Abstract
While it has been shown that epigenetics accounts for a portion of the variability of complex traits linked to interactions with the environment, the real contribution of epigenetics to phenotypic variation remains to be assessed. In recent years, a growing number of studies have revealed that epigenetic modifications can be transmitted across generations in several animal species. Numerous studies have demonstrated inter- or multi-generational effects of changing environment in birds, but very few studies have been published showing epigenetic transgenerational inheritance in these species. In this review, we mention work conducted in parent-to-offspring transmission analyses in bird species, with a focus on the impact of early stressors on behaviour. We then present recent advances in transgenerational epigenetics in birds, which involve germline linked non-Mendelian inheritance, underline the advantages and drawbacks of working on birds in this field and comment on future directions of transgenerational studies in bird species.
Keywords: transgenerational epigenetics, birds, non-mendelian inheritance, environment, behaviour
Introduction
A portion of the variability of complex traits is affected by interactions with the environment, through epigenetic phenomena [1, 2]. Thus, besides genetic variability, external influences that affect early life stages (pre and postnatal) can have enormous consequences on the adult phenotype [3]. Epigenetic marks programmed during embryogenesis are mostly maintained throughout development, and thus, less susceptible to environmental changes later in life [4]. However, the absolute contribution of epigenetics to phenotypic variation remains to be assessed, and deciphering the part of genetics and epigenetics in phenotype construction, under different conditions, still encounters challenges [5, 6]: appropriate tools to analyze a high number of epigenetic markers, to be associated with phenotypic variability, are lacking in these species; clear identification of genetic effects in farm animals, which are mostly not inbred, need expensive genomic analyses; furthermore, environmentally induced epigenetic modifications can induce genetic mutations [7]. These challenges represent important concerns in animal breeding, and there is a clear need for concerted research work in these areas [8, 9]. Among the environmental influences acting on parental animals, several factors are reported to affect epigenetic processes during early development of the offspring, such as endocrine disrupting chemicals [10], inorganic chemicals [11], nutritional compounds [12, 13] and stressful conditions [14].
Epigenetics is the study of molecules that attach to the genome and maintain this interaction in a mitotically stable manner, thereby regulating gene expression [15]. Epigenetic modifications include DNA methylation or hydroxymethylation of CG dinucleotides, chemical modifications of histones, interaction of DNA with small RNAs, or states of chromatin condensation [1, 16]. Research on epigenetics has permeated several fields of biological research, from molecular biology to evolution [17]. Altering epigenetics states in some genomic regions can have drastic phenotypic consequences such as changes in the coat color [12] or increased disease susceptibility [3, 18]. A variety of model organisms have been used in epigenetic research, including laboratory rodents [10, 12, 13], invertebrates [19], plants [20] or yeast specimens [21] .
In recent years, a growing number of studies have revealed that epigenetic modifications can be transmitted across generations [6], but the debate continues on how epigenetic marks that are acquired from environmental exposure can be transmitted beyond the exposed generations, via ‘transgenerational epigenetic inheritance’ [22, 23]. A germline-dependent epigenetic effect, through histone modifications, DNA methylation or small RNAs, has been identified in various animals including worms and mammals (see [24–31]). However, in spite of the importance of epigenetic mechanisms affecting phenotypic traits and gene expression, few epigenetic studies in farm animals have taken place, even though in the production environment parents and offspring may live under a variety of environmental conditions, which generate phenotypic variability even in highly genetically homogeneous groups of individuals.
While numerous studies have demonstrated inter- or multi-generational effects in birds, very few have shown that germline-dependent epigenetic mechanisms are causally involved in non-DNA sequence-based inheritance in these species. Epigenetic mechanisms are far less known in birds than in mammals, and some processes may be quite different: it seems, for instance, that there is no genomic imprinting in birds [32–34]. Thus, our knowledge has to be improved with regards to the biological importance of these economically important species. In this review, we briefly mention work conducted in parent-to-offspring transmission analyses in birds. Further, we present recent advances in transgenerational epigenetics in birds, underline the advantages and drawbacks of working on birds in this field and comment on future directions of transgenerational studies in bird species.
Examples of Inter- and Multi-generational Studies in Birds
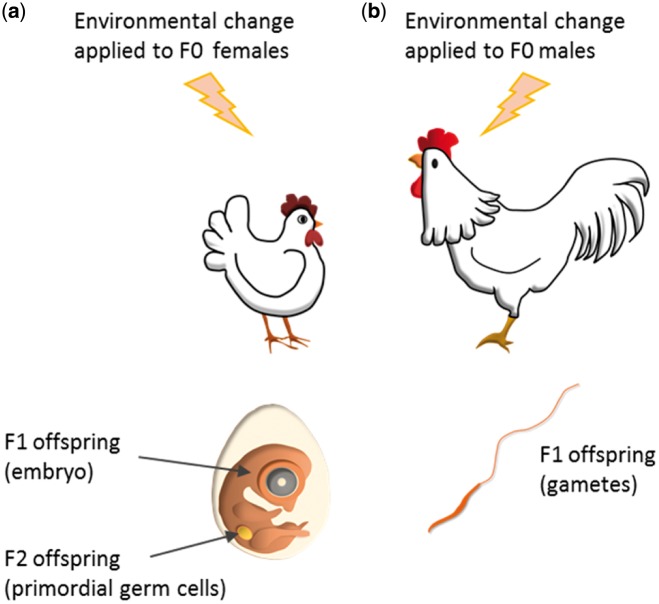
In birds, the future embryo development (F1 generation) and its primordial germ cells (PGCs) (F2 generation) are directly affected by the egg components, depending on the mother's environment. These egg components include the quality and quantity of nutrients and concentrations of yolk hormones [35]. In birds as in mammals [36], inter-generational (from mother to offspring) or multi-generational (from father to offspring and from mother to grand-offspring) effects cannot be considered as transgenerational effects (from mother to G3 or from father to G2) (Fig. 1) [37].
