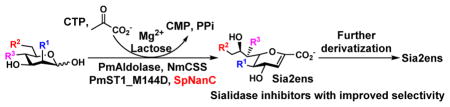
Abstract
Sialidase transition state analog inhibitor 2,3-dehydro-2-deoxy-N-acetylneuraminic acid (Neu5Ac2en, DANA) has played a leading role in developing clinically used anti-influenza virus drugs. Taking advantage of the Neu5Ac2en-forming catalytic property of Streptococcus pneumoniae sialidase SpNanC, an effective one-pot multienzyme (OPME) strategy has been developed to directly access Neu5Ac2en and its C-5, C-9, and C-7-analogs from N-acetylmannosamine (ManNAc) and analogs. The obtained Neu5Ac2en analogs can be further derivatized at various positions to generate a larger inhibitor library. Inhibition studies demonstrated improved selectivity of several C-5- or C-9-modified Neu5Ac2en derivatives against several bacterial sialidases. The study provides an efficient enzymatic method to access sialidase inhibitors with improved selectivity.
Keywords: biocatalysis, enzymatic synthesis, Neu5Ac2en, sialidase, sialidase inhibitor
Graphical Abstract
Sialic acids are common terminal monosaccharides on the carbohydrate moieties of mammalian cell surface glycoconjugates and play important biological roles. Sialidases or neuraminidases, the exoglycosidases that catalyze the cleavage of the terminal sialic acids, are widely spread in vertebrates and microbes residing in or infecting vertebrates.1–6 Viral and bacterial sialidases are attractive targets for designing inhibitors as potent antimicrobial therapeutics.7–11 The sialidase transition state analog, 2,3-dehydro-2-deoxy-N-acetylneuraminic acid (Neu5Ac2en or DANA), has been used as an important probe for structural and mechanistic studies of sialidases.12–19 It was also a lead compound for rational design of clinical anti-influenza virus drugs Relenza (Zanamivir or 4-deoxy-4-guanidino-Neu5Ac2en)20–22 and Tamiflu (Oseltamivir).23–24 Recently, designing and synthesizing 2,3-dehydro-2-deoxy-sialic acids (Sia2ens) and their derivatives have been actively pursued for developing anti-viral and anti-bacterial therapeutics as well as tools for functional studies of human sialidase isoforms.25–27 Except for a few examples of de novo synthesis of Zanamivir aiming at reducing the cost,28–29 chemical modification of selectively protected sialic acids has been a common strategy to obtain Sia2ens.30–34 For synthesizing Neu5Ac2en analogs, derivatization can be introduced before34 or after33, 35 the formation of the C2–C3 double bond. These methods require multiple protection and deprotection steps. In comparison, biocatalysis has advantages of high efficiency, environmental friendliness, and excellent regio- and stereo-selectivity without protecting group manipulation and has been increasingly used for synthesizing carbohydrates and derivatives. In this work, we aim to develop an effective enzymatic method that allows the formation of Sia2ens directly from the corresponding sialic acids or six-carbon precursors in one pot.
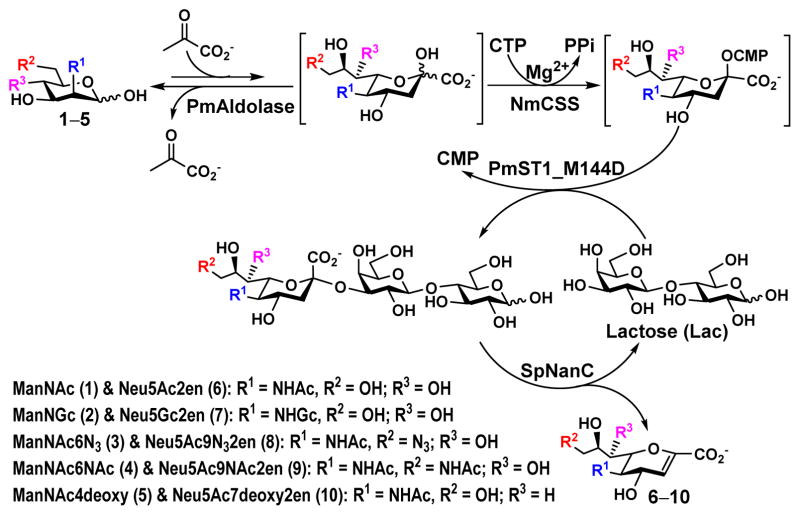
Streptococcus pneumoniae sialidase SpNanC, one of the three sialidases (SpNanA, SpNanB, and SpNanC) identified from hundreds of Streptococcus pneumoniae strains,36 was chosen for synthesizing Sia2en and analogs. SpNanC specifically recognizes α2–3-linked sialosides and catalyzes the formation of the general sialic acid transition state analog inhibitor Neu5Ac2en, which can be further hydrated by SpNanC to form N-acetylneuraminic acid (Neu5Ac).37–38 Combining the Neu5Ac2en-forming property of SpNanC and the highly efficient one-pot multienzyme (OPME) sialoside formation strategy39 that we developed previously, a new OPME system has been developed that can be used to produce a diverse array of Sia2en derivatives directly from sialic acid derivatives or their six-carbon precursors.
