Abstract
Hypothesis
Intraoperative, intracochlear electrocochleography (ECochG) will provide a means to monitor cochlear hair cell and neural response during cochlear implant (CI) electrode insertion. Distinct patterns in the insertion track can be characterized.
Background
Conventional CI surgery is performed without a means of actively monitoring cochlear hair cell and neural responses. Intracochlear ECochG obtained directly through the CI may be a source of such feedback. Understanding the patterns observed in the “insertion track” is an essential step toward refining intracochlear ECochG as a tool that can be used to assist in intraoperative decision making and prognostication of hearing preservation.
Methods
Intracochlear ECochG was performed in 17 patients. During electrode insertion, a 50-ms tone burst acoustic stimulus was delivered with a frequency of 500 Hz at 110 dB SPL. The ECochG response was monitored from the apical-most electrode. The amplitude of the first harmonic was plotted and monitored in near real time by the audiologist-surgeon team during CI electrode insertion.
Results
Three distinct patterns in first harmonic amplitude change were observed across subjects during insertion: Type A (52%), overall increase in amplitude from the beginning of insertion until completion; Type B (11%), a maximum amplitude at the beginning of insertion, with a decrease in amplitude as insertion progressed to completion; and Type C (35%), comparable amplitudes at the beginning and completion of the insertion with the maximum amplitude mid-insertion.
Conclusion
Three ECochG patterns were observed during electrode advancement into the cochlea. Ongoing and future work will broaden our scope of knowledge regarding the relationship among these patterns, the presence of cochlear trauma, and functional outcomes related to hearing preservation.
Keywords: Cochlear implants, Electrocochleography, Hearing preservation
Using conventional approaches, cochlear implant (CI) surgery does not currently allow for real-time, continuous feedback indicative of the impact of electrode insertion on the physiologic health of the organ of Corti. To facilitate the goals of minimizing electrode insertion trauma and optimizing electrode placement for electro-acoustic stimulation strategies, intraoperative feedback reflective of cochlear micromechanics is desirable during CI surgery.
One potential source of feedback from the cochlea during CI surgery is electrocochleography (ECochG). ECochG was first introduced 75 years ago as a near-field method for capturing acoustically evoked cochlear and auditory nerve responses (1). Interest in ECochG as a means of assessing cochlear micromechanics before, during, and after CI electrode placement from the vantage point of the round window (2–4) and from within the cochlea itself (5–10) has inspired a number of studies in recent years.
The ECochG response consists of several convolved components that can be teased apart by windowing and filtering the raw signal (11,12). These consist of the cochlear microphonic (CM), the summation potential, the compound action potential (CAP), and the auditory nerve neurophonic (ANN). A line of animal model work (12,13) has characterized the origins and characteristics of these components of the raw ECochG response. The CM reflects the alternating current generated by stereo-cilia of the outer hair cells of the cochlea and closely follows the acoustic stimulus’ waveform (13–15). The summation potential is thought to represent a direct current shift of the receptor potential, principally of the hair cells (inner and outer) (16,17). The CAP and ANN both reflect cochlear nerve activity: the CAP is observed in response to the onset/offset of the acoustic stimulus; the ANN is reflective of the phase-locked response of auditory nerve fibers to the stimulus, especially important at low frequencies where the CAP is less prominent (2,18,19).
Existing work utilizing intracochlear ECochG has demonstrated its general feasibility and ease of application during CI electrode insertion (5,9,10), its relationship to preoperative hearing levels (19), and its potential to predict postoperative hearing levels and electrode position (20). The purpose of the present study was to characterize the ECochG patterns that the audiologist-surgeon team observes in the “insertion track”—the “live feed” received directly from the CI electrode during its advancement into the cochlea.
METHODS
Inclusion Criteria
The participating institutions’ internal review boards reviewed and approved this study for adult and pediatric participants. Patients who independently selected an Advanced Bionics CI (Valencia, CA, U.S.A.) with a HiRes90K internal receiver-stimulator were prospectively invited to participate in this study. Either a HighFocus 1J or a HiFocus Mid Scala electrode was used based on the surgeon’s preference. Residual low-frequency hearing was not an inclusion criterion, but was present for several patients.
