Abstract
Purpose of review
Ubiquitously-expressed small GTPase Rap1 is a key modulator of integrin- and cadherin-regulated processes. In endothelium Rap1 promotes angiogenesis and endothelial barrier (EB) function, acting downstream from cAMP-activated Rap1GEF, Epac. Recent in vivo studies in mouse models have provided more information about the physiological role of Rap1 in vessel development and after birth under normal and pathologic conditions. Important molecular details of dynamic regulation of EB are uncovered.
Recent findings
Rap1 is not essential for initial vessel formation but is critical for vessel stabilization, as double knockout of the two Rap1 isoforms leads to hemorrhage and embryonic lethality. After development, Rap1 is not required for EB maintenance but is critical for NO production and endothelial function. Radil and Afadin mediate Rap1 effects on EB function by regulating connection with Rho GTPases, actomyosin cytoskeleton and cell-cell adhesion receptors.
Summary
Rap1 is critically required for NO release and normal endothelial function in vivo. Mechanistic studies lead to a novel paradigm of Rap1 as a critical regulator of EC shear stress responses and endothelial homeostasis. Increased understanding of molecular mechanisms underlying EB regulation may identify novel pharmacological targets for retinopathies and conditions with altered EB function or when increased EB is desired.
Keywords: endothelial homeostasis, shear stress sensing, vessel formation, mechanotransduction, signal transduction
INTRODUCTION
Evolutionarily conserved and ubiquitously expressed small GTPase Rap1 has been well characterized as a key modulator of integrin- and cadherin-regulated processes. Spatio-temporally regulated by a network of guanine nucleotine exchange factors (GEFs) and GTPase activating protein (GAPs), in a context-specific manner, GTP-bound Rap1 interacts with a number of effectors controlling integrin-mediated cell-ECM adhesion, cell-cell junction formation and cellular polarity (1, 2). In endothelium Rap1 is a key positive regulator of angiogenesis (3, 4) and an important regulator of endothelial barrier (EB) (5) acting as a mediator of elevated cAMP/cAMP-dependent Rap1 GEF, Epac activation (6). Recent research has increased the understanding of the role of Rap1 in vascular development, normal and pathological angiogenesis and EB enhancement under pathological conditions in vivo, and molecular mechanisms underlying dynamic regulation of EB with a particular emphasis on the connection with Rho GTPases and the actomyosin cytoskeleton. Importantly, recent research uncovered a novel and critically important physiological role of Rap in regulation of NO release and endothelial function and implicated Rap1 as a key regulator of mechanotransduction in endothelial cells (ECs) by regulation of shear stress-mediated signaling in response to flowing blood.
Vascular development and angiogenesis
Vessel development and developmental angiogenesis
The role of Rap1a and Rap1b, the two Rap1 isoforms present in higher organisms in vascular development has been studied in several mouse models. Single global knockout of either Rap1 isoform leads to embryonic lethality of the majority of embryos and bleeding (7–9), defects in hematopoiesis, neurological and immune responses and defective angiogenesis (10, 11), also present in endothelial lineage-restricted (Tie2-Cre) Rap1b knockouts (12). While the development of mice lacking single Rap1 isoform in the endothelium is normal, EC-restricted knockout of both isoforms leads to developmental abnormality and lethality of ~50% embryos at mid-gestation, while the remaining embryos appear normal. However, all double knockout embryos die before E13.5, likely due to hemorrhage (13). Therefore, Rap1 in endothelium is indispensable for vascular stability and the formation of functional vasculature. Interestingly, mice with one Rap1a allele (EC-Rap1a+/−Rap1b−/−) exhibited embryonic hemorrhage and significantly reduced viability at weaning, a phenotype absent in mice with one remaining Rap1b allele (EC-Rap1a−/−Rap1b+/−), signifying relative importance of the Rap1b isoform for normal vessel formation. Remarkably, post-development, deletion of both Rap1 isoforms does not have a deleterious effect on vessel permeability or gross vessel and cell-cell junction morphology (8). Therefore, Rap1 is not absolutely required for maintenance of existing cell-cell junctions and preservation of EB.
Interestingly, murine EC-specific knockout of Ras-interacting protein 1 (Rasip1), a 104kD effector of H-Ras, K-Ras and Rap1 (14), leads to a more severe vascular embryonic phenotype with focal hemorrhage and lethality due to vessels unable to sustain circulation beyond mid-gestation (15, 16). Implicated as a junctional effector of the Epac-Rap1 signaling axis, the Rasip1-ArhGAP-RhoA pathway had been previously suggested to regulate EC tubulogenesis and establishment of endothelial polarity (15), however patent vessels are formed in EC-Rasip1 KO mice, to collapse later in development (16). Therefore, unlike Rap1, Rasip1 is critical for early vasculogenesis.
Rap1 is also a positive regulator of angiogenesis in vivo (previously reviewed in (3, 4), with an adaptor protein Afadin acting as one of its effectors. Afadin, a 205 kD adaptor molecule localized to cell-cell junctions connects actin cytoskeleton with cell-cell junctional receptors. In addition to RA domain through which it binds Rap1 and a PDZ domain through which it interacts with transmembrane proteins (17), afadin contains forkhead-associated and dilute domains, also shared with a relative, Rasip1 (14).
