Abstract
Early life exposures to endocrine-disrupting chemicals (EDCs) have been associated with physiological changes of endocrine-sensitive tissues throughout postnatal life. Although hormones play a critical role in skeletal growth and maintenance, the effects of prenatal EDC exposure on adult bone health are not well understood. Moreover, studies assessing skeletal changes across multiple generations are limited. In this article, we present previously unpublished data demonstrating dose-, sex-, and generation-specific changes in bone morphology and function in adult mice developmentally exposed to the model estrogenic EDC bisphenol A (BPA) at doses of 10 μg (lower dose) or 10 mg per kg bw/d (upper dose) throughout gestation and lactation. We show that F1 generation adult males, but not females, developmentally exposed to bisphenol A exhibit dose-dependent reductions in outer bone size resulting in compromised bone stiffness and strength. These structural alterations and weaker bone phenotypes in the F1 generation did not persist in the F2 generation. Instead, F2 generation males exhibited greater bone strength. The underlying mechanisms driving the EDC-induced physiological changes remain to be determined. We discuss potential molecular changes that could contribute to the EDC-induced skeletal effects, with an emphasis on epigenetic dysregulation. Furthermore, we assess the necessity of intact sex steroid receptors to mediate these effects. Expanding future assessments of EDC-induced effects to the skeleton may provide much needed insight into one of the many health effects of these chemicals and aid in regulatory decision making regarding exposure of vulnerable populations to these chemicals.
Keywords: endocrine disrupting chemicals (EDCs), bisphenol A (BPA), estrogen, bone, epigenetics, multigenerational
Introduction
The developmental origins of health and disease (DOHaD) hypothesis suggests growth conditions in utero are capable of affecting health trajectories throughout postnatal life [1]. Since its inception, the hypothesis has been applied to a variety of environmental perturbations and therapeutic areas [1, 2]. While human and rodent studies have demonstrated links between adverse early life events and increased disease risk in adulthood, the underlying mechanisms driving these changes remain unclear. The role of epigenetic dysregulation as a mediator of later life disease, however, has become an intensely investigated area of research.
The potential transmission of phenotypes across generations, known as multi- and transgenerational inheritance, has also become an important topic of discussion within the DOHaD field, particularly in mammalian systems. The manifestation of phenotypes across generations with direct exposure to the initial stimulus is known as a multigenerational effect. In the case of a gestating mother [designated the filial (F) 0 generation], an exposure could affect the mother, the developing fetus (F1 generation), and the germ cells of the fetus (F2 generation). Although the F0–F2 generations are all directly exposed to the original stimulus, the exposure occurs during distinct life stages, each possessing a unique set of molecular pathways that could be susceptible to the initial insult. As a result, the outcomes associated with the exposure may vary across generations. If effects persist to a generation with no direct exposure, the F3 generation in this example, this would be considered a transgenerational effect.
Public health concerns regarding endocrine-disrupting chemicals (EDCs) have risen given the mounting evidence of disorders in humans, wildlife, and laboratory animals linked to developmental EDC exposure [3]. Interestingly, a commonly observed phenomenon in EDC exposure studies is the occurrence of sex-specific effects [2]. One EDC that has been in the public eye is bisphenol A (BPA), a ubiquitous chemical commonly used in polycarbonate plastics and epoxy resins that has been best studied for its estrogenic activity [2]. Although BPA has relatively low binding affinity for the classical estrogen receptors α and β (ERα and ERβ, respectively), it is capable of inducing effects of comparable magnitude to endogenous estrogens in a variety of tissue types [4, 5]. Reports have also suggested that BPA may be mediating its effects via nonclassical ERs such as G protein-coupled receptor (GPER) and estrogen-related receptor (ERR) [6]. While BPA-induced phenotypes have been assessed in a variety of endocrine-sensitive tissues [2], the skeletal system has been relatively overlooked. Importantly, much of the variation in adult bone mass is established by young adulthood, suggesting bone function in later life greatly depends on proper skeletal development in early life [7]. Bone health in offspring is known to be modifiable by maternal nutrition and lifestyle [8, 9], but the effects of maternal EDC exposure on offspring skeletal development are not well-studied.
Human case studies and genetic mouse models have demonstrated critical roles for sex steroid signaling in bone remodeling [10–14]. Single and double ER knockout (KO) studies have suggested that ERα plays the predominant role in maintaining bone mass in both male and female mice [14]. While deletion of ERα reduced cortical bone thickness and cortical bone mineral density in both male and female mice, loss of ERβ alone had no significant effects on bone mass, and ERα/β null mice displayed bone phenotypes comparable to ERα single KO mice [15]. In addition to the classical receptors, the nonclassical GPER and two members of the ERR family, ERRα and ERRγ, have also been implicated in bone growth [10–13]. While not the focus of this article, androgens, independent of aromatization into estrogen, bind to their cognate receptor and stimulate additional bone mass acquisition in male mice [14]. Given the presence of multiple sex steroid receptors in the skeletal system, it is likely that EDC exposure could interfere with skeletal health.
Here, we discuss the evidence for EDC-induced changes in skeletal strength, with an emphasis on BPA. We present previously unpublished data from our own BPA exposure studies demonstrating dose-, sex-, and generation-specific differences in skeletal morphology and mechanical function following gestational and lactational exposure to BPA. Moreover, we describe, for the first time, multigenerational assessments of skeletal strength following BPA exposure transmitted through the maternal germline. In an extended discussion, we compare BPA-induced skeletal alterations with those of other well-established exogenous estrogens and discuss potential molecular mechanisms contributing to the observed phenotypes, with a focus on epigenetic dysregulation.
Effects of Developmental BPA Exposure on Adult Bone Health Vary by Dose, Sex, and Generation
In our exposure paradigm, C57BL/6J virgin female mice (designated the F0 generation) were chronically exposed to BPA through the diet at one of two doses: 10 μg or 10 mg per kg bw/d, referred to as lower and upper dose BPA, respectively. Serum assessments demonstrated that circulating BPA levels in F0 dams were comparable with those reported in humans [16, 17]. Exposures began 2 weeks prior to mating and continued through gestation and lactation, after which F1 offspring were weaned onto a control diet at postnatal day (PND) 21. F0 and F1 generation females were mated to unexposed males to produce F1 and F2 generations, respectively.
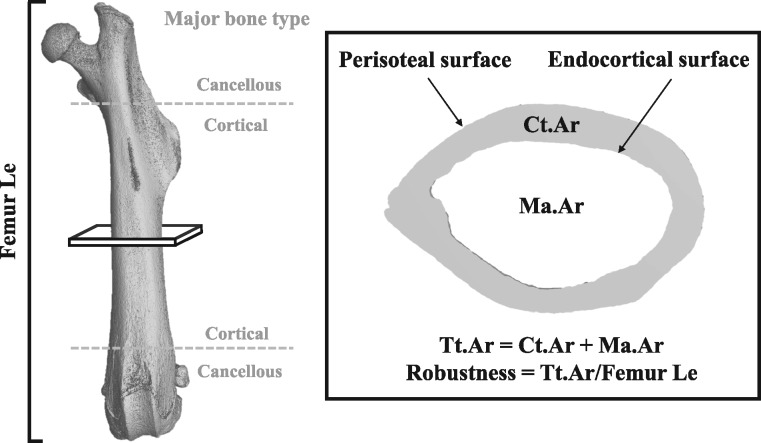
To understand the functional implications of developmental BPA exposure on adult skeletal strength, we focused our analyses on the mid-diaphysis of the femur, a region consisting primarily of cortical bone. Because cortical bone is most often used to predict bone strength, assessments in this area would directly correspond to the region analyzed in four-point bending tests for mechanical strength. We first examined femoral structure to identify biomechanical changes that may contribute to alterations in bone strength (Fig. 1, Table 1). Femur length was comparable across groups and sex, suggesting longitudinal growth, an endochondral process, was not impaired by BPA exposure. On the other hand, lateral growth, as measured by cross-sectional area of the femur, appeared to be affected in a sex- and dose-specific manner (Table 1). Total cross-sectional area, which depends on osteoblast function, was significantly reduced in upper dose males. Robustness, the ratio of total cross-sectional area to femur length and a parameter predictive of bone strength [18], was reduced in upper dose BPA male mice (Table 1). The moderately reduced cortical area of BPA-exposed males was appropriately adapted for the narrower structure (Table 1). Total cross-sectional area, robustness, and cortical area were unaffected in F1 female mice. However, upper dose BPA-exposed F1 female mice displayed reduced tissue mineral density while males were unaffected (Table 1). Thus, developmental BPA exposure is associated with dose- and sex-specific alterations in femoral morphological parameters that could influence bone strength.