Intraoperative Setup and Surgical Approach
The methodological specifics of ECochG are presented in detail elsewhere (9,10). In brief, acoustic stimuli used to elicit the ECochG response were created using the NI DAQ system (NI DAQ 6216, National Instruments Corporation; Austin, TX, U.S.A.) paired with an audio amplifier (Sony PHA-2, Sony Corporation, New York, NY, U.S.A.) and presented through ER-3A insert earphones (Etymotic Research, Inc.; Elk Grove Village, IL, U.S.A.). An ER-7 probe microphone was used to monitor the stimulus level in the external auditory canal (EAC). The ECochGresponse was measured using the Advanced Bionics Clinical Programming Interface-II, Platinum Series Sound Processor, and Universal Headpiece (Valencia, CA, U.S.A.).
After anesthetic induction and patient positioning, the insert earphone and probe microphone were placed in the EAC. The surgical site was prepared and the nonsterile EAC and pinna were isolated from the surgical field by folding the auricle forward with transparent adhesive draping material. Care was taken to ensure that the sound tube/insert earphone was not crimped.
Acquisition Parameters and Physiologic Analysis
After positioning of the CI receiver/stimulator, but before electrode insertion, the external transmitting coil was coupled to the receiver/stimulator. During insertion, a 50-ms tone burst acoustic stimulus was delivered via the insert earphone with a 5-ms onset/offset ramp time and a 40-ms plateau. The stimulus rate was set at five averages per second. A stimulus frequency of 500 Hz at 110 dB SPL was presented during electrode insertion. The surgeon and audiologist communicated closely through the electrode insertion to ascertain the approximate location of the electrode at a given time.
Stimulus delivered to the EAC was calibrated in situ to evaluate the stimulus level. The CI electrode was introduced via the round window and advanced to achieve a full insertion. The ECochG signal was recorded from the apical-most electrode throughout insertion. The ECochG responses were recorded for 4 to 80 presentations at a time, consisting of 2 to 40 rarefactions and 2 to 40 condensations. This range was contingent on the signal-to-noise ratio: depending on how large the response signal was, as few as four and as many as 80 presentations were attempted before the program advanced to test the next intensity level. The CI’s neural response imaging amplifier provided a gain of 1,000, and data was recorded through back-telemetry. A “difference curve” was created by subtracting condensation and rarefaction waveforms from each other. The spectral energy and amplitude of the first harmonic of the ongoing response were then calculated using fast Fourier transformation analysis. The fast Fourier transformation amplitude of the first harmonic of the recorded response was then plotted on a time/amplitude graph, which was observed by the audiologist-surgeon team during electrode insertion. To ensure that responses were not the result of noise artifact from equipment in the operating room, whenever possible, a “no sound run” concluded the recording session in most patients. This was performed by clamping of the tube delivering the acoustic stimulus. If a genuine ECochG response is being observed, the signal disappears with this maneuver.
RESULTS
Seventeen subjects (5 pediatric, 12 adult) were included in our final analysis. The level of preoperative sensorineural hearing loss as indicated by audiometric thresholds ranged from moderate to profound. All electrode insertions were performed by either a round window or an extended round window approach and all were full insertions. Table 1 shows demographic and audiological characteristics (pure-tone average [PTA]) of our sample.
TABLE 1.
Preoperative pure-tone averages and electrocochleographic characteristics during electrode insertion
| Subject | Age | PTA (0.5, 1, 2 kHz) dB HL | Amplitude (μV)
|
dB Change
|
Pattern Type (A–C) | |||
|---|---|---|---|---|---|---|---|---|
| RW Amplitude (μV) | Max Peak (μV) | Final Amplitude (μV) | RW—Peak | Peak—End | ||||
| V1 | >=18 | 85 | 0.37 | 11.31 | 11.31 | 30 | 0 | A |
| V2 | >=18 | 73 | 6.92 | 13.47 | 8.24 | 5 | −5 | C |
| V3 | >=18 | 75 | 2.75 | 20.67 | 9.09 | 18 | −8 | A |
| V4 | >=18 | 82 | 0.94 | 22.77 | 19.95 | 28 | −1 | A |
| V5 | >=18 | 75 | 1.68 | 46.81 | 31.31 | 40 | −4 | A |
| V6 | >=18 | 57 | 1.05 | 53.40 | 31.38 | 34 | −5 | A |
| V7 | >=18 | 73 | 0.67 | 4.15 | 3.06 | 16 | −3 | A |
| V8 | >=18 | 95 | 1.89 | 5.08 | 4.73 | 9 | −1 | A |
| O1 | <18 | 100 | 0.31 | 1.20 | 0.56 | 12 | −6 | C |
| O2 | >=18 | 120 | 48.47 | 48.47 | 16.55 | 0 | −17 | B |
| O3 | <18 | 82 | 4.00 | 5.46 | 2.15 | 3 | −17 | B |
| O4 | >=18 | 93 | 9.75 | 36.12 | 9.37 | 11 | − | C |
| O5 | <18 | 93 | 1.28 | 19.82 | 4.38 | 24 | −12 | C |
| O6 | >=18 | 73 | 10.24 | 65.79 | 7.06 | 15 | −18 | C |
| O7 | <18 | 93 | 0.40 | 3.32 | 1.22 | 18 | −8 | C |
| O8 | >=18 | 103 | 1.43 | 55.21 | 29.05 | 0 | −7 | A |
| O9 | <18 | 55 | 2.42 | 40.10 | 40.10 | 24 | 0 | A |
PTA indicates pure-tone average.
