SUMMARY
Spectraplakins are large molecules that cross-link F-actin and microtubules (MTs). Mutations in spectraplakins yield defective cell polarization, aberrant focal adhesion dynamics, and dystonia. We present the 2.8 Å crystal structure of the hACF7 EF1-EF2-GAR MT-binding module and delineate the GAR residues critical for MT binding. The EF1-EF2 and GAR domains are autonomous domains connected by a flexible linker. The EF1-EF2 domain is an EFβ-scaffold with two bound Ca2+ ions that straddles a N-terminal α-helix. The GAR domain has a unique α/β sandwich fold that coordinates Zn2+. While the EF1-EF2 domain is not sufficient for MT-binding, the GAR domain is and likely enhances EF1-EF2-MT engagement. Residues in a conserved basic patch, distal to the GAR domain’s Zn2+ binding site, mediate MT binding.
Keywords: Spectraplakin, ACF7, GAR, EF-Hand, Microtubule, Actin
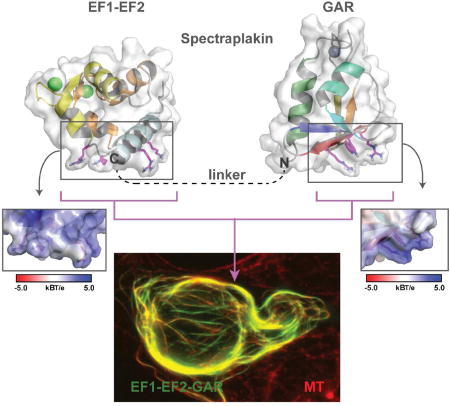
Spectraplakins are F-actin-microtubule (MT) cross-linkers critical for polarized cell migration. How spectraplakins bind MTs is poorly understood. Lane et al. present the structure of the spectraplakin EF1-EF2 and GAR domains. The GAR domain uses basic determinants to bind MTs and likely enhances EF1-EF2-MT engagement.
INTRODUCTION
The dynamic MT and F-actin cytoskeletal networks create a complex cellular infrastructure critical for basic processes including cellular migration, focal adhesion dynamics, dendrite formation, and intracellular trafficking. These biological processes dually engage the actin and MT networks and require coordinated polarity and dynamics. Underlying cytoskeletal coordination and coupling are a set of MT-F-actin cytoskeletal cross-linkers. Determining how cross-linkers engage, coordinate, and regulate cytoskeletal dynamics is an active area of research.
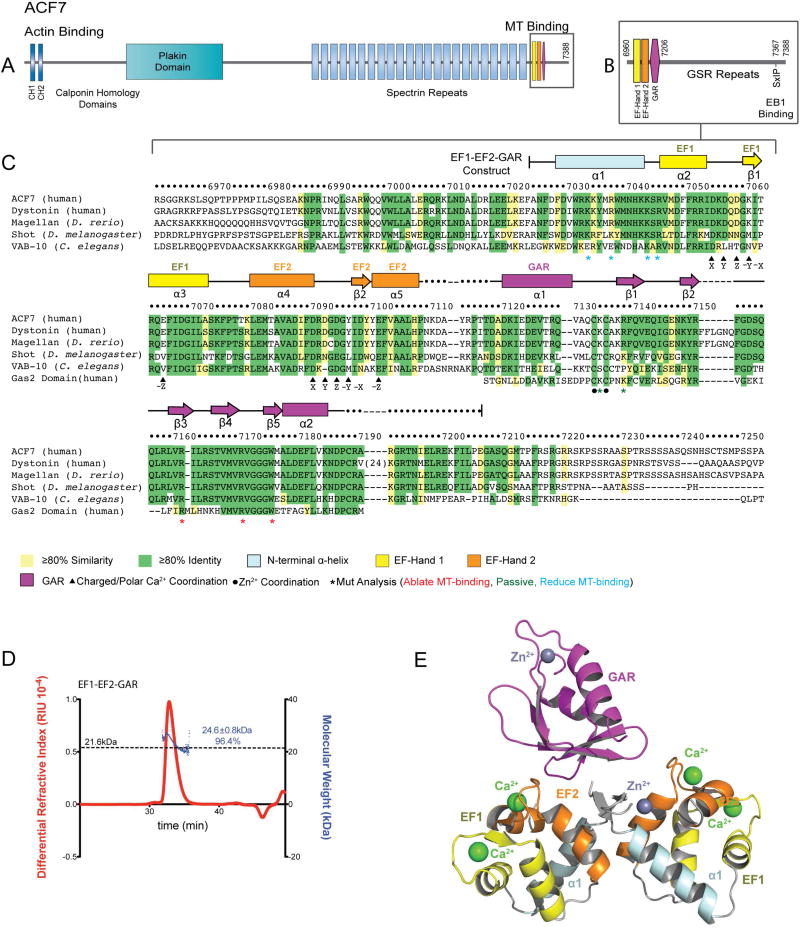
Spectraplakins are F-actin-MT cross-linkers (Yang et al., 1999). There are two mammalian spectraplakins: MT actin cross-linking factor 1 (MACF1 or ACF7) (Byers et al., 1995) and bullous pemphigoid antigen 1 (BPAG1/MACF2/dystonin) (Stanley et al., 1981; Brown et al., 1995; Guo et al., 1995). Drosophila and C. elegans have a single spectraplakin, Short stop (Van Vactor et al., 1993) and VAB-10 (Bosher et al., 2003) respectively. Spectraplakins contain diverse domains and motifs including: N-terminal F-actin-binding calponin-homology (CH) domains (Karakesisoglou et al., 2000; Yang et al., 1996), a central plakin domain and spectrin repeats, and a C-terminal MT-binding region that includes two Ca2+-binding EF-Hands, a Growth arrest specific 2 (Gas2)-related (GAR) domain (Leung et al., 1999; Sun et al., 2001), a Gly-Ser-Arg repeat (GSR) region, and an EB1-binding SxIP motif (Slep et al., 2005; Subramanian et al., 2003) (Figure 1A,B). These domains create a bivalent protein architecture that couples F-actin and MTs.
Figure 1. Architecture of the ACF7 EF1-EF2-GAR Module.
(A) hACF7 domain architecture and zoom view (B) of the MT-binding region. (C) Sequence alignment of the EF1-EF2-GAR module from human, D. rerio, D. melanogaster, and C. elegans spectraplakins, and human Gas2. hACF7 EF1-EF2-GAR 2° structure and residue number are depicted above, residues mutated are indicated below. (D) SEC-MALS analysis of the EF1-EF2-GAR module. The molecular weight of a monomer is indicated by the dashed line. The peak measured accounts for 96.4% of the total mass eluted. (E) Model of the EF1-EF2-GAR modules observed in the ASU. See also Figure S1.
Deleting or mutating spectraplakins yields dramatic phenotypes that vary depending on the organism and cell type. Knocking out ACF7 in mice causes pre-implantation lethality (Kodama et al., 2003), while disruption of BPAG1 causes early-onset postnatal death (Yang et al., 1996). Lack of functional BPAG1 in epithelial tissue causes bullous pemphigoid (Mueller et al., 1989), while the absence of BPAG1 in neuronal cells causes dystonia (Brown et al., 1995; Guo et al., 1995). In each of these disease states, cells exhibit gross changes in cytoskeletal organization (Suozzi et al., 2012). Knocking out ACF7 or BPAG1 in cells yields defects in cellular polarization (Prokop et al., 1998), migration (Kodama et al., 2003; Wu et al., 2011), intracellular vesicular trafficking (Liu et al., 2003), and focal adhesion dynamics (Wu et al., 2008; Yue et al., 2016). Collectively, spectraplakins play critical roles in many core biological processes.
While the structure and actin-binding mechanism of the spectraplakin CH domain has been described (Yue et al., 2016), we lack an understanding of spectraplakin-MT interactions. Within the C-terminal EF1-EF2-GAR-GSR-SxIP region, the GAR domain is sufficient for MT binding (Leung et al., 1999; Sun et al., 2001). Whether the EF1-EF2 domain plays a direct role in MT binding is not known, but deleting the domain affects MT-co-localization (Applewhite et al., 2013), and functionally, the EF-Hands play roles in MT-dependent processes: neuronal extension and path finding (Bottenberg et al., 2009; Lee and Kolodziej, 2002; Lee et al., 2007; Sanchez-Soriano et al., 2009). Here, we present the 2.8 Å crystal structure of the EF1-EF2-GAR module. The EF1-EF2 domain is bound to two Ca2+ ions and is tethered to the GAR domain by a flexible linker. The GAR domain has a novel α/β-sandwich fold that coordinates zinc. The GAR domain is the principal mediator of direct MT binding and likely enhances EF1-EF2-MT binding. We map EF1-EF2 and GAR MT-binding determinants to conserved basic patches distal to each domain’s divalent binding region.
