Abstract
Ample evidence suggests that environmental and occupational exposure to bisphenol A (BPA) and phthalate, two chemicals widely used in the plastics industry, disturbs homeostasis of innate immunity and causes inflammatory diseases. However, the underlying molecular mechanisms of these toxicants in the regulation of macrophage inflammatory functions remain poorly understood. In this study, we addressed the effect of chronic exposure to BPA or phthalate at levels relevant to human exposure, either in vitro or in vivo, on the inflammatory reprograming of peritoneal macrophages. Our studies revealed that BPA and phthalates adversely affected expression levels of the proinflammatory cytokines and mediators in response to lipopolysaccharide stimulation. Exposure to these toxicants also affected gene expression of scavenger receptors and phagocytic capacity of peritoneal macrophages. Our studies revealed that the epigenetic inhibitors differentially modulated target gene expression in these cells. Further analysis revealed that certain histone modification enzymes were aberrantly expressed in response to BPA or phthalate exposure, leading to alteration in the levels of H3K36 acetylation and dimethylation, two chromatin modifications that are critical for transcriptional efficacy and accuracy. Our results further revealed that silencing of H3K36-specific methyltransferase Smyd2 expression or inhibition of SMYD2 enzymatic activity attenuated H3K36 dimethylation and enhanced interleukin-6 and tumor necrosis factor-α expression but dampened the phagocytic capacity of peritoneal macrophages. In summary, our results indicate that peritoneal macrophages are vulnerable to BPA or phthalate at levels relevant to human exposure. These environmental toxicants affect phenotypic programming of macrophages via epigenetic mechanisms involving SMYD2-mediated H3K36 modification.
BPA and phthalate adversely affect the inflammatory and phagocytic activities of peritoneal resident macrophages via downregulation of SMYD2-mediated H3K36 methylation.
Recent evidence suggests that tissue macrophages originate from the progenitor cells in the yolk sac and fetal liver during embryo development. These progenitor cells migrate to different parts of the body, undergo neonatal expansion, and then develop into mature resident macrophages in adults [reviewed by Gomez Perdiguero et al. (1)]. Resident macrophages in tissues play critical roles in innate immunity and host defense. These immune cells engulf and digest almost any kind of harmful materials, such as pathogens, damaged or stressed cells, activated lymphocytes, cellular debris, and even cancerous cells, in a process termed phagocytosis. In addition, macrophages are critically involved in inflammation progression from initiation to resolution in adults [reviewed by Gomez Perdiguero et al. (1) and Davies et al. (2)]. In response to the stimulatory cues in microenvironments, macrophages undergo reprogramming and display a spectrum of overlapping phenotypes, from the inflammatory to the anti-inflammatory, that exert different functions during the process of inflammation and tissue remodeling in adults [reviewed by Novak and Koh (3)]. Any perturbation in the microenvironment will lead to aberrant macrophage activation that contributes to the pathogenesis of a variety of chronic inflammatory diseases.
Macrophage inflammatory activation is tightly controlled by the feedforward and feedback inhibitory signaling mechanisms. In response to inflammatory stimuli, macrophages undergo reprogramming by producing proinflammatory cytokines/mediators [e.g., tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, and nitric oxide synthase (NOS2 or inducible NOS)] and molecules that are critical for phagocytosis of damaged tissues or microorganisms. Meanwhile, feedback inhibitory loops such as the IL-10–activated signal transducer and activator of transcription (STAT) 3–suppressor of cytokine signaling 3 pathway are engaged to restrain the amplitude of inflammation and avoid excess toxicity and tissue damage (4).
Growing evidence suggests that environmental and occupational exposure to toxicants such as BPA and phthalates adversely affects macrophage function by aberrant production of the proinflammatory cytokines (5–7). However, the definitive roles and the underlying molecular mechanisms of these environmental toxicants in the regulation of macrophage reprogramming remain to be addressed. In this study, we have chronically exposed peritoneal macrophages during perinatal development or in adults to BPA or phthalates at levels relevant to human exposure. Macrophages were then activated in vitro to assess their inflammatory and phagocytic capacities. Our studies revealed that BPA and phthalate at levels relevant to human exposure adversely affected the phenotypic reprogramming of peritoneal macrophages. We found that exposure to these environmental toxicants led to changes in the chromatin landscape of the macrophage genome, indicating involvement of epigenetic mechanisms in macrophage phenotypic reprogramming.
Materials and Methods
Reagents
Bisphenol A (BPA; catalog no. 239658), di-(2-ethylhexyl) phthalate (DEHP; catalog no. 80030), 5-aza-2′-deoxycytidine (DAC; catalog no. A3656), trichostatin A (TSA; catalog no. T1952), lipopolysaccharides (LPSs; catalog no. L2654), yellow-green fluorescence–labeled, amine-modified polystyrene latex beads (catalog no. L1030), and tocopherol-stripped corn oil (catalog no. C8267) were purchased from Sigma-Aldrich (St. Louis, MO). Mono(2-ethylhexyl) phthalate (MEHP; catalog no. ALR-138N) was purchased from AccuStandard (New Haven, CT). Interferon-γ (IFN-γ; Cyt-358), interleukin (IL)-4 (Cyt-282), and IL-10 (Cyt-497) were purchased from Prospect-Tany TechnoGene Ltd. (Rehovot, Israel). LLY-507 was purchased from MedChem Express (Monmouth Junction, NJ). Antibodies against histone deacetylase (HDAC)1, HDAC2, HDAC3, HDAC4, and HDAC6, and trimethylation of histone H3 lysine 4 (H3K4me3) and H3K36me2 were obtained from Cell Signaling Technology (Danvers, MA), antibodies against H3K36ac and H3K36me3 were obtained from Active Motif (Carlsbad, CA), and antibody against F4/80 was purchased from eBioscience (San Diego, CA) (Table 1).
Table 1.
Antibodies Used in This Study
| Peptide/Protein Target | Manufacturer, Catalog No. | Species; Monoclonal or Polyclonal | Dilution | RRID |
|---|---|---|---|---|
| HDAC1 | Cell Signaling Technology, 2062 | Rabbit; polyclonal | 1:200 | AB_2118523 |
| HDAC2 | Cell Signaling Technology, 2540 | Rabbit; polyclonal | 1:200 | AB_2116822 |
| HDAC3 | Cell Signaling Technology, 2632 | Rabbit; polyclonal | 1:200 | AB_331545 |
| HDAC4 | Cell Signaling Technology, 2072 | Rabbit; polyclonal | 1:200 | AB_2232915 |
| HDAC6 | Cell Signaling Technology, 2162 | Rabbit; polyclonal | 1:200 | AB_2279600 |
| H3K4me3 | Cell Signaling Technology, 9727 | Rabbit; polyclonal | 1:200 | AB_561095 |
| H3K36ac | Active Motif, 39379 | Rabbit; polyclonal | 1:200 | AB_2614977 |
| H3K36Me2 | Cell Signaling Technology, 2901 | Rabbit; monoclonal | 1:100 | AB_1030983 |
| H3K36me3 | Active Motif, 61101 | Rabbit; polyclonal | 1:200 | AB_2615073 |
| F4/80 | eBiosciences, 14-4801-82 | Rabbit; monoclonal | 1:200 | AB_467558 |
Abbreviation: RRID, Research Resource Identifier.
Experimental animals
All experiments involving animals were conducted in accordance with US National Institutes of Health standards for the use and care of animals. The animal protocols were approved by the University of Illinois Institutional Animal Care and Use Committee. The long-term goal of our study is to investigate the contribution of peritoneal macrophages on female reproductive diseases; hence, CD-1 female mice were chosen as the resource of peritoneal macrophages (purchased from Charles River, Wilmington, MA). Mice were kept in a standard light-controlled animal room (12 hours of daylight) at 23°C to 25°C in polypropylene cages and provided Teklad Rodent Diet 2016 by Harlan (Indianapolis, IN) and reverse osmosis–treated water in glass bottles, ad libitum, to minimize exposure to estrogenic chemicals from food, water, and cages.