Figure 1:
major bone types and morphological parameters assessed by nano-computed tomography (nanoCT) in our exposure study. The shaft of the long bone (between the gray dotted lines) is primarily composed of cortical bone, while the distal ends contain more cancellous bone. Cross-sectional bone assessments (inset) in the mid-diaphyseal region of the femur included: Ct.Ar, cortical area (gray shaded region); Ma.Ar, marrow area (inner white region); Tt.Ar, total cross-sectional area (gray + inner white regions); Femur Le, femur length.
Table 1:
percent difference in bone traits by exposure group (lower dose or upper dose) compared to the sex matched control group
| F1 males |
F1 females |
F2 males |
F2 females |
|||||
|---|---|---|---|---|---|---|---|---|
| Traits | Lower | Upper | Lower | Upper | Lower | Upper | Lower | Upper |
| N | 5 | 15 | 4 | 12 | 18 | 21 | 4 | 12 |
| BW (g) | 3.5% | −0.4% | 9.8% | −6.7% | −0.7% | 1.4% | 9.1% | 0.4% |
| Femur Le (mm) | −1.7% | −0.3% | 0.8% | −1.6% | 0.3% | −1.2% | −0.3% | 0.0% |
| Tt.Ar (mm2) | −9.0% | −7.1% | 2.2% | 0.3% | 1.7% | −2.6% | −3.5% | −4.7% |
| Robustness (mm) | −7.3% | −6.9% | 2.2% | 1.4% | 1.4% | −1.3% | −1.6% | −4.6% |
| Ct.Ar (mm2) | −3.0% | −2.2% | 2.2% | −3.0% | 2.3% | −0.1% | 1.7% | −6.8% |
| Ma.Ar (mm2) | −8.7% | −6.3% | 0.5% | 1.8% | 0.1% | −3.2% | −6.4% | −0.2% |
| TMD (mgHA/cc) | −0.8% | 0.7% | −0.2% | −1.9% | 0.4% | 0.6% | −0.9% | 2.3% |
| Stiffness (N/mm) | −19.6% | −15.9% | 9.8% | −7.6% | 12.8% | 13.7% | 9.2% | −18.2% |
| ML (N) | −13.0% | −17.0% | 9.1% | 1.7% | 14.0% | 6.4% | 9.0% | −13.4% |
| PYD (mm) | −0.6% | 12.0% | −12.0% | 40.2% | −5.3% | 16.8% | 72.0% | −26.9% |
| Work (Nm-m) | −10.6% | −7.3% | 4.2% | 36.6% | 13.4% | 21.9% | 45.0% | −32.1% |
BW and Femur Le are adjusted by age (days). All other traits have been adjusted for BW and age except Ct.Ar and TMD that have been adjusted for BW, age, and robustness. Bold font and shaded cell indicates significant differences (P < 0.05) relative to the sex-matched controls (F1 male: N = 13; F1 female: N = 8, F2 male: N = 18, F2 female: N = 9). Values were calculated from means determined with general linear model ANOVAs.
Abbreviations: BW, body weight; Ct.Ar, cortical area; Femur Le, femur length; HA, hydroxyapatite; Ma.Ar, marrow area, ML, maximum load (in Newtons); PYD, post-yield displacement; TMD, tissue mineral density; Tt.Ar, total cross-sectional area; Work, work-to-fracture.
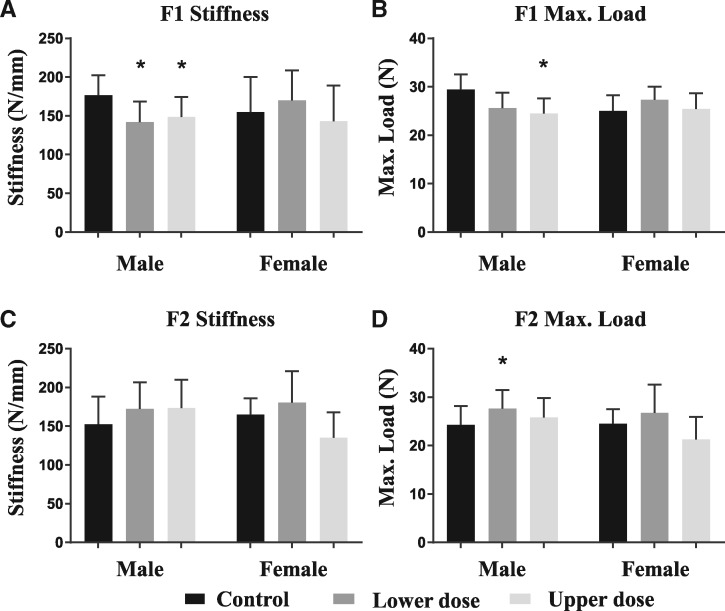
We next asked whether these morphological changes were sufficient in magnitude to elicit changes in mechanical function. Individuals with narrow bones have historically shown a higher risk of fracturing throughout life [19, 20]. Because long bone stiffness and strength are proportional to the fourth and third powers of external bone size, respectively, we hypothesized that the small reductions in external bone size in BPA-exposed males would affect whole bone strength. In females, the reduced tissue mineral density could also be predictive of enhanced susceptibility to fracture [21]. Using four-point bending to assess the maximum load (i.e. strength) femurs could withstand prior to fracture, only BPA-exposed F1 males demonstrated reduced whole bone stiffness and strength compared to controls (Fig. 2, Table 1). Given the nonlinear relationship between outer bone size and whole bone strength, it is not surprising that lower dose BPA-exposed F1 males also exhibited impaired whole bone mechanical function. F1 female bone stiffness and strength, on the other hand, were not significantly affected by BPA exposure.
Figure 2:
mean stiffness and maximum load for males and females by generation after adjustments for body mass and age from general linear model ANOVAs. Error bars are standard deviations. The first three bars in each graph represent the males for that generation while the last three represent the females. Controls are in black, lower dose in dark grey, and upper dose in light grey. The most pronounced difference is evident for first generation males because they have reduced stiffness and maximum load to the levels at or below those for females of the same generation. The adult mouse was the unit of measurement. Moving left to right through the bars on each graph A and B: F1 generation males (N= 13, 5, and 15) and F1 generation females (N= 8, 4, and 12). C and D: F2 generation males (N= 18, 18, and 21) and F2 generation females (N= 9, 4, and 12). The number of litters represented in each treatment group, moving left to right through the bars on each graph A and B: F1 generation males (N= 3, 3, and 5) and F1 generation females (N = 2, 1, and 3). C and D: F2 generation males (N = 7, 4, and 7) and F2 generation females (N = 3, 1, and 4).
BPA-induced multi- and transgenerational effects in nonskeletal endpoints have been reported by our laboratory and others [2]. Therefore, we next asked whether the BPA-induced skeletal changes in the F1 generation could also be transmitted to the subsequent (F2) generation. The reduced femoral stiffness, strength, and robustness in BPA-exposed F1 males were not observed in F2 generation males (Fig. 2, Table 1). Conversely, F2 lower dose BPA males showed increases in whole bone strength relative to controls. Unlike the F1 generation, the altered strength in the F2 lower dose males cannot be explained by any of the assessed femoral cross-sectional parameters (Table 1). The functional changes in the F2 lower dose males could be an adaptive response. Alternatively, as mentioned previously, the distinct molecular pathways susceptible to environmental perturbations at each developmental stage could drive different phenotypic outcomes. The F2 females showed no significant difference in any assessed morphological parameter compared with F2 female controls (Table 1).
In summary, developmental exposure to BPA in our exposure paradigm resulted in sex-, dose-, and generation-specific changes in morphology and function. Although some morphological changes were identified in F1 and F2 generation females, these alterations were not sufficient to generate a change in bone strength. The bone findings are consistent with our previous studies that demonstrate male-specific effects associated with developmental BPA exposure [22, 23]. We acknowledge, however, that the small sample and litter size in the lower dose F1 and F2 generation females in this study increases the possibility of a false negative finding. Most striking are the functional changes in BPA-exposed males, with deficits in F1 generation femur strength being directly associated with a narrower bone structure. Given that outer bone surface expansion of the mid-diaphysis largely depends on osteoblastic activity and to a lesser extent osteoclastic activity, the data suggest that BPA in our exposure model exerts effects in F1 generation males by suppressing osteoblast activity on the periosteal surface (Fig. 1). Future investigations assessing osteoblast number and function will provide greater insight into the sex-specific mechanism of BPA-induced skeletal pathophysiology.
