Abstract
To understand the roles of plant vacuoles, we have purified and characterized a major soluble protein from vacuoles of radish (Raphanus sativus cv Tokinashi-daikon) taproots. The results showed that it is a novel radish vacuole Ca2+-binding protein (RVCaB). RVCaB was released from the vacuolar membrane fraction by sonication, and purified by ion exchange and gel filtration column chromatography. RVCaB is an acidic protein and migrated on sodium dodecyl sulfate-polyacrylamide gel with an apparent molecular mass of 43 kD. The Ca2+-binding activity was confirmed by the 45Ca2+-overlay assay. RVCaB was localized in the lumen, as the protein was recovered in intact vacuoles prepared from protoplasts and was resistant to trypsin digestion. Plant vacuoles store Ca2+ using two active Ca2+ uptake systems, namely Ca2+-ATPase and Ca2+/H+ antiporter. Vacuolar membrane vesicles containing RVCaB accumulated more Ca2+ than sonicated vesicles depleted of the protein at a wide range of Ca2+ concentrations. A cDNA (RVCaB) encoding a 248-amino acid polypeptide was cloned. Its deduced sequence was identical to amino acid sequences obtained from several peptide fragments of the purified RVCaB. The deduced sequence is not homologous to that of other Ca2+-binding proteins such as calreticulin. RVCaB has a repetitive unique acidic motif, but not the EF-hand motif. The recombinant RVCaB expressed in Escherichia coli-bound Ca2+ as evidenced by staining with Stains-all and migrated with an apparent molecular mass of 44 kD. These results suggest that RVCaB is a new type Ca2+-binding protein with high capacity and low affinity for Ca2+ and that the protein could function as a Ca2+-buffer and/or Ca2+-sequestering protein in the vacuole.
The calcium ions have been demonstrated to control a variety of cellular processes with a high degree of spatial and temporal precision. In cells of all organisms, a complicated mechanism exists to control Ca2+ in a localized fashion. Ca2+-ATPase, Ca2+/H+ antiporter, Ca2+/Na+ antiporter, and Ca2+ channel are involved in regulation of the cytosolic Ca2+ concentration (Sanders et al., 1999; Sze et al., 2000). In addition to these elements, several kinds of calcium-binding proteins (CaBPs) are thought to mediate the Ca2+ signal transduction (Mackrill, 1999), some with a low affinity for Ca2+, binding it only at millimolar concentrations, and some with a high affinity, binding it in the nanomolar to micromolar range. In the cytosol, resting free Ca2+ levels are kept extremely low and small increments of Ca2+ perform second-messenger functions in cooperation with CaBPs and membrane transport systems for Ca2+. CaBPs in the cytosol and organelles function as signaling proteins, Ca2+ buffers, and Ca2+-sequestering proteins.
Many kinds of CaBPs have been isolated and cloned from plants. Calreticulin is one of the better-characterized proteins among plant CaBPs. Calreticulin has been detected as an abundant protein with a molecular mass ranging from 50 to 60 kD, and has been subsequently cloned from several species (Chen et al., 1994; Denecke et al., 1995; Hassan et al., 1995; Napier et al., 1995; Dresselhaus et al., 1996; Coughlan et al., 1997). Plant calreticulin is located in the endoplasmic reticulum (ER) lumen as a complex with other proteins and is estimated to function as a molecular chaperone together with calnexin (Crofts and Denecke, 1998). Calreticulins contain the ER retention signal K(H)DEL at the carboxyl terminus.
Annexin proteins are located in membranes and have a particular property of calcium-dependent binding to acidic phospholipids. Annexins have been isolated and cloned from several plants (Battey et al., 1996; Seals and Randall, 1997; Kovacs et al., 1998; Lim et al., 1998; Shin and Brown, 1999). Their molecular masses have been reported to be 30 to 42 kD. Although their exact function is unclear, it has been suggested that plant annexins are involved in a variety of cellular processes: cell wall maturation (Proust et al., 1999), cell elongation (Shin and Brown, 1999), exocytosis (Carroll et al., 1998), communication between the cytoskeleton and membranes (Calvert et al., 1996), and response to stress (Gidrol et al., 1996).
In plant cells the central vacuole occupies a large part of the cell volume and serves as a primary pool of Ca2+. The vacuolar membrane has two distinct active transport systems for Ca2+: Ca2+-ATPase and Ca2+/H+ antiporter (Sanders et al., 1999; Sze et al., 2000). Although no CaBP has been reported to be localized in vacuoles, CaBPs may contribute to the incorporation and storage of Ca2+ if they are localized in vacuoles. We found a CaBP (radish [Raphanus sativus] vacuole Ca2+-binding protein [RVCaB]) in the vacuolar membrane fraction of radish taproots. The 43-kD protein was easily released from membranes by sonication. In this work we demonstrated that RVCaB stimulated the incorporation of Ca2+ by vacuolar membrane vesicles and that the protein was localized in vacuoles. We have cloned the cDNA encoding radish RVCaB. The deduced sequence indicates that RVCaB is a unique CaBP. We discuss the molecular properties and physiological significance of RVCaB.
