Abstract
Puerarin has long been used as a traditional Chinese medicine, which possesses various physiological properties, including anti-inflammation, anti-oxidative, anti-diabetic and anti-cancer activities. The aim of the current study was to investigate the effect of puerarin on delayed-type hypersensitivity (DTH) induced by ovalbumin (OVA) in mice and explore its underlying mechanisms. The results showed that puerarin significantly attenuated DTH, resulting from a decrement in footpad swelling, reduction in inflammatory cell as well as a decline in anti-OVA IgG in serum. In the homogenized supernatant of footpad tissues, the classic Th1-cytokines, including interferon (IFN)-γ was suppressed following puerarin treatment. Furthermore, a high dose of puerarin inhibited interleukin (IL)-4 production, the classic Th2-cytokine. The concanavalin A stimulation and MTT assays indicated a suppressive effect of puerarin on Th1 response via decreasing IFN-γ production in OVA-primed lymphocytes. Detailed studies revealed that puerarin modulated the Th1/Th2 balance in DTH responses, attributing to lower T-bet/GATA binding protein-3 mRNA and protein level ratios, which led to the shift change of IFN-γ/IL-4 with puerarin treatment. These findings demonstrate that puerarin alleviated inflammation in DTH triggered by OVA application via curbing inflammatory cytokines by modulating the Th1/Th2 balance. These results suggest that puerarin may be an alternative therapeutic option for the treatment of DTH.
Keywords: puerarin, inflammation, ovalbumin, delayed-type hypersensitivity, Th1/Th2
Introduction
Two subsets of Th cells, named Th1 and Th2, play a key role in the regulation of the adaptive immunity (1). Th1 cells majorly present pro-inflammatory cytokines, including interferon (IFN)-γ, interleukin (IL)-2 and tumor necrosis factor (TNF)-α, which activate CD8+ T cells and macrophages, thus promoting cellular immune reactions. Conversely, Th2 cells largely generate anti-inflammatory cytokines, such as IL-4, IL-10 and IL-13, as well as stimulate mast cells, eosinophils and B cells, thereby enhancing allergic responses and humoral immunity (2). The imbalance between Th1 and Th2 cells in development and regulation participates in various immune disorders including hypersensitivity (3).
Delayed-type hypersensitivity (DTH), known as type IV hypersensitivity reaction, has been demonstrated to be a Th1-driven immune response, and is associated with contact allergy with the activity of inflammatory Th1 lineage secreting IFN-γ (4). Because the hypersensitivity is also a useful approach for evaluating cell-mediated immune responses that migrates to the inflammatory site, the experimental model of ovalbumin (OVA)-induced DTH is widely used to assess the immunomodulation and anti-inflammatory effects of a serious of new substances and materials on T-cell immune response (5,6).
As the principal bioactive ingredient, puerarin generally is isolated from the roots of Pueraria lobate. Until now, puerarin has been widely indicated to possess a broad range of pharmacological activities, including anti-cardiotoxicity anti-neurotoxicity, anti-oxidative, anti-diabetic, anti-tumor and anti-inflammatory effects (7,8). A previous study showed that puerarin inhibited the activation of NF-kB pathway and TNF-α productio in peripheral blood mononuclear cells in asthma patients, suggesting its protective effect in allergic disease (9). Utilizing a mouse model of allergic asthma, a recent study indicated that puerarin treatment attenuated OVA-induced airway inflammation by the regulation of eotaxin-3, which suggested its potential application in allergic inflammation although the detailed mechanism was unknown (10).
In this study, our results indicated that puerarin treatment alleviated OVA-induced DTH by modulating Th1/Th2 balance, resulting from the limitation of Th1 response accompanying inhibitive production of IFN-γ. These findings suggest puerarin would be effective alternative to eliminate inflammation associated with DTH.
Materials and methods
Animals
Female C57BL/6 mice weighing 18–20 g, were purchased from Hubei Research Center of Laboratory Animals (Wuhan, China) and housed in an air-conditioned room (23±0.5°C, 12 h light/dark cycle) with free access to food and water. All experimental procedures were carried out in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publication no. 80–23, revised in 1996), and animal handling followed the dictates of the Animal Care and Use Committee of Hubei University of Chinese Medicine (SYXK-2012-0067).