Qualitatively, intracochlear ECochG was obtained without difficulty and with minimal added time beyond that of conventional CI surgery. As detailed elsewhere (9,10), one advantage of this method of intracochlear ECochG and related methods (5) is that there is not a need for additional instrumentation or pause of surgical progress beyond coupling of the external transmitting coil to the receiver/stimulator.
The principal outcome of interest in this study was the pattern of first harmonic amplitude of the ECochG during insertion. Response was measured from the apical-most electrode during electrode advancement starting with co-chlear entry (round window) and ending with completion of insertion. Table 1 shows the amplitude (uV) characteristics across the cohort and changes in amplitude (dB change) at three distinct points in time: 1) initiation of insertion just inside the round window, 2) at the maximum amplitude observed during insertion, and 3) final amplitude at completion of insertion. Using the amplitudes at each interval, we then calculated the amplitude change (dB change) between point 1 (round window) to point 2 (maximum) and then from point 2 (maximum) to point 3 (final).
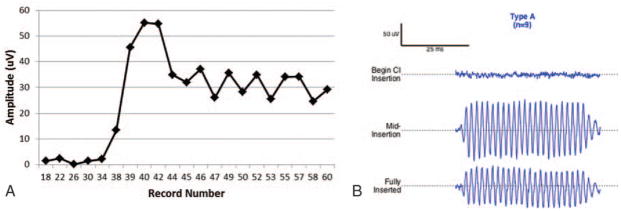
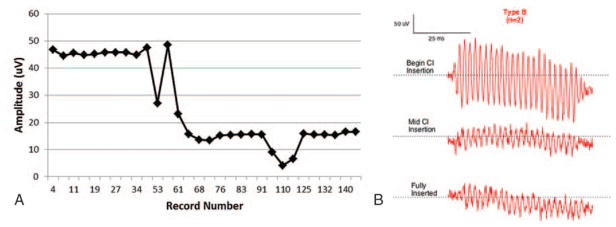
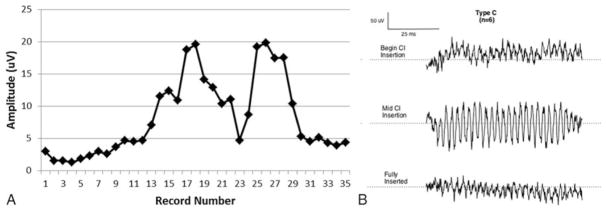
Using this approach, three distinct patterns were observed across subjects during insertion: Types A, B, and C (Table 1, last column). The amplitude characteristics of the Type A pattern (Fig. 1, A and B) was an overall increase in amplitude as measured from the beginning of insertion until completion. Nine participants (52%) exhibited this pattern, the most commonly observed in our sample. The mean amplitude of Type A patterns at point 1 (inside the round window) was 1.47 μV with final mean amplitude at point 3 of 20 μV. The Type B pattern (Fig. 2, A and B) was distinguished by a maximum amplitude (mean = 29.97 μV) at the beginning of insertion, just inside round window, with a decrease in amplitude as insertion progressed to completion (mean = 9.36 μV). This pattern was the least commonly observed in our sample, noted in two participants (11%). The final pattern, Type C (Fig. 3, A and B), was characterized by amplitudes (mean = 4.82 μV) at the start of insertion that were comparable to amplitudes at the completion of the insertion (mean = 5.14 μV) with a max amplitude (mean = 23.3 μV) being reached mid-insertion. This was the second most common pattern, observed in six participants (35%). Finally, the mean preoperative PTA for each group was 78 dB HL for Type A, 101 dB HL for Type B, and 88 dB HL for Type C. Patients whose insertion tracings did not emerge from the noise floor were considered “no response” and were not considered in the final analysis.