RESULTS
The hACF7 EF1-EF2-GAR Module is Monomeric
To gain structural insight into the spectraplakin C-terminal EF1-EF2-GAR MT-binding module (Figure 1A–C) we expressed and purified hACF7 residues 7024–7206. We analyzed this construct (formula weight = 21.6 kDa) using size exclusion chromatography with multi-angle light scattering (SEC-MALS) to determine its oligomeric state. hACF7 EF1-EF2-GAR eluted as a single peak with an experimentally determined mass of 24.6 kDa (Figure 1D), indicating the module is monomeric in solution.
Overall Structure of the EF1-EF2-GAR Module
We grew crystals of the hACF7 EF1-EF2-GAR module (space group P3121) and collected a single wavelength anomalous dispersion (SAD) data set at the zinc K edge. Experimental electron density allowed model building for two EF1-EF2 domains (Chains A and B) and a single GAR domain (Chain C) in the asymmetric unit (ASU)(Figure 1E). We refined the structure to 2.8 Å resolution (Rfree = 0.279; crystallographic statistics presented in Table 1). There was insufficient electron density to resolve a 9-residue stretch that connects the EF1-EF2 and GAR domains. The lack of density for the second GAR domain and the linker that joins it to the EF1-EF2 domain suggests that the EF1-EF2 and GAR domains are tethered and have relative independent freedom to sample space. In support, limit trypsin proteolysis of the EF1-EF2-GAR module in solution yielded products with molecular weights that paralleled those of the independent domains, indicative that the bridging linker is accessible (Figure S1A). SDS-PAGE analysis of harvested crystals revealed minimal proteolysis (Figure S1B). This suggests that the second GAR domain is present in the lattice, but is not ordered, and likely resides in the ~40 Å diameter channels that contribute to 66% of the crystal space not occupied by the model (Figure S1C). The shortest distances between the first and last residues modeled of the GAR and EF1-EF2 domains respectively are 15.5 Å (EF1-EF2 chain A in the ASU), 12.5 Å (EF1-EF2 chain A, symmetry mate X–Y,−Y,−Z+2/3), and 11.6 Å (EF1-EF2 chain B, symmetry mate −Y,X–Y,Z+1/3)(Figure S1D). Because we could not determine which EF1-EF2 domain was associated with the GAR domain modeled, the GAR domain was given an independent chain ID. Several of the prime interactions between the EF1-EF2 domains and the GAR domain involve hydrophobic residues at the N-terminal region of each EF1-EF2 domain that were added in cloning, suggesting that an interaction between the GAR domain and the EF1-EF2 domains is not physiological (Figure S1E). Additionally, in the lattice, the GAR domain engages an EF1-EF2:EF1-EF2 crystal packing interface that would not occur in the module’s monomeric solution state (Figure 1D). We used SEC-MALS to test whether the EF1-EF2 domain and the GAR domain interact in trans (expressed and purified as separate peptides and tested at a 2:1 molar ratio) but did not detect an interaction (Figure S1F). Thus, we do not view interactions between the EF1-EF2 domains and the GAR domain in our crystal structure to be biologically relevant. Accordingly, we present and interpret the structure of each domain independently.
Table 1.
Crystallographic Data and Refinement Statistics
| H.s. ACF7 EF1-EF2-GAR | |
|---|---|
| Data Collection | |
| Beamline | 23-ID, GM/CA-CAT, APS, ANL |
| Wavelength (Å) | 1.28295 |
| Space group | P3121 |
| Cell: a,b,c (Å) | 92.98, 92.98, 90.45 |
| Resolution (Å) | 41.35 – 2.80 (2.90 – 2.80) |
| # Reflections: total/unique | 244648 (11546)/ 11285 (1131) |
| Completeness (%) | 100 (100) |
| <|I/σ|> | 27.08 (2.62) |
| Multiplicity | 21.2 (18.6) |
| Rmerge | 0.1295 (1.161) |
| CC1/2 | 0.999 (0.858) |
| Refinement | |
| Refinement Resolution (Å) | 41.35 – 2.80 (2.90 – 2.80) |
| Rwork | 0.256 (0.358) |
| Rfree | 0.279 (0.445) |
| Rmsd bond lengths (Å) | 0.003 |
| Rmsd bond angles (°) | 0.75 |
| Average B-factor (Å2) | 84.30 |
| Rfree test set size (%) | 10.08 |
| Rfree test set count | 1138 |
| Mean B-factors (A): overall/protein/water | 84.30/84.30/65.70 |
| Ramachandran analysis: favored/ allowed (%) | 94.74/5.26 |
| PDB accession code | 5VE9 |
Rmerge = ΣhklΣi|Ii(hkl) − <I(hkl)>|/ΣhklΣiIi(hkl).
Rwork = Σ|Fc − Fo|)/ΣFo.
Values in parentheses indicate statistics for the highest-resolution bin.
The hACF7 EF1-EF2 Domain is a Canonical EFβ-scaffold
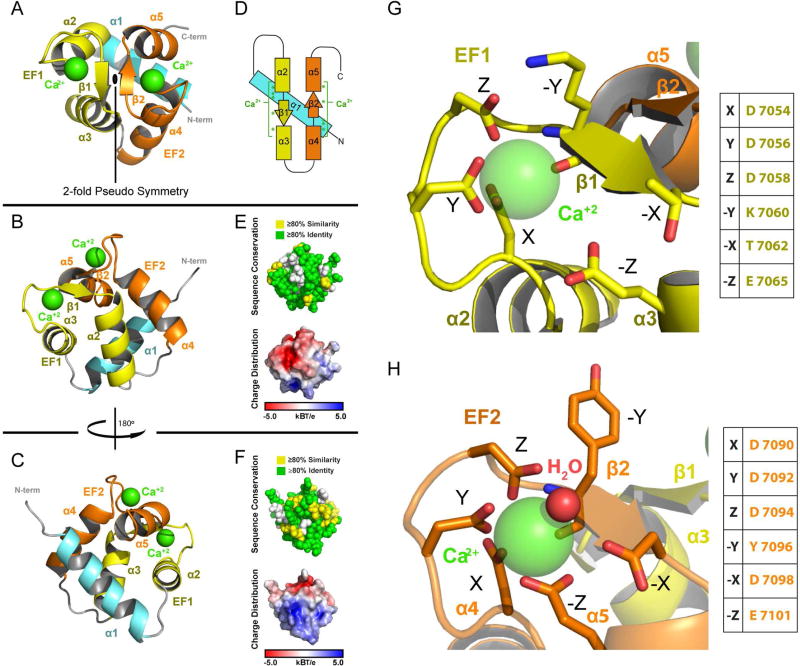
The EF1-EF2 domain consists of an N-terminal α1-helix followed by two EF-hands that each coordinate Ca2+ using a helix-loop-helix motif (Figure 2A–C). The two EF-hands are related by a prototypical two-fold pseudo symmetry axis, packing against one another to form a four helix-bundle (Denessiouk et al., 2014; Figure 2A). The loop regions within each EF-hand engage one another through backbone hydrogen bonding to form a two-stranded anti-parallel β-sheet across the pseudo two-fold axis, yielding an EFβ-scaffold as observed in calmodulin (Babu et al., 1988). The N-terminal α-helix is arranged orthogonal to, and wedged between, the helices of each EF-Hand in a mode most similar to that observed in the structure of calcyphosine (Dong et al., 2008; Figure S2A). The positioning of the ACF7 EF1-EF2 α1 helix is also reminiscent of the mode by which calcium-bound calmodulin EF3-EF4 engages target peptides (Chen et al., 2016; Figure S2B).
Figure 2. EF1-EF2 Domain Structure.