Exogenous Estrogens Induce Overlapping but Distinct Effects on Skeletal Physiology
The limited studies assessing skeletal health following developmental exposure to BPA and other exogenous estrogens have reported variable outcomes (Table 2). Due to the heterogeneity in experimental design among the published reports, it is difficult to discern the source(s) of discrepancy. Differences such as rodent strain, dose, route of administration, duration of exposure, age of assessment, and bone type could all contribute to variable findings in the literature (Table 2). Regardless, these reports provide evidence for skeletal susceptibility to exogenous estrogenic EDCs.
Table 2:
skeletal consequences following early life exposure to exogenous estrogen or estrogenic EDCs
| Estrogen or EDC | Rodent strain | Dose(s); route of administration | Exposure window | Age of assessment | Bone | Sex | Bone mass | Bone strength | Ref |
|---|---|---|---|---|---|---|---|---|---|
| BPA | C57BL/6J | 10 μg/kg/d; osmotic pump | E11–PND12 | 13–23 wks | Femur | M | ↔ | ↔ | [6] |
| F | ↔ | ↔ | |||||||
| BPA | Fischer 344 | 0.5 or 50 μg/kg bw/d; drinking water | E3.5–PND22 | 5 wks | Femur | Ma | ↓ | ↔ | [24] |
| F | ↔ | ↔ | |||||||
| BPA | Wistar | 0.025, 0.250, 5, or 50 mg/kg bw/d; gavage | E7–PND22 | 12 wks | Femur | Ma | ↑/↔ | ↔ | [25] |
| F | ↔ | ↔ | |||||||
| BPA | Wistar Han | 5 μg/kg/d; gavage | E0–PND110 (continuous) | PND110 | Vertebra | M | ↔ | ND | [26] |
| F | ↔ | ND | |||||||
| DES | C57BL/6J | 0.1, 1, 10 μg/kg/d; injection | E11–E14 | 4 mos | Femur | M | ↔ | ND | [40] |
| F | ↔ | ND | |||||||
| Vertebra | M | ↔ | ND | ||||||
| Fa | ↓/↑ | ND | |||||||
| DES | CD-1 | 0.1, 2.5, 5, 10, 50, or 100 μg/kg bw/d; injection | E9–E16 | 7–9 mos | Femur | M | ND | ND | [9] |
| F | ↔ | ND | |||||||
| Vertebra | M | ND | ND | ||||||
| F | ↑ | ND | |||||||
| DES | CD-1 | 2 μg/d; injection | PND1–PND5 | 12–14 mos | Femur | M | ND | ND | [31] |
| F | ↑ | ND | |||||||
| Vertebra | M | ND | ND | ||||||
| F | ↑ | ND | |||||||
| DES | CD-1 | 2 mg/kg bw/d; injection | PND1–PND5 | 4 mos | Femura | M | ↓ | ↔ | [29] |
| F | ↑ | ↑ | |||||||
| Vertebra | M | ↓ | ↓ | ||||||
| F | ↑ | ↑ | |||||||
| DES | C57BL/Tw | 3 μg/d; injection | PND1–PND5 | 15 mos | Femur | M | ↓ | ND | [30] |
| F | ND | ND | |||||||
| Pelvis | M | ↓ | ND | ||||||
| F | ND | ND | |||||||
| DES | C57BL/6J | 0.1 μg/kg/d; osmotic pump | E11–PND12 | 13–23 wks | Femur | M | ↔ | ↓ | [6] |
| F | ↔ | ↔ | |||||||
| EE | C57BL/6J | 0.01, 0.1, or 1 μg/kg/d; osmotic pump | E11–PND12 | 10 wks | Femur | M | ND | ND | [6] |
| Fa | ↔ | ↓ | |||||||
| EE | Sprague-Dawley | 0, 2, 10, or 50 ppb (∼0–6 μg/kg bw/d); in diet | E0-PND70 (continuous) | 10 wks | Femur | M | ↔ | ND | [36] |
| F | ↔ | ND | |||||||
| Vertebra | M | ↔ | ND | ||||||
| Fa | ↓ | ND | |||||||
| Tibia | M | ND | ND | ||||||
| F | ↔ | ND | |||||||
| EB | C57BL/6J | 100 μg; injection | PND1 | 16 wks | Femur | M | ↓ | ↓ | [37] |
| F | ↔ | ↔ | |||||||
| Vertebra | M | ↓ | ND | ||||||
| F | ↔ | ND | |||||||
| EB | Fischer CDF | 1 mg/kg/d; injection | E19–PND7 | 12 wks | Femur | M | ↓ | ↔ | [38] |
| F | ND | ND | |||||||
| Vertebra | M | ↓ | ↔ | ||||||
| F | ND | ND | |||||||
| Tibia | M | ↓ | ND | ||||||
| F | ND | ND |
Abbreviations: BPA, bisphenol A; DES, diethylstilbestrol; E, embryonic day; EB, estradiol benzoate; EE, ethinyl estradiol; F, female; M, male; ND, not determined; PND, postnatal day; ↑, increase; ↓, decrease; ↔, no change.
aNonmonotonic effect.
Three other studies have assessed changes in femoral morphology and function following early life exposure to BPA, each with its own unique combination of morphological changes [6, 24, 25]. However, unlike our study, none of the previously reported morphological changes were large enough to produce effects on bone strength (Table 2). Compared with the other studies, our doses and the age of assessment are roughly equivalent. Unique to our study, though, is the route of BPA administration and duration of exposure. Our chronic exposure included the entire gestational and lactational periods. Therefore, our exposure window could be targeting a critical period in early development in which BPA exposure exerts its effects on bone strength that is not captured in the other studies. While our study evaluated BPA-induced multigenerational skeletal effects transmitted through the maternal germline, one other study assessed multigenerational outcomes occurring through the paternal germline [26]. When male mice continuously exposed to BPA beginning at the time of conception were mated to unexposed females, their offspring did not exhibit any changes in bone morphology relative to controls, suggesting the skeletal effects of BPA exposure are limited to the F1 generation [26]. It is not known, however, if the affected female could have transmitted skeletal health aberrations to the F2 generation.
While BPA is structurally similar to estrogen, its pleiotropic effects suggest that its mechanisms of action may not be limited to pathways mediated by estrogen [2]. Not surprisingly, when compared to other established estrogenic chemicals, notably diethylstilbestrol (DES), estradiol benzoate (EB), and ethinyl estradiol (EE), the effects of BPA demonstrate some parallel, but also unique, changes (Table 2).
DES, a synthetic estrogen with high affinity for ER, was prescribed to pregnant women in the United States from the 1940s to the 1970s in an attempt to reduce the risk of miscarriage [27]. Both DES-exposed children and grandchildren have been reported to be at increased risk for several reproductive health-related outcomes [28], providing evidence for EDC-induced multigenerational effects in humans [2]. Unfortunately, the skeletal health consequences of DES exposure are not well-defined in humans. In rodent models, DES exposure-induced skeletal alterations have generally been sex-specific (Table 2). When affected, bone mass in males tends to be reduced [29, 30], similar to what we observed in our BPA exposure model. However, DES-exposed females tend to display increases in bone mass [9, 29, 31], while BPA-exposed females in our study were minimally affected by the exposure. It is worth noting that the route of DES administration in the majority of these studies occurred through injection, which may augment the effect of DES (Table 2). Consistent with our BPA exposure model, the morphological changes associated with DES exposure did not always predict functional changes (Table 2), again emphasizing the need to validate physiological changes in the presence of EDC-induced morphological differences.
EE and EB are synthetic estrogens most commonly used in human oral contraceptive pills and in promoting livestock weight gain, respectively [32–35]. Following developmental EE exposure, Pelch and colleagues observed a reduction in femoral strength in adult female mice that could not be explained by altered morphology [6]. The authors speculated that differences in mineral composition of EE-exposed bones may have contributed to the compromised bone strength. In a second study, continuous exposure to EE from conception throughout adult life sex-specifically decreased vertebral bone mass in female mice, while femoral and tibial mass were not affected [36]. Unfortunately, no mechanical testing was performed to determine the functional consequences associated with these morphological changes. Of all exogenous estrogen studies, the effects of BPA in our study are most similar to those following an acute administration of EB on PND1 (Table 2), which resulted in male-specific reductions in bone mass and strength, while females were unaffected [37]. A second study using a greater dose of EB coupled with a longer exposure again resulted in reduced bone mass in males but did not translate to significant changes in bone function [38]. In addition to technical variability in study design, the hallmark nonmonotonic nature of EDCs supports the possibility of distinct effects at different doses [2, 22, 23].