FIG. 1.
Type A. A, Insertion tract of subject O8 obtained during electrode insertion. Full insertion was reached at record number 46. B, Raw ECochG waveforms (500 Hz tone burst stimulus) for three distinct points during insertion: beginning of electrode insertion, mid-insertion, and at full insertion.
FIG. 2.
Type B. A, Insertion tract of subject O2 obtained during electrode insertion. Full insertion was reached at record number 131. B, Raw ECochG waveforms (500 Hz tone burst stimulus) for three distinct points during insertion: beginning of electrode insertion, mid-insertion, and at full insertion.
FIG. 3.
Type C. A, Insertion tract of subject O5 obtained during electrode insertion. Full insertion was reached at record number 32. B, Raw ECochG waveforms (500 Hz tone burst stimulus) for three distinct points during insertion: beginning of electrode insertion, mid-insertion, and at full insertion.
DISCUSSION
Intracochlear ECochG offers the potential for near real-time, continuous feedback reflective of cochlear micro-mechanics during CI electrode insertion (5,8–10,19–21). Intraoperative ECochG from the round window (2–4) and from within the cochlea itself is desirable with the long-term goal identifying and minimizing cochlear trauma and ensuring proper electrode placement during CI electrode insertion. The primary goal of this study was to characterize and further our understanding of the patterns acquired in the running feedback that the audiologist-surgeon team monitors during advancement of a CI electrode.
Three distinct ECochG amplitude patterns as measured from the apical CI electrode during the course of insertion were observed in this sample. The most commonly observed pattern, Type A, was observed in 52% (9/17) of insertion tracings and is characterized by an overall increase in the ECochG amplitude with the lowest point corresponding to initiation of insertion at the round window and the maximum upon completion of insertion. The second most common pattern, Type C was observed in 35% (6/17) and is characterized by maximum amplitude at mid-insertion followed by a return to baseline by completion of insertion. Least commonly observed, Type B, is characterized by a steady decrease in amplitude from the maximum amplitude at the round window. Insertion tracings that were flat throughout insertion (no significant responses above noise floor) from start to finish were not included in the present analyses. This “no response” group is important to consider, and may itself be considered a fourth pattern. Theoretical explanations for absence of response include severity of hearing loss or an abnormally large amount of electrical noise masking the ECochG signal.
While our sample size is currently small, it is important to note that the patients in our sample with the best preoperative PTAs tended to demonstrate the Type A pattern; those with the poorest patterns exhibited the Type C pattern. Why patients with the poorest overall preoperative PTA should demonstrate the highest 1st harmonic amplitudes at initiation of electrode advancement is currently not clear. One possibility may relate to the fact that many patients in this sample had an unknown etiology of hearing loss. Auditory neuropathy spectrum disorder may confer a picture of large hair cell response on ECochG but poor PTA preoperatively (4). The possibility of electrical artifact is also important to consider in interpreting this pattern; however, we have reason to believe that this configuration is not artifactual. First, electrical noise artifact was monitored in the raw signal and, whenever possible, recording sessions concluded with a “no sound run,” whereby the tube presenting the auditory stimulus was clamped to rule out artifact from “room noise.” The device itself has been shown by Koka et al. (10) to be a negligible source of electrical artifact. Further investigation is called for in this area.
The current data are complementary to the findings recently reported by Campbell et al. (5) in their account of initial experiences with intracochlear ECochG. Similarly, Bester et al. (19) recently detailed their characterization of intracochlear ECochG patterns observed during CI surgery. Both of these works use a Neural Response Telemetry adaptation with the Cochlear CI422 (Cochlear Corp, Ltd., Sydney, Australia). They are differentiated from the present work by several factors. First, Bester et al. performed recordings from different locations through their CI electrode after it was inserted as opposed to during the act of insertion, as presented here. Second, their cohort consisted of participants all with residual low-frequency hearing, rather than all comers, which describes our sample. Third, technical differences in the way ECochG is recorded between these recent studies and our study are worth noting. These are discussed in detail elsewhere (9,10). In brief, we use longer acquisition times (54.5-ms) compared with the 10 or 20-ms previously described (5) and a more gradual onset/offset ramp (5-ms) compared with the 1-ms previously reported (5,21). The value of longer acquisition time is that it brings the more apical, low frequency-serving, regions of the cochlea into greater resolution. The value of a slower onset/offset ramp is that fewer “onset neurons” are activated (i.e., the CAP), which can otherwise confuse interpretation of CM and ANN spectral distortions.