(A–C) Structure of the EF1-EF2 domain in different orientations. Following the N-terminal α-helix (cyan) are the EF1 (yellow) and EF2 (orange) helix-turn-helix motifs. Calcium atoms are shown in green. (D) Secondary structure topology of the EF1-EF2 domain showing the location of residues that coordinate calcium (green asterisks). (E,F) The EF1-EF2 domain illustrating cross-species conservation as delineated in Figure 1C (above, spherical representation) and displaying electrostatic surface potential (below, surface representation). Domain orientation in (E) and (F) correspond to the orientation in (B) and (C) respectively. (G, H) Zoom views of the EF1 and EF2 calcium binding sites. Canonical EF-Hand residues are indicated. See also Figure S2.
The EF1-EF2 domain is highly conserved across spectraplakins, with approximately 60% of residues identical across human, zebrafish, fruit fly, and nematode homologs (Figure 1C and 2E,F). Conservation maps across the surface of the domain, as well as the core, which consists of numerous aromatic residues. The EF1-EF2 domain has a polarized electrostatic surface potential: the face containing the Ca2+ coordinating residues is negatively charged while the opposite face, where the N-terminal α1-helix is positioned, is positively charged (Figure 2E,F). An extensive network of conserved aromatic residues dominates the hydrophobic core. While a subset of the aromatic network is conserved across EF-Hand-containing proteins (Denessiouk et al., 2014), the network is surprisingly expanded in ACF7 and may contribute to domain stabilization.
The EF1-EF2 Domain Coordinates Two Ca2+ Ions
The structure of the ACF7 EF1-EF2 domain reveals two canonical EF-Hand Ca2+ binding sites, each with a bound Ca2+ ion (Figure 2G,H). For frame of reference, our discussion uses the twelve-residue X·Y·Z·−Y·−X· ·−Z EF-hand nomenclature for residues involved in calcium binding (Kretsinger and Nockolds, 1973). EF-Hand structures typically coordinate Ca2+ using a pentagonal bipyramid geometry that involves seven oxygen atoms. While the coordination sphere in EF2 is canonical, the coordination sphere in EF1 is more relaxed. We discuss the coordination geometry of each EF-Hand in detail below.
In EF1 and EF2, residues X, Y, Z and −Y are positioned on the loop of the helix-loop-helix motif (Figure 2G,H). Residue –Z is located on the second α-helix of the motif. For both EF1 and EF2, residues X, Y, Z are aspartic acid residues and adhere to a prototypical DxDxDG calcium-binding loop sequence (Rigden and Galperin, 2004). The glycine residue found at position Z+1 in each EF-Hand uses its backbone amide to stabilize the side chain carboxyl group of the aspartic acid at position X, and imparts the flexibility necessary for the backbone carbonyl of the residue at position –Y to coordinate the Ca2+ ion. Collectively, residues at positions X, Y, Z and –Y contribute key oxygen atoms to the coordination geometry of each Ca2+ ion. In EF2, additional oxygen atoms are contributed by a glutamic acid residue at position –Z and from a water molecule stabilized by a glutamic acid residue at position –X, forming the archetypal pentagonal bipyramid coordination geometry (Figure 2H). In contrast, in EF1, the glutamic acid residue at position –Z is pulled back from the calcium binding site, and the serine at position –X is angled away, preventing direct, or water-mediated coordination of the calcium respectively (Figure 2G). The δ-carboxyl oxygens of the bidentate EF1 –Z glutamic acid are 4.7–5.9 Å from the Ca2+ ion, an atypical, extended distance that far exceeds the average distance of 2.37–2.43 Å found in other EF-Hand crystal structures (Zheng et al., 2008). As noted, this is not the case for the δ-carbonyl oxygens of the EF2 −Z glutamic acid residue, which are positioned 2.4–2.5 Å from the Ca2+ ion. Previous studies involving calmodulin and troponin C have demonstrated that the −Z residue plays important roles in calcium binding as well as target protein binding (Gagné et al., 1997; Gao et al., 1993; Maune et al., 1992). Intriguingly, the identity of the EF1 –Z residue is not conserved across spectraplakins (Figure 1C), suggesting that it does not play a key role in calcium binding, consistent with our structure. While our structure, which was crystallized in the presence of 1–2 mM calcium, has calcium bound to both EF-hands, it is likely that differences in the coordination geometries of EF1 and EF2 differentially affect their respective affinities for calcium. This may enable the domain to adopt distinct structural states across different calcium concentrations that regulate its binding to other factors or determinants within ACF7 itself.
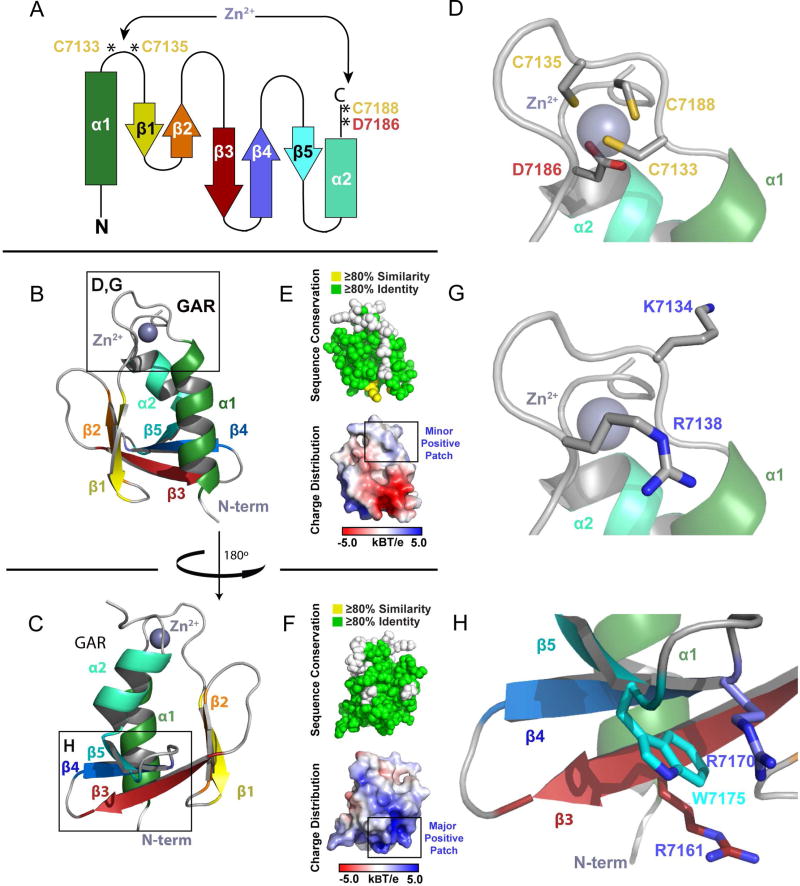
The GAR Domain is an α/β Sandwich that Coordinates Zinc
The structure of the GAR domain reveals a novel zinc-binding α/β fold. The GAR domain initiates with an N-terminal α-helix (α1) followed by a five-stranded anti-parallel β-sheet (β1–5) and a C-terminal α-helix (α2) positioned anti-parallel to α1 (Figure 3A–C). The two α-helices pack against the β-sheet to form an α/β sandwich. The α1-β1 loop and the C-terminal loop flanking α2 contain the highly conserved residues C7133, C7135, D7186 and C7188 that coordinate the bound zinc ion (Figures 1C, 3D). A Dali server search for homologous domains shows the highest degree of similarity to the frataxin (FXN) family of iron chaperones that play key roles in iron homeostasis (Roman et al., 2013; Figure S2C). In contrast to the GAR domain, FXN has a 6-stranded β-sheet, lacks a homologous zinc binding site, and uses a distinct, surface exposed region to bind iron (Bencze et al., 2007).
Figure 3. The GAR Domain is a Novel α/β Sandwich that Coordinates Zinc.
(A) Secondary structure topology of the GAR domain α/β sandwich showing the location of the residues that coordinate zinc. (B) Ribbon diagram of the GAR domain as colored in A. (C) The GAR domain as shown in (B), rotated 180° about the y-axis. (D) Zoom view of the zinc binding site boxed in (B), showing the Cys2-Asp-Cys residues that coordinate zinc. (E,F) The GAR domain illustrating cross-species conservation as delineated in Figure 1C (above, spherical representation) and displaying electrostatic surface potential (below, surface representation). Domain orientation in (E) and (F) correspond to the orientation in (B) and (C) respectively. (G,H) Zoom view of two positively charged regions on the GAR domain, corresponding to the boxed regions in (B) and (C) respectively. See also Figure S2.