Peritoneal macrophage preparation and culture condition
Peritoneal macrophages were isolated and prepared as described previously. Briefly, peritoneal cells were exuded from peritoneal cavities of adult female mice with ice-cold Hanks balanced salt solution (HBSS) (Thermo Fisher Scientific, Waltham, MA). The pooled cells were washed by centrifugation and resuspended in Dulbecco’s modified Eagle medium/F12 culture medium supplemented with 10% fetal bovine serum and plated in 12-well plates (Corning Life Sciences, Lowell, MA) at a density of 1 to 2 × 106 per well. The nonadherent cells were removed 2 hours after incubation, and the remaining cells (>95% of cells are macrophages by our estimation) were cultured in fresh medium supplemented with vehicle (dimethyl sulfoxide), variable concentrations of BPA and MEHP (the bioactive metabolite of DEHP in vivo), or 10 nM of 17β-estradiol (E2) (n = 3 per treatment) for 2 days. For inflammatory activation, peritoneal macrophages were cultured in the presence of IFN-γ (150 U/mL) plus a suboptimal dose of LPS (10 ng/mL) for 12 hours. For IL-10–mediated anti-M1 activation, peritoneal macrophages were cultured for 12 hours in the presence of 50 ng/mL IL-10 along with IFN-γ (150 U/mL) plus LPS (10 ng/mL). For the epigenetic inhibitors studies, 4 hours prior to activation, the cells were pretreated with HDAC inhibitor TSA (25 nM), DNA methyltransferase (DNMT) inhibitor , DAC (50 nM), or histone methyltransferase EZH2 inhibitor 3-deazaneplanocin A (DZNep) (5 µM), respectively. For the methyltransferase inhibitor studies, peritoneal macrophages were cultured in the presence of 1 µM of LLY-507 for 24 hours and then subjected to inflammatory activation.
Chronic BPA and phthalate exposure protocol in female mice
The chronic exposure protocol was performed as described in our earlier studies (8, 9). Briefly, BPA and DEHP were dissolved in dimethyl sulfoxide and then diluted in tocopherol-stripped corn oil. Female mice were exposed to BPA or DEHP at 0.2 to 200 μg/kg by three equal feedings in a day (corresponding to 0.6 to 600 μg/kg/d) by placing a micropipette with the dosing solution at one side of the mouth. Exposure volumes were adjusted daily based on body weight (1 µL/g). The dose of 20 μg/kg of BPA or phthalate is relevant to exposure in occupational workers and is close to the dose that is considered safe for human consumption (i.e., 50 µg/kg/d) (10, 11).
For developmental exposure, female CD1 mice at 8 weeks of age were housed with fertile male mice. Pregnancy was confirmed by the appearance of vaginal sperm plug and designated as gestational day (GD)1. At GD9, the pregnant female mice were randomly assigned to six groups (6 to 8 mice per group) and exposed to vehicle, BPA, or DEHP at 0.6 to 600 μg/kg/d until GD17. These mice were allowed to deliver naturally, and the day of delivery was designated as postnatal day (PND)0. Beginning at PND1 until PND10, all of the pups were further exposed to BPA or DEHP orally at the same level as that of their mothers. At PND42, peritoneal macrophages were harvested from female mice, cell numbers were determined, and cells were stimulated without or with IFN-γ (150 U/mL) and LPS (10 ng/mL) in culture for 12 hours. The exposure window for these toxicants covers the periods of fetal macrophage specification, tissue colonization, and expansion (1, 2). For adult exposure, CD1 female mice (PND42) were randomly separated into three groups and chronically exposed to vehicle, BPA, or DEHP at 60 µg/kg/d (10 mice/group) for 3 weeks. Peritoneal macrophages were harvested separately and stimulated without (control) or with IFN-γ (150 U/mL) and LPS (10 ng/mL) for 12 hours.
It is estimated that human daily intake levels of BPA and DEHP are within 0.043 to 14.7 μg/kg/d and 16 to 135 μg/kg/d, respectively. The level of biologically active, unconjugated BPA measured in human serum ranges from 0.5 to 10 ng/mL, with an average level of ∼2 ng/mL. Levels of MEHP, a bioactive metabolite of DEHP, in human serum ranges from 1.4 to 537 ng/mL (10–12). Pharmacokinetic studies of BPA in adult and neonatal CD1 female mice have shown a linear relationship between serum levels and an oral dose of BPA ranging from 2 to 100,000 μg/kg. Oral administration of 400 μg/kg BPA resulted in a maximal unconjugated serum BPA level of 3.28 ng/mL, with an average area under the curve over a 24-hour peroid (AUC0–24) of 0.7 ng/mL (13, 14). Based on our observations, we estimate that repetitive oral exposure to BPA at 0.2 to 200 µg/kg, three times a day, would correspond to a serum BPA level of 1.64 ng/mL at 1 hour after each ingestion, and the average AUC0–24 would correspond to 1.05 ng/mL. Hence, it is plausible that the exposure levels in this study are relevant to human exposure.
Quantitative real-time polymerase chain reaction analysis
Quantitative polymerase chain reaction (qPCR) analysis was performed as described previously (8). Primer sequences corresponding to specific target genes used in qPCR are listed in Supplemental Table 1. For each target gene, the Δ cycle threshold (ΔCt) value was determined by the geometric mean of Ct values derived from three independent measurements after normalization to the geometric mean of Ct values obtained from three different housekeeping genes (Rplp0, Gapdh, and Actb). The comparative Ct (ΔΔCt) was calculated as the difference between the ΔCt values of the experimental and control samples. The fold change of gene expression in each sample relative to a control was computed as 2–ΔΔCt. The relative gene expression level was expressed as the average fold change ± standard error (SD) from at least three independent experiments.
Immunofluorescence
Immunofluorescence was performed as described previously (15). Briefly, peritoneal macrophages were fixed with 10% formalin solution (Sigma-Aldrich) and permeated with 0.25% Triton X-100 in PBS. After blocking with 10% normal serum, cells were incubated with the corresponding primary antibodies and then with Alexa Fluor® 488–conjugated secondary antibodies against rabbit or mouse immunoglobulin G heavy and light chain, respectively (Invitrogen, Carlsbad, CA). Cells were counterstained in antifade mounting medium with 4′,6-diamidino-2-phenylindole (H-1200; Vector Laboratories, Burlingame, CA), examined under a confocal fluorescence microscope (BX51; Olympus, Central Valley, PA), and photographed randomly at three different locations under the same setting. Images with the same magnification were analyzed by ImageJ software (ImageJ 1.49; National Institutes of Health, Bethesda, MD). Briefly, the nuclear area was determined by 4′,6-diamidino-2-phenylindole staining, and the fluorescence intensities were determined by the average gray values measured within the nuclear areas. The relative levels of protein expression were quantified by comparing the intensities of the stained cells from three independent samples (>500 cells per sample).
Detection of the inflammatory cytokines and chemokines
The levels of mouse proinflammatory cytokines IL-6, IL-10, monocyte chemoattractant protein 1/chemokine (C-C motif) ligand 2 (CCL2), IFN-γ, TNF-α, and IL-12 active heterodimer (IL-12p70) in the cell culture supernatants were detected using a Cytometric Bead Array Kit (552364; BD Biosciences, San Jose, CA) following the manufacturer’s instructions. This kit allows measurement of the selected cytokines simultaneously with limits of detection of 5, 17.5, 52.7, 2.5, 7.3, or 10.7 pg/mL, respectively. Briefly, equal volumes of test samples and diluted standards (20 to 5000 pg/mL) were mixed with the capture beads and the mouse inflammation detection reagent in assay tubes. After incubation for 2 hours at room temperature, the captured beads were washed extensively by centrifugation and analyzed by flow cytometry (The Roy J. Carver Biotechnology Center, University of Illinois at Urbana-Champaign). Acquisition was performed with Accuri™ C6 Flow Cytometer (BD Biosciences, San Jose, CA). The cytokine levels were expressed as the mean ± SD from three independent samples (pg/mL supernatant).
