Abstract
DNA methylation is closely associated with aberrant epigenetic changes. Previous studies have identified various genes associated with non-small cell lung cancer (NSCLC), but the precise combination responsible for its etiology is still debated. The aim of the present study was to select a new set of NSCLC-related genes using methylation-sensitive high-resolution melting. The promoter methylation status of six selected genes, consisting of protocadherin γ subfamily B, 6 (PCDHGB6), homeobox A9 (HOXA9), O6-methylguanine-DNA methyltransferase (MGMT), microRNA (miR)-126, suppressor of cytokine signaling 3 (SOCS3) and Ras association domain family member 5, also termed NORE1A, was evaluated in 54 NSCLC patients. From these samples, genome-wide DNA was extracted and bisulfite conversion was performed along with fluorogenic quantitative polymerase chain reaction to detect methylation values of the six selected promoters. The present results revealed frequent methylation on PCDHGB6, HOXA9 and miR-126, which contrasted with infrequent methylation on MGMT. The results indicated no methylation on either SOCS3 or NORE1A. The sensitivity and specificity of the methylation assessment were 85.2 and 81.5%, respectively, and the analysis results were validated by pyrosequencing. Furthermore, minute comparison of the association between DNA methylation and clinical features was performed. Overall, these results may provide potential information for the development of better clinical diagnostics and more targeted and effective therapies for NSCLC.
Keywords: methylation, high-resolution melting, CpG, lung cancer, diagnosis
Introduction
Lung cancer is among the most widely diagnosed cancers and the leading cause of cancer-associated mortality worldwide (1). Human lung cancer is an umbrella term that predominantly includes small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), with the latter comprising over 80–85% of all lung cancer diagnoses (2). The majority of NSCLC patients are diagnosed at either the mid- or terminal stages, resulting in low survival rates (3).
Currently, only small improvements in the treatment of NSCLC have been made, with early diagnosis considered one of the most effective means of improving five-year survival rates (4). The presence of validated prognostic biomarkers has the potential to provide a valuable tool for this early diagnosis (4). Intensive work over previous years has shown that aberrant DNA methylation in the promoter regions containing CpG-rich areas (termed CpG islands) is one of the most well-defined epigenetic changes found in human cancers (5). Not only could this feature be utilized to distinguish cancer cells from normal tissue, but it could also be exploited for use in early cancer diagnosis.
At present, there are various methylation detection methods that are in use, including methylation-specific polymerase chain reaction (6), methylight (7), methylation-sensitive high-resolution melting (MS-HRM) (8), pyrosequencing (9), and microarray technologies (10). Known as a sensitive and more efficient technology for detecting single nucleotide variations (11), MS-HRM is considered to be a novel technology that would provide clinically-relevant sensitivity and rapidity for gene methylation screening. MS-HRM identifies PCR-amplified products by monitoring the melting temperature (Tm) of the double-stranded DNA helix (12). This process does not require product movement into another system, which ensures a closed-tube procedure, and makes it an appropriate method for methylation detection in patient populations.
In the present study, six target genes, consisting of protocadherin γ subfamily B, 6 (PCDHGB6), homeobox A9 (HOXA9), O6-methylguanine-DNA methyltransferase (MGMT), microRNA (miR)-126, suppressor of cytokine signaling 3 (SOCS3) and Ras association domain family member 5, also termed NORE1A, were selected to analyze the promoter methylation status for NSCLC detection, based on previous studies (4,13–16). PCDHGB6 is located on chromosome 5, and is a member of the protocadherin γ gene cluster and has an immunoglobulin-like organization (17). Hypermethylation of PCDHGB6 has been found to be significantly associated with stage I NSCLC (18). Overexpression of HOXA9 has been shown to significantly inhibit invasion of cell lines and may therefore be a potential gene marker for NSCLC diagnosis (19). miR-126 is a metastasis-suppressing gene and has been shown to be downregulated in a variety of inherited diseases (20). As a tumor suppressor, the promoter hypermethylation of miR-126 in lung tumors has a key role in decreased expression of miR-126, leading to oncogenesis of lung tissue (21,22). The translation product of MGMT genes is O6-methylguanine-DNA-methylotrans, which is both a critical enzyme in repairing DNA alkylation damages and is considered a common DNA repair gene (23). In primary lung carcinomas, MGMT inactivation caused by promoter methylation has been shown to be more prevalent in advanced stages (24). The SOCS3 gene is a tumor suppressor gene, with aberrant methylation of SOCS3 occurring frequently in several types of human cancers (25,26). Similarly, NORE1A also performs as a tumor suppressor and has been shown to be generally inactivated in cancer tumors (27). Finally, hypermethylation of CpG islands in the NORE1A promoter has been found in primary tumors, including NSCLC and SCLC (28).
In the present study, the promoter methylation status in the target sequences of six selected genes was analyzed using MS-HRM as the technology platform. Through the establishment of standard curves of control DNA samples, the gene methylation status of 54 lung cancer tissue samples and 54 corresponding non-tumorous tissue (NT) samples was investigated. As a result, the present study found frequent methylation on PCDHGB6, HOXA9 and miR-126, infrequent methylation on MGMT, and no methylation on either SOCS3 or NORE1A. The combination of PCDHGB6, HOXA9, miR-126 and MGMT reached 85.2% sensitivity and 81.5% specificity, with an area under the curve (AUC) value of 0.891. In addition, high consistency was shown between MS-HRM and pyrosequencing. Furthermore, potential clinical values have been excavated in the early diagnosis of NSCLC.
Materials and methods
Patient samples
In the present study, 54 pairs of lung cancer and adjacent NT samples were obtained from 54 patients who underwent surgical resection from January 2014 to June 2014 at the Department of Pathology, Shanghai Zhongshan Hospital (Shanghai, China). Of these patients, 12 were diagnosed with squamous cell carcinoma and 42 were diagnosed with adenocarcinoma. All these samples were obtained with informed consent and were stored at −80°C until later total DNA extraction. This research was approved by the Institutional Review Board of Shanghai Zhongshan Hospital (Shanghai, China).
