Abstract
Gastric cancer most commonly occurs in East Asia, and China accounts for more than half the of world's gastric cancer burden. Despite the efficacy of chemotherapy for patients, this treatment leads to significant patient inconvenience, toxicity and cost. The present study aimed to assess a non-toxic agent, glabridin, as a future chemotherapeutic approach for treating gastric cancer. Using cell proliferation, apoptosis, invasion, and colony formation assays, it was determined that glabridin alone, or in combination with 5-fluorouracil (5-FU), inhibited MKN-45 cell proliferation and invasion, and increased apoptosis. These effects were accompanied by downregulation of p16, E-cadherin and apoptosis regulator Bcl-2 protein, and upregulation of N-cadherin, apoptosis regulator BAX and caspases 3, 8 and 9. The results demonstrated that glabridin may inhibit the malignant proliferation of the human gastric cancer MKN-45 cell line and enhance the efficiency of 5-FU. The data indicate that the p16, and potentially the p16/cyclin-dependent kinase 4/cyclin D1 pathway, may be a novel target for gastric cancer therapy.
Keywords: gastric cancer, glabridin, apoptosis, invasion, 5-FU
Introduction
Gastric cancer is the third leading cause of cancer-associated mortalities worldwide (1), with a particularly high incidence rate in China (2). Surgical resection is the principal method of treatment, however, the prognosis of patients with gastric cancer is poor (3). The post-operative recurrence rate is as high as 50–70%, with a 5-year survival rate of 20–50%. Consequently, novel methods other than surgery are being investigated (4,5). In recent years, traditional Chinese medicines, including licorice (Glycyrrhiza glabra) and Xanthium bitter ginseng, are gaining more attention in the treatment of tumors, as they have less severe side effects (6). Licorice is primarily cultivated in Iran, China, Russia, Spain and India. In traditional Chinese medicine, the roots and rhizomes of a variety species of the perennial herb licorice are used for the treatment of a number of conditions, including fatigue, asthma and excessive phlegm production, and for relieving drug toxicity (7). One study demonstrated that Chinese licorice inhibits the growth of HepG2 cells by arresting cell proliferation and the subsequent induction of apoptosis (8).
Glabridin is an active isoflavane located in the hydrophobic fraction of licorice root (9,10); in humans and mice, it can be easily incorporated into gut cells and released to the basolateral surface in an aglycone form (11,12). Glabridin exhibits a number of biological activities, including modulation of the quantity and function of lymphocytes, inhibition of the antibody formation of IgE, effects against inflammatory mediator and proinflammatory cytokines, and induction of pharmacologic activities against inflammation and allergy (9–19). Studies have reported that glabridin also exhibits properties of growth inhibition against a number of types of human cancer, including breast and liver cancer, and hepatocellular carcinoma (13–19). Enhanced cancer chemotherapy efficiency via inhibition of P-glycoprotein and multidrug resistance protein 1 synthesis has also been demonstrated (20).
In the present study, the gastric cancer MKN-45 cell line was used to investigate the effects of glabridin, either alone or in conjunction with the commonly administered gastric cancer chemotherapeutic 5-fluorouracil (5-FU). The effects of glabridin and 5-FU on MKN-45 cells were evaluated, including cell proliferation, invasion, colony formation and the number of cells undergoing apoptosis. Therefore, our aim is to further determine the effect of glabridin in combination with 5-FU on the proliferation, invasion and apoptosis of MKN-45 cells and to further investigate the intrinsic mechanism by which glabridin plus 5-FU affects MKN-45 cells. Hope to explore new ways for the clinical treatment of gastric cancer.
Materials and methods
Reagents
Cell culture reagents were purchased from Gibco; Thermo Fisher Scientific Inc. (Waltham, MA, USA). Unless otherwise stated, all other reagents were from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). The cell counting kit-8 (CCK-8) for assessing cell proliferation was purchased from Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). Annexin V-FITC/PI Apoptosis Detection Kit and binding buffer, were obtained from Nanjing KeyGen Biotech. Co. Ltd. (Nanjing, China). The TRIzol® reagent was purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA) and the ReverTra Ace® qPCR RT kit was purchased from Toyobo Co., Ltd. (Osaka, Japan). Primers were synthesized by Beijing Genomics Institute (Shenzhen, China). 5-FU was purchased from Sigma-Aldrich; Merck KGaA. Glabridin was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Cell culture
Human gastric cancer MKN-45 cells, purchased from the Chinese Academy of Sciences Cell Bank (Shanghai, China) were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 µg/ml ampicillin, and 0.1 mg/ml streptomycin at 37°C in 5% CO2.
Cell proliferation assay
Cells were plated in 96-well plates (Falcon; BD Biosciences, Franklin Lakes, NJ, USA) at a density of 1×104 cells per well. After 24 h, different concentrations of glabridin (0, 6, 12, 25, 30 and 40 µM) and 5-FU (0, 0.01, 0.05, 0.1 0.2 and 1 mM) were added and the cells were cultured for a further 48 h. The CCK-8 staining solution was then diluted (1:10), added to the 96-well plate and cultured for 1–2 h in an incubator at 37°C, according to the manufacturer's protocol. The intensity of the color developed was detected using a microplate reader at 570 nm. All assays were performed with five replicates. And empty culture medium was used as a blank control group.
Cell colony formation assay
A total of 500 MKN-45 cells were seeded in a 6-well plate and treated with glabridin (25 µM), 5-FU (0.1 mM) or glabridin combined with 5-FU for 10 days. The cell culture medium was replaced every 2–3 days. When a visible colony appeared in the 6-well plate, the culture was terminated and the cells were washed twice in PBS. Next, the cells were fixed with methanol for 15 min and stained with Giemsa Stain Solution (cat. no. RFT200-WHI; Biomart, Co., Ltd., Beijing, China) for 10 min at room temperature. The number of cell clones was counted under the microscope (>50 clones validated; Leica, Germany), and 4 independent experiments were performed.
Cell apoptosis assay
In total, 1×106 MKN-45 cells were treated with glabridin (25 µM), 5-FU (0.1 mM) or glabridin combined with 5-FU for 3 days. The drug-treated cells were digested with trypsin and washed twice with PBS. Next, 100 µl Binding Buffer and 10 µl fluorescein isothiocyanate (FITC)-labeled Annexin-V (20 µg/ml) were added and incubated at room temperature for 30 min. Propidium iodide (5 µl; 50 µg/ml) was added and the cells were incubated for 5 min in the dark. Next, 400 µl Binding Buffer was added and the results were immediately quantified by FACScan flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). A total of 3 independent experiments were performed.