Nitrite oxide assay
Peritoneal macrophages (2 × 105 cells per well) were seeded in 48-well plates and cultured in the presence or absence of BPA or MEHP for 48 hours (n = 6 per treatment). At 12 hours after IFN and LPS activation, the cell culture supernatants were harvested to determine the nitrite levels using Griess reagent following the manufacturer’s instructions (catalog no. G4410; Sigma-Aldrich). The relative nitrite level in each treatment group compared with the vehicle-treated controls was expressed as average percentage ± SD.
Phagocytosis assay
Amine-modified polystyrene latex beads (fluorescent yellow-green, 1.0 μm mean particle size) were incubated with macrophages in culture at a ratio of 10:1 for 30 minutes at 37°C. The unbound beads were washed away by HBSS, and the cells were counterstained with high-affinity F-actin probe (ActinRed 555 ReadyProbes reagent, R37112; Thermo Fisher Scientific). The fluorescent beads ingested by macrophages were examined under a confocal fluorescence microscope (BX51; Olympus) and photographed randomly at three different locations under the same setting. The fluorescence intensities in the cells were analyzed by ImageJ 1.49 (National Institutes of Health, Bethesda, MD). Briefly, the intracellular areas were determined by F-actin labeling, and the fluorescence intensities were determined by the average gray values measured within the cells and expressed as average percentage ± SD from three independent replicates compared with the vehicle-treated controls.
Small interfering RNA transfection
Peritoneal macrophages (2 × 105 cells per well) were seeded in 24-well plates. After extensive washing with HBSS, the attached cells were transfected with 20 nm of mouse Symd2 small interfering RNA (siRNA) SMARTpool (M-051174-00-0005) or ON-TARGETplus control siRNA (D-001810-10-05; Dharmacon, Lafayette, CO) following the manufacturer’s protocol (INTERFERin; Polyplus-transfection, New York, NY). At 24 hours after transfection, these cells were stimulated with 150 U/mL IFN and 10 ng/mL LPS and harvested at 12 hours for qPCR analysis to assess the relative level of gene expression or at 4 hours for immunofluorescence to assess the level of H3K36 dimethylation.
Statistical analysis
All numerical values were obtained from at least three independent samples and analyzed by one-way analysis of variance followed by the Dunnett test when comparisons were made between a control group and more than one experimental group or by Student t test for single comparison (GraphPad Prism 5.0; GraphPad Software, Inc., San Diego, CA). Data were expressed as mean ± SD. Statistical significance was defined as P < 0.05.
Results
Impact of BPA or phthalate exposure at levels relevant to human exposure on the inflammatory activation of peritoneal macrophages in vitro
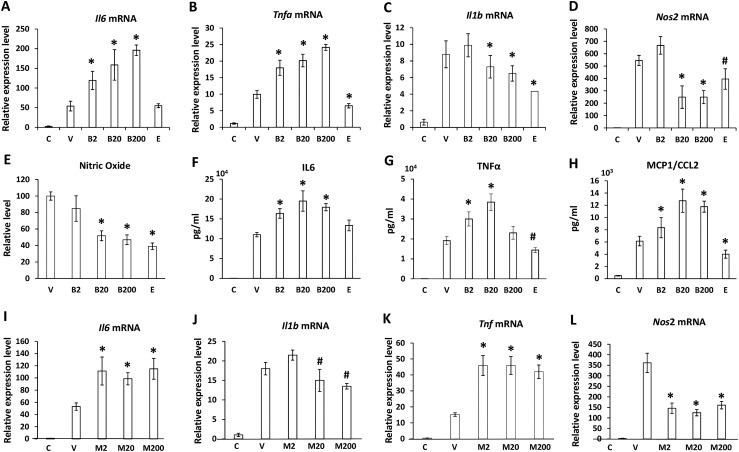
To investigate the impact of BPA and phthalate exposure on the inflammatory functions of macrophages, we collected the naïve resident macrophages from the peritoneal cavities of 20 adult female mice (16). These cells were cultured in 12-well plates in the absence or presence of 0.2 to 200 ng/mL BPA or MEHP, a bioactive metabolite of DEHP, or 10 nm E2 for 48 hours and subjected to inflammatory activation with 150 U/mL of IFN-γ and a suboptimal dose of LPS at 10 ng/mL (n = 3). The cell lysates and the culture supernatants were harvested separately to determine the relative level of gene expression and secretion of the inflammatory cytokines by qPCR and flow cytometry–based immunoassays, respectively. As shown in Fig. 1, in response to E2, messenger RNA (mRNA) and protein levels corresponding to the proinflammatory cytokines and mediators, such as Tnf, Il1b, Ccl2, and Nos2, were downregulated, indicating an anti-inflammatory role of E2 in peritoneal macrophages, as suggested previously (17). A negligible effect on inflammatory cytokine expression was observed in cells in response to exposure to BPA or MEHP at 0.2 ng/mL (data not shown). Both toxicants at 2 to 200 ng/mL adversely affected production of proinflammatory cytokines/mediators in the peritoneal macrophages in response to LPS stimulation. Whereas gene expression levels of Il6 (Fig. 1A and 1I) and Tnf (Fig. 1B and 1K) were potentiated, expression levels of Il1b (Fig. 1C and 1J) and Nos2 (Fig. 1D and 1L) were attenuated significantly. BPA also enhanced protein secretion of IL-6 (Fig. 1F), TNF-α (Fig. 1G), and monocyte chemoattractant protein 1/CCL2 (Fig. 1H) but suppressed NOS2 enzymatic activity in the cells, as indicated by reduced levels of nitric oxide production (Fig. 1E). We also investigated the impact of BPA or phthalate exposure on IL-10–mediated response in the inflammatory peritoneal macrophages. Our results showed that the levels of IL-10 mRNA expression and protein secretion were significantly elevated in response to BPA, MEHP, or E2 exposure (Supplemental Fig. 1A–1C). In addition, no appreciable changes were observed in the levels of Il1b, Il6, and Tnf mRNA expression in these cells, indicating that the IL-10–mediated anti-inflammatory response remains intact in peritoneal macrophages in response to BPA or phthalate exposure (Supplemental Fig. 1D).
Figure 1.
BPA or phthalate exposure in vitro adversely affects the inflammatory phenotype of adult peritoneal macrophages. Peritoneal macrophages exuded from adult female mice were pooled and cultured in the presence of 2, 20, or 200 ng/mL BPA (B2, B20, B200), MEHP (M2, M20, M200), 10 nM estradiol (E), or vehicle (V) for 48 hours, followed by inflammatory activation with 150 U/mL IFN-γ and 10 ng/mL LPS for an additional 12 hours. Naïve macrophages without treatment served as unstimulated controls (C). (A–D and I–L) The relative level of gene expression was assessed by qPCR. (E) Griess assay was performed to measure nitric oxide levels in the cultural supernatants. (F–H) Flow cytometry–based cytometric bead arrays were performed to measure the cytokine levels in the culture supernatants. Values represent average fold induction (± SD) in the treated samples in comparison with the nonstimulated controls (C) from three independent treatments. Statistical significance in BPA- or MEHP-exposed samples vs vehicle-treated controls were analyzed by Student t test. *P < 0.01; #P < 0.05.