DNA extraction and bisulfite conversion
No more than 30 mg of tissue was obtained using sterilized operating scissors. Tissue was then ground in tissue lyser (Jingxin, Shanghai, China) for 80 sec at 65 Hz. Whole-genome DNA extraction was then performed using ALL Prep DNA/RNA Mini kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions. Sodium bisulfite was used to convert the extracted DNA using an EZ DNA Methylation-Gold kit (Zymo Research Corp., Irvine, CA, USA), in which ~500 ng of DNA was used as the proper addition, according to specification. The final elution volume was 10 µl.
MS-HRM assay
Converted DNA was amplified using a LightCycler 480 (Roche Diagnostics GmbH, Mannheim, Germany). The whole reaction volume was 10 µl, which consisted of, using the MGMT gene as an example: 1 ng/µl DNA template, 1X Master Mix (Roche Diagnostics GmbH), 0.5 µM primers and 4 mM magnesium ions. Polymerase chain reaction (PCR)-grade water was used to bring the final reaction volume to 10 µl. The detailed amplification thermocycling protocol was set to the following: Preheat for 10 min at 95°C before starting a 50-cycle process involving 10 sec at 95°C, 20 sec at a temperature between 61 to 55°C for 20 sec (temperature dropped 2.2°C per sec), and 20 sec at 72°C. The following MS-HRM melting protocol was used: Heating at 95°C for 1 min, followed by 40°C for 1 min (29), 65°C for 1 sec, and continuous heating to 95°C at a ramp rate of 0.02°C per second. To ensure veracity and repeatability, each reaction was conducted in duplicate. 5-Aza-dc-treated Jurkat Genomic DNA and CpG Methylated Jurkat Genomic DNA (New England Biolabs, Ipswich, MA, USA) that had been subjected to bisulfite conversion were used as fully methylated and unmethylated control DNA samples, respectively. The former was added into the latter in gradient proportions (0, 5, 10, 25, 50, 75, 90 and 100%) and used as artificial DNA standards of different methylation levels. These DNA standards were used in each assay to establish gradient standard curves. These curves were then used to evaluate the methylation levels of the tumor and normal samples.
Pyrosequencing validation
In the present study, pyrosequencing was used for 3 of the 6 genes (PCDHGB6, HOXA9 and miR-126), to validate the accuracy of the present results. Pyrosequencing was performed at Shanghai Medical College, Fudan University (Shanghai, China). Table I shows the basic primer information for both MS-HRM and pyrosequencing. Primers were designed by MethPrimer (http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi), which is used for designing methylation PCR primers. Subsequent to bisulfite conversion, cytosine was converted to thymine (except for CpG islands) and the newfound sequence was used as the template for primer design.
Table I.
Primers for MS-HRM and pyrosequencing.
| A, MS-HRM | ||||
|---|---|---|---|---|
| Primer | Sequence, 5′-3′ | Amplicon length, bp | Ensembl version | Genomic region |
| PCDHGB6 | 171 | ENST00000520790 | CHR5:140787402-140787572 | |
| Forward | AATTTGAGGGGGATGTATATTT | |||
| Reverse | AAAATCCCAAACCAAAAACT | |||
| HOXA9 | 118 | ENST00000343483 | CHR7:27200705-27200822 | |
| Forward | GAGTTGTGGTTGTTTTTTTTTG | |||
| Reverse | ACCTTTCAAAACTCCTTCCTC | |||
| MGMT | 110 | ENST00000482653 | chr10:131155459-131155568 | |
| Forward | GCGTTTCGGATATGTTGGGATAGT | |||
| Reverse | AACGACCCAAACACTCACCAAA | |||
| miR-126 | 93 bp | ENST00000362291 | CHR9:139564092-139564184 | |
| Forward | TGGGTTGGTTTTTGTTAGG | |||
| Reverse | TAACCCTCACCTACTCCACAA | |||
| SOCS3 | 105 bp | ENST00000330871 | chr17:76354057-76354161 | |
| Forward | GAAGGTTTTTTTGTGGATTTTA | |||
| Reverse | ACTAAACCCCCTCRAATCC | |||
| NORE1A | 174 | ENST00000367117 | chr1:206680666-206680839 | |
| Forward | GGAATTTTGTAGTTGTTTTAGGTG | |||
| Reverse | CCTTTAAAAAAACCRCAAC | |||
| B, Pyrosequencing | ||||
| Primer | Sequence, 5′-3′ | Amplicon length, bp | Ensembl version | Genomic region |
| PCDHGB6 | 116 | ENST00000520790 | CHR5:140787402-140787572 | |
| Forward | AATTTGAGGGGGATGTATATTT | |||
| Reverse | biotin-AAAATCCCAAACCAAAAACT | |||
| Sequencing primer | GAATTTAAAATGAAAAAT | |||
| HOXA9 | 101 | ENST00000343483 | CHR7:27200705-27200822 | |
| Forward | GAGTTGTGGTTGTTTTTTTTTG | |||
| Reverse | biotin-ACCTTTCAAAACTCCTTCCTC | |||
| Sequencing primer | TTTTGGGTTTTGTATTTTTT | |||
| miR-126 | 93 | ENST00000362291 | CHR9:139564092-139564184 | |
| Forward | TGGGTTGGTTTTTGTTAGG | |||
| Reverse | biotin-TAACCCTCACCTACTCCACAA | |||
| Sequencing primer | TGGGTTGGTTTTTGTTAGG | |||
PCDHGB6, protocadherin γ subfamily B, 6; HOXA9, homeobox A9; MGMT, O6-methylguanine-DNA methyltransferase; miR-126, microRNA-126; SOCS3, suppressor of cytokine signaling 3; NORE1A, Ras association domain family member 5; MS-HRM, methylation-sensitive high-resolution melting.
Statistical analysis
SPSS v17.0 (SPSS, Inc., Chicago, IL, USA) was used for all statistical analysis. The dependence between the HRM-assessed methylation status and temperature difference was analyzed using simple linear regression. To verify the sensitivity and specificity of high and low methylation status, the receiver-operating characteristic (ROC) curve was generated and the AUC was calculated to obtain the highest specificity and sensitivity. Differences between tumor and surrounding tissue were evaluated by t-test and P<0.05 was considered to indicate a statistically significant difference. Additionally, Pearson's correlation coefficient was evaluated to examine the consistency between HRM assay and pyrosequencing of PCDHGB6, HOXA9 and miR-126.