As shown in Scheme 1, four enzymes are used in the OPME system to produce Sia2ens from the corresponding six-carbon precursors of sialic acids. Sialic acid aldolase from Pasteurella multocida (PmAldolase)40 catalyzes the formation of sialic acid from its six-carbon monosaccharide precursor and pyruvate. CMP-sialic acid synthetase from Neisseria meningitidis (NmCSS)41 catalyzes the conversion of cytidine 5′-triphosphate (CTP) and sialic acid for the formation CMP-sialic acid, which is used together with an acceptor such as lactose by Pasteurella multocida sialyltransferase 1 M144D mutant (PmST1_M144D)42–43 for the synthesis of the corresponding α2–3-linked sialosides, providing the substrates for SpNanC for the production of target Sia2ens.
Scheme 1.
One-pot Multienzyme (OPME) Synthesis of Sia2ens
To obtain SpNanC as a key biocatalyst for the OPME synthesis of Sia2ens, full length SpNanC from Streptococcus pneumonia TIGR4 was cloned in pMAL-c4X vector and expressed as an N-terminal maltose-binding protein (MBP)-fused and C-terminal His6-tagged recombinant enzyme in Escherichia coli BL21(DE3) cells. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis showed that Ni2+-affinity column-purified MBP-SpNanC-His6 was presented as two bands with a major band at about 80 kDa corresponding to the protein without the MBP-tag and a faint band at around 120 kDa corresponding to the expected size of the full-length fusion protein. N-Terminal protein sequencing of the major 80 kDa-band indicated that the MBP-tag and the first twenty-six amino acids (26 aa) of SpNanC were cleaved during the expression and purification. As the resulting protein retained activity, the N-terminal 26 aa-truncated protein was re-cloned in pET22b(+) vector as a C-His6-tagged recombinant enzyme (Δ26SpNanC-His6). Approximately 108 mg of Ni2+-column purified Δ26SpNanC-His6 (abbreviated as SpNanC, Figure S1) could be routinely obtained from one liter Escherichia coli culture.
SpNanC was active in a pH range of 5.5–9.0 with an optimal activity at pH 6.5 (Figure S2). It did not require a metal ion for activity and the addition of a reducing reagent dithiothreitol (DTT) did not affect its activity (Figure S3). Kinetics studies using Neu5Acα2–3GalβpNP as the substrate indicated that SpNanC was a highly active sialidase (kcat/KM = 150 s−1 mM−1 with KM = 2.7 ± 0.5 mM and kcat = 406.8 ± 39.0 s−1) compared to a previously reported recombinant SpNanC using α2–3-sialyllactose as the substrate (kcat/KM = 0.29 s−1 mM−1 with KM = 1.08 ± 0.35 mM, kcat = 0.313 ± 0.052 s−1).37
Substrate specificity studies of SpNanC using a library of para-nitrophenol (pNP)-tagged α2–3-linked sialosides44–47 indicated that the enzyme could tolerate various modifications at C-5, C-9, and C-7 of Neu5Ac (Table 1). Different from what was described in a previous report,48 α2–3-linked sialosides containing N-glycolylneuraminic acid (Neu5Gc), a non-human sialic acid form,49 or its C-9 derivatives were also suitable substrates for the enzyme. α2–3-Linked sialosides containing 2-keto-3-deoxynonulsonic acid (Kdn) or its derivatives with modifications at C-5 or C-9 were not effective substrates of SpNanC. Among compounds tested, two of the sialosides containing C7-modified Kdn (Kdn7OMeα2–3GalβpNP and Kdn7deoxyα2–3GalβpNP) could be cleaved off by SpNanC with low yields (23% and 10% respectively in reactions using 3 μg of SpNanC).
Table 1.