RESULTS
Purification and Biochemical Properties of RVCaB
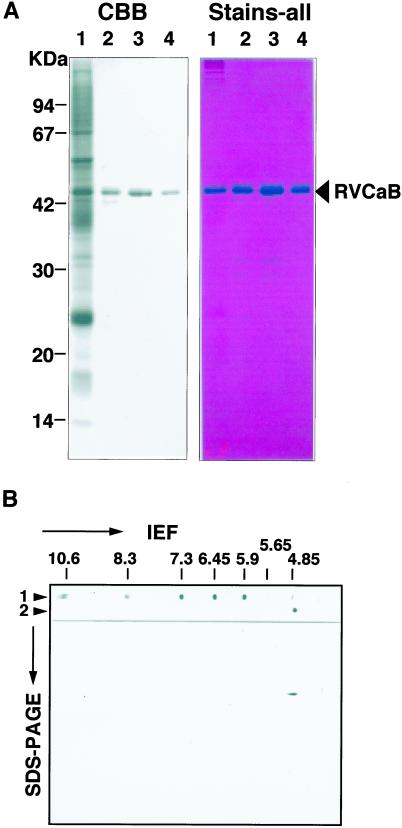
Most of the RVCaB protein in the vacuolar membrane fraction was easily solubilized by sonication. As shown in Figure 1A, RVCaB was the major protein in the soluble fraction obtained after sonication and was purified by ion-exchange chromatography and gel filtration. About 100 μg of RVCaB was obtained in a highly purified form from 2 kg of taproot. The purified RVCaB showed a band at 43 kD on SDS-PAGE and gave a single spot with an pI of 4.8 (Fig. 1B). The RVCaB protein was eluted as a 52-kD protein on column chromatography of Superdex 200HR and migrated as a protein of less than 67 kD on gradient gel of PAGE under non-denaturing conditions (data not shown). The results suggest that RVCaB exists as a monomer or dimer under physiological conditions.
Figure 1.
Purified RVCaB protein of 43 kD has a pI of 4.8. A, The gel was stained with Coomassie Blue (CBB, left) or Stains-all (Stains-all, right). Lane 1, A vacuolar membrane fraction (30 μg) prepared from radish taproots; lane 2, supernatant (4 μg) obtained after sonication of the membrane fraction; lanes 3 and 4, eluates after QAE-Toyopearl chromatography (4 μg) and Sephacryl S-100 HR column chromatography (3 μg), respectively. B, Two-dimensional PAGE of RVCaB. The purified RVCaB was subjected to isoelectric focusing (IEF) and then SDS-PAGE. The gel was stained with Coomassie Blue. Lane 1, IEF of pI marker proteins. Values on the top indicate their pI. Lane 2, IEF of the purified RVCaB.
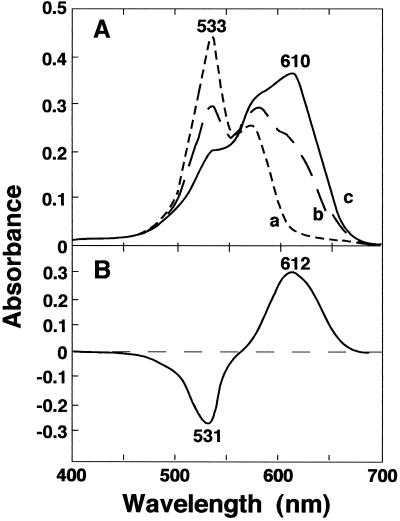
The RVCaB protein was clearly stained blue with Stains-all (Fig. 1A), which has been used for the identification of CaBPs (Campbell et al., 1983). Most of the CaBPs with acidic motifs to bind Ca2+, such as calreticulin (Krause et al., 1990) and calsequestrin (Campbell et al., 1983), stain blue with Stains-all, whereas other proteins stain pink and the color fades away quickly in the light. Figure 2 shows the absorption spectra of the mixture of purified RVCaB and Stains-all. Sharma and Balasubramanian (1991) reported that CaBPs can be separated into three groups from absorption spectra with Stains-all: proteins that induce a J band (600–650 nm), proteins that induce a γ band (500–520 nm), and proteins that induce the J and γ bands. Binding of Stains-all with RVCaB resulted in only the J band at 610 nm (Fig. 2). The spectral property of RVCaB was similar to those of parvalbumin (Caday and Steiner, 1985) and β-crystallin (Sharma et al., 1989), but not to those of calsequestrin or calmodulin (Campbell et al., 1983). It has been proposed that the J band occurs when the dye is bound to anionic sites that are present in the globular or compact conformations of the proteins (Sharma and Balasubramanian, 1991).
Figure 2.
The complex of RVCaB with Stains-all shows unique absorption spectra. The purified RVCaB was incubated at room temperature for 20 min in the dark in a 1.0-mL solution of 2 mm MOPS [3-(N-morpholino)-propanesulfonic acid]-KOH buffer, pH 7.2, containing 5 μm Stains-all and 30% (w/v) ethylene glycol, and then the absorption spectra was taken with a spectrophotometer. A, Line a, spectrum of the Stains-all solution without RVCaB; lines b and c, spectra of a Stains-all solution mixed with RVCaB of 6 μg (line b) and 10 μg (line c). B, Difference spectrum of a Stains-all solution mixed with 10 μg of RVCaB against the control solution without RVCaB. The RVCaB concentration was calculated to be about 0.33 μm.