Results
MS-HRM melting curves for each gene
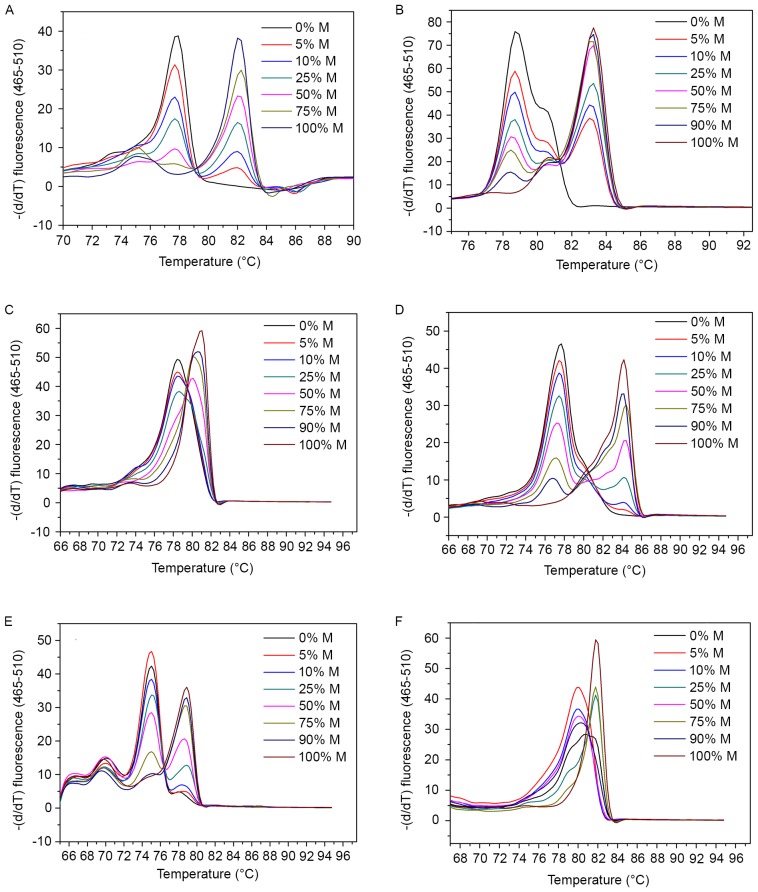
In the present MS-HRM assay, melting temperature was obtained as a Tm value in the Tm calling analysis. This refers to the temperature at which half of the double-stranded DNA melts into single strands, thus undergoing a sharp decline in fluorescence intensity (8,9). Fig. 1 shows the melting curves of control samples of the six genes in the Tm calling analysis. A gradient of diluted methylated DNA with unmethylated DNA (0–100%) subsequent to bisulfite conversion were used for each gene as controls. Melting profiles of all these gene promoters showed higher Tm in methylated controls and lower Tm in controls without methylation. Among them, Tm values of SOCS3 gene exhibited the largest span, from 77 to 84°C (Fig. 1D), whereas small temperature differences were found in HOXA9 and miR-126 as less than 2°C (Fig. 1C and F). Based on the difference in melting temperatures between the amplicons, single nucleotides can be distinguished (30).
Figure 1.
Melting curves for (A) protocadherin γ subfamily B, 6, (B) O6-methylguanine-DNA methyltransferase, (C) homeobox A9, (D) suppressor of cytokine signaling 3, (E) Ras association domain family member 5 and (F) microRNA-126 in the methylation-sensitive high-resolution melting assay. Artificially prepared methylation gradient ratios were used to evaluate the methylation index of each sample. The horizontal axis indicates different melting temperatures of gradient-dilution standard DNA samples and vertical axis indicates the corresponding slopes. Methylated DNA that contains cytosines has an increased melting temperature than unmethylated DNA.
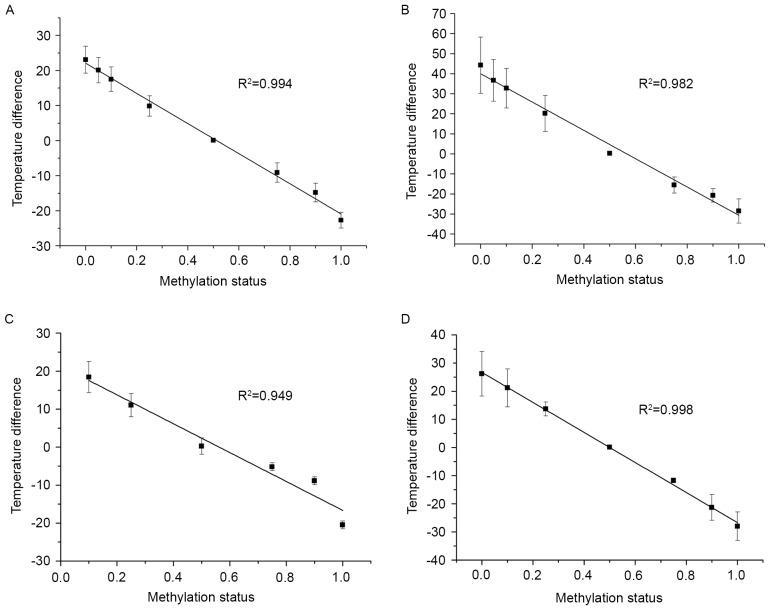
To further explore this association, a correlational analysis was conducted between temperature difference and methylation status using a simple linear regression (Fig. 2). Every standard curve yielded a corresponding temperature difference value. As shown in Fig. 2, there was a negative correlation between the methylation status of DNA standards in a series of dilutions and temperatures. HOXA9, PCDHGB6, MGMT and miR-126 exhibited good liner association with significant R2 values of 0.994, 0.982, 0.949 and 0.998, respectively. From these data, accurate methylation degrees could be calculated.
Figure 2.
Simple linear regression of (A) homeobox A9, (B) protocadherin γ subfamily B, 6, (C) O6-methylguanine-DNA methyltransferase and (D) microRNA-126. The horizontal axis indicates the percentage of methylation value of the gradient controls, and the vertical axis indicates the temperature difference obtained from the methylation-sensitive high-resolution melting assay. The error bars represent the standard deviation for at least three repeated experiments.