Substrate Specificity of SpNanC
| Substrate | Percentage conversion (%) |
|---|---|
| Neu5Acα2–3GalβpNP | 85.1 ± 2.4 |
| Neu5AcFα2–3GalβpNP | 74.8 ± 3.2 |
| Neu5AcOMeα2–3GalβpNP | 81.8 ± 1.7 |
| Neu5AcN3α2–3GalβpNP | 87.9 ± 1.6 |
| Neu5Ac9Fα2–3GalβpNP | 75.6 ± 3.9 |
| Neu5Ac9OMeα2–3GalβpNP | 88.5 ± 5.1 |
| Neu5Ac9deoxyα2–3GalβpNP | 93.5 ± 3.1 |
| Neu5Ac7Fα2–3GalβpNP | 91.0 ± 4.3 |
| Neu5Ac7OMeα2–3GalβpNP | 96.5 ± 1.7 |
| Neu5Ac7deoxyα2–3GalβpNP | 91.2 ± 1.8 |
| Neu5Ac7N3α2–3GalβpNP | 65.9 ± 1.1 |
| Neu5Gcα2–3GalβpNP | 80.6 ± 6.7 |
| Neu5Gc9Fα2–3GalβpNP | 72.3 ± 2.6 |
| Neu5Gc9OMeα2–3GalβpNP | 75.0 ± 1.9 |
| Neu5Gc9deoxyα2–3GalβpNP | 85.5 ± 1.4 |
| Neu5Gc9N3α2–3GalβpNP | 89.5 ± 3.5 |
| Kdnα2–3GalβpNP | 4.50 ± 0.45 |
| Kdn5Fα2–3GalβpNP | 0.48 ± 0.00 |
| Kdn5N3α2–3GalβpNP | 0.32 ± 0.45 |
| Kdn5deoxyα2–3GalβpNP | 5.31 ± 0.23 |
| Kdn5OMeα2–3GalβpNP | 1.53 ± 1.02 |
| Kdn9Fα2–3GalβpNP | 3.70 ± 1.14 |
| Kdn9OMeα2–3GalβpNP | 4.02 ± 1.36 |
| Kdn7Fα2–3GalβpNP | 0.56 ± 0.11 |
| Kdn7OMeα2–3GalβpNP | 23.4 ± 3.3 |
| Kdn7deoxyα2–3GalβpNP | 10.1 ± 0.0 |
| Kdn7N3α2–3GalβpNP | 4.50 ± 0.45 |
The substrate promiscuities of SpNanC (Table 1) and the enzymes involved in the one-pot multienzyme (OPME) synthesis of sialosides40–43 that we reported previously39 provide an opportunity for synthesizing Sia2ens directly from the six-carbon precursors of sialic acids. As SpNanC has a dual function of forming Neu5Ac2en and further hydrating it to form Neu5Ac,37–38 the amount of SpNanC used in the one-pot four-enzyme reaction needs to be controlled. Small-scale reactions using ManNAc (1) as the starting material followed by thin layer chromatography (TLC) and mass spectroscopy (MS) analyses suggested that a concentration of SpNanC in the range of 0.004–0.04 mg/mL was optimal for reactions using 10 mM of ManNAc. Due to its low cost, commercially available lactose was chosen as the sialyltransferase acceptor. As shown in Scheme 1, lactose was regenerated in the SpNanC-catalyzed Sia2en-production reaction and was needed only for a catalytic amount. An optimal concentration of lactose was found to be 0.25 equivalent (2.5 mM) of ManNAc where it provided a good balance of reaction rate and the consumption of reagents. A Tris-HCl buffer with pH 7.5 was chosen for the Sia2en-production OPME reaction to balance the activities of enzymes involved including PmAldolase (good activity in pH 6.0–9.0),40 NmCSS (good activity in pH 7.0–10.0),50 PmST1_M144D (good α2–3-sialyltransferase activity in pH 6.0–10.0 similar to PmST142), and SpNanC (good activity in pH 6.0–7.5) (Figure S2). Under the optimal conditions determined by small-scale reactions, preparative-scale reactions were carried out and the amounts of the purified products were used to calculate the synthetic yields. Neu5Ac2en (6) was synthesized from ManNAc (1) in 72% yield (Table 2). Similar reaction conditions were used to synthesize 2,3-dehydro-2-deoxy-N-glycolylneuraminic acid (Neu5Gc2en, 7)34 from Neu5Gc precursor, N-glycolylmannosamine (ManNGc, 2).41 To our surprise, only a trace amount of the desired product was obtained. The slower formation of Neu5Gc-containing sialosides was identified to be the cause for the low yield. To improve the yield, an alternative two-step process was developed. In this case, a one-pot three-enzyme sialylation reaction39 was carried out for overnight using equal molar amounts of lactose and ManNGc before the addition of SpNanC. Using this two-step process, the yield for Neu5Gc2en (7) was improved to 60% (Table 2). A similar two-step process applied for Neu5Ac2en (6) production led to the improvement of the yield to 82% from 72% using the one-step process.
