaD hypothesis. The ex vivo development allows for precise control of developmental toxicant exposures and, although sexual maturity is not as rapid as in rodent models, zebrafish reach sexual maturity in 3–4 months. Emerging zebrafish research identifying the later in life effects of developmental toxicant exposure have investigated metals, polychlorinated dibenzodioxins, and the herbicide atrazine. Baker et al. (2013) described disruptions in adult skeletal structure and reproduction in zebrafish after sublethal exposure to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) and later identified microscopic and transcriptomic alterations in male testes to help elucidate the mechanism of inheritable TCDD-related infertility (Baker et al., 2016). Developmental exposure to sublethal concentrations of cadmium lengthened the time spent in the bottom during a NTT and altered the activity of antioxidant systems through a suspected alteration in DNA methyltransferase activity (Ruiter et al., 2016). Developmental exposure to atrazine was linked to alterations in male and female gonad and brain transcriptome profiles, changes in female neurotransmitter levels, and decreased spawning in adult zebrafish (Wirbisky et al., 2015, 2016a). The identification of other developmental toxicants that cause later life effects and the elucidation of their mechanisms of action represent a major area for exploration.
Multi- and Transgenerational Studies
An emerging area of toxicology somewhat related to the DOHaD paradigm is multi- and transgenerational toxicity. This field studies the effects of xenobiotic exposure in the descendants of the generation originally exposed. Many of the chemicals implicated in multi- and transgenerational toxicity are not genotoxic and epigenetic alterations are thought to be the main mechanism for inherited toxicity, possibly as a result of changes to DNA methylation in developing germ cells (reviewed by Nilsson and Skinner, 2015). There is significant public health implications for heritable, epigenetic toxicity (reviewed by Marczylo et al., 2016) and zebrafish can model multi- and transgenerational toxicity due to the ease of developmental exposure, similarities in epigenetic regulation and metabolic pathways, and relatively short generational period, with sexual maturity occurring around 3–4 months.
When mapping multi- and transgenerational effects, the original exposure conditions determine the type of exposure to the following generations (Figure 3). Multigenerational toxicology studies are concerned with effects observable in generations originally exposed to the xenobiotic, whether as an adult (F0), as a fetus (F1), or as a primordial germ cell (F2) in the developing fetus with mammalian in utero exposure. In transgenerational studies, the effects in subsequent generations (F3 and beyond in mammals) are of interest. These generations did not have direct exposure to the xenobiotic and adverse effects observed are hypothesized to be due to epigenetic alterations. Rather than dosing pregnant animals, the zebrafish model has external fertilization and development that allow for in vitro exposure of F0 embryos, and because of this, the F2 generation is considered transgenerational in comparison to the F3 generation in mammals. One limitation of the model, though, is the inability to study placental effects on toxicity.
