Abstract
To date, there have been a limited number of useful biomarkers for the screening and monitoring of B-cell non-Hodgkin's lymphoma (B-NHL), which leads to the impetus to discover novel biomarkers for the disease. In the present study, gas chromatography-mass spectrometry (GC-MS) combined with head-space solid-phase micro-extraction (HS-SPME) was employed to analyze the volatile metabolites in the urine samples of 131 subjects. The subjects were divided into 4 main groups: Aggressive B-NHL, indolent B-NHL, benign lymphatic diseases patients and healthy volunteers. The differences of the concentrations of the potential biomarkers among the groups were assessed by non-parametric Wilcoxon's test. The ability of the potential biomarkers to discriminate between the four aforementioned groups was evaluated by receiver operating characteristic curves (ROC). The present study indicated that 4-heptanone, 2-methylpyrazine, 2-methylbutanal, 2,6-dimethyl-7-octen-2-ol and decanoic acid may serve as potential biomarkers for B-NHL. The area under the curve (AUC) values of single potential biomarker ranged from 0.634 to 0.901. The diagnostic models established with combined biomarkers exhibited higher diagnostic values (AUC, 0.824–0.968) compared with the models established with single biomarkers. The present study indicated that urinary volatile metabolites might be potential biomarkers for screening and monitoring of B-NHL.
Keywords: B-cell non-Hodgkin lymphoma, urine, volatile metabolites, screening, monitoring
Introduction
Non-Hodgkin's lymphoma (NHL) is the most common hematological cancer with a rapidly increasing incidence among adults. In adults, ~85% of cases of NHL were of B cell origin (1). A lack of specific clinical symptoms and clear risk factors hinders the early diagnosis of B-cell non-Hodgkin's lymphomas (B-NHL), which results in poor prognoses (2). Population-based screening undoubtedly contributes to earlier diagnosis and improved prognosis. However, this is unfeasible for B-NHL due to a lack of useful screening methods. During the course of the treatment for B-NHL, the therapeutic effects need to be monitored by clinicians regularly. Following treatment, the disease needs to be monitored in long-term follow-up in case of recurrence or progression. At present, apart from computed tomography (CT), magnetic resonance imaging (MRI) and other imaging methods, tumor biomarkers are often employed to screen malignant diseases, monitor treatment responses and assess the status of the disease, including α-fetoprotein for liver cancer and prostate-specific antigen for prostate cancer (3,4). Compared with imaging methods, tumor biomarkers are easier and more economical. Unfortunately, the sensitivities and specificities of the commonly used biomarkers for lymphoma (e.g., lactate dehydrogenase and β 2-microglobulin) are limited (5,6).
B-NHL consists of aggressive and indolent subtypes according to its clinical features. Indolent lymphoma accounts for nearly one-third of NHL cases, and it is considered incurable (7). Indolent lymphoma includes low-grade (grade 1–2) follicular lymphoma (FL), chronic lymphocyte leukemia/small lymphocytic lymphoma, mucosa-associated lymphoid tumors (MALT), minority of mantle cell lymphoma (MCL) and lymphoplasmacytic lymphoma (8). Aggressive B-NHL comprises diffuse large B-cell lymphoma (DLBCL), most cases of MCL, grade 3 FL and Burkitt's lymphoma (9). Occasionally, several indolent subtypes may transform into aggressive subtypes. For example, low grade FL often changes into aggressive B-NHL, which for most of the cases are DLBCL, spontaneously. This transformation is referred to as transformed lymphoma (TL) and has a reported incidence of 10–70% (10). Since the outcomes of the two subtypes are very different, it is of clinical significance to monitor the transformation during long-term follow-up. However, pathological examination is not suitable for the long-term follow-up of TL due to its invasiveness. Therefore, a new non-invasive method should be developed to monitor the transformation.
Metabonomic analysis, a brand-new approach, employs comprehensive metabolic profiling methods to provide systemic views of the disordered biological processes of diseases. Urine is considered ideal for metabonomic analysis as sampling of urine is non-invasive, and it is relatively easy to obtain sufficient volume for analysis (11). Since cancer often induces severe metabolic disorders, the comprehensive analysis of metabolites in urine may help to discover useful biomarkers for cancer (12,13). Silva et al (14) observed lower levels of hexanoic acid in the urine of patients with cancer compared with healthy controls using solid phase micro-extraction (SPME) in combination with gas chromatography-mass spectrometry (GC-MS). Guadagni et al (15) also detected higher concentrations of hexanal in the urine samples of patients with lung cancer compared with healthy controls using head-space SPME-GC-MS. Here, we present a pilot study using head-space SPME-GC-MS to assess the possibility of volatile metabolites in urine to satisfy the aims stated above.
Materials and methods
Recruitment of subjects
Between March 2014 and June 2014, urine samples were collected from 101 previously untreated patients [71 male and 30 female, average age 56.6 (37–78) years old] with a diagnosis of lymphatic disease and 30 healthy volunteers [21 male and 9 female, average age 57.1 (38–76) years old] at the First Affiliated Hospital of Anhui Medical University, (Hefei, China). The diagnoses of lymphatic diseases (B-NHL and benign lymphatic diseases) were confirmed by pathology. The clinical stage of the B-NHL was determined according to the Ann Arbor staging system (16). The prognosis of B-NHL was assessed using International Prognostic Index (IPI) score. The 30 healthy volunteers employed in the present study were family members of the patients. The exclusion criteria for the present study included: i) History of other types of cancer; ii) pregnant or lactating female; iii) presence of metabolic diseases, including diabetes; iv) liver or urinary diseases; v) smoker or drinker; vi) use of specific drugs, including antibiotics, hormones and non-steroid anti-inflammatory drugs. A questionnaire was designed to investigate the dietary habits of the subjects, and no significant difference was observed among the groups. All subjects have signed an informed consent to participate in the present study. The present study was approved by the Ethics Committee of Anhui Medical University, and the use of human urine samples was in accordance with the Guidelines of the Declaration of Helsinki.