While most of the studies published thus far have reported small changes in skeletal morphology that do not necessarily translate into functional differences, these studies have also been limited in the type of bone (i.e. femur) assessed. Femur, tibia, and vertebrae are composed of different proportions of cortical and cancellous bone, which have been shown to be differentially affected in sex steroid receptor KO mice [14]. Cancellous bone, also known as spongy or trabecular bone, forms a web-like network within the marrow cavity and serves as the site of active bone remodeling; it is found in greater proportions at the ends of joints (Fig. 1) and in vertebrae [36, 39]. In studies that have assessed bone mass and strength in multiple bone types (Table 2), vertebrae are often the most susceptible to exposure [9, 29, 36, 38, 40]. Future BPA studies, therefore, would benefit from more comprehensive investigations of cortical and cancellous bone health in male and female offspring. The careful coordination of the morphologies and material properties of these bone components is crucial for bone functional homeostasis. Furthermore, molecular investigations are required to determine the underlying mechanistic changes driving the associated phenotypic consequences.
A Role for Epigenetic Regulation in Skeletal Dysfunction
Tremendous progress has been made in understanding the molecular mechanisms responsible for bone metabolism, with epigenetic mechanisms emerging as key regulators of skeletal homeostasis. This section will discuss the role of two major epigenetic regulatory mechanisms in bone formation: DNA methylation and histone post-translational modifications (PTMs). Of note is the extensive crosstalk that occurs between epigenetic modifications and signaling pathways involved in bone formation [2, 41, 42], adding further complexity to the identification of the driving factor(s) responsible for EDC-induced skeletal phenotypes. While in vitro studies in isolated cell populations provide a useful starting point for identifying mechanisms of EDC action on bone [39, 43], the extensive paracrine signaling that occurs between bone cell types requires in vivo validation to demonstrate functional significance. In addition, because tissue-specific transcriptional coregulators could be disrupted by estrogenic EDCs as opposed to epigenetic regulators or ERs themselves, care must be taken in generalizing EDC-induced effects across tissues.
DNA Methylation
DNA methylation involves the covalent addition of a methyl group to a cytosine base, most commonly in a CpG dinucleotide context in mammals [2]. Depending on the genomic region of the mark, DNA methylation can be associated with either gene repression or serve as a marker of an actively transcribed region [2]. DNA methyltransferases (DNMTs) add the methyl group to the cytosine; loss of DNA methylation can occur in a passive, replication-dependent manner or through an active process of methylcytosine oxidation by the ten eleven translocation (TET) family of enzymes [44]. If EDCs alter the activity of DNA methylation-associated enzymes, the consequences could be widespread. Global changes in DNA methylation can influence genomic stability as well as critical processes such as cell fate determination [45].
Multiple genes and processes have been reported to be regulated by DNA methylation. With the proper activation of key lineage-determining factors, mesenchymal stem cells (MSCs) can differentiate into various cell types, including the bone-forming osteoblasts. Runt-related transcription factor 2 (RUNX2) is critical to the regulation of early osteoblast commitment [14]. In primary human bone marrow-derived stromal cells, DNA methylation at the RUNX2 promoter was inversely correlated with its expression [43]. Target genes of RUNX2, including osteoblast differentiation markers osteocalcin, osteopontin, and osterix have also been reported to undergo changes in DNA methylation during differentiation [46–48].
Sclerostin (Sost/SOST), a paracrine factor expressed by osteocytes, or terminally differentiated osteoblasts, suppresses Wnt/β-catenin signaling in osteoblasts and subsequent bone formation [39]. Increased DNA methylation at the SOST promoter is associated with reduced expression in primary human cancellous bone [42], and both DNA methylation and expression have been reported to strongly correlate with fracture risk in humans [49]. Essential to osteoclastogenesis, the catabolic process in bone metabolism, is the activation of the receptor activator of nuclear factor kappa-B (RANK)/RANK ligand (RANKL) pathway. Activation of this pathway and subsequent osteoclast formation can be disrupted by a decoy ligand, osteoprotegerin (OPG) [50]. RANKL and OPG are both paracrine factors expressed by osteoblasts, and increased DNA methylation in their promoters is associated with reduced expression in rodent and in human osteoblast cell lines, respectively [42, 51].
Reports on estrogenic EDC-induced changes in bone DNA methylation in vivo are sparse. Nevertheless, estrogen and BPA have been reported to increase OPG expression in human osteoblasts and decrease Rank expression in murine pre-osteoclast cells in vitro [50, 52]. By reducing the availability of the RANK ligand or its receptor, both estrogen and BPA induce a net loss in RANK/RANKL signaling, ultimately impairing osteoclast activity. DES administration also impairs osteoclast activity in ovariectomized mice by simultaneously inducing OPG and suppressing RANKL expression, thus restoring ovariectomy-induced bone loss [53]. It is not known whether these changes in expression are due to epigenetic dysregulation and warrant further investigation.
The necessity of intact classical and/or nonclassical ERs to mediate these EDC-induced skeletal changes remains unclear. Estrogen-bound ERα and/or ERRα are both capable of stimulating and repressing RUNX2 activity in human and rodent osteoblasts in a developmental stage-specific manner [54–56], suggesting EDCs could similarly interfere with these processes. Moreover, whether ER-mediated signaling can influence DNA methylation in bone is not known. Work in nonskeletal tissues suggest that the expression of DNMTs and ERs is mutually regulated in humans and rodents, although directionality may vary given the tissue type, available coregulators, and the transformed state of the cells [57–60]. Developmental BPA exposure has been reported to increase DNMT expression, increase DNA methylation in the promoter of the ERα-encoding gene, Esr1, and reduce Esr1 gene transcription in a sex- and brain region-specific manner in BALB/c mice [61]. Whether these relationships translate to the skeletal system remains to be explored.
Histone Post-Translational Modifications
The most widely researched epigenetic regulators in the context of bone are enzymes that add or remove histone PTMs [46]. Histone PTMs, including methylation and acetylation, are epigenetic marks capable of influencing gene expression through alterations in chromatin accessibility as well as the recruitment or prevention of transcription factor binding that is sensitive to the modification [62]. As a family of epigenetic regulators, histone-modifying enzymes exhibit broad activity across multiple cell types, and mouse genetic deletion studies have demonstrated a role for many of these enzymes in skeletal development and homeostasis [41, 43, 46].
In MSCs, trimethylation of lysine residue 27 of histone H3 (H3K27me3) plays a critical role in lineage specification [46]. The histone methyltransferase responsible for this mark, Enhancer of Zeste 2 (EZH2), is a subunit of the Polycomb Repressive Complex 2 (PRC2) [46]. A mesenchyme-specific deletion of Ezh2 resulted in heterozygous and homozygous offspring displaying thinner cortical bone and compromised bone function [63]. In addition to deleterious effects in the skeleton, Ezh2 null mice displayed significant increases in marrow adipose, providing further evidence for EZH2 as a critical switch in MSC fate commitment [63]. Conversely, pharmacological inhibition of EZH2 in skeletally mature, ovariectomized mice alleviated estrogen-depleted bone loss [64], suggesting timing of disrupted EZH2 function as well as circulating hormone levels may influence the directionality of responses.
Among the histone-modifying enzymes, histone deacetylases (HDACs) have received the most attention for their role in bone development. HDACs are a large family of enzymes responsible for the removal of acetyl groups from histone tails and other proteins [62]. As a result, enzymes that recognize the acetyl mark can no longer bind, the interaction strength between the negatively charged DNA and positively charged histones increases, and transcription is reduced [62]. Frequently reported HDACs to be altered following estrogen and/or EDC exposure in nonskeletal tissues [65–67] are the class I HDACs (HDAC1, HDAC2, and HDAC3) and will be discussed here. While many other HDACs have also been reported to regulate bone development [41, 43, 46], their potential susceptibility to EDCs is unknown. Of note, HDAC-mediated regulation of osteogenesis has been suggested to occur via both deacetylation-dependent [68] and deacetylation-independent [69] mechanisms.
Because class I HDACs are widely expressed, constitutive loss of these enzymes results in embryonic (HDAC1 and HDAC3) or perinatal (HDAC2) lethality, underscoring the necessity for tissue-specific gene deletion models to study their precise roles in bone development [41]. In vitro evidence suggests that HDAC1 regulates osteoblast differentiation via physical interactions with RUNX2, subsequently decreasing RUNX2 target gene transcription and osteogenesis [68]. Whether HDAC1 is physically obstructing RUNX2 binding to DNA or its deacetylase activity is restricting chromatin accessibility around RUNX2 target genes remains to be determined. In dental pulp stem cells, HDAC2 depletion resulted in reduced mRNA expression of osteocalcin, a late-stage osteoblast differentiation marker, suggesting a role for HDAC2 in osteogenesis [70]. In vivo, males and females with a conditional deletion of Hdac3 in pre-osteoblastic cells demonstrated reduced trabecular and cortical bone density and thinner cortical bone, which were associated with reduced osteoblast number [71]. Concurrently, HDAC3 depletion increased marrow fat, suggesting HDAC3 also serves as a MSC lineage commitment switch [71].