Figure 3.
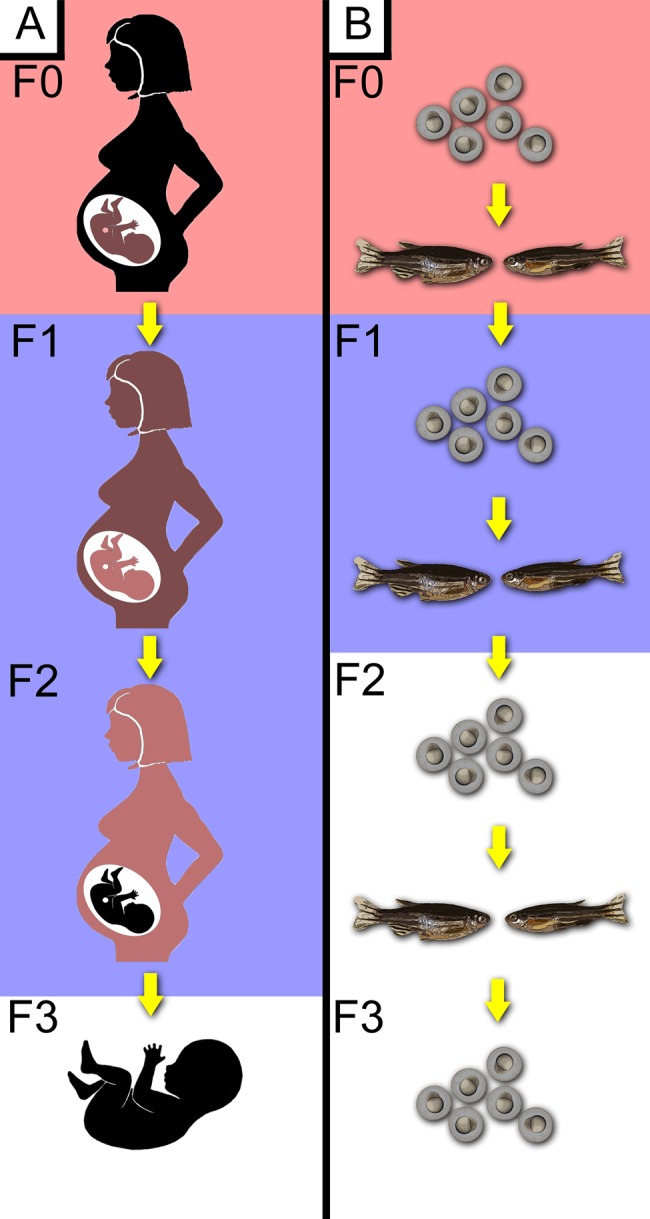
Multi- and transgenerational studies in humans and the zebrafish. Xenobiotic exposure during mammalian pregnancy (A) exposes the mother (F0), the fetus (F1), and the primordial germ cells within the fetus (F2) to potential direct toxic effects. Although the F1 and F2 generations have xenobiotic exposure, the F3 generation is the first generation without exposure, although toxicity may continue through inherited epigenetic toxicity. In zebrafish (B), developmental exposure to a F0 generation also exposes the germ cells, resulting in indirect xenobiotic exposure to the F1 generation. The F2 generation and beyond lack xenobiotic exposure, but may have an altered epigenome. Top blocks represent direct exposure to xenobiotics. The middle blocks represent multigenerational toxicity, where exposure occurred during germ cell stages. The white background represents transgenerational toxicity, where the subjects had no direct xenobiotic exposure and any toxic effects are expected to from epigenetic alterations.
A few studies using zebrafish for multi- and transgenerational toxicity work are published to date. Wirbisky et al. (2016b) observed morphological alterations in F1 larvae after the F0 generation had developmental exposure to atrazine, suggesting that atrazine has multigenerational toxicity. In another multigenerational study, Liu et al. (2016) fed adult female zebrafish a diet derived from wild-caught walleye fish contaminated with methylmercury and found increased malformations, decreased visual responses, and altered gene expression in their offspring. Olsvik et al. (2014) used the zebrafish model system to evaluate transgenerational changes to DNA methylation after TCDD and methylmercury exposure and described only modest effects. Knecht et al. (2017) found that developmental exposure of an F0 zebrafish generation to benzo[a]pyrene caused altered behavior in the F0 and F2 generations and altered physical parameters in both generations. Skeletal and reproductive alterations associated with TCDD exposure were found to persist through the F1 and F2 generations (Baker et al., 2014).
FUTURE DIRECTIONS
The zebrafish is a flexible model that fits between in vitro models and mammalian rodent models of toxicity. The strengths of the zebrafish model assure that developmental toxicity assays will continue to screen for chemical or drug toxicity. Advances in high-throughput technology will increase the scale, repeatability, and endpoints associated with xenobiotic screens. Although larval behavioral assays are already relatively automated, increased utilization and standardization of adult behavioral assays will provide high quality results and demonstrate functional outcomes of xenobiotic exposure. Finally, the incorporation of the DOHaD hypothesis and the recognition of toxicant induced, heritable epigenetic changes offer expanded avenues for toxicological research.