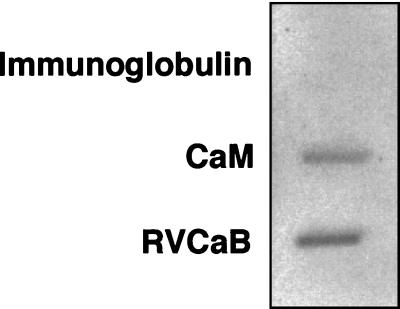
The ability of RVCaB to bind Ca2+ was confirmed by the 45Ca2+ overlay analysis (Fig. 3). A membrane sheet blotted with the purified RVCaB was incubated with 45Ca2+ and then rinsed with a buffer containing 5 mm MgCl2 and 60 mm KCl. The purified RVCaB reproducibly gave a clear positive signal of 45Ca2+. Calmodulin showed a positive signal, but immunoglobulin, a negative control, gave no signal. The accurate amount of RVCaB could not be determined in this experiment by reason that RVCaB was only partially trapped on the membrane filter. This low recovery may be due to the highly acidic nature of RVCaB as discussed later. Thus the number of the bound 45Ca2+ per RVCaB could not be determined.
Figure 3.
RVCaB binds Ca2+ in 45Ca2+ overlay assay. The purified RVCaB (2 μg) was slot-blotted to a polyvinylidene difluoride membrane, and then the membrane sheet was subjected to 45Ca2+ overlay assay with 1.8 MBq 45Ca2+ as CaCl2 (37 GBq mmol−1). Calmodulin from bovine brain (2 μg, CaM) and immunoglobulin (2 μg) were also applied to the membrane as a positive and negative control of CaBPs, respectively.
Location of RVCaB in Vacuoles
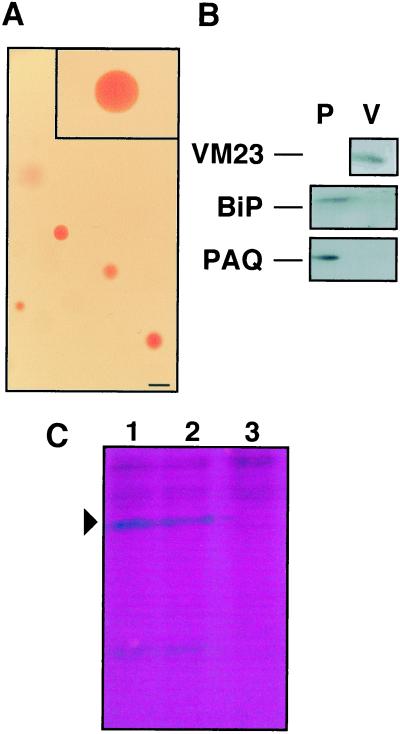
We isolated intact vacuoles and examined them for the presence of RVCaB. Intact vacuoles were prepared by treatment of protoplasts of etiolated shoots with potassium dextran sulfate (Fig. 4A). The isolated vacuole fraction contained not only RVCaB, but also the vacuolar membrane aquaporin VM23 as expected (Fig. 4B). In an immunoblot we could not detect the plasma membrane aquaporin or BiP, a marker protein of ER lumen. The activities of NADPH-cytochrome c-reductase (ER) and inosine diphosphatase (Golgi apparatus) were scarcely detected in the isolated vacuoles. Thus the obtained vacuoles were not contaminated with other organelles.
Figure 4.
RVCaB is recovered in intact vacuoles. A, Vacuoles were isolated from protoplasts of radish hypocotyls and then stained with neutral red. Bar represents 100 μm. B, The isolated protoplasts (P) and vacuoles (V) were subjected to immunoblotting with antibodies to the vacuolar membrane aquaporin (VM23), the plasma membrane aquaporin (PAQ1), and the ER lumenal protein (BiP). C, The isolated vacuoles (500 μL) were treated with trypsin (2 pmol) at 37°C for 30 min and then subjected to SDS-PAGE. The gel was stained with Stains-all. Lane 1, Vacuoles without trypsin treatment; lane 2, vacuoles treated with trypsin, lane 3, vacuoles treated with trypsin in the presence of 0.1% (w/v) Triton X-100.
Next we determined the trypsin susceptibility of RVCaB in the isolated vacuoles to test whether RVCaB exists inside the vacuoles. RVCaB was not digested by trypsin as shown in Figure 4C. About 80% of RVCaB was retained as 43-kD protein in vacuoles without detergent, but the protein was completely digested by treatment with trypsin in the presence of 0.1% (w/v) Triton X-100. This indicates that RVCaB is localized in the vacuole and is not on the cytosolic surface of vacuole.
Stimulus Effect of RVCaB on Ca2+ Uptake by Vacuolar Membrane Vesicles
Vacuolar membrane vesicles exhibited a relatively high activity of Ca2+ uptake driven by ATP or inorganic pyrophosphate. ATP drives Ca2+-ATPase directly and Ca2+/H+ antiporter indirectly through a proton gradient generated by H+-ATPase (Sze et al., 1999, 2000). H+-ATPase is the main proton pump of radish taproot vacuolar membranes (Maeshima et al., 1996). In this study the Ca2+ uptake into vacuolar membrane vesicles of radish taproots was determined with ATP and 45Ca2+ by a method established for mung bean vacuolar membranes (Ueoka-Nakanishi et al., 1999, 2000). Radish vacuolar membrane vesicles actively incorporated Ca2+ in an ATP-dependent manner, and Ca2+ accumulated in vesicles was released immediately after addition of a Ca2+ ionophore A23187. The total ATP-dependent Ca2+ uptake activity of radish vacuolar membrane vesicles, which contained RVCaB, was 4.5 nmol mg−1 min−1. The value was lower than that of vesicles from mung bean hypocotyls (21 nmol mg−1 min−1; Ueoka-Nakanishi et al., 1999). The difference may be due to the levels of Ca2+-ATPase, Ca2+/H+ antiporter, and H+-ATPase in the membrane.
