Abstract
Consensus has yet to be achieved on whether obesity is inexorably tied to poor fitness. We tested the hypothesis that appropriate reference of cardiopulmonary exercise testing (CPET) variables to lean body mass (LBM) would eliminate differences in fitness between high-BMI (≥ 95th percentile, n = 72, 50% female) and normal-BMI (< 85th percentile, n = 142, 49% female), otherwise-healthy children and adolescents typically seen when referencing body weight. We measured body composition with dual x-ray absorptiometry (DXA) and CPET variables from cycle ergometry using both peak values and submaximal exercise slopes (peak VO2, ΔVO2/ΔHR, ΔWR/ΔHR, ΔVO2/ΔWR, and ΔVE/ΔVCO2). In contrast to our hypothesis, referencing to LBM tended to lessen, but did not eliminate, the differences (peak VO2 [p < .004] and ΔVO2/ΔHR [p < .02]) in males and females; ΔWR/ΔHR differed between the two groups in females (p = .041) but not males (p = .1). The mean percent predicted values for all CPET variables were below 100% in the high-BMI group. The pattern of CPET abnormalities suggested a pervasive impairment of O2 delivery in the high-BMI group (ΔVO2/ΔWR was in fact highest in normal-BMI males). Tailoring lifestyle interventions to the specific fitness capabilities of each child (personalized exercise medicine) may be one of the ways to stem what has been an intractable epidemic.
Keywords: obesity, physical fitness, slopes, peak VO2, aerobic
Physical fitness and physical activity are essential components in both the diagnosis and treatment of the overweight and obese child and adolescent (8,15,23). Despite this, evidence-based guidelines do not yet exist on how best to assess and track physical fitness in this population. Recently, Hansen and coworkers (21) reviewed a number of studies focused on assessing fitness from cardiopulmonary exercise testing (CPET) in obese adolescents. They were unable to find consensus among these studies, and reached the following conclusion: “Whether cardiopulmonary anomalies during maximal exercise testing would occur in obese adolescents remains uncertain. Studies are therefore warranted to examine the cardiopulmonary response during maximal exercise testing in obese adolescents” (p. 894).
The purpose of this research was to begin to examine the relationship between elements of body composition (obtained by dual x-ray absorptiometry [DXA]) and key physiologic variables obtained from gas exchange and heart rate (HR) monitoring during CPET in obese children and adolescents. CPET primarily measures cardiovascular and respiratory fitness, which we summarize as the ability of the heart, lungs, and blood vessels to support gas exchange, nutrients, and the removal of byproducts of energy metabolism (largely through ATP turnover) in the working muscles. In typical CPET, gas exchange measured at the mouth (i.e., oxygen uptake [VO2], carbon dioxide production [VCO2], and ventilation [VE]) increases as the workload progresses and reflects gas exchange in the skeletal muscle (44). Not surprisingly, muscle mass is a major determinant of peak exercise gas exchange (9).
Measuring fitness in children (whether normal weight, overweight, or obese) is complicated because muscle and fat mass and hormonal regulation of metabolism and growth change rapidly in children and adolescents (11,19,40). Consequently, any physiological variable derived from CPET must be scaled to some index of body size and maturational status. In the obese child, useful scaling of CPET variables is further confounded because body fat (virtually metabolically inactive during exercise) may obscure the effect of the metabolically active muscle tissue when CPET is normalized to body mass.
Traditional CPET in both children and adults relies on some assessment of peak or maximal oxygen uptake using protocols in which the effort performed increases progressively. Maximal exercise tests are, by definition, highly dependent on the willingness of each child to continue exercise at relatively high work rates (WR) when dyspnea, muscle fatigue, and other stress sensations are commonly experienced. In some cases, investigators purposefully do not exhort obese children during progressive exercise testing. For example, Salvadego and coworkers (34) studied exercise in a group of obese, otherwise healthy adolescents and stopped exercise when the participant achieved a HR of 180 bpm. The authors noted, “A true maximal test was not performed to avoid the cardiovascular risks associated with maximal exercise in obese subjects” (p. R1299). Further, several studies suggest that obese children and adolescents perceive high-intensity exercise differently than normal-weight controls (3,37). It is not surprising that the plateau in oxygen uptake, the classical physiologic proof that VO2max had been reached, is found in relatively small proportions of normal-weight or obese children and adolescents (6).
Consequently, in the current study, in addition to the VO2max or peak VO2, we used variables obtained from the submaximal portions of CPET (Figure 1; such as dynamic slopes of gas exchange and HR during progressive exercise) which we recently demonstrated might prove useful when peak VO2 or VO2max values were questionable (9). Unlike peak VO2, which is derived from no more than a few data points at the end of a progressive exercise test, the CPET slopes were calculated from a response continuum of gas exchange, WR, and HR variables throughout a large portion of the progressive exercise test. We found that information derived from the analysis of the submaximal CPET slopes may augment peak VO2 data by identifying specific determinants of the exercise response that relate uniquely to ventilatory and/ or cardiovascular clinical status of the child or adolescent undergoing testing.
Figure 1.
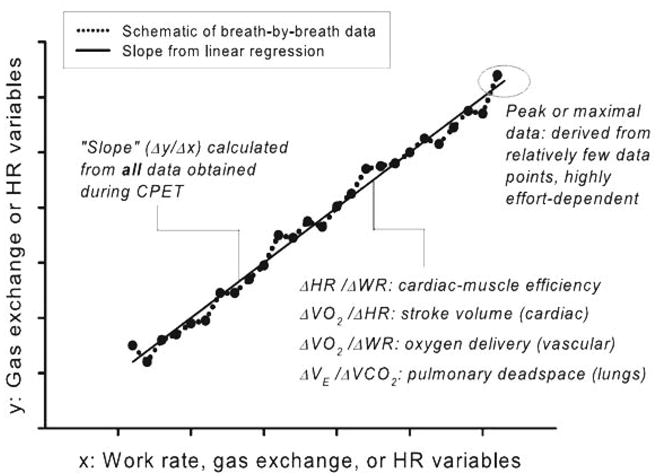
Cardiopulmonary exercise testing (CPET) slopes and peak values. This is a schematic generalization of the determination of slopes of key CPET variables from a ramp-type cycle ergometry, progressive exercise protocol. Unlike the peak VO2 or VO2max, which rely on a relatively small set of data obtained during very heavy exercise, the slopes are derived from a much larger set of data obtained throughout the exercise protocol. See Cooper et al. (9). HR = heart rate; WR = work rate.