The GAR Domain Uses a Tetrahedral Zinc Coordination Environment
The GAR domain binds zinc using a tetrahedral coordination geometry. Zinc is an abundant metal in proteins, and is thought to be a co-factor in ~10% of proteins (Laitaoja et al., 2013) where it plays either a structural or enzymatic role. Zn2+ ions with a structural role are usually coordinated by four side chains, while in enzymatic roles, the zinc coordination sphere usually consists of three side chains and a water molecule. As the GAR domain employs three cysteine residues and an aspartic acid residue (Figure 3D), it is likely that zinc plays a structural role. In support, we found that mutating cysteine residues in the coordination sphere to serine (individual or pairs) compromised the domain’s solubility when expressed in E. coli (data not shown). While the Cys2-Asp-Cys coordination sphere is invariant across spectraplakin homologs, its occurrence outside of spectraplakins is rare, with Cys4 or Cys2-His-Cys coordination spheres being more prevalent (Laitaoja et al., 2013). The GAR domain’s conserved coordination sphere suggests that the zinc binding region confers a function that cannot be accommodated by the more common coordination spheres.
The GAR Domain has a Conserved Basic Patch Distal to the Zinc Binding Site
The GAR domain surface is highly conserved with a notable exception being the residues in the α1-β1 loop not involved in zinc coordination (Figure 3E,F). Surface potential analysis reveals distinct patches of positive and negative charge. Many MT-associated proteins use a positively charged surface to bind the negatively charged MT lattice. The GAR domain has two basic regions (Figure 3G,H). The dominant basic patch spans β3-β5 includes the invariant arginine residues R7161 and R7170 that sandwich a conserved tryptophan, W7175 (Figure 3H). This region represents the highest, contiguous segment of conservation across spectraplakin GAR domains (Figure 1C), and extends to the homologous Gas2 MT-binding domain (Figure 1). A second minor basic patch is situated near the zinc-binding site (Figure 3G) and is composed of two positively charged residues (R7138, K7134) that are conserved across vertebrates, but are variable across invertebrates.
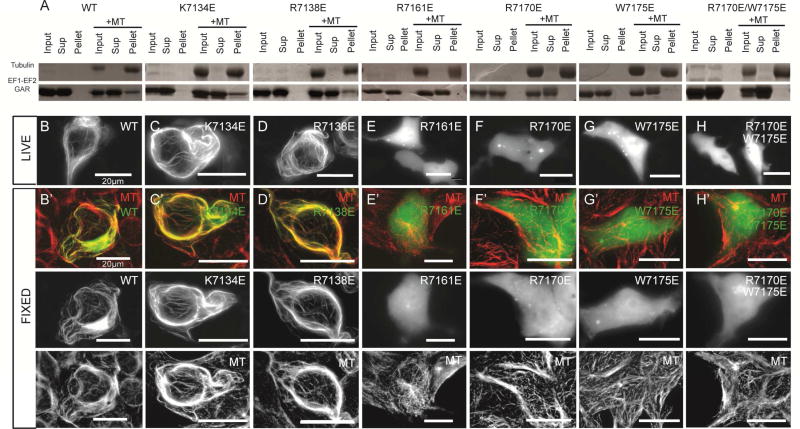
The GAR β3-β5 Region Mediates MT Binding
We next set out to map the GAR domain’s MT binding surface. Based on the acidic surface of MTs, we hypothesized that one of the GAR domain’s two basic regions (Figure 3E,F) mediates MT binding. We assayed the ability of EF1-EF2-GAR constructs to co-sediment with taxol-stabilized MTs in vitro. WT EF1-EF2-GAR showed robust MT co-sedimentation activity (Figure 4A). We introduced charge reversal mutations to test the involvement of each of the GAR domains’ basic regions. Inverting the charge of basic residues proximal to the zinc binding site (K7134E and R7138E; Figure 3E,G) did not affect MT co-sedimentation activity (Figure 4A). In contrast, negative charges introduced into the β3-β5 region (individual point mutations: R7161E, R7170E, W7175E, and double mutant: R7170E/W7175E; Figure 3F,H) completely ablated MT co-sedimentation activity (Figure 4A). The circular dichroism (CD) signature and thermal melt profiles of the WT and mutant EF1-EF2-GAR constructs were similar, indicating that these mutations did not compromise domain fold or stability (Figure S3A,B). Our co-sedimentation assays demonstrate that mutations in the GAR domain are sufficient to ablate EF1-EF2-GAR MT binding, indicating that the EF1-EF2 domain is not sufficient for direct MT binding. We did not detect MT co-sedimentation activity for an ACF7 EF1-EF2 construct, though an ACF7 GAR construct (as well as a GST-dimerized GAR construct) showed robust MT-co-sedimentation activity that could be ablated when R7170E and W7175E mutations were introduced (Figure S4A). While the EF1-EF2 domain was not sufficient for MT binding in vitro, our assay did not rule out the possibility that a low affinity interaction between the EF1-EF2 domain and MTs could occur if tethered to the MT lattice via GAR domain MT binding. To test this, we analyzed whether a dimerized GST-EF1-EF2 construct showed enhanced affinity for the MT lattice. The GST-EF1-EF2 construct cosedimented with MTs while a GST control construct did not. Negative charges introduced into a conserved basic patch delineated by EF1-EF2 α1 and the α1- α2 loop (pairwise point mutations: K7033E/R7037E and K7043E/R7045E) reduced the GST-EF1-EF2 construct’s MT co-sedimentation activity (Figure S4A) but did not affect domain fold as determined using CD (Figure S3C), implicating this region in MT binding.
Figure 4. The GAR β3-β5 Basic Region Mediates MT Binding.
(A) MT co-sedimentation assays of WT and mutant EF1-EF2-GAR constructs. (B–H) Live cell analysis of GFP-EF1-EF2-GAR WT and mutant constructs in HEK293 cells. (B’-H’) Analysis of GFP-EF1-EF2-GAR WT and mutant constructs for MT co-localization in fixed HEK293 cells. All scale bars: 20 µm. See also Figures S3, S4.
We next examined whether the EF1-EF2-GAR MT-binding determinants we delineated using MT co-sedimentation assays were responsible for MT-binding in HEK293 cells. We found that a GFP-EF1-EF2-GAR construct co-localized with MTs in live and fixed cells (Figure 4B, B’) consistent with previous studies (Leung et al., 1999; Sun et al., 2001). GFP-EF1-EF2-GAR overexpression caused MT bundling, a common artifact of overexpressing a MT binding protein in cells (Sousa et al., 2007). In contrast, both a GFP-EF1-EF2 construct as well as a dimerized GFP-GST-EF1-EF2 construct failed to co-localize with MTs (Figure S4B). While our GST-EF1-EF2 construct showed MT binding activity in vitro, we hypothesize that cellular salt concentrations and competition from other MT binding proteins prevented GST-EF1-EF2 MT binding in cells. We next tested whether mutating basic residues in the GAR domain would ablate MT binding in cells. Charge reversal mutations in the zinc binding region (K7134E and R7138E) did not affect MT binding in cell culture (Figure 4C,C’,D,D’). In contrast, negative charges introduced in the GAR domain’s β3-β5 region (R7161E, R7170E, W7175E, and R7170E/W7175E) completely abrogated the ability of the EF1-EF2-GAR construct to co-localize with MTs (Figure 4E,E’ – H,H’). In summary, a basic, conserved region spanning β3-β5 is involved in GAR MT binding (Figure S4C). Driven to the MT via the GAR domain, EF1-EF2 likely engages the MT using basic residues in α1 and the α1- α2 loop (Figure S4D). MT engagement using both the EF1-EF2 and GAR domains aligns with previous work that implicated EF1-EF2 in MT association (Applewhite et al., 2013; Kapur et al., 2012).