Materials and equipment
Methanol standards (purity ≥99.0%) and 30 quantitatively volatile metabolites (purity ≥95.0–99.5%) were purchased from 7 different chemical reagent companies (Aladdin Co., Ltd, Shanghai, China (http://www.aladdin-e.com); Energy-Chemical Co., Ltd., Shanghai, China (https://www.energy-chemical.com); Alfachina Co., Ltd., Shanghai, China (http://www.alfachina.cn); Brain Biotecnology Co., Ltd., Shanghai, China (http://www.brain-biot.com); Sinopharm Chemical Reagent Co., Ltd., Shanghai, China; Xibao Biotecnology Co., Ltd., Nanjing, China (http://seebio.biomart.cn); Zhongwei Chemical Co., Ltd, Beijing, China (http://www.wechem.cn). The pH values of samples were tested using a portable pH test pen (Hengaodeyiqiyibiao Co., Ltd., Beijing, China). The 30 ml sterile glass vials were purchased from Sanyuanhuaboyiqi Co., Ltd., (Hefei, China). The 20 ml GC vials that were capped with Teflon (PTFE) septa were obtained from (Shimadzu Corporation, Kyoto, Japan). SPME manual holder and the carboxen-polydimethylsiloxane (CAR-PDMS) fiber were produced from Sigma-Aldrich, (Merck KGaA, Darmstadt, Germany). GC-MS QP 2010 Plus equipped with HP-5MS column and NIST 05 library was obtained from Shimadzu Corporation. The HP-5MS column (size × I.D., 30 m × 0.25 mm; thickness, 0.25 µ.) was obtained from Agilent Technologies, Inc., (Santa Clara, CA, USA).
Sample collection and preparation
Each subject (either patient or healthy control) fasted and did not drink water overnight. The next day, the subject was requested to pass mid-stream morning urine into a 30 ml sterile glass vial, which was sealed thereafter. The samples were analyzed on the same day of sample collection (transported at 4°C to the laboratory and analyzed within 1 h of collection). In the laboratory, 10 ml urine samples were transported into GC vials that were previously added with 3 g sodium chloride. Either 0.1 ml 5 mol/l HCl, 5 mol/l NaOH or ultra-pure water was added into the GC vials depending on the required final pH as described in a previous study (17). In this way, acids and sulfur compounds are better extracted at an acidic pH, whereas the extraction of alcohols and heterocyclic compounds in an alkaline environment is favored.
SPME procedure
The volatile metabolites in the headspace of urine were extracted using CAR-PDMS fiber as described in a previous study (18). Briefly, SPME fibers were pre-conditioned by inserting them into the GC injector port as recommended by the manufacturer (Sigma-Aldrich; Merck KGaA). The GC vials containing urine samples were sealed and placed in an oven (Shimadzu Corporation) at 40°C for 30 min with vibration. Next, the vials were equilibrated in the same oven without vibration for 30 min. Following equilibration, static extraction in the headspace of urine was performed for 30 min using the CAR-PDMS fiber. At the end of the extraction time, the fiber was inserted into the GC injector port for the thermal desorption of volatile metabolites.
GC-MS analyses
The GC injector port temperature was 250°C. The carrier gas used for GC was helium with a flow rate of 1 ml/min, and the splitless mode was used. The temperature profile of the column oven was set as follows: i) 35°C for 2 min; ii) increase in temperature for 6°C/min until 150°C, iii) increase in temperature for 12°C/min until 250°C, and the temperature is maintained for 3 min. The ion source temperature of the mass spectrometer was 200°C, and the detector operated in a mass range of 40–350 m/z. The chromatographic data sets were analyzed as described in previous studies by the present authors (19,20). Using the GC-MS Post Run software (GCMS Solution, Shimadzu Corporation), peak detection and volatile metabolite identification were carried out. The identification of each volatile metabolite was achieved by comparing the fragmentation patterns (i.e., presence and intensity of the signals) with those in the NIST 2005 library, and this was verified by evaluating the retention time using standard compounds, when available. The fragment ion m/z values of the identified volatile metabolites that have the highest abundance and the matching percentages in the NIST library were assessed. Additionally, the occurrence rates of the volatile metabolites in urine samples were assessed.
Samples for quantitation of volatile metabolite and method validation
Calibration samples were prepared in 20 ml GC vials containing a set of six volumes of standard metabolites (i.e., 30 volatile metabolites that are present in 100% of the urine samples) prepared in methanol, 10 ml urine (from a healthy volunteer) and 3 g sodium chloride. These samples were used to establish the calibration curves in order to quantify the volatile metabolites in the urine samples. The calibration samples were not prepared by adding the metabolite standards into water due to the complexity of the components of human urine as the complex components of urine may have a notable effect on the quantification of target volatile metabolites. Either 0.1 ml 5 mol/l HCl, 5 mol/l NaOH or ultra-pure water was added into the GC vials containing calibration samples depending on the required final pH. The control samples were prepared with 10 ml urine and 3 g sodium chloride (NaCl). Similar to the preparation of the calibration samples, either 0.1 ml 5mol/l HCl, 5 mol/l NaOH or ultra-pure water was also added into control samples depending on the required final pH. Blank samples were prepared by adding 10 ml ultra-pure water into 20 ml GC vials (previously added with 3 g sodium chloride and either 0.1 ml l 5 mol/l HCl, 5 mol/l NaOH or ultra-pure water. Calibration samples were analyzed together with the control samples, for the quantification of volatile metabolites. The blank samples were adopted for the evaluation of contamination in the environment, accuracy, precision, detection and quantification limits.
Quantification of volatile metabolites and method validation
Calibration curves were established as described in a previous study (15). The chromatographic peak areas of target volatile metabolites in the calibration samples were subtracted with areas from the control samples. The subtraction is required due to the endogeneity of the volatile metabolites. Here, limit of detection (LOD) and limit of quantification (LOQ) of the detection method are defined as the concentration of the volatile metabolite that results in a noise ratio of 3 and 10, respectively. Due to the endogenous nature of the volatile metabolites, blank samples were used instead of control samples. The signal to noise ratios of the chromatographic peaks of the blank samples that were added with decreasing concentrations of volatile metabolites were measured to evaluate the LODs and LOQs of this detection method. The accuracy of the detection method was evaluated by the percentage of deviation between the calculated concentration value (from the calibration curve) and the actual value of standard added in the blank samples. The precision of the detection method was calculated as relative standard deviation (RSD) of the peak area values by detecting blank samples that were added with a standard of constant concentration at three different days (1st, 7 and 30th day; 3 times per day).
Statistical analyses
To avoid bias and over-fitting of the data, urine samples were coded prior to data acquisition and randomized using MATLAB (R2008a; MathWorks, MA, USA). The volatile metabolites that exhibited significant differences (P-value=0.05) among the groups were determined from the head-space SPME-GC-MS results using the SPSS 23.0 package for Windows (SPSS, Inc., Chicago, IL, USA) by the non-parametric Wilcoxon's test. Receiver operating characteristic (ROC) curves were used to evaluate the diagnostic values of the potential biomarkers. Binary logistic regression analyses were performed to construct ROC curves of the combination of potential biomarkers. The concentrations of volatile biomarkers that exhibited statistically significant differences between the groups were included as variables for the ROC curves, and the groups were used as the dependent variables.
