Abstract
An outbreak of familial monkeypox occurred in the Central African Republic in 2015/2016 by 3 transmission modes: familial, health care–related, and transport-related. Ten people (3 children and 7 adults) were infected. Most presented with cutaneous lesions and fever, and 2 children died. The viral strain responsible was a Zaire genotype strain.
Keywords: Central African Republic, monkeypox, nosocomial outbreak
Monkeypox is an infectious disease caused by the monkeypox virus (MPXV), which belongs to the Orthopoxvirus genus of the Poxviridae family. The main clinical signs of monkeypox in humans are maculopapular lesions that initially arise on the face, in most cases, and rapidly spread in a centrifugal manner over the entire body. It can be difficult to differentiate this disease from smallpox and chickenpox on clinical grounds [1]. Most human monkeypox infections occur in Central Africa, mainly in the Democratic Republic of the Congo (DRC), but cases have also been reported in West Africa [2–6]. Human infections seem to occur after contact with animals with suspected infection, through biological fluids, a bite, or the consumption of bush meat (rodents or primates), but its spread in human populations is caused by human-to-human transmission [7]. The precise animal reservoir of this zoonosis remains unknown [1]. This virus was identified for the first time in the United States in 2003, following the importation of rodents from Ghana [8]. We report here an outbreak of monkeypox in the Central African Republic (CAR) transmitted by 3 modes: familial, health care–related, and transport-related.
MATERIAL AND METHODS
Organization of the Epidemiological Investigation of the Outbreak
There have been previous outbreaks of monkeypox in the CAR. Thus, the Ministry of Health set up community relays in all regions to raise public awareness of this disease, such that any suspect cases be rapidly and systematically declared. Moreover, standardized tools for data collection were developed and validated by the Ministry of Health and the World Health Organization (WHO). These tools were systematically used for the notification of suspect cases, to collect biological samples for diagnosis, and for data collection in the field by the investigative team. A mission composed of an investigative team of the Ministry and the WHO was commissioned to go into the outbreak area. They performed interviews with health officials of the local health care teams. They also looked at the consultation registers, health records, and hospitalization forms to complete the epidemiological data.
Isolation of the Monkeypox Virus, DNA Extraction, and Molecular Assays
The monkeypox virus was isolated and amplified by 1 passage of intracranial inoculation in the brains of newborn mice using pus or scab homogenates from patients, as previously described [9]. After dilution biopsy in sterile water, DNA extraction was performed using the QIAmp viral DNA minikit, according to the manufacturer’s instructions. Extracted DNA was stored at –20°C until subsequent analyses. DNA was quantified using the Qubit dsDNA BR Assay kit with the Qubit 2.0 fluorimeter (Life Technologies, CA). The monkeypox virus was detected in extracted DNA from pus, scab and/or blood samples, and after isolation using quantitative and conventional polymerase chain reaction (PCR), as previously described [10, 11].
Ethical Considerations
The study was approved by the Medical Ethics Committee of the Central African Republic and the Scientific Committee of the Institut Pasteur de Bangui (IPB), and written parental consent was obtained for all included patients.
Sequence Accession Number
The 2 partial sequences of ATI and HA are available in the DDBJ/EMBL/GenBank database under accession numbers MF437051 and MF437052.
RESULTS
Clinical Aspects
From December 2015 to January 2016, 10 cases of monkeypox were identified and reported in the Bakouma and Bangassou subprefectures of the Mbomou province in the eastern region of the CAR (Supplement Table 1). The first 2 clinical cases occurred in children age 5 and 9 years from a family of hunters residing in the Madigui village, roughly 10 kilometers from the town of Bakouma. The primary case (ie, index case), a 9-year-old boy, developed a rash on December 5, 2015, but the precise diagnosis of monkeypox was not confirmed until December 28, 2015. The index case fell sick after killing and cutting up a rodent known locally as “cibissi” and identified as Thryonomis. Indeed, this rodent is 1 of the game animals frequently consumed as bush meat in this area. It was trapped and removed from the trap by the child, transported back to the village, and cut up. The index case was the only 1 who had been in contact with the potentially infected biological fluids when the meat was raw. The parents were unable to give further information on the exact number of days between his contact with the rodent and the onset of the disease. His younger brother, age 5 years, developed symptoms on December 10, 2015. Both children were initially treated at the Bakouma Health Center, supported by Catholic Relief Services (CRS), on December 13, 2015, and were then transferred and admitted to the regional hospital in Bangassou, supported by Médecins Sans Frontières (MSF), on December 17, 2015. Patients were transferred in a prefectural hospital run by MSF, which had qualified personnel and adequate infrastructure for better care. Indeed, the first center did not possess any antibiotics, and the first aid health staff did not know of the disease because the qualified nurses had returned to Bangui for security reasons. Indeed, these nurses performed first aid (taking the temperature, taking blood samples, and removing the patient’s clothes) without wearing individual protective equipment, such as gloves and a mask. It was only after the first nurse became ill that the security measures were reinforced. Several other family members contracted the disease during the same period, including the boys’ mother, a 15-month-old brother, and a maternal aunt. In addition, 5 individuals outside the family developed signs of the disease: 1 hospital nurse and 1 doctor, 1 CRS health center nurse who accompanied the patients during transfer to the hospital, and 2 individuals who transported the patients to the hospital. Seven of these 10 individuals were hospitalized, and the 2 younger brothers of the primary case, who were 15 months and 5 years old, died from this disease.