Afadin, regulates VEGF and S1P signaling and modulates postnatal retinal angiogenesis (18), but is not essential for developmental endothelial angiogenesis (19). It is, however, essential for normal embryonic development of lymphatic vessels in mice as its deficiency in Tie2-Cre driven conditional KO mice leads to severe subcutaneous edema, lymphatic vessel malformation, disruption of VE-cadherin junctions, and embryonic lethality after day E15.5. Underlying the lymphangiogenic defects are disrupted VE-cadherin junctions in vivo and elevated Rho activity in lymphatic ECs (but not vascular ECs) in vitro (19). This suggests cell-type specificity of Afadin as a Rap1 effector.
In vivo studies have defined physiological significance of Rap1 in endothelium: during development, Rap1, with its partners Rasip1 and, in lymphatic vessels, Afadin, are essential for full development of functional, stable vessels. After blood vessels form, Rap1 is not essential for the adhesive function and normal gross vessel morphology, but plays a major role in endothelial homeostasis by transducing shear stress signals critical for EC function, as described below.
Angiogenic processes
Rap1 promotes cellular processes underlying angiogenic response, including integrin-mediated adhesion, migration, tube formation (3) and formation of tip cells at the leading edge of a developing vascular bed (12, 20) and in ECs in vitro (21) (Figure 1). A recent study identified Polo-like Kinase 2 (Plk2) as a regulator of VEGF-dependent endothelial tip cells progression in zebrafish in vivo and in lamellipodia formation in HUVECs (22). Plk2, a member of the serine-threonine kinase family containing a conserved polo domain responsible for its localization and involved in regulation of cell cycle, had been previously implicated in promoting Rap1 activity in regulation of synaptic plasticity (23). Plk2 phosphorylation promotes PDZ-GEF activity towards Rap1 in neurons, and similarly, Plk2 binds to PDZ-GEF to regulate Rap1 localization and activity in ECs during lamellipodia formation. where it is required for angiogenesis in vivo (22). Interestingly, PDZ-GEF is critical for vessel formation and its deletion leads to early lethality in mice (24). Therefore, Plk2-PDZ-GEF-Rap1 signaling axis is essential for angiogenesis.
Figure 1. Rap1 is critical for shear stress sensing, angiogenic responses and control of endothelial barrier.
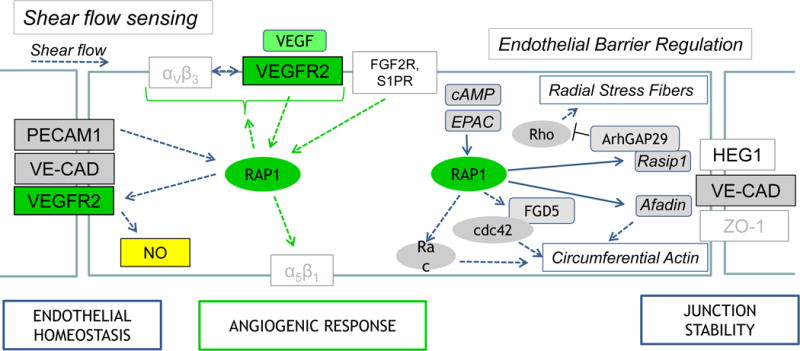
Rap1, activated by shear stress, is required for NO production downstream from the mechanosensing complex comprised of PECAM-1, VE-cadherin (VE-CAD) and VEGFR2. By controlling NO release, Rap1 signaling regulates endothelial homeostasis. Rap1 regulates angiogenic responses by promoting VEGF Receptor 2 (VEGFR2) activation in a process dependent on integrin αVβ3, and as a downstream effector of angiogenic growth factor receptors (FGFR2, S1PR and VEGFR2); integrin α5β1 is only one of the downstream effectors, others are not shown. Activated by cAMP-dependent GEF, Epac, Rap1 promotes junctional stability via its effectors Rasip1 and Radil (not shown) which, translocated to junctions by Heart-of-Glass (HEG1) receptor, inhibit Rho-mediated Radial Stress Fiber formation, while cdc42 and Afadin promote circumferential actin bundle formation increasing junctional stability. Afadin also promotes junctional tightening by mediating attachment between tight and adherens junctions. Other Rap1 effectors, such as Krit1 (not shown) are also involved in this process.
Pathological angiogenesis in the retina
While a positive regulator of developmental angiogenesis, Rap1’s role in pathological angiogenesis appears more complex, as Rap1is involved in vessel stabilizing and inhibitory processes. Pathological processes of inflammation, oxidation and angiogenesis in retinal pigmented epithelial (RPE) and choroidal endothelial cells (CECs) underlie the development of neovascular age-related macular degeneration (AMD), a leading cause of blindness in the elderly (25). Visual acuity loss occurs when activated CECs migrate through Bruch’s membrane and across the retinal pigment epithelium (RPE) into the sensory retina to proliferate into choroidal neovascularization (CNV) (26). Epac-mediated activation of Rap1 in both these cellular compartments leads to decreased CNV. In RPEs, Epac1-dependent Rap1a activation promotes RPE barrier formation by inhibiting NADPH oxidase-generated ROS (27). By a similar mechanism in CECs, activated Rap1a inhibits NADPH oxidase -dependent ROS production induced by proinflammatory stimuli (28). In both cases, Rap1 exerts these effects by inhibiting Rac1-dependent NADPH oxidase activity. Therefore, activating Rap1a has been proposed as a novel therapeutic approach for AMD (26).