Figure 1:
The maternal environment directly impacts F1 and F2 offspring while the paternal environment only impacts F1 offspring. (a) A change in the maternal environment can affect egg components and thus may impact F1 individuals. However, as these F1 developing offspring bear the PGCs that will lead to differentiated gametes, the change in maternal environment may also impact F2 individuals. Thus only the effects observed on the F3 individuals will be considered as transgenerational effects. (b) A change in the paternal environment only affects its own gametes that will lead to the F1 generation. The effects observed on the F2 individuals will be considered as transgenerational effects
Parent–Offspring Transmission
Giving an exhaustive view of the impact of the parental environment on the offspring phenotype is out of the scope of this mini-review, and several articles have given examples of such studies [38–46]. Therefore, we only present recent papers, which focus mainly on behavioural consequences.
Evidence of the influence of maternal stress on offspring phenotypes has been shown in chickens [47]. Factors such as food deprivation, physical restraint or social isolation of parental laying hen were shown to have significant effects on the offspring's hypothalamus-pituitary-adrenal-axis response by elevation of corticosterone (CORT), a stress-related hormone. Furthermore, early life stress in the parents affected several aspects of behaviour (cognition and adaptive response). In a more applied, on-farm study in laying hens, de Haas et al. [48] showed that offspring from parent stock with high basal CORT were more anxious and more likely to develop severe feather pecking (FP) behaviour in the first weeks of life than offspring from parents with lower basal CORT levels. Additionally, changes in fatty acid concentrations in the mother diet of laying hens modified egg mass, yolk-lipid composition, yolk-hormone concentration, weight at hatch and fear of novelty in the offspring [49]. In Japanese quail, another selected bird species, simulating maternal stress by injection of eggs with CORT resulted in an attenuated stress response in their offspring, with maternal pre-natal stress being more important on the offspring’s physiological response than post-natal stress [50]. In chickens, CORT levels in the yolk are not elevated due to stress, although stress affects other yolk hormones, such as testosterone and estrogens; the importance of pre-natal stress may be a particularity of precocial species of birds, self-sufficient at hatching [50]. These examples confirm in birds (as oviparous species) the direct influence of the maternal environment on offspring’s phenotypes.
Parent-Grand-Offspring Transmission
In zebra finches, plasma CORT was elevated by oral treatment of mothers with high plasma CORT, resulting in an increase of grand-offspring growth rate and a sex-biased offspring mortality [51]. In ducks, a study showed that maternal methionine deficiency affected grand-progeny phenotypes through the paternal path of transmission (e.g. body-weight and lipid metabolism components) [52].
These studies add new evidence regarding the already known essential role of prenatal maternal provisioning in shaping offspring’s phenotype. This role may be confounded with other maternal effects such as direct additive genetic effects, mitochondrial inheritance or sexual chromosome-linkage. A recent study has disentangled prenatal maternal effects from these sources of confounding variation in quail by using reciprocal crosses, allowing to observe individuals with similar genotypes but different maternal egg investment [53]. The grand-parental transmission of environmental effects described here, especially when shown to be paternally inherited, suggests a non-genetic germline-linked inheritance in birds.
Multigenerational Epigenetics and Selection
The aforementioned studies have been performed on experimental or farm populations and recent work extends the intergenerational effect, probably of epigenetic origin, to wild populations. In house sparrows, for example, parental age has a negative effect on offspring lifetime fitness [54]. The authors of this study showed that this effect was not due to environmental factors such as senescence in parental care behaviour, as they used a cross-fostering design and observed that the lifetime reproductive success depended on the age of the genetic parent but not on the age of the foster parent. These findings show that transgenerational effect of parental age may impact population dynamics, potentially through epigenetic phenomena [54]. In another work conducted on natural populations of Darwin finches, DNA methylation marks were shown to be accumulated during evolution, while the phylogenetic distance was not associated with the number of genetic mutations, at least for copy number variations. While other genetic variations such as single nucleotide polymorphisms cannot be ruled out, this study opens the possibility for the involvement of epigenetic changes in the speciation of natural populations [55]. Finally, results obtained from an artificial selection experiment in great tits showed that methylation levels near the transcription start site of the DRD4 gene (dopamine receptor D4, known to be associated with personality traits in several species) were associated with exploratory behaviour [56]. However, these differential epigenetic marks obtained after 4 generations of selection may have emerged due to genetic selection. They, nevertheless, seem to be associated with the variability of the selected trait, while no functional genetic variant acting at the protein level has been detected [56]. These findings again support the hypothesis of non-genetic inheritance in birds.