DTH
Mice were immunized s.c. with 50 µg OVA (Worthington Biochemical Corp., Lakewood, NJ, USA) in 100 µl PBS-CFA emulsion (50 µl complete freund's adjuvant) (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). After 6 days, mice were challenged s.c. 200 µg heat-aggregated OVA in 20 µl of PBS in the left hind footpad and in the right footpad with PBS only. After 24 h, OVA-specific DTH was determined by footpad swelling according to the difference in the footpad thickness between OVA-challenged footpad and PBS-injected control footpad.
Groups
48 Mice were randomly assigned to six different groups: Control group (not immunized), DTH group (OVA-induced DTH), Puerarin (Pue) + DTH group (low-, middle- and high-dose puerarin was used during repeated OVA application, respectively), Dexamethasone (Dex) + DTH group (dexamethasone treatment during repeated OVA application).
Puerarin treatment
In the therapeutic treatment protocol (Fig. 1A), mice were treated with puerarin (Sigma-Aldrich; Merck KGaA) via intraperitoneal injection for continuous 7 days (8 mg/kg for the low dose, 40 mg/kg for the middle dose and 200 mg/kg for the high dose) (11,12). As a positive control, dexamethasone (1 mg/kg) (Sigma-Aldrich; Merck KGaA) was administered intraperitoneally from day 1 to day 7.
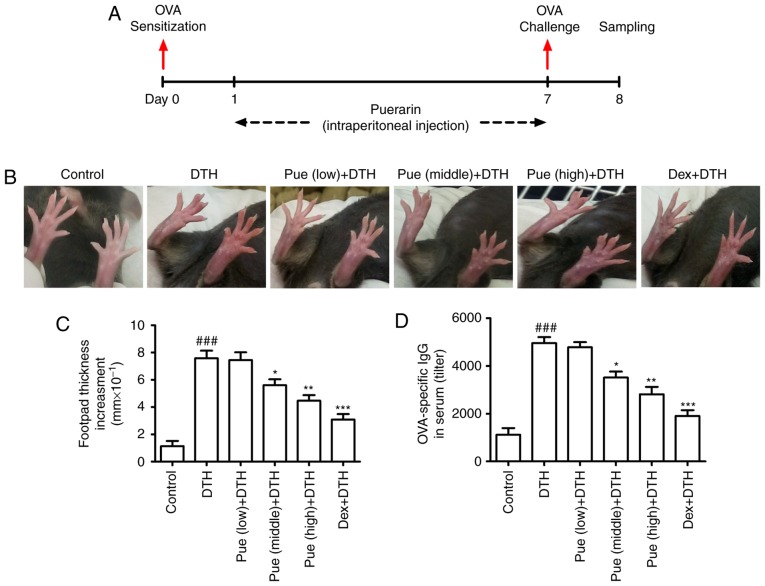
Figure 1.
Puerarin treatment attenuates ovalbumin (OVA)-induced delayed-type hypersensitivity (DTH) in mice. (A) C57BL/6 mice were sensitized with OVA on day 1 and challenged after 6 days. Simultaneously, different concentration of puerarin was used for continuous one week. (B) Morphological changes of the footpad swelling and (C) The increment of swelling was shown, respectively. (D) Effect of puerarin on the OVA-specific antibody in serum of different group. *P<0.05, **P<0.01 and ***P<0.001, comparison with the DTH group. ###P<0.001, comparison with the Control group. Dex, Dexamethasone.
Histological analysis
The left hind footpads of mice in the different group were removed and fixed in 4% paraformaldehyde. Fixed tissues were embedded in paraffin for 24 h and serially sectioned to a thickness of 5 µm for histological analysis. Tissue sections were stained with hematoxylin and eosin (H&E; Sigma-Aldrich; Merck KGaA) and examined for histopathological change. Pictures were captured using a Nikon Eclipse Ti-S microscope (Nikon, Tokyo, Japan). A minimum of three sections per animal experimentation was examined, and ten visual fields of each sample were randomly selected to observe the inflammatory cell infiltration in a blinded manner. The evaluation was performed at ×200 magnification.
Sear collection and anti-OVA IgG assay
Sera of mice in different groups were collected at the termination of experiment for the detection of anti-OVA IgG. Blood from the internal veins of mice in the different group was collected. After centrifugation at 12,000 × g for 10 min at 4°C, the serum was used for the ELISA assay of anti-OVA IgG. The levels of serum anti-OVA IgG were determined using the ELISA kit (3011) (Chondrex, Seattle, WA, USA) with a micro-plate reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Enzyme-linked immunosorbent assay (ELISA)
The left footpad samples of different group were weighed and homogenized in 1 ml of tissue protein extraction reagent containing a protease inhibitor cocktail (Pierce; Thermo Fisher Scientific, Inc.). Homogenates were then centrifuged at 12,000 × g for 15 min at 4°C to obtain the supernatant. Cytokine such as IFN-γ (MIF00) and IL-4 (M4000B) were measured with the ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturer's instruction.