On the other hand, excessive neovascularization of the retina is associated with proliferative retinopathies, such as diabetic and retinopathy of prematurity (ROP). Studies in Junctional Adhesion Molecule (JAM)-C KO mice, which negatively regulates Rap1 activation in endothelium (29), revealed that activation of Rap1 via inhibition of JAM-C, promotes endothelial adhesion. This occurs via integrin β1 and tip cell formation of vessels in the avascular area and vessel normalization in ROP, and has been proposed as a therapeutic approach for ROP (20). These studies emphasize context-specific effects of Rap1 on cellular and physiological processes.
Novel physiological function of Rap1: regulation of vascular tone, NO release and EC shear stress responses
Rap1 in endothelium controls vascular tone and NO release
Our recent analysis of tissue-specific knockout mice revealed that, via separate mechanisms in smooth muscle and endothelium, Rap1 controls vascular tone and is required for maintenance of normal blood pressure (8, 30). We found that total Rap1b-deficiency leads to hypertension and pathological cardiac hypertrophy. We discovered that in smooth muscle, Rap1-deficiency leads to increased contractility under basal conditions and decreased cAMP-dependent relaxation in response to Rap1 activator, Epac1 (30). Interestingly, we also found that selective deletion of both Rap1 isoforms in endothelium leads to an even more severe phenotype of hypertension, cardiac hypertrophy, and early lethality of male mice, the most severe endothelial phenotype described so far (4). Endothelium plays a key role in response to increased blood flow by releasing nitric oxide, which leads to vessel relaxation. Defective endothelial release of dilatory NO in response to elevated blood flow leads to hypertension. We found that deletion of both Rap1 isoforms in endothelium leads to a severe attenuation of NO release in response to shear stress and severely inhibits NO-dependent vasodilation (8). Underlying defective NO release is impaired ability of Rap1-KO EC to sense shear stress via endothelial junctional mechanosensing complex involving VE-cadherin, as described below. These findings point to a novel, critical role of Rap1 in regulation of endothelial homeostasis, as decreased NO bioavailability leads to endothelial dysfunction, which is associated with proinflammatory states and underlies the pathogenesis of cardiovascular disease (31).
Mechanotransduction of shear stress signals from flowing blood
In addition to promoting VEGF-dependent VEGFR2-mediated angiogenic responses and VEGF-dependent VEGFR2 activation (12), our recent research identified Rap1 as a key regulator of shear stress-induced VEGFR2 activation (8) (Figure 1). Essential EC responses, including NO release, are significantly regulated by shear stress of flowing blood (32). The mechanisms of sensing shear stress involve the formation of endothelial mechanosensing complex comprised of PECAM-1, VE-cadherin and VEGFR2 (33), which is required for release of NO, a key regulator of endothelial homeostasis. We found that in response to shear stress, Rap1, activated downstream from PECAM-1, promotes the formation of the mechanosensing complex, VEGFR2 transactivation, association with PI3K and downstream signaling to eNOS activation and NO production (8) (Figure 1). While the exact molecular details are not fully understood, Afadin-knockout leads to a similar signaling defect in PI3K-VEGFR2 association in ECs, and a defect in post-natal VEGF-dependent angiogenesis in vivo (18). Additionally, a mechanism of Rap1-mediated regulation of VEGFR2 downstream signaling to PI3K involving Afadin has been proposed (34). Mechanistically, this finding establishes a novel paradigm for Rap1 as a regulator of mechanotransduction in ECs that may be applicable to other cell types; as Rap1 activation in response to turbulence has been reported in hematopoietic cells (35) and in response to stretching in permeabilized fibroblasts (36). Physiological significance of this novel Rap1 function as a regulator of mechanosensing is clinically significant because hemodynamic shear stress is physiologically the most significant NO inducer. Defects in NO bioavailability lead to EC dysfunction, and are associated with CV disease (37, 38). Furthermore, defective shear stress sensing in EC-specific Rap1 knockout embryos may underlie physiologic/hemodynamic abnormality that leads to hemorrhage and lethality (13).
C. Dynamic regulation of EB
Since the discovery of the effect of activated Rap1 on promoting EC barrier function (39–42), research has focused on the underlying mechanisms, especially in response to elevated cAMP. cAMP, one of the most potent stabilizers of EB, acts via Rap1 GEF Epac and PKA in signaling pathways that converge on Rac1 (6, 43, 44) (Figure 1). It is now better understood that Rap1 activity is dynamically regulated during different stages of barrier control (5), via specific GEFs and upstream activators. Once activated Rap1, regulates EB function via two major mechanisms: (1) directly via enforcement of VE-adhesiveness (Rap1 is also activated downstream from VE-cadherin) and VE-mediated endothelial junction integrity, and (2) indirectly, via dynamic regulation of the actomyosin cytoskeleton and Rho-dependent tension.