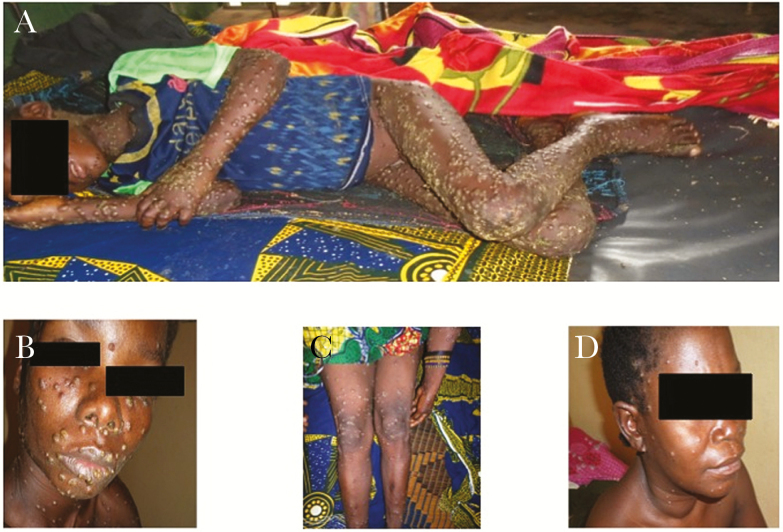
Clinically, the history of the primary case (a 9-year-old boy) began with fever and headaches on December 2, 2015 (Figure 1A). A rash appeared on the head and rapidly disseminated over the entire body. On hospital admission, the rash was vesiculopapular with umbilications, pruritic, and involved the palms of the hands and soles of the feet. The patient was treated with oral antibiotics to prevent a cutaneous bacterial infection. The patient’s condition gradually improved, although the fever persisted for more than 1 week, with the skin lesions drying up after approximately 1 week. One of the patient’s younger siblings, a 5-year-old boy, who developed his first symptoms 5 days after the onset of rash of the older brother, was also hospitalized on December 17, 2015. His skin lesions were flat and confluent and involved the palms of the hands and soles of the feet. He presented fever and cervical adenitis, severe facial edema, and bilateral conjunctivitis and was treated with intravenous antibiotics and tetracycline eye ointment. Oral and genital lesions developed during hospitalization, and the pruritus got worse on promethazine. Although his chest was clear on admission, the patient developed signs of pulmonary edema (transient pulmonary rates and oxygen desaturation), treated with furosemide and oxygen, and profound hypothermia. His condition rapidly worsened, and he died on December 25, 2015. Moreover, these 2 patients tested negative for HIV and malaria.
Figure 1.
Disseminated cutaneous lesions consisting of macules, papules, and vesicles on the entire body of the primary case (case 1) (1A), on the face (1B) and legs (1C) of the mother (case 4), and rash and cervical lymph node of the hospital nurse (case 5) (1D).