Results
Clinical characteristics of enrolled subjects
A total of 131 subjects were recruited in the present study. The subjects were divided into four groups. Subjects with aggressive B-NHL were included in the first group. A total of 35 patients with DLBCL were recruited in this group. The second group consisted of 33 subjects with indolent B-NHL (23 cases with low grade FL and 10 cases with MALT). The third group included 33 reactive hyperplastic lymphadenitis patients. The fourth group included 30 healthy controls. There was no difference in the distribution of age and sex among the groups (P>0.05). The details of the subjects are stated in Table I.
Table I.
Clinical characteristics of enrolled subjects.
| A, B-NHL | ||
|---|---|---|
| Characteristics | AB, (n=35) | IB, (n=33) |
| Age, years, mean, (range) | 54.3 (37–71) | 56.7 (40–78) |
| Sex | ||
| Male | 24 | 24 |
| Female | 11 | 9 |
| CD20 | ||
| Positive (+) | 20 | 12 |
| Negative (−) | 15 | 21 |
| Stage | ||
| Early (I–II) | 27 | 22 |
| Advanced (III–IV) | 8 | 11 |
| IPI score | ||
| 0–2 | 19 | 25 |
| 3–5 | 16 | 8 |
| B, Non-lymphoma | ||
| Characteristics | BLD, (n=33) | HC, (n=30) |
| Age, years, mean, (range) | 58.9 (40–75) | 57.1 (38–76) |
| Sex | ||
| Male | 23 | 21 |
| Female | 10 | 9 |
| CD20 | ||
| Positive (+) | – | – |
| Negative (−) | – | – |
| Stage | ||
| Early (I–II) | – | – |
| Advanced (III–IV) | – | – |
| IPI score | ||
| 0–2 | – | – |
| 3–5 | – | – |
AB, aggressive B-NHL; B-NHL, B-cell non-Hodgkin's lymphoma; IB, indolent B-NHL; BLD, benign lymphatic diseases; HC, healthy control; IPI, International Prognostic Index.
Qualitative and quantitative analyses of volatile metabolites
A total of 227 volatile metabolites were identified under acidic, basified and unmodified pH. The identified volatile metabolites included various chemical families: Aldehydes, ketones, acids, alcohols, benzene derivatives, phenols, esters, furan and sulfur-containing compounds. A total of 35 of the 227 volatile metabolites were present in 100% of the urine samples. A total of 125 volatile metabolites were detected in the acidic samples, and 22 of the metabolites were ubiquitous. A total of 102 metabolites were detected in the basified urine samples and 19 of the metabolites were present in 100% of the urine samples. A total of 85 volatile metabolites were detected in the unmodified pH urine samples, and 22 of the metabolites were ubiquitous. A total of 7 volatile metabolites were present independent of pH values. These 7 metabolites were acetone, 2-butanone, 4-heptanone, dimethyl disulfide, furan, 2-methylbutanal and 2-methylpyrazine.
The relative heights of the mass spectra of the ubiquitous volatile metabolites in the samples and NIST library were similar indicating the absence of disruptors. Out of the 35 ubiquitous volatile metabolites, 1 was excluded because of chromatographic column bleed. A total of 2 metabolites were excluded due to the unavailability of a high-purity calibration standard, and the other 2 metabolites were considered as environmental contamination as the abundances of these metabolites were similar in urine and blank samples. Therefore, 30 volatile metabolites were further analyzed (Table II).
Table II.
Ubiquitous volatile metabolites identified under acidic/basified/unmodified pH in the urine samples.
| Ubiquitous or not | ||||||
|---|---|---|---|---|---|---|
| Name | CAS no. | Chemical group | m/z | Acidic | Basified | Unmodified |
| 2-Butanone | 78-93-3 | Ketone | 43 | Y | Y | Y |
| 4-Heptanone | 123-19-3 | Ketone | 43 | Y | Y | Y |
| Furan | 110-00-9 | Furan | 68 | Y | Y | Y |
| Acetone | 67-64-1 | Ketone | 43 | Y | Y | Y |
| 2-Methylbutanal | 96-17-3 | Aldehyde | 41 | Y | Y | Y |
| 2-Methylpyrazine | 109-08-0 | Pyrazine | 94 | Y | Y | Y |
| Dimethyl disulfide | 624-92-0 | Sulfide | 94 | Y | Y | Y |
| 2,6-Dimethyl-7-octen-2-ol | 17,042-16-9 | Ketone | 59 | N | Y | Y |
| Decanoic acid | 334-48-5 | Acid | 60 | Y | N | Y |
| 6-Methyl-3-heptanone | 624-42-0 | Ketone | 57 | N | Y | Y |
| Methylpropyldisulfide | 2,179-60-4 | Thioether | 80 | Y | N | Y |
| Phenol | 108-95-2 | Phenol | 94 | Y | N | N |
| Methylphenol | 620-17-7 | Phenol | 107 | Y | N | N |
| Benzaldehyde | 100-52-7 | Aldehyde | 77 | Y | N | Y |
| α-Calacorene | 21,391-99-1 | Alkene | 157 | Y | N | N |
| 1,2-Dihydro-1,1,6-trimethylnaphthalene | 30,364-38-6 | Naphthaline | 157 | Y | N | N |
| 2,5-Dimethylfuran | 625-86-5 | Furan | 96 | Y | N | N |
| Nonanal | 124-19-6 | Aldehyde | 57 | Y | N | Y |
| 3-Nonen-2-one | 14,309-57-0 | Ketone | 55 | N | Y | Y |
| Furfural | 98-01-1 | Aldehyde | 96 | Y | N | Y |
| Hexanal | 66-25-1 | Aldehyde | 44 | Y | N | Y |
| Indole | 120-72-9 | Indole | 117 | N | Y | Y |
| Methanethiol | 74-93-1 | Alcohol | 47 | N | Y | Y |
| 2-Methyl-3-phenyl-2-propenal | 101-39-3 | Aldehyde | 145 | Y | N | N |
| 3-Methyl-1-butanol | 123-51-3 | Alcohol | 55 | N | Y | Y |
| 2-Ethyl-5-methylfuran | 1,703-52-2 | Furan | 95 | N | Y | Y |
| 6-Methyl-5-hepten-2-one | 110-93-0 | Ketone | 43 | N | Y | Y |
| 2-Pentanone | 107-87-9 | Ketone | 43 | N | Y | N |
| 3-Methyl-3-buten-2-one | 814-78-8 | Ketone | 43 | N | Y | N |
| 1,2,4-Trimethylbenzene | 95-63-6 | Benzene | 105 | N | Y | N |
CAS no., chemical abstracts service identifier.