Cell invasion assays
A total of 1×105 MKN-45 cells were suspended in 200 µl serum-free RPMI-1640 medium, and seeded into the Matrigel pre-coated Transwell upper chamber. A total of 500 µl RPMI-1640 medium containing 10% FBS with or without glabridin (25 µM), 5-FU (0.1 mM) or glabridin combined with 5-FU was added to the lower chamber of a 24-well plate fitted with insert and cultured in an incubator at 37°C in 5% CO2. The culture medium was discarded following a 72-h incubation and the Matrigel and the cells that did not pass through the upper surface of the insert were removed with a wet cotton swab. Cells were subsequently stained with 0.1% crystal violet for 10 min and observed using a microscope (Leica, Microsystems GmbH, Wetzlar, Germany), and the number of cells penetrating the artificial basement membrane were counted. Each experiment was repeated 3 times and averaged as the experimental results for statistical analysis.
Caspase assays
A total of 1×106 MKN-45 cells were incubated in a 6-well plate with or without glabridin (25 µM), 5-FU 0.1 mM) or glabridin combined with 5-FU for 3 days. The cells from the different treatment groups were harvested and washed twice with PBS. The cells were subsequently lysed on ice in a 50-µl lysis buffer for 30 min. The cells were centrifuged at 4°C, 12,000 × g, for 10 min, and the supernatant protein concentration was quantified using the BCA protein assay kit (cat. no. 23252; Thermo Fisher Scientific, Inc.). The fluorescence substrates of caspase-3 (BF3100), −8 (BF4100) and −9 (BF10100) (all R&D Systems China, Co., Ltd.) were added to the protein samples and incubated at 37°C for 1 h. The absorbance was detected using a microplate reader at 405 nm. A standard curve provided by the kit was used to calculate the activity of caspase-3, −8 and −9.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
A total of 1×106 MKN-45 cells were collected and total RNA was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The optical density (OD)260 and OD280 values in the extracted RNA were determined using a spectrophotometer and the ratio was between 1.8–2.1. cDNA was obtained by 3 µg RNA reverse transcription using a ReverTra Ace qPCR RT kit (Toyobo Co. Ltd.) according to the manufacturer's protocol. Furthermore, qPCR of the cDNA was performed to analyze the gene expression levels of glyceraldehyde 3-phosphate dehydrogenase (GADPH), vascular endothelial growth factor (VEGF), apoptosis regulator BAX (Bax), apoptosis regulator Bcl-2 (Bcl-2), cyclin D1, epidermal growth factor receptor (EGFR), proliferation marker protein Ki-67 (Ki-67), matrix metalloproteinase (MMP)-9, MMP-2, metalloproteinase inhibitor 2 (TIMP-2) and E-cadherin using the ReverTra Ace qPCR RT kit with SYBR-Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA) in a GFX96 Real-time system (Bio-Rad Laboratories, Inc.). The mixture consisted of 10 µl SYBR-Green Mix, 1 µl forward, 1 µl reverse primers, 1 µl diluted cDNA and 7 µl nuclease-free waters. The reaction process was performed at 95°C for 2 min, followed by 40 cycles of 95°C for 10 sec and 61°C for 30 sec. All the primers used are shown in Table I and GAPDH was used as the control gene. The 2−ΔΔCq (14) method was used to calculate the relative mRNA expression of target genes.
Table I.
Primer sequences used for reverse transcription-quantitative polymerase chain reaction.
| Gene | Forward primer | Reverse primer | Product length, bp |
|---|---|---|---|
| GAPDH | 5′-TGAACGGGAAGCTCACTGG-3′ | 5′-TCCACCACCCTGTTGCTGTA-3′ | 307 |
| VEGF | 5′-ATTATGCGGATCAAACCTC-3′ | 5′-ATTTCTTGCGCTTTCGTT-3′ | 157 |
| Bax | 5′-CCCGAGAGGTCTTTTTCCGAG-3′ | 5′-CCAGCCCATGATGGTTCTGAT-3′ | 155 |
| Bcl-2 | 5′-CCTGGGCAATTCCGCATT-3′ | 5′-AACAGGCCACGTAAAGCAAC-3′ | 158 |
| Cyclin D1 | 5′-GCTGCGAAGTGGAAACCATC-3′ | 5′-CCTCCTTCTGCACACATTTGAA-3′ | 135 |
| EGFR | 5′-AGGCACGAGTAACAAGCTCAC-3′ | 5′-ATGAGGACATAACCAGCCACC-3′ | 177 |
| Ki-67 | 5′-AGAAGACCTGCTACTCCAAAGA-3′ | 5′-AGTTTGCGTGGCCTGTACTAA-3′ | 70 |
| MMP-9 | 5′-ACTACTGTGCCTTTGAGTCC-3′ | 5′-AGAATCGCCAGTACTTCCCA-3′ | 115 |
| MMP-2 | 5′-ACTCTGGACTTAGACCGCTTG-3′ | 5′-ACAGGTTGCAGCTCTCCTTG-3′ | 217 |
| TIMP-2 | 5′-ACCCCTGTTCGCTTCCTGT-3′ | 5′-GGGTCAAATGCTTCCACGAT-3′ | 196 |
| E-cadherin | 5′-GCTAACGTCGTAATCACCAC-3′ | 5′-AATGCCATCGTTGTTCACTG-3′ | 141 |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; VEGF, vascular endothelial growth factor; Bax, apoptosis regulator BAX; Bcl-2, apoptosis regulator Bcl-2; EGFR, epidermal growth factor receptor; Ki-67, proliferation marker protein Ki-67; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase.