Given the lack of data demonstrating estrogen-mediated modulation of EZH2 or the class I HDACs in the context of bone, we discuss reports in nonskeletal tissues as a proof of concept. The Ezh2 promoter contains multiple functional estrogen response elements, and its expression in the mammary gland of ovariectomized rats is increased upon estradiol administration [72]. To our knowledge, no studies have examined the relationship between classical or nonclassical ERs and class I HDACs in nontransformed systems. However, in cancer cell models of the breast, another well-established estrogen-sensitive tissue, it has been suggested that HDAC1 negatively regulates ERα activity by binding to multiple functional domains or by reducing transcription factor accessibility to the Esr1 gene promoter [73]. Whether estrogenic EDCs target histone modifying enzymes in bone and/or require intact ERs to mediate their effects requires further investigation.
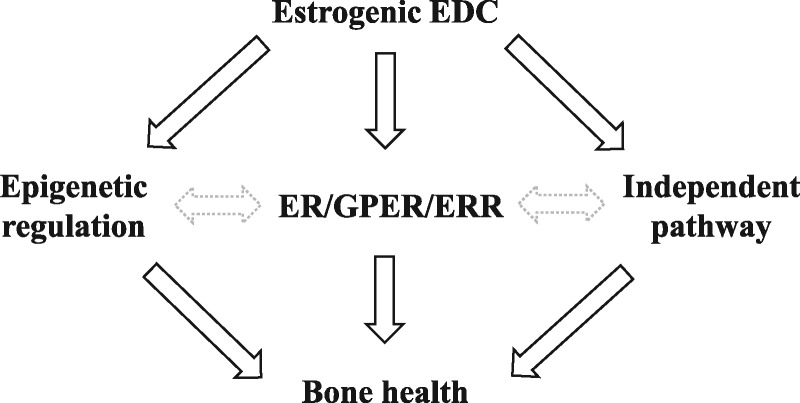
Much work is needed to determine the role of epigenetics in EDC-induced skeletal dysfunction. Based on the available literature, it is clear that epigenetic machinery is susceptible to EDC exposure in nonskeletal tissue types [2, 61]. Further, other studies have demonstrated a critical role for epigenetic regulation in proper skeletal development and maintenance [41, 43, 46]. No study to date, however, has directly assessed epigenetic involvement in the skeletal system following developmental EDC exposure. Future investigations that address this research question will provide critical insights into the mechanism(s) of EDC action on bone biology. In addition, whether the classical and/or nonclassical receptors act up- or downstream of the epigenetic modifications, if at all, in the context of bone require further study (Fig. 3). Given the paucity of information on EDC-induced molecular changes in bone, especially those related to epigenetic dysregulation, countless opportunities for novel discoveries exist.
Figure 3:
proposed pathways of estrogenic EDC-induced skeletal health effects. While EDCs can act to disrupt epigenetic regulation, and have been shown to elicit effects through classical and nonclassical estrogen receptors, whether these effects are also found in bone remain to be determined. Moreover, the link between epigenetic regulation and ER/GPER/ERR signaling in bone are not well-defined. Given the pleiotropic effects of EDCs, direct and indirect pathways independent of epigenetics and hormone receptors could also affect bone health. Solid black lines: more established connections in the currently available literature. Gray dashed lines: Less established connections that remain to be explored. ER, estrogen receptor; GPER, G protein-coupled receptor; ERR, estrogen-related receptor.
Final Considerations and Conclusion
While EDC-induced skeletal effects could be disrupting pathways in bone cells directly, it is also possible that the effects on skeletal health are secondary to other physiological changes. For example, EDCs that target MSCs and promote adipocyte differentiation at the expense of osteoblast formation would indirectly compromise bone health. Phthalates and tributyltin, two nonestrogenic EDCs, have been best studied for their ability to activate the epigenetically modifiable master regulator of adipogenesis, peroxisome proliferator activated receptor γ (PPARγ), and divert MSCs toward an adipogenic fate [74]. Additionally, adverse metabolic health in both the gestating mother and offspring have been associated with poor bone health in animal models and humans [75–78]. In our BPA exposure model, we previously reported BPA-induced gestational diabetes in F0 dams and adverse metabolic health outcomes in F1 and F2 offspring [22], suggesting BPA-induced skeletal system dysfunction could be a secondary effect of perturbed metabolic health. Assessing both metabolic and skeletal postnatal outcomes following early life EDC exposure may provide clues regarding the target cell(s) of EDC action: the co-occurrence of metabolic and skeletal alterations would warrant further investigation in progenitor cell populations while physiological changes in only one system would point toward effects in more differentiated cell types.
A greater understanding of the epigenetic reprogramming events that occur during pre-implantation embryo and fetal germ cell development will also provide greater mechanistic insight into the transmission of phenotypes to the F1 and F2 generation offspring, respectively. While the F1 zygote undergoes a global wave of epigenetic reprogramming to establish an initial pluripotent state, its germ cells undergo a second wave of reprogramming in order to reset marks in the gametes according to the sex of the developing embryo [79]. Of note, the global loss of DNA methylation in fetal germ cells, known as primordial germ cells (PGCs), is more extensive than the reprogramming following fertilization, including the erasure of previously protected imprints and some repetitive transposable elements [79]. Additionally, the identification of epigenetic modification enzymes that function exclusively during one wave of reprogramming [80] suggests unique epigenetic reprogramming mechanisms exist within each generation that may yield differential susceptibility to environmental triggers. The failure to properly erase, establish, and/or maintain these epigenetic marks during either stage of reprogramming could, therefore, contribute to distinct maladaptive, or possibly adaptive, responses to later life environment.
Although not discussed in great detail here, the molecular basis for frequently reported sex-specific responses to EDC exposure also requires further investigation. In the case of male-specific phenotypes such as our BPA exposure model, it is possible that systemic androgens are being affected. In addition to directly measuring circulating sex steroids in adult rodents, future assessments of skeletal phenotypes prior to puberty would provide further indication for the requirement of circulating hormones to elicit sex-specific skeletal effects. On the other hand, sexually dimorphic development during gestation may allow for sex-specific effects independent of pubertal and adult sex steroid actions. Placentas associated with male fetuses exhibit distinct adaptation strategies in the face of environmental perturbations relative to female-associated placentas [81]. Female fetuses are also protected from endogenous, and possibly exogenous, estrogens in the maternal milieu via alpha-fetoprotein binding, subsequently reducing estrogen-mediated actions on target cells [82]. Furthermore, sex-specific DNA methylation profiles are already apparent at birth [58], suggesting distinct fetal epigenetic programming in males and females that could be differentially impacted by EDC exposure. Finally, several genes encoding histone-modifying enzymes are located on sex chromosomes, providing other potential targets for sex-biased susceptibility to EDCs [83].
In summary, there is sufficient evidence in the literature, and results described here, to suggest developmental exposure to estrogenic EDCs can disrupt skeletal strength, but the underlying mechanisms driving these changes remain unclear. Additionally, the expression and/or activity of a variety of epigenetic enzymes, classical ERs, and nonclassical ERs are susceptible to estrogenic EDCs in vitro and in nonskeletal tissues in vivo. However, the extent to which these effects translate to the skeletal system and influence skeletal function remains to be determined (Fig. 3). Future coordinated efforts across research laboratories to systematically evaluate the impact of various exposure routes, genetic background, and outcome variables will aid in defining the molecular and physiological effects of BPA and other EDCs on skeletal strength. Ultimately, a more comprehensive analysis of the effects of EDC exposure on whole organism physiology may help guide future public policy and regulation of these chemicals and others.