Potential biomarkers and their diagnostic values for B-NHL
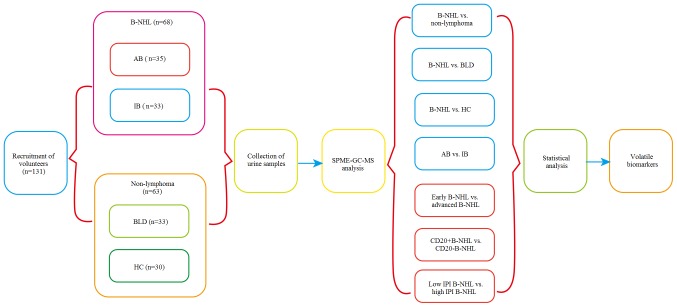
Various cross-comparisons between the groups were carried out as follows: i) B-NHL (aggressive B-NHL and indolent B-NHL; n=68) vs. non-lymphoma (benign lymphatic disease and healthy control; n=63); ii) B-NHL (n=68) vs. BLD (benign lymphatic disease; n=33); iii) B-NHL (n=68) vs. healthy control (n=30); iv) aggressive B-NHL (n=35) vs. indolent B-NHL (n=33); v) CD20+ B-NHL (n=32) versus CD20− B-NHL (n=36); vi) early-stage B-NHL (n=49) vs. advanced B-NHL (n=19); (7) low IPI score (0–2) B-NHL (n=44) vs. high IPI score (3–5) B-NHL (n=24). The comparisons are indicated in Fig. 1.
Figure 1.
Flowchart of the study. B-NHL, B-cell non-Hodgkin's lymphoma; AB, aggressive B-NHL; IB, indolent B-NH; BLD, benign lymphatic diseases; HC, healthy control; IPI, International Prognostic Index.
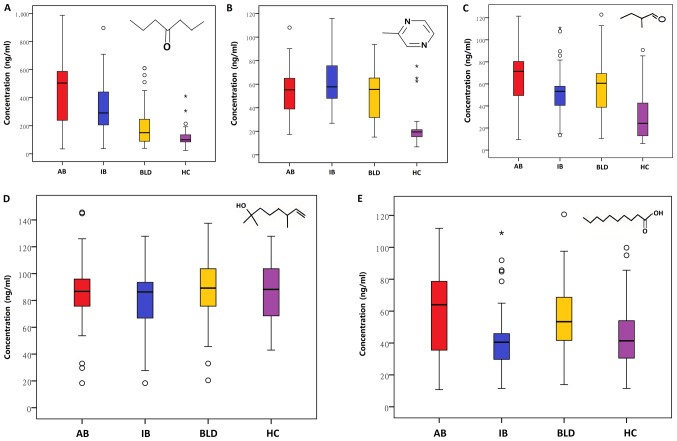
A total of 3 volatile metabolites (4-heptanone, 2-methylpyrazine and 2-methylbutanal) were detected to significantly discriminate B-NHL from non-lymphoma. The mean concentrations of these metabolites ranged from 24.76 to 494.27 ng/ml in the four groups (Fig. 2 and Table III). The concentrations of the 3 volatile metabolites also were detected to be significantly different between patients with B-NHL and healthy controls (Table III). Notably, the concentration of 4-heptanone was significantly higher in patients with B-NHL compared with those with BLD (Table III). In addition, the concentrations of the 3 volatile metabolites (4-heptanone, 2-methylbutanal and decanoic acid) were significantly different between aggressive B-NHL and indolent B-NHL (Table III). The mean concentrations of the 3 volatile metabolites ranged from 45.50 to 494.27 ng/ml in the aggressive B-NHL and indolent B-NHL groups (Table III). Two volatile metabolites (4-heptanone and 2,6-dimethyl-7-octen-2-ol) were detected to able to significantly discriminate early-stage lymphoma from advanced lymphoma. A total of 2 volatile metabolites (2-methylbutanal and decanoic acid) were able to distinguish the patients with CD20− B-NHL and CD20+ B-NHL with significantly higher concentration levels in patients with CD20− B-NHL (Table III). Additionally, 2-methylpyrazine was detected at higher concentration in patients with high IPI score B-NHL compared with those with low IPI score B-NHL (Table III).
Figure 2.
Levels of volatile biomarkers in urine among the groups. Box-whisker plots of the concentrations of (A) 4-heptanone, (B) 2-methylpyrazine, (C) 2-methylbutanal, (D) 2,6-dimethyl-7-octen-2-ol and (E) decanoic acid. The data are presented as the median value (black line), interquartile range (box), and 5th and 95th percentiles (whiskers). AB, aggressive B-NHL; IB, indolent B-NHL; BLD, benign lymphatic diseases; HC, healthy control. The circles represent values >1.5 times the amount of the interquartile value and ≤3 times the amount of the interquartile value. *Represent values >3 times the amount of the interquartile value.
Table III.
Identified VOCs with significant statistical differences between the study groups in urine samples from healthy volunteers, patients with B-NHL and patients with BLD.
| Concentration, M ± SDa (ng/ml) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B-NHL | Non-lymphoma | P-value (Wilcoxon's non-parametric test) | |||||||||
| Volatile metabolite | AB | IB | BLD | HC | B-NHL vs. non-lymphoma | B-NHL vs. BLD | B-NHL vs. HC | AB vs. IB | CD20+ vs. CD20 | Early-stage | Low IPI vs. high IPI |
| 4-Heptanone | 494.27±239.65 | 356.09±208.59 | 206.31±165.40 | 119.86±76.96 | <0.001 | 0.002 | <0.001 | 0.036 | 0.858 | <0.001 | 0.864 |
| 2-Methylpyrazine | 55.78±21.83 | 61.26±19.92 | 51.99±20.43 | 24.76±20.24 | <0.001 | 0.238 | <0.001 | 0.177 | 0.659 | 0.098 | 0.005 |
| 2-Methylbutanal | 66.26±30.15 | 51.25±23.60 | 58.42±25.37 | 31.43±23.03 | 0.005 | 0.131 | <0.001 | 0.029 | <0.001 | 0.095 | 0.165 |
| 2,6-Dimethyl-7-octen-2-ol | 94.56±40.18 | 82.02±30.63 | 92.92±35.64 | 87.91±22.48 | 0.428 | 0.680 | 0.978 | 0.334 | 0.798 | 0.003 | 0.218 |
| Decanoic acid | 60.34±26.31 | 45.50±22.21 | 58.22±24.82 | 45.98±21.40 | 0.127 | 0.447 | 0.06 | 0.017 | <0.001 | 0.156 | 0.258 |
The highest level of concentration was selected when volatile metabolite was detected in the same urine sample with different pHs. B-NHL, B-cell non-Hodgkin's lymphoma; IPI, International Prognostic Index; AB, aggressive B-NHL; IB, indolent B-NH; BLD, benign lymphatic diseases; HC, healthy control; M, mean; SD, standard deviation.