Genetics and Epigenetics of Domesticated Birds’ Welfare
Animal welfare has high societal and scientific priority, and the public is becoming increasingly concerned about how animals, including domesticated birds, are treated. To study gene × epigenetics × environment interaction in the field of animal welfare represents a great challenge [57]. Under commercial conditions, birds such as turkeys, ducks, quail or chickens, are exposed to a number of stressors during early development, such as hatching without maternal contact, transportation, heat and cold stress, or separation from social mates. These early stressors have impact on behaviour and physiology probably also through epigenetic mechanisms that affect the next generation [58], for example, via alteration in steroid hormones deposited in the yolk. Yolk steroids may provide powerful maternal signals for adaptive modifications of offspring development in response to the environmental conditions [35]. Likewise, epigenetic marks in peripheral cells could be a feasible way to measure past detrimental conditions, since it has recently been shown that epigenetic marks in red blood cells can identify rearing conditions in hens [59].
Many behaviours in the chicken are affected by early environmental factors and maternal hormones, such as learning, food choice, stress response [44] and neophobia [49]. Likely also maladaptive redirected FP could be influenced by early environment. FP occurs when chickens are unable to perform foraging behaviour [60], and in animals which are anxious and have a high pecking motivation [61]. FP chickens peck at and pull out the feathers of recipient birds, sometimes followed by eating the removed feathers [62]. FP is a detrimental behaviour in chickens that represents both serious economic and welfare problem: feather loss affects negatively animal health and egg production, increases feed consumption and can lead to increased mortality rates [60, 63–65]. Pulling out feathers causes pain [66] and severe FP may develop into cannibalism [62]. Interestingly, genetic mutations affecting FP were found in laying hens: Flisikowski et al. [67] performed an association study in high and low FP lines and found an association between DEAF1, a gene for a regulatory factor of the serotonergic system, and FP. Further, mutations in the dopamine D4 receptor were detected. Similarly, Biscarini et al. [68] found an association between the gene for the serotonin receptor HTR2C and feather damage, which was significant across a population of nine pure-bred selection lines.
The genetic contribution to the development of this behaviour was demonstrated by successful divergent selection [69–71]. However, epigenetic processes behind the development of damaging pecking remain largely unknown, although highly plausible considering the dependence of these behaviours on early environmental conditions. One of the aims of a recently formed research network, i.e. the COST Action GroupHouseNet (www.grouphousenet.eu), is to contribute to the clarification of these aspects [72]. This COST Action aims to reduce damaging behaviour in pigs and laying hens. One working group focuses on genetic and epigenetic approaches to reduce damaging behaviour. Prevention of parental stress and stress during early life of animals could be an important way to reduce the risk of these damaging behaviours developing. Whether the impact of early stressors has transgenerational consequences on behaviour remains to be evaluated.
Transgenerational Non-Genetic Inheritance in Quail
Very few transgenerational studies have been published in farm animals [73], especially farm chickens [40], and to the best of our knowledge, only one experimental study has been published in transgenerational inheritance in domesticated birds until the F3 generation [74]. To test for the existence of transgenerational transmission of an environmental effect in birds, two quail ‘epilines’ were produced using fertilized eggs from the same founder population, the high social reinstatement quail line that was developed by divergent selection on social motivation [75]. A methylation modifier (Genistein, an endocrine disruptor present in soybean products) was injected (Epi+ epiline) or not (Epi- epiline) into the eggs before incubation. To evaluate the persistence of a putative effect of the treatment, three generations were produced from the founders with exact parallel pedigrees between the Epi+ and Epi- epilines. Mirrored single-pair mating was performed to minimize between-line genetic variability by maintaining similar ancestor contributions across generations in each epiline. So observed differences were likely to be due, at least in part, to epigenetic transgenerational inheritance. Indeed, after three generations of breeding without further injection, several traits were affected, such as age at first egg (delayed by 8 days) and behavioural traits (reduction of birds’ reaction to social isolation, measured as the total distance travelled when isolated in a test room).
Advantages and Drawbacks of Transgenerational Studies in Birds
A major obstacle in transmitting epigenetic information through DNA methylation from one generation to the next is the epigenetic reprogramming, that is, the demethylation-remethylation of most of the CG sites. Little is known in birds about epigenetic reprogramming, and a reasonable hypothesis is that events occur in the same way as it happens in mammals, where two developmental periods exist in which major epigenetic changes occur in the genome [76, 77]. One is after fertilization, when an initial reduction in DNA methylation is followed by re-methylation at the time of blastocyst implantation [77]. Another period of epigenetic reprogramming occurs during the migration of primordial germ cells (PGCs) towards their final establishment in the gonads [78]. During this migration, major demethylation of the genome also occurs followed by re-methylation [76–78]. Both of these periods of resetting of methylation patterns are windows of sensitivity to environmental exposures, as the methylome is less subject to changes later in life in response to environmental changes [1]. Interfering with the resetting period of PGCs, however, has different implications than interfering with the resetting period of pre-implantation embryos. Because the germ line has the ability of transmitting epigenetic marks to next generations, altered DNA methylation patterns produced in the germ line can be transgenerationally perpetuated, probably having escaped from epigenetic reprogramming [15]. Recent studies in rodents have shown that exposure to a variety of substances can alter methylation patterns in the germ line that may be transmitted to future generations, while associating with the emergence of altered phenotypes [79–82]. Unravelling the periods of epigenetic programming in various animals could help us to understand where and how epigenetic transgenerational inheritance works.