Table 2.
Sia2ens Obtained by OPME Synthesis
| Substrate | Product | Yielda | Yieldb |
|---|---|---|---|
ManNAc (1)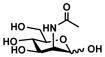
|
Neu5Ac2en (6)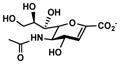
|
72% | 82% |
ManNGc (2)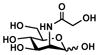
|
Neu5Gc2en (7)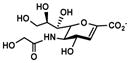
|
<5%c | 60% |
ManNAc6N3 (3)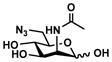
|
Neu5Ac9N32en (8)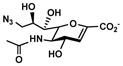
|
61% | 79% |
ManNAc6NAc (4)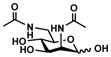
|
Neu5Ac9NAc2en (9)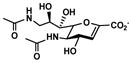
|
63% | 71% |
ManNAc4deoxy (5)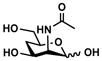
|
Neu5Ac7deoxy2en (10)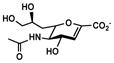
|
11% | 25% |
One-step process.
Two-step process.
Estimated by TLC analysis.
Other than Neu5Gc2en which is a C-5 derivative of Neu5Ac2en, two C-9 derivatives of Neu5Ac2en were successfully synthesized. Neu5Ac9N32en (8)33, 35 containing a 9-azido group was synthesized from ManNAc6N3 (3)42 with a yield of 61% using the one-step process. The two-step process improved the yield to 79%. On the other hand, Neu5Ac9NAc2en (9)33 containing a 9-acetamido group was synthesized from ManNAc6NAc (4)51 in a yield of 63% using the one-step process and a yield of 71% was obtained using the two-step process. Neu5Ac9NAc2en (9) is a more stable analog of Neu5Ac9OAc2en51 and is a promising inhibitor against sialidases that prefer to cleave 9-O-acetyl Neu5Ac.
The OPME system was also used for synthesizing Neu5Ac7deoxy2en (10), a C-7 derivative of Neu5Ac2en.52–53 From ManNAc4deoxy (5),47 Neu5Ac7deoxy2en (10) was obtained in a low 11% yield using the one-step process and the two-step process improved the yield to 25%. The lower yield was due to the lower efficiency in sialoside formation.
In general, the one-pot process was time efficient and the reactions were usually completed in 2–6 h. In comparison, the two-step process required a longer 12–20 h duration but usually led to improved yields, especially for sialic acid modifications causing the slowing down of the sialoside formation process. The enzymatic approach compared favorably to the traditional chemical methods which used organic solvents and involved multiple protection and deprotection steps. For example, chemical synthesis of Neu5Ac9NAc2en (9) from Neu5Ac involved nine steps and multiple purification processes with an overall yield of 9–14%.33 For the chemoenzymatic approach described here, ManNAc6NAc (4) was chemically synthesized from ManNAc via ManNAc6N3 (3)42 intermediate in three steps with a 48% overall yield.51 Considering the 63% or 71% yield for the one-step or two-step OPME synthetic process (Table 2), the overall yield for Neu5Ac9NAc2en (9) was 30% or 34% starting from ManNAc without the need of purifying the intermediates of enzymatic reactions.
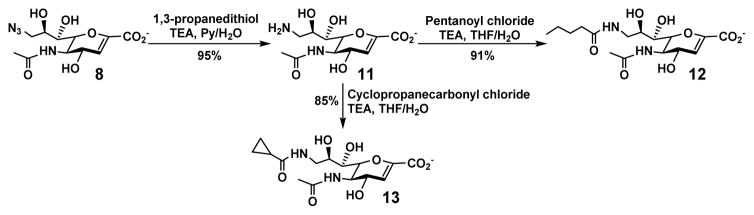
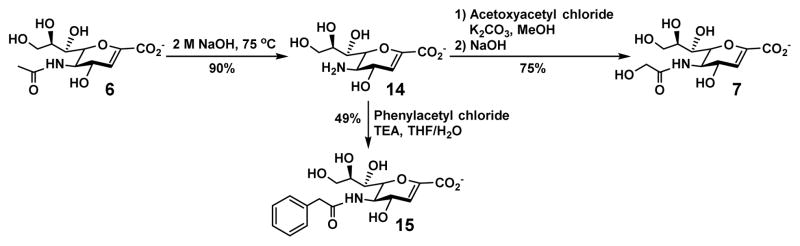
The C9-azido group of Neu5Ac9N32en (8) allowed easy derivatization via Neu5Ac9NH22en (11) which was obtained by reduction of the azido group to an amino group using 1,3-propanedithiol (Scheme 2). Installation of various acyl groups was achieved conveniently to form Neu5Ac9NPent2en (12)33 with an improved selectivity against human NEU133 and Neu5Ac9NCyclopro2en (13)33 with a higher selectivity against viral sialidases.54 The C-5 position of Neu5Ac2en (6) was also easily derivatized by removal of the N-acetyl group using an aqueous sodium hydroxide solution (2 M) to produce Neu2en (14) with a free amino group at C-5 which allowed facile acylation (Scheme 3). Neu5Gc2en (7) and Neu5PhAc2en (15)55 were conveniently obtained by this approach.