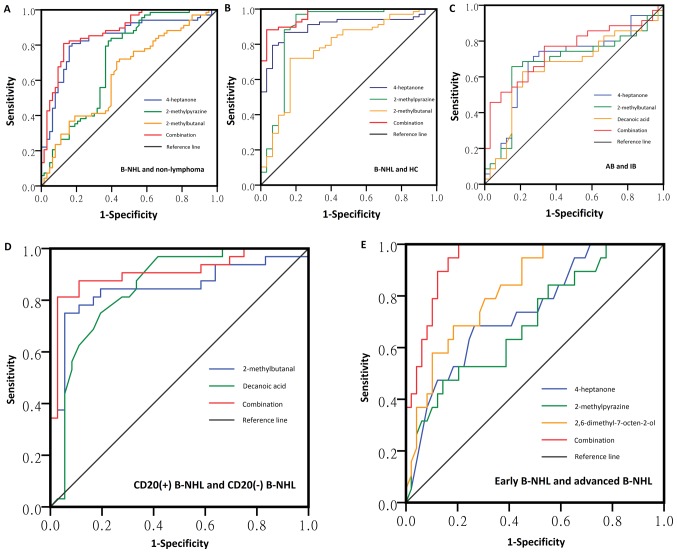
The applicability of the potential biomarkers identified in the Wilcoxon's test was assessed with ROC curve (Fig. 3 and Table IV). The sensitivities of the potential biomarkers ranged from 62.9 to 97.1% and the specificities ranged from 55.6 to 91.0%. Their AUC values ranged from 0.634 to 0.901. In order to construct ROC curves of a combination of the different biomarkers, binary logistical regression analyses were performed. The AUC values ranged from 0.824 to 0.968. Therefore, diagnostic models that use a combination of different biomarkers may be preferable.
Figure 3.
ROC curves of volatile biomarkers for the diagnosis of (A) B-NHL from non-lymphoma, (B) B-NHL from healthy control, (C) aggressive B-NHL from indolent B-NHL, (D) CD20+ B-NHL from CD20− B-NHL, (E) early-stage B-NHL from advanced B-NHL using different combinations of 4-heptanone, 2-methylpyrazine, 2-methylbutanal, 2,6-dimethyl-7-octen-2-ol and decanoic acid. The AUC values are 0.878, 0.968, 0.824, 0.908 and 0.941, respectively. AUC, area under curve; B-NHL, B-cell non-Hodgkin's lymphoma; ROC, receiver operating characteristic.
Table IV.
ROC curves of potential volatile biomarkers.
| Volatile metabolite | Cut-off value | Sensitivity | Specificity | AUC | P-value | AUC 95% CI |
|---|---|---|---|---|---|---|
| 4-Heptanone | ||||||
| B-NHL vs. non-lymphoma | 224.27 | 0.794 | 0.841 | 0.835 | <0.001 | 0.763–0.907 |
| B-NHL vs. BLD | 198.02 | 0.809 | 0.758 | 0.775 | <0.001 | 0.677–0.874 |
| B-NHL vs. HC | 144.87 | 0.868 | 0.867 | 0.901 | <0.001 | 0.838–0.964 |
| AB vs. IB | 440.38 | 0.686 | 0.758 | 0.687 | 0.008 | 0.555–0.818 |
| Early-stage vs. advanced | 412.45 | 0.684 | 0.735 | 0.742 | 0.002 | 0.613–0.870 |
| 2-Methylpyrazine | ||||||
| B-NHL vs. non-lymphoma | 38.79 | 0.824 | 0.619 | 0.714 | <0.001 | 0.623–0.805 |
| B-NHL vs. HC | 21.78 | 0.971 | 0.800 | 0.885 | <0.001 | 0.792–0.978 |
| Low IPI vs. high IPI | 66.05 | 0.696 | 0.818 | 0.765 | <0.001 | 0.634–0.897 |
| 2-Methylbutanal | ||||||
| B-NHL vs. non-lymphoma | 42.86 | 0.721 | 0.556 | 0.634 | 0.008 | 0.539–0.730 |
| B-NHL vs. HC | 42.86 | 0.721 | 0.833 | 0.777 | <0.001 | 0.673–0.881 |
| AB vs. IB | 69.94 | 0.743 | 0.909 | 0.798 | <0.001 | 0.678–0.919 |
| CD20+ vs. CD20− | 69.59 | 0.750 | 0.840 | 0.848 | <0.001 | 0.746–0.950 |
| 2,6-Dimethyl-7-octen-2-ol | ||||||
| Early-stage vs. advanced | 333.77 | 0.737 | 0.694 | 0.734 | 0.003 | 0.608–0.860 |
| Decanoic acid | ||||||
| AB vs. IB | 48.04 | 0.629 | 0.788 | 0.657 | 0.026 | 0.523–0.792 |
| CD20+ vs. CD20− | 40.70 | 0.780 | 0.910 | 0.851 | <0.001 | 0.874–0.986 |
| Combined ROC models | ||||||
| B-NHL vs. non-lymphoma | – | – | – | 0.878 | <0.001 | 0.819–0.937 |
| B-NHL vs. HC | – | – | – | 0.968 | <0.001 | 0.938–0.997 |
| AB vs. IB | – | – | – | 0.824 | <0.001 | 0.806–0.912 |
| CD20+ vs. CD20− | – | – | – | 0.908 | <0.001 | 0.830–0.986 |
| Early-stage vs. advanced | – | – | – | 0.941 | <0.001 | 0.890–0.992 |
AUC, area under the curve; B-NHL, B-cell non-Hodgkin's lymphoma; AB, aggressive B-NHL; IB, indolent B-NH; BLD, benign lymphatic diseases; CI, confidence interval; HC, healthy control; IPI, International Prognostic Index; ROC, receiver operating characteristic.
Linearity, LOD, LOQ precision and accuracy
The LOD, LOQ, linear coefficient (R2) and linear range for each potential volatile biomarker are presented in Table V. For the identified volatile biomarkers, the LODs ranged from 0.8 to 4.8 ng/ml, and the LOQs ranged from 2.5 to 9.5 ng/ml, which demonstrated that the detection method was sensitive. The RSD values for intra-and inter-day precision were <5%, which demonstrated that the detection method was reliable. The accuracy of detection method was also evaluated, and this ranged from 97 to 108% (Table V).
Table V.