Materials and Methods
Exposure and Husbandry
Animal studies were performed in accordance with protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee and have been described previously [16]. Briefly, 6–10-week old virgin female C57BL/6J mice (designated the F0 generation) were randomly assigned to one of three diets: (i) control, modified AIN-93G diet (TD.95092 with 7% corn oil substituted for 7% soybean oil; Envigo); (ii) lower dose BPA, modified AIN-93G supplemented with 50 μg/kg diet of BPA (TD.110337; Envigo); or (iii) upper dose BPA, modified AIN-93G supplemented with 50 mg/kg diet of BPA (TD.06156; Envigo). These doses were estimated to result in exposures of 10 µg BPA/kg bw/day and 10 mg BPA/kg bw/day, respectively [16]. In this exposure model, we previously reported dose-dependent increases in serum levels of unconjugated BPA in gestating dams, ranging from 0.7 ± 0.2 ng/ml in control dams to 2.0 ± 0.4 ng/ml in upper dose BPA-exposed dams [16]. The relatively low level of detectable BPA in serum of the control treatment group suggests that control animals are not entirely free of BPA exposure. Care was taken to minimize BPA exposure from other sources, such as the use of polypropylene, as opposed to polycarbonate, water bottles. However, other environmental sources of BPA, including drinking water itself and indoor air [84], may be contributing to the low background levels of BPA in our study. Importantly, animals randomly assigned to diets containing BPA still received greater BPA exposure than controls. Concentrations of parent BPA in human maternal plasma has been reported to range from 0.3 to 18.9 ng/ml [17], suggesting the doses of BPA in our exposure paradigm are representative of human exposure levels. Exposures began 2 weeks prior to mating and lasted throughout gestation and lactation (PND21), when F1 offspring were weaned onto control diets for the remainder of the study. Unexposed C57BL/6J male mice were mated with F0 and F1 females to generate F1 and F2 offspring, respectively.
Skeletal Morphology and Whole Bone Mechanical Properties
Same-sex siblings were used within a treatment group, and litter numbers are indicated in the legend of Fig. 2. The decision to use same-sex siblings was based on our previously published study [16] demonstrating a high degree of intra-litter variability, suggesting that the observed changes determined in one animal are not necessarily representative of other animals in the litter. Clustering analysis demonstrated that animals within a litter did not tend to cluster with one another more than other nonsibling animals within a treatment group (data not shown). Therefore, the individual adult animal was used as the unit of measurement. Additional analysis was performed using the dam as the unit of measurement (i.e. averaging all values of offspring from one litter into a single data point). We confirmed the differences in skeletal parameters across treatment groups exhibited the same trends, and the average value within a treatment group was not due to one deviant litter biasing the group average (data not shown).
Treatment group allocation and sex of each specimen remained blinded until quantification was complete. Left femurs of adult male and female F1 and F2 (13–22 weeks) were excised, cleaned of soft tissue, and stored at −40°C in phosphate-buffered saline (PBS). Body weights (BWs) were recorded prior to euthanasia. Since this was an ad-hoc study, a more limited age specification was not achievable. However, the 13–22 week time point represents an adult age when bone mass and external size show very little age-related change [85]. Cross-sectional morphology was quantified as described previously [86, 87]. After imaging, left femurs were loaded to failure using previously described methods for four-point bending [87–89]. Load–deflection curves were used to calculate stiffness (S), maximum load (ML), post-yield deflection (PYD), and work to fracture (work). Whole bone strength is used synonymously with ML.
Statistical Analysis
Prior to unblinding and statistical analyses, all traits were adjusted for previously established covariates known to be associated with skeletal morphology and whole bone mechanical properties [87, 89]. By using adjusted traits for the statistical analysis, we were able to minimize the contribution of the covariates in our determination of exposure-associated effects on skeletal outcomes. The adjustment was performed by GLM ANOVAs (i.e. a generalized version of a multiple regression model). The exact covariates used in each model varied depending on the trait being modeled. For BW and femur length: age was the only covariate included in the model. For total area (Tt.Ar), marrow area (Ma.Ar), robustnesss, and the mechanical traits: age and BW were covariates in the model. Finally, for cortical area (Ct.Ar) and tissue mineral density (TMD): age, BW, and robustness were included as covariates, as our previous work reported positive and negative associations of Ct.Ar and TMD with robustness, respectively [89]. Adjusting Ct.Ar and TMD for BW, age, and robustness allowed us to determine whether BPA exposure affected the ability of the system to accommodate for variations in external bone size.
Following adjustments, samples were unblinded for treatment group and sex, and statistical analysis was performed with a single factor (i.e. treatment) general linear model (GLM) ANOVA (Minitab 16.2.3; Minitab Inc, State College, PA, USA) for each sex and generation combination. Adjusted traits by treatment group were compared back with their sex-matched controls individually using Student’s t-test. A standard significance threshold of P < 0.05 was used in all cases.
Acknowledgements
Research reported in this publication was supported by the National Institutes of Health (AR065424, AR069620 to K.J.J., K99ES022244 to M.S., T32ES019851 to F.X., and ES023284 to M.S.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Due to space limitations, we frequently cited review articles – we apologize to those researchers whose original work was not directly referenced.
Conflict of interest statement. None declared.
References
- 1. Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ.. Weight in infancy and death from ischaemic heart disease. Lancet (London, England) 1989;2:577–80. [DOI] [PubMed] [Google Scholar]
- 2. Xin F, Susiarjo M, Bartolomei MS.. Multigenerational and transgenerational effects of endocrine disrupting chemicals: a role for altered epigenetic regulation? Semin. Cell Dev. Biol 2015;43:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO. Global assessment of the state-of-the-science of endocrine disruptors, WHO, 2013. http://www.who.int/ipcs/publications/new_issues/endocrine_disruptors/en/ (accessed 19 September 2017).
- 4. Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB.. The pancreatic β-cell as a target of estrogens and xenoestrogens: implications for blood glucose homeostasis and diabetes. Mol. Cell. Endocrinol. 2009;304:63–8. [DOI] [PubMed] [Google Scholar]
- 5. Watson CS, Bulayeva NN, Wozniak AL, Alyea RA.. Xenoestrogens are potent activators of nongenomic estrogenic responses. Steroids 2007;72:124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pelch KE, Carleton SM, Phillips Cl, Nagel SC.. Developmental exposure to xenoestrogens at low doses alters femur length and tensile strength in adult mice. Biol. Reprod. 2012;86:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Q, Cheng S, Alén M, Seeman E.. Finnish Calex Study Group. Bone’s structural diversity in adult females is established before puberty. J. Clin. Endocrinol. Metab. 2009;94:1555–61. [DOI] [PubMed] [Google Scholar]
- 8. Holroyd C, Harvey N, Dennison E, Cooper C.. Epigenetic influences in the developmental origins of osteoporosis. Osteoporos. Int. 2012;23:401–10. [DOI] [PubMed] [Google Scholar]
- 9. Migliaccio S, Newbold RR, Bullock BC, Jefferson WJ, Sutton FG, McLachlan JA, Korach KS.. Alterations of maternal estrogen levels during gestation affect the skeleton of female offspring. Endocrinology 1996;137:2118–25. [DOI] [PubMed] [Google Scholar]
- 10. Ford J, Hajibeigi A, Long M, Hahner L, Gore C, Hsieh J-T, Clegg D, Zerwekh J, Oz OK.. GPR30 deficiency causes increased bone mass, mineralization, and growth plate proliferative activity in male mice. J. Bone Miner. Res. 2011;26:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mårtensson UEA, Salehi SA, Windahl S, Gomez MF, Swärd K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grände P-O, et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 2009;150:687–98. [DOI] [PubMed] [Google Scholar]