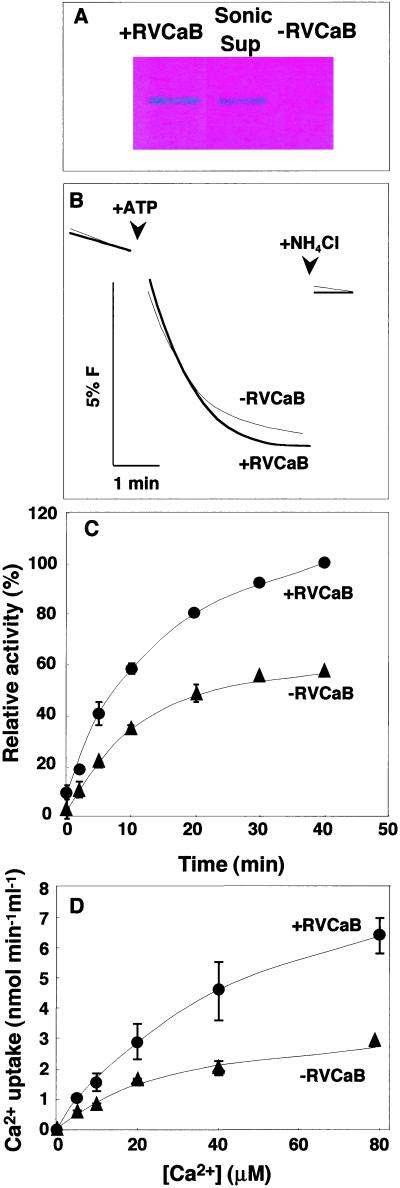
The ATP-dependent Ca2+ uptake activity was compared between the membrane vesicles with and without RVCaB. RVCaB in the vesicle was depleted by a brief sonication. After centrifugation, the membrane vesicles were suspended to the same volume of the original solution to normalize vesicle populations and used as the −RVCaB vesicles. RVCaB was not retained in the −RVCaB preparation and recovered in the supernatant fraction after sonication (Fig. 5A). There was no difference in the ATP-dependent H+ transport activity and H+ leakage between the two vesicle preparations (Fig. 5B). Therefore, it was judged that neither the brief sonication of the vesicles nor RVCaB affected the activity of H+-ATPase.
Figure 5.
RVCaB stimulates the Ca2+ uptake into vacuolar membrane vesicles. The vacuolar membrane vesicles with (+RVCaB) or without (−RVCaB) were prepared from radish taproots and then assayed for Ca2+ uptake activity. A, Detection of RVCaB. The membrane vesicles before (+RVCaB) and after sonication (−RVCaB) and the supernatant fraction after sonication (Sup) were subjected to SDS-PAGE and then the gel was stained with Stains-all. B, ATP-dependent H+ transport activity of the vesicles with (+RVCaB, thick line) and without RVCaB (−RVCaB, fine line) was measured as the rate of fluorescence (F) quenching of acridine. At the indicated time, NH4Cl was added at 1 mm to collapse a proton gradient. C, The Ca2+ uptake activity was assayed in a medium containing 100 μm CaCl2 (45Ca2+) by the filtration method. Black circles, +RVCaB; black triangles, −RVCaB. D, Vacuolar membrane vesicles with (+RVCaB, black circles) and without RVCaB (−RVCaB, black triangles) were assayed for Ca2+ uptake activity at indicated concentrations of CaCl2 for 2 min. The values correspond to the initial rates of Ca2+ uptake.
The total amount of Ca2+ incorporated into vesicles was doubled by the presence of RVCaB (Fig. 5C). In this experiment unspecific binding of Ca2+ to the vesicles was omitted by subtraction of the activity without ATP from the activity with ATP. Because the Ca2+ uptake was linear for the first several minutes, we determined the amount of Ca2+ incorporated into vesicles for 2 min and considered the values as the initial rates of the Ca2+ uptake. The initial rate in the presence of RVCaB was two times greater than that in the absence of RVCaB, even at high concentrations of Ca2+ (Fig. 5D).