The data from our earlier study of CPET slopes in children and adolescents with a body mass index (BMI) below the 95th percentile showed that many of these variables, like peak or maximal VO2, were highly correlated with muscle mass. A number of studies have shown that when lean body mass is appropriately considered, CPET measures in children and adolescents with obesity disorders are largely within normal ranges (12,18). In contrast, other studies of exercise gas exchange responses in obese children suggest that obesity impairs fitness, even when the data are normalized to some estimate of muscle mass (34) and, as Hansen and coworkers noted (21), much additional work is needed to develop useful approaches to assessing cardiorespiratory fitness in the child or adolescent with obesity. We reasoned that using all of the data obtained during CPET (i.e., peak, maximal, and submaximal), along with DXA estimates of muscle mass, would help more accurately assess fitness in the obese child or adolescent.
Methods
High Body Mass Index Volunteers
We identified 72 children and adolescents aged 8–18 with no serious health conditions whose BMI was ≥ 95th percentile. These participants had a DXA measurement within 30 days of the exercise protocol. The high-BMI participants had participated in 3 UC Irvine Institutional Review Board (IRB) approved pediatric exercise research studies (focused on physical fitness and childhood obesity) where a CPET was performed in our laboratory.
Normal Body Mass Index Volunteers
We used the CPET and body composition data from 142 children and adolescents whose BMI was ≤ 85th percentile as described previously (9). The normal-BMI group participants had participated in 5 UC Irvine IRB approved pediatric exercise research studies, 3 of which were the same as the high-BMI participants, in the same laboratory using identical protocols.
Anthropometric Measurement and Body Composition
Standard, calibrated scales and stadiometers were used to determine weight and height (Table 1). Body composition, including lean body mass (LBM), fat mass, and percent body fat (% fat), were determined by DXA using a Hologic QDR 4500 densitometer (Hologic Inc., Bedford, MA). Participants were scanned in light clothing while lying supine. On the day of each test, the DXA instrument was calibrated using the procedures provided by the manufacturer and DXA scans were performed and analyzed using pediatric software.
Table 1. Anthropometric Data in the Study Participants.
| Variable | Female | Male | ||
|---|---|---|---|---|
|
|
|
|||
| Normal-BMI | High-BMI | Normal-BMI | High-BMI | |
| # of subjects | 70 | 36 | 72 | 36 |
| Height (cm) | 153.0 ± 14.4 | 156.6 ± 11.2 | 157.5 ± 17.7 | 159.9 ± 17.1 |
| Weight (kg) | 44.6 ± 13.0 | 78.3 ± 21.8 | 48.4 ± 16.9 | 74.9 ± 23.3 |
| BMI (kg/m2) | 18.6 ± 2.9 | 31.2 ± 5.1 | 18.8 ± 2.8 | 28.5 ± 4.1 |
| BMI percentile | 45.5 ± 26.4 | 98.1 ± 1.1 | 47.0 ± 23.9 | 97.7 ± 1.4 |
| Lean body mass (kg) | 33.8 ± 9.7 | 46.7 ± 12.4 | 40.3 ± 15.3 | 49.9 ± 17.7 |
| Fat mass (kg) | 10.9 ± 4.4 | 32.0 ± 10.8 | 8.0 ± 3.1 | 25.5 ± 8.8 |
| % body fat | 24.1 ± 5.3 | 40.3 ± 5.3 | 17.3 ± 5.7 | 34.4 ± 7.2 |
Note. Data are presented as mean ± SD. BMI = body mass index.
Measurement of Peak VO2 and Slopes
Each participant performed a ramp-type progressive cycle ergometry using the SensorMedics metabolic system (Ergoline 800S, Yorba Linda, CA) and gas exchange was measured breath-by-breath. We used peak VO2 which was calculated as the maximum of 30-second averages (calculated in 6-s intervals) over the last 2 minutes of exercise. There is currently no validated, universally-accepted respiratory exchange ratio (RER) cutoff in children for the determination of peak VO2. We used RER ≥ 1.0, a criterion recently used in a large study by Rowland and coworkers (33). For subjects who participated in more than one study, one test was randomly selected. Appropriate assents and consents were obtained from each participant and his or her parent or guardian by each study. From the WR, HR, and gas exchange measurements obtained during CPET, the slopes were calculated using standard linear regression. We used simple linear approximations (y = α + β • x, in which β is the slope and α is the y-intercept).
Statistical Analysis
The linear regression model was applied to evaluate how body mass (weight and LBM) affects CPET variables (peak VO2, ΔVO2/ΔHR, ΔWR/ΔHR, and ΔVE/ΔVCO2) and the 2-sample t test was used to compare the difference in slope between high-BMI and normal-BMI participants for males and females separately. The analysis of variance (ANOVA) was applied to compare difference in ΔVO2/ΔWR, peak HR, peak RER, and O2-pulse between the 4 gender by BMI groups. The post hoc comparisons were adjusted using Bonferroni's method. Descriptive statistics are presented with mean and standard deviation (SD). All analyses were performed using SAS 9.4 (Cary, NC) and the significance level was set at .05.
Results
Subject Characteristics
Of the 72 high-BMI participants, 50% were males, 46% were Caucasian, 28% were Hispanic, 19% were Asian, and 7% were African-American. Of the 142 normal-BMI participants, 51% were males, 61% were Caucasian, 18% were Hispanic, 19% were Asian, and 2% were African-American.