Impact of BPA or phthalate exposure in vivo on the inflammatory reprogramming of peritoneal macrophages
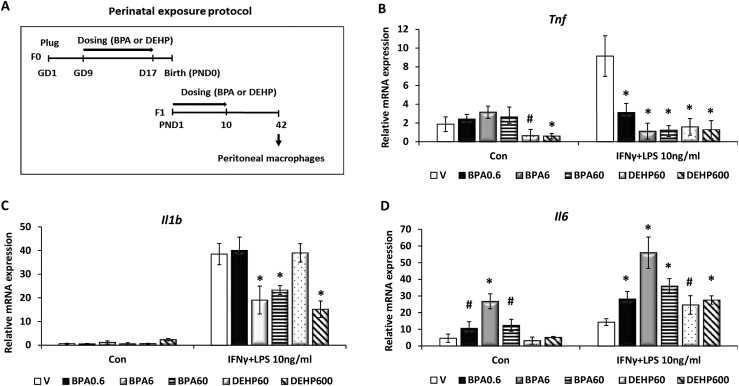
To investigate the impact of chronic BPA or phthalate exposure on the inflammatory functions of peritoneal macrophages in vivo, female mice were exposed to these chemicals perinatally as described in the section Materials and Methods, and the inflammatory activation was assessed in vitro. Briefly, BPA at 0, 0.2, 2, and 20 μg/kg and DEHP at 20 or 200 µg/kg were administered to pregnant female mice (n = 6 to 8 mice per treatment group) during GD9 to GD17 and then to neonatal female pups from PND1 to PND10 three times in a day (corresponding to 0.6 to 600 μg/kg/d). On PND42, peritoneal macrophages were harvested from two or three female mice per littermate and subjected to inflammatory activation with IFN-γ and LPS for 12 hours (Fig. 2A). qPCR was used to assess gene expression of the inflammatory cytokines. Our results revealed that Tnf and Il1b expression was suppressed in response to BPA or DEHP exposure (Fig. 2B and 2C), whereas Il6 expression was enhanced significantly in the activated macrophages (Fig. 2D). In the unstimulated naive macrophages, however, BPA enhanced the basal level of Il6 expression, whereas DEHP suppressed Tnf mRNA induction (Fig. 2B and 2D).
Figure 2.
Perinatal BPA or phthalate exposure to female mice impairs the inflammatory phenotype of adult peritoneal macrophages. (A) Perinatal exposure protocol. CD1 pregnant female mice were orally exposed to vehicle (V); 0.6, 6, 60, or 600 μg/kg/d of BPA; or DEHP from GD9 to GD17 (six to eight mice per group). After natural delivery, pups were further exposed to the same dose of chemicals as the dams from PND1 to PND10. At PND42, peritoneal macrophages were harvested from female littermates and stimulated without (Con) or with IFN-γ and LPS (10 ng/mL) for 12 hours. (B–D) Total RNAs were purified from these cells, and the relative levels of gene expression corresponding to (B) Tnf, (C) Il1b, and (D) Il6 were assessed by qPCR. Values represent average fold induction (± SD). Statistical significance in BPA- or DEHP-exposed samples vs vehicle-treated controls was analyzed by Student t test. *P < 0.01; #P < 0.05.
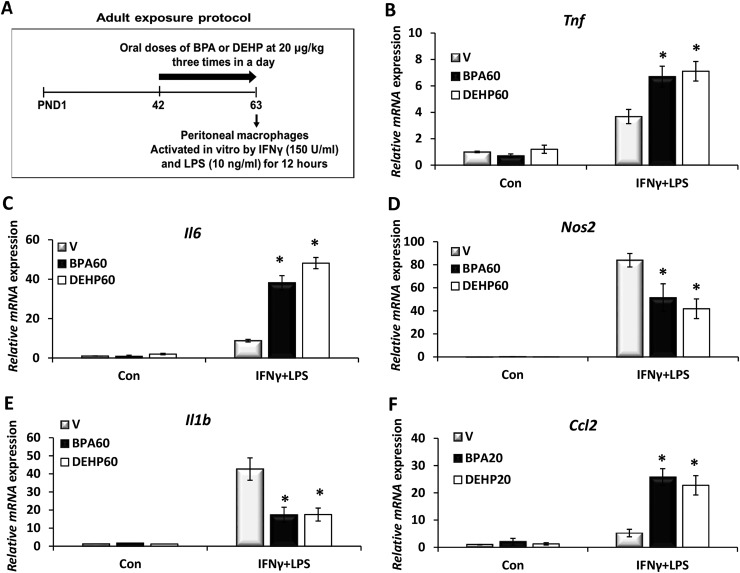
To further investigate the impact of chronic BPA or phthalate exposure on peritoneal macrophage function in adults, female mice at PND42 were orally dosed with vehicle, BPA, or DEHP at 20 μg/kg/d, three times a day, for 3 weeks (n = 10 mice per group). Peritoneal macrophages were harvested from these mice and activated in culture for 12 hours (Fig. 3A). The relative gene expression levels of the proinflammatory cytokines were assessed by qPCR. BPA and DEHP had minimal effects on proinflammatory cytokine expression in the unstimulated cells (Fig. 3). However, in response to the inflammatory activation, expression of Tnf (Fig. 3B), Il6 (Fig. 3C), and Ccl2 (Fig. 3F) was increased, whereas Nos2 (Fig. 3D) and Il1b expression (Fig. 3E) were suppressed significantly in BPA- or DEHP-exposed cells. Collectively, our results show that the inflammatory function of peritoneal macrophages is susceptible to exposure to an environmental toxicant such as BPA and phthalates.
Figure 3.
Chronic BPA or phthalate exposure to adult female mice impairs the inflammatory phenotype of peritoneal macrophages. (A) Adult exposure protocol. CD-1 female mice (PND42) were orally exposed to vehicle (V), 60 μg/kg/d of BPA, or DEHP for 3 weeks (n = 10). Peritoneal macrophages were harvested and stimulated without (Con) or with IFN-γ and LPS (10 ng/mL) for 12 hours. (B–F) Total RNAs were purified from these cells, and the relative levels of gene expression corresponding to (B) Tnf, (C) Il6, (D) Nos2, (E) Il1b, and (F) Ccl2 were assessed by qPCR. Values represent average fold induction (± SD) in three independent experiments. Statistical significance in BPA- or DEHP-exposed samples vs vehicle-treated controls was analyzed by Student t test. *P < 0.01.
BPA or phthalate impairs the phagocytic activity of peritoneal macrophages
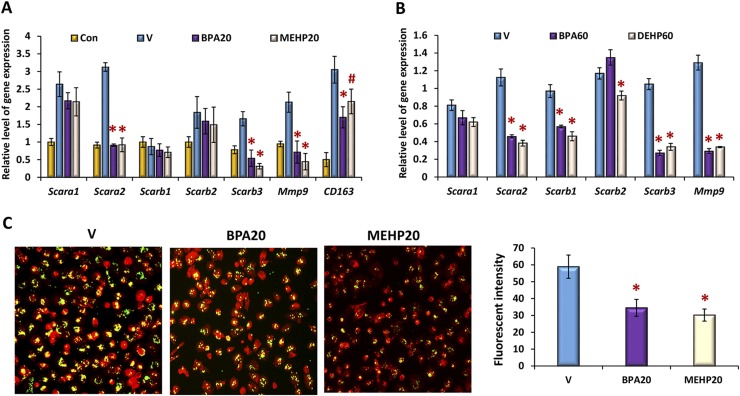
To investigate the impact of BPA and phthalate exposure on the phagocytic activity of peritoneal macrophages, we examined the expression of molecules that are critical for phagocytosis (18). Our results showed that the expression levels of mRNA corresponding to the scavenger receptors Scara2/Marco, Scarb1, Scarb3/CD36, CD163, and Mmp9 were attenuated significantly in peritoneal macrophages that were exposed to BPA or phthalate either in vitro or in vivo (Fig. 4A and 4B). We next assessed the phagocytic activity of these cells by examining the ingestion rate to latex beads. Peritoneal macrophages were cultured in the presence of 20 ng/mL of BPA or MEHP for 2 days, followed by inflammatory stimulation. These cells were then incubated with fluorescence-labeled latex beads for 30 minutes, counterstained with red-phalloidin, and visualized under a fluorescence microscope. The images of these cells were photographed, and the fluorescence intensities (green) were analyzed by the Image J program. Our results revealed that the intake rates of fluorescence-labeled beads were reduced significantly in BPA- or MEHP-exposed cells in comparison with vehicle-treated controls, indicating an impaired phagocytosis of inflammatory peritoneal macrophages in response to chemical exposure (Fig. 4C).