Because in mammals the period of migration of PGCs involves major epigenetic reprogramming in their genome, it is expected that a similar phenomenon occurs in birds. However in chickens, PGCs migration occurs partially in extraembryonic tissues. After egg laying, chicken PGCs migrate outwards from the anterior part of the embryo (germinal crescent) towards the extraembryonic tissue, while blood vessels are being formed [83]. Once the circulatory system starts to be active, PGCs migrate inside the embryo through the newly formed blood vessels, finally reaching the genital ridges at around 60 h after egg laying [83, 84]. Recent research in chickens has determined changes in gene expression of DNA methyltransferases that suggest the occurrence of a major epigenetic reprogramming of PGCs during their migration [85]. However, a precise description of the time of epigenetic reprogramming of PGCs is still lacking in birds. This lack of knowledge compared to mammals remains a limitation to study transgenerational epigenetic inheritance in birds. Furthermore, in heavily selected bird species, these factors may have a significant input on the sire or dam line, and respective offspring crossbred generations, because of the high number of offspring issued from a single parent. Thus, a better understanding of these phenomena may help in the improvement of breeding systems, by taking into account the parental environment (e.g. diet, temperature and so on) to increase offspring performances.
Other hypotheses have been stated about the germ-line vector of non-genetic inheritance, as histone marks [86] or non-coding RNAs [27], the latter gathering more and more interest [25]. Also in these fields, much work has to be done to improve our knowledge in birds.
The major drawback, however, may be the outbred nature of most of the available lines: inbred strains are an ideal tool to avoid genetic variability and thus to have an unbiased interpretation of epigenetic variations [87]. As in the earlier quail example, it is not straightforward to discriminate epigenetic from genetic effects when analyzing outbred lines. But inbred lines may be less susceptible to epigenetic transgenerational phenomena than outbred ones, as shown in rodents [79]. If this phenomenon also exists in birds, it may thus be more advantageous than disadvantageous to analyze outbred lines, in order to observe significant transgenerational effects in these species. Another advantage of using outbred lines is that they are genetically more similar to wild species and thus more informative about what could be observed in nature. So a well-designed study including high-throughput genotyping or sequencing may be an effective way to decipher transgenerational inheritance in birds. The obstacle of this kind of studies is the large number of individuals that have to be raised in order to be able to observe statistically significant epigenetic effects, taking into account the genetic variability. Conversely, as a part of the variability of epigenetic marks is under genetic control, as shown in human [88, 89], such designs would help deciphering the genetic-based epigenetic variability.
Compared to mammals, birds have several advantages with regards to the study of transgenerational epigenetic inheritance. One major advantage is the oviparity: the embryo develops outside of the mother, and the maternal influence relies on the egg composition as provided during oviposition. So the developing environment (as temperature and humidity) of a whole batch can be strictly controlled to minimize inter-individual environmental variability. Following hatching, one can avoid parent-offspring cohabitation thanks to the precocial nature of farm bird species where offspring are totally independent of their parents and minimize confounding postnatal effects; rearing in large groups further reduces the cage effect as sometimes observed in rodents [87].
Finally, avian species have short generation times compared to other farm animals, an advantage when dealing with transgenerational studies. Also, the number of offspring is not restricted to available womb space prenatally or litter sizes postnatally. Importantly, the number of available bird genome assemblies is growing, and several species may be used for epigenetic studies involving high-throughput sequencing.
Among production animals, chickens have recently been suggested as a promising model for epigenetic studies for several reasons [41]. Chicken (Gallus gallus) genome has been sequenced [90, 91], their embryos can be easily accessed during times in which epigenetic (re)programming is reported in other vertebrates [41], and they have been an important model for translational research with implications in human health and physiology [92]. In addition to chicken, Japanese quail (Coturnix japonica) is a very important species in avian research [93] and is an ideal model for experimental studies on transgenerational epigenetics involving high-throughput sequencing, thanks to its short generation time and a high-quality genome assembly.
Finally, genome wide studies in Great tit (Parus major) and Zebra finch (Taeniopygia guttata) have proven that these two species may also be valuable models for epigenetic studies: they play a major role in our knowledge of behavioural, ecological and evolutionary mechanisms, domains where epigenetic mechanisms are probably important players; moreover, as they are well-studied model species, genomic tools (such as genome assembly) are available [94–96].
Future Directions
The number of transgenerational studies in birds should increase in the coming years. The avian scientific community has a wealth of species suitable for this kind of studies, from experimental lines to natural populations.
Concerning applied research in the field, future work should pave the way to improved models in genetic selection. In recent years, new selection schemes incorporating molecular information have been implemented or are under development, in different farm species (genomic selection, marker-assisted selection). So far the information taken into account in these programs corresponds to DNA nucleotide variations. On the contrary, the part played by the inherited epigenetic variability in shaping phenotypes over generations is still unknown. The integration of epigenetic phenomena in enriched models should improve both the accuracy of the prediction models and their effectiveness in animal selection. The results will ultimately condition the work to be conducted in other animal species of agricultural interest.
As underlined earlier, a precise knowledge of the dynamic of the epigenetic reprogramming of PGCs and early embryo is absent in birds. This is a very important field of investigation for the future: indeed, the identification of developmental periods of major epigenetic reprogramming in laboratory rodents was a fundamental step in epigenetic research [76].
For most of the studies described earlier, molecular epigenetic analyses are missing, and future work has to combine well-defined animal designs and thorough molecular analyses, including DNA methylation, histone marks analyses and non-coding RNA sequencing. Performing these analyses in pure tissues is necessary, as the epigenetic landscape is strongly tissue-specific. Moreover, deciphering the genetic from the epigenetic relative weight of such inheritance is challenging, and designs have to be built to minimize and/or accurately account for the genetic variability.