Assessing gene methylation frequency in tumor and normal tissues
According to the generated standard curves, 54 pairs of tumor and NT tissues were investigated. Methylation levels of each gene are shown in Fig. 3. From the experimental results, PCDHGB6 methylation was found in 35 of the 54 tumors (64.8%) and HOXA9 methylation was found in 23 of the 54 tumors (42.6%). miR-126 also had a high methylation frequency (68.5%), but methylation of miR-126 was found to be nonspecific in normal tissue, with a frequency of 46.3%. Notably, no promoter methylation of either SOCS3 or NORE1A was identified in the tumor or normal tissue. As for MGMT, which has been affected in several malignancies that include colorectal and lung cancer, did not show any prominent impact (14 positive samples out of the 54 tumor samples) in the early diagnosis of NSCLC.
Figure 3.
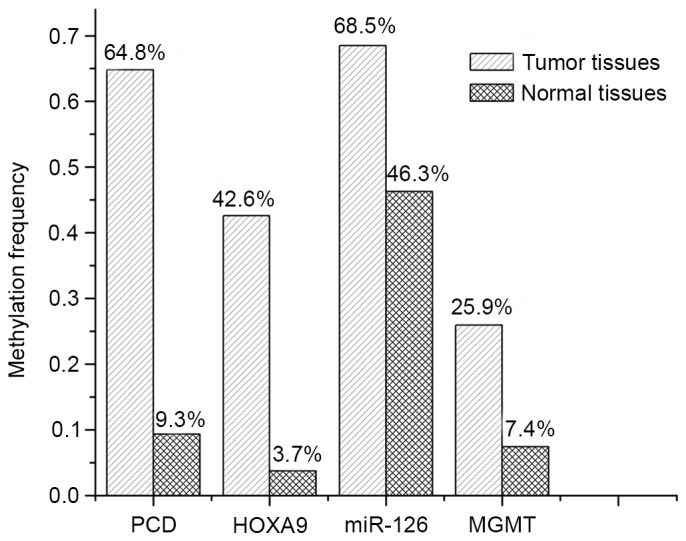
Methylation frequency of genes evaluated in the methylation-sensitive high-resolution melting assay. PCD, protocadherin γ subfamily B, 6; HOXA9, homeobox A9; MGMT, O6-methylguanine-DNA methyltransferase; miR-126, microRNA-126.
Validation of methylation status by pyrosequencing
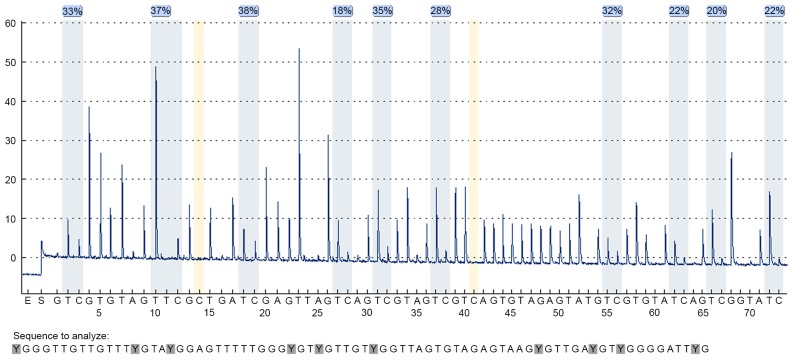
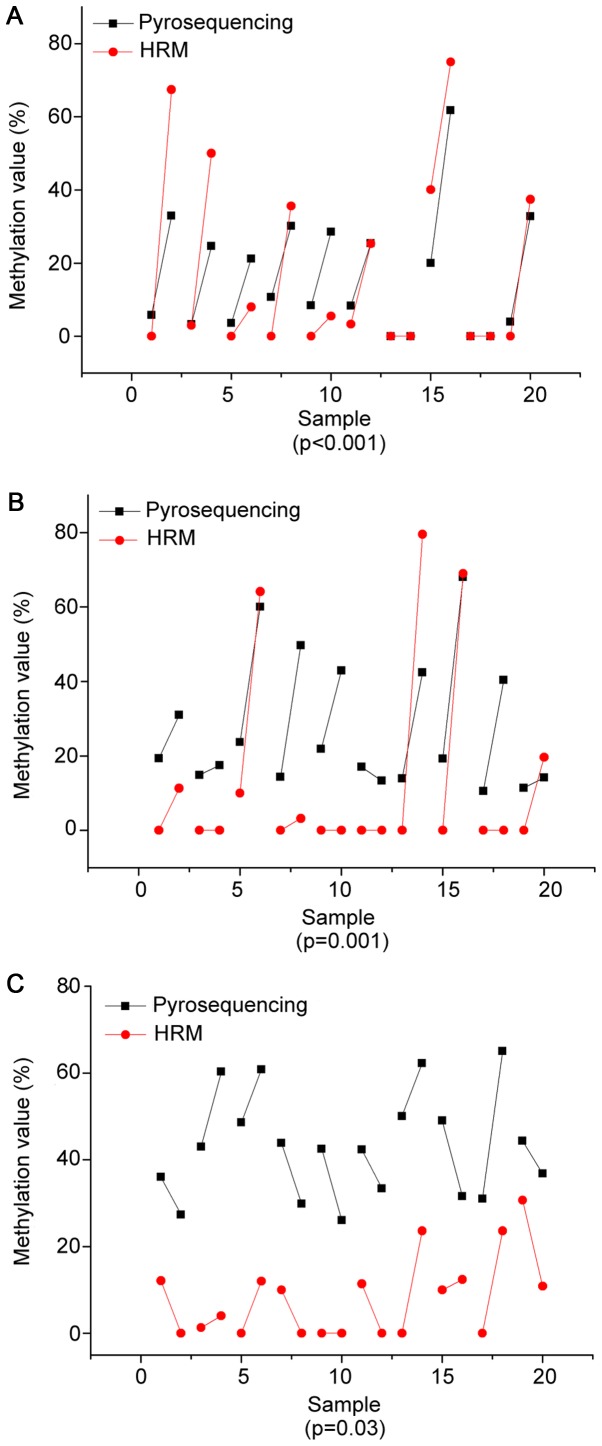
The precise methylation values obtained from our linear regression analysis were then compared with pyrosequencing. In the present study, methylation values were derived from an average of all the CpG sites. Ten pairs of malignant and control tissue from each selected DNA sequence were chosen to detect methylation degrees of PCDHGB6, HOXA9 and miR-126 by pyrosequencing with 10, 6 and 5 possible mutations of CpG sites, respectively. Fig. 4 illustrates the pyrosequencing results of PCDHGB6 from one tumor tissue sample. The blue sections indicate successful detection of CpG sites. Fig. 5 shows comparisons of methylation degrees, as tested by MS-HRM and pyrosequencing. Every two samples joined by a line represent a pair of tumor and para-carcinoma tissue excised from the same patient. As shown, PCDHGB6 had the most closely associated values of the three genes, while HOXA9 came second. The sensitivity of assessing miR-126 methylation by MS-HRM was generally lower than by pyrosequencing. To examine statistical significance, Pearson's correlation coefficient was evaluated for PCDHGB6, HOXA9 and miR-126, deriving values of P<0.001, P=0.001 and P=0.03, respectively. Collectively, these results demonstrated a statistically significant coincident tendency between MS-HRM and pyrosequencing (P<0.05).