Figure 4.
BPA or phthalate exposure modulates the phagocytic capacity of peritoneal macrophages. Peritoneal macrophages were exposed to BPA or phthalates (A) in vitro at 20 ng/mL or (B) in vivo at 60 µg/kg/d, as described in Figs. 1 and 3. The relative levels of gene expression for phagocytic molecules were monitored by qPCR. Values represent the average fold induction of gene expression (± SD) in BPA or phthalate-exposed samples vs vehicle-treated controls (V). (C) The exposed peritoneal macrophages and the corresponding controls (V) were subjected to inflammatory activation for 12 hours and then incubated with yellow-green fluorescence–labeled latex beads for 30 minutes. After extensive washing, the cells were counterstained with red-phalloidin, and the fluorescence intensities within cells (>500 cells) were quantified by Image J and expressed as mean ± SD. Statistical significance in BPA or phthalate-exposed samples vs vehicle-treated controls was analyzed by Student t test. *P < 0.01; #P < 0.05. Con, unstimulated controls.
BPA or phthalate modulates chromatin landscape of peritoneal macrophages during reprogramming of inflammatory phenotype
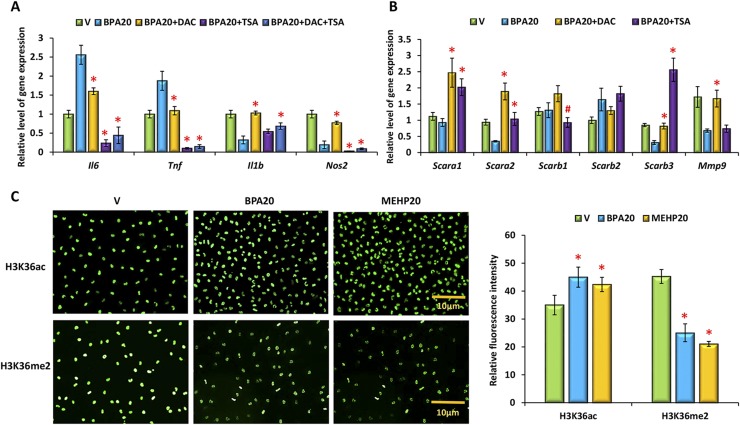
Previous studies indicated that environmental toxicants, including BPA and phthalates, are epigenetic modifiers. To investigate whether BPA or phthalate exposure could modulate inflammatory gene expression by affecting the epigenetic landscape of the macrophage genome, we used specific epigenetic inhibitors to block chromatin modification. Peritoneal macrophages were cultured in the presence of BPA at 20 ng/mL for 2 days. Four hours prior to inflammatory stimulation, these cells were pretreated with a suboptimal dose of the HDAC inhibitor TSA (25 nM), the DNA methyltransferase inhibitor DAC (50 nM), a histone methyltransferase EZH2 inhibitor DZNep (5 µM), or a combination of DAC and TSA, respectively. Total RNAs were purified from these cells, and gene expression levels were assessed by qPCR analysis. The epigenetic inhibitors differentially regulated target gene expression (Fig. 5A). Whereas DZNep at 5 µM exhibited a minimal effect on expression of these genes (data not shown), DAC suppressed Il6 and Tnf expression and rescued Il1b and Nos2 expression in BPA-exposed peritoneal macrophages. TSA abolished Il6, Tnf, and Nos2 expression but had minimal effect on Il1b mRNA expression. We also monitored expression levels of the molecules that are critical for phagocytosis. Our qPCR results showed that DAC and TSA enhanced Scara1, Scara2, Scarb3, and Mmp9 expression in BPA-exposed macrophages (Fig. 5B).
Figure 5.
BPA or phthalate modulates target gene expression in inflammatory peritoneal macrophages via chromatin modification. Peritoneal macrophages were cultured in the presence of vehicle (V), BPA, or MEHP at 20 ng/mL for 48 hours and then subjected to inflammatory activation for an additional 12 hours. (A and B) Four hours before inflammatory stimulation, cells were pretreated with vehicle, TSA (25 nM), DAC (50 nM), or a combination of TSA and DAC. The relative level of gene expression was assessed by qPCR. Values represent the average fold induction of gene expression (± SD) in BPA or phthalate-exposed samples vs vehicle-treated controls. (C) H3K36 acetylation and dimethylation was assessed by immunofluorescence using the specific antibodies. The fluorescence intensities within the nuclei were quantified by Image J and expressed as mean ± SD. Statistical significance in BPA or phthalate-exposed samples vs vehicle-treated controls was analyzed by Student t test (>500 cells) from three independent experiments. *P < 0.01; #P < 0.05.
We next monitored expression of HDACs and histone methylation in peritoneal macrophages in response to BPA or MEHP exposure by immunofluorescence analysis. The staining intensities in the nuclei of these cells were quantified by Image J. We found that both BPA and MEHP enhanced HDAC3, HDAC4, and HDAC6 expression but did not affect HDAC1 and HDAC2 expression in these cells. Regarding histone methylation, BPA and MEHP had no apparent effect on the trimethylation of H3K4, H3K27, and H3K36 (Supplemental Fig. 2). Our studies revealed that these toxicants markedly modulated the global levels of H3K36 acetylation and dimethylation. Although H3K36 acetylation was enhanced slightly, we found that dimethylation of H3K36 was attenuated significantly in inflammatory macrophages upon BPA or phthalate exposure (Fig. 5C).
To understand better the cause of alteration in the level of H3K36 methylation in the inflammatory peritoneal macrophages, we examined the expression of well-known methyltransferases and demethylases that are specific for H3K36 (19, 20). Our results showed that expression levels of Nsd2, Setmar, and Smyd2, which catalyze H3K36 dimethylation, were affected by BPA or MEHP exposure. In addition, expression of H3K36 demethylases Kdm2a, Kdm4a, and Kdm8 was altered in these cells (Supplemental Fig. 3). These results clearly indicate that these environmental toxicants have the potential to modify the chromatin landscape of macrophages and affect the transcription of target genes that are critical for inflammatory response and phagocytosis.
Suppression of SMYD2-mediated H3K36 dimethylation impairs the phagocytic capacity of peritoneal macrophages
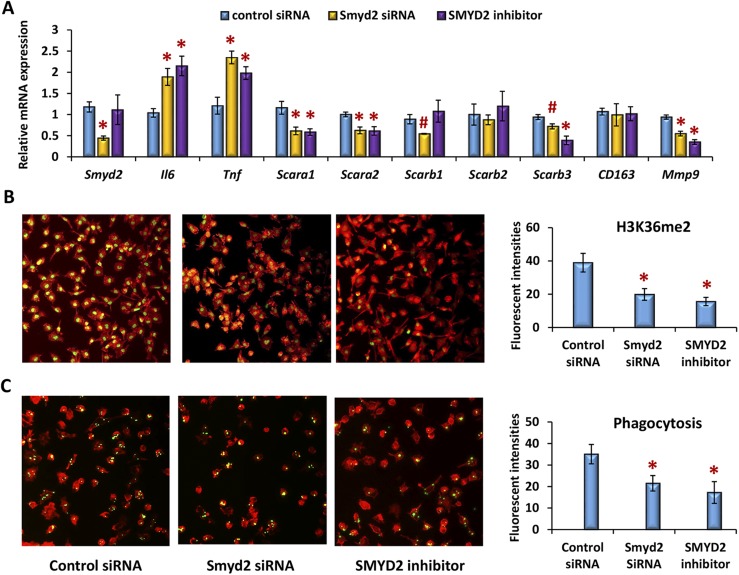
SMYD2 is a protein-lysine N-methyltransferase that methylates both histones and nonhistone proteins and specifically catalyzes dimethylation of histone H3K36 (21). Recent studies showed that SMYD2 is downregulated by LPS in inflammatory macrophages. SMYD2 also suppresses gene expression of Tnf and Il6 by subsiding H3K36 dimethylation in their promoters (22). To investigate the impact of SMYD2-mediated H3K36 methylation on the phagocytic activity of peritoneal macrophages, we used Smyd2 siRNA-mediated silencing and an SMYD2-specific inhibitor (LLY-507) in the cells (23). As expected, silencing of Smyd2 mRNA expression or inhibition of SMYD2 enzymatic activity attenuated H3K36 dimethylation and enhanced Il6 or Tnf gene expression significantly (Fig. 6A and 6B). In addition, expression levels of scavenger receptors (e.g., Scara1, Scara2, Scarb1, Scarb3, and Mmp9) were impaired in these cells (Fig. 6A). Inhibition of SMYD2-mediated functions led to an ∼50% reduction in the phagocytic activity of peritoneal macrophages to fluorescence-labeled latex beads (Fig. 6C), indicating that suppression of SMYD2-mediated H3K36 methylation contributes, at least in part, to the impaired phagocytosis of peritoneal macrophages in response to an environmental toxicant exposure.