Method validation of each potential biomarker (ng/ml).
| Volatile metabolite | LOD | LOQ | Linear range | Coefficient (R2) | Precision (RSD), % | Accuracy (%) |
|---|---|---|---|---|---|---|
| 4-Heptanone | 2.6 | 7.5 | 10–600 | 0.992 | 3.8 | 105 |
| 2-Methylpyrazine | 0.9 | 2.5 | 5–100 | 0.995 | 2.5 | 108 |
| 2-Methylbutanal | 1.5 | 2.8 | 5–150 | 0.999 | 1.8 | 99 |
| 2,6-Dimethyl-7-octen-2-ol | 4.8 | 9.5 | 10–250 | 0.997 | 4.8 | 97 |
| Decanoic acid | 0.8 | 2.5 | 5–100 | 0.995 | 2.3 | 102 |
LOD, limit of detection; LOQ, limit of quantification; RSD, relative standard deviation.
Discussion
The majority of patients with B-NHL were diagnosed at an advanced stage resulting in poor prognoses because of being asymptomatic at early stages of the disease (21). For example, 70–75% of patients with DLBCL were diagnosed at an advanced stage (22), and only 25% of patients with FL were diagnosed at early stage (23). The patients with aggressive B-NHL that were diagnosed at early stages of the disease not only had improved prognosis compared with patients diagnosed at an advanced stage (5-year overall survival, 90 vs. <70%) but also had advantages of reduced dosages, toxicities of therapy and less economic burden (24–26). In the present study, the concentration levels of 3 volatile metabolites (4-heptanone, 2-methylpyrazine and 2-methylbutanal) in urine samples from patients with B-NHL were significantly higher compared with non-lymphoma subjects, and the concentration of 1 of the metabolites (4-heptanone) was significantly different between early and advanced stages of lymphoma, indicating that 4-heptanone may be a potentially useful biomarker for screening of B-NHL.
The prognosis of TL is quite poor. A retrospective study indicated a median overall survival (OS) of 1.2 years from the time of transformation (27). The misdiagnosis of TL delays opportunities for treatment. In the present study, 4-heptanone, 2-methylbutanal and decanoic acid were identified as novel volatile biomarkers with satisfactory accuracy to differentiate aggressive B-NHL from indolent B-NHL. Therefore, it is possible to monitor the transformation of indolent lymphoma using volatile biomarkers in urine.
In the present study, volatile metabolites in urine were identified to be capable of discerning the critical features of B-NHL, including CD20+/CD20−, early/advanced stage and low/high IPI scores. The sensitivities of the metabolites ranged from 68.4 to 78.0%, and the specificity values ranged from 69.4 to 91.0%. Currently, the determination of clinical stage of B-NHL is primarily based on expensive imaging methods, including CT, MRI, positron emission tomography-CT and invasive pathologic analysis of bone marrow samples. However, the detection of volatile metabolites in urine is cheap and noninvasive, which means a better method for determination of clinical stage of B-NHL. The present classification of CD20 status mainly relies on immunohistochemistry, which is subjective and requires a suitable quality of lymphoma tissue and qualified pathology physicians (28). Due to the lack of expertise in pathology, the false judgment of CD20 is encountered in hospitals (21). By contrast, volatile metabolites in urine, as an objective diagnostic method, may be a valuable method for improving the diagnostic accuracy of CD20 status. Moreover, different volatile metabolites were detected in in urine samples from B-NHL patients with low and high IPI scores, which indicate that volatile biomarkers in urine have the potential to predict the prognosis of B-NHL.
The latent volatile biomarkers may origin from a variety of endogenous biochemical pathways and exogenous sources (environmental pollutions, food, tobacco and alcohol) (29). For example, increased reactive oxygen species can attack the polyunsaturated fatty acids in the cell membranes and generate volatile organic compounds (VOCs), which result in a process known as oxidative stress (30). In the present study, the concentrations of 3 VOCs (4-heptanone, 2-methylpyrazine and 2-methylbutanal) were significantly different between the B-NHL group and healthy control group. However, only 1 VOC, 4-heptanone, was able to distinguish B-NHL from benign lymphatic disease. This result can be explained as benign lymphatic diseases have the level of oxidative stress similar to B-NHL. In terms of endogenous sources, it has been proposed that new VOCs can be produced or the levels of VOCs can change in pathological processes (31). The VOCs could be biomarkers for early diagnoses of malignancies based on the hypothesis that tumors have marked metabolic abnormalities even in the early stages (32). To reduce the confounders from exogenous contamination, smokers and drinkers were excluded in the present study. Furthermore, no significant differences in dietary habits of the volunteers were observed among the groups.
It has been reported that 4-heptanone is a β-oxidation product of 2-ethylhexanoic acid from plasticizers in a study on the in vivo metabolism of humans (33). Since plasticizers are considered as carcinogen (34), 4-heptanone can be an exogenous biomarker for B-NHL. Hanai et al (35) detected increased concentrations of 2-methylpyrazine in the urine of human lung tumor-bearing mice. Nevertheless, 2-methylpyrazine was not considered as a suitable candidate for lung cancer biomarker, since it was not likely to be released from lung cancer cell. A study by Calejo et al (36) revealed that 2-methylbutanal had a higher level of concentration in the urine samples of smokers compared with non-smokers. The authors suggested that 2-methylbutanal as a potentially useful biomarker to identify smoking habits. However in the present study, smokers were excluded, which indicates that there are different sources of 2-methylbutanal. Previous studies indicated that abnormal expression of 10-formyltetrahydrofolate dehydrogenase and alcohol dehydrogenase in cancer may increase aldehyde levels (37,38). However, the association between the enzymes and 2-methylbutanal has not been demonstrated. Another potential biomarker, 2,6-dimethyl-7-octen-2-ol, had been identified in urine samples from patients with prostate cancer, and the diagnostic value of 2,6-dimethyl-7-octen-2-ol was similar to PSA (39). Decanoic acid was detected in several types of fats and may be responsible for the mitochondrial proliferation associated with the ketogenic diet (40,41). To date, decanoic acid has not been reported to be a biomarker of cancer. In the present study, significant differences in the concentration of decanoic acid were detected between the different subtypes of B-NHL.
There are several limitations in the present study. Firstly, all the volunteers in the present study were non-smokers and non-drinkers from the same place and of the same ethnic origin. Therefore, diverse populations should be tested to evaluate the effects of these confounding factors in further studies. Secondly, the sample size of the present study is limited. In many other disease-screening studies (e.g., lung cancer) that asses the levels of volatile metabolites, subtypes of lung cancer are often pooled in one group due to the limitation of sample size (42–44). For the same reason, different subtypes of B-NHL were pooled to differentiate B-NHL from healthy control and to distinguish aggressive B-NHL from indolent B-NHL. Even biomarkers with high diagnostic values were found in our study, caution is needed to explain the results. Taken together, the present study indicated that volatile metabolites in urine might be potentially used as biomarkers for screening and monitoring of B-NHL. The possibility of volatile biomarkers in urine samples for the screening of different subtypes of B-NHL will be assessed in future studies with larger sample sizes.