Preparation of spleen lymphocytes
Spleen was collected and the mononuclear cells were isolated based on the method of density gradient centrifugation using the Ficoll-Hypaque (Sigma-Aldrich; Merck KGaA) (13–15). Spleen mononuclear cells were isolated by density gradient centrifugation (2,000 × g for 30 min) (Ficoll-Hypaque density 1.077 g/ml) and incubated overnight at 37°C in complete RPMI-1640 medium containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.). Nonadherent cells were harvested as spleen lymphocytes, which majorly includes T cells.
ConA stimulation assay
Spleen lymphocytes (2×105) were isolated and co-cultured with ConA (5 mg/ml) (Sigma-Aldrich; Merck KGaA) at 37°C. After 24 h, the ConA stimulation assay was analyzed.
MTT assay
Spleen lymphocytes (5×105) were separated and co-cultured with OVA (100 µg/ml) at 37°C for 24 h. MTT (Sigma-Aldrich; Merck KGaA) working solution (0.5 mg/ml) was added to each well. After 4 h, the plates were centrifugated and dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) was added for MTT assay. The absorbance was detected with a plate reader (hermo Fisher Scientific, Inc.).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA from spleen lymphocytes in the different group was extracted using a Total RNA kit (Tiangen Biotechnology Co., Ltd., Beijing, China). The super Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV; BioTeke, Beijing, China) was used for DNA synthesis. qPCR was performed using the SYBR-Green Master Mix system (Tiangen Biotechnology Co., Ltd.). Following primers were used: T-bet: Forward, 5′-TGCCTGCAGTGCTTCTAACA-3′ and Reverse, 5′-TGCCCCGCTTCCTCTCCAACCAA-3′; GATA-3: Forward, 5′-GAAGGCATCCAGACCCGAAAC-3′ and Reverse, 5′-ACCCATGGCGGTGACCATGC-3′; IFN-γ: Forward, 5′-AGCGGCTGACTGAACTCAGATTGTAG-3′ and Reverse, 5′-GTCGCTTCGTTGATCACAA-3′; IL-4: Forward, 5′-TCAACCCCCAGCTAGTTGTC-3′ and Reverse, 5′-TGTTCTTCGTTGCTGTGAGG-3′; GAPDH: Forward, 5′-AACTTTGGCATTGTGGAAGG-3′ and Reverse, 5′-ACACATTGGGGGTAGGAACA-3′. The thermal cycling parameters for the PCR: denaturation at 95°C for 5 min followed by 40 cycles at 95°C for 30 sec, 55°C for 30 sec and 72°C for 30 sec. The relative expression of mRNA was calculated according to 2−∆∆Cq method.
Western blotting
Spleen lymphocytes were isolated and lyzed with the buffer (Cell Signaling Technology, Inc., Beverly, Massachusetts, USA). The total protein concentration in the supernatants was determined using a BCA Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.). The protein (30 µg) were separated by 12% (w/v) sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA) using a wet transfer method. The membranes were blocked with 5% skim milk for 2 h at room temperature and then incubated overnight at 4°C with the primary rabbit anti-mouse antibodies against T-bet (sc-21003), GATA-3 (sc-9009) or GAPDH (sc-25778; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The membranes were incubated with secondary antibody conjugated to horseradish peroxidase for 2 h (sc-2317; Santa Cruz Biotechnology, Inc.) for 2 h at room temperature, and the label proteins were detected with chemiluminescence detection regent (Cell Signaling Technology, Inc.) and visualized with the detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Optical density for each band was assessed using ImageJ analysis software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data were expressed as mean ± standard deviation. Statistical analysis was performed with one-way analysis of variance followed by Duncan's multiple range tests. SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA) was used for all calculations. P<0.05 was considered to indicate a statistically significant difference.