There are many potential future directions and areas to broaden our knowledge regarding intracochlear ECochG from the CI electrode. Having identified the patterns witnessed during electrode insertion, the next step is to identify what structural and/or physiological changes may be associated with them. Confirmation of electrode scalar position (i.e., scala tympani versus scala vestibule) using postoperative high-resolution computed tomography is one method of achieving this goal, toward which preliminary work is underway (20). Additionally, there is the need to identify preoperative demographic and audiological factors that may predict these patterns. With interest in predicting success of hearing preservation, there is a need to correlate the ongoing response patterns observed here with their associated postoperative audiograms. Going forward, a long-term goal will be studying clinical outcomes following electrode insertions that were guided by ECochG feedback. In such patients, the audiologist-surgeon team may be confronted with several hypothetical scenarios: 1) an ECochG pattern suggestive of irreversible trauma, 2) a pattern suggestive of impending, but reversible trauma, or 3) a pattern consistent with the presence of functional hair cells, which may lead the surgeon to limit insertion depth or overlap functional regions, as appropriate.
In interpreting the data reported here, there are several important limitations to bear in mind. First, given that this study was performed by multiple surgeons across two institutions, there is inherent heterogeneity in surgical technique. Additionally, although all other equipment and technical aspects were consistent, both 1J and Mid Scala electrodes were used, as appropriate. Beyond individual differences in the technique, it is worth noting that although electrode insertion was performed as slowly as possible, the insertion itself was not modified in any way on the basis of ECochG feedback. The present sample of CI patients is also fairly heterogeneous, including both pediatric and adult patients and variable levels of preoperative hearing. Importantly, etiology of hearing loss was not known in all patients. Inclusion of certain potential etiologies, such as auditory neuropathy spectrum disorder, may have influenced the patterns observed, as discussed above. Future work will be needed to better characterize how patterns in the ongoing response are influenced by specific pathologies resulting in hearing loss.
CONCLUSIONS
Intracochlear ECochG has the potential to provide the audiologist-surgeon team with near real-time feedback that is not currently possible during conventional CI surgery. In this study, we characterized the three most commonly observed patterns during electrode advancement into the cochlea. Ongoing and future work will broaden our scope of knowledge regarding the relationship between the ECochG response during CI electrode insertion and functional outcomes related to hearing preservation.
Footnotes
The authors disclose no conflicts of interest.
M.S.H. (none); W.J.R. (none); C.K.G. (supported by NIH/NIDCD 1-F30-DC-015168); B.P.O. (none); J.T.H. (none); R.T.D. (none); K.K. (AB employee); R.F.L. (consultant for Advanced Bionics, Medtronics, and Ototronix); D.C.F. (research contracts with Med-El and Cochlear Corp.); O.F.A. (consultant for AB, Cochlear, Med El).