Scheme 2.
Derivatization at C-9 of Neu5Ac9N32en (8)
Scheme 3.
Derivatization at C-5 of Neu5Ac2en (6)
The obtained Sia2ens except for Neu5Ac9N32en (8)35 were tested as inhibitors against several glycoside hydrolase GH33 family sialidases and a glycosyltransferase GT80 sialyltransferase with α2–3-sialidase activity categorized in the Carbohydrate Active Enzyme (CAZy) database (www.cazy.org).56–58 These include human cytosolic sialidase hNEU234 and several bacterial sialidases such as recombinant sialidases cloned from Streptococcus pneumoniae (SpNanA,59 SpNanB,59 and SpNanC60), Pasteurella multocida (PmST1, a bacterial sialyltransferase which also has α2–3-sialidase activity),42 Bifidobacterium infantis (BiNanH2),61 as well as commercially available sialidases from Arthrobacter ureafaciens (AuSialidase), Clostridium perfringens (CpNanH and CpNanI), and Vibrio cholerae (VcSialidase). Inhibition studies using Neu5Acα2–3GalβpNP as the sialidase substrate44, 62–63 and 0.1 mM of each inhibitor showed (Table S1) that Neu5Ac2en (6) and its derivatives at C-5 (7, 15), C-9 (9, 12, 13), and C-7 (10) were not effective inhibitors against SpNanB, SpNanC, CpNanH,64–65 or PmST1. On the other hand, among all compounds tested, Neu5Ac2en (6) was the least selective one. At 0.1 mM, it had more than 50% inhibition against SpNanA, AuSialidase, CpNanI, VcSialidase, BiNanH2, and hNEU2. C9-Derivatization of Neu5Ac2en (compounds 9, 12, 13) lowered the inhibitory activity against AuSialidase, VcSialidase, and hNEU2. In comparison, C5-derivatization of Neu5Ac2en also improved the inhibitory selectivity. For example, at 0.1 mM, Neu5Gc2en (7) retained more than 50% inhibition against only VcSialidase and hNEU2, similar to that reported previously.34 Neu5PhAc2en (15) retained inhibitory activity against hNEU2 only, but not any bacterial sialidases tested. The previously identified selectivity of bacterial sialidases toward C7-deoxy Neu5Ac based on substrate specificity studies47 was confirmed by the selective inhibitory activity of Neu5Ac7deoxy2en (10) against bacterial sialidases including SpNanA, AuSialidase, and CpNanI, but not hNEU2. Nevertheless, it was not an effective inhibitor against VcSialidase or BiNanH2.
For inhibitors with more than 65% inhibitory activity against certain sialidases at 0.1 mM concentration, IC50 values were obtained. As shown in Table 3, derivatization at C-9 (compounds 9, 12, 13) did not significantly alter the inhibitory activity of Neu5Ac2en (6) against SpNanA or BiNanH2 and the IC50 values retained in the ranges of 6.8–11.0 μM and 29–40 μM, respectively. The C7-deoxy modification in Neu5Ac7deoxy2en (10) did not alter the inhibition activity against SpNanA, but decreased inhibitory activity against AuSialidase resulting in an IC50 value of 17 μM (increased from 8.1 μM).
Table 3.
IC50 Values of Sia2ens Against Bacterial Sialidases SpNanA, AuSialidase, and BiNanH2
| Sialidases | IC50 values of different inhibitors (μM) | ||||
|---|---|---|---|---|---|
| 6 | 9 | 10 | 12 | 13 | |
| SpNanA | 10.0±0.2 | 11.0±0.2 | 10.7± 0.4 | 10.5±0.5 | 6.8±0.2 |
| AuSialidase | 8.1±0.3 | - | 17 ± 1 | - | - |
| BiNanH2 | 40±2 | 33±1 | - | 34±2 | 29±2 |
In conclusion, using the special property of SpNanC-catalyzed reaction, a new one-pot multienzyme strategy has been successfully developed for the synthesis of sialidase transition state analog inhibitors, 2,3-dehydro-2-deoxy-sialic acids (Sia2ens), and analogs. Such compounds can be further derivatized at various positions. Inhibition studies demonstrated improved selectivity of several Neu5Ac2en (6) derivatives with modifications at C-5, C-9, or C-7 against several bacterial sialidases.