Results
Puerarin treatment attenuates OVA-induced DTH in mice
Firstly, mice model with OVA-induced DTH was established with or without puerarin treatment (Fig. 1A). In comparison with control group, repeated OVA application induced an obvious amplification of footpad swelling (Fig. 1B). Compared to the DTH group, puerarin treatment, middle and high dose, inhibited the increment in the swelling thickness (Fig. 1C). Additionally, puerarin treatment also significantly lowered the levels of OVA-IgG in serum (Fig. 1D), suggesting the protective role of puerarin on DTH. As the positive control, Dex showed obvious inhibitory effect on the footpad swelling and the level of OVA-specific antibody in serum compared with those in DTH group.
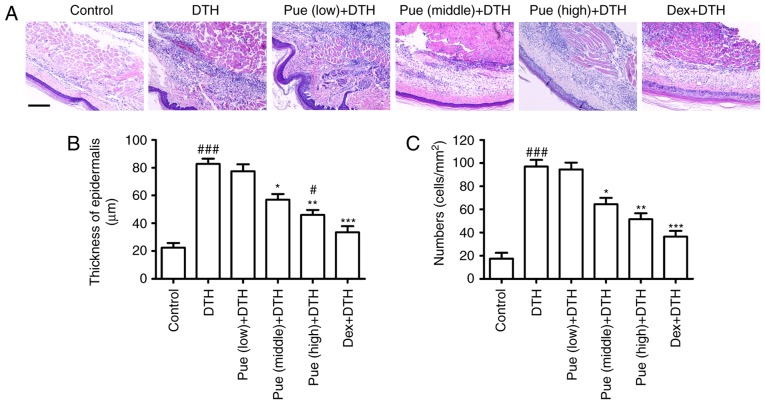
Puerarin decreases local inflammatory cell infiltration
Inflammatory cells were evidently increased following OVA-challenge in the dermal tissue (Fig. 2). Compared to the DTH group, puerarin evidently inhibited the increment of epidermis thickness (Fig. 2B) and reduced inflammatory cell infiltration (Fig. 2C), which diminished the number of inflammatory cells recruited to the footpad associated with the local inflammation.
Figure 2.
Puerarin (Pue) deduces the infiltration of inflammatory cell. Footpad tissues were obtained and stained (H&E). (A) Histological changes in the different groups were shown. Scar bar=20 µm. (B) The increment of epidermal thickness and (C) inflammatory cells infiltration were statistically analyzed, respectively. *P<0.05, **P<0.01 and ***P<0.001, comparison with the delayed-type hypersensitivity (DTH) group. #P<0.05, ###P<0.001, comparison with the Control group. Dex, Dexamethasone.
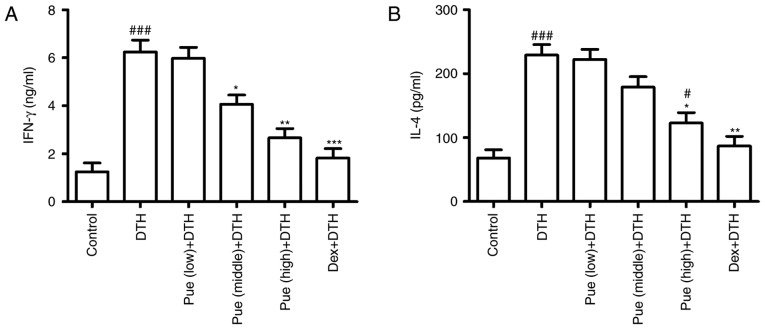
Puerarin suppresses inflammation associated with OVA-induced DTH
Following puerarin treatment, cytokine associated with inflammation in OVA-induced DTH was investigated. IFN-γ, the classic Th1-type cytokines, was found to be greatly down-regulated in puerarin group compared to that in the DTH group (Fig. 3A), suggesting the anti-inflammatory effect of puerarin on DTH. Interestingly, only high dose of puerarin showed suppressive effect in IL-4 production (Fig. 3B), suggesting its anti-allergic potential of puerarin.
Figure 3.
Puerarin (Pue) suppresses inflammation in ovalbumin (OVA)-induced delayed-type hypersensitivity (DTH). The homogenized supernatant was obtained according to the method mentioned in the Materials. (A) The secretion of IFN-γ and (B) IL-4 were measured by ELISA and statistically analyzed. *P<0.05, **P<0.01 and ***P<0.001, comparison with the DTH group. #P<0.05, ###P<0.001, comparison with the Control group. Dex, Dexamethasone.