DISCUSSION
Our work illuminates the structures of spectraplakin EF1-EF2 and GAR domains. While the EF1-EF2 domain is not sufficient for MT binding in cells, the GAR domain is and likely enhances the ability of the EF1-EF2 domain to engage the MT lattice. A flexible linker between the domains would enable each domain to engage unique sites on the MT lattice. Once the GAR domain is bound to the MT lattice, the flexible linker could enable the EF1-EF2 domain to sample equivalent binding sites on the MT lattice, should a subset be sterically blocked by other MT associated proteins. The GAR domain’s dominant role in MT binding is consistent with the fact that the GAR domain, and not the EF1-EF2 domain, is found in the MT-binding Gas2 protein family. We have mapped GAR domain MT binding determinants to a conserved basic region distal to the Zn2+ binding site and EF1-EF2 MT binding determinants to a basic region opposite its calcium binding sites. The spectraplakin C-terminal MT-binding region consists of the MT-binding EF1-EF2-GAR module, a GSR repeat region with MT-lattice binding activity, and an EB1-binding SxIP motif. This tripartite composition suggests complex MT interactions that may involve MT plus end loading followed by MT-lattice binding. The ability to form distinct, multivalent interactions with a MT presents a means to tune spectraplakin-MT binding so that cross-linking activity can be spatially regulated. Consistent with this, GSK3β kinase activity spatially regulates GSR-MT interactions (Wu et al., 2011). Interestingly, the Drosophila Shot N-terminal CH domains bind the EF1-EF2-GAR module and limit MT lattice binding without affecting MT plus end tracking activity (Appelwhite et al., 2013). Future work will investigate whether an interaction between the EF1-EF2-GAR module and the CH domains is conserved in ACF7 and if so, how this interaction structurally limits MT-binding.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for reagents should be directed to the Lead Contact, Kevin C. Slep (kslep@bio.unc.edu).
METHOD DETAILS
Cloning, Expression, and Protein Purification of Crystallized hACF7 Construct
hACF7 EF1-EF2-GAR (Isoform 1 residues 7024–7206 Sequence: ANFDFKKYMRWMNHKKSRVMDFFRRIDKDQDGKITRQEFIDGILASKFPTTKLEMTAVA DIFDRDGDGDVWRYIDYYEFVAALHPNKDAYRPTTDADKIEDEVTRQVAQCKCAKRFQV EQIGENKYRFGDSQQLRLVRILRSTVMVRVGGGWMALDEFLVKNDPCRARGRTNIELREK FILPE) was subcloned into pET28 (Novagen) with a PreScission (GE Healthcare) cleavage site (LEVLFQ/GP), expressed in BL21 DE3 E. coli grown in Luria broth (50 µg/ml kanamycin) at 37°C and induced at OD600=0.8 with 0.1 mM isopropyl-β-D-thiogalactoside for 16 hrs at 20°C. Cells were isolated by centrifugation at 2900xg, resuspended in 250 ml buffer A (25 mM Tris, pH 8.0, 300 mM NaCl, 10 mM imidazole, 100 µM ZnCl2, 2 mM CaCl2, 0.1% β-mercaptoethanol (β-ME)), and stored at −20°C. The cells were thawed and sonicated with the addition of 1 mM PMSF as a protease inhibitor, centrifuged at 26900xg for 50 mins, and then purified using Ni2+-NTA (QIAGEN) chromatography (5 ml CV). The supernatant was loaded on the column by gravity at 4°C and then subsequently washed with 150 ml of buffer A. The protein was eluted by fractionation (5 ml) via FPLC (GE Healthcare) with a gradient of 10–300mM (0–100%) imidazole over 50 fractions. The protein eluted off the column between 70–115 mM imidazole. The fractions were pooled and buffer exchanged into Buffer C (25 mM HEPES (pH 8.0), 50 mM NaCl, 0.1% β-ME, 100 µM ZnCl2, 2 mM CaCl2). The hexahistidine tag was cleaved by PreScission protease (GE Healthcare) at 4°C for 18hrs. The cleaved protein was loaded onto an SP-sepharose (GE Healthcare) column (5 ml) and eluted off by fractionation (5 ml) via FPLC (GE Healthcare) with a gradient of 50mM-1M NaCl (0–100%) over 50 fractions. The protein eluted off the column between 250–400 mM NaCl. Protein eluted off between 300–400 mM NaCl was pooled and exchanged into 25 mM HEPES (pH 8.0), 150 mM NaCl, 0.1% β-ME, 100 µM ZnCl2, 2 mM CaCl2, concentrated to 10 mg/ml using a 10kDa cut-off centrifugal filter (Millipore), flash frozen in liquid nitrogen, and stored at −80°C.
Cloning, Expression, and Protein Purification of Additional hACF7 Constructs
hACF7 EF1-EF2-GAR wild-type/mutants (residues 7018–7206), EF1-EF2 wild-type/mutants (residues 7018–7116), and GAR (residues 7117–2706) were each subcloned into pET28 (Novagen) with a PreScission cleavage site (LEVLFQ/GP), pDEST15, and/or pGEX-62 (GAR only) (GE Healthcare). pDEST15 was subcloned using the Gateway pENTR/D-TOPO system (Invitrogen). pET28 with PreScission cleavage site and pGEX-62 were subcloned using restriction enzymes NdeI/HindIII and BamH1/EcoRI, respectively (see table for primers). Following cloning the protein constructs were expressed in BL21 DE3 E. coli grown in Luria broth in the appropriate antibiotic (50 µg/ml kanamycin: pET28, 50 µg/ml ampicillin: pDEST15/pGEX-62) at 37°C and induced at OD600≈0.8 with 0.1 mM isopropyl-β-D-thiogalactoside for 16 hrs at 20°C. Cells were harvested, resuspended in 250 ml buffer A (25 mM Tris, pH 8.0, 300 mM NaCl, 100 µM ZnCl2, 2 mM CaCl2, 0.1% β-mercaptoethanol (β-ME)), and stored at −20°C. For proteins with the hexahistidine tag, buffer A also included10 mM imidazole. Following thawing, sonication with the addition of 1 mM PMSF, centrifugation at 26900xg for 50 mins, these proteins were purified either by Ni2+-NTA (QIAGEN) or glutathione-S-sepharose (GE Healthcare) chromatography. For each hACF7 EF1-EF2-GAR (residues 7018–7206) and hACF7 EF1-EF2 (residues 7018–7116) construct expressed using pET28 with a PreScission site, the hexahistidine tag was cleaved by PreScission protease (GE Healthcare) at 4°C for 18hrs. The hexahistidine tag on the hACF7 GAR construct (residues 7117–2706) was unable to be cleaved by the PreScission protease. GST-tagged proteins were batch eluted using buffer A supplemented with 25 mM glutathione, pH 8.0. Proteins purified using Ni2+-NTA chromatography were batch eluted using buffer B (buffer A supplemented with 300 mM Imidazole). The proteins were exchanged into buffer A, concentrated to between 5–100 mg/ml using the appropriate size cut-off centrifugal filter (Millipore), then flash frozen in liquid nitrogen and stored at −80°C.
Crystallization of hACF7 EF1-EF2-GAR and Structure Determination
hACF7 EF1-EF2-GAR (residues 7024–7206, 10.0 mg/ml) was crystallized by the hanging drop method using a mother liquor (1 ml) containing 0.2 M KCl, 20% PEG3350. A 2 µl aliquot of hACF7 EF1-EF2-GAR at 10.0 mg/ml in crystallization buffer (25mM HEPES (pH 8.0), 150 mM NaCl, 0.1% β-ME, 100 µM ZnCl2, 2 mM CaCl2) was added to 2 µl of mother liquor at a 1:1 ratio on a siliconized glass coverslip (Hampton Research) and then sealed to reach equilibrium with the 1 ml mother liquor at 20°C in VDX plates (Hampton Research). Approximately 10 days following set up, crystals were harvested and transferred to perfluoropolyether oil (Mitegen, LV Cryo Oil) and flash frozen in liquid nitrogen. A zinc SAD peak diffraction data set (1.28295 Å) was collected using the 23-ID beamline (Advanced Photon Source, Argonne National Laboratory) at 100K. HKL2000 (Otwinowski and Minor, 1997), PHENIX (Adams et al., 2010), and Coot (Emsley et al., 2010) were used to integrate and scale diffraction data, solve and refine, and build the structure, respectively. Diffraction data was scaled and processed to 2.8 Å-resolution based on CC1/2 and Chi2 values in the final shell (2.90–2.80 Å) which dropped off significantly beyond 2.80 Å. Zn2+ and Ca2+ ions were identified and used to generate initial experimental, density-modified electron density maps (PHENIX). An initial model was generated using AutoBuild (PHENIX). Reiterative building in Coot followed by refinement runs using phenix.refine (PHENIX) were performed using individual B-factor refinement, experimental phase restraints against a maximum-likelihood Hendrickson-Lattman target, optimized x-ray/stereochemistry weight/ADP weight, and translation libration screw-motion refinement (12 TLS groups). Rfree was calculated using 10% of the data randomly excluded from refinement. The final model includes two hACF7 EF1-EF2 domains (residues 7024–7108, Chains A and B), a single GAR domain (residues 7118–7191, Chain C), 4 Ca2+ ions, 2 Zn2+ ions, and 11 water molecules (Rfree = 0.279). One Zn2+ ion was bound to the GAR domain by 3 cysteines (C7133, C7135, C7188) and an aspartic acid (D7186). A second Zn2+ ion interacted with a histidine residue introduced during cloning, as well as D7019 and R7032. Figures were generated using PyMOL (Schrödinger) and APBS (Baker et al., 2001).