In birds, the issues related to the estimation of the importance of epigenetic variability, its inheritance and the putative genetic control of these phenomena, go far beyond animal production and deal with fundamental biological questions related to phenotype formation. As in mammals [87], bird studies must thus be set up to answer different but complementary scientific questions, as ‘which part of the phenotypic variability is caused by epigenetic phenomena?’, ‘to what extent is epigenetic marks variability governed by the genome?’, ‘how is the environment able to modify germ cells epigenetic marks?’ ‘which germline-specific epigenetic mark is able to transfer the effect of a changing environment to the offspring?’
Acknowledgements
This article is partially based upon work from COST Action CA15134 Synergy for preventing damaging behaviour in group housed pigs and chickens (GroupHouseNet), supported by COST (European Cooperation in Science and Technology). C.G.B. greatly appreciates funding by the ERC advanced grant GeneWell (322206) to Per Jensen.
Conflict of interest statement. None declared.
References
- 1. Feil R, Fraga MF.. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet 2012; 13:97–109. [DOI] [PubMed] [Google Scholar]
- 2. Skinner Mk. Environmental epigenetics and a unified theory of the molecular aspects of evolution: a neo-Lamarckian concept that facilitates neo-Darwinian evolution. Genome Biol Evol 2015; 7:1296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guerrero-Bosagna C, Skinner Mk.. Environmentally induced epigenetic transgenerational inheritance of phenotype and disease. Mol Cell Endocrinol 2012; 354:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skinner Mk. Role of epigenetics in developmental biology and transgenerational inheritance. Birth Defects Res C Embryo Today 2011; 93:51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ibeagha-Awemu EM, Zhao X.. Epigenetic marks: regulators of livestock phenotypes and conceivable sources of missing variation in livestock improvement programs. Front Genet 2015; 6:302.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miska EA, Ferguson-Smith AC.. Transgenerational inheritance: models and mechanisms of non-DNA sequence-based inheritance. Science 2016; 354:59–63. [DOI] [PubMed] [Google Scholar]
- 7. Skinner MK, Guerrero-Bosagna C, Haque MM.. Environmentally induced epigenetic transgenerational inheritance of sperm epimutations promote genetic mutations. Epigenetics 2015; 10:762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goddard ME, Whitelaw E.. The use of epigenetic phenomena for the improvement of sheep and cattle. Front Genet 2014; 5:247.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sinclair KD, Rutherford KM, Wallace JM, Brameld JM, Stoger R, Alberio R, Sweetman D, Gardner DS, Perry VE, Adam CL.. Epigenetics and developmental programming of welfare and production traits in farm animals. Reprod Fertil Dev 2016; 28:1443–1478. [DOI] [PubMed] [Google Scholar]
- 10. Susiarjo M, Sasson I, Mesaros C, Bartolomei MS.. Bisphenol a exposure disrupts genomic imprinting in the mouse. PLoS Genet 2013; 9:e1003401.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kile ML, Houseman EA, Baccarelli A, Quamruzzaman Q, Rahman M, Mostofa G, Cardenas A, Wright Ro, Christiani DC.. Effect of prenatal arsenic exposure on DNA methylation and leukocyte subpopulations in cord blood. Epigenetics 2014;9:774.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dolinoy DC, Huang D, Jirtle RL.. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA 2007; 104:13056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guerrero-Bosagna CM, Sabat P, Valdovinos FS, Valladares LE, Clark SJ.. Epigenetic and phenotypic changes result from a continuous pre and post natal dietary exposure to phytoestrogens in an experimental population of mice. BMC Physiol 2008; 8:17.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fagiolini M, Jensen CL, Champagne FA.. Epigenetic influences on brain development and plasticity. Curr Opin Neurobiol 2009; 19:207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skinner MK, Manikkam M, Guerrero-Bosagna C.. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab 2010; 21:214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Denham J, Marques FZ, O'Brien BJ, Charchar FJ.. Exercise: putting action into our epigenome. Sports Med 2014; 44:189–209. [DOI] [PubMed] [Google Scholar]
- 17. Stein R, Davis D.. Epigenetics: a fascinating field with profound research, clinical, & public health implications. Am Biol Teach 2012; 74:213–23. [Google Scholar]
- 18. Guerrero-Bosagna C, Jensen P.. Globalization, climate change, and transgenerational epigenetic inheritance: will our descendants be at risk? Clin Epigenetics 2015; 7:8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lyko F, Foret S, Kucharski R, Wolf S, Falckenhayn C, Maleszka R.. The honey bee epigenomes: differential methylation of brain DNA in queens and workers. PLoS Biol 2010; 8:e1000506.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manning K, Tor M, Poole M, Hong Y, Thompson AJ, King GJ, Giovannoni JJ, Seymour GB.. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet 2006; 38:948–52. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Q, Yoon Y, Yu Y, Parnell EJ, Garay JA, Mwangi MM, Cross FR, Stillman DJ, Bai L.. Stochastic expression and epigenetic memory at the yeast HO promoter. Proc Natl Acad Sci USA 2013; 110:14012–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jablonka E, Raz G.. Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Q Rev Biol 2009; 84:131–76. [DOI] [PubMed] [Google Scholar]