References
- 1.Eggermont JJ. Ups and downs in 75 years of electrocochleography. Front Syst Neurosci. 2017;11:1–21. doi: 10.3389/fnsys.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzpatrick DC, Campbell AP, Choudhury B, et al. Round window electrocochleography just before cochlear implantation: Relationship to word recognition outcomes in adults. Otol Neurotol. 2014;35:64–71. doi: 10.1097/MAO.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Formeister EJ, McClellan JH, Merwin WH, 3rd, et al. Intraoperative round window electrocochleography and speech perception outcomes in pediatric cochlear implant recipients. Ear Hear. 2015;36:249–60. doi: 10.1097/AUD.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 4.Riggs WJ, Roche JP, Giardina CK, et al. Intraoperative electrocochleographic characteristics of auditory neuropathy spectrum disorder in cochlear implant subjects. Front Neurosci. 2017;11:416. doi: 10.3389/fnins.2017.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell L, Kaicer A, Sly D, et al. Intraoperative real-time cochlear response telemetry predicts hearing preservation in co-chlear implantation. Otol Neurotol. 2016;37:332–8. doi: 10.1097/MAO.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 6.Radeloff A, Shehata-Dieler W, Scherzed A, et al. Intraoperative monitoring using cochlear microphonics in cochlea implant patients with residual hearing. Otol Neurotol. 2012;33:348–54. doi: 10.1097/MAO.0b013e318248ea86. [DOI] [PubMed] [Google Scholar]
- 7.Calloway NH, Fitzpatrick DC, Campbell AP, et al. Intracochlear electrocochleography during cochlear implantation. Otol Neurotol. 2014;35:1451–7. doi: 10.1097/MAO.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 8.Acharya AN, Tavora-Vieira D, Rajan GP. Using the implant electrode array to conduct real-time intraoperative hearing monitoring during pediatric cochlear implantation: Preliminary experiences. Otol Neurotol. 2016;37:148–53. doi: 10.1097/MAO.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 9.Harris MS, Riggs WJ, Koka K, et al. Real-time intracochlear electrocochleography obtained directly through a cochlear implant. Otol Neurotol. 2017;38:e107–13. doi: 10.1097/MAO.0000000000001425. [DOI] [PubMed] [Google Scholar]
- 10.Koka K, Saoji AA, Litvak LM. Electrocochleography in cochlear implant recipients with residual hearing: Comparison with audiometric thresholds. Ear Hear. 2017;38:e161–7. doi: 10.1097/AUD.0000000000000385. [DOI] [PubMed] [Google Scholar]
- 11.Choudhury B, Fitzpatrick DC, Buchman CA, et al. Intraoperative round window recordings to acoustic stimuli from cochlear implant patients. Otol Neurotol. 2012;33:1507–15. doi: 10.1097/MAO.0b013e31826dbc80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forgues M, Koehn HA, Dunnon AK, et al. Distinguishing hair cell from neural potentials recorded at the round window. J Neurophysiol. 2014;11:580–93. doi: 10.1152/jn.00446.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verpy E, Weil D, Leibovici M, et al. Stereocilin-deficient mice reveal the origin of cochlear waveform distortions. Nature. 2008;13:255–8. doi: 10.1038/nature07380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohmer H, Kinarti R, Gafni M. The source along the basilar membrane of the cochlear microphonic potential recorded by surface electrodes in man. Electroencephalogr Clin Neurophysiol. 1980;49:506–14. doi: 10.1016/0013-4694(80)90393-4. [DOI] [PubMed] [Google Scholar]
- 15.Patuzzi RB, Yates GK, Johnstone BM. Outer hair cell receptor current and sensorineural hearing loss. Hear Res. 1989;42:47–72. doi: 10.1016/0378-5955(89)90117-2. [DOI] [PubMed] [Google Scholar]
- 16.Durrant JD, Wang J, Ding DL, Salvi RJ. Are inner or outer hair cells the source of summating potentials recorded from the round window? J Acoust Soc Am. 1998;104:370Y7. doi: 10.1121/1.423293. [DOI] [PubMed] [Google Scholar]
- 17.Zheng XY, Ding DL, McFadden SL, et al. Evidence that inner hair cells are the major source of cochlear summating potentials. Hear Res. 1997;113:76–88. doi: 10.1016/s0378-5955(97)00127-5. [DOI] [PubMed] [Google Scholar]
- 18.Lichtenhan JT, Cooper NP, Guinan JJ., Jr A new auditory threshold estimation technique for low frequencies: Proof of concept. Ear Hear. 2013;34:42–51. doi: 10.1097/AUD.0b013e31825f9bd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bester CW, Campbell L, Dragovic A, et al. Characterizing electrocochleography in cochlear implant recipients with residual low-frequency hearing. Front Neurosci. 2017;11:141. doi: 10.3389/fnins.2017.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connell BP, Holder JT, Dwyer RT, et al. Intra- and postoperative electrocochleography may be predictive of final electrode position and postoperative hearing preservation. Front Neurosci. 2017;11:291. doi: 10.3389/fnins.2017.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalbert A, Huber A, Veraguth D, et al. Assessment of cochlear trauma during cochlear implantation using electrocochleography and cone beam computed tomography. Otol Neurotol. 2016;37:446–53. doi: 10.1097/MAO.0000000000000998. [DOI] [PubMed] [Google Scholar]