Zebrafish have great potential for mechanistic toxicology and will likely be utilized to a greater extent in the future. The first part of this prediction is based on the foreseeable increase in transgenic models created through CRISPR-Cas9 genome editing systems. Increased genomic editing has the potential to help the zebrafish community provide tools to link the results of screening and behavioral tests to mechanisms of action or molecular initiating events in the adverse outcome pathway framework. The second part of the prediction is based on the recognized need for better information on zebrafish toxicokinetics and the similarities to and differences from human and other mammalian models. Metabolomics (or the comprehensive evaluation of metabolites and other small molecules within a cell, tissue, or organism) offers the ability to evaluate both the amount of toxicant and its metabolite present in tissues and organisms and possible alterations in metabolic function based on altered metabolite flux (Hasin et al., 2017). Recently Kirla et al. (2016) found that characterizing toxicokinetics is important to explain behavioral differences between zebrafish larvae and mammals with cocaine exposure. The waterborne route of exposure, used due to the inherent limitations of the aquatic zebrafish model, caused differences in cocaine uptake as compared with inhalation or intravenous routes studied in mammals. Metabolomics techniques have the ability to expand information about the absorption, distribution, metabolism, and excretion of significant toxicants.
In summary, the zebrafish is a small, but exceedingly robust model organism, providing an economical balance between in vitro assays and more complex, mammalian organisms. The many advantages of the model allow for easy adoption of new techniques, technologies, and fields of study. Although more research is required to better correlate aspects of zebrafish toxicokinetics after xenobiotic exposures, zebrafish hold great potential for models of translational toxicology.
FUNDING
This work was supported by the National Institutes of Health, National Institute of Environmental Health Sciences (R15 ES019137) and the National Institute for Occupational Safety and Health (T42/OH008672).
REFERENCES
- Ablain J., Durand E. M., Yang S., Zhou Y., Zon L. I. (2015). A CRISPR/Cas9 vector system for tissue-specific gene disruption in zebrafish. Dev. Cell 32, 756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad F., Richardson M. K., Noldus L. P. J. J., Tegelenbosch R. A. J. (2012). Zebrafish embryos and larvae in behavioural assays. Behaviour 149, 1241–1281. [Google Scholar]
- Albadri S., Del Bene F., Revenu C. (2017). Genome editing using CRISPR/Cas9-based knock-in approaches in zebrafish. Methods 121–122, 77–85. [DOI] [PubMed] [Google Scholar]
- Auer T. O., Duroure K., De Cian A., Concordet J. P., Del Bene F. (2014). Highly efficient CRISPR/Cas9-mediated knock-in in zebrafish by homology-independent DNA repair. Genome Res. 24, 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J., Oliveri A., Levin E. D. (2013). Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res. C Embryo Today 99, 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. B., Yee J. S., Meyer D. N., Yang D., Baker T. R. (2016). Histological and transcriptomic changes in male zebrafish testes due to early life exposure to low level 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. Zebrafish 13, 413.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. R., Peterson R. E., Heideman W. (2013). Early dioxin exposure causes toxic effects in adult zebrafish. Toxicol. Sci. 135, 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. R., Peterson R. E., Heideman W. (2014). Using zebrafish as a model system for studying the transgenerational effects of dioxin. Toxicol. Sci. 138, 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambino K., Chu J. (2017). Chapter nine - Zebrafish in toxicology and environmental health In Current Topics in Developmental Biology (Sadler K. C., Ed.), Vol. 124, pp. 331–367. Academic Press, Cambridge, MA, USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse G. D., Peterson R. T. (2017). Development of an opioid self-administration assay to study drug seeking in zebrafish. Behav. Brain Res. 335, 158–166. [DOI] [PubMed] [Google Scholar]