Body Composition
The LBM and body fat averages were, respectively, 33.8 ± 9.7 kg and 10.9 ± 4.4 kg for normal-BMI girls, 40.3 ± 15.3 kg and 8 ± 3.1 kg for normal-BMI boys, 46.7 ± 12.4 kg and 32 ± 10.8 kg for high-BMI girls, and 49.9 ± 17.7 kg and 25.5 ± 8.8 kg for high-BMI boys, The high-BMI participants had much higher percent body fat compared with the normal-BMI participants and, as seen in Figure 2, this pattern was observed across the age span of our participants.
Figure 2.
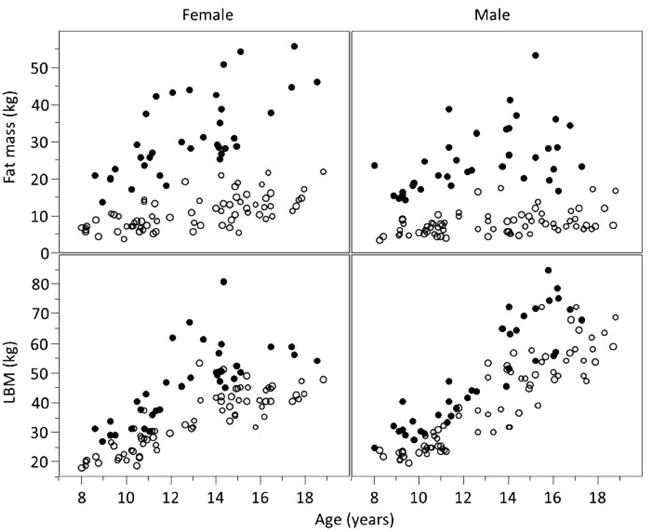
Scatter plot of fat mass and lean body mass (LBM) vs age. Open circles represent individual with a normal body mass index (BMI); closed circles represent individual with a high BMI. The high-BMI groups had substantially greater fat mass than normal-BMI. Lean body mass tended to be greater in the high-BMI, but to a lesser degree than fat mass.
Cardiopulmonary Exercise Testing Variables
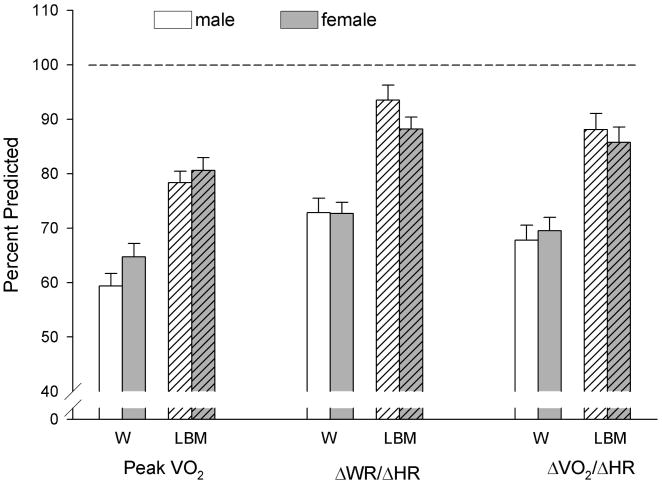
As shown in Figures 3–5, the CPET variables were highly correlated with both weight and LBM in the normal- and high-BMI participants. Differences between males and females were also noted in these variables. When using body weight, the differences between the normal- and high-BMI groups were most salient. The CPET variables were all significantly different between normal- and high-BMI groups (peak VO2: p < .0001; ΔVO2/ΔHR: p < .002; and ΔWR/ΔHR: p < .01). Referencing these CPET variables to LBM tended to lessen the differences. The slopes were still significantly different between normal- and high-BMI groups for peak VO2 (p < .004) and ΔVO2/ΔHR (p < .02). The ΔWR/ΔHR differed between the 2 groups in females (p = .041) but not males (p = .1). The regression line from the normal-BMI subjects was then applied to the high-BMI subjects to calculate the percent predicted CPET value. Figure 6 shows that the percent predicted of CPET for the majority of high-BMI subjects were below 100% predicted in all cases and ranged between 39% and 125% when referenced to body weight and between 57% and 143% when referenced to LBM.
Figure 3.
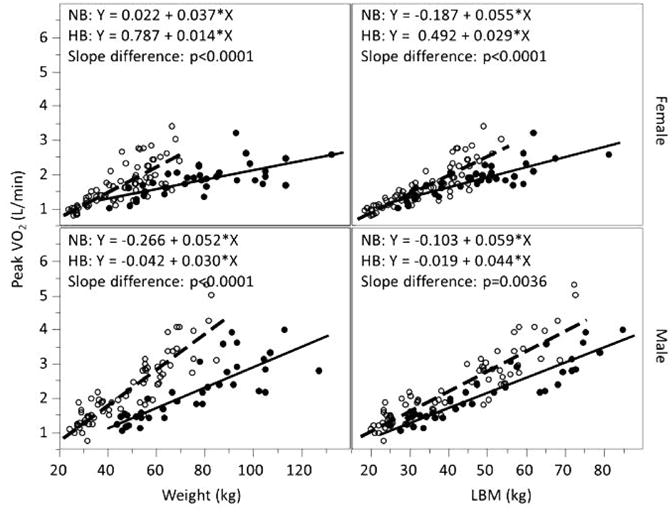
Peak VO2 and weight and lean body mass (LBM) in normal- and high-BMI male and female children and adolescents. Open circles represent individual with normal body mass index (BMI); closed circles represent individual with high BMI; dashed line represents linear regression line for normal-BMI group with equation labeled as NB; solid line represents linear regression line for high-BMI group with equation labeled as HB. When referenced to body weight, peak VO2 values tended to be much lower in high-BMI participants. Referencing to LBM reduced values, but did not eliminate the differences.
Figure 5.