SEC-MALS
hACF7 EF1-EF2-GAR (residues 7024–7206, 8 and 5 mg/ml,100 µl), His-GAR (residues 7117–7206,1 mg/ml,100 µl), EF1-EF2 (residues 7018–7116, 2.5 mg/ml, 100µl), His-GAR + EF1-EF2 (same concentrations as individual experiments, ≈2:1 molar ratio EF1-EF2:GAR) were injected onto a Superdex 200 10/300 GL size exclusion column (GE Healthcare) at 0.5 ml/min in running buffer (25 mM Tris, pH 8.0, 300 mM NaCl, 0.1% β-mercaptoethanol, and 0.2 g/liter sodium azide) and then passed serially through a UV detector, a light scattering instrument (DAWN HELEOS II; Wyatt Technology), and a refractometer (Optilab rEX; Wyatt Technology). The light scattering and refractive index data were used to calculate the weight-averaged molar mass using Wyatt Astra V software (Wyatt Technology). Data were processed with ASTRA software and plotted using Prism 6. hACF7 EF1-EF2-GAR samples injected at 8 mg/ml and 5 mg/ml eluted as a single Gaussian peak with an experimentally determined molecular weight of 24.6±0.8 and 26.4±0.8 kDa respectively, corresponding to a predominantly monomeric population (99.1% and 96.4% respectively). This is reiterated with both hACF7 His-GAR (14.2±1.8 kDa, 98.0%) and hACF7 EF1-EF2 (16.4±1.4 kDa, 100%), which both yield a single Gaussian peak when analyzed individually. The combined hACF7 His-GAR and EF1-EF2 also produced a single peak (100%, 13.8±0.4 kDa) corresponding to no interaction between the domains. Theoretical values suggesting no interaction were generated by the addition of the differential refractive index for hACF7 His-GAR and EF1-EF2 run individually. The approximate 2-fold molar excess of hACF7 EF1-EF2 and His-GAR were incubated overnight at 4°C to allow for sufficient time to potentially interact.
MT Co-sedimentation Assays
Taxol-stabilized MTs were prepared and co-sedimentation assays conducted as described (Campbell and Slep, 2011). The proteins of interest were buffer exchanged into BRB80 (80 mM PIPES, 1 mM MgCl2, 1 mM EGTA pH to 6.8 (adjusted with KOH)) using a suitable molecular-weight cutoff centrifugal filter (Millipore). Following buffer exchange, the proteins were centrifuged at 100,000xg for 7 mins at 4°C to pellet and remove any unstable protein. 10 µM taxol-stabilized MTs, BRB80, 1 mM GTP, 20 µM taxol, and 20 µM protein of interest was incubated at room temperature for 20 min. As a control, samples that lacked MTs were also incubated for 20 min. 100 µl of the 120 µl reaction mixture was layered on top of a 150 µl, 40% glycerol cushion and centrifuged at 100,000xg for 30 min at room temperature. A supernatant fraction was collected from the top of the sample and than the glycerol cushion was washed 3x with BRB80. Following the wash step, the pellet fraction was collected from below the glycerol cushion by resuspension of the remaining protein in 100 µl BRB80. Supernatant and pellet samples were analyzed by SDS-PAGE using R-250 Brilliant blue coomassie stain (ThermoFisher) to assay for co-sedimentation activity.
Circular Dichroism
Each protein sample was diluted to a final concentration of 0.1 mg/ml in 10 mM sodium phosphate buffer (pH 7.5), 50 mM NaFl. CD spectra (185–260 nm) of hACF7 EF1-EF2-GAS2 (residues 7018–7206) (wild-type, R7161E, R7170E, W4042E, R7170E/W7175E) and EF1-EF2 (residues 7018–7116, wild-type, K7033E/R7037E, K7043E/R7045E) were collected at 20°C with a time per point of 1.25 s using a 1-mm path-length cuvette in duplicate. Individual spectra were averaged and smoothed with a moving window of 4 points in the Chirascan-plus software. The direct signal output (mdegs) from the Chirascan-plus CD spectrometer (Applied Photophysics) was converted to molar elipticity (deg cm2 dmol−1) using Excel. For each hACF7 EF1-EF2-GAS2 construct, data for a range of temperatures was also collected. The CD signal (mdeg) was recorded at 208 and 222 nm (two minima associated with helical secondary structure) along a temperature gradient from 20–94°C with a temperature step size of 1°C using a 1-mm path-length cuvette. A spectrum was taken at 94°C (not shown) to ensure that the protein was unfolded. The time per point was 1.25 s and the temperature tolerance was 0.2°C. Each temperature ramp was smoothed with a moving window of 6 points in the Chirascan-plus software. The stability of each mutant was quantified by observing the unfolding vs. temperature at 208/222 nm. The Tm was calculated by delineating the inflection point of the curve (maximum of the first derivative). Since Tm is assumed to be independent of concentration, the units were not converted from mdeg to molar elipticity. Each CD melt curve was repeated once to ensure reproducibility.
HEK293 Cell Expression Plasmids
hACF7 EF1-EF2-GAR wild-type/mutants (residues 7018–7206) and EF1-EF2 (residues 7018–7116) constructs were subcloned using the Gateway pENTR/D-TOPO system (Invitrogen) into a destination vector with an N-terminal GFP tag (pcDNA-DEST53 plasmid) under a CMV constitutively active promoter (Invitrogen). Mutant constructs were generated using a KOD Xtreme site-directed mutagenesis protocol (Novagen) and confirmed by DNA sequencing. Modification of a pDEST53 vector subcloned with EF1-EF2 was used to generate the GFP-GST-EF1-EF2 (residues 7018–7116) construct. The GST+linker from pGEX-6p2 (GE Healthcare) with modified 5’ and 3’ extensions was amplified by PCR and used as “primers” for KOD Xtreme amplification of GST-EF1-EF2 (residues 7018–7116) in pDEST53. The terminal vector contained the intact linkers of both pDEST53 and pGEX-6p2, providing enough flexibility and linker distance to enable each domain to engage homologous sites on the microtubule lattice. The GST addition was confirmed by DNA sequencing.
Cell Culture and Transfection
HEK293 cells (ThermoFisher) were cultured in DMEM high-glucose media (Gibco) supplemented with 10% FBS serum (Gibco) and a 1x Anti-Anti (Gibco) at 37°C and at 5% C02. Cells were seeded onto acid-washed glass coverslips and transfected once they reached 60–80% confluence. Seeds were obtained by suspending 60–80% confluent cells with 0.5% Trypsin with EDTA following by neutralization by supplemented DMEM. hACF7 GFP-EF1-EF2-GAR (residues 7018–7206) and GFP-EF1-EF2 (residues 7018–7116) constructs (under control of a constitutively active human cytomegalovirus immediate-early (CMV) promoter) were transfected using the ViaFect Transfection Reagent (Promega) as described by the manufacturer’s protocol. Each transfection used 1 µg of plasmid DNA diluted into DMEM to a final volume of 100 µl. ViaFect Transfection Reagent was then added at a 3:1 reagent:DNA ratio and incubated for 15–20 mins before being added to the cells.