- Brannen K. C., Panzica-Kelly J. M., Danberry T. L., Augustine-Rauch K. A. (2010). Development of a zebrafish embryo teratogenicity assay and quantitative prediction model. Birth Defects Res. B Dev. Reprod. Toxicol. 89, 66–77. [DOI] [PubMed] [Google Scholar]
- Ducharme N. A., Peterson L. E., Benfenati E., Reif D., McCollum C. W., Gustafsson J.-Å., Bondesson M. (2013). Meta-analysis of toxicity and teratogenicity of 133 chemicals from zebrafish developmental toxicity studies. Reprod. Toxicol. 41, 98–108. [DOI] [PubMed] [Google Scholar]
- Ducharme N. A., Reif D. M., Gustafsson J.-A., Bondesson M. (2015). Comparison of toxicity values across zebrafish early life stages and mammalian studies: Implications for chemical testing. Reprod. Toxicol. 55, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D., Wang C., Xi Z., Zhou Y., Wang Y., Zuo Z. (2017). Early-life benzo[a]pyrene exposure causes neurodegenerative syndromes in adult zebrafish (Danio rerio) and the mechanism involved. Toxicol. Sci. 157, 74–84. [DOI] [PubMed] [Google Scholar]
- Garcia G. R., Noyes P. D., Tanguay R. L. (2016). Advancements in zebrafish applications for 21st century toxicology. Pharmacol. Ther. 161, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibert Y., Trengove M. C., Ward A. C. (2013). Zebrafish as a genetic model in pre-clinical drug testing and screening. Curr. Med. Chem. 20, 2458–2466. [DOI] [PubMed] [Google Scholar]
- Goessling W., Sadler K. C. (2015). Zebrafish: An important tool for liver disease research. Gastroenterology 149, 1361–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales A. P., Yeh J. R. (2014). Cas9-based genome editing in zebrafish. Methods Enzymol. 546, 377–413. [DOI] [PubMed] [Google Scholar]
- Green J., Collins C., Kyzar E. J., Pham M., Roth A., Gaikwad S., Cachat J., Stewart A. M., Landsman S., Grieco F. et al. , (2012). Automated high-throughput neurophenotyping of zebrafish social behavior. J. Neurosci. Methods 210, 266–271. [DOI] [PubMed] [Google Scholar]
- Hasin Y., Seldin M., Lusis A. (2017). Multi-omics approaches to disease. Genome Biol. 18, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen A. C., Schug T. T., Collman G., Heindel J. J. (2015). Evolution of DOHaD: The impact of environmental health sciences. J. Dev. Origins Health Dis. 6, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel J. J., Balbus J., Birnbaum L., Brune-Drisse M. N., Grandjean P., Gray K., Landrigan P. J., Sly P. D., Suk W., Cory Slechta D. et al. , (2015). Developmental origins of health and disease: Integrating environmental influences. Endocrinology 156, 3416–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. J., Teraoka H., Heideman W., Peterson R. E. (2005). Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 86, 6–19. [DOI] [PubMed] [Google Scholar]
- Hisano Y., Sakuma T., Nakade S., Ohga R., Ota S., Okamoto H., Yamamoto T., Kawahara A. (2015). Precise in-frame integration of exogenous DNA mediated by CRISPR/Cas9 system in zebrafish. Sci. Rep. 5, 8841.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horzmann K. A., Freeman J. L. (2017). Toxicogenomic Evaluation Using the Zebrafish Model System. Encyclopedia of Analytical Chemistry. 1–19. [Google Scholar]
- Howe K., Clark M. D., Torroja C. F., Torrance J., Berthelot C., Muffato M., Collins J. E., Humphray S., McLaren K., Matthews L. et al. , (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtt M. E., Cappon G. D., Browning A. (2003). Proposal for a tiered approach to developmental toxicity testing for veterinary pharmaceutical products for food-producing animals. Food Chem. Toxicol. 41, 611–619. [DOI] [PubMed] [Google Scholar]