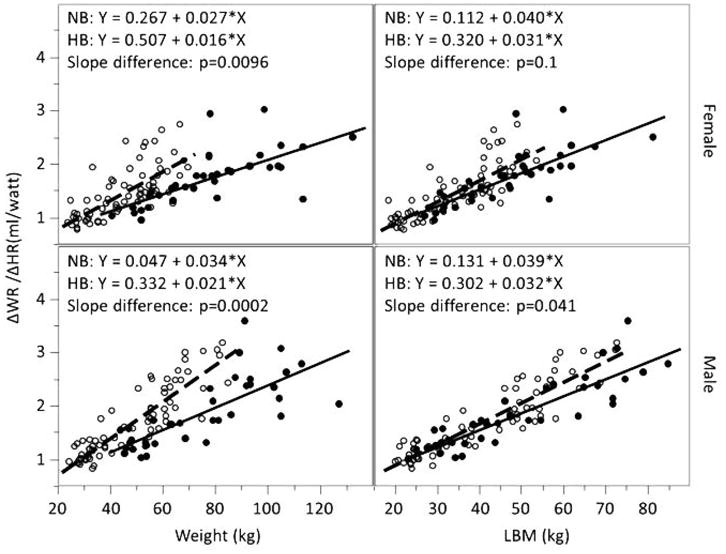
ΔWR/ΔHR and weight and lean body mass (LBM) in normal- and high-BMI male and female children and adolescents. Open circles represent individual with normal body mass index (BMI); closed circles represent individual with high BMI; dashed line represents linear regression line for normal-BMI group with equation labeled as NB; solid line represents linear regression for high-BMI group with equation labeled as HB. When referenced to body weight, ΔWR/ΔHR values tended to be much lower in high-BMI participants. Referencing to LBM eliminated the differences.
Figure 6.
Percent predicted values of peak VO2, ΔWR/ΔHR, and ΔVO2/ΔHR in high body mass index (BMI) males and females. We used linear regressions from the normal-BMI participants to determine expected values of peak VO2, ΔWR/ΔHR, and ΔVO2/ΔHR using either weight (W) or lean body mass (LBM). Consistent with the data shown in Figures 3–5, referencing to LBM reduced differences but values were still lower in the high-BMI participants despite the improvement.
ΔVE/ΔVCO2 decreased with weight and LBM and the slope difference was signifcant (p < .03) between the high- and normal-BMI girls but not between boys (p > .45).
There was a significant difference (p = .0003) in ΔVO2/ΔWR among the 4 gender by BMI groups. The post hoc comparisons showed that normal-BMI boys (10.8 ± 1.6 mL/min/W) had a small but significantly higher ΔVO2/ΔWR than high-BMI boys (10.0 ± 1.5; adjusted p = .047), normal-BMI girls (9.8 ± 1.2; adjusted p < .001), and high-BMI girls (9.9 ± 1.4; adjusted p = .017).
There were also significant differences in peak HR (p = .019), peak RER (p < .0001), and O2 pulse (p < .0001) among the groups. The high-BMI girls had significantly lower HR (183.3 ± 10.7 beat/min; adjusted p < .05) than normal-BMI girls (189.3 ± 10.1), high-BMI boys (189.1 ± 10.0), and normal-BMI boys (189.9 ± 10.2). The peak RER was significantly lower for high-BMI girls (1.10 ± 0.05) and high-BMI boys (1.10 ± 0.06) compared with normal-BMI girls (1.15 ± 0.07; adjusted p < .005) and normal-BMI boys (1.14 ± 0.08; adjusted p < .03). The normal-BMI girls had significantly lower O2 pulse (8.9 ± 3.2 mL/beat; adjusted p < .01) than boys (normal-BMI: 12.0 ± 5.1; high-BMI: 11.6 ± 4.6), while the high-BMI girls (10.2 ± 2.7; adjusted p > .25) had no difference with any group.
Discussion
This exploratory research provides new insights into the relationship between physical fitness and body composition in children and adolescents with normal- and high-BMI and likely to suffer from lifespan health disorders related to obesity. Strengths of the study included the simultaneous measurement of body composition and CPET using both maximal and submaximal gas exchange and HR variables in a sizeable cohort of children and adolescents. The lack of consensus regarding fitness levels in obese children and adolescents as well as earlier observations from this and other laboratories led us to hypothesize that apparent reductions in fitness in high-BMI children and adolescents compared with normal-BMI participants would be largely eliminated if the CPET values were referenced to LBM rather than weight. Although referencing these values to LBM lessened the discrepancies in fitness between the 2 groups, the data suggested that the child or adolescent with a BMI ≥ 95th percentile has a substantial risk of reduced fitness as well. The reduced fitness in high-BMI participants was detected by submaximal variables derived from CPET, namely, the ΔWR/ΔHR and the ΔVO2/ΔHR. The differences between high- and normal-BMI groups were also seen when using peak VO2 to an even greater degree than for the submaximal values.
A number of recent studies have pointed out that while a high-BMI is suggestive of obesity (defined in terms of percent body fat), high-BMI is not interchangeable with obesity disorders as in some cases the high BMI results from muscle mass rather than fat mass (31,32,43). Our data showed that the high-BMI group had inordinately high body fat mass compared with normal-BMI children and adolescents (Figure 2). In parallel, muscle mass was higher in the high-BMI children and, as Ervin and coworkers recently noted, heavier body weight in children and adolescents was associated with increased muscular strength but only in those tests of strength that did not involve moving all of body weight (14). The observation of systematic increases in lean body mass in high-BMI subjects (with and without elevated body fat) highlights the importance of referencing muscle mass per se when attempting to compare fitness levels in overweight children and adolescents.