Western blot analysis
A total of 1×106 MKN-45 cells were incubated with indicated concentrations of glabridin (25 µM), 5-FU (0.1 mM) or glabridin combined with 5-FU for 3 days. The cell culture medium was discarded and the cells were washed twice with PBS. Next, the cells were lysed in 200 µl radioimmunoprecipitation assay buffer (cat. no. P0013B; Beyotime Institute of Biotechnology, Haimen, China). The concentration of the extracted protein was determined using the bicinchoninic acid protein quantification method and 10% SDS-PAGE was performed with 40 µg of protein sample and transferred to a polyvinylidene difluoride (PVDF) membrane, and then 5% skimmed milk powder was added and incubation occurred for 1 h at room temperature. The PVDF membrane was then incubated at 4°C overnight with anti-cyclin-dependent kinase inhibitor 2A (CDKN2A)/p16INK4a antibody (cat. no. ab108349; dilution, 1:1,000), anti-Bax antibody (cat. no. ab32503; dilution, 1:1,000), anti-Bcl-2 antibody (cat. no. ab32124; dilution, 1:1,000), anti-E-cadherin antibody (cat. no. ab76055; dilution, 1:500), anti-N-cadherin antibody (cat. no. ab76057; dilution, 1:500) and anti-β-actin antibody (cat. no. ab8226; dilution, 1:1,000) (all Abcam, Cambridge, UK), and washed with Tris-buffered saline plus Tween-20 (TBST) 3–5 times. The corresponding horseradish peroxidase-conjugated secondary antibodies (cat. nos. ab6721 and ab6789; dilution, 1:2,000; both Abcam) were used and incubated for 1 h at room temperature, respectively. The membranes were washed three times with TBST, incubated with ECL solution (cat. no. 34580; Thermo Fisher Scientific Inc. Waltham, MA, USA) and analyzed using a gel imaging system (Biox-vision, Co., Ltd., Anhui, China). Western blotting quantification was estimated using ImageJ software (version 1.42q; National Institutes of Health, Bethesda. MD, USA).
Statistical analysis
One-way analysis of variance (ANOVA) and the Kruskal-Wallis test were used for data analysis, and Dunnett's t-test was used following ANOVA. P<0.05 was considered to indicate a statistically significant difference. All data were analyzed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) and had a minimum of three independent experimental repeats.
Results
Glabridin combined with 5-FU inhibits MKN-45 cell proliferation
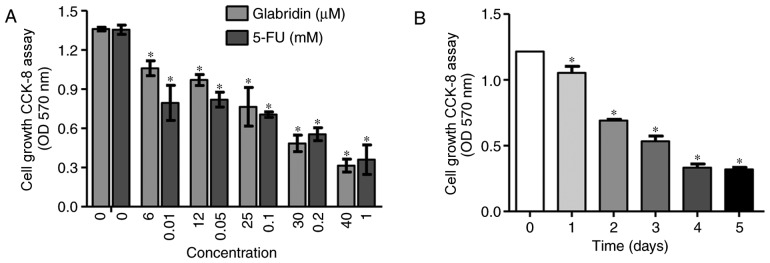
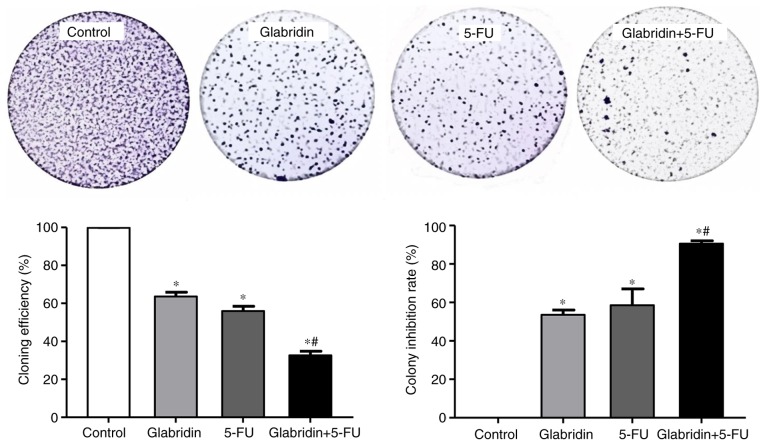
The present study examined the effects of glabridin and 5-FU on gastric cancer cell proliferation. MKN-45 cells were treated with different concentrations of glabridin (0, 6, 12, 25, 30 and 40 µM) and 5-FU (0, 0.01, 0.05, 0.1 0.2 and 1 mM) for 48 h (Fig. 1A). MKN-45 cells also were treated with 25 µM glabridin alone for 1–5 days (Fig. 1B). The results demonstrated that glabridin and 5-FU inhibited the proliferation of MKN-45 cells in a dose-and time-dependent manner. Based on these preliminary experimental results, it was confirmed that glabridin may inhibit gastric cancer cell proliferation. As demonstrated in Fig. 1B, at the end of the 5-day incubation period, glabridin was effective in significantly inhibiting the growth of the MKN-45 cells. This is supported by a number of studies where 5-FU or glabridin was applied to tumor cells, such as in the study by Khazraei-Moradian et al (21), which demonstrated that licorice protein fractions are capable of inhibiting the proliferation of gastrointestinal cancer cell lines. Additionally, Zhou et al (22) identified that curcumin enhances the effects of 5-FU and oxaliplatin in inducing the apoptosis of gastric cancer cells in vitro and in vivo (22). Although 5-FU or glabridin was able to inhibit the proliferation and induce the apoptosis of the gastric cancer cell lines cited in the previous studies, 5-FU and glabridin used at varying concentrations (14,15,17). Therefore, following analysis of these studies and the preliminary experimental results of the present study (Fig. 1), a concentration of 0.1 mM 5-FU and 25 µM glabridin were used in the subsequent experiments. In the colony formation assay, the results also demonstrated that a combination of glabridin and 5-FU treatment could significantly inhibit colony formation when compared with glabridin or 5-FU alone (Fig. 2).
Figure 1.
Cell growth inhibition in gastric cancer MKN-45 cells. (A) Dose-dependent inhibition of growth of MKN-45 cell in response to glabridin and 5-FU alone. (B) MKN-45 cell growth following treatment with 25 µM glabridin for 1–5 days. Data was obtained from at least three independent experiments. *P<0.05 (vs. control group) was considered to indicate a statistically significant difference. OD, optical density; 5-FU, 5-fluorouracil; CCK-8, cell counting kit-8.
Figure 2.