Figure 4.
A total of 10 CpG sites analyzed in protocadherin γ subfamily B, 6. Blue sections indicated passed detection results.
Figure 5.
Comparison of MS-HRM and pyrosequencing in (A) protocadherin γ subfamily B, 6 (B) homeobox A9 and (C) microRNA-126 genes. HRM, methylation-sensitive high-resolution melting.
Association between clinical features of NSCLC patients with tumor tissue methylation
The association between clinical manifestations of NSCLC and the MS-HRM analysis for selected genes are shown in Table II (31). Based on the present study, the methylation frequency of PCDHGB6 and HOXA9 increased with disease progression, while the methylation frequency of PCDHGB6 was higher than that of HOXA9 in every disease stage. For PCDHGB6, the frequency was at 53.8% at stage I, with the ratio reaching 83.3% at later stages. In the case of HOXA9 gene, the ratio increased between 38.5% at stage I to 66.7% at stages III–IV. When compared with the PCDHGB6 and HOXA9 genes, MGMT showed no evident association with tumor-node-metastasis (TNM) stage and possessed low detection rates in both normal and tumor tissue. For the miR-126 gene, methylation frequency was significantly increased in early stages, with 73.1% methylation frequency in stage I. Additionally, results from all selected genes showed that males appeared to be more susceptible than females. In the case of histopathological classification, these four genes appeared to have increased sensitivity in squamous cell carcinoma, with detection rates of ≥75%. PCDHGB6 and miR-126 were both reliable biomarkers for the detection of adenocarcinoma, with positive rates of 54.8 and 61.9%, respectively.
Table II.
The association between clinical manifestations of patients and study results of selected genes from methylation-sensitive high-resolution melting analysis.
| Histopathological classification, n (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methylation frequency, n (%) | Stage, n (%) | Age, n (%) | Gender, n (%) | |||||||||
| Squamous | ||||||||||||
| Gene | Tumor tissue | Normal tissue | I | II | III–IV | ≤40 years | >40 years | Male | Female | Adenocarcinoma | cell carcinoma | |
| Total | 54 (100.0) | 54 (100.0) | 26 (100.0) | 22 (100.0) | 6 (100.0) | 10 (100.0) | 44 (100.0) | 32 (100.0 | 22 (100.0) | 42 (100.0) | 12 (100.0) | |
| PCDHGB6 | 35 (64.8) | 5 (9.3) | 14 (53.8) | 17 (77.3) | 5 (83.3) | 7 (70.0) | 30 (68.2) | 25 (78.1) | 10 (45.5) | 23 (54.8) | 12 (100.0) | |
| HOXA9 | 23 (42.6) | 2 (3.7) | 10 (38.5) | 9 (41.0) | 4 (66.7) | 3 (30.0) | 20 (45.5) | 17 (53.1) | 6 (27.3) | 14 (33.3) | 9 (75.0) | |
| MGMT | 14 (25.9) | 4 (7.4) | 7 (26.9) | 3 (13.6) | 1 (16.7) | 6 (60.0) | 7 (15.9) | 9 (28.1) | 4 (18.2) | 10 (23.8) | 2 (16.7) | |
| miR-126 | 37 (68.5) | 25 (46.3) | 19 (73.1) | 16 (72.7) | 2 (33.3) | 4 (40.0) | 33 (75.0) | 20 (62.5) | 16 (72.7) | 26 (61.9) | 9 (75.0) | |
PCDHGB6, protocadherin γ subfamily B, 6; HOXA9, homeobox A9; MGMT, O6-methylguanine-DNA methyltransferase; miR-126, microRNA-126.
Joint detection of selected genes
A multi-gene analysis was then conducted to evaluate the sensitivity and specificity of methylation. Results were analyzed by ROC curve. As shown in Table III, values were calculated using SPSS software, with various combinations used to acquire the best result. PCDHGB6 had the highest sensitivity (66.7%) out of the four genes, with 75.9% of the patients having at least two methylated genes. Finally, the combination of four genes resulted in the highest sensitivity and specificity (85.2 and 81.5%, respectively), with the AUC being 0.891 (Fig. 6).
Table III.
Analysis of different combinations of four selected genes.
| Methylation frequency, % | ||||||
|---|---|---|---|---|---|---|
| Genes | Tumor tissues | Normal tissues | Sensitivity, % | Specificity, % | AUC | P-value |
| PCDHGB6 | 64.8 | 9.3 | 66.7 | 90.7 | 0.796 | P<0.001 |
| HOXA9 | 42.6 | 3.7 | 42.6 | 96.3 | 0.694 | P<0.001 |
| MGMT | 25.9 | 7.4 | 25.9 | 94.4 | 0.594 | P=0.059 |
| miR-126 | 68.5 | 46.3 | 38.9 | 90.7 | 0.658 | P<0.001 |
| PCDHGB6, HOXA9 | 75.9 | 11.1 | 75.9 | 88.9 | 0.835 | P<0.001 |
| PCDHGB6, HOXA9, miR-126 | 94.4 | 53.7 | 81.5 | 85.2 | 0.872 | P<0.001 |
| PCDHGB6, HOXA9, MGMT | 81.5 | 18.5 | 81.5 | 81.5 | 0.850 | P<0.001 |
| PCDHGB6, HOXA9, MGMT, miR-126 | 98.1 | 55.5 | 85.2 | 81.5 | 0.891 | P<0.001 |
PCDHGB6, protocadherin γ subfamily B, 6; HOXA9, homeobox A9; MGMT, O6-methylguanine-DNA methyltransferase; miR-126, microRNA-126; AUC, area under the curve.