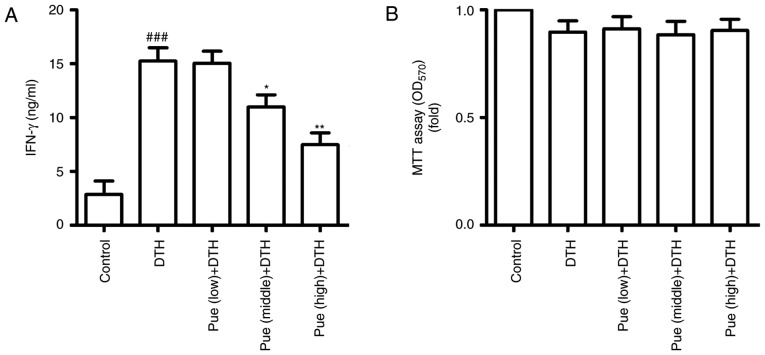
Puerarin inhibits Th1 response
Further, we observed the effect of puerarin on Th1 response based on spleen lymphocytes. Following ConA stimulation, IFN-γ was increased in the DTH group, but puerarin treatment significantly inhibited the secretion of IFN-γ (Fig. 4A). In addition, cell proliferation was no difference between DTH group and puerarin group by the MTT assay (Fig. 4B). These results suggest that puerarin can restrain Th1 response, which inhibited the activation of Th1 cells and led to deduce IFN-γ production, thereby the regulation of inflammation in OVA-induced DTH.
Figure 4.
Puerarin (Pue) treatment inhibits Th1 response. (A) Spleen lymphocytes were isolated and cultured in the presence of ConA (5 mg/ml). After 24 h, the supernatant was collected and the secretion of IFN-γ was detected by ELISA. (B) Spleen lymphocytes were obtained and cultured following stimulation with ovalbumin (OVA) (50 µg/ml). After 24 h, cell proliferation was conducted for MTT assay. *P<0.05 and **P<0.01, comparison with the delayed-type hypersensitivity (DTH) group. ###P<0.001, comparison with the Control group. Dex, Dexamethasone.
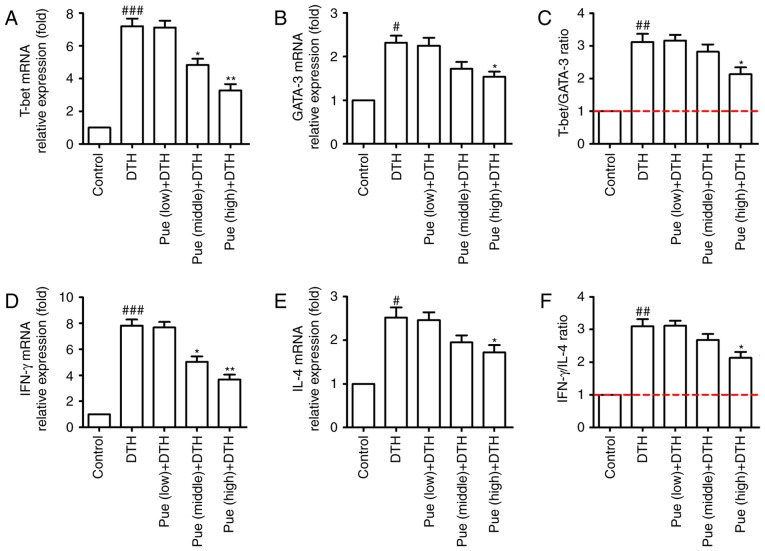
Effect of puerarin in Th1 and Th2 differentiation
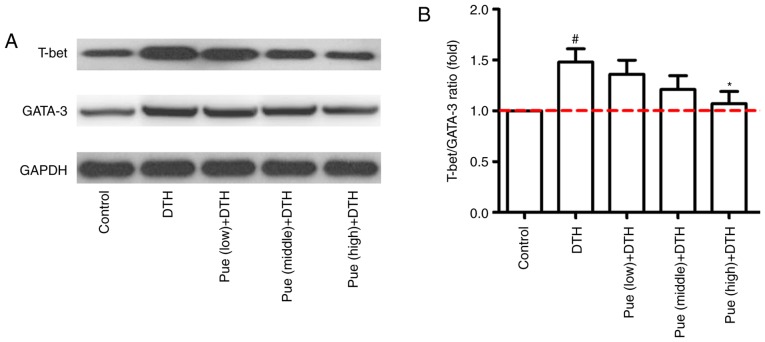
Lastly, the effect of puerarin on the transcription factors such as T-bet and GATA-3 were investigated, which directly or indirectly regulate Th1 and Th2 differentiation. As shown in Fig. 5A-C, middle and high concentration of puerarin inhibited the expression of T-bet in mRNA level, whereas only high dose of puerarin showed suppressive effect for GATA-3 (Fig. 5A-C). In addition, the similar tendency was found for the expression of IFN-γ and IL-4 in the molecular level (Fig. 5D-F). Compared with that in the control group, the expression of T-bet protein was clearly increased in the DTH group. However, high dose of puerarin significantly reduced the level of T-bet and GATA-3 protein (Fig. 6A and B). These results suggest that puerarin could regulate a shift of Th1 polarization to Th1/Th2 balance, which is related to the decrement of T-bet in mRNA and protein levels and subsequently inhibitive IFN-γ production.