Immunofluorescence Microscopy
HEK293 (ThermoFisher) cells transfected with hACF7 GFP-EF1-EF2-GAR (residues 7018–7206, wild-type/mutant), GFP-EF1-EF2 (residues 7018–7116), or GFP-GST-EF1-EF2 (residues 7018–7116) constructs were fixed or imaged live 14–16 hrs post transfection. Cells were fixed with 4% paraformaldehyde in PHEM (5 mM HEPES, 60 mM PIPES pH 7.0, 10 mM EGTA, and 2 mM MgCl2) for 20 min. PBS-Triton (PBST)(0.5%) and PHEM-Triton (0.2%) supplemented with 1% normal bovine serum (Fisher Scientific) were used to permeabilize and block the cells, respectively. Antibodies were diluted in PHEM supplemented with 1% normal bovine serum. Antibodies used for immunofluorescence included a mouse monoclonal anti-α-tubulin antibody (clone DM1a, T6199; 1:500) (Sigma-Aldrich) and a Cy3-α-mouse conjugated secondary antibody (1:500) (Jackson ImmunoResearch Laboratories). Nuclei were stained with 4’, 6-diamidino-2-phenylindole (1:2000) (Molecular Probes, Invitrogen). Cells were mounted in fluorescence mounting medium (Dako). Images were acquired using an Eclipse Ti microscope with a 100x oil NA-1.45 objective (Nikon) by a Coolsnap HQ camera (Photometrics), driven by NIS Elements software (Nikon). Representative images were processed using Photoshop CS5 (Adobe Systems) and ImageJ (National Institutes of Health).
Trypsin digest of hACF7 EF1-EF2-GAR, EF1-EF2, His-GAR
hACF7 EF1-EF2-GAR (residues 7024–7206), EF1-EF2 (residues 7018–7116), and His-GAR (residues 7117–7206) trypsin digests were all done at room temperature using the following protocol: 20 µg (8 µl) of trypsin (Gibco) was added to 50 µg of the protein of interest (1:2.5 trypsin:hACF7) in 17 µl HEPES buffer (25 mM HEPES (pH 8.0), 100 mM NaCl, 0.1% β-ME). Samples incubated at room temperature for 0.1, 5, 10, 20, 30, 40, 60, 90, 120, and 160 mins (hACF7 EF1-EF2-GAR only for 160 mins). At each time point, SDS was added and the reaction was boiled for 20 mins to quench the digest and samples were maintained on ice. Once the final sample was quenched, time points were immediately analyzed via SDS-PAGE. Samples with trypsin or protein alone were also incubated for the entirety of the experiment at room temperature as controls.
Analysis of the hACF7 EF1-EF2-GAR construct following crystallization
All crystals analyzed via SDS-PAGE were grown in PEG3350 and KCl (as described above) and were harvested approximately 4 months after setup. The crystals were isolated from the remaining solubilized protein before analysis. Each sample consisted of a 2 µl aliquot of hACF7 EF1-EF2-GAR (residues 7024–7206) at 10.0 mg/ml in crystallization buffer (25mM HEPES (pH 8.0), 150 mM NaCl, 0.1% β-ME, 100 µM ZnCl2, 2 mM CaCl2) combined with 2 µl of the mother liquor at a 1:1 ratio on a siliconized glass coverslip (Hampton Research) and then sealed to reach equilibrium with the 1 ml mother liquor at 20°C in VDX plates (Hampton Research). Crystals were harvested, crushed in 20 µl of crystallization buffer, SDS loading buffer added, boiled for 15 mins, and then analyzed using SDS-PAGE. The resuspended crystals were comparatively run against thawed, frozen stocks (−80°C) of hACF7 EF1-EF2-GAR (residues 7024–7206), EF1-EF2 (residues 7018–7116), and His-GAR (residues 7117–7206), revealing an intact EF1-EF2-GAR.
DATA AND SOFTWARE AVAILABILITY
The coordinates and structure factors for the human ACF7 EF1-EF2-GAR structure have been deposited in the RCSB Protein Data Bank under ID code 5VE9.
Supplementary Material
HIGHLIGHTS.
-
-
The EF1-EF2 and GAR domains are autonomous domains connected by a flexible linker.
-
-
The GAR domain is a Zn2+-binding α/β sandwich that is sufficient for MT binding.
-
-
The GAR domain uses a conserved basic patch, distal to its Zn2+ site, to bind MTs.
-
-
GAR-MT binding likely enhances EF1-EF2-MT engagement.
Acknowledgments
We thank A. Tripathy for technical assistance. Work was supported by NIH grants R03HD084980 and R01GM094415 (to K.C.S.), T32GM008570 (to the UNC Molecular and Cellular Biophysics Program), and R37AR27883 (to E.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information includes STAR Methods and four figures.
AUTHOR CONTRIBUTIONS
T.R.L., E.F., and K.C.S. designed research; T.R.L. performed research. T.R.L. and K.C.S. wrote the manuscript. E.F. commented on the manuscript.
References
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applewhite DA, Grode KD, Duncan MC, Rogers SL. The actin-microtubule cross-linking activity of Drosophila Short stop is regulated by intramolecular inhibition. Mol. Biol. Cell. 2013;24:2885–2893. doi: 10.1091/mbc.E12-11-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu YS, Bugg CE, Cook WJ. Structure of calmodulin refined at 2.2 Å resolution. J. Mol. Biol. 1988;204:191–204. doi: 10.1016/0022-2836(88)90608-0. [DOI] [PubMed] [Google Scholar]
- Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencze KZ, Yoon T, Millán-Pacheco C, Bradley PB, Pastor N, Cowan JA, Stemmler TL. Human frataxin: iron and ferrochelatase binding surface. Chem. Commun. 2007:1798–1800. doi: 10.1039/b703195e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher JM, Hahn BS, Legouis R, Sookhareea S, Weimer RM, Gansmuller A, Chisholm AD, Rose AM, Bessereau JL, Labouesse M. The Caenorhabditis elegans vab-10 spectraplakin isoforms protect the epidermis against internal and external forces. J. Cell Biol. 2003;161:757–768. doi: 10.1083/jcb.200302151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenberg W, Sanchez-Soriano N, Alves-Silva J, Hahn I, Mende M, Prokop A. Context-specific requirements of functional domains of the Spectraplakin Short stop in vivo. Mech. Dev. 2009;126:489–502. doi: 10.1016/j.mod.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Brown A, Bernier G, Mathieu M, Rossant J, Kothary R. The mouse dystonia musculorum gene is a neural isoform of bullous pemphigoid antigen 1. Nat. Genet. 1995;10:301–306. doi: 10.1038/ng0795-301. [DOI] [PubMed] [Google Scholar]
- Byers TJ, Beggs AH, McNally EM, Kunkel LM. Novel actin crosslinker superfamily member identified by a two step degenerate PCR procedure. FEBS Lett. 1995;368:500–504. doi: 10.1016/0014-5793(95)00722-l. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Slep KC. αβ-Tubulin and microtubule-binding assays. Methods Mol. Biol. 2011;777:87–97. doi: 10.1007/978-1-61779-252-6_6. [DOI] [PubMed] [Google Scholar]
- Chen Y, Clarke OB, Kim J, Stowe S, Kim Y-K, Assur Z, Cavalier M, Godoy-Ruiz R, von Alpen DC, Manzini C, et al. Structure of the STRA6 receptor for retinol uptake. Science. 2016;353 doi: 10.1126/science.aad8266. aad8266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denessiouk K, Permyakov S, Denesyuk A, Permyakov E, Johnson MS. Two structural motifs within canonical EF-hand calcium-binding domains identify five different classes of calcium buffers and sensors. PLoS One. 2014:9. doi: 10.1371/journal.pone.0109287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Li X, Lou Z, Xu X, Su D, Zhou X, Zhou W, Bartlam M, Rao Z. Crystal-Structure and Biochemical Characterization of Recombinant Human Calcyphosine Delineates a Novel EF-Hand-Containing Protein Family. J. Mol. Biol. 2008;383:455–464. doi: 10.1016/j.jmb.2008.08.048. [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagné SM, Li MX, Sykes BD. Mechanism of direct coupling between binding and induced structural change in regulatory calcium binding proteins. Biochemistry. 1997;36:4386–4392. doi: 10.1021/bi963076+. [DOI] [PubMed] [Google Scholar]