Supplementary Material
Acknowledgments
This work was partially supported by National Institutes of Health (NIH) Common Fund grant U01GM120419 and NIH grant R01AI130684.
Footnotes
Notes
Y.L., H.Y., and X.C. are co-founders of Glycohub, Inc., a company focused on the development of carbohydrate-based reagents, diagnostics, and therapeutics. Glycohub, Inc. played no role in the design, execution, interpretation, or publication of this study.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscatal.xxxx
Cloning, expression, purification, and characterization of SpNanC; detailed synthetic procedures, nuclear magnetic resonance (NMR) spectroscopy and high-resolution mass spectrometry (HRMS) data, and NMR spectra for products (PDF)
References
- 1.Taylor G. Curr Opin Struct Biol. 1996;6:830–837. doi: 10.1016/s0959-440x(96)80014-5. [DOI] [PubMed] [Google Scholar]
- 2.Monti E, Preti A, Venerando B, Borsani G. Neurochem Res. 2002;27:649–663. doi: 10.1023/a:1020276000901. [DOI] [PubMed] [Google Scholar]
- 3.Corfield T. Glycobiology. 1992;2:509–521. doi: 10.1093/glycob/2.6.509. [DOI] [PubMed] [Google Scholar]
- 4.Lewis AL, Lewis WG. Cell Microbiol. 2012;14:1174–1182. doi: 10.1111/j.1462-5822.2012.01807.x. [DOI] [PubMed] [Google Scholar]
- 5.Air GM, Laver WG. Proteins. 1989;6:341–356. doi: 10.1002/prot.340060402. [DOI] [PubMed] [Google Scholar]
- 6.Colman PM. Protein Sci. 1994;3:1687–1696. doi: 10.1002/pro.5560031007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Von Itzstein M. Nat Rev Drug Discov. 2007;6:967–974. doi: 10.1038/nrd2400. [DOI] [PubMed] [Google Scholar]
- 8.Air GM, Ghate AA, Stray SJ. Adv Virus Res. 1999;54:375–402. doi: 10.1016/s0065-3527(08)60372-3. [DOI] [PubMed] [Google Scholar]
- 9.Meanwell NA, Krystal M. Drug Discov Today. 1996;1:388–397. doi: 10.1016/s1359-6446(00)01500-2. [DOI] [PubMed] [Google Scholar]
- 10.Islam T, Von Itzstein M. Adv Carbohydr Chem Biochem. 2007;61:293–352. doi: 10.1016/S0065-2318(07)61006-3. [DOI] [PubMed] [Google Scholar]
- 11.Du J, Cross TA, Zhou HX. Drug Discov Today. 2012;17:1111–1120. doi: 10.1016/j.drudis.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varghese JN, McKimm-Breschkin JL, Caldwell JB, Kortt AA, Colman PM. Proteins. 1992;14:327–332. doi: 10.1002/prot.340140302. [DOI] [PubMed] [Google Scholar]
- 13.Wei DQ, Du QS, Sun H, Chou KC. Biochem Biophys Res Commun. 2006;344:1048–1055. doi: 10.1016/j.bbrc.2006.03.210. [DOI] [PubMed] [Google Scholar]
- 14.Yuan P, Thompson TB, Wurzburg BA, Paterson RG, Lamb RA, Jardetzky TS. Structure. 2005;13:803–815. doi: 10.1016/j.str.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Chavas LM, Tringali C, Fusi P, Venerando B, Tettamanti G, Kato R, Monti E, Wakatsuki S. J Biol Chem. 2005;280:469–475. doi: 10.1074/jbc.M411506200. [DOI] [PubMed] [Google Scholar]
- 16.Burmeister WP, Henrissat B, Bosso C, Cusack S, Ruigrok RW. Structure. 1993;1:19–26. doi: 10.1016/0969-2126(93)90005-2. [DOI] [PubMed] [Google Scholar]
- 17.Xu G, Potter JA, Russell RJ, Oggioni MR, Andrew PW, Taylor GL. J Mol Biol. 2008;384:436–449. doi: 10.1016/j.jmb.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 18.Newstead SL, Potter JA, Wilson JC, Xu G, Chien CH, Watts AG, Withers SG, Taylor GL. J Biol Chem. 2008;283:9080–9088. doi: 10.1074/jbc.M710247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crennell SJ, Garman EF, Laver WG, Vimr ER, Taylor GL. Proc Natl Acad Sci U S A. 1993;90:9852–9856. doi: 10.1073/pnas.90.21.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Itzstein M, Wu WY, Kok GB, Pegg MS, Dyason JC, Jin B, Van Phan T, Smythe ML, White HF, Oliver SW. Nature. 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 21.von Itzstein M, Wu WY, Jin B. Carbohydr Res. 1994;259:301–305. doi: 10.1016/0008-6215(94)84065-2. [DOI] [PubMed] [Google Scholar]