Figure 6.
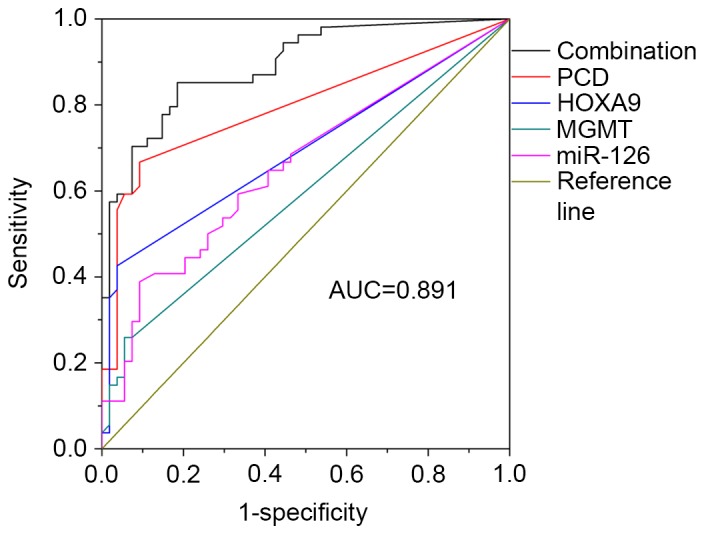
Receiver-operating characteristic curve of methylation levels of multi-gene analysis. PCD, protocadherin γ subfamily B, 6; HOXA9, homeobox A9; MGMT, O6-methylguanine-DNA methyltransferase; miR-126, microRNA-126; AUC, area under the curve.
Discussion
In the present study, a new combination of target promoter sequences for the diagnosis of NSCLC was obtained by MS-HRM analysis. MS-HRM enabled evaluation of the PCR amplicon by monitoring gradient changes in fluorescence correlated with sequence-dependent melting properties (32,33). Thus, an accurate melting status of the PCR amplicon may be identified by mixing an intercalating dye with the product and monitoring fluorescence intensity. In the present study, 54 pairs of tumor and surrounding tissues were selected from NSCLC patients that ranged between early and advanced TNM stages. Through the HRM diagnostic system, the promoter methylation status of a series of possible genes associated with NSCLC, consisting of PCDHGB6, HOXA9, MGMT, miR-126, SOCS3 and NORE1A, was determined.
Among these tested target genes, Tm values of the SOCS3 gene exhibited the largest span (77–84°C), whereas the HOXA9 and miR-126 genes showed small temperature differences. In addition, and increased Tm difference between methylated and unmethylated samples indicated a richer CpG content in selected loci. Based on the establishment of standard curves that showed highly correlated relations, PCDHGB6 methylation was found in 35 of the 54 tumors (64.8%) and HOXA9 methylation was found in 23 of the 54 tumors (42.6%). These values correspond with previous studies (4,34,35) that showed a close association between methylation and NSCLC. miR-126 also had a high methylation frequency (68.5%), and has been found to be significant at stage I–II, indicating that it would be more sensitive for use in early diagnosis. The only complication was that methylation of miR-126 was found to exhibit lower specificity for normal tissues, with a frequency of 46.3%. This may be due to the presence of methylated CpG distributed in the normal sequence. Compared with the three hypermethylated genes (PCDHGB6, HOXA9 and miR-126), it was found that MGMT had a lower detection rate, with 14 of 54 tumor samples (25.9%) found to be MGMT methylation-positive. However, this is still of clinical value due to the ubiquity of MGMT methylation in both early and advanced TNM stages. The promoter methylation observed in SOCS3 and NORE1A showed no correlation in NSCLC diagnosis. In addition, results from all selected genes exhibited the tendency that males were more susceptible than females, which may derive from the increased incidence of smoking in the male population (36). In the case of histopathological classification, methylation of these four genes (PCDHGB6, HOXA9, MGMT and miR-126) were more likely to occur in squamous cell carcinoma, while PCDHGB6 and miR-126 both appeared to be reliable biomarkers for adenocarcinoma, and are therefore important for the typing of NSCLC at diagnosis.
Pyrosequencing is a new DNA sequencing technique and is a modification of combined bisulfite restriction analysis (37). Pyrosequencing applies to the analysis of known, short nucleotide sequences and it is advantageous in terms of its accuracy, rapidity and repeatability (38). These qualities make it a gold standard for evaluating degrees of methylation (39). Pyrosequencing was used to examine the accuracy of MS-HRM methylation patterns observed in PCDHGB6, HOXA9 and miR-126 by MS-HRM. The present results demonstrated high consistency between HRM and pyrosequencing data (P<0.05 for all three genes). It should be noted that the sensitivity of assessing miR-126 methylation by MS-HRM was generally decreased compared with pyrosequencing. One possible reason for this is that methylation sites are distributed on normally selected positions, while by default the MS-HRM assay regards methylation of normal tissue as zero. All of these aforementioned results demonstrate the feasibility of evaluating heterogeneous promoter methylation by MS-HRM.
Finally, the combination of PCDHGB6, HOXA9, miR-126 and MGMT reached an AUC value of 0.891, with 85.2% sensitivity and 81.5% specificity. This indicated a significant association between this diagnostic system and NSCLC pathology. Overall, these results demonstrate that not only does methylation assessment have statistical significance, but also that conjoint analysis has improved sensitivity and specificity compared with a single gene.
In conclusion, a significant joint testing of relevant target genes was established to evaluate clinical status of NSCLC by MS-HRM analysis. This research indicates that early diagnosis of NSCLC is feasible through the monitoring of promoter methylation using an effective combination of related genes. It provides a potential valuable and economical method for clinical applications.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- SCLC
small cell lung cancer
- NSCLC
non-small cell lung cancer
- HRM
high-resolution melting
- MS-HRM
methylation-sensitive high-resolution melting
- Tm
melting temperature
- NT
non-tumorous tissue
- ROC
receiver operating characteristic
- AUC
area under the curve
Funding
The present study was supported by grants from the National Science Foundation of China (grant no. 61571429), MOST of China (grant no. 2017YFA0205300), the STS Project of the Chinese Academy of Sciences (grant no. KFJ-STS-SCYD-120) and the Science and Technology Commission of Shanghai Municipality (grant no. 16410711800).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
MH and LF conceived and designed the study. LF, ZH, CZ and BY performed the experiments. LS analyzed the patient data regarding the TNM stage and histopathological classification. ZQ and ZJ participated in the data collection and preparation of the manuscript. LF, WZ and ZL analyzed and interpreted experimental data. LF wrote the paper. MH, ZQ and ZJ revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All samples were obtained with informed consent of the participants. This research was approved by the Institutional Review Board of Shanghai Zhongshan Hospital (Shanghai, China).