- Hwang W. Y., Fu Y., Reyon D., Maeder M. L., Kaini P., Sander J. D., Joung J. K., Peterson R. T., Yeh J.-R. J. (2013a). Heritable and precise zebrafish genome editing using a CRISPR-cas system. PLoS One 8, e68708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W. Y., Fu Y., Reyon D., Maeder M. L., Tsai S. Q., Sander J. D., Peterson R. T., Yeh J. R. J., Joung J. K. (2013b). Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irion U., Krauss J., Nusslein-Volhard C. (2014). Precise and efficient genome editing in zebrafish using the CRISPR/Cas9 system. Development 141, 4827–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao L.-E., Wente S. R., Chen W. (2013). Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. U.S.A. 110, 13904–13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff A. V., ed. (2017). The rights and wrongs of zebrafish-Behavioral phenotyping of zebrafish pp. 1-327. Springer International Publishing, Cham, Switzerland. [Google Scholar]
- Kalueff A. V., Echevarria D. J., Homechaudhuri S., Stewart A. M., Collier A. D., Kaluyeva A. A., Li S., Liu Y., Chen P., Wang J. et al. , (2016). Zebrafish neurobehavioral phenomics for aquatic neuropharmacology and toxicology research. Aquat. Toxicol. 170, 297–309. [DOI] [PubMed] [Google Scholar]
- Kalueff A. V., Gebhardt M., Stewart A. M., Cachat J. M., Brimmer M., Chawla J. S., Craddock C., Kyzar E. J., Roth A., Landsman S. et al. , (2013). Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 10, 70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dynam. 203, 253–310. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Hisano Y., Kawahara A., Higashijima S. (2014). Efficient generation of knock-in transgenic zebrafish carrying reporter/driver genes by CRISPR/Cas9-mediated genome engineering. Sci. Rep. 4, 6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirla K. T., Groh K. J., Steuer A. E., Poetzsch M., Banote R. K., Stadnicka-Michalak J., Eggen R. I. L., Schirmer K., Kraemer T. (2016). From the cover: Zebrafish larvae are insensitive to stimulation by cocaine: Importance of exposure route and toxicokinetics. Toxicol. Sci. 154, 183–193. [DOI] [PubMed] [Google Scholar]
- Knecht A. L., Truong L., Marvel S. W., Reif D. M., Garcia A., Lu C., Simonich M. T., Teeguarden J. G., Tanguay R. L. (2017). Transgenerational inheritance of neurobehavioral and physiological deficits from developmental exposure to benzo[a]pyrene in zebrafish. Toxicol. Appl. Pharmacol. 329, 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok F. O., Shin M., Ni C.-W., Gupta A., Grosse A S., van Impel A., Kirchmaier B. C., Peterson-Maduro J. et al. , (2015). Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev. Cell 32, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokel D., Dunn T. W., Ahrens M. B., Alshut R., Cheung C. Y., Saint-Amant L., Bruni G., Mateus R., van Ham T. J., Shiraki T. et al. , (2013). Identification of nonvisual photomotor response cells in the vertebrate hindbrain. J. Neurosci. 33, 3834–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Zhao L., Page-McCaw P. S., Chen W. (2016). Zebrafish genome engineering using the CRISPR-Cas9 System. Trends Genet. 32, 815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Klingler R. H., Wimpee B., Dellinger M., King-Heiden T., Grzybowski J., Gerstenberger S. L., Weber D. N., Carvan M. J. 3rd, (2016). Maternal methylmercury from a wild-caught walleye diet induces developmental abnormalities in zebrafish. Reprod. Toxicol. (Elmsford, N.Y.) 65, 272–282. [DOI] [PubMed] [Google Scholar]
- MacPhail R. C., Brooks J., Hunter D. L., Padnos B., Irons T. D., Padilla S. (2009). Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology 30, 52–58. [DOI] [PubMed] [Google Scholar]
- MacPhail R. C., Hunter D. L., Irons T. D., Padilla S. (2011) Locomotion and Behavioral Toxicity in Larval Zebrafish: Background, Methods, and Data, in Zebrafish: Methods for Assessing Drug Safety and Toxicity (P. McGrath Ed.), John Wiley & Sons, Inc, Hoboken, NJ, USA. [Google Scholar]