Rap1, via discrete effectors spatially controls actomyosin contractility to regulate EB
EB function is dynamically regulated by rearrangement of the actin cytoskeleton, with circumferential actin bundles (CAB) promoting adherens junction (AJ) formation and EC junction tightening, while the induction of permeability leads to formation of radial stress fibers (RSF) connected to punctate AJs (45). The balance between the two types of SFs, punctate AJs, generating “cytoskeletal tension”, and CABs attached to linear junctions and dependent on non-muscle IIB (NM-IIB) binding, generating “junctional tension” (5), determines EB function. This balance is differentially regulated by external factors; inflammatory stimuli promote RSFs and junction dissociation, while factors leading to elevation of membrane-associated cAMP promote CABs. In epithelial cells NM-IIB localization at cell-cell contacts and CAB formation was shown to be regulated by Rap1 (46). A similar mechanism was recently implicated in ECs (47, 48). In ECs Rap1 induces NM-II activation at cell-cell contacts and CAP formation through FGD5-dependent activation of the Cdc42-MRCK pathway. In response to cAMP elevation, Rap1 suppresses NM-II activation by Rho-ROCK pathway, preventing ROCK-dependent RSF induction. This process is mediated by Rap1 effectors Rasip1 and its close relative, a more widely expressed 117 kD Ras-association and dilute domain-containing protein (Radil), originally identified as Rap1a effector in promoting integrin-mediated cell adhesion and migration (49). Upon Rap1 activation Rasip1 and Radil, via their RA domains associate with Rap1, and with Heart of Glass (HEG1) receptor, which transports them to the plasma membrane (50), where they form a multimeric complex with RhoGAP ArhGAP29. This then inhibits Rho signaling and reduces actomyosin-induced tension on AJs (47, 48, 51) (Figure 1). In vivo phenotype of Rasip1 KO mice is consistent with the proposed model, with altered junctional actin organization and altered localization of actin-bundling protein nonmuscle myosin heavy chain IIB and junctional remodeling (16).
Rap1 promotes junctional adhesiveness via Afadin
Scaffolding properties of Afadin play a role in connecting actomyosin cytoskeleton to cadherins and between AJs and tight junctions. Afadin, in a Rap1-dependent manner, promotes barrier enhancement by mediating a cross talk between tight junctions and AJ by interacting with ZO1–1 and p120-catenin, respectively (52, 53). Activated by C3G during barrier recovery after thrombin dissolution, Rap1, via Afadin, leads to enhanced interaction between AJ proteins VE-cadherin and p120-catenin, stimulating AJ reannealing and leading to inhibition of Rho in a Rac1-Rac1 GEF, TIAM-dependent manner (53) (Figure 1). Thus, by binding both stress fibers and the cortical actin ring, Afadin acts as an important sensor of mechanical forces reinforcing EC junctions (54). In epithelial cells, together with ZO1, Afadin regulates tension and maintains junction architecture in response to changes in contractility (55).
Rap1 enhances EC barrier under inflammatory conditions
In vitro stimulation of Epac1 in cultured ECs promotes barrier tightening and prevents increased permeability in vivo. However, Epac activation does not lead to reduced basal permeability in intact microvessels under normal conditions; strong evidence supports the notion that under resting conditions, cAMP sustains the EB primarily via PKA-dependent mechanisms, whereas the Epac pathway is more relevant for stimulated barrier protection (6, 43). In vivo studies have revealed the relative role of Rap1 in EC barrier maintenance in normal and challenged conditions. While in adult vasculature Rap1 is not essential for EB maintenance, as evidenced by normal appearance of EC junctions in vessels from Rap1-deficient mice (8), Rap1 is important for regulation of EB stability under pathophysiological conditions. In vivo studies have demonstrated the barrier protective role of cAMP-Epac-Rap1a signaling in a mouse model of acute lung injury (ALI) in response to prostacyclin (56) or mechanical injury (57). Interestingly this protective function of Rap1a in ALI is mediated by Krev/Rap1 Interaction Trapped 1 (Krit1), a 81 kD multi-domain protein encoded by CCM1 gene, and implicated in autosomal forms of Cerebral Cavernous Malformation, a vascular defect responsible for seizures and stroke (58) and previously shown to localize to cell-cell junctions in a Rap1-dependent manner (59). Therefore, while Krit1, with preferential expression in small vessels with well defined tight junctions may have distinct from Rap1 functions, particularly during development (4, 13) Rap1a-Krit1 axis may act as potential new target for treatment of acute vascular barrier dysfunction (57). Epac-Rap1 axis has also been shown to prevent exotoxin-3 endothelial permeability and bacterial transmigration across vascular wall in bacterial infection by Pseudomonas aeruginosa, a leading agent of nosocomial infections (60). Because most strains are multiresistant to anti-biotherapy, targeting the Epac-Rap1 axis may offer an attractive strategy aimed at limiting the action of its major virulence factors.