Consent for publication
All identifying information has been removed from the manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hubaux R, Thu KL, Vucic EA, Pikor LA, Kung SH, Martinez VD, Mosslemi M, Becker-Santos DD, Gazdar AF, Lam S, Lam WL. Microtubule affinity-regulating kinase 2 is associated with DNA damage response and cisplatin resistance in non-small cell lung cancer. Int J Cancer. 2015;137:2072–2082. doi: 10.1002/ijc.29577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters S, Adjei AA, Gridelli C, Reck M, Kerr K, Felip E. ESMO Guidelines Working Group: Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii56–64. doi: 10.1093/annonc/mds226. [DOI] [PubMed] [Google Scholar]
- 3.Dillman RO, Herndon J, Seagren SL, Eaton WL, Jr, Green MR. Improved survival in stage III non-small-cell lung cancer: Seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996;88:1210–1215. doi: 10.1093/jnci/88.17.1210. [DOI] [PubMed] [Google Scholar]
- 4.Sandoval J, Mendez-Gonzalez J, Nadal E, Chen G, Carmona FJ, Sayols S, Moran S, Heyn H, Vizoso M, Gomez A, et al. A prognostic DNA methylation signature for stage I non-small-cell lung cancer. J Clin Oncol. 2013;31:4140–4147. doi: 10.1200/JCO.2012.48.5516. [DOI] [PubMed] [Google Scholar]
- 5.Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: Anovel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eads CA, Danenberg KD, Kawakami K, Saltz LB, Blake C, Shibata D, Danenberg PV, Laird PW. Methy Light: A high-throughput assay to measure DNA methylation. Nucleic Acids Res. 2000;28:e32. doi: 10.1093/nar/28.8.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du Y, Zhou Y, Wu Q. MS-HRM to detect serum DNA methylation of intrauterine growth retardation children. Engineering. 2012;4:106–109. doi: 10.4236/eng.2012.410B027. [DOI] [Google Scholar]
- 9.Doerks T, Copley RR, Schultz J, Ponting CP, Bork P. Systematic identification of novel protein domain families associated with nuclear functions. Genome Res. 2002;12:47–56. doi: 10.1101/gr.203201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gitan RS, Shi H, Chen CM, Yan PS, Huang TH. Methylation-specific oligonucleotide microarray: A new potential for high-throughput methylation analysis. Genome Res. 2002;12:158–164. doi: 10.1101/gr.202801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wojdacz TK. Methylation-sensitive high-resolution melting in the context of legislative requirements for validation of analytical procedures for diagnostic applications. Expert Rev Mol Diagn. 2012;12:9. doi: 10.1586/erm.11.88. [DOI] [PubMed] [Google Scholar]
- 12.Wojdacz TK, Dobrovic A. Methylation-sensitive high resolution melting (MS-HRM): A new approach for sensitive and high-throughput assessment of methylation. Nucleic Acids Res. 2007;35:e41. doi: 10.1093/nar/gkm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf P, Hu YC, Doffek K, Sidransky D, Ahrendt SA. O6-Methylguanine-DNA methyltransferase promoter hypermethylation shifts the p53 mutational spectrum in non-small cell lung cancer. Cancer Res. 2001;61:8113–8117. [PubMed] [Google Scholar]
- 14.Watanabe K, Emoto N, Hamano E, Sunohara M, Kawakami M, Kage H, Kitano K, Nakajima J, Goto A, Fukayama M, et al. Genome structure-based screening identified epigenetically silenced microRNA associated with invasiveness in non-small-cell lung cancer. Int J Cancer. 2012;130:2580–2590. doi: 10.1002/ijc.26254. [DOI] [PubMed] [Google Scholar]
- 15.Boosani CS, Agrawal DK. Methylation and microRNA-mediated epigenetic regulation of SOCS3. Mol Biol Rep. 2015;42:853–872. doi: 10.1007/s11033-015-3860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irimia M, Fraga MF, Sanchez-Cespedes M, Esteller M. CpG island promoter hypermethylation of the Ras-effector gene NORE1A occurs in the context of a wild-type K-ras in lung cancer. Oncogene. 2004;23:8695–8699. doi: 10.1038/sj.onc.1207914. [DOI] [PubMed] [Google Scholar]
- 17.Wang KH, Lin CJ, Liu CJ, Liu DW, Huang RL, Ding DC, Weng CF, Chu TY. Global methylation silencing of clustered proto-cadherin genes in cervical cancer: Serving as diagnostic markers comparable to HPV. Cancer Med. 2015;4:43–55. doi: 10.1002/cam4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haller F, Zhang JD, Moskalev EA, Braun A, Otto C, Geddert H, Riazalhosseini Y, Ward A, Balwierz A, Schaefer IM, et al. Combined DNA methylation and gene expression profiling in gastrointestinal stromal tumors reveals hypomethylation of SPP1 as an independent prognostic factor. Int J Cancer. 2015;136:1013–1023. doi: 10.1002/ijc.29088. [DOI] [PubMed] [Google Scholar]
- 19.Son JW, Jeong KJ, Jean WS, Park SY, Jheon S, Cho HM, Park CG, Lee HY, Kang J. Genome-wide combination profiling of DNA copy number and methylation for deciphering biomarkers in non-small cell lung cancer patients. Cancer Lett. 2011;311:29–37. doi: 10.1016/j.canlet.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Saito Y, Friedman JM, Chihara Y, Egger G, Chuang JC, Liang G. Epigenetic therapy upregulates the tumor suppressor microRNA-126 and its host gene EGFL7 in human cancer cells. Biochem Biophys Res Commun. 2009;379:726–731. doi: 10.1016/j.bbrc.2008.12.098. [DOI] [PubMed] [Google Scholar]