Figure 6.
Suppression of SMYD2-mediated H3K36 dimethylation impairs the phagocytic capacity of peritoneal macrophages. Peritoneal macrophages of adult CD-1 female mice were transfected with 20 nm of Smyd2-specific siRNA oligonucleotides and the corresponding controls or treated with 1 µM of the SMYD2-specific inhibitor LLY-507 for 24 hours. These cells were subjected to inflammatory activation for an additional 12 hours. (A) qPCR analysis was used to assess the relative level of gene expression. (B) Immunofluorescence was performed to evaluate H3K36 dimethylation. (C) Cells were incubated with yellow-green fluorescence–labeled latex beads for 30 minutes and counterstained with red-phalloidin to assess the phagocytic activity. The green fluorescence intensities within cells (>500 cells) were quantified by Image J and expressed as mean ± SD (n = 3) from three independent experiments. Statistical significance was analyzed by Student t test. *P < 0.01; #P < 0.05.
Discussion
Recent studies suggest that exposure to an environmental toxicant, such as BPA or phthalate, adversely affects innate immunity, including the inflammatory response of macrophages. The effects, however, are debatable due to the levels of exposure and the varied response to inflammatory stimulation. In this study, we performed in vitro and ex vivo studies to address the effect of these chemicals at levels relevant to human exposure on reprogramming of the inflammatory peritoneal macrophages. Consistent with recent observations obtained from human and fish macrophages (5, 24), we found that, in response to a suboptimal level of LPS, these toxic chemicals differentially modulate LPS-induced macrophage production of inflammatory cytokines/mediators. Whereas TNF and IL-6 expression levels are enhanced, the levels of IL-1β and NOS2 are attenuated simultaneously. Although the underlying molecular mechanism of this regulation remains unclear, LPS-dependent TNF-α and IL-6 production was significantly potentiated with a coincident downregulation of nitric oxide production in macrophages in response to priming with substimulatory LPS doses (25, 26). It is conceivable that BPA and phthalates may affect LPS-dependent macrophage reprogramming via modulating the common regulatory pathways that control expression of the respective bioactive cytokines/mediators, which are critical for inflammation amplitude and phagocytosis of peritoneal macrophages during inflammation.
E2 is known to affect the immune system via estrogen receptor (ER)-α– or ER-β–dependent and –independent mechanisms (27). Earlier studies have shown that E2 exposure to primary murine peritoneal macrophages suppresses the inflammatory cytokine production and accelerates the resolution of inflammation (28–30). Both ER-α and ER-β mRNA and protein are detectable in human macrophages (31). However, the biological activities of these receptors in mouse peritoneal resident macrophages remain unclear. Using transgenic mouse models in which ER-α– or ER-β–driven Cre recombinase mouse lines were crossed with Ai9-RFP mice (32), we observed that ER-β activity is barely detectable in mouse peritoneal resident macrophages (Supplemental Fig. 4), indicating ER-α as the primary receptor mediating E2 action in mouse peritoneal macrophages. This notion is supported by the observation that ablation of ER-α enhances TNF-α secretion and bacterial killing by murine macrophages (33). Activation of ER-α facilitates IL-10–dependent inflammation deactivation and polarizes macrophages to alternative activation (17, 28). Our studies showed that chronic BPA or phthalate exposure in adult peritoneal macrophages potentiates production of the proinflammatory cytokines such as IL-6 and TNF-α. BPA and phthalates are considered weak estrogens due to their low binding affinity to ER-α and ER-β (34, 35). It is possible that BPA and phthalates may interfere with ER-α–dependent gene expression in these cells, because early studies showed that antiestrogen compound inhibited BPA-induced production of the proinflammatory cytokines (24, 36). A recent study has also shown that pretreatment of E2 reduces the bioavailability of BPA in the uterus after ingestion of 50 µg/kg of BPA by female rodents (37). In addition, BPA and phthalates could act via alternative mechanisms regulating inflammatory macrophages, such as activation of PPAR or P38 and AKT-mediated MAPK pathways (7, 38).
Chromatin landscape modification through histone methylation, acetylation, and DNA methylation is critical for macrophage activation and polarization (39–41). Disturbing the balance of these modifications would lead to aberrant inflammatory responses. Accumulating evidence suggests that environmental toxicants are able to modulate long-term gene expression via histone tail modification in a variety of cell types (42, 43). In this study, we report that chronic BPA and phthalate exposure to female mice during early development adversely affects the inflammatory function of peritoneal macrophages later in life. Using epigenetic inhibitors, we further show the involvement of epigenetic modifications in the regulation of the inflammatory gene expression in peritoneal macrophages in response to BPA or phthalate exposure. DNMT inhibitor attenuated BPA/phthalate-induced Il6 and Tnf expression but rescued BPA or phthalate-mediated suppression of Il1b and Nos2 expression. HDAC inhibitor, on the other hand, abolished Il6, Tnf, and Nos2 expression. These observations suggest that BPA and phthalates could modulate the epigenetic landscape of an individual genomic locus and adversely influence transcriptional efficacy of each target gene in macrophages. Previous studies showed that HDAC family members are dynamically induced in macrophages in response to LPS stimulation (44). It is known that HDAC4 and HDAC6 regulate macrophage inflammatory functions primarily though deacetylation of nonhistone proteins (45, 46). HDAC3, however, deacetylates thousands of genomic loci (mostly at enhancer sites) and regulates LPS-responsive genes via the IFN-β–STAT1 signaling pathway (47).
Our studies showed that BPA and phthalate impairs Smyd2 expression and H3K36 methylation during phenotypic reprogramming of peritoneal macrophages in response to LPS stimulation. Early studies showed that SMYD2 promotes differentiation of hematopoietic progenitors into B lymphocyte and myeloid lineages during early development. In mature macrophages, however, SMYD2 facilitates dimethylation of H3K36 specifically at the promoters of proinflammatory genes, such as Il6 and Tnf, to suppress their transcriptional capacity (22). The molecular mechanisms by which BPA and phthalates modulate individual target gene expression via H3K36me2-mediated chromatin modification remain elusive. Ample evidence suggests that H3K36me2/3 interacts with DNMTs and HDACs at the coding region and resets local chromatin landscape in the wake of RNAPII (48, 49). Alteration in chromatin architecture at the coding regions affects genomic stability and RNA polymerase II (RNAP II)–mediated transcription, including production of long noncoding RNAs, whose expression is associated with numerous human diseases (50, 51) and environmental exposure (52, 53). Yeast cells lacking Smyd2 expression or H3K36 dimethylation display a short lifespan with an increased intragenic transcription in response to nutrient stress (54). It is plausible that environmental toxicants may impair inflammatory functions by affecting transcription integrity and stability. The SMYD2/H3K36me2-mediated changes of mRNA and long noncoding RNA expression profiles in peritoneal macrophages in response to environmental toxicants are under active investigation in our laboratory.