Figure 5.
Effect of puerarin (Pue) on Th1 and Th2 differentiation. Spleen lymphocytes were isolated and total RNA were extracted and quantitated. (A) Quantitative analysis of T-bet and (B) GATA-3 was handled, and (C) the ratio of T-bet/GATA-3 was calculated. (D) Quantitative analysis of IFN-γ and (E) IL-4 was handled and (F) the ratio of IFN-γ/IL-4 was calculated. *P<0.05 and **P<0.01, comparison with the delayed-type hypersensitivity (DTH) group. #P<0.05, ##P<0.01 and ###P<0.001, comparison with the Control group.
Figure 6.
Effect of puerarin (Pue) on the protein expression of T-bet and GATA-3. Mice with ovalbumin (OVA)-induced delayed-type hypersensitivity (DTH) were treated with different concentrations of puerarin (low, middle and high dose). Spleen lymphocytes were isolated and the proteins expression were analyzed by western blotting. (A) The expression of T-bet and GATA-3 proteins was detected by western blotting, and a representative result was shown. (B) The ratio of T-bet/GATA-3 was statistically analyzed. *P<0.05, comparison with the DTH group. #P<0.05, comparison with the Control group.
Discussion
Although further development of puerarin has drawn substantial attention in natural product research, the direct targets and the underlying mechanisms of its bioactivity remain unclear. The present study shows that puerarin alleviates OVA-induced DTH via cytokine inhibition by modulating Th1/Th2 balance, which might provide a new perspective for comprehensive understanding of its biological effects.
Increasing evidence indicates the anti-inflammatory effect of puerarin in different disease. Using a murine model of Parkinson's disease, puerarin significantly alleviated injury in dopaminergic neurons, which was attributed to its anti-apoptotic and anti-inflammatory activities, resulting in inhibition of cleaved Caspase-3 and Bax as well as reduction of inducible nitric oxide synthase (16). In addition, puerarin attenuated inflammation and oxidation in mice with collagen antibody-induced arthritis, which was associated with reducing production of pro-inflammatory cytokines such as IL-6 and TNF-α (17). In a sepsis model using H9c2 cardiomyocytes stimulated with lipopolysaccharide (LPS), puerarin treatment attenuated the expression of TNF-α and IL-β in mRNA and protein levels. Cytokine inhibition, especially Th1-type cytokines including TNF-α and IL-β, contributed to the anti-inflammatory effect of puerarin (18). In line with above results, our study showed puerarin restrained the footpad swelling (Fig. 1B and C), inflammatory cell infiltration in skin tissues (Fig. 2) triggered by OVA application. Importantly, puerarin treatment significantly hampered IFN-γ production (Fig. 3A), classic Th1-type cytokine, which suggested its anti-inflammatory effect was associated with inhibiting Th1 response. The speculate also was confirmed by the ConA stimulation assay and MTT assay (Fig. 4). Following puerarin treatment, IFN-γ was decreased and Th1 response was hindered in the presence of ConA.