Cell colony formation assay. MKN-45 cells were treated with glabridin, 5-FU, glabridin plus 5-FU or control medium. The number of cell clones was counted under the microscope (>50 clones validated), and four separate experiments were performed for average. Randomly select 5 different fields to be counted in each well, and four separate experiments were performed for average. Data was obtained from at least three independent experiments. *P<0.05 (vs. control group) or #P<0.05 (glabridin +5-FU vs. 5-FU) was considered to indicate a statistically significant difference. 5-FU, 5-fluorouracil.
Glabridin combined with 5-FU promotes MKN-45 cell apoptosis
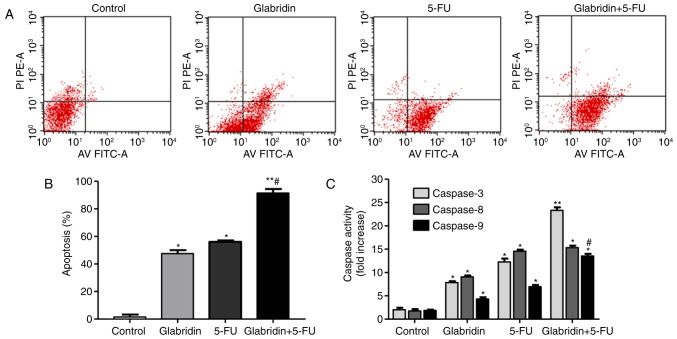
Apoptotic cell death was identified by flow cytometric analysis of Annexin V-FITC-stained apoptotic cells. The results of the present study demonstrated that the apoptosis of MKN-45 cells was observed following treatment with 8 µg/ml glabridin, 0.1 mM 5-FU and the combined treatment, respectively. Additionally, glabridin plus 5-FU significantly induced the apoptosis of the MKN-45 cells compared with glabridin or 5-FU alone groups (Fig. 3). As shown in Fig. 3B, the apoptotic percentages of the glabridin, 5-FU, combination and control groups were approximately 55.37±3.13, 56.16±2.08, 93.88±4.05 and 2.35±0.61%, respectively. To investigate the apoptotic mechanisms in cells treated with glabridin and 5-FU, the activity of caspases −3, −8 and −9 were assayed using caspase activity assay kits. As shown in Fig. 3C, caspases −3, −8 and −9 were all activated following induction with glabridin, 5-FU or glabridin plus 5-FU. In comparison with the control, caspase-3, −8, and −9 levels were demonstrated to be significantly higher in the glabridin, 5-FU and combination groups (P<0.01). Compared with the activity in the combination group, caspase-3 and −9 activities were identified to be significantly lower (P<0.01) in the glabridin and 5-FU groups. As a result, it was observed that glabridin had an enhanced effect on the MKN-45 cells by increasing the activity of caspase-3, −8, and −9, which are precursors of apoptosis (Fig. 3C).
Figure 3.
Assessment of apoptosis of MKN-45 cells treated with glabridin and 5-FU alone or glabridin plus 5-FU. (A) Flow cytometric analysis of each group of MKN-45 cells stained with Annexin V-FITC and PI. (B) MKN-45 cell apoptosis percentage. (C) Fold increase of caspase-3, −8 and −9 induced by glabridin, 5-FU and glabridin plus 5-FU. Data were obtained from at least three independent experiments. *P<0.05 (vs. control group) or #P<0.05 (glabridin+5-FU vs. -FU) was considered to indicate a statistically significant difference. 5-FU, 5-fluorouracil; PI, propidium iodide; PE, phycoerythrin; AV, Annexin V; FITC, fluorescein isothiocyanate.
Glabridin combined with 5-FU inhibits the invasion of MKN-45 cells
Cell invasion assays were conducted to further examine the effect of glabridin, 5-FU and the two-drug combination on cell invasion. In these experiments, it was demonstrated that glabridin combined with 5-FU significantly decreased the chemotaxis and invasion of MKN-45 cells compared with that of the controls (Fig. 4). The quantity of invading cells was also reduced by glabridin and 5-FU in a Matrigel invasion assay (Fig. 4A). The number of invading cells was 69.67% (control), 40.96% (glabridin), 28.70% (5-FU) and 15.23% (glabridin combined with 5-FU) (Fig. 4B).
Figure 4.
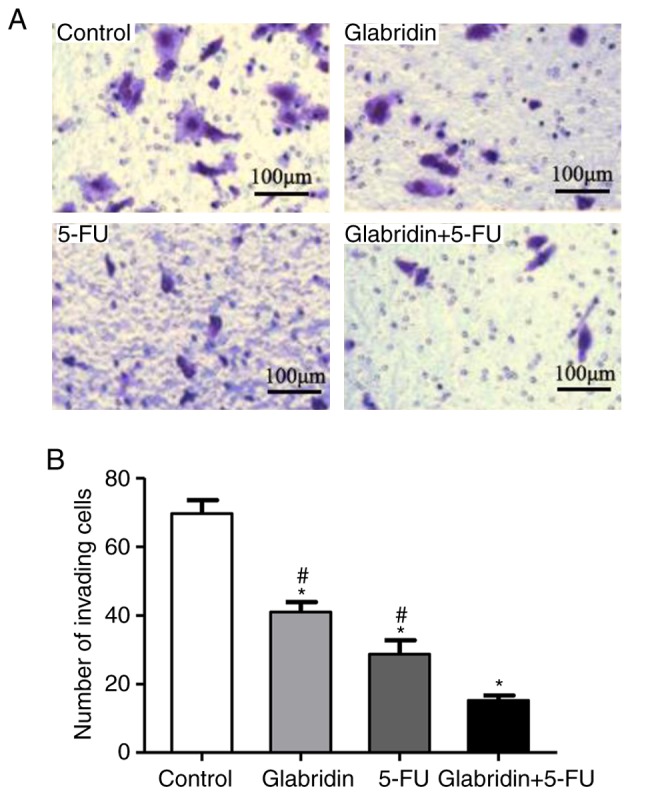
MKN-45 cell invasion is influenced by glabridin and 5-FU. (A) MKN-45 cells were plated on Matrigel-coated membranes for invasion assays and incubated for 48 h. (B) The number of invasive cells in different treatment groups. Data were obtained from at least three independent experiments. *P<0.05 (vs. control group) or #P<0.05 (glabridin+5-FU vs. 5-FU or glabridin) was considered to indicate a statistically significant difference. 5-FU, 5-fluorouracil.