The impaired phagocytic activity of macrophages coupled with aberrant inflammatory activation is involved in pathogenesis of many inflammation-associated diseases, including endometriosis, whose etiology involves failure in the clearance of endometrial cells from the peritoneal cavity (55). Our current study showed that, in response to BPA or phthalates, peritoneal macrophages exhibit impairment in phagocytic activity and enhancement in inflammatory responses. It is conceivable that macrophage dysfunction would create a permissive environment in the peritoneal cavity that is favorable for endometrial cell survival, attachment, and invasion, thus promoting establishment and growth of endometrial implants at ectopic sites. The contribution of environmental toxicants to the increased susceptibility of women to endometriosis is under active investigation in our laboratory.
In summary, our current studies revealed that peritoneal macrophages are vulnerable to BPA or phthalates at levels relevant to human exposure. These environmental toxicants are able to alter Smyd2 gene expression and H3K36 modification, which impairs chromatin stability over the transcribed genes and affects transcriptional efficiency and fidelity. Identification of H3K36-mediated genetic and epigenetic changes in macrophages will help us to understand better the molecular mechanisms by which BPA, phthalates, and other environmental toxicants modulate macrophage function that ultimately contribute to the increased susceptibility of women to endometriosis and other chronic inflammation–associated diseases.
Acknowledgments
We thank Dr. CheMyong Ko at the Department of Comparative Biosciences, University of Illinois at Urbana-Champaign, for ESR1-iCre Ai9 and ESR2-iCre Ai9 transgenic mice and Yanfen Li at the Department of Bioengineering, University of Illinois at Urbana-Champaign, for fluorescence imaging analysis.
Financial Support: This research was supported by a University of Illinois at Urbana-Champaign–Office of the Vice Chancellor for Research (OVCR) award, by National Institutes of Health, National Institute of Environmental Health Sciences Grants R21 ES024198 (to Q.L.), P01 ES 022848 (to J.A.F.), R21 ES026388 (to R.A.N. and Q.L.), and US Environmental Protection Agency Grant RD83 543401 (to J.A.F.).
Disclosure Summary:
The authors have nothing to disclose.
Glossary
Abbreviations:
- BPA
bisphenol A
- Ccl2
C-C motif chemokine ligand 2
- Ct
cycle threshold
- DAC
5-Aza-2′-deoxycytidine
- DEHP
di-(2-ethylhexyl)phthalate
- DNMT
DNA methyltransferase
- DZNep
3-deazaneplanocin A
- E2
17β-estradiol
- ER
estrogen receptor
- GD
gestational day
- HBSS
Hanks balanced salt solution
- HDAC
histone deacetylase
- IFN-γ
interferon-γ
- IL
interleukin
- LPS
lipopolysaccharides
- MEHP
mono-(2-ethylhexyl)phthalate
- mRNA
messenger RNA
- NOS
nitric oxide synthase
- PND
postnatal day
- qPCR
quantitative polymerase chain reaction
- SD
standard error
- STAT
signal transducer and activator of transcription
- TNF
tumor necrosis factor
- TSA
trichostatin A
References
- 1. Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol. 2013;183(5):1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berlato C, Cassatella MA, Kinjyo I, Gatto L, Yoshimura A, Bazzoni F. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J Immunol. 2002;168(12):6404–6411. [DOI] [PubMed] [Google Scholar]
- 5. Liu Y, Mei C, Liu H, Wang H, Zeng G, Lin J, Xu M. Modulation of cytokine expression in human macrophages by endocrine-disrupting chemical Bisphenol-A. Biochem Biophys Res Commun. 2014;451(4):592–598. [DOI] [PubMed] [Google Scholar]
- 6. Teixeira D, Marques C, Pestana D, Faria A, Norberto S, Calhau C, Monteiro R. Effects of xenoestrogens in human M1 and M2 macrophage migration, cytokine release, and estrogen-related signaling pathways. Environ Toxicol. 2016;31(11):1496–1509. [DOI] [PubMed] [Google Scholar]
- 7. Bølling AK, Ovrevik J, Samuelsen JT, Holme JA, Rakkestad KE, Mathisen GH, Paulsen RE, Korsnes MS, Becher R. Mono-2-ethylhexylphthalate (MEHP) induces TNF-α release and macrophage differentiation through different signalling pathways in RAW264.7 cells. Toxicol Lett. 2012;209(1):43–50. [DOI] [PubMed] [Google Scholar]
- 8. Li Q, Davila J, Kannan A, Flaws JA, Bagchi MK, Bagchi IC. Chronic exposure to bisphenol A affects uterine function during early pregnancy in mice. Endocrinology. 2016;157(5):1764–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berger A, Ziv-Gal A, Cudiamat J, Wang W, Zhou C, Flaws JA. The effects of in utero bisphenol A exposure on the ovaries in multiple generations of mice. Reprod Toxicol. 2016;60:39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kavlock R, Barr D, Boekelheide K, Breslin W, Breysse P, Chapin R, Gaido K, Hodgson E, Marcus M, Shea K, Williams P. NTP-CERHR Expert Panel update on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol. 2006;22(3):291–399. [DOI] [PubMed] [Google Scholar]
- 11. Chapin RE, Adams J, Boekelheide K, Gray LE Jr, Hayward SW, Lees PS, McIntyre BS, Portier KM, Schnorr TM, Selevan SG, Vandenbergh JG, Woskie SR. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83(3):157–395. [DOI] [PubMed] [Google Scholar]
- 12. Hines CJ, Hopf NB, Deddens JA, Silva MJ, Calafat AM. Estimated daily intake of phthalates in occupationally exposed groups. J Expo Sci Environ Epidemiol. 2011;21(2):133–141. [DOI] [PubMed] [Google Scholar]
- 13. Doerge DR, Twaddle NC, Woodling KA, Fisher JW. Pharmacokinetics of bisphenol A in neonatal and adult rhesus monkeys. Toxicol Appl Pharmacol. 2010;248(1):1–11. [DOI] [PubMed] [Google Scholar]
- 14. Taylor JA, Vom Saal FS, Welshons WV, Drury B, Rottinghaus G, Hunt PA, Toutain PL, Laffont CM, VandeVoort CA. Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: relevance for human exposure [published correction appears in Environ Health Perspect. 2011;119(4):429]. Environ Health Perspect. 2011;119(4):422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Y, Adur MK, Kannan A, Davila J, Zhao Y, Nowak RA, Bagchi MK, Bagchi IC, Li Q. Progesterone alleviates endometriosis via inhibition of uterine cell proliferation, inflammation and angiogenesis in an immunocompetent mouse model. PLoS One. 2016;11(10):e0165347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ray A, Dittel BN. Isolation of mouse peritoneal cavity cells. J Vis Exp. 2010;(35):1488. [DOI] [PMC free article] [PubMed]
- 17. Campbell L, Emmerson E, Williams H, Saville CR, Krust A, Chambon P, Mace KA, Hardman MJ. Estrogen receptor-alpha promotes alternative macrophage activation during cutaneous repair. J Invest Dermatol. 2014;134(9):2447–2457. [DOI] [PubMed] [Google Scholar]
- 18. Gordon S. Phagocytosis: an immunobiologic process. Immunity. 2016;44(3):463–475. [DOI] [PubMed] [Google Scholar]
- 19. Kuo AJ, Cheung P, Chen K, Zee BM, Kioi M, Lauring J, Xi Y, Park BH, Shi X, Garcia BA, Li W, Gozani O. NSD2 links dimethylation of histone H3 at lysine 36 to oncogenic programming. Mol Cell. 2011;44(4):609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blackledge NP, Zhou JC, Tolstorukov MY, Farcas AM, Park PJ, Klose RJ. CpG islands recruit a histone H3 lysine 36 demethylase. Mol Cell. 2010;38(2):179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown MA, Sims RJ III, Gottlieb PD, Tucker PW. Identification and characterization of Smyd2: a split SET/MYND domain-containing histone H3 lysine 36-specific methyltransferase that interacts with the Sin3 histone deacetylase complex. Mol Cancer. 2006;5(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu G, Liu G, Xiong S, Liu H, Chen X, Zheng B. The histone methyltransferase Smyd2 is a negative regulator of macrophage activation by suppressing interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) production. J Biol Chem. 2015;290(9):5414–5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nguyen H, Allali-Hassani A, Antonysamy S, Chang S, Chen LH, Curtis C, Emtage S, Fan L, Gheyi T, Li F, Liu S, Martin JR, Mendel D, Olsen JB, Pelletier L, Shatseva T, Wu S, Zhang FF, Arrowsmith CH, Brown PJ, Campbell RM, Garcia BA, Barsyte-Lovejoy D, Mader M, Vedadi M. LLY-507, a cell-active, potent, and selective inhibitor of protein-lysine methyltransferase SMYD2. J Biol Chem. 2015;290(22):13641–13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang M, Qiu W, Chen B, Chen J, Liu S, Wu M, Wang KJ. The in vitro immune modulatory effect of bisphenol A on fish macrophages via estrogen receptor α and nuclear factor-κB signaling. Environ Sci Technol. 2015;49(3):1888–1895. [DOI] [PubMed] [Google Scholar]