- 21.Wang P, Yang D, Zhang H, Wei X, Ma T, Cheng Z, Hong Q, Hu J, Zhuo H, Song Y, et al. Early detection of lung cancer in serum by a panel of MicroRNA biomarkers. Clin Lung Cancer. 2015;16:313–319.e1. doi: 10.1016/j.cllc.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Crawford M, Brawner E, Batte K, Yu L, Hunter MG, Otterson GA, Nuovo G, Marsh CB, Nana-Sinkam SP. MicroRNA-126 inhibits invasion in non-small cell lung carcinoma cell lines. Biochem Biophys Res Commun. 2008;373:607–612. doi: 10.1016/j.bbrc.2008.06.090. [DOI] [PubMed] [Google Scholar]
- 23.Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha VV, Baylin SB, Herman JG. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. New Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 24.Wu JY, Wang J, Lai JC, Cheng YW, Yeh KT, Wu TC, Chen CY, Lee H. Association of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation with p53 mutation occurrence in non-small cell lung cancer with different histology, gender and smoking status. Ann Surg Oncol. 2008;15:3272–3277. doi: 10.1245/s10434-008-0078-9. [DOI] [PubMed] [Google Scholar]
- 25.Barclay JL, Anderson ST, Waters MJ, Curlewis JD. SOCS3 as a tumor suppressor in breast cancer cells and its regulation by PRL. Int J Cancer. 2009;124:1756–1766. doi: 10.1002/ijc.24172. [DOI] [PubMed] [Google Scholar]
- 26.Rigby RJ, Simmons JG, Greenhalgh CJ, Alexander WS, Lund PK. Suppressor of cytokine signaling 3 (SOCS3) limits damage-induced crypt hyper-proliferation and inflammation-associated tumorigenesis in the colon. Oncogene. 2007;26:4833–4841. doi: 10.1038/sj.onc.1210286. [DOI] [PubMed] [Google Scholar]
- 27.Moshnikova A, Frye J, Shay JW, Minna JD, Khokhlatchev AV. The growth and tumor suppressor NORE1A is a cytoskeletal protein that suppresses growth by inhibition of the ERK pathway. J Biol Chem. 2006;281:8143–8152. doi: 10.1074/jbc.M511837200. [DOI] [PubMed] [Google Scholar]
- 28.Hesson L, Dallol A, Minna JD, Maher ER, Latif F. NORE1A, a homologue of RASSF1A tumour suppressor gene is inactivated in human cancers. Oncogene. 2003;22:947–954. doi: 10.1038/sj.onc.1206191. [DOI] [PubMed] [Google Scholar]
- 29.Lin TC, Jiang SS, Chou WC, Hou HA, Lin YM, Chang CL, Hsu CA, Tien HF, Lin LI. Rapid assessment of the heterogeneous methylation status of CEBPA in patients with acute myeloid leukemia by using high-resolution melting profile. J Mol Diagn. 2011;13:514–519. doi: 10.1016/j.jmoldx.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman M, Blyth BJ, Hussey DJ, Jardine D, Sykes PJ, Ormsby RJ. Sensitive quantitative analysis of murine LINE1 DNA methylation using high resolution melt analysis. Epigenetics. 2012;7:92–105. doi: 10.4161/epi.7.1.18815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X, Dai W, Kwong DL, Szeto CY, Wong EH, Ng WT, Lee AW, Ngan RK, Yau CC, Tung SY, Lung ML. Epigenetic markers for noninvasive early detection of nasopharyngeal carcinoma by methylation-sensitive high resolution melting. Int J Cancer. 2015;136:E127–E135. doi: 10.1002/ijc.29192. [DOI] [PubMed] [Google Scholar]
- 32.Wojdacz TK, Møller TH, Thestrup BB, Kristensen LS, Hansen LL. Limitations and advantages of MS-HRM and bisulfite sequencing for single locus methylation studies. Expert Rev Mol Diagn. 2010;10:575–580. doi: 10.1586/erm.10.46. [DOI] [PubMed] [Google Scholar]
- 33.Zhu J, Yao X. Use of DNA methylation for cancer detection: Promises and challenges. Int J Biochem Cell Biol. 2009;41:147–154. doi: 10.1016/j.biocel.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Ma Y, Bai Y, Mao H, Hong Q, Yang D, Zhang H, Liu F, Wu Z, Jin Q, Zhou H, et al. A panel of promoter methylation markers for invasive and noninvasive early detection of NSCLC using a quantum dots-based FRET approach. Biosens Bioelectron. 2016;85:641–648. doi: 10.1016/j.bios.2016.05.067. [DOI] [PubMed] [Google Scholar]
- 35.Hwang JA, Lee BB, Kim Y, Hong SH, Kim YH, Han J, Shim YM, Yoon CY, Lee YS, Kim DH. HOXA9 inhibits migration of lung cancer cells and its hypermethylation is associated with recurrence in non-small cell lung cancer. Mol Carcinog. 2015;54(Suppl 1):E72–E80. doi: 10.1002/mc.22180. [DOI] [PubMed] [Google Scholar]
- 36.Huang T, Chen X, Hong Q, Deng Z, Ma H, Xin Y, Fang Y, Ye H, Wang R, Zhang C, et al. Meta-analyses of gene methylation and smoking behavior in non-small cell lung cancer patients. Sci Rep. 2015;5:8897. doi: 10.1038/srep08897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colella S, Shen L, Baggerly KA, Issa JP, Krahe R. Sensitive and quantitative universal Pyrosequencing methylation analysis of CpG sites. Biotechniques. 2003;35:146–150. doi: 10.2144/03351md01. [DOI] [PubMed] [Google Scholar]
- 38.Candiloro IL, Mikeska T, Dobrovic A. Assessing combined methylation-sensitive high resolution melting and pyrosequencing for the analysis of heterogeneous DNA methylation. Epigenetics. 2011;6:500–507. doi: 10.4161/epi.6.4.14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quillien V, Lavenu A, Karayan-Tapon L, Carpentier C, Labussiere M, Lesimple T, Chinot O, Wager M, Honnorat J, Saikali S, et al. Comparative assessment of 5 methods (methylation-specific polymerase chain reaction, MethyLight, pyrosequencing, methylation-sensitive high-resolution melting and immunohistochemistry) to analyze O6-methylguanine-DNA-methyltranferase in a series of 100 glioblastoma patients. Cancer. 2012;118:4201–4211. doi: 10.1002/cncr.27392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.