Direct Sequences of the Purified RVCaB and cDNA Cloning
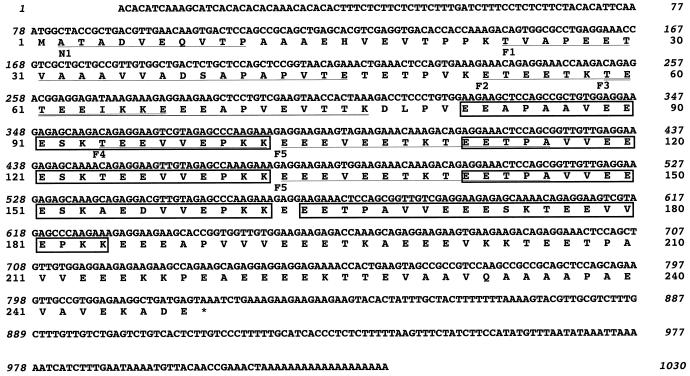
To determine the primary sequence of RVCaB, we obtained the sequences of the amino terminal and internal parts of the purified RVCaB and then cloned its cDNA from poly(A)+ RNA of radish hypocotyls. The cloned cDNA (RVCaB) consists of 1,011 bp upstream the polyadenylate tail, which includes a 77-bp 5′-non-coding region, a 744-bp open reading frame encoding 248 amino acids, and a 190-bp 3′-non-coding region (Fig. 6). The N-terminal sequence that was directly determined for the purified RVCaB was xATADVEQVTP (x, undetermined residue) (Fig. 6). The first Met residue may be chemically modified. The amino acid sequences of tryptic fragments were also determined. These sequences were identical with the sequence deduced from the cDNA (Fig. 6). Thus we concluded that an open reading frame started from the first ATG codon and encoded a full sequence of RVCaB. The molecular mass and the pI were calculated to be 27.1 kD and 3.94, respectively. It should be noted that there is no membrane-spanning domain in RVCaB (Fig. 6).
Figure 6.
cDNA cloning reveals a unique amino acid sequence of RVCaB. The nucleotide sequence of cDNA (accession no. AB035900) for RVCaB and the deduced amino acid sequence are shown. The N-terminal sequence (N1) of the purified RVCaB and the internal tryptic fragments (F1–F5) were determined directly. N1, ATADVEQVTP. F1, TVAPEETVAAAVVADDAPAPVTE. F2, ETEETKTETEEIKKE. F3, TETEEIKKEEEAPVEVTTK. F4, TEEVVEPKK. F5, EEEVEExKxEExPA (x, undetermined residue). The underlined amino acid residues were identical to those of the direct sequences obtained from the purified RVCaB. Repeat sequences [EET(A) PAV(A)VEEESKT(A)EE(D)VVEPKK] are boxed.
There was no EF-hand motif, a helix-turn-helix structure, in RVCaB. Annexin family proteins have a region known as the endonexin fold (GxGTDE). RVCaB possesses neither this motif nor the ER-retention signal K(H)DEL. Judging from the primary sequence, RVCaB is not a member of the EF-hand protein family or the annexin family. We found some unique motifs in the sequence of RVCaB. A long sequence [EET(A)PAV(A)VEEESKT(A)EE(D)VVEPKK]repeats four times. This sequence is not homologous to the common repeat sequence of calreticulins (KPEDWD; Chen et al., 1994). The repeat sequence in RVCaB seems to be a candidate for the Ca2+-binding motif of RVCaB. The Ca2+-binding capacity of this motif should be determined by in vitro experiments.
Since RVCaB does not contain a cleavable peptide at the N- or C-termini, a question arises on the targeting to the vacuole. Most luminal proteins in vacuoles possess vacuolar-sorting signal in the N- or C-terminal propeptides, and these prosequences are cleaved off in the vacuole (Matsuoka and Neuhaus, 1999). However, some vacuolar proteins, such as phytohemagglutinin (von Schaewen and Chrispeels, 1993) and legumin (Saalbach et al., 1991) are synthesized without propeptides. α-Mannosidase, which is located on the inner surface of the vacuolar membrane, has no cleavable signal sequence (Yoshihisa and Anraku, 1990). As well as these unprocessed vacuolar proteins, RVCaB may have a targeting signal in its mature protein.
Heterologous Expression of RVCaB in Escherichia coli
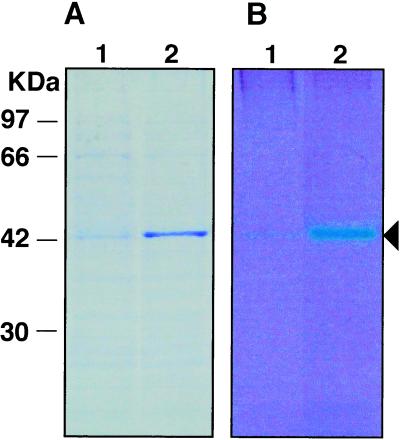
The molecular mass (27.1 kD) predicted from the cDNA was substantially lower than that determined by SDS-PAGE (43 kD). Acidic proteins have been reported to migrate slowly on SDS-PAGE (Vorum et al., 1998). To determine whether the difference is due to this acidic nature or not, we attempted to express of RVCaB in E. coli. The recombinant RVCaB had an additional 14-amino acid sequence, which was originated from the vector used for recombination, at the N-terminal part of RVCaB. The recombinant protein migrated on SDS-PAGE with an apparent molecular mass of 44 kD (Fig. 7). Furthermore, the protein bound Ca2+ as it was clearly stained blue with Stains-all. Therefore, it was confirmed that the RVCaB clone is a full-length cDNA for RVCaB.
Figure 7.
Recombinant RVCaB stains blue with Stains-all. The cell lysate from E. coli transformed with RVCaB, which were treated with (lane 2) or without (lane 1) isopropyl thio-β-d-galactoside, were separated by SDS-PAGE. The gel was stained with Coomassie Blue (A) or Stains-all (B). An arrowhead indicates the position of recombinant RVCaB.