A converse approach of inhibiting Rap1 has been proposed as a strategy to increase stem cell engraftment in dystrophic muscle (61). Inhibition of endothelial junctional protein JAM-A was found to promote engraftment of mesoangioblasts, vessel associated-stem cells, by preventing Rap1 activation in Epac1/2-dependent manner. Thus, pharmacological inhibition of JAM-A-Epac-Rap1 signaling may offer a novel strategy for stem-based therapies of muscular dystrophy.
Future directions
Studies in disease models in vivo point to an important role of Rap1 in dynamic regulation of EB and vessel integrity and implicate Rap1with its effectors as potential therapeutic targets. While most studies have focused on cAMP-mediated regulation of Rap1, protective in EB, recent discovery of Rap1 as a critical regulator of shear stress-induced responses and NO release, justify further investigation into the role of Rap1 in pathologies associated with defective shear stress responses and underlying cardiovascular disease (38, 62). Furthermore, as a positive, upstream regulator of VEGF- and shear stress induced VEGFR2 activation and signaling (8, 12), Rap1 signaling may be exploited in pathologies associated with abnormal VEGF signaling, such as retinopathies, diabetes, and tumor angiogenesis. In addition to pathology-associated conditions, relative importance of the various Rap1 signaling pathways in endothelium is likely to depend on vessel type and location and, therefore, ought to be examined in small and large vessels.
Conclusions
Exciting advances have been made in understanding biological functions of Rap1 and their significance in vascular biology. The complexity of Rap1 signaling is becoming unraveled with the identification of specific molecular targets mediating Rap1 responses. Targeting Rap signaling is emerging as a novel therapeutic approach in cardiovascular pathologies associated with retinopathies, inflammation, diabetes and cancer. Emerging Rap1 function as a critical regulator of EC shear stress responses justifies further investigation into the role of Rap1 in endothelial dysfunction-associated cardiovascular pathologies.
Bullet points.
In endothelium in vivo, Rap1 and its effectors: Rasip1 and, in lymphatic vessels, Afadin, are essential for development of functional, stable vessels.
After blood vessels form, Rap1 is not essential for endothelial barrier or vessel maintenance, but is critical for NO release, endothelial function, normal blood pressure and normal life span in mice.
In response to elevated cAMP, Rap1 promotes endothelial barrier tightening by spatially controlling cdc42- and rac –regulated actomyosin cytoskeleton contractility and architecture at cell-cell junctions.
Epac-Rap1 signaling axis offers EB protection under pathological conditions of increased vascular leakiness and emerges as a novel therapeutic strategy.
Rap1 is critically required for the formation of endothelial mechanosensing complex and for transmitting EC responses to shear stress, thereby regulating EC homeostasis.
Acknowledgments
We thank Dr. Sribalaji Lakshmikanthan’s for help with figure preparation and Ms. Shana Maker - for proof-reading the manuscript.
Financial support
The work was funded by NIH grant HL111582.
This work was supported by NIH grant HL111582
Footnotes
Conflicts of interest
None.
ANNOTATED REFERENCES
Starred references are original of papers of particular (*), or high interest (**) published within last three years.
- 1.Gloerich M, Bos JL. Regulating Rap small G-proteins in time and space. Trends in Cell Biology. 2011;21(10):615–23. doi: 10.1016/j.tcb.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Roberts OL, Dart C. CAMP signalling in the vasculature: The role of Epac (exchange protein directly activated by cAMP) Biochemical Society Transactions. 2014;42(1):89–97. doi: 10.1042/BST20130253. [DOI] [PubMed] [Google Scholar]
- 3.Chrzanowska-Wodnicka M. Regulation of angiogenesis by a small GTPase Rap1. Vascular Pharmacology. 2010;53(1–2):1–10. doi: 10.1016/j.vph.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Chrzanowska-Wodnicka M. Distinct functions for Rap1 signaling in vascular morphogenesis and dysfunction. Experimental Cell Research. 2013;319(15):2350–9. doi: 10.1016/j.yexcr.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pannekoek WJ, Post A, Bos JL. Rap1 signaling in endothelial barrier control. Cell Adhesion and Migration. 2014;8(2):100–7. doi: 10.4161/cam.27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlegel N, Waschke J. CAMP with other signaling cues converges on Rac1 to stabilize the endothelial barrier - A signaling pathway compromised in inflammation. Cell and Tissue Research. 2014;355(3):587–96. doi: 10.1007/s00441-013-1755-y. [DOI] [PubMed] [Google Scholar]