- 23. Heard E, Martienssen RA.. Transgenerational epigenetic inheritance: myths and mechanisms. Cell 2014; 157:95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burggren WW. Dynamics of epigenetic phenomena: intergenerational and intragenerational phenotype ‘washout’. J Exp Biol 2015; 218:80–7. [DOI] [PubMed] [Google Scholar]
- 25. Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, Feng GH, Peng H, Zhang X, Zhang Y, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2016; 351:397–400. [DOI] [PubMed] [Google Scholar]
- 26. Daxinger L, Whitelaw E.. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat Rev Genet 2012; 13:153–62. [DOI] [PubMed] [Google Scholar]
- 27. Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM.. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci 2014; 17:667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grandjean V, Fourre S, De Abreu DA, Derieppe MA, Remy JJ, Rassoulzadegan M.. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci Rep 2015; 5:18193.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL.. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 2013; 33:9003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodgers AB, Morgan CP, Leu NA, Bale TL.. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci USA 2015; 112:13699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soubry A. Epigenetic inheritance and evolution: a paternal perspective on dietary influences. Prog Biophys Mol Biol 2015; 118:79–85. [DOI] [PubMed] [Google Scholar]
- 32. Zhuo Z, Lamont SJ, Abasht B.. RNA-Seq analyses identify frequent allele specific expression and no evidence of genomic imprinting in specific embryonic tissues of chicken. Sci Rep 2017; 7:11944.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Q, Li K, Zhang D, Li J, Xu G, Zheng J, Yang N, Qu L.. Next-generation sequencing techniques reveal that genomic imprinting is absent in day-old Gallus gallus domesticus brains. PLoS One 2015;10:e0132345.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fresard L, Leroux S, Servin B, Gourichon D, Dehais P, Cristobal MS, Marsaud N, Vignoles F, Bed'hom B, Coville JL, et al. Transcriptome-wide investigation of genomic imprinting in chicken. Nucleic Acids Res 2014; 42:3768–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Groothuis TG, Muller W, von Engelhardt N, Carere C, Eising C.. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci Biobehav Rev 2005; 29:329–52. [DOI] [PubMed] [Google Scholar]
- 36. Skinner MK. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics 2011; 6:838–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Skinner MK. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod Toxicol 2008; 25:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Berghof TV, Parmentier HK, Lammers A.. Transgenerational epigenetic effects on innate immunity in broilers: an underestimated field to be explored? Poult Sci 2013; 92:2904–13. [DOI] [PubMed] [Google Scholar]
- 39. Dixon LM, Sparks NH, Rutherford KM.. Early experiences matter: a review of the effects of prenatal environment on offspring characteristics in poultry. Poult Sci 2016; 95:489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Feeney A, Nilsson E, Skinner MK.. Epigenetics and transgenerational inheritance in domesticated farm animals. J Anim Sci Biotechnol 2014; 5:48.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fresard L, Morisson M, Brun JM, Collin A, Pain B, Minvielle F, Pitel F.. Epigenetics and phenotypic variability: some interesting insights from birds. Genet Sel Evol 2013; 45:16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Houdelier C, Pittet F, Guibert F, de Margerie E, Lumineau S.. Non-genetic Inheritance in Birds: transmission of behaviour from mother to offspring. Non Genetic Inheritance 2013;1:62–8. [Google Scholar]
- 43. Jensen P. Transgenerational epigenetic effects on animal behaviour. Prog Biophys Mol Biol 2013; 113:447–54. [DOI] [PubMed] [Google Scholar]
- 44. Jensen P. Adding ‘epi-’ to behaviour genetics: implications for animal domestication. J Exp Biol 2015; 218:32–40. [DOI] [PubMed] [Google Scholar]
- 45. Morisson M, Coustham V, Frésard L, Collin A, Zerjal T, Métayer-Coustard S, Bodin L, Minvielle F, Brun J-M, Pitel F, Nutritional programming and effect of ancestor diet in birds In: Patel V, Preedy V (eds.), Handbook of Nutrition, Diet, and Epigenetics. Cham:Springer International Publishing, 2017, 1–18. [Google Scholar]
- 46. Rodenburg BT, de Haas EN.. Of nature and nurture: the role of genetics and environment in behavioural development of laying hens. Curr Opin Behav Sci 2016; 7:91–4. [Google Scholar]
- 47. Ericsson M, Henriksen R, Belteky J, Sundman AS, Shionoya K, Jensen P.. Long-term and transgenerational effects of stress experienced during different life phases in chickens (Gallus gallus). PLoS One 2016;11:e0153879.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Haas EN, Bolhuis JE, Kemp B, Groothuis TG, Rodenburg TB.. Parents and early life environment affect behavioral development of laying hen chickens. PLoS One 2014; 9:e90577.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Haas EN, Calandreau L, Baeza E, Chartrin P, Palme R, Darmaillacq AS, Dickel L, Lumineau S, Houdelier C, Denis I, et al. Lipids in maternal diet influence yolk hormone levels and post-hatch neophobia in the domestic chick. Dev Psychobiol 2017; 59:400–9. [DOI] [PubMed] [Google Scholar]