The observation that both CPET submaximal values and peak VO2 were lower in the high-BMI group despite referencing to LBM is particularly salient. VO2max or peak VO2, considered to be the “gold standard” of fitness (42), may by itself be difficult to interpret in any child with a chronic disease or condition. For example, in a recent large study of children and adolescents (mean age 12.3 y) who had undergone the Fontan correction for congenital heart disease during childhood, only 166 of 411 patients (40%) achieved an acceptable VO2max using current criteria (30). As noted earlier, some researchers are hesitant to cajole and push these children as hard as they would normal-weight children (34). Further, obese children may not push themselves as hard as normal-weight children in the high-intensity range of exercise that typifies peak or maximal VO2. Shim and coworkers (37), in obese children with asthma, and Marinov and coworkers (28), in otherwise healthy obese children, for example, noted greater sense of breathlessness at high WRs than in normal-BMI children. Salvadego et al. (35), in a recent elegant study, showed that by reducing the work of breathing using heliox, obese adolescents improved their rate of perceived exertion during high-intensity exercise.
In our study, the high-BMI boys and girls tended to have slightly lower peak HR and RER than did the normal-BMI participants. This might reflect the reluctance of the high-BMI participants to push themselves at high WRs when exercise becomes stressful. Alternatively, there may be physiological obesity-related mechanisms as well. For example, lower peak HR could result from autonomic impairment in heart rate control associated with obesity (13). Lower RER could be caused by increased work of breathing at high WRs leading to relatively reduced CO2 output near peak exercise.
The ΔWR/ΔHR and ΔVO2/ΔHR slopes reflect a useful physiological response supplying the clinician with insights (albeit, indirect) into cardiac function and muscle perfusion (9). Resting VO2 and VO2 at unloaded (0-W) pedaling are elevated in obese subjects because of their greater body mass and the work associated with it (5). Once pedaling on the ergometer has commenced and remains at a constant rate as work increases, this particular effect of body mass will have less of an influence on the slopes of CPET parameters because the work of moving the legs themselves (in contrast to the work imposed by the ergometer) does not change as exercise progresses.
Submaximal CPET slopes, ΔWR/ΔHR and the ΔVO2/ΔHR, were less than expected even when referenced to LBM. There is increasing evidence that the matching of tissue perfusion with local metabolic demand during exercise is impaired in obesity even before frank atherosclerotic vascular disease develops (1). Recent studies have shown that even at rest, subclinical abnormalities in vascular and cardiac function are detectable using sophisticated imaging techniques in obese youth (10,36). Moreover, Sunagawa and coworkers (39) suggested in 1984 that an abnormal VO2-HR slope could indicate cardiac impairment during exercise in children. Data from both human and animal models indicate that this impairment results from interrelated mechanisms of chronic inflammation, neuroadrenergic dysregulation, altered fiber-type distribution in skeletal muscle, and endothelial damage (17,20,26). Brun and coworkers (7) demonstrated interactions between abdominal obesity, circulating lipids, and increased blood viscosity. The latter phenomenon could potentially inhibit oxygen delivery, even in the range of submaximal exercise. Other factors, such as impaired peripheral nervous system function associated with obesity (24), could also contribute to the lower than expected exercise responses in many high-BMI children and adolescents.
Muscle hypertrophy occurs in many children and adolescents with obesity disorders, possibly in response to the abnormalities in matching perfusion to muscle demand. The increased muscle mass, which we observed in our high-BMI participants, can obscure abnormalities in exercise responses measured by traditional CPET when normalized to muscle mass; but there is evidence that the hypertrophic muscle in obese patients is abnormal with respect to voluntary fatigue and relative strength (27). Substantiating the hypothesis that inordinately high levels of body fat could impair oxygen delivery was an observation we made in a recent study of children and adolescents with BMI < 95th percentile (12). Even in this group, we found that percent body fat was significantly, albeit to a very small degree, inversely correlated with VO2peak, ΔWR/ΔHR, and ΔVO2/ΔHR, suggesting that exercise testing might prove to be an early indicator of vascular dysfunction in the overweight or obese child.
One key assumption in interpreting CPET variables is that the change in WR measured on the cycle ergometer accounts for virtually all of the increase in external work performed as exercise progresses. In obesity, this assumption may not hold (2). For example, the work of breathing may substantially increase nonlinearly (even as WR increases linearly) because respiratory rate and tidal volumes increase as exercise progresses and the work of breathing in the obese individual is substantially greater because of chest wall mass and the resistive effects on the diaphragm of increased visceral abdominal fat. The study of Salvadego et al. (35) supports the notion that work of breathing is inordinately high and increases nonlinearly in obese subjects. We did not observe substantial abnormalities in the ΔVE/ΔVCO2, suggesting that despite an increase in work of breathing, abnormalities in dead space or respiratory control that do influence the ΔVE/ΔVCO2 in lung diseases such as cystic fibrosis (29), respiratory physiology remained largely intact in the high-BMI group.
In this context, our data may actually have under-estimated the impairment in fitness in the high-BMI group. We found that the ΔVO2/ΔWR was actually higher in the normal-BMI boys than in the high-BMI groups. ΔVO2/ΔWR is the inverse of the thermodynamic work efficiency, which had been assumed to be independent of body size as it was proposed to represent the stoichiometry of ATP rephosphorylation during exercise (45). However, it has become clear that the ΔVO2/ΔWR can be influenced by cardiac output and muscle blood flow and perfusion (41), and not solely by the oxygen required to rephosphorylate ADP. In the case of the high-BMI group in our study, if the work of breathing increased nonlinearly as ergometer work increased (again, as hinted in the Salvadego study [35]), then the lower ΔVO2/ΔWR in the high-BMI individuals could indicate impaired oxygen delivery since the actual work performed would be greater than the ΔWR gauged solely from the cycle ergometer.
As recently noted by Hansen and coworkers (21), consensus regarding CPET in obese adolescents has yet to be reached. There may be differences in the effect of obesity on CPET as children age. For example, earlier work Goran et al. (18) and Salvadego et al. (34) found that when normalized to fat free mass, peak VO2 in high-BMI prepubertal children did not differ from controls. Neither of these earlier studies included adolescents. Interestingly, in our data of the high-BMI subjects, we observed a mild inverse correlation (r = –.36) between percent predicted peak VO2 (referenced to LBM) and age, suggesting the hypothesis that peak exercise values are more affected in adolescents than in younger children. The correlation between age and VO2/HR and WR/HR slopes (referencing to LBM) were −.3 and −.19, respectively. When referencing to weight, the correlations were between −.05 and −.15.