- 12. Cardelli M, Aubin JE.. ERRγ is not required for skeletal development but is a RUNX2-dependent negative regulator of postnatal bone formation in male mice. PLoS One 2014;9:e109592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delhon I, Gutzwiller S, Morvan F, Rangwala S, Wyder L, Evans G, Studer A, Kneissel M, Fournier B.. Absence of estrogen receptor-related-α increases osteoblastic differentiation and cancellous bone mineral density. Endocrinology 2009;150:4463–72. [DOI] [PubMed] [Google Scholar]
- 14. Vanderschueren D, Laurent MR, Claessens F, Gielen E, Lagerquist MK, Vandenput L, Börjesson AE, Ohlsson C.. Sex steroid actions in male bone. Endocr. Rev. 2014;35:906–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, Resche-Rigon M, Gaillard-Kelly M, Baron R.. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone 2002;30:18–25. [DOI] [PubMed] [Google Scholar]
- 16. Susiarjo M, Sasson I, Mesaros C, Bartolomei Ms.. Bisphenol A exposure disrupts genomic imprinting in the mouse. PLoS Genet. 2013;9:e1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schönfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I.. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 2002;110:A703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhola S, Chen J, Fusco J, Duarte GF, Andarawis-Puri N, Ghillani R, Jepsen KJ.. Variation in childhood skeletal robustness is an important determinant of cortical area in young adults. Bone 2011;49:799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beck TJ, Ruff CB, Mourtada FA, Shaffer RA, Maxwell-Williams K, Kao GL, Sartoris DJ, Brodine S.. Dual-energy X-ray absorptiometry derived structural geometry for stress fracture prediction in male U.S. Marine Corps recruits. J. Bone Miner. Res. 2009;11:645–53. [DOI] [PubMed] [Google Scholar]
- 20. Szulc P, Munoz F, Duboeuf F, Marchand F, Delmas PD.. Low width of tubular bones is associated with increased risk of fragility fracture in elderly men – the MINOS study. Bone 2006;38:595–602. [DOI] [PubMed] [Google Scholar]
- 21. Small Re. Uses and limitations of bone mineral density measurements in the management of osteoporosis. MedGenMed 2005;7:3. [PMC free article] [PubMed] [Google Scholar]
- 22. Susiarjo M, Xin F, Bansal A, Stefaniak M, Li C, Simmons RA, Bartolomei MS.. Bisphenol A exposure disrupts metabolic health across multiple generations in the mouse. Endocrinology 2015;156:2049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bansal A, Rashid C, Xin F, Li C, Polyak E, Duemler A, van der Meer T, Stefaniak M, Wajid S, Doliba N, et al. Sex- and dose-specific effects of maternal bisphenol a exposure on pancreatic islets of first- and second-generation adult mice offspring. Environ. Health Perspect. 2017;125:97022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lind T, Lejonklou MH, Dunder L, Rasmusson A, Larsson S, Melhus H, Lind PM.. Low-dose developmental exposure to bisphenol A induces sex-specific effects in bone of Fischer 344 rat offspring. Environ. Res. 2017;159:61–8. [DOI] [PubMed] [Google Scholar]
- 25. Lejonklou MH, Lind T, Rasmusson A, Larsson S, Melhus H, Lind PM.. Developmental low-dose exposure to bisphenol A results in gender-specific and non-monotonic effects on Fischer F344 rat bone. Toxicol. Lett. 2015;238:S255. [Google Scholar]
- 26. Auxietre T-A, Dumontier M-F, Balguy I, Frapart Y, Canivenc-Lavier M-C, Berges R, Boudalia S, Auger J, Corvol M-T, Savouret J-F.. Sub-NOAEL amounts of vinclozolin and xenoestrogens target rat chondrogenesis in vivo. Biochimie 2014;99:169–77. [DOI] [PubMed] [Google Scholar]
- 27. Hilakivi-Clarke L. Maternal exposure to diethylstilbestrol during pregnancy and increased breast cancer risk in daughters. Breast Cancer Res. 2014;16:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmidt CW. Uncertain inheritance transgenerational effects of environmental exposures. Environ. Health Perspect. 2013;121:A298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaludjerovic J, Ward WE.. Diethylstilbesterol has gender-specific effects on weight gain and bone development in mice. J. Toxicol. Environ. Health Part A 2008;71:1032–42. [DOI] [PubMed] [Google Scholar]
- 30. Fukazawa Y, Nobata S, Katoh M, Tanaka M, Kobayashi S, Ohta Y, Hayashi Y, Iguchi T.. Effect of neonatal exposure to diethylstilbestrol and tamoxifen on pelvis and femur in male mice. Anat. Rec. 1996;244:416–22. [DOI] [PubMed] [Google Scholar]
- 31. Migliaccio S, Newbold RR, Bullock BC, McLachlan JA, Korach KS.. Developmental exposure to estrogens induces persistent changes in skeletal tissue. Endocrinology 1992;130:1756–8. [DOI] [PubMed] [Google Scholar]
- 32. Sitruk-Ware R. Hormonal contraception and thrombosis. Fertil. Steril. 2016;106:1289–94. [DOI] [PubMed] [Google Scholar]
- 33. Strufaldi R, Pompei LM, Steiner ML, Cunha EP, Ferreira JAS, Peixoto S, Fernandes CE.. Effects of two combined hormonal contraceptives with the same composition and different doses on female sexual function and plasma androgen levels. Contraception 2010;82:147–54. [DOI] [PubMed] [Google Scholar]
- 34. Cavalieri J, Hepworth G, Macmillan K.. Ovarian follicular development in Holstein cows following synchronisation of oestrus with oestradiol benzoate and an intravaginal progesterone releasing insert for 5–9 days and duration of the oestrous cycle and concentrations of progesterone following ovulation. Anim. Reprod. Sci. 2004;81:177–93. [DOI] [PubMed] [Google Scholar]
- 35. Rumsey TS, Hammond AC, McMurtry JP.. Response to reimplanting beef steers with estradiol benzoate and progesterone: performance, implant absorption pattern, and thyroxine status. J. Anim. Sci. 1992;70:995–1001. [DOI] [PubMed] [Google Scholar]
- 36. Hotchkiss CE, Weis C, Blaydes B, Newbold R, Delclos KB.. Multigenerational exposure to ethinyl estradiol affects bone geometry, but not bone mineral density in rats. Bone 2008;43:110–8. [DOI] [PubMed] [Google Scholar]
- 37. Connelly KJ, Larson EA, Marks DL, Klein RF.. Neonatal estrogen exposure results in biphasic age-dependent effects on the skeletal development of male mice. Endocrinology 2015;156:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fagnant HS, Uzumcu M, Buckendahl P, Dunn MG, Shupper P, Shapses SA.. Fetal and neonatal exposure to the endocrine disruptor, methoxychlor, reduces lean body mass and bone mineral density and increases cortical porosity. Calcif. Tissue Int. 2014;95:521–9. [DOI] [PubMed] [Google Scholar]
- 39. Regard JB, Zhong Z, Williams BO, Yang Y.. Wnt signaling in bone development and disease: making stronger bone with Wnts. Cold Spring Harb. Perspect. Biol. 2012;4:a007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Al Rowas S, Gawri R, Haddad R, Almaawi A, Chalifour LE, Antoniou J, Mwale F.. Effect of in utero exposure to diethylstilbestrol on lumbar and femoral bone, articular cartilage, and the intervertebral disc in male and female adult mice progeny with and without swimming exercise. Arthritis Res. Ther. 2012;2:s-0032-1319931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bradley EW, McGee-Lawrence ME, Westendorf JJ.. Hdac-mediated control of endochondral and intramembranous ossification. Crit. Rev. Eukar. Gene Expr. 2011;21:101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Husain A, Jeffries MA.. Epigenetics and bone remodeling. Curr. Osteoporos. Rep. 2017;15:450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Westendorf JJ. Histone deacetylases in control of skeletogenesis. J. Cell Biochem. 2007;102:332–340. [DOI] [PubMed] [Google Scholar]
- 44. Kohli RM, Zhang Y.. TET enzymes, TDG and the dynamics of DNA demethylation. Nature 2013;502:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schübeler D. Function and information content of DNA methylation. Nat 2015;517:321. [DOI] [PubMed] [Google Scholar]
- 46. Gordon JAR, Stein JL, Westendorf JJ, van Wijnen AJ.. Chromatin modifiers and histone modifications in bone formation, regeneration, and therapeutic intervention for bone-related disease. Bone 2015;81:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sepulveda H, Villagra A, Montecino M.. Tet-mediated DNA demethylation is required for SWI/SNF-dependent chromatin remodeling and histone-modifying activities that trigger expression of the Sp7 osteoblast master gene during mesenchymal lineage commitment. Mol. Cell Biol. 2017;37:e00177-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Andrés MC, Kingham E, Imagawa K, Gonzalez A, Roach Hi, Wilson DI, Oreffo ROC.. Epigenetic regulation during fetal femur development: DNA methylation matters. PLoS One 2013;8:e54957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reppe S, Noer A, Grimholt RM, Halldórsson BV, Medina-Gomez C, Gautvik VT, Olstad OK, Berg JP, Datta H, Estrada K, et al. Methylation of bone SOST, its mRNA, and serum sclerostin levels correlate strongly with fracture risk in postmenopausal women. J. Bone Miner. Res. 2015;30:249–56. [DOI] [PubMed] [Google Scholar]