- 50. Zimmer C, Larriva M, Boogert NJ, Spencer KA.. Transgenerational transmission of a stress-coping phenotype programmed by early-life stress in the Japanese quail. Sci Rep 2017; 7:46125.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khan N, Peters RA, Richardson E, Robert KA.. Maternal corticosterone exposure has transgenerational effects on grand-offspring. Biol Lett 2016;12:20160627.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brun JM, Bernadet MD, Cornuez A, Leroux S, Bodin L, Basso B, Davail S, Jaglin M, Lessire M, Martin X, et al. Influence of grand-mother diet on offspring performances through the male line in Muscovy duck. BMC Genet 2015; 16:145.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pick JL, Ebneter C, Hutter P, Tschirren B.. Disentangling genetic and prenatal maternal effects on offspring size and survival. Am Nat 2016; 188:628–39. [DOI] [PubMed] [Google Scholar]
- 54. Schroeder J, Nakagawa S, Rees M, Mannarelli ME, Burke T.. Reduced fitness in progeny from old parents in a natural population. Proc Natl Acad Sci USA 2015; 112:4021–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Skinner MK, Gurerrero-Bosagna C, Haque MM, Nilsson EE, Koop JA, Knutie SA, Clayton DH.. Epigenetics and the evolution of Darwin's Finches. Genome Biol Evol 2014; 6:1972–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Verhulst EC, Mateman AC, Zwier MV, Caro SP, Verhoeven KJ, van Oers K.. Evidence from pyrosequencing indicates that natural variation in animal personality is associated with DRD4 DNA methylation. Mol Ecol 2016; 25:1801–11. [DOI] [PubMed] [Google Scholar]
- 57. Jensen P. Behaviour epigenetics–the connection between environment, stress and welfare. Appl Anim Behav Sci 2014; 157:1–7. [Google Scholar]
- 58. Goerlich VC, Nätt D, Elfwing M, Macdonald B, Jensen P.. Transgenerational effects of early experience on behavioral, hormonal and gene expression responses to acute stress in the precocial chicken. Horm Behav 2012; 61:711–8. [DOI] [PubMed] [Google Scholar]
- 59. Pértille F, Brantsæter M, Nordgreen J, Coutinho Ll, Janczak Am, Jensen P, Guerrero-Bosagna C.. DNA methylation profiles in red blood cells of adult hens correlate with their rearing conditions. J Exp Biol 2017; 220:3579–87. [DOI] [PubMed] [Google Scholar]
- 60. Rodenburg TB, van Krimpen MM, de Jong IC, de Haas EN, Kops MS, Riedstra BJ, Nordquist RE, Wagenaar JP, Bestman M, Nicol CJ.. The prevention and control of feather pecking in laying hens: identifying the underlying principles. Worlds Poult Sci J 2013; 69:361–74. [Google Scholar]
- 61. de Haas EN, Nielsen BL, Buitenhuis AJ, Rodenburg TB.. Selection on feather pecking affects response to novelty and foraging behaviour in laying hens. Appl Anim Behav Sci 2010; 124:90–6. [Google Scholar]
- 62. Savory C. Feather pecking and cannibalism. Worlds Poult Sci J 1995; 51:215–9. [Google Scholar]
- 63. Glatz P, Productivity and Profitability of Caged Layers with Poor Feather Cover. Tech. Report No. SAR-6A. Rural Industries Development and Corporation, Barton Act. South Australia: 1998: 32. [Google Scholar]
- 64. Mills A, Faure J, Williams J, Feather loss and egg production in broiler breeders and layers In: Annales De Zootechnie. INRA/EDP Sciences, 1988; 37:pp.133–142. [Google Scholar]
- 65. Yamak U, Sarica M.. Relationships between feather score and egg production and feed consumption of different layer hybrids kept in conventional cages. Archiv Fur Geflügelkunde 2012; 76:31–7. [Google Scholar]
- 66. Gentle M. Neuroma formation following partial beak amputation (beak trimming) in the chicken. Res Vet Sci 1986; 41:383–5. [PubMed] [Google Scholar]
- 67. Flisikowski K, Schwarzenbacher H, Wysocki M, Weigend S, Preisinger R, Kjaer JB, Fries R.. Variation in neighbouring genes of the dopaminergic and serotonergic systems affects feather pecking behaviour of laying hens. Anim Genet 2009; 40:192–9. [DOI] [PubMed] [Google Scholar]
- 68. Biscarini F, Bovenhuis H, Parmentier HK, Van Der Poel JJ, Rodenburg TB.. Van Arendonk JAM. Across-line SNP associations study of plumage condition in laying hens. Behav Genet 2010; 40: 715–27. [DOI] [PubMed] [Google Scholar]
- 69. Kjaer JB, Sørensen P, Su G.. Divergent selection on feather pecking behaviour in laying hens (Gallus gallus domesticus). Appl Anim Behav Sci 2001;71:229–39. [DOI] [PubMed] [Google Scholar]
- 70. Su G, Kjaer JB, Sorensen P.. Variance components and selection response for feather-pecking behavior in laying hens. Poult Sci 2005; 84:14–21. [DOI] [PubMed] [Google Scholar]
- 71. Rodenburg TB, Koene P.. Comparison of individual and social feather pecking tests in two lines of laying hens at ten different ages. Appl Anim Behav Sci 2003; 81:133–48. [Google Scholar]
- 72. Rodenburg T, Berk J, Dimitrov I, Edgar J, van der Eijk J, Estevez I, Ferrante V, de Haas E, Kostal L, Liaubet L, The GroupHouseNet COST Action: exploiting European synergy to reduce feather pecking in laying hens In: Xth European Symposium on Poultry Welfare. Book of Abstracts, p. 41. 19–22 June 2017. Ploufragan, France, World’s Poultry Science Journal, 2017. [Google Scholar]