Despite this improvement in the specificity of CPET to identify poor fitness using slopes and LBM reference approaches, our data suggest that a significantly high proportion of children and adolescents with high BMI have less than predicted fitness. The pervasiveness of impaired physiological fitness in obese children has been observed by others using approaches to measure cardiorespiratory fitness that do not involve maximal testing. For example, Salvadego and coworkers (34) recently quantified the response time of VO2 at the onset of exercise in a group of obese adolescents and found the response times to be prolonged compared with normal-weight controls. They further observed that VO2 continued to increase in some obese adolescents during constant (unchanging) WR exercise testing at levels of exercise where VO2 did not increase in nonobese subjects. The authors speculated that the gas exchange data suggested impairment of skeletal muscle oxidative metabolism. Qualitatively similar prolongation of exercise recovery times were also found recently in obese prepubertal children by a number of investigators (25,38).
Our data highlight the need to accurately reference CPET data to some estimate of muscle mass, the key driving tissue in exercise testing. In children and adolescents with obesity disorders, many subjects have apparently normal fitness, and one must wonder whether imposing additional physical interventions on these children would be productive. However, our data do suggest that in otherwise healthy, high-BMI children and adolescents, there seems to be a deleterious effect of increased body fat on CPET. Moreover, access to sophisticated CPET and DXA measures of fitness and body composition are not typically available to primary care providers. Simpler and less expensive approaches will be necessary if we are to provide clinicians with useful biomarkers to diagnose and treat obesity disorders. Given the complexities of obesity disorders in children and adolescents, tailoring lifestyle interventions to the specific fitness capabilities of each child (personalized exercise medicine [4,16,22]) may be one of the ways to stem what has been an intractable epidemic.
Figure 4.
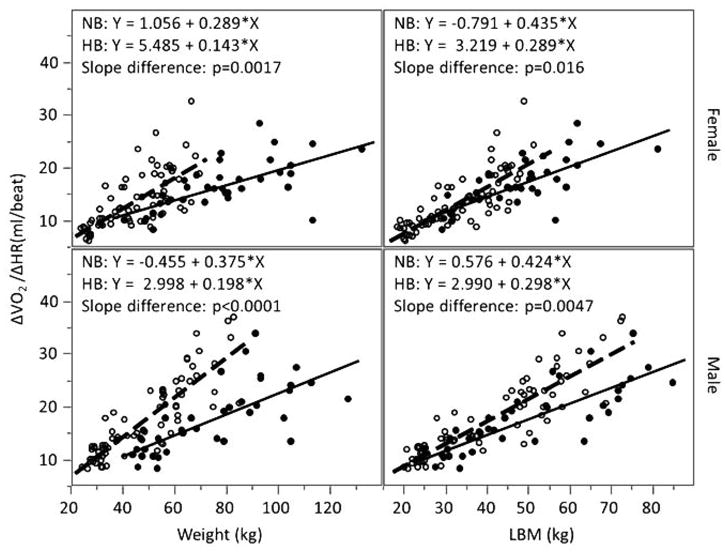
ΔVO2/ΔHR and weight and lean body mass (LBM) in normal- and high-BMI male and female children and adolescents. Open circles represent individual with normal body mass index (BMI); closed circles represent individual with high BMI; dashed line represents linear regression line for normal-BMI group with equation labeled as NB; solid line represents linear regression line for high-BMI group with equation labeled as HB. When referenced to body weight, peak ΔVO2/ΔHR values tended to be much lower in high-BMI participants. Referencing to LBM reduced values, but did not eliminate the differences.
Contributor Information
Dan M. Cooper, Department of Pediatrics, Pediatric Exercise and Genomic Research Center, University of California-Irvine, Irvine, CA
Szu-Yun Leu, Institute for Clinical and Translational Science, University of California-Irvine, Irvine, CA.
Candice Taylor-Lucas, Department of Pediatrics, Pediatric Exercise and Genomic Research Center, University of California-Irvine, Irvine, CA.
Kim Lu, Department of Pediatrics, Pediatric Exercise and Genomic Research Center, University of California-Irvine, Irvine, CA.
Pietro Galassetti, Department of Pediatrics, Pediatric Exercise and Genomic Research Center, University of California-Irvine, Irvine, CA.
Shlomit Radom-Aizik, Department of Pediatrics, Pediatric Exercise and Genomic Research Center, University of California-Irvine, Irvine, CA.