Acknowledgements
The authors would like to thank the staff of Anhui Institute for Food and Drug Control for their technical assistance.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81472750), the Nature Science Research Project of Anhui province (grant no. 1508085MH171) and the Scientific Research Project for Young and Middle-aged Staff by Wannan Medical College (grant no. KY85880317).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
QLH, LW and CL made substantial contributions to acquisition and analyses of data. They also drafted the manuscript and revised it critically for important intellectual content. LLH and YZZ were major contributors in method design and GC-MS operation. HL made substantial contributions to study conception and design. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Anhui Medical University (no. 20140141), and the use of human urine samples was in accordance with the Guidelines of the Declaration of Helsinki. All subjects provided informed consent to participate in the present study.
Consent for publication
No identifying information of the patients or volunteers were included in our manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Ondrejka SL, His ED. Pathology of B-cell lymphomas: Diagnosis and biomarker discovery. Cancer Treat Res. 2015;165:27–50. doi: 10.1007/978-3-319-13150-4_2. [DOI] [PubMed] [Google Scholar]
- 3.Filella X, Foj L. Prostate cancer detection and prognosis: From prostate specific antigen (PSA) to exosomal Biomarkers. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17111784. pii: E1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sauzay C, Petit A, Bourgeois AM, Barbare JC, Chauffert B, Galmiche A, Houessinon A. Alpha-foetoprotein (AFP): A multi-purpose marker in hepatocellular carcinoma. Clin Chim Acta. 2016;463:39–44. doi: 10.1016/j.cca.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Toth DF, Raderer M, Wadsak W, Karanikas G. Beta-2 microglobulin as a diagnostic parameter in non-Hodgkin lymphoma: A comparative study with FDG-PET. Anticancer Res. 2013;33:3341–3345. [PubMed] [Google Scholar]
- 6.William BM, Bongu NR, Bast M, Bociek RG, Bierman PJ, Vose JM, Armitage JO. The utility of lactate dehydrogenase in the follow up of patients with diffuse large B-cell lymphoma. Rev Bras Hematol Hemoter. 2013;35:189–191. doi: 10.5581/1516-8484.20130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leslie LA, Skarbnik AP, Bejot C, Stives S, Feldman TA, Goy AH. Targeting indolent non-Hodgkin lymphoma. Expert Rev Hematol. 2017;10:299–313. doi: 10.1080/17474086.2017.1303374. [DOI] [PubMed] [Google Scholar]
- 8.van den Brand M, Scheijen B, Hess CJ, van Krieken JHJ, Groenen PJTA. Pathways towards indolent B-cell lymphoma-Etiology and therapeutic strategies. Blood Rev. 2017;31:426–435. doi: 10.1016/j.blre.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Sweetenham JW. Following aggressive B-cell lymphoma. Blood. 2015;125:3673–3674. doi: 10.1182/blood-2015-04-641738. [DOI] [PubMed] [Google Scholar]
- 10.Yuen AR, Kamel OW, Halpern J, Horning SJ. Long-term survival after histologic transformation of low-grade follicular lymphoma. J Clin Oncol. 1995;13:1726–1733. doi: 10.1200/JCO.1995.13.7.1726. [DOI] [PubMed] [Google Scholar]
- 11.Amann A, Costello Bde L, Miekisch W, Schubert J, Buszewski B, Pleil J, Ratcliffe N, Risby T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J Breath Res. 2014;8:034001. doi: 10.1088/1752-7155/8/3/034001. [DOI] [PubMed] [Google Scholar]
- 12.Bouatra S, Aziat F, Mandal R, Guo AC, Wilson MR, Knox C, Bjorndahl TC, Krishnamurthy R, Saleem F, Liu P, et al. The human urine metabolome. PLoS One. 2013;8:e73076. doi: 10.1371/journal.pone.0073076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lacy Costello B, Amann A, Al-Kateb H, Flynn C, Filipiak W, Khalid T, Osborne D, Ratcliffe NM. A review of the volatiles from the healthy human body. J Breath Res. 2014;8:014001. doi: 10.1088/1752-7155/8/1/014001. [DOI] [PubMed] [Google Scholar]
- 14.Silva CL, Passos M, Camara JS. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br J Cancer. 2011;105:1894–1904. doi: 10.1038/bjc.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guadagni R, Miraglia N, Simonelli A, Silvestre A, Lamberti M, Feola D, Acampora A, Sannolo N. Solid-phase microextraction-gas chromatography-mass spectrometry method validation for the determination of endogenous substances: Urinary hexanal and heptanal as lung tumor biomarkers. Anal Chim Acta. 2011;701:29–36. doi: 10.1016/j.aca.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 16.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971;31:1860–1861. [PubMed] [Google Scholar]
- 17.Banday KM, Pasikanti KK, Chan EC, Singla R, Rao KV, Chauhan VS, Nanda RK. Use of urine volatile organic compounds to discriminate tuberculosis patients from healthy subjects. Anal Chem. 2011;83:5526–5534. doi: 10.1021/ac200265g. [DOI] [PubMed] [Google Scholar]
- 18.Mills GA, Walker V. Headspace solid-phase microextraction profiling of volatile compounds in urine: Application to metabolic investigations. Journal of chromatography. J Chromatogr B Biomed Sci Appl. 2001;753:259–268. doi: 10.1016/S0378-4347(00)00554-5. [DOI] [PubMed] [Google Scholar]
- 19.Qin T, Liu H, Song Q, Song G, Wang HZ, Pan YY, Xiong FX, Gu KS, Sun GP, Chen ZD. The screening of volatile markers for hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2010;19:2247–2253. doi: 10.1158/1055-9965.EPI-10-0302. [DOI] [PubMed] [Google Scholar]
- 20.Song G, Qin T, Liu H, Xu GB, Pan YY, Xiong FX, Gu KS, Sun GP, Chen ZD. Quantitative breath analysis of volatile organic compounds of lung cancer patients. Lung Cancer. 2010;67:227–231. doi: 10.1016/j.lungcan.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Ansell SM. Non-Hodgkin Lymphoma: Diagnosis and Treatment. Mayo Clin Proc. 2015;90:1152–1163. doi: 10.1016/j.mayocp.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Sundararajan S, Puvvada S, Persky DO. Limited stage aggressive non-hodgkin lymphoma: What is optimal therapy? Curr Treat Options Oncol. 2016;17:45. doi: 10.1007/s11864-016-0424-2. [DOI] [PubMed] [Google Scholar]