- MacRae C. A., Peterson R. T. (2015). Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 14, 721–731. [DOI] [PubMed] [Google Scholar]
- Marczylo E. L., Jacobs M. N., Gant T. W. (2016). Environmentally induced epigenetic toxicity: potential public health concerns. Crit Rev Toxicol 468, 676-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin S. D., Petit M. A., Duvignacq M. C., Sumpter J. P. (2017). Towards improved behavioural testing in aquatic toxicology: Acclimation and observation times are important factors when designing behavioural tests with fish. Chemosphere 180, 430–436. [DOI] [PubMed] [Google Scholar]
- Nilsson E. E., Skinner M. K. (2015). Environmentally induced epigenetic transgenerational inheritance of disease susceptibility. Transl. Res. 165, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Murakami S., Ashikawa Y., Sasagawa S., Umemoto N., Shimada Y., Tanaka T. (2015). Zebrafish as a systems toxicology model for developmental neurotoxicity testing. Congenit Anom. (Kyoto) 55, 1–16. [DOI] [PubMed] [Google Scholar]
- Olsvik P. A., Williams T. D., Tung H-s., Mirbahai L., Sanden M., Skjaerven K. H., Ellingsen S. (2014). Impacts of TCDD and MeHg on DNA methylation in zebrafish (Danio rerio) across two generations. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 165(Suppl C), 17–27. [DOI] [PubMed] [Google Scholar]
- Padilla S., Corum D., Padnos B., Hunter D. L., Beam A., Houck K. A., Sipes N., Kleinstreuer N., Knudsen T., Dix D. J. et al. , (2012). Zebrafish developmental screening of the ToxCast Phase I chemical library. Reprod. Toxicol. (Elmsford, N.Y.) 33, 174–187. [DOI] [PubMed] [Google Scholar]
- Padilla S., Hunter D. L., Padnos B., Frady S., MacPhail R. C. (2011). Assessing locomotor activity in larval zebrafish: Influence of extrinsic and intrinsic variables. Neurotoxicol. Teratol. 33, 624–630. [DOI] [PubMed] [Google Scholar]
- Peterson R. T., Macrae C. A. (2012). Systematic approaches to toxicology in the zebrafish. Annu. Rev. Pharmacol. Toxicol. 52, 433–453. [DOI] [PubMed] [Google Scholar]
- Phillips J. B., Westerfield M. (2014). Zebrafish models in translational research: Tipping the scales toward advancements in human health. Dis. Model Mech. 7, 739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulak R. (2016). Tools for automating the imaging of zebrafish larvae. Methods 96, 118–126. [DOI] [PubMed] [Google Scholar]
- Reif D. M., Truong L., Mandrell D., Marvel S., Zhang G., Tanguay R. L. (2016). High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch. Toxicol. 90, 1459–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennekamp A. J., Peterson R. T. (2015). 15 years of zebrafish chemical screening. Curr. Opin. Chem. Biol. 24, 58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiter S., Sippel J., Bouwmeester M. C., Lommelaars T., Beekhof P., Hodemaekers H. M., Bakker F., van den Brandhof E. J., Pennings J. L., van der Ven L. T. (2016). Programmed effects in neurobehavior and antioxidative physiology in zebrafish embryonically exposed to cadmium: Observations and hypothesized adverse outcome pathway framework. Int. J. Mol. Sci. 17, 1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Kishi S. (2013). Molecular and chemical genetic approaches to developmental origins of aging and disease in zebrafish. Biochim. Biophys. Acta Mol. Basis Dis. 1832, 1362–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug T. T., Barouki R., Gluckman P. D., Grandjean P., Hanson M., Heindel J. J. (2013). PPTOX III: Environmental stressors in the developmental origins of disease–evidence and mechanisms. Toxicol. Sci. 131, 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selderslaghs I. W. T., Van Rompay A. R., De Coen W., Witters H. E. (2009). Development of a screening assay to identify teratogenic and embryotoxic chemicals using the zebrafish embryo. Reprod. Toxicol. 28, 308–320. [DOI] [PubMed] [Google Scholar]