References
- 1.Aggoun Y. Obesity, metabolic syndrome, and cardiovascular disease. Pediatr Res. 2007;61(6):653–659. doi: 10.1203/pdr.0b013e31805d8a8c. [DOI] [PubMed] [Google Scholar]
- 2.Babb TG. Obesity: challenges to ventilatory control during exercise–a brief review. Respir Physiol Neurobiol. 2013;189(2):364–370. doi: 10.1016/j.resp.2013.05.019. doi:10.1016/j. resp.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belanger K, Breithaupt P, Ferraro ZM, et al. Do obese children perceive submaximal and maximal exertion differently? Clin Med Insights Pediatr. 2013;7:35–40. doi: 10.4137/CMPed.S12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouchard C, Antunes-Correa LM, Ashley EA, et al. Personalized preventive medicine: genetics and the response to regular exercise in preventive interventions. Prog Cardiovasc Dis. 2015;57(4):337–346. doi: 10.1016/j.pcad.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray GA, Whipp BJ, Koyal SN, Wasserman K. Some respiratory and metabolic effects of exercise in moderately obese men. Metabolism. 1977;26(4):403–412. doi: 10.1016/0026-0495(77)90107-X. [DOI] [PubMed] [Google Scholar]
- 6.Breithaupt P, Adamo KB, Colley RC. The HALO submaximal treadmill protocol to measure cardio-respiratory fitness in obese children and youth: a proof of principle study. Appl Physiol Nutr Metab. 2012;37(2):308–314. doi: 10.1139/h2012-003. [DOI] [PubMed] [Google Scholar]
- 7.Brun JF, Varlet-Marie E, Fedou C, Raynaud de ME. Body composition and exercise performance as determinants of blood rheology in middle-aged patients exhibiting the metabolic syndrome. Clin Hemorheol Microcirc. 2011;49(1-4):215–223. doi: 10.3233/CH-2011-1471. PubMed. [DOI] [PubMed] [Google Scholar]
- 8.Conn VS, Hafdahl A, Phillips LJ, Ruppar TM, Chase JA. Impact of physical activity interventions on anthropometric outcomes: systematic review and meta-analysis. J Prim Prev. 2014;35(4):203–215. doi: 10.1007/s10935-014-0352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper DM, Leu SY, Galassetti P, Radom-Aizik S. Dynamic interactions of gas exchange, body mass, and progressive exercise in children. Med Sci Sports Exerc. 2014;46(5):877–886. doi: 10.1249/MSS.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cote AT, Phillips AA, Harris KC, Sandor GG, Panagiotopoulos C, Devlin AM. Obesity and arterial stiffness in children: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2015;35(4):1038–1044. doi: 10.1161/ATVBAHA.114.305062. [DOI] [PubMed] [Google Scholar]
- 11.Eliakim A, Barstow TJ, Brasel JA, et al. Effect of exercise training on energy expenditure, muscle volume, and maximal oxygen uptake in female adolescents. J Pediatr. 1996;129(4):537–543. doi: 10.1016/S0022-3476(96)70118-X. [DOI] [PubMed] [Google Scholar]
- 12.Eliakim A, Burke GS, Cooper DM. Fitness, fatness, and the effect of training assessed by magnetic resonance imaging and skinfold-thickness measurements in healthy adolescent females. Am J Clin Nutr. 1997;66(2):223–231. doi: 10.1093/ajcn/66.2.223. PubMed. [DOI] [PubMed] [Google Scholar]
- 13.Eliakim A, Nemet D, Zaldivar F, McMurray RG, Culler FL, Galassetti P, Cooper DM. Reduced exercise-associated response of the GH-IGF-I axis and catecholamines in obese children and adolescents. J Appl Physiol (1985) 2006;100(5):1630–1637. doi: 10.1152/japplphysiol.01072.2005. [DOI] [PubMed] [Google Scholar]
- 14.Ervin RB, Fryar CD, Wang CY, Miller IM, Ogden CL. Strength and body weight in US children and adolescents. Pediatrics. 2014;134(3):e782–e789. doi: 10.1542/peds.2014-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escalante Y, Saavedra JM, Garcia-Hermoso A, Dominguez AM. Improvement of the lipid profile with exercise in obese children: a systematic review. Prev Med. 2012;54(5):293–301. doi: 10.1016/j.ypmed.2012.02.006. doi:10.1016/j. ypmed.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Faulkner MS, Michaliszyn SF, Hepworth JT, Wheeler MD. Personalized exercise for adolescents with diabetes or obesity. Biol Res Nurs. 2014;16(1):46–54. doi: 10.1177/1099800413500064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frisbee JC, Goodwill AG, Butcher JT, Olfert IM. Divergence between arterial perfusion and fatigue resistance in skeletal muscle in the metabolic syndrome. Exp Physiol. 2011;96(3):369–383. doi: 10.1113/expphysiol.2010.055418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goran M, Fields DA, Hunter GR, Herd SL, Weinsier RL. Total body fat does not influence maximal aerobic capacity. Int J Obes Relat Metab Disord. 2000;24(7):841–848. doi: 10.1038/sj.ijo.0801241. [DOI] [PubMed] [Google Scholar]
- 19.Graves LE, Batterham AM, Foweather L, et al. Scaling of peak oxygen uptake in children: a comparison of three body size index models. Med Sci Sports Exerc. 2013;45(12):2341–2345. doi: 10.1249/MSS.0b013e31829bfa79. [DOI] [PubMed] [Google Scholar]
- 20.Hallsten K, Yki-Jarvinen H, Peltoniemi P, et al. Insulin-and exercise-stimulated skeletal muscle blood flow and glucose uptake in obese men. Obes Res. 2003;11(2):257–265. doi: 10.1038/oby.2003.39. [DOI] [PubMed] [Google Scholar]
- 21.Hansen D, Marinus N, Remans M, et al. Exercise tolerance in obese vs. lean adolescents: a systematic review and meta-analysis. Obes Rev. 2014;15(11):894–904. doi: 10.1111/obr.12202. [DOI] [PubMed] [Google Scholar]
- 22.Hecksteden A, Kraushaar J, Scharhag-Rosenberger F, Theisen D, Senn S, Meyer T. Individual response to exercise training - a statistical perspective. J Appl Physiol (1985) 2015;118(12):1450–1459. doi: 10.1152/japplphysiol.00714.2014. [DOI] [PubMed] [Google Scholar]