As we know, IFN-γ is involved in the allergic inflammation, such as asthma and DTH (19,20). However, it is worth noting that IL-4 also participates in the immune progress (21,22). The Th1/Th2 paradigm assumes that a dominance of Th1 cell activation and an inadequate Th2 cell response are responsible for the development of inflammation, suggesting the balance of Th1/Th2 is important to regulate immune response (23–25). As the regulatory factor on Th differentiation, T-bet and GATA-3 has shown the modulating effect in Th1 and Th2 cells, respectively. T-bet controls the development and differentiation of Th1 cells as well as induces the synthesis of IFN-γ, but presents a negative regulation on Th2 cells (26). In this study, puerarin treatment (middle and high dose, respectively) declined the expression of T-bet and IFN-γ in mRNA and protein levels, but middle dose of puerarin showed few effect on the expression of GATA-3 and IL-4 (Fig. 5). Additionally, the ratio of T-bet/GATA-3 also showed the similar trend as IFN-γ/IL-4 ratio (Th1/Th2 ratio) (Fig. 6). Based on these results, we speculated that puerarin treatment could inhibit the expression of T-bet in mRNA and protein levels, resulting in the repression of Th1 differentiation, which was responsible for the immune-switch between Th1 and Th2 cells. Interestingly, high dose of puerarin also restrained the expression of GATA-3 mRNA and IL-4 secretion as the similar effect of dexamethasone, suggesting its anti-allergic potential of puerarin on DTH. If other T-cell subtype (such as regulatory T cells), transcription factors (such as NF-κB) or signaling pathway (such as MAPK pathway) participates in the immuno-shift between Th1 and Th2 cells in the DTH responses triggered by repeated-OVA administration, further studies are required to clarify the facts.
In summary, puerarin treatment plays a regulator role between Th1 and Th2 cells, which restores Th1/Th2 balance through facilitating an immune-shift from Th1 cells to Th2 cells on OVA-induced DTH, accompanying regulation of footpad swelling, anti-OVA IgG, IFN-γ/IL-4 production, Th1 response and T-bet/GATA-3 ratio. These findings suggest the potential therapeutic utility of puerarin as a candidate in treating Th1-mediated inflammation disorders such as DTH, although its detailed mechanism needs further elucidation.
References
- 1.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Neurath MF, Finotto S, Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nat Med. 2002;8:567–573. doi: 10.1038/nm0602-567. [DOI] [PubMed] [Google Scholar]
- 3.Kidd P. Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- 4.Kobayashi K, Kaneda K, Kasama T. Immunopathogenesis of delayed-type hypersensitivity. Microsc Res Tech. 2001;53:241–245. doi: 10.1002/jemt.1090. [DOI] [PubMed] [Google Scholar]
- 5.Hernández A, Yager JA, Wilkie BN, Leslie KE, Mallard BA. Evaluation of bovine cutaneous delayed-type hypersensitivity (DTH) to various test antigens and a mitogen using several adjuvants. Vet Immunol Immunopathol. 2005;104:45–58. doi: 10.1016/j.vetimm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Jacysyn JF, Abrahamsohn IA, Macedo MS. Modulation of delayed-type hypersensitivity during the time course of immune response to a protein antigen. Immunology. 2001;102:373–379. doi: 10.1046/j.1365-2567.2001.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou YX, Zhang H, Peng C. Puerarin: A review of pharmacological effects. Phytother Res. 2014;28:961–975. doi: 10.1002/ptr.5083. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Lam TN, Zuo Z. Radix Puerariae: An overview of its chemistry, pharmacology, pharmacokinetics and clinical use. J Clin Pharmacol. 2013;53:787–811. doi: 10.1002/jcph.96. [DOI] [PubMed] [Google Scholar]
- 9.Liu XJ, Zhao J, Gu XY. The effects of genistein and puerarin on the activation of nuclear factor-kappaB and the production of tumor necrosis factor-alpha in asthma patients. Pharmazie. 2010;65:127–131. [PubMed] [Google Scholar]
- 10.Wang J, Zhang T, Ma C, Wang S. Puerarin attenuates airway inflammation by regulation of eotaxin-3. Immunol Lett. 2015;163:173–178. doi: 10.1016/j.imlet.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Mahdy HM, Mohamed MR, Emam MA, Karim AM, Abdel-Naim AB, Khalifa AE. The anti-apoptotic and anti-inflammatory properties of puerarin attenuate 3-nitropropionic-acid induced neurotoxicity in rats. Can J Physiol Pharmacol. 2014;92:252–258. doi: 10.1139/cjpp-2014-0133. [DOI] [PubMed] [Google Scholar]