- Gao ZH, Krebs J, VanBerkum MFA, Tang WJ, Maune JF, Means AR, Stull JT, Beckingham K. Activation of four enzymes by two series of calmodulin mutants with point mutations in individual Ca2+ binding Sites. J. Biol. Chem. 1993;268:20096–20104. [PubMed] [Google Scholar]
- Guo L, Degenstein L, Dowling J, Yu QC, Wollmann R, Perman B, Fuchs E. Gene targeting of BPAG1: Abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell. 1995;81:233–243. doi: 10.1016/0092-8674(95)90333-x. [DOI] [PubMed] [Google Scholar]
- Kapur M, Wang W, Maloney MT, Millan I, Lundin VF, Tran T-A, Yang Y. Calcium tips the balance: a microtubule plus end to lattice binding switch operates in the carboxyl terminus of BPAG1n4. EMBO Rep. 2012;13:1021–1029. doi: 10.1038/embor.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakesisoglou I, Yang Y, Fuchs E. An epidermal plakin that integrates actin and microtubule networks at cellular junctions. J. Cell Biol. 2000;149:195–208. doi: 10.1083/jcb.149.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama A, Karakesisoglou I, Wong E, Vaezi A, Fuchs E. ACF7: An essential integrator of microtubule dynamics. Cell. 2003;115:343–354. doi: 10.1016/s0092-8674(03)00813-4. [DOI] [PubMed] [Google Scholar]
- Kretsinger RH, Nockolds CE. Carp muscle calcium-binding protein. II. Structure determination and general description. J. Biol. Chem. 1973;248:3313–3326. [PubMed] [Google Scholar]
- Laitaoja M, Valjakka J, Jänis J. Zinc coordination spheres in protein structures. Inorg. Chem. 2013;52:10983–10991. doi: 10.1021/ic401072d. [DOI] [PubMed] [Google Scholar]
- Lee S, Kolodziej Pa. Short Stop provides an essential link between F-actin and microtubules during axon extension. Development. 2002;129:1195–1204. doi: 10.1242/dev.129.5.1195. [DOI] [PubMed] [Google Scholar]
- Lee S, Nahm M, Lee M, Kwon M, Kim E, Zadeh AD, Cao H, Kim H-J, Lee ZH, Oh SB, et al. The F-actin-microtubule crosslinker Shot is a platform for Krasavietz-mediated translational regulation of midline axon repulsion. Development. 2007;134:1767–1777. doi: 10.1242/dev.02842. [DOI] [PubMed] [Google Scholar]
- Leung CL, Sun D, Zheng M, Knowles DR, Liem RKH. Microtubule actin cross-linking factor (MACF): A hybrid of dystonin and dystrophin that can interact with the actin and microtubule cytoskeletons. J. Cell Biol. 1999;147:1275–1285. doi: 10.1083/jcb.147.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JJ, Ding J, Kowal AS, Nardine T, Allen E, Delcroix JD, Wu C, Mobley W, Fuchs E, Yang Y. BPAG1n4 is essential for retrograde axonal transport in sensory neurons. J. Cell Biol. 2003;163:223–229. doi: 10.1083/jcb.200306075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maune JF, Klee CB, Beckingham K. Ca2+ binding and conformational change in two series of point mutations to the individual Ca2+-binding sites of calmodulin. J. Biol. Chem. 1992;267:5286–5295. [PubMed] [Google Scholar]
- Mueller S, Klaus-Kovtun V, Stanley JR. A 230-kD basic protein is the major bullous pemphigoid antigen. J. Invest. Dermatol. 1989;92:33–38. doi: 10.1111/1523-1747.ep13070476. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Prokop A, Uhler J, Roote J, Bate M. The kakapo mutation affects terminal arborization and central dendritic sprouting of Drosophila motorneurons. J. Cell Biol. 1998;143:1283–1294. doi: 10.1083/jcb.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigden DJ, Galperin MY. The DxDxDG motif for calcium binding: Multiple structural contexts and implications for evolution. J. Mol. Biol. 2004;343:971–984. doi: 10.1016/j.jmb.2004.08.077. [DOI] [PubMed] [Google Scholar]
- Roman EA, Faraj SE, Cousido-Siah A, Mitschler A, Podjarny A, Santos J. Frataxin from Psychromonas ingrahamii as a model to study stability modulation within the CyaY protein family. Biochim. Biophys. Acta - Proteins Proteomics. 2013;1834:1168–1180. doi: 10.1016/j.bbapap.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Sanchez-Soriano N, Travis M, Dajas-Bailador F, Gonçalves-Pimentel C, Whitmarsh AJ, Prokop A. Mouse ACF7 and Drosophila Short stop modulate filopodia formation and microtubule organisation during neuronal growth. J. Cell Sci. 2009;122:2534–2542. doi: 10.1242/jcs.046268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slep KC, Rogers SL, Elliott SL, Ohkura H, Kolodziej PA, Vale RD. Structural determinants for EB1-mediated recruitment of APC and spectraplakins to the microtubule plus end. J. Cell Biol. 2005;168:587–598. doi: 10.1083/jcb.200410114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa A, Reis R, Sampaio P, Sunkel CE. The Drosophila CLASP homolog, MAST/Orbit regulates the dynamic behaviour of interphase microtubules by promoting the pause state. Cell Motil Cytoskeleton. 2007;64:605–620. doi: 10.1002/cm.20208. [DOI] [PubMed] [Google Scholar]
- Stanley JR, Hawley-Nelson P, Yuspa SH, Shevach EM, Katz SI. Characterization of bullous pemphigoid antigen: A unique basement membrane protein of stratified squamous epithelia. Cell. 1981;24:897–903. doi: 10.1016/0092-8674(81)90115-x. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Prokop A, Yamamoto M, Sugimura K, Uemura T, Betschinger J, Knoblich JA, Volk T. Shortstop recruits EB1/APC1 and promotes microtubule assembly at the muscle-tendon junction. Curr. Biol. 2003;13:1086–1095. doi: 10.1016/s0960-9822(03)00416-0. [DOI] [PubMed] [Google Scholar]
- Sun D, Leung CL, Liem RK. Characterization of the microtubule binding domain of microtubule actin crosslinking factor (MACF): identification of a novel group of microtubule associated proteins. J. Cell Sci. 2001;114:161–172. doi: 10.1242/jcs.114.1.161. [DOI] [PubMed] [Google Scholar]
- Suozzi KC, Wu X, Fuchs E. Spectraplakins: Master orchestrators of cytoskeletal dynamics. J. Cell Biol. 2012;197:465–475. doi: 10.1083/jcb.201112034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vactor D, Sink H, Fambrough D, Tsoo R, Goodman CS. Genes that control neuromuscular specificity in Drosophila. Cell. 1993;73:1137–1153. doi: 10.1016/0092-8674(93)90643-5. [DOI] [PubMed] [Google Scholar]
- Wu X, Kodama A, Fuchs E. ACF7 Regulates Cytoskeletal-Focal Adhesion Dynamics and Migration and has ATPase Activity. Cell. 2008;135:137–148. doi: 10.1016/j.cell.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Shen QT, Oristian DS, Lu CP, Zheng Q, Wang HW, Fuchs E. Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3β. Cell. 2011;144:341–352. doi: 10.1016/j.cell.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Bauer C, Strasser G, Wollman R, Julien JP, Fuchs E. Integrators of the cytoskeleton that stabilize microtubules. Cell. 1999;98:229–238. doi: 10.1016/s0092-8674(00)81017-x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Dowling J, Yu QC, Kouklis P, Cleveland DW, Fuchs E. An essential cytoskeletal linker protein connecting actin microfilaments to intermediate filaments. Cell. 1996;86:655–665. doi: 10.1016/s0092-8674(00)80138-5. [DOI] [PubMed] [Google Scholar]
- Yue J, Zhang Y, Liang WG, Gou X, Lee P, Liu H, Lyu W, Tang WJ, Chen SY, Yang F, et al. In vivo epidermal migration requires focal adhesion targeting of ACF7. Nat Commun. 2016;7:11692. doi: 10.1038/ncomms11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Chruszcz M, Lasota P, Lebioda L, Minor W. Data mining of metal ion environments present in protein structures. J. Inorg. Biochem. 2008;102:1765–1776. doi: 10.1016/j.jinorgbio.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.