DISCUSSION
We purified and characterized a major Ca2+-binding protein from the vacuole. The RVCaB protein may exist as a monomeric or dimeric form judging from the results of gel permeation column chromatography and PAGE under non-denaturing conditions. The full-length cDNA for RVCaB encodes a 248-amino acid protein. Its calculated molecular mass of 27.1 kD is different from the apparent size of 43 kD on SDS-PAGE. This difference may be due to its electrochemical property, because the recombinant RVCaB expressed in E. coli exhibited the same size as the purified RVCaB.
RVCaB Is a Ca2+-Binding Protein in the Vacuole
The vacuole localization of RVCaB was confirmed by the experiment using isolated intact vacuoles. RVCaB was retained in the isolated vacuoles and RVCaB proteins in the vacuolar fraction were resistant to trypsin. Most RVCaB was recovered in the vacuolar membrane fraction, not in the cytosolic or ER fractions. RVCaB was hardly detected in the post-microsomal fraction. These results suggest that RVCaB is weakly associated with the inner surface of vacuolar membranes.
The 45Ca2+ overlay assay demonstrated that RVCaB bound Ca2+, although its kinetic properties could not be determined. Stimulation of the Ca2+ uptake activity supports the role of RVCaB in accumulating vacuolar Ca2+. The membrane vesicles incorporated Ca2+ in an ATP-dependent manner, and the amount of Ca2+ was increased in the vesicles containing RVCaB. RVCaB did not affect the vacuolar H+-ATPase activity. Thus we suggest that Ca2+ binding by RVCaB enhances the Ca2+ content stored in radish vacuoles in vivo. By binding Ca2+, RVCaB may effectively lower the free Ca2+ concentration inside the vacuole. This will lower the Ca2+ gradient thus allowing the vacuolar Ca2+-ATPase and Ca2+/H+ antiporter to remain active. The present results would support the role of the vacuole as a reservoir of Ca2+.
We calculated kinetic parameters from the present results. The net amount of Ca2+ incorporated into membrane vesicles for 40 min, which was stimulated by RVCaB (0.35 μg), was 200 pmol (Fig. 5C). On the assumption that the increment of Ca2+ content was due to the binding by RVCaB, the number of Ca2+ bound per RVCaB was roughly calculated to be 15. This means that the binding capacity of RVCaB for Ca2+ is high. Also, it should be noted that the stimulation by RVCaB was observed at high Ca2+ concentrations of more than 40 μm (Fig. 5D). At these concentrations, most of the Ca2+ uptake can be attributed to the Ca2+/H+ antiporter as reported previously (Schumaker and Sze, 1986; Blackford et al., 1990; Ueoka-Nakanishi et al., 1999). The antiporter has been reported to accumulate Ca2+ at concentrations of up to at least 100-fold over the outside under a pH gradient of 2 between the vesicles and the buffer (Schumaker and Sze, 1986). Therefore, the Ca2+ concentration in the vesicles may reach millimolar concentrations. Judging from the present and previous studies, the affinity of RVCaB for Ca2+ may be low. In conclusion we suggest that RVCaB is a low-affinity and high-capacity CaBP. This protein may assist the Ca2+ transporters by lowering the free concentration of Ca2+ and as a result the total content of Ca2+ in the vacuole may be increased.
Furthermore, we should consider the other mechanisms to explain the high Ca2+ uptake activity after sonication (Fig. 5). There is a possibility that an inhibitor of the Ca2+ transporter was released from the membrane by sonication. The inhibitor of Ca2+-ATPase and Ca2+/H+ antiporter in the vacuole has not been reported. In mung bean vacuolar membranes the Ca2+ uptake activity was not changed by sonication. Therefore, it is hard to think that radish vacuolar membranes possess such an inhibitor. The second explanation is that RVCaB interacted with Ca2+-ATPase and Ca2+/H+ antiporter directly and activates them in the vacuole. This may be unlikely, because the hydrophilic, functional main parts of the transporters are exposed to the cytosol. The present study does not exclude the possibility that RVCaB plays a role in calcium signaling in the vacuole. Further studies by the reconstituted vesicles are necessary to examine these possibilities and to understand the physiological meanings of the association of RVCaB to the vacuolar-membrane inner surface.
RVCaB Is Distinct from Other Ca2+-Binding Proteins
Various Ca2+-binding proteins have been found in plants and characterized at the biochemical and molecular level. CaBPs are classified into four groups: the EF-hand protein family, the ER intraluminal CaBP group, the annexin family, and a fourth group that includes Ca2+-ATPase and Ca2+/H+ antiporter. The EF-hand family is the largest group of CaBPs and its members function as “buffer” proteins that act by controlling the cytosolic Ca2+ concentration or as “modulator” proteins that regulate other proteins in a Ca2+-dependent manner (Kretsinger, 1997; Kawasaki et al., 1998). It is evident from the absence of EF-hand motif that RVCaB is not a member of the EF-hand family.