Cell apoptosis-, cycle progression-, invasion- and angiogenesis-related genes are influenced by glabridin and 5-FU
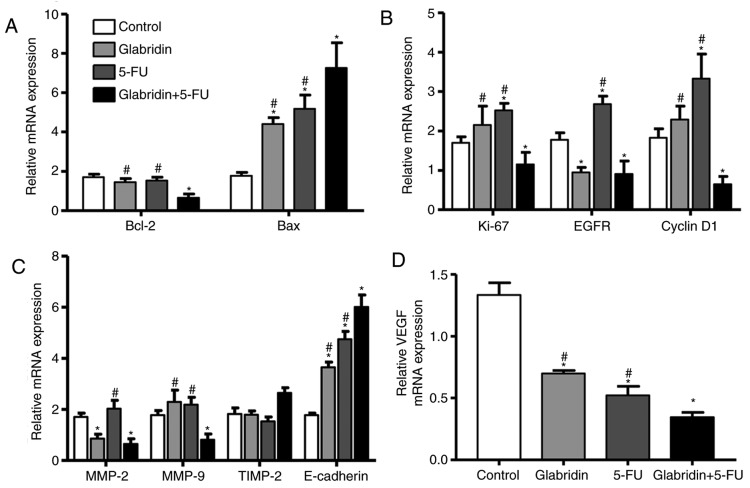
The results of the present study confirmed that glabridin combined with 5-FU decreases proliferation and colony formation, and promotes the apoptosis of MKN-45 cells. These results influenced the decision to examine the effects of glabridin, 5-FU and the two-drug combination on the gene expression of MKN-45 cells. Glabridin combined with 5-FU significantly weakened the expression of the anti-apoptotic gene Bcl-2 and increased expression of the pro-apoptotic gene Bax (Fig. 5A). These results suggest that glabridin combined with 5-FU may promote apoptosis of MKN-45 cells by upregulating the Bax gene and downregulating the Bcl-2 gene.
Figure 5.
Determining the amount of mRNA expression by reverse transcription-quantitative polymerase chain reaction. (A) Apoptosis-, (B) cell cycle progression-, (C) invasion- and (D) angiogenesis-related genes are influenced by glabridin, 5-FU and glabridin plus 5-FU. *P<0.05 (vs. control group) or #P<0.05 (glabridin+5-FU vs. 5-FU or glabridin) was considered to indicate a statistically significant difference. VEGF, vascular endothelial growth factor; 5-FU, 5-fluorouracil; Bcl-2, apoptosis regulator Bcl-2; Bax, apoptosis regulator BAX; Ki-67, proliferation marker protein Ki-67; EGFR, epidermal growth factor receptor; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase.
To understand the regulation of genes by glabridin with regard to cell proliferation and colony formation, RT-qPCR was performed to examine the relative mRNA expression levels of EGFR, cyclin D1 and Ki-67. It was found that glabridin significantly decreased mRNA expression of EGFR, but had no effect on mRNA expression of Ki-67 and cyclin D1 compared with the control (Fig. 5B). Glabridin combined with 5-FU significantly downregulated the expression of Ki-67, EGFR and cyclin D1 gene compared with the control. This data demonstrates that glabridin plus 5-FU could induce MKN-45 cell apoptosis and inhibit cell cycle progression by reducing mRNA expression of cyclin D1, EGFR and Ki-67.
Additionally, regulation of the MMP-2, TIMP-2, MMP-9 and E-cadherin genes for MKN-45 cell invasion was examined. Glabridin alone significantly downregulated mRNA expression of MMP-2, and glabridin and 5-FU significantly upregulated mRNA expression of E-cadherin compared with the control. Notably, glabridin in conjunction with 5-FU could significantly alter the mRNA expression of the MMP-2, MMP-9 and E-cadherin genes (Fig. 5C). The effect of glabridin and 5-FU on the expression of the VEGF gene was examined, and it was found that the mRNA expression of VEGF was most significantly decreased by treatment of glabridin plus 5-FU (Fig. 5D). Taken together, the results of the present study suggested that glabridin combined with 5-FU may inhibit gastric cancer cell invasion, and angiogenesis by regulation of MMP-2, MMP-9, E-cadherin and VEGF genes.
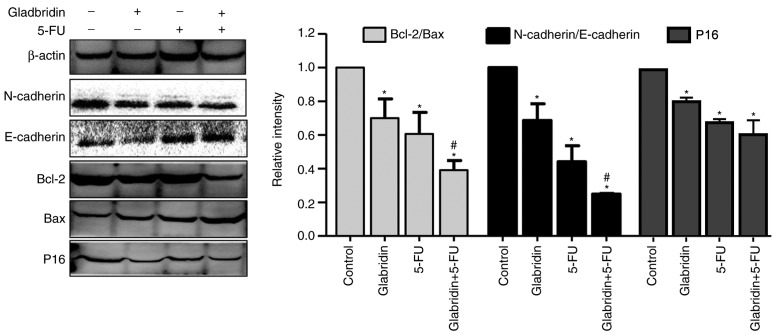
Effects of glabridin combined with 5-FU on the protein expression of MKN-45 cells
RT-qPCR analysis demonstrated that glabridin plus 5-FU can affect the proliferation, invasion and apoptosis capabilities of MKN-45 cells. p16 is a key regulatory protein for the cell cycle, with western blot analysis revealing that the expression of p16 in the glabridin-treated MKN-45 cells was significantly decreased compared with that in the control group (Fig. 6). The results demonstrated that glabridin alone and combined with 5-FU could reduce the expression of p16 protein. Western blot analysis also demonstrated that the apoptosis proteins Bcl-2 and Bax were affected in glabridin-treated MKN-45 cells, and that the glabridin plus 5-FU combination synergistically decreased the Bcl-2/Bax protein ratio (Fig. 6). Similarly, compared with the 5-FU-treated MKN-45 cells group, the expression of N-cadherin/E-cadherin was significantly decreased in the glabridin combination with 5-FU-treated MKN-45 cells group. Cadherins are a class of transmembrane proteins whose primary function is to form an adherent junction to bind cells within the tissue (23). The results revealed that the glabridin in conjunction with 5-FU may affect the apoptosis, proliferation and invasion of cancer cells by altering the expression of Bcl-2, Bax, E-cadherin, N-cadherin and p16 proteins.