- 73. Braunschweig M, Jagannathan V, Gutzwiller A, Bee G.. Investigations on transgenerational epigenetic response down the male line in F2 pigs. PLoS One 2012; 7:e30583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Leroux S, Gourichon D, Leterrier C, Labrune Y, Coustham V, Riviere S, Zerjal T, Coville JL, Morisson M, Minvielle F, et al. Embryonic environment and transgenerational effects in quail. Genet Sel Evol 2017; 49:14.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mills AD, Faure JM.. Divergent selection for duration of tonic immobility and social reinstatement behavior in Japanese quail (Coturnix japonica) chicks. J Comp Psychol 1991;105:25–38. [DOI] [PubMed] [Google Scholar]
- 76. Hackett JA, Surani MA.. Beyond DNA: programming and inheritance of parental methylomes. Cell 2013; 153:737–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Reik W, Dean W, Walter J.. Epigenetic reprogramming in mammalian development. Science 2001; 293:1089–93. [DOI] [PubMed] [Google Scholar]
- 78. Lees-Murdock DJ, Walsh CP.. DNA methylation reprogramming in the germ line. Epigenetics 2008; 3:5–13. [DOI] [PubMed] [Google Scholar]
- 79. Guerrero-Bosagna C, Covert TR, Haque MM, Settles M, Nilsson EE, Anway MD, Skinner MK.. Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reprod Toxicol 2012; 34:694–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK.. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS One 2010;5:e13100.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Manikkam M, Guerrero-Bosagna C, Tracey R, Haque MM, Skinner MK.. Transgenerational actions of environmental compounds on reproductive disease and epigenetic biomarkers of ancestral exposures. PLoS One 2012; 7:e31901.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Skinner MK, Manikkam M, Tracey R, Guerrero-Bosagna C, Haque MM, Nilsson E.. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med 2013; 11:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nakamura Y, Yamamoto Y, Usui F, Mushika T, Ono T, Setioko AR, Takeda K, Nirasawa K, Kagami H, Tagami T.. Migration and proliferation of primordial germ cells in the early chicken embryo. Poult Sci 2007; 86:2182–93. [DOI] [PubMed] [Google Scholar]
- 84. De Melo Bernardo A, Sprenkels K, Rodrigues G, Noce T, Chuva De Sousa Lopes SM.. Chicken primordial germ cells use the anterior vitelline veins to enter the embryonic circulation. Biol Open 2012; 1:1146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rengaraj D, Lee BR, Lee SI, Seo HW, Han JY.. Expression patterns and miRNA regulation of DNA methyltransferases in chicken primordial germ cells. PLoS One 2011; 6:e19524.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ragunathan K, Jih G, Moazed D.. Epigenetics. Epigenetic inheritance uncoupled from sequence-specific recruitment. Science 2015; 348:1258699.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bohacek J, Mansuy IM.. A guide to designing germline-dependent epigenetic inheritance experiments in mammals. Nat Methods 2017; 14:243–9. [DOI] [PubMed] [Google Scholar]
- 88. Bell JT, Pai AA, Pickrell JK, Gaffney DJ, Pique-Regi R, Degner JF, Gilad Y, Pritchard JK.. DNA methylation patterns associate with genetic and gene expression variation in HapMap cell lines. Genome Biol 2011; 12:R10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gibbs JR, van der Brug MP, Hernandez DG, Traynor BJ, Nalls MA, Lai SL, Arepalli S, Dillman A, Rafferty IP, Troncoso J, et al. Abundant quantitative trait loci exist for DNA methylation and gene expression in human brain. PLoS Genet 2010; 6:e1000952.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. International Chicken Genome Sequencing C. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 2004; 432:695. [DOI] [PubMed] [Google Scholar]
- 91. Rubin CJ, Zody MC, Eriksson J, Meadows JR, Sherwood E, Webster MT, Jiang L, Ingman M, Sharpe T, Ka S, et al. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature 2010; 464:587–91. [DOI] [PubMed] [Google Scholar]
- 92. Kain KH, Miller JW, Jones-Paris CR, Thomason RT, Lewis JD, Bader DM, Barnett JV, Zijlstra A.. The chick embryo as an expanding experimental model for cancer and cardiovascular research. Dev Dyn 2014; 243:216–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Minvielle F. What are quail good for in a chicken-focused world? Worlds Poult Sci J 2009; 65:601–8. [Google Scholar]
- 94. Laine VN, Gossmann TI, Schachtschneider KM, Garroway CJ, Madsen O, Verhoeven KJ, de Jager V, Megens HJ, Warren WC, Minx P, et al. Evolutionary signals of selection on cognition from the great tit genome and methylome. Nat Commun 2016; 7:10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Steyaert S, Diddens J, Galle J, De Meester E, De Keulenaer S, Bakker A, Sohnius-Wilhelmi N, Frankl-Vilches C, Van der Linden A, Van Criekinge W, et al. A genome-wide search for epigenetically [corrected] regulated genes in zebra finch using MethylCap-seq and RNA-seq. Sci Rep 2016; 6:20957.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Whitney O, Pfenning AR, Howard JT, Blatti CA, Liu F, Ward JM, Wang R, Audet JN, Kellis M, Mukherjee S, et al. Core and region-enriched networks of behaviorally regulated genes and the singing genome. Science 2014; 346:1256780.. [DOI] [PMC free article] [PubMed] [Google Scholar]