Calreticulin and calsequestrin belong to the ER intraluminal CaBP group. The apparent molecular masses of plant calreticulin proteins range from 53 to 60 kD (Hassan et al., 1995; Napier et al., 1995; Crofts and Denecke, 1998). Plant calreticulins have been reported to be located in the ER lumen and function as a Ca2+-binding molecular chaperone (Crofts and Denecke, 1998). Because there are differences in the molecular masses, intracellular localization, and the primary sequences, RVCaB is not a counterpart of calreticulin in radish. The absence of the ER retention signal in RVCaB also suggests that it is not an ER luminal protein.
Proteins of the annexin family have been identified and cloned from various plants (Battey et al., 1996; Calvert et al., 1996; Proust et al., 1999; Shin and Brown, 1999). Annexin proteins belong to a ubiquitous family of Ca2+-dependent phospholipid-binding proteins. A 42-kD annexin has been reported to be located on the cytosolic surface of the vacuole and function in vesicle fusion during vacuole biogenesis (Seals and Randall, 1997). This annexin had no effect on the Ca2+ accumulation by membrane vesicles. In general, annexins bind to the membrane reversibly by addition of Ca2+ at less than 1 μm, whereas the purified RVCaB did not bind to the vacuolar or ER membranes even in the presence of 1 mm Ca2+ (data not shown). We examined the binding capacity of RVCaB to the phospholipid micelles of phosphatidylcholine, phosphatidylserine, and phosphatidylinositol in the presence of 1 mm Ca2+, but no RVCaB associated with liposomes (data not shown). The absence of the endonexin fold also indicates that RVCaB does not belong to the annexin family. Therefore, we concluded that RVCaB is a novel CaBP with a unique primary structure. The precise Ca2+-binding motif in RVCaB remains to be determined.
MATERIALS AND METHODS
Preparation of Vacuolar Membrane Fractions
About 2 kg of the parenchyma tissue of radish (Raphanus sativus cv Tokinashi-daikon) taproot was homogenized in 2 L of a grinding medium using a grater. The grinding medium contained 0.25 m sorbitol, 2 mm EGTA, 10 μm (p-amidinophenyl) methanesulfonyl fluoride hydrochloride, 1% (w/v) polyvinylpyrrolidone, 2 mm dithiothreitol (DTT), and 50 mm Tris-HCl, pH 7.5. Vacuolar membranes were prepared as described previously (Maeshima and Yoshida, 1989; Maeshima, 1992). The homogenate was filtered and centrifuged at 3,600g for 10 min. The supernatant was centrifuged at 100,000g for 20 min. The precipitate was resuspended in 0.5 m Suc and 1 mm EGTA containing 10 mm Tris-HCl, pH 7.5, 1 mm DTT, and 1 mm MgCl2 (Tris-DM), and was used as a microsomal membrane fraction. The microsomal membranes were placed in a centrifuge tube and overlaid with the same volume of Tris-DM buffer containing 0.25 m sorbitol and 1 mm EGTA. After centrifugation at 100,000g for 30 min, vacuolar membrane vesicles forming a band at the interface between the two solutions were collected and suspended in two volumes of 10 mm Tris-HCl buffer, pH 7.5, 1 mm DTT, and 5% (w/v) glycerol (Tris-DG), containing 0.25 m sorbitol. After centrifugation at 100,000g for 30 min, the precipitate was suspended in a small volume of the same buffer, and used as the vacuolar membrane fraction. All steps were performed at 4°C.
Purification of RVCaB
Vacuolar membranes were subjected to sonic oscillation at 70 kHz for 90 s and then centrifuged at 100,000g for 40 min. The clear supernatant was applied to a column (gel volume, 3 mL) of QAE-Toyopearl (Tosoh, Tokyo) pre-equilibrated with Tris-DG buffer. The column was washed with 10 mL of Tris-DG buffer containing 0.2 m KCl and 2 mL of the buffer containing 0.3 m KCl, and then RVCaB was eluted with 10 mL of the buffer containing 0.35 m KCl. The amount of RVCaB in the fractions was estimated by SDS-PAGE. The peak fractions were collected and applied to a column (1.6 × 70 cm) of Sephacryl S-100 HR (Amersham Pharmacia Biotech, Buckinghamshire, UK) pre-equilibrated with Tris-DG buffer. Gel filtration was done at a flow rate of 30 mL h−1. The eluate was collected in 1.5-mL fractions. To determine the molecular mass of RVCaB under non-denaturing conditions, the purified RVCaB was subjected to column chromatography of Superdex 200HR (1.0 × 30 cm) pre-equilibrated with 10 mm MES [2-(N-morpholino)-ethanesulfonic acid]-KOH, pH 5.5, and 150 mm KCl. Bovine serum albumin (67 kD), ovalbumin (43 kD), and ribonuclease A (14 kD) were used as protein standards.
Amino Acid Sequence Analysis
Protein sequencing was performed as described previously (Rosenfeld et al., 1992). RVCaB was completely purified by SDS-PAGE of the preparation after ion-exchange column chromatography. The purified RVCaB was partially digested with trypsin to obtain polypeptide fragments. The amino-terminal sequences of the purified RVCaB and the fragments were determined with an 476A sequencer (Applied Biosystems, Foster City, CA).