Figure 6.
Protein expression of MKN-45 cells was determined by western blotting in the presence or absence of glabridin and 5-FU. E-cadherin and Bax expression was significantly increased in the presence of glabridin or 5-FU, and reached a maximum in MKN-45 cells in the presence of glabridin combined with 5-FU. N-cadherin, Bcl-2 and p16 expression was significantly decreased in the presence of glabridin or 5-FU, and reached the lowest level in MKN-45 cells treated with glabridin combined with 5-FU. β-actin was used as a loading control. Data was obtained from a minimum of three independent experiments. *P<0.05 (vs. control group) or #P<0.05 (glabridin+5-FU vs. 5-FU) was considered to indicate a statistically significant difference. The molecular weights of E-cadherin and N-cadherin were 97 and 140 KDa, respectively; thus, the molecular weight was so large that the exposure time was extended. Therefore, the background of the E- and N-cadherin bands appears granulated and different from the background of the other bands. The same membrane was used. 5-FU, fluorouracil; Bcl-2, apoptosis regulator Bcl-2; Bax, apoptosis regulator BAX.
Discussion
Gastric cancer incidence and mortality have increased worldwide, and according to the latest report by the World Health Organization, the incidence of gastric cancer in the global incidence of malignancy ranks fifth mortality rate ranks third (24). Hu et al reported that that the annual number of new cases of gastric cancer in China is 400,000, accounting for 42% of the global gastric cancer cases, with a mortality rate of ~300,000 (25,26). Chemotherapy is the principal treatment for patients with gastric cancer. However, it produces a number of adverse side effects such as damage to normal, functional cells. This has culminated in the requirement for the development of drugs with fewer adverse side effects and increased efficiency. Glabridin is an isoflavane, a type of isoflavonoid, which is found in the root extract of licorice (Glycyrrhiza glabra) (27). The effects of glabridin as a phytoestrogen for the treatment of breast and endometrial cancer have been investigated (13,17,28). Furthermore, a number of studies have demonstrated that glabridin has anti-proliferative, anti-invasion and antitumor angiogenesis capabilities in breast cancer cells, endometrial cancer and hepatocarcinoma cells (17–19). The results of the present study indicate that glabridin either alone or in conjunction with 5-FU may significantly inhibit colony formation and invasion, and promote the apoptosis of MKN-45 cells.
The p16 protein is an important factor in cell cycle regulation and differentiation. Previous studies have identified that the p16 gene is a tumor suppressor gene that participates in the development of multiple tumors (29,30). It has been reported that p16 specifically inhibits the activity of CDK4, and prevents cells from the G1 phase entering into the S phase. When the expression of p16 is decreased, CDK4 expression is upregulated, and p16 combined with cyclin D1 can promote cell mitosis, thereby promoting cell proliferation, leading to tumor formation (31–34). Benassi et al (31) investigated the mechanisms regulating cell-cycle progression in human osteosarcomas and Retinoblastoma-associated protein (pRb)/p16/CDK4 expression was analyzed in 39 high-grade osteosarcomas; the data confirmed the important role of the pRb/p16/CDK4 pathway in osteosarcoma development (31). Notably, it was identified in the present study that glabridin reduces the proliferation and colony formation of MKN-45 cells. This result validates those of previous studies, which hypothesized an inhibitory role for glabridin in various types of cancer cells (35–37). However, compared with 5-FU, the present study unexpectedly identified that the combination of glabridin and 5-FU was more effective in significantly inhibiting the proliferation of MKN-45 cells. According to the experimental results, we hypothesize that glabridin plus 5-FU may involve a combination of increased expression of p16 protein and reduced mRNA expression of the cyclin D1, EGFR and Ki-67 genes, although the molecular mechanisms responsible for these actions have yet to be established. Therefore, we hypothesize that glabridin may inhibit gastric cancer cell proliferation through the p16/CDK4/cyclin D1 pathway.
The results of the present study demonstrated that glabridin and 5-FU are capable of up or downregulating several key genes, including Bcl-2, Bax, MMP-9, VEGF, MMP-2 and E-cadherin, all of which are important regulators of cell invasion, proliferation and apoptosis. The experimental results confirm that glabridin or glabridin with 5-FU inhibited MKN-45 cell proliferation and invasion, and suggest that the combination of glabridin and 5-FU may inhibit the invasion of cancer cells by downregulating the MMP-9 and MMP-2 genes. The role of these downstream pathway mediators in gastric cancer is presently unclear. Caspase and Bcl-2 families serve important roles in apoptosis. It has previously been reported caspase-3, −8, and −9 activation exhibits a vital role in early apoptosis, as regulated by a range of factors, including the Bcl-2 protein family (22). The mitochondria-mediated apoptotic pathway is also regulated by members of the Bcl-2 family (38) and is dependent on the balance of the anti-apoptotic protein Bcl-2 and the pro-apoptotic protein Bax. The present study indicated that glabridin shifts the equilibrium of Bcl-2 family members toward apoptosis and elevates the expression of Bax and procaspases 3, 8 and 9. The results demonstrated that the glabridin plus 5-FU combination synergistically decreased the Bcl-2/Bax protein ratio, making the MKN-45 cells more susceptible to apoptosis.
In summary, glabridin alone or in combination with 5-FU inhibits MKN-45 cell proliferation, survival and invasion. The data suggest that using glabridin alone or in conjunction with 5-FU may be an effective therapeutic strategy to eliminate gastric cancer cells via the induction of apoptosis. The present study also suggests that the p16/CDK4/cyclin D1 pathway may be a novel target site for gastric cancer therapeutics, and the combined use of glabridin and 5-FU chemotherapy may pose a useful approach to treat gastric cancer. However, a limitation of the present study is that only one gastric cancer cell line was used, although it successfully demonstrated that glabridin plus 5-FU can effectively promote apoptosis or inhibit proliferation and invasion of the MKN-45 cell line by different methods, including assessment of mRNA and protein levels. Ultimately, the intrinsic mechanism of glabridin plus 5-FU inhibiting the proliferation and invasion of MKN-45 cells requires further experimental verification. Furthermore, due of the high cost of antibodies, a number of the PCR genes were not further validated at the protein level, consequently these may be included in the next phase of research.