- 23.Hills AP, Dengel DR, Lubans DR. Supporting public health priorities: recommendations for physical education and physical activity promotion in schools. Prog Cardiovasc Dis. 2015;57(4):368–374. doi: 10.1016/j.pcad.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Isojarvi H, Keinanen-Kiukaanniemi S, Kallio M, et al. Exercise and fitness are related to peripheral nervous system function in overweight adults. Med Sci Sports Exerc. 2010;42(7):1241–1245. doi: 10.1249/MSS.0b013e3181cb8331. [DOI] [PubMed] [Google Scholar]
- 25.Laguna M, Aznar S, Lara MT, Lucia A, Ruiz JR. Heart rate recovery is associated with obesity traits and related cardiometabolic risk factors in children and adolescents. Nutr Metab Cardiovasc Dis. 2013;23(10):995–1001. doi: 10.1016/j.numecd.2012.10.002. doi:10.1016/j. numecd.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Lazzer S, Salvadego D, Porcelli S, et al. Skeletal muscle oxygen uptake in obese patients: functional evaluation by knee-extension exercise. Eur J Appl Physiol. 2013;113(8):2125–2132. doi: 10.1007/s00421-013-2647-2. [DOI] [PubMed] [Google Scholar]
- 27.Maffiuletti NA, Jubeau M, Munzinger U, et al. Differences in quadriceps muscle strength and fatigue between lean and obese subjects. Eur J Appl Physiol. 2007;101(1):51–59. doi: 10.1007/s00421-007-0471-2. [DOI] [PubMed] [Google Scholar]
- 28.Marinov B, Kostianev S, Turnovska T. Ventilatory efficiency and rate of perceived exertion in obese and non-obese children performing standardized exercise. Clin Physiol Funct Imaging. 2002;22(4):254–260. doi: 10.1046/j.1475-097X.2002.00427.x. [DOI] [PubMed] [Google Scholar]
- 29.Moser C, Tirakitsoontorn P, Nussbaum E, Newcomb R, Cooper DM. Muscle size and cardiorespiratory response to exercise in cystic fibrosis. Am J Respir Crit Care Med. 2000;162(5):1823–1827. doi: 10.1164/ajrccm.162.5.2003057. [DOI] [PubMed] [Google Scholar]
- 30.Paridon SM, Mitchell PD, Colan SD, et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol. 2008;52(2):99–107. doi: 10.1016/j.jacc.2008.02.081. doi:10.1016/j. jacc.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 31.Petranovic MZ, Tomas Z, Skaric-Juric T, Milicic J, Narancic NS. Are the physically active adolescents belonging to the “at risk of overweight” BMI category really fat? Coll Antropol. 2013;37(Suppl. 2):131–138. PubMed. [PubMed] [Google Scholar]
- 32.Reichert FF, Baptista Menezes AM, Wells JC, Carvalho DS, Hallal PC. Physical activity as a predictor of adolescent body fatness: a systematic review. Sports Med. 2009;39(4):279–294. doi: 10.2165/00007256-200939040-00002. [DOI] [PubMed] [Google Scholar]
- 33.Rowland T, Hagenbuch S, Pober D, Garrison A. Exercise tolerance and thermoregulatory responses during cycling in boys and men. Med Sci Sports Exerc. 2008;40(2):282–287. doi: 10.1249/mss.0b013e31815a95a7. [DOI] [PubMed] [Google Scholar]
- 34.Salvadego D, Lazzer S, Busti C, et al. Gas exchange kinetics in obese adolescents. Inferences on exercise tolerance and prescription. Am J Physiol Regul Integr Comp Physiol. 2010;299(5):R1298–R1305. doi: 10.1152/ajpregu.00038.2010. [DOI] [PubMed] [Google Scholar]
- 35.Salvadego D, Sartorio A, Agosti F, et al. Acute respiratory muscle unloading by normoxic helium-O(2) breathing reduces the O(2) cost of cycling and perceived exertion in obese adolescents. Eur J Appl Physiol. 2015;115(1):99–109. doi: 10.1007/s00421-014-2993-8. [DOI] [PubMed] [Google Scholar]
- 36.Shah RV, Abbasi SA, Neilan TG, et al. Myocardial tissue remodeling in adolescent obesity. J Am Heart Assoc. 2013;2(4):e000279. doi: 10.1161/JAHA.113.000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shim YM, Burnette A, Lucas S, et al. Physical deconditioning as a cause of breathlessness among obese adolescents with a diagnosis of asthma. PLoS One. 2013;8(4):e61022. doi: 10.1371/journal.pone.0061022. doi:10.1371/journal. pone.0061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simhaee D, Corriveau N, Gurm R, et al. Recovery heart rate: an indicator of cardiovascular risk among middle school children. Pediatr Cardiol. 2013;34(6):1431–1437. doi: 10.1007/s00246-013-0667-7. [DOI] [PubMed] [Google Scholar]
- 39.Sunagawa H, Honda S, Mizoguchi Y, Yoshii K, Iwao H. Physiological significance of the slope of the regression equation between oxygen consumption and heart rate in exercise testing. Jpn Circ J. 1984;48(12):1399–1401. doi: 10.1253/jcj.48.1399. [DOI] [PubMed] [Google Scholar]
- 40.Tolfrey K, Barker A, Thom JM, Morse CI, Narici MV, Batterham AM. Scaling of maximal oxygen uptake by lower leg muscle volume in boys and men. J Appl Physiol (1985) 2006;100(6):1851–1856. doi: 10.1152/japplphysiol.01213.2005. [DOI] [PubMed] [Google Scholar]
- 41.Troutman WB, Barstow TJ, Galindo AJ, Cooper DM. Abnormal dynamic cardiorespiratory responses to exercise in pediatric patients after Fontan procedure. J Am Coll Cardiol. 1998;31:668–673. doi: 10.1016/S0735-1097(97)00545-7. [DOI] [PubMed] [Google Scholar]
- 42.Vanhees L, Lefevre J, Philippaerts R, et al. How to assess physical activity? How to assess physical fitness? Eur J Cardiovasc Prev Rehabil. 2005;12(2):102–114. doi: 10.1097/00149831-200504000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Varekova R, Vareka I. How to estimate overweight in pubescent asthmatics? Adv Med Sci. 2013;58(2):331–337. doi: 10.2478/ams-2013-0013. [DOI] [PubMed] [Google Scholar]
- 44.Wasserman K. Breathing during exercise. N Engl J Med. 1978;298(14):780–785. doi: 10.1056/NEJM197804062981408. [DOI] [PubMed] [Google Scholar]
- 45.Whipp BJ, Davis JA, Torres F, Wasserman K. A test to determine parameters of aerobic function during exercise. J Appl Physiol. 1981;50(1):217–221. doi: 10.1152/jappl.1981.50.1.217. PubMed. [DOI] [PubMed] [Google Scholar]