- 7.Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC. Rap1b is required for normal platelet function and hemostasis in mice. Journal of Clinical Investigation. 2005;115(8):2296–-. doi: 10.1172/JCI22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakshmikanthan S, Zheng X, Nishijima Y, Sobczak M, Szabo A, Vasquez-Vivar J, et al. Rap1 promotes endothelial mechanosensing complex formation, NO release and normal endothelial function. EMBO Reports. 2015;16(5):628–37. doi: 10.15252/embr.201439846. **Using endothelial-specific Rap1a;Rap1b-KO mice, describes a novel, physiologically important function of Rap1 in regulation of endothelial NO release and endothelial function and describes the mechanisms underlying shear-stress-induced NO release. Establishes a paradigm of novel role of Rap1 in transmitting mechanical signals in endothelium. Demonstrates that Rap1 is not essential for endothelial junction maintenance, but with a severe inhibition of endothelial vasodilation and sudden, early death in male mice, describes one of the most severe Rap1-KO phenotypes in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Yan J, De P, Chang H-C, Yamauchi A, Christopherson KW, II, et al. Rap1a Null Mice Have Altered Myeloid Cell Functions Suggesting Distinct Roles for the Closely Related Rap1a and 1b Proteins. J Immunol. 2007;179(12):8322–31. doi: 10.4049/jimmunol.179.12.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chrzanowska-Wodnicka M, Kraus AE, Gale D, White GC, 2nd, Vansluys J. Defective angiogenesis, endothelial migration, proliferation, and MAPK signaling in Rap1b-deficient mice. Blood. 2008;111(5):2647–56. doi: 10.1182/blood-2007-08-109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan J, Li F, Ingram DA, Quilliam LA. Rap1a is a key regulator of fibroblast growth factor 2-induced angiogenesis and together with Rap1b controls human endothelial cell functions. Molecular and Cellular Biology. 2008;28(18):5803–10. doi: 10.1128/MCB.00393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakshmikanthan S, Sobczak M, Chun C, Henschel A, Dargatz J, Ramchandran R, et al. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin (alpha)v(beta)3. Blood. 2011;118(7):2015–26. doi: 10.1182/blood-2011-04-349282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chrzanowska-Wodnicka M, White GC, Quilliam LA, Whitehead KJ. Small GTPase Rap1 is essential for mouse development and formation of functional vasculature. PLoS ONE. 2015;10(12) doi: 10.1371/journal.pone.0145689. * Describes developmental phenotypes of total and endothelial-specific phenotypes of Rap1-knockout mice and demonstrates that while not essential for intial vessel formation, Rap1 is critical for vessel stability. Because of the implication in the Cerebral Cavernous Malformation syndrome via interaction with CCM1 product, Krit1, the comparison is made to embryonic phenotype of CCM1-KO mice. In contrast with the latter, no defect in branchial arch formation is detected in EC-Rap1 KO mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitin NY, Ramocki MB, Zullo AJ, Der CJ, Konieczny SF, Taparowsky EJ. Identification and characterization of rain, a novel Ras-interacting protein with a unique subcellular localization. Journal of Biological Chemistry. 2004;279(21):22353–61. doi: 10.1074/jbc.M312867200. [DOI] [PubMed] [Google Scholar]
- 15.Xu K, Sacharidou A, Fu S, Chong D, Skaug B, Chen Z, et al. Blood Vessel Tubulogenesis Requires Rasip1 Regulation of GTPase Signaling. Developmental Cell. 2011;20(4):526–39. doi: 10.1016/j.devcel.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson CW, Parker LH, Hall CJ, Smyczek T, Mak J, Crow A, et al. RASIP1 regulates vertebrate vascular endothelial junction stability through EPAC1-RAP1 signaling. Blood. 2013;122(22):3678–90. doi: 10.1182/blood-2013-02-483156. ** Demonstrates imporatance of Rasip1 in vascular development in vivo and as an Epac-Rap1 effector promoting EB formation in ECs in vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, et al. Afadin: A novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. Journal of Cell Biology. 1997;139(2):517–28. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tawa H, Rikitake Y, Takahashi M, Amano H, Miyata M, Satomi-Kobayashi S, et al. Role of Afadin in Vascular Endothelial Growth Factor- and Sphingosine 1-Phosphate-Induced Angiogenesis. Circ Res. 2010;106(11):1731–42. doi: 10.1161/CIRCRESAHA.110.216747. [DOI] [PubMed] [Google Scholar]
- 19.Majima T, Takeuchi K, Sano K, Hirashima M, Zankov DP, Tanaka-Okamoto M, et al. An Adaptor Molecule Afadin Regulates Lymphangiogenesis by Modulating RhoA Activity in the Developing Mouse Embryo. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0068134. * Describes lymphatic vessel defect in EC-specific Afadin KO mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Economopoulou M, Avramovic N, Klotzsche-von Ameln A, Korovina I, Sprott D, Samus M, et al. Endothelial-specific deficiency of junctional adhesion molecule-C promotes vessel normalisation in proliferative retinopathy. Thrombosis and Haemostasis. 2015;114(6):1241–9. doi: 10.1160/TH15-01-0051. [DOI] [PubMed] [Google Scholar]
- 21.Carmona G, Gottig S, Orlandi A, Scheele J, Bauerle T, Jugold M, et al. Role of the small GTPase Rap1 for integrin activity regulation in endothelial cells and angiogenesis. Blood. 2009;113(2):488–97. doi: 10.1182/blood-2008-02-138438. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Fang L, Zhan R, Hegarty JM, Ren J, Hsiai TK, et al. Polo-like kinase 2 regulates angiogenic sprouting and blood vessel development. Developmental Biology. 2015;404(2):49–60. doi: 10.1016/j.ydbio.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K, Lee Y, Rozeboom A, Lee JY, Udagawa N, Hoe HS, et al. Requirement for Plk2 in Orchestrated Ras and Rap Signaling, Homeostatic Structural Plasticity, and Memory. Neuron. 2011;69(5):957–73. doi: 10.1016/j.neuron.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei P, Satoh T, Edamatsu H, Aiba A, Setsu T, Terashima T, et al. Defective vascular morphogenesis and mid-gestation in mice lacking RA-GEF-1. Biochemical and Biophysical Research Communications. 2007;363(1):106–12. doi: 10.1016/j.bbrc.2007.08.149. [DOI] [PubMed] [Google Scholar]