- 22.Hayden FG, Treanor JJ, Betts RF, Lobo M, Esinhart JD, Hussey EK. Jama. 1996;275:295–299. [PubMed] [Google Scholar]
- 23.Kim CU, Lew W, Williams MA, Liu H, Zhang L, Swaminathan S, Bischofberger N, Chen MS, Mendel DB, Tai CY. J Am Chem Soc. 1997;119:681–690. doi: 10.1021/ja963036t. [DOI] [PubMed] [Google Scholar]
- 24.Kim CU, Lew W, Williams MA, Wu H, Zhang L, Chen X, Escarpe PA, Mendel DB, Laver WG, Stevens RC. J Med Chem. 1998;41:2451–2460. doi: 10.1021/jm980162u. [DOI] [PubMed] [Google Scholar]
- 25.Chen GY, Chen X, King S, Cavassani KA, Cheng J, Zheng X, Cao H, Yu H, Qu J, Fang D, Wu W, Bai XF, Liu JQ, Woodiga SA, Chen C, Sun L, Hogaboam CM, Kunkel SL, Zheng P, Liu Y. Nat Biotechnol. 2011;29:428–435. doi: 10.1038/nbt.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen GY, Brown NK, Wu W, Khedri Z, Yu H, Chen X, van de Vlekkert D, D’Azzo A, Zheng P, Liu Y. eLife. 2014;3:e04066. doi: 10.7554/eLife.04066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albohy A, Zhang Y, Smutova V, Pshezhetsky AV, Cairo CW. ACS Med Chem Lett. 2013;4:532–537. doi: 10.1021/ml400080t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nitabaru T, Kumagai N, Shibasaki M. Angew Chem Int Ed. 2012;51:1644–1647. doi: 10.1002/anie.201108153. [DOI] [PubMed] [Google Scholar]
- 29.Tian J, Zhong J, Li Y, Ma D. Angew Chem Int Ed. 2014;53:13885–13888. doi: 10.1002/anie.201408138. [DOI] [PubMed] [Google Scholar]
- 30.Kiefel MJ, von Itzstein M. Chem Rev. 2002;102:471–490. doi: 10.1021/cr000414a. [DOI] [PubMed] [Google Scholar]
- 31.Laborda P, Wang SY, Voglmeir J. Molecules. 2016;21 doi: 10.3390/molecules21111513. pii: E1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hemeon I, Bennet AJ. Synthesis. 2007;2007:1899–1926. [Google Scholar]
- 33.Magesh S, Moriya S, Suzuki T, Miyagi T, Ishida H, Kiso M. Bioorg Med Chem Lett. 2008;18:532–537. doi: 10.1016/j.bmcl.2007.11.084. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Cao H, Yu H, Chen Y, Lau K, Qu J, Thon V, Sugiarto G, Chen X. Mol BioSyst. 2011;7:1060–1072. doi: 10.1039/c0mb00244e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khedri Z, Li Y, Cao H, Qu J, Yu H, Muthana MM, Chen X. Org Biomol Chem. 2012;10:6112–6120. doi: 10.1039/c2ob25335f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pettigrew MM, Fennie KP, York MP, Daniels J, Ghaffar F. Infect Immun. 2006;74:3360–3365. doi: 10.1128/IAI.01442-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu G, Kiefel MJ, Wilson JC, Andrew PW, Oggioni MR, Taylor GL. J Am Chem Soc. 2011;133:1718–1721. doi: 10.1021/ja110733q. [DOI] [PubMed] [Google Scholar]
- 38.Owen CD, Lukacik P, Potter JA, Sleator O, Taylor GL, Walsh MA. J Biol Chemy. 2015;290:27736–27748. doi: 10.1074/jbc.M115.673632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu H, Chokhawala HA, Huang S, Chen X. Nat Protoc. 2006;1:2485–2492. doi: 10.1038/nprot.2006.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Yu H, Cao H, Lau K, Muthana S, Tiwari VK, Son B, Chen X. Appl Microbiol Biotechnol. 2008;79:963–970. doi: 10.1007/s00253-008-1506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu H, Yu H, Karpel R, Chen X. Bioorg Med Chem. 2004;12:6427–6435. doi: 10.1016/j.bmc.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 42.Yu H, Chokhawala H, Karpel R, Yu H, Wu B, Zhang J, Zhang Y, Jia Q, Chen X. J Am Chem Soc. 2005;127:17618–17619. doi: 10.1021/ja0561690. [DOI] [PubMed] [Google Scholar]