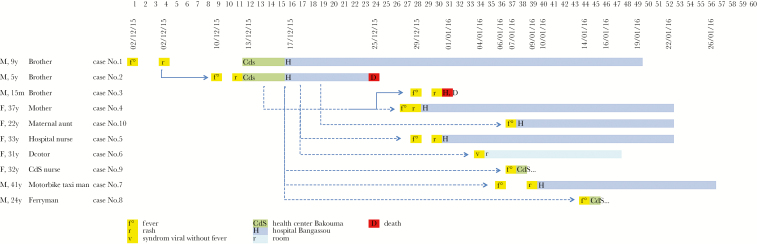
The boys’ mother began to complain of malaise, fever, and aching muscles on December 28, 2015. The following day, a few vesicles appeared on her arms and legs. The pruritic skin lesions then spread to her entire body, except for her palms and feet. Cervical and inguinal lymphadenopathy and mucous membrane involvement were observed (Figure 1, B and C). She was still breastfeeding her younger child, who was 15 months old. This child presented 2 cutaneous lesions on December 29, 2015, without involvement of the palms or the hands or the soles of the feet, or lymphadenopathy. Two days later, he developed fever and a few pruritic vesicles, treated with promethazine. This child slept more and breastfed less than usual, but, otherwise, his general condition remained good. He died suddenly, 3 days after the onset of the first symptoms, on January 1, 2016, after a brief period of agitation and hypotonia, although he had breastfed and appeared clinically well on the morning of his death. On the same day, a hospital nurse who had cared for these sick children arrived at the hospital with a vesicular rash. This rash had been preceded by fever 1 day earlier. These lesions, strongly resembling the lesions seen in the primary case, spread over the entire body, and bulky cervical nodes were detected (Figure 1D). On January 4, 2015, the doctor involved in the care of the patients presented flu-like symptoms, without fever, and several (<20) vesico-papular lesions. On January 7, 2016, the CRS health center nurse who accompanied the patients during transport from the health center to the hospital developed fever, a rash, and lymphadenopathy. Likewise, the taxi driver from the CRS, who transported the first cases by motorbike to the hospital, developed fever on January 6, 2016, and was admitted on January 10, 2016, with a maculopapular rash on the head and palms of his hands and small cervical nodes. Four days later, on January 14, 2016, the ferryboat driver who transported the patients across a river became symptomatic. Finally, the maternal aunt was hospitalized on January 16, 2016, with a fever and a maculopapular rash. All the clinical and epidemiological data, including exposure and the hypothetical pattern of viral transmission (epidemiological chain), are summarized in Figure 2 and Supplementary Table 1. However, except for the 2 transmissions between the index case and case No. 2 and between cases No. 3 and No. 4, the other people (Nos 5 to 10) may have been contaminated by either the index case or case No. 2. Indeed, no information allows us to assert which of these sources was at the origin of the contamination, given that they were together in the different hospitals and that they had contact with the same people. Furthermore, it is almost impossible to predict the exact day on which the ferryboat driver was contaminated because there was significant travel of the family members to both the village and hospital, and they were obliged to cross by ferry. However, based on the information we have, there were either 2 generations of transmission, if the mother and the other people were contaminated by the index case, or 3 generations, if these same people were contaminated by case No. 2. Indeed, in this case, the third generation would have been between the mother and the 15-month infant. Although we cannot be 100% certain, the nurses, other members of the family, and different ferryboat drivers were probably contaminated by either the index case or case No. 2 and not between themselves.
Figure 2.
Hypothetical pattern of viral transmission during the human monkeypox outbreak in Central Africa in 2015/2016. Cases are shown according to the date of disease onset. The number of the case, description of family ties with the index case, sex, and age for each infected person are shown on the left. The index case corresponds to number 1. The number of days after the estimated beginning of symptoms for the index case are shown at the top. Full arrows show confirmed transmission, whereas dotted arrows correspond to potential transmission. Indeed, we cannot be certain about the potential infectious contact or the date of infection because of the significant travel of the family members and ferryboat driver toward the village and hospital.
Virological Results
Virological investigations were performed on blood, scabs, vesicles, or pus samples from 4 people, including the primary case, at the Institut Pasteur de Bangui (CAR). However, the monkeypox virus was only detected in blood or pus samples from patients No. 1, 2, and 4 by quantitative and conventional PCR. The virus was isolated following intracranial inoculation of pus or scab homogenates that were positive for MPXV during the first molecular investigation in newborn mice in the laboratory. The cycle threshold (computed tomography from quantitative PCR) values obtained ranged from 16 to 30 for DNA extracted from primary samples and 13 to 26 for DNA extracted from the brains of inoculated mice. Sequencing of the amplicons obtained for the hemagglutinin (HA) gene and part of the A-type, including the (ATI) gene, from human biological and mouse samples showed that the same Zaire genotype strain was responsible for all 4 cases. Based on the molecular data obtained from the partial HA (328 bp) and ATI gene (836 bp) sequences, this MPXV strain, isolated in 2015, was identical to a strain detected in the DRC in 2008 [12]. However, it differed from the strains identified in 2 previously reported cases of monkeypox in the CAR in 2001 and 2010, based on the sequence of the ATI gene, which displayed 2 nucleotide variations [13]. Indeed, these nucleotide variations resulted in 2 modifications of the amino acid sequence at positions 650 (L to R) and 655 (R to K), based on the reference sequence used (KP849469, A27L gene). Further studies based on longer sequences will be necessary to better understand circulation of the virus in this region. The Bangassou region is only separated from the DRC by the Mbomou River. However, this physical barrier does not hamper population migration on either side of the river or economic exchange inside the region. Indeed, there is strong evidence to suggest that populations from both regions have shared temporary fishing camps, which may have provided an opportunity for viral transmission from 1 population to the other.