- 25.Bird AC. Therapeutic targets in age-related macular disease. J Clin Invest. 2010;120(9):3033–41. doi: 10.1172/JCI42437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Elizabeth Hartnett M. Regulation of signaling events involved in the pathophysiology of neovascular AMD. Molecular Vision. 2016;22:189–202. [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Jiang Y, Shi D, Quilliam LA, Chrzanowska-Wodnicka M, Wittchen ES, et al. Activation of Rap1 inhibits NADPH oxidase-dependent ROS generation in retinal pigment epithelium and reduces choroidal neovascularization. FASEB Journal. 2014;28(1):265–74. doi: 10.1096/fj.13-240028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Fotheringham L, Wittchen ES, Hartnett ME. Rap1 GTPase inhibits tumor necrosis factor-α-induced choroidal endothelial migration via NADPH oxidase- and NF-κB-dependent activation of Rac1. American Journal of Pathology. 2015;185(12):3316–25. doi: 10.1016/j.ajpath.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orlova VV, Economopoulou M, Lupu F, Santoso S, Chavakis T. Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VE-cadherin-mediated cell-cell contacts. J Exp Med. 2006 doi: 10.1084/jem.20051730. jem.20051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lakshmikanthan S, Zieba BJ, Ge ZD, Momotani K, Zheng X, Lund H, et al. Rap1b in smooth muscle and endothelium is required for maintenance of vascular tone and normal blood pressure. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34(7):1486–94. doi: 10.1161/ATVBAHA.114.303678. * Describes hypertensive phenotype in Rap1b-KO mice leading to cardiac hypertrophy, and underlying phenotypes: increased contractility and decreased cAMP-Epac induced smooth muscle relaxation and partial inhibition of NO-dependent endothelium-mediated vasodilation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42(7):1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, Yuge A, Rajah AM, Unek G, Rinaudo PF, Maltepe E. LIMK1 Regulates Human Trophoblast Invasion/Differentiation and Is Down-Regulated in Preeclampsia. The American Journal of Pathology. 2014;184(12):3321–31. doi: 10.1016/j.ajpath.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437(7057):426–31. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 34.Zankov DP, Ogita H. Actin-tethered junctional complexes in angiogenesis and lymphangiogenesis in association with vascular endothelial growth factor. BioMed Research International. 2015:2015. doi: 10.1155/2015/314178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Bruyn KM, Zwartkruis FJ, de Rooij J, Akkerman J-WN, Bos JL. The Small GTPase Rap1 Is Activated by Turbulence and Is Involved in Integrin {alpha}IIb{beta}3-mediated Cell Adhesion in Human Megakaryocytes. J Biol Chem. 2003;278(25):22412–7. doi: 10.1074/jbc.M212036200. [DOI] [PubMed] [Google Scholar]
- 36.Tamada M, Sheetz MP, Sawada Y. Activation of a Signaling Cascade by Cytoskeleton Stretch. Developmental Cell. 2004;7(5):709–18. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34(10):2191–8. doi: 10.1161/ATVBAHA.114.303422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nature Reviews Molecular Cell Biology. 2009;10(1):53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kooistra MRH, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Letters. 2005;579(22):4966–72. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- 40.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105(5):1950–5. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- 41.Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. Rap1 GTPase Inhibits Leukocyte Transmigration by Promoting Endothelial Barrier Function. J Biol Chem. 2005;280(12):11675–82. doi: 10.1074/jbc.M412595200. [DOI] [PubMed] [Google Scholar]
- 42.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, et al. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Molecular and Cellular Biology. 2005;25(1):136–46. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curry FRE, Adamson RH. Tonic regulation of vascular permeability. Acta Physiol. 2013;207(4):628–49. doi: 10.1111/apha.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frye M, Dierkes M, Küppers V, Vockel M, Tomm J, Zeuschner S, et al. Interfering with VE-PTP stabilizes endothelial junctions in vivo via Tie-2 in the absence of VE-cadherin. Journal of Experimental Medicine. 2015;212(13):2267–87. doi: 10.1084/jem.20150718. * Identifies Rap1 as a downstream effector of Tie2 stabilizing endothelial junctions in a VE-cadherin-independent manner, acting upstream of Rac1-mediated junction stabilitzation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoelzle MK, Svitkina T. The cytoskeletal mechanisms of cell-cell junction formation in endothelial cells. Molecular Biology of the Cell. 2012;23(2):310–23. doi: 10.1091/mbc.E11-08-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, et al. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol. 2010;12(7):696–702. doi: 10.1038/ncb2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ando K, Fukuhara S, Moriya T, Obara Y, Nakahata N, Mochizuki N. Rap1 potentiates endothelial cell junctions by spatially controlling myosin I activity and actin organization. Journal of Cell Biology. 2013;202(6):901–16. doi: 10.1083/jcb.201301115. **Provides a mechanistic explaination of Rap1-mediated EB tightening by explaining the molecular basis of Rap1-mediated circumferential actin bundle formation and inhibition of radial stress fiber formation. Demonstrates that Rap1 potentiates EC junctions by spatially controlling nonmuscle-II activity through activation of the Cdc42-MRCK pathway and suppression of the Rho-ROCK pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Post A, Pannekoek WJ, Ross SH, Verlaan I, Brouwer PM, Bos JL. Rasip1 mediates Rap1 regulation of Rho in endothelial barrier function through ArhGAP29. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(28):11427–32. doi: 10.1073/pnas.1306595110. **Identifies Rasip/Radil binding target, a Rho GAP ArhGAP29 as a Rap1 effector and proposes a model through which Rap1 regulates EB tightness by controling Rho-mediated contractility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smolen GA, Schott BJ, Stewart RA, Diederichs S, Muir B, Provencher HL, et al. A rap GTPase interactor, RADIL, mediates migration of neural crest precursors. Genes and Development. 2007;21(17):2131–6. doi: 10.1101/gad.1561507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Kreuk BJ, Gingras AR, Knight JDR, Liu JJ, Gingras AC, Ginsberg MH. Heart of glass anchors rasip1 at endothelial cell-cell junctions to support vascular integrity.) eLife. 2016 Jan;2016(5) doi: 10.7554/eLife.11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Post A, Pannekoek WJ, Ponsioen B, Vliem MJ, Bos JL. Rap1 spatially controls ArhGAP29 to inhibit Rho signaling during endothelial barrier regulation. Molecular and Cellular Biology. 2015;35(14):2495–502. doi: 10.1128/MCB.01453-14. * Identifies Radil, alongside Rasip1 as members of Rap1-dependent complex inhibiting Rho contractily and barrier tightening. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Birukova AA, Fu P, Wu T, Dubrovskyi O, Sarich N, Poroyko V, et al. Afadin controls p120-catenin-ZO-1 interactions leading to endothelial barrier enhancement by oxidized phospholipids. Journal of Cellular Physiology. 2012;227(5):1883–90. doi: 10.1002/jcp.22916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Birukova AA, Tian X, Tian Y, Higginbotham K, Birukov KG. Rap-afadin axis in control of Rho signaling and endothelial barrier recovery. Molecular Biology of the Cell. 2013;24(17):2678–88. doi: 10.1091/mbc.E13-02-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.García-Ponce A, Citalán-Madrid AF, Velázquez-Avila M, Vargas-Robles H, Schnoor M. The role of actin-binding proteins in the control of endothelial barrier integrity. Thrombosis and Haemostasis. 2015;113(1):20–36. doi: 10.1160/TH14-04-0298. [DOI] [PubMed] [Google Scholar]
- 55.Choi W, Acharya BR, Peyret G, Fardin M-A, Mège R-M, Ladoux B, et al. Remodeling the zonula adherens in response to tension and the role of afadin in this response. The Journal of Cell Biology. 2016;213(2):243–60. doi: 10.1083/jcb.201506115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Birukova AA, Meng F, Tian Y, Meliton A, Sarich N, Quilliam LA, et al. Prostacyclin post-treatment improves LPS-induced acute lung injury and endothelial barrier recovery via Rap1. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2015;1852(5):778–91. doi: 10.1016/j.bbadis.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meliton A, Meng F, Tian Y, Shah AA, Birukova AA, Birukov KG. Role of krev interaction trapped-1 in prostacyclin-induced protection against lung vascular permeability induced by excessive mechanical forces and thrombin receptor activating peptide 6. Am J Respir Cell Mol Biol. 2015;53(6):834–43. doi: 10.1165/rcmb.2014-0376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Revencu N, Vikkula M. Cerebral cavernous malformation: new molecular and clinical insights. J Med Genet. 2006;43(9):716–21. doi: 10.1136/jmg.2006.041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glading A, Han J, Stockton RA, Ginsberg MH. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J Cell Biol. 2007;179(2):247–54. doi: 10.1083/jcb.200705175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bouillot S, Attrée I, Huber P. Pharmacological activation of Rap1 antagonizes the endothelial barrier disruption induced by exotoxins ExoS and ExoT of Pseudomonas aeruginosa. Infection and Immunity. 2015;83(5):1820–9. doi: 10.1128/IAI.00010-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giannotta M, Benedetti S, Tedesco FS, Corada M, Trani M, D’Antuono R, et al. Targeting endothelial junctional adhesion molecule-A/EPAC/Rap-1 axis as a novel strategy to increase stem cell engraftment in dystrophic muscles. EMBO Molecular Medicine. 2014;6(2):239–58. doi: 10.1002/emmm.201302520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiological Reviews. 2011;91(1):327–87. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