PAGE and Staining with Stains-All
SDS-PAGE in 12% (w/v) polyacrylamide gel was carried out by the method of Laemmli (1970). Two-dimensional gel electrophoresis was conducted in glass tubes (0.1 × 6.5 cm) for 4 h at 240 V. Gels contained 5% (w/v) acrylamide, 0.25% (w/v) bisacrylamide, 2% (v/v) Ampholine (Amersham Pharmacia Biotech), and 6.5% (w/v) glycerol. The buffers for cathode and anode were 40 mm NaOH and 10 mm H3PO4, respectively. The gel was overlaid on the second SDS gel. After electrophoresis, the gel was fixed and then stained with Coomassie Blue. One-dimensional electrophoresis was done in a 4% to 15% linear gradient polyacrylamide gel at 120 V for 18 h using non-denaturing conditions (Maeshima, 1990). In this non-denaturing system, the gel and electrode buffers contained neither SDS nor detergent. Proteins were visualized by staining with Coomassie Blue.
To detect the Ca2+-binding proteins, polyacrylamide gels were stained with the metachromatic cationic carbocyanine dye Stains-all (Sigma Aldrich, Tokyo; Campbell et al., 1983). After electrophoresis, the gel was fixed with 25% (v/v) isopropyl alcohol and 30 mm Tris. Then the gel was stained in the dark for 24 h with 0.0025% (w/v) Stains-all, 25% (v/v) isopropyl alcohol, 7.5% (v/v) formamide, and 30 mm Tris, pH 8.8. For spectral analysis, an aliquot of the purified RVCaB was dissolved in 1 mL of 2 mm MOPS-KOH, pH 7.2, 30% (v/v) ethylene glycol, and 5 mm Stains-all as described by Caday and Steiner (1985). After incubation in the dark for 30 min, absorption spectra were taken with a spectrophotometer.
Immunoblotting
The antibodies to vacuolar membrane aquaporin VM23 were prepared (Maeshima, 1992). The antibody against one (PAQ1) of the radish plasma membrane aquaporins was also prepared (Suga et al., 2000). The antibody to BiP was a kind gift from Dr. Mikio Nishimura (National Institute for Basic Biology, Japan). Immunoblotting was performed by the standard method using horseradish peroxidase-linked protein A and chemiluminescent reagents (Amersham Pharmacia Biotech).
Ca2+-Binding Assay of RVCaB
The 45Ca2+ overlay assay was carried out by the method of Maruyama et al. (1984). The purified RVCaB was slot-blotted to poly(vinylidene difluoride) membrane using a slot-blot apparatus (Bio-Rad, Hercules, CA). The membrane sheet was washed four times with 10 mm MES-KOH, pH 6.5, 5 mm MgCl2, and 60 mm KCl. Then the membrane was incubated in the same buffer supplemented with 1.8 MBq 45Ca2+ as CaCl2 (37 GBq mmol−1, Amersham Pharmacia Biotech) at 23°C for 10 min. The membrane was washed three times in 50% (w/v) ethanol and dried at room temperature. An autoradiogram of the 45Ca2+-labeled proteins on the membrane was obtained by exposure to an x-ray film for 3 d at −80°C.
Ca2+ Transport Assay
Ca2+ uptake into membrane vesicles was measured by the filtration method (Ueoka-Nakanishi et al., 1999). Two preparations of vacuolar membranes with and without RVCaB were assayed for Ca2+ uptake activity. The vesicles prepared as described above were used as the vesicles with RVCaB. To prepare the vesicles without RVCaB, the vacuolar membrane fraction was sonicated briefly at 70 kHz for 30 s and then centrifuged at 100,000g for 40 min. The precipitate was suspended Tris-DG containing 0.25 m sorbitol to make up the same volume of the original membrane suspension to normalize vesicle populations. Ca2+ transport activity was assayed at 22°C in 100 μL of a medium consisting of 0.25 m sorbitol, 5 mm MES-Tris, pH 7.2, 50 mm KCl, 0.5 mm DTT, 3 mm MgCl2, 100 μm CaCl2 ([45Ca] 37 kBq mL−1), and 1 mm Tris-ATP, pH 7.5. Reaction was initiated by the addition of ATP at 1 mm. After 2 min, the mixture was filtered through a presoaked 0.45-μm nitrocellulose filter (13 mm in diameter). The filter was washed twice with 200 μL of 0.25 m sorbitol, 5 mm MES-Tris, pH 7.2, 50 mm KCl, 0.5 mm DTT, 0.25 mm MgCl2, and 1 mm EGTA. The radioactivity associated with the filter was measured with a liquid scintillation counter. Background values obtained after incubation without ATP were subtracted from corresponding values obtained in the presence of ATP.
Preparation of Intact Vacuoles
Radish shoots (1 g) from the etiolated 4-d-old seedlings were incubated with 5 mL of an enzyme solution that contained 2% (w/v) cellulase Onozuka RS (Yakult Pharmaceutical, Japan), 0.5% (w/v) Macerozyme R-10 (Yakult), 0.03% (w/v) Pectolyase (Kyowa Hakko, Tokyo), 50 mm MES-KOH, pH 5.5, 2 mm DTT, and 0.4 m mannitol. After incubation for 3 h at 30°C, the suspension was filtered through two layers of Miracloth (Calbiochem, La Jolla, CA) and then centrifuged at 100g for 5 min. The supernatant fraction (1 mL) was mixed with 1 mL of 0.2 mg/mL diethylaminoethyl-dextran and incubated for 20 min at