- 25. Zhang X, Morrison DC. Lipopolysaccharide-induced selective priming effects on tumor necrosis factor alpha and nitric oxide production in mouse peritoneal macrophages. J Exp Med. 1993;177(2):511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hirohashi N, Morrison DC. Low-dose lipopolysaccharide (LPS) pretreatment of mouse macrophages modulates LPS-dependent interleukin-6 production in vitro. Infect Immun. 1996;64(3):1011–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Villa A, Rizzi N, Vegeto E, Ciana P, Maggi A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci Rep. 2015;5(1):15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ghisletti S, Meda C, Maggi A, Vegeto E. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol. 2005;25(8):2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu L, Wang Z. Estrogen attenuates lipopolysaccharide-induced nitric oxide production in macrophages partially via the nongenomic pathway. Cell Immunol. 2013;286(1-2):53–58. [DOI] [PubMed] [Google Scholar]
- 31. Toniolo A, Fadini GP, Tedesco S, Cappellari R, Vegeto E, Maggi A, Avogaro A, Bolego C, Cignarella A. Alternative activation of human macrophages is rescued by estrogen treatment in vitro and impaired by menopausal status. J Clin Endocrinol Metab. 2015;100(1):E50–E58. [DOI] [PubMed] [Google Scholar]
- 32. Cacioppo JA, Koo Y, Lin PC, Osmulski SA, Ko CD, Ko C. Generation of an estrogen receptor beta-iCre knock-in mouse. Genesis. 2016;54(1):38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lambert KC, Curran EM, Judy BM, Lubahn DB, Estes DM. Estrogen receptor-alpha deficiency promotes increased TNF-alpha secretion and bacterial killing by murine macrophages in response to microbial stimuli in vitro. J Leukoc Biol. 2004;75(6):1166–1172. [DOI] [PubMed] [Google Scholar]
- 34. Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24(2):199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147(6, Suppl):S56–S69. [DOI] [PubMed] [Google Scholar]
- 36. Couleau N, Falla J, Beillerot A, Battaglia E, D’Innocenzo M, Plançon S, Laval-Gilly P, Bennasroune A. Effects of endocrine disruptor compounds, alone or in combination, on human macrophage-like THP-1 cell response. PLoS One. 2015;10(7):e0131428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pollock T, deCatanzaro D. Presence and bioavailability of bisphenol A in the uterus of rats and mice following single and repeated dietary administration at low doses. Reprod Toxicol. 2014;49:145–154. [DOI] [PubMed] [Google Scholar]
- 38. Rakkestad KE, Holme JA, Paulsen RE, Schwarze PE, Becher R. Mono(2-ethylhexyl) phthalate induces both pro- and anti-inflammatory responses in rat alveolar macrophages through crosstalk between p38, the lipoxygenase pathway and PPARalpha. Inhal Toxicol. 2010;22(2):140–150. [DOI] [PubMed] [Google Scholar]
- 39. Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications [published correction appears in Nature. 20083;451(7174):102]. Nature. 2007;447(7147):972–978. [DOI] [PubMed] [Google Scholar]
- 40. Serrat N, Sebastian C, Pereira-Lopes S, Valverde-Estrella L, Lloberas J, Celada A. The response of secondary genes to lipopolysaccharides in macrophages depends on histone deacetylase and phosphorylation of C/EBPβ. J Immunol. 2014;192(1):418–426. [DOI] [PubMed] [Google Scholar]
- 41. Yang X, Wang X, Liu D, Yu L, Xue B, Shi H. Epigenetic regulation of macrophage polarization by DNA methyltransferase 3b. Mol Endocrinol. 2014;28(4):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Singh S, Li SS. Epigenetic effects of environmental chemicals bisphenol A and phthalates. Int J Mol Sci. 2012;13(8):10143–10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu S, Zhu J, Li Y, Lin T, Gan L, Yuan X, Xu M, Wei G. Dynamic effect of di-2-(ethylhexyl) phthalate on testicular toxicity: epigenetic changes and their impact on gene expression. Int J Toxicol. 2010;29(2):193–200. [DOI] [PubMed] [Google Scholar]
- 44. Aung HT, Schroder K, Himes SR, Brion K, van Zuylen W, Trieu A, Suzuki H, Hayashizaki Y, Hume DA, Sweet MJ, Ravasi T. LPS regulates proinflammatory gene expression in macrophages by altering histone deacetylase expression. FASEB J. 2006;20(9):1315–1327. [DOI] [PubMed] [Google Scholar]
- 45. Wang B, Liu TY, Lai CH, Rao YH, Choi MC, Chi JT, Dai JW, Rathmell JC, Yao TP. Glycolysis-dependent histone deacetylase 4 degradation regulates inflammatory cytokine production. Mol Biol Cell. 2014;25(21):3300–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yan B, Xie S, Liu Z, Ran J, Li Y, Wang J, Yang Y, Zhou J, Li D, Liu M. HDAC6 deacetylase activity is critical for lipopolysaccharide-induced activation of macrophages. PLoS One. 2014;9(10):e110718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen X, Barozzi I, Termanini A, Prosperini E, Recchiuti A, Dalli J, Mietton F, Matteoli G, Hiebert S, Natoli G. Requirement for the histone deacetylase Hdac3 for the inflammatory gene expression program in macrophages. Proc Natl Acad Sci USA. 2012;109(42):E2865–E2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dhayalan A, Rajavelu A, Rathert P, Tamas R, Jurkowska RZ, Ragozin S, Jeltsch A. The Dnmt3a PWWP domain reads histone 3 lysine 36 trimethylation and guides DNA methylation. J Biol Chem. 2010;285(34):26114–26120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gilbert TM, McDaniel SL, Byrum SD, Cades JA, Dancy BC, Wade H, Tackett AJ, Strahl BD, Taverna SD. A PWWP domain-containing protein targets the NuA3 acetyltransferase complex via histone H3 lysine 36 trimethylation to coordinate transcriptional elongation at coding regions. Mol Cell Proteomics. 2014;13(11):2883–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81(1):145–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, Poliakov A, Cao X, Dhanasekaran SM, Wu YM, Robinson DR, Beer DG, Feng FY, Iyer HK, Chinnaiyan AM. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47(3):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karlsson O, Baccarelli AA. Environmental health and long non-coding RNAs. Curr Environ Health Rep. 2016;3(3):178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhou Z, Liu H, Wang C, Lu Q, Huang Q, Zheng C, Lei Y. Long non-coding RNAs as novel expression signatures modulate DNA damage and repair in cadmium toxicology. Sci Rep. 2015;5(1):15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McDaniel SL, Hepperla AJ, Huang J, Dronamraju R, Adams AT, Kulkarni VG, Davis IJ, Strahl BD. H3K36 methylation regulates nutrient stress response in saccharomyces cerevisiae by enforcing transcriptional fidelity. Cell Reports. 2017;19(11):2371–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Capobianco A, Rovere-Querini P. Endometriosis, a disease of the macrophage. Front Immunol. 2013;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]