- 50. Khalid AB, Krum SA.. Estrogen receptors alpha and beta in bone. Bone 2016;87:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Delgado-Calle J, Sañudo C, Fernández AF, García-Renedo R, Fraga Mf, Riancho JA.. Role of DNA methylation in the regulation of the RANKL-OPG system in human bone. Epigenetics 2012;7:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hwang JK, Min KH, Choi KH, Hwang YC, Jeong I-K, Ahn KJ, Chung H-Y, Chang JS.. Bisphenol A reduces differentiation and stimulates apoptosis of osteoclasts and osteoblasts. Life Sci. 2013;93:367–72. [DOI] [PubMed] [Google Scholar]
- 53. Liu M, Xiao GG, Rong P, Zhang Z, Dong J, Zhao H, Li H, Li Y, Pan J, Liu H, et al. Therapeutic effects of radix dipsaci, pyrola herb, and Cynomorium songaricum on bone metabolism of ovariectomized rats., BMC Complement. Altern. Med 2012;12:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Khalid O, Baniwal SK, Purcell DJ, Leclerc N, Gabet Y, Stallcup MR, Coetzee GA, Frenkel B.. Modulation of Runx2 activity by estrogen receptor-α: implications for osteoporosis and breast cancer. Endocrinology 2008;149:5984–5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McCarthy TL, Chang W-Z, Liu Y, Centrella M.. Runx2 integrates estrogen activity in osteoblasts. J. Biol. Chem. 2003;278:43121–9. [DOI] [PubMed] [Google Scholar]
- 56. Carnesecchi J, Vanacker J-M.. Estrogen-related receptors and the control of bone cell fate. Mol. Cell Endocrinol. 2016;432:37–43. [DOI] [PubMed] [Google Scholar]
- 57. Issa JP, Zehnbauer BA, Civin CI, Collector MI, Sharkis SJ, Davidson NE, Kaufmann SH, Baylin SB.. The estrogen receptor CpG island is methylated in most hematopoietic neoplasms. Cancer Res. 1996;56:973–77. [PubMed] [Google Scholar]
- 58. Ratnu VS, Emami MR, Bredy TW.. Genetic and epigenetic factors underlying sex differences in the regulation of gene expression in the brain. J. Neurosci. Res. 2017;95:301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yamagata Y, Asada H, Tamura I, Lee L, Maekawa R, Taniguchi K, Taketani T, Matsuoka A, Tamura H, Sugino N.. DNA methyltransferase expression in the human endometrium: down-regulation by progesterone and estrogen. Hum. Reprod. 2009;24:1126–1132. [DOI] [PubMed] [Google Scholar]
- 60. Cui M, Wen Z, Yang Z, Chen J, Wang F.. Estrogen regulates DNA methyltransferase 3B expression in Ishikawa endometrial adenocarcinoma cells. Mol. Biol. Rep. 2009;36:2201–2207. [DOI] [PubMed] [Google Scholar]
- 61. Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA.. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc. Natl. Acad. Sci. U. S. A 2013;110:9956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kouzarides T. Chromatin modifications and their function. Cell 2007;128:693–705. [DOI] [PubMed] [Google Scholar]
- 63. Hemming S, Cakouros D, Codrington J, Vandyke K, Arthur A, Zannettino A, Gronthos S.. EZH2 deletion in early mesenchyme compromises postnatal bone microarchitecture and structural integrity and accelerates remodeling. FASEB J. 2017;31:1011–1027. [DOI] [PubMed] [Google Scholar]
- 64. Dudakovic A, Camilleri ET, Riester SM, Paradise CR, Gluscevic M, O'Toole TM, Thaler R, Evans JM, Yan H, Subramaniam M, et al. Enhancer of zeste homolog 2 inhibition stimulates bone formation and mitigates bone loss caused by ovariectomy in skeletally mature mice. J. Biol. Chem. 2016;291:24594–24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jefferson WN, Chevalier DM, Phelps JY, Cantor AM, Padilla-Banks E, Newbold RR, Archer TK, Kinyamu HK, Williams CJ.. Persistently altered epigenetic marks in the mouse uterus after neonatal estrogen exposure. Mol. Endocrinol. 2013;27:1666–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kumar D, Thakur MK.. Effect of perinatal exposure to bisphenol-A on DNA methylation and histone acetylation in cerebral cortex and hippocampus of postnatal male mice. J. Toxicol. Sci. 2017;42:281–289. [DOI] [PubMed] [Google Scholar]
- 67. Fortress AM, Kim J, Poole RL, Gould TJ, Frick KM.. 17β-Estradiol regulates histone alterations associated with memory consolidation and increases Bdnf promoter acetylation in middle-aged female mice. Learn. Mem. 2014;21:457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee HW, Suh JH, Kim AY, Lee YS, Park SY, Kim JB.. Histone deacetylase 1-mediated histone modification regulates osteoblast differentiation. Mol. Endocrinol. 2006;20:2432–43. [DOI] [PubMed] [Google Scholar]
- 69. Jensen ED, Schroeder TM, Bailey J, Gopalakrishnan R, Westendorf JJ.. Histone deacetylase 7 associates with Runx2 and represses its activity during osteoblast maturation in a deacetylation-independent manner. J. Bone Miner. Res. 2007;23:361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Paino F, La Noce M, Tirino V, Naddeo P, Desiderio V, Pirozzi G, De Rosa A, Laino L, Altucci L, Papaccio G.. Histone deacetylase inhibition with valproic acid downregulates osteocalcin gene expression in human dental pulp stem cells and osteoblasts: evidence for HDAC2 involvement. Stem Cells 2014;32:279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Razidlo DF, Whitney TJ, Casper ME, McGee-Lawrence ME, Stensgard BA, Li X, Secreto FJ, Knutson SK, Hiebert SW, Westendorf JJ.. Histone deacetylase 3 depletion in osteo/chondroprogenitor cells decreases bone density and increases marrow fat. PLoS One 2010;5:e11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bhan A, Hussain I, Ansari KI, Bobzean SAM, Perrotti LI, Mandal SS.. Histone methyltransferase EZH2 is transcriptionally induced by estradiol as well as estrogenic endocrine disruptors bisphenol-A and diethylstilbestrol. J. Mol. Biol 2014;426:3426–41. [DOI] [PubMed] [Google Scholar]
- 73. Hervouet E, Cartron P-F, Jouvenot M, Delage-Mourroux R.. Epigenetic regulation of estrogen signaling in breast cancer. Epigenetics 2013;8:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bateman ME, Strong AL, McLachlan JA, Burow ME, Bunnell BA.. The effects of endocrine disruptors on adipogenesis and osteogenesis in mesenchymal stem cells: a review. Front. Endocrinol. 2017;7:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gower BA, Casazza K.. Divergent effects of obesity on bone health. J. Clin. Densitom. 2013;16:450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hamrick MW, Della-Fera MA, Baile CA, Pollock NK, Lewis RD.. Body fat as a regulator of bone mass: experimental evidence from animal models. Clin. Rev. Bone Miner. Metab. 2009;7:224–229. [Google Scholar]
- 77. Namgung R, Tsang RC.. Factors affecting newborn bone mineral content: in utero effects on newborn bone mineralization. Proc. Nutr. Soc. 2000;59:55–63. [DOI] [PubMed] [Google Scholar]
- 78. Kovacs CS, Kronenberg HM.. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr. Rev. 1997;18:832–872. [DOI] [PubMed] [Google Scholar]
- 79. Saitou M, Kagiwada S, Kurimoto K.. Epigenetic reprogramming in mouse pre-implantation development and primordial germ cells. Development 2012;139:15–31. [DOI] [PubMed] [Google Scholar]
- 80. Saitou M, Yamaji M.. Primordial germ cells in mice. Cold Spring Harb. Perspect. Biol. 2012;4:a008375.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Clifton Vl. Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta 2010;31:S33–9. [DOI] [PubMed] [Google Scholar]
- 82. McHenry J, Carrier N, Hull E, Kabbaj M.. Sex differences in anxiety and depression: role of testosterone. Front. Neuroendocrinol. 2014;35:42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Carrel L, Brown Cj.. When the lyon(ized chromosome) roars: ongoing expression from an inactive X chromosome. Phil. Trans. R. Soc. B 2017;372:20160355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Integrated Risk Information System (IRIS). Bisphenol A (CASRN 80-05-7), 1988. https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm? substance_nmbr=356 (accessed 31 August 2017).
- 85. Price C, Herman BC, Lufkin T, Goldman HM, Jepsen KJ.. Genetic variation in bone growth patterns defines adult mouse bone fragility. J. Bone Miner. Res. 2005;20:1983–91. [DOI] [PubMed] [Google Scholar]
- 86. Smith LM, Bigelow EMR, Nolan BT, Faillace ME, Nadeau JH, Jepsen KJ.. Genetic perturbations that impair functional trait interactions lead to reduced bone strength and increased fragility in mice. Bone 2014;67:130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Smith L, Bigelow EMR, Jepsen KJ.. Systematic evaluation of skeletal mechanical function. Curr. Protoc. Mouse Biol. 2013;3:39–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jepsen KJ, Goldstein SA, Kuhn JL, Schaffler MB, Bonadio J.. Type-I collagen mutation compromises the post-yield behavior of Mov13 long bone. J. Orthop. Res. 1996;14:493–9. [DOI] [PubMed] [Google Scholar]
- 89. Schlecht SH, Jepsen KJ.. Functional integration of skeletal traits: an intraskeletal assessment of bone size, mineralization, and volume covariance. Bone 2013;56:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]