- Shah A. N., Davey C. F., Whitebirch A. C., Miller A. C., Moens C. B. (2015). Rapid reverse genetic screening using CRISPR in zebrafish. Nat. Methods 12, 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipes N. S., Padilla S., Knudsen T. B. (2011). Zebrafish: As an integrative model for twenty-first century toxicity testing. Birth Defects Res. C Embryo Today 93, 256–267. [DOI] [PubMed] [Google Scholar]
- Stevens J. S., Padilla S., DeMarini D. M., Hunter D. L., Martin W. K., Thompson L. C., Gilmour M. I., Hazari M. S., Farraj A. K. (2018). Zebrafish locomotor responses reveal irritant effects of fine particulate matter extracts and a role for TRPA1. Toxicol. Sci. 161, 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A. M., Grieco F., Tegelenbosch R. A. J., Kyzar E. J., Nguyen M., Kaluyeva A., Song C., Noldus L. P. J. J., Kalueff A. V. (2015). A novel 3D method of locomotor analysis in adult zebrafish: Implications for automated detection of CNS drug-evoked phenotypes. J. Neurosci. Methods 255, 66–74. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Walker C., Dower N., Knauber D., Singer F. (1981). Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 291, 293–296. [DOI] [PubMed] [Google Scholar]
- Tian J., Hu J., Chen M., Yin H., Miao P., Bai P., Yin J. (2017). The use of mrp1-deficient (Danio rerio) zebrafish embryos to investigate the role of Mrp1 in the toxicity of cadmium chloride and benzo[a]pyrene. Aquat. Toxicol. 186, 123–133. [DOI] [PubMed] [Google Scholar]
- Truong L., Bugel S. M., Chlebowski A., Usenko C. Y., Simonich M. T., Simonich S. L. M., Tanguay R. L. (2016). Optimizing multi-dimensional high throughput screening using zebrafish. Reprod. Toxicol. 65, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L., Reif D. M., St Mary L., Geier M. C., Truong H. D., Tanguay R. L. (2014). Multidimensional in vivo hazard assessment using zebrafish. Toxicol. Sci. 137, 212–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney G. K., Sood R., Burgess S. M. (2015). Understanding and Editing the Zebrafish Genome. Adv. Genet. 92, 1–52. [DOI] [PubMed] [Google Scholar]
- Volz D. C., Hipszer R. A., Leet J. K., Raftery T. D. (2015). Leveraging embryonic zebrafish to prioritize toxcast testing. Environ. Sci. Technol. Lett. 2, 171–176. [Google Scholar]
- Wiley D. S., Redfield S. E., Zon L. I. (2017). Chapter 23 - Chemical screening in zebrafish for novel biological and therapeutic discovery In Methods in Cell Biology (Detrich H. W., Westerfield M., Zon L. I., Eds.), Vol. 138, pp. 651–679. Academic Press, Cambridge, MA, USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirbisky S. E., Sepulveda M. S., Weber G. J., Jannasch A. S., Horzmann K. A., Freeman J. L. (2016a). Embryonic atrazine exposure elicits alterations in genes associated with neuroendocrine function in adult male zebrafish. Toxicol. Sci. 153, 149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirbisky S. E., Weber G. J., Sepúlveda M. S., Lin T.-L., Jannasch A. S., Freeman J. L. (2016b). An embryonic atrazine exposure results in reproductive dysfunction in adult zebrafish and morphological alterations in their offspring. Sci. Rep. 6, 21337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirbisky S. E., Weber G. J., Sepulveda M. S., Xiao C., Cannon J. R., Freeman J. L. (2015). Developmental origins of neurotransmitter and transcriptome alterations in adult female zebrafish exposed to atrazine during embryogenesis. Toxicology 333, 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A.V., Nuñez J.K., Doudna J. A. (2016). Biology and applications of CRISPR systems: Harnessing nature’s toolbox for genome engineering. Cell 164, 29–44. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Qin W., Lu X., Xu J., Huang H., Bai H., Li S., Lin S. (2017). Programmable base editing of zebrafish genome using a modified CRISPR-Cas9 system. Nat. Commun. 8, 118.. [DOI] [PMC free article] [PubMed] [Google Scholar]