- 23.Filippi AR, Ciammella P, Ricardi U. Limited stage follicular lymphoma: Current role of radiation therapy. Mediterr J Hematol Infect Dis. 2016;8:e2016041. doi: 10.4084/mjhid.2016.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gine E, Sehn LH. Diffuse large B-cell lymphoma: Should limited-stage patients Be treated differently? Hematol Oncol Clin North Am. 2016;30:1179–1194. doi: 10.1016/j.hoc.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Till BG, Li H, Bernstein SH, Fisher RI, Burack WR, Rimsza LM, Floyd JD, DaSilva MA, Moore DF, Jr, Pozdnyakova O, et al. Phase II trial of R-CHOP plus bortezomib induction therapy followed by bortezomib maintenance for newly diagnosed mantle cell lymphoma: SWOG S0601. Br J Haematol. 2016;172:208–218. doi: 10.1111/bjh.13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phan J, Mazloom A, Medeiros LJ, Zreik TG, Wogan C, Shihadeh F, Rodriguez MA, Fayad L, Fowler N, Reed V, et al. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncol. 2010;28:4170–4176. doi: 10.1200/JCO.2009.27.3441. [DOI] [PubMed] [Google Scholar]
- 27.Montoto S, Davies AJ, Matthews J, Calaminici M, Norton AJ, Amess J, Vinnicombe S, Waters R, Rohatiner AZ, Lister TA. Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J Clin Oncol. 2007;25:2426–2433. doi: 10.1200/JCO.2006.09.3260. [DOI] [PubMed] [Google Scholar]
- 28.Chan JK, Kwong YL. Common misdiagnoses in lymphomas and avoidance strategies. Lancet Oncol. 2010;11:579–588. doi: 10.1016/S1470-2045(09)70351-1. [DOI] [PubMed] [Google Scholar]
- 29.Ramos AG, Anton AP, Sanchez MDN, Pavon JLP, Cordero BM. Urinary volatile fingerprint based on mass spectrometry for the discrimination of patients with lung cancer and controls. Talanta. 2017;174:158–164. doi: 10.1016/j.talanta.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Shestivska V, Rutter AV, Sulé-Suso J, Smith D, Španěl P. Evaluation of peroxidative stress of cancer cells in vitro by real-time quantification of volatile aldehydes in culture headspace. Rapid Commun Mass Spectrom. 2017;31:1344–1352. doi: 10.1002/rcm.7911. [DOI] [PubMed] [Google Scholar]
- 31.Anton Perez A, Ramos AG, Del Nogal Sanchez M, Pavon JL, Cordero BM, Pozas AP. Headspace-programmed temperature vaporization-mass spectrometry for the rapid determination of possible volatile biomarkers of lung cancer in urine. Anal Bioanal Chem. 2016;408:5239–5246. doi: 10.1007/s00216-016-9618-5. [DOI] [PubMed] [Google Scholar]
- 32.Rios-Arrabal S, Artacho-Cordon F, Leon J, Román-Marinetto E, Del Mar Salinas-Asensio M, Calvente I, Núñez MI. Involvement of free radicals in breast cancer. Springer Plus. 2013;2:404. doi: 10.1186/2193-1801-2-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker V, Mills GA. Urine 4-heptanone: A beta-oxidation product of 2-ethylhexanoic acid from plasticisers. Clin Chim Acta. 2001;306:51–61. doi: 10.1016/S0009-8981(01)00390-4. [DOI] [PubMed] [Google Scholar]
- 34.Karami S, Boffetta P, Brennan P, Stewart PA, Zaridze D, Matveev V, Janout V, Kollarova H, Bencko V, Navratilova M, et al. Renal cancer risk and occupational exposure to polycyclic aromatic hydrocarbons and plastics. J Occup Environ Med. 2011;53:218–223. doi: 10.1097/JOM.0b013e31820a40a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanai Y, Shimono K, Oka H, Baba Y, Yamazaki K, Beauchamp GK. Analysis of volatile organic compounds released from human lung cancer cells and from the urine of tumor-bearing mice. Cancer Cell Int. 2012;12:7. doi: 10.1186/1475-2867-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calejo I, Moreira N, Araujo AM, Carvalho M, Bastos Mde L, de Pinho PG. Optimisation and validation of a HS-SPME-GC-IT/MS method for analysis of carbonyl volatile compounds as biomarkers in human urine: Application in a pilot study to discriminate individuals with smoking habits. Talanta. 2016;148:486–493. doi: 10.1016/j.talanta.2015.09.070. [DOI] [PubMed] [Google Scholar]
- 37.Krupenko SA, Oleinik NV. 10-formyltetrahydrofolate dehydrogenase, one of the major folate enzymes, is down-regulated in tumor tissues and possesses suppressor effects on cancer cells. Cell Growth Differ. 2002;13:227–236. [PubMed] [Google Scholar]
- 38.Ma S, Chan KW, Lee TK, Tang KH, Wo JY, Zheng BJ, Guan XY. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6:1146–1153. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- 39.Khalid T, Aggio R, White P, De Lacy Costello B, Persad R, Al-Kateb H, Jones P, Probert CS, Ratcliffe N. Urinary Volatile Organic Compounds for the Detection of Prostate Cancer. PLoS One. 2015;10:e0143283. doi: 10.1371/journal.pone.0143283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes SD, Kanabus M, Anderson G, Hargreaves IP, Rutherford T, O'Donnell M, Cross JH, Rahman S, Eaton S, Heales SJ. The ketogenic diet component decanoic acid increases mitochondrial citrate synthase and complex I activity in neuronal cells. J Neurochem. 2014;129:426–433. doi: 10.1111/jnc.12646. [DOI] [PubMed] [Google Scholar]
- 41.Malapaka RR, Khoo S, Zhang J, Choi JH, Zhou XE, Xu Y, Gong Y, Li J, Yong EL, Chalmers MJ, et al. Identification and mechanism of 10-carbon fatty acid as modulating ligand of peroxisome proliferator-activated receptors. J Biol Chem. 2012;287:183–195. doi: 10.1074/jbc.M111.294785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazzone PJ, Wang XF, Lim S, Choi H, Jett J, Vachani A, Zhang Q, Beukemann M, Seeley M, Martino R, Rhodes P. Accuracy of volatile urine biomarkers for the detection and characterization of lung cancer. BMC Cancer. 2015;15:1001. doi: 10.1186/s12885-015-1996-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gasparri R, Santonico M, Valentini C, Sedda G, Borri A, Petrella F, Maisonneuve P, Pennazza G, D'Amico A, Di Natale C, et al. Volatile signature for the early diagnosis of lung cancer. J Breath Res. 2016;10:016007. doi: 10.1088/1752-7155/10/1/016007. [DOI] [PubMed] [Google Scholar]
- 44.Handa H, Usuba A, Maddula S, Baumbach JI, Mineshita M, Miyazawa T. Exhaled breath analysis for lung cancer detection using ion mobility spectrometry. PLoS One. 2014;9:e114555. doi: 10.1371/journal.pone.0114555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.