In addition to the 10 cases described above, possible sporadic monkeypox cases, strongly suspected on clinical grounds, were reported to the Institut Pasteur de Bangui in 2012 and 2015 (Supplemental Figure S1). These 5 suspected cases from Batangafo (2 cases) and Bria (3 cases, including 1 death) were familial. Virological confirmation of these infections was not possible due to the difficulties in obtaining high-quality biological samples in such remote and dangerous regions (there was a civil war at the time).
CONCLUSION
We describe here the first health care– and transport-related transmission outbreak of monkeypox in the CAR. Several other similar outbreaks have been reported, especially in the DRC, South Sudan, and the northern part of the Republic of the Congo [5]. This outbreak and the small number of sporadic cases reported here, and previously in the CAR, occurred in remote areas, which have a very poor medical infrastructure. The country’s health care system is in very poor condition for many reasons, including frequent armed conflicts over many years. Similar situations prevail in the DRC and the Republic of the Congo. In these countries and those of the surrounding area, such as Sudan, there appears to be a potential for larger and more frequent outbreaks in the future, including outbreaks involving health care–related transmission, as the proportion of people vaccinated against smallpox is gradually decreasing because anti–smallpox vaccination was halted in 1978 and the current average life expectancy is 39–40 years in the CAR. Further studies are necessary and efforts are already being made to screen DNA from various animal species living in these areas for the presence of monkeypox viruses to obtain insight into the wildlife reservoirs of this zoonotic disease.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
Author contributions. B. S., G. F. K., and S. M. G. O. performed all experimental studies. E. L., C. J., I. Z., M. V. H., J. P. F., T. D. K., and C. M. looked after all patients. All authors analyzed the data. E. N., A. G., and N. B. wrote the first version of manuscript, which was completed by E. L., C. J., I. Z., and M. V. H. All authors read and approved the final manuscript.
Financial support. This study was supported by the Institut Pasteur de Bangui, the Institut Pasteur of Paris, France (Projet ACIP A-02-2013), and the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (LabEx) research program. The CIRMF is supported by the Government of Gabon, Total-Fina-Elf Gabon, and the Ministère de la Coopération Française. The funders had no role in study design, data analysis, or preparation of the manuscript.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014;58:260–7. [DOI] [PubMed] [Google Scholar]
- 2. Formenty P, Muntasir MO, Damon I et al. . Human monkeypox outbreak caused by novel virus belonging to Congo Basin clade, Sudan, 2005. Emerg Infect Dis. 2010;16:1539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Learned LA, Reynolds MG, Wassa DW et al. . Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am J Trop Med Hyg. 2005;73:428–34. [PubMed] [Google Scholar]
- 4. Nakazawa Y, Emerson GL, Carroll DS et al. . Phylogenetic and ecologic perspectives of a monkeypox outbreak, southern Sudan, 2005. Emerg Infect Dis. 2013;19:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nolen LD, Osadebe L, Katomba J et al. . Extended human-to-human transmission during a monkeypox outbreak in the Democratic Republic of the Congo. Emerg Infect Dis. 2016;22:1014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khodakevich L, Widy-Wirski R, Arita I et al. . Monkey pox virus infection in humans in the Central African Republic. Bull Soc Pathol Exot Filiales. 1985;78:311–20. [PubMed] [Google Scholar]
- 7. Jezek Z, Grab B, Szczeniowski M et al. . Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull World Health Organ. 1988;66:459–64. [PMC free article] [PubMed] [Google Scholar]
- 8. Reed KD, Melski JW, Graham MB et al. . The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–50. [DOI] [PubMed] [Google Scholar]
- 9. Saluzzo JF, Gonzalez JP, Herve JP, Georges AJ. Epidemiological study of arboviruses in the Central African Republic: demonstration of Chikungunya virus during 1978 and 1979. Bull Soc Pathol Exot. 1980;73:390–9. [PubMed] [Google Scholar]
- 10. Meyer H, Ropp SL, Esposito JJ. Gene for A-type inclusion body protein is useful for a polymerase chain reaction assay to differentiate orthopoxviruses. J Virol Methods. 1997;64:217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Panning M, Asper M, Kramme S et al. . Rapid detection and differentiation of human pathogenic orthopox viruses by a fluorescence resonance energy transfer real-time PCR assay. Clin Chem. 2004;50:702–8. [DOI] [PubMed] [Google Scholar]
- 12. Kugelman JR, Johnston SC, Mulembakani PM et al. . Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg Infect Dis. 2014;20:232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berthet N, Nakouné E, Whist E et al. . Maculopapular lesions in the Central African Republic. Lancet. 2011;378:1354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.