Acknowledgements
The authors would like to thank Dr. Sandhya Mana for providing comments on earlier versions of the manuscript.
Funding
No funding was received.
Availability of data and materials
The authors declare that materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality.
Authors' contributions
XL conceived and designed the study. LZ and HC conducted experiments; MW, XS, JZ and FD analyzed the data. JZ also drafted the manuscript. XL and LZ designed the experiments and revised the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kásler M, Ottó S, Kenessey I. The current situation of cancer morbidity and mortality in the light of the national cancer registry. Orv Hetil. 2017;158:84–89. doi: 10.1556/650.2017.30654. (In Hungarian) [DOI] [PubMed] [Google Scholar]
- 2.Sun X, Liu W, Wu S, Han H, Lin Y, Dai X. The morbidity and mortality trend and prediction of lung cancer in residents of Nangang District of Harbin in China during the past 10 years. Zhongguo Fei Ai Za Zhi. 2005;8:514–517. doi: 10.3779/j.issn.1009-3419.2005.06.06. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 3.Fukunaga S, Nagami Y, Shiba M, Ominami M, Tanigawa T, Yamagami H, Tanaka H, Muguruma K, Watanabe T, Tominaga K, et al. Long-term prognosis of expanded-indication differentiated-type early gastric cancer treated with endoscopic submucosal dissection or surgery using propensity score analysis. Gastrointest Endosc. 2017;85:143–152. doi: 10.1016/j.gie.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 4.Dittmar Y, Schüle S, Koch A, Rauchfuss F, Scheuerlein H, Settmacher U. Predictive factors for survival and recurrence rate in patients with node-negative gastric cancer-a European single-centre experience. Langenbecks Arch Surg. 2015;400:27–35. doi: 10.1007/s00423-014-1226-2. [DOI] [PubMed] [Google Scholar]
- 5.Greenhill C. Gastric cancer. Metformin improves survival and recurrence rate in patients with diabetes and gastric cancer. Nat Rev Gastroenterol Hepatol. 2015;12:124. doi: 10.1038/nrgastro.2015.9. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Ye M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice) J Chromatogr A. 2009;1216:1954–1969. doi: 10.1016/j.chroma.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 7.Yang R, Yuan BC, Ma YS, Zhou S, Liu Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm Biol. 2017;55:5–18. doi: 10.1080/13880209.2016.1225775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Liu Z, Meng R, Shi C, Guo N. Antioxidative and anticancer properties of Licochalcone A from licorice. J Ethnopharmacol. 2017;198:331–337. doi: 10.1016/j.jep.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 9.Zhang LP, Li JG. Glabridin reduces lipopolysaccharide-induced lung injury in rats by inhibiting p38 mitogen activated protein kinase/extracellular regulated protein kinases signaling pathway. Zhonghua Yi Xue Za Zhi. 2016;96:3893–3897. doi: 10.3760/cma.j.issn.0376-2491.2016.48.009. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Sui H, Zhao Q, Zhang X, Wang W. Enhanced skin permeation of Glabridin using eutectic mixture-based nanoemulsion. Drug Deliv Transl Res. 2017;7:325–332. doi: 10.1007/s13346-017-0359-6. [DOI] [PubMed] [Google Scholar]
- 11.Ito C, Oi N, Hashimoto T, Nakabayashi H, Aoki F, Tominaga Y, Yokota S, Hosoe K, Kanazawa K. Absorption of dietary licorice isoflavan glabridin to blood circulation in rats. J Nutr Sci Vitaminol (Tokyo) 2007;53:358–365. doi: 10.3177/jnsv.53.358. [DOI] [PubMed] [Google Scholar]
- 12.Cao J, Chen X, Liang J, Yu XQ, Xu AL, Chan E, Wei D, Huang M, Wen JY, Yu XY, et al. Role of P-glycoprotein in the intestinal absorption of glabridin, an active flavonoid from the root of Glycyrrhiza glabra. Drug Metab Dispos. 2007;35:539–553. doi: 10.1124/dmd.106.010801. [DOI] [PubMed] [Google Scholar]
- 13.Ye X, Jiang F, Li Y, Mu J, Si L, Wang X, Ning S, Li Z. Glabridin attenuates the migratory and invasive capacity of breast cancer cells by activating microRNA-200c. Cancer Sci. 2014;105:875–882. doi: 10.1111/cas.12426. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Tsai YM, Yang CJ, Hsu YL, Wu LY, Tsai YC, Hung JY, Lien CT, Huang MS, Kuo PL. Glabridin inhibits migration, invasion, and angiogenesis of human non-small cell lung cancer A549 cells by inhibiting the FAK/rho signaling pathway. Integr Cancer Ther. 2011;10:341–349. doi: 10.1177/1534735410384860. [DOI] [PubMed] [Google Scholar]
- 15.Tamir S, Eizenberg M, Somjen D, Stern N, Shelach R, Kaye A, Vaya J. Estrogenic and antiproliferative properties of glabridin from licorice in human breast cancer cells. Cancer Res. 2000;60:5704–5709. [PubMed] [Google Scholar]
- 16.Jiang F, Mu J, Wang X, Ye X, Si L, Ning S, Li Z, Li Y. The repressive effect of miR-148a on TGF beta-SMADs signal pathway is involved in the glabridin-induced inhibition of the cancer stem cells-like properties in hepatocellular carcinoma cells. PLoS One. 2014;9:e96698. doi: 10.1371/journal.pone.0096698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang F, Li Y, Mu J, Hu C, Zhou M, Wang X, Si L, Ning S, Li Z. Glabridin inhibits cancer stem cell-like properties of human breast cancer cells: An epigenetic regulation of miR-148a/SMAd2 signaling. Mol Carcinog. 2016;55:929–940. doi: 10.1002/mc.22333. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh MJ, Lin CW, Yang SF, Chen MK, Chiou HL. Glabridin inhibits migration and invasion by transcriptional inhibition of matrix metalloproteinase 9 through modulation of NF-κB and AP-1 activity in human liver cancer cells. Br J Pharmacol. 2014;171:3037–3050. doi: 10.1111/bph.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Retraction statement: ‘Glabridin attenuates the migratory and invasive capacity of breast cancer cells by activating microRNA-200c’. Xianqing Ye, Fei Jiang, Yuan Li, Juan Mu, Lu Si, Xingxing Wang, Shilong Ning, Zhong L.. Cancer Sci. 2015;106:125. doi: 10.1111/cas.12578. by. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nabekura T, Yamaki T, Ueno K, Kitagawa S. Inhibition of P-glycoprotein and multidrug resistance protein 1 by dietary phytochemicals. Cancer Chemother Pharmacol. 2008;62:867–873. doi: 10.1007/s00280-007-0676-4. [DOI] [PubMed] [Google Scholar]