- 43.Sugiarto G, Lau K, Qu J, Li Y, Lim S, Mu S, Ames JB, Fisher AJ, Chen X. ACS Chem Biol. 2012;7:1232–1240. doi: 10.1021/cb300125k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chokhawala HA, Yu H, Chen X. Chembiochem. 2007;8:194–201. doi: 10.1002/cbic.200600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao H, Li Y, Lau K, Muthana S, Yu H, Cheng J, Chokhawala HA, Sugiarto G, Zhang L, Chen X. Org Biomol Chem. 2009;7:5137–5145. doi: 10.1039/b916305k. [DOI] [PubMed] [Google Scholar]
- 46.Khedri Z, Muthana MM, Li Y, Muthana SM, Yu H, Cao H, Chen X. Chem Commun. 2012;48:3357–3359. doi: 10.1039/c2cc17393j. [DOI] [PubMed] [Google Scholar]
- 47.Khedri Z, Li Y, Muthana S, Muthana MM, Hsiao CW, Yu H, Chen X. Carbohydr Res. 2014;389:100–111. doi: 10.1016/j.carres.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parker RB, McCombs JE, Kohler JJ. ACS Chem Biol. 2012;7:1509–1514. doi: 10.1021/cb300241v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bardor M, Nguyen DH, Diaz S, Varki A. J Biol Chem. 2005;280:4228–4237. doi: 10.1074/jbc.M412040200. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, Yu H, Cao H, Muthana S, Chen X. Appl Microbiol Biotechnol. 2012;93:2411–2423. doi: 10.1007/s00253-011-3579-6. [DOI] [PubMed] [Google Scholar]
- 51.Khedri Z, Xiao A, Yu H, Landig CS, Li W, Diaz S, Wasik BR, Parrish CR, Wang LP, Varki A, Chen X. ACS Chem Biol. 2017;12:214–224. doi: 10.1021/acschembio.6b00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zbiral E, Schreiner E, Christian R, Kleineidam RG, Schauer R. Liebigs Annalen der Chemie. 1989;1989:159–165. [Google Scholar]
- 53.Honda T, Masuda T, Yoshida S, Arai M, Kobayashi Y, Yamashita M. Bioorg Med Chem Lett. 2002;12:1921–1924. doi: 10.1016/s0960-894x(02)00328-1. [DOI] [PubMed] [Google Scholar]
- 54.Magesh S, Sriwilaijaroen N, Moriya S, Ando H, Miyagi T, Suzuki Y, Ishida H, Kiso M. Int J Med Chem. 2011;2011:539245 1–7. doi: 10.1155/2011/539245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chand P, Babu YS, Rowland SR, Lin T-H. WO2002076971. Biocryst Pharmaceuticals, Inc; 2002
- 56.Henrissat B. Biochem J. 1991;280:309–316. doi: 10.1042/bj2800309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henrissat B, Bairoch A. Biochem J. 1993;293:781–788. doi: 10.1042/bj2930781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Henrissat B, Bairoch A. Biochem J. 1996;316:695–696. doi: 10.1042/bj3160695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tasnima N, Yu H, Li Y, Santra A, Chen X. Org Biomol Chem. 2016;15:160–167. doi: 10.1039/c6ob02240e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li W, Xiao A, Li Y, Yu H, Chen X. Carbohydr Res. 2017;451:51–58. doi: 10.1016/j.carres.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sela DA, Li Y, Lerno L, Wu S, Marcobal AM, German JB, Chen X, Lebrilla CB, Mills DA. J Biol Chem. 2011;286:11909–11918. doi: 10.1074/jbc.M110.193359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eschenfelder V, Brossmer R. Carbohydr Res. 1987;162:294–297. doi: 10.1016/0008-6215(87)80224-0. [DOI] [PubMed] [Google Scholar]
- 63.Kodama H, Baum LG, Paulson JC. Carbohydr Res. 1991;218:111–119. doi: 10.1016/0008-6215(91)84090-2. [DOI] [PubMed] [Google Scholar]
- 64.Peter R, Reinhard G, Roland S. Biol Chem Hoppe-Seyler. 1995;376:569–576. [Google Scholar]
- 65.Li J, McClane BA. Appl Environ Microbiol. 2014;80:1701–1709. doi: 10.1128/AEM.03440-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.