- 12.Zhang D, Ma G, Hou M, Zhang T, Chen L, Zhao C. The Neuroprotective effect of Puerarin in acute spinal cord injury rats. Cell Physiol Biochem. 2016;39:1152–1164. doi: 10.1159/000447822. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Ouyang L, Liang Z, Chen J, Yu Q, Jeza VT, Gong Y, Shen G, Weng X, Wu X. CD8(low)CD28(-) T cells: A human CD8 T-suppressor subpopulation with alloantigen specificity induced by soluble HLA-A2 dimer in vitro. Cell Transplant. 2015;24:2129–2142. doi: 10.3727/096368914X683575. [DOI] [PubMed] [Google Scholar]
- 14.Yu Q, Zhang L, Ouyang L, Gong Y, Liang Z, Shen G, Weng X, Wu X. A similarity in peptide cross-reactivity between alloantigen- and nominal antigen-induced CD8+ T cell responses in vitro. Immunogenetics. 2013;65:173–184. doi: 10.1007/s00251-012-0668-3. [DOI] [PubMed] [Google Scholar]
- 15.Weng X, Lu S, Zhong M, Liang Z, Shen G, Chen J, Wu X. Allo-restricted CTLs generated by coculturing of PBLs and autologous monocytes loaded with allogeneic peptide/HLA/IgG1-Fc fusion protein. J Leukoc Biol. 2009;85:574–581. doi: 10.1189/jlb.0408242. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Xiong J, Liu S, Wang L, Huang J, Liu L, Yang J, Zhang G, Guo K, Zhang Z, et al. Puerarin protects dopaminergic neurons in Parkinson's disease models. Neuroscience. 2014;280:88–98. doi: 10.1016/j.neuroscience.2014.08.052. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Wang W, Jin X, Shen J, Hu W, Jiang T. Puerarin attenuates inflammation and oxidation in mice with collagen antibody-induced arthritis via TLR4/NF-κB signaling. Mol Med Rep. 2016;14:1365–1370. doi: 10.3892/mmr.2016.5357. [DOI] [PubMed] [Google Scholar]
- 18.Yuan Y, Zhou H, Wu QQ, Li FF, Bian ZY, Deng W, Zhou MQ, Tang QZ. Puerarin attenuates the inflammatory response and apoptosis in LPS-stimulated cardiomyocytes. Exp Ther Med. 2016;11:415–420. doi: 10.3892/etm.2015.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, Huff R, Pilewski J, Holguin F, Kolls J, et al. High IFN-γ and low SLPI mark severe asthma in mice and humans. J Clin Invest. 2015;125:3037–3050. doi: 10.1172/JCI80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Chen Z, Chen T, Yi T, Zheng Z, Fan H, Chen Z. Berberine attenuates inflammation associated with delayed-type hypersensitivity via suppressing Th1 response and inhibiting apoptosis. Inflammation. 2017;40:221–231. doi: 10.1007/s10753-016-0472-6. [DOI] [PubMed] [Google Scholar]
- 21.Hirose K, Iwata A, Tamachi T, Nakajima H. Allergic airway inflammation: Key players beyond the Th2 cell pathway. Immunol Rev. 2017;278:145–161. doi: 10.1111/imr.12540. [DOI] [PubMed] [Google Scholar]
- 22.Ashraf MI, Shahzad M, Shabbir A. Oxyresveratrol ameliorates allergic airway inflammation via attenuation of IL-4, IL-5 and IL-13 expression levels. Cytokine. 2015;76:375–381. doi: 10.1016/j.cyto.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Hirahara K, Nakayama T. CD4+ T-cell subsets in inflammatory diseases: Beyond the Th1/Th2 paradigm. Int Immunol. 2016;28:163–171. doi: 10.1093/intimm/dxw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bui TT, Piao CH, Kim SM, Song CH, Shin HS, Lee CH, Chai OH. Citrus tachibana leaves ethanol extract alleviates airway inflammation by the modulation of Th1/Th2 imbalance via inhibiting NF-κB signaling and histamine secretion in a mouse model of allergic asthma. J Med Food. 2017;20:676–684. doi: 10.1089/jmf.2016.3853. [DOI] [PubMed] [Google Scholar]
- 25.Peine M, Rausch S, Helmstetter C, Fröhlich A, Hegazy AN, Kühl AA, Grevelding CG, Höfer T, Hartmann S, Löhning M. Stable T-bet(+) GATA-3(+) Th1/Th2 hybrid cells arise in vivo, can develop directly from naive precursors and limit immunopathologic inflammation. PLoS Biol. 2013;11:e1001633. doi: 10.1371/journal.pbio.1001633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yagi R, Zhu J, Paul WE. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int Immunol. 2011;23:415–420. doi: 10.1093/intimm/dxr029. [DOI] [PMC free article] [PubMed] [Google Scholar]