- 21.Khazraei-Moradian S, Ganjalikhani-Hakemi M, Andalib A, Yazdani R, Arasteh J, Kardar GA. The effect of licorice protein fractions on proliferation and apoptosis of gastrointestinal cancer cell lines. Nutr Cancer. 2017;69:330–339. doi: 10.1080/01635581.2017.1263347. [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, Wang W, Li P, Zheng Z, Tu Y, Zhang Y, You T. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in inducing gastric cancer cell apoptosis both in vitro and in vivo. Oncol Res. 2016;23:29–34. doi: 10.3727/096504015X14452563486011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alimperti S, Andreadis ST. CDH2 and CDH11 act as regulators of stem cell fate decisions. Stem Cell Res. 2015;14:270–282. doi: 10.1016/j.scr.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saika K, Sobue T. Cancer statistics in the world. Gan To Kagaku Ryoho. 2013;40:2475–2480. (In Japanese) [PubMed] [Google Scholar]
- 25.Zeng H, Zheng R, Zhang S, Zuo T, Xia C, Zou X, Chen W. Esophageal cancer statistics in China, 2011: Estimates based on 177 cancer registries. Thorac Cancer. 2016;7:232–237. doi: 10.1111/1759-7714.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita T, Kajiyama K, Hiraga Y, Takahashi K, Tamura Y, Mizutani K. Isoflavan derivatives from Glycyrrhiza glabra (licorice) Heterocycles. 1996;43:581–588. doi: 10.3987/COM-95-7296. [DOI] [Google Scholar]
- 28.Vaya J, Belinky PA, Aviram M. Antioxidant constituents from licorice roots: Isolation, structure elucidation and antioxidative capacity toward LDL oxidation. Free Radic Biol Med. 1997;23:302–313. doi: 10.1016/S0891-5849(97)00089-0. [DOI] [PubMed] [Google Scholar]
- 29.Fulmer CG, Hoda RS, Pirog EC, Park KJ, Holcomb K. Cytomorphology of gastric-type cervical adenocarcinoma on a ThinPrep Pap test: Report of a p16-positive tumor case. Diagn Cytopathol. 2016;44:710–713. doi: 10.1002/dc.23498. [DOI] [PubMed] [Google Scholar]
- 30.Kosemehmetoglu K, Ardic F, Karslioglu Y, Kandemir O, Ozcan A. p16 expression predicts neoadjuvant tumor necrosis in osteosarcomas: Reappraisal with a larger series using whole sections. Hum Pathol. 2016;50:170–175. doi: 10.1016/j.humpath.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 31.Benassi MS, Molendini L, Gamberi G, Ragazzini P, Sollazzo MR, Merli M, Asp J, Magagnoli G, Balladelli A, Bertoni F, Picci P. Alteration of pRb/p16/cdk4 regulation in human osteosarcoma. Int J Cancer. 1999;84:489–493. doi: 10.1002/(SICI)1097-0215(19991022)84:5<489::AID-IJC7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 32.Dobashi Y, Goto A, Fukayama M, Abe A, Ooi A. Overexpression of cdk4/cyclin D1, a possible mediator of apoptosis and an indicator of prognosis in human primary lung carcinoma. Int J Cancer. 2004;110:532–541. doi: 10.1002/ijc.20167. [DOI] [PubMed] [Google Scholar]
- 33.Maelandsmo GM, Flørenes VA, Hovig E, Oyjord T, Engebraaten O, Holm R, Børresen AL, Fodstad O. Involvement of the pRb/p16/cdk4/cyclin D1 pathway in the tumorigenesis of sporadic malignant melanomas. Br J Cancer. 1996;73:909–916. doi: 10.1038/bjc.1996.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossi KA, Markwalder JA, Seitz SP, Chang CH, Cox S, Boisclair MD, Brizuela L, Brenner SL, Stouten PF. Understanding and modulating cyclin-dependent kinase inhibitor specificity: Molecular modeling and biochemical evaluation of pyrazolopyrimidinones as CDK2/cyclin A and CDK4/cyclin D1 inhibitors. J Comput Aided Mol Des. 2005;19:111–122. doi: 10.1007/s10822-005-1778-x. [DOI] [PubMed] [Google Scholar]
- 35.Lee SK, Park KK, Park JH, Lim SS, Chung WY. The inhibitory effect of roasted licorice extract on human metastatic breast cancer cell-induced bone destruction. Phytother Res. 2013;27:1776–1783. doi: 10.1002/ptr.4930. [DOI] [PubMed] [Google Scholar]
- 36.Jo EH, Hong HD, Ahn NC, Jung JW, Yang SR, Park JS, Kim SH, Lee YS, Kang KS. Modulations of the Bcl-2/Bax family were involved in the chemopreventive effects of licorice root (Glycyrrhiza uralensis Fisch) in MCF-7 human breast cancer cell. J Agric Food Chem. 2004;52:1715–1719. doi: 10.1021/jf035012t. [DOI] [PubMed] [Google Scholar]
- 37.Jo EH, Kim SH, Ra JC, Kim SR, Cho SD, Jung JW, Yang SR, Park JS, Hwang JW, Aruoma OI, et al. Chemopreventive properties of the ethanol extract of chinese licorice (Glycyrrhiza uralensis) root: Induction of apoptosis and G1 cell cycle arrest in MCF-7 human breast cancer cells. Cancer Lett. 2005;230:239–247. doi: 10.1016/j.canlet.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 38.Brahmbhatt H, Oppermann S, Osterlund EJ, Leber B, Andrews DW. Molecular pathways: Leveraging the BCL-2 interactome to kill cancer cells-mitochondrial outer membrane permeabilization and beyond. Clin Cancer Res. 2015;21:2671–2676. doi: 10.1158/1078-0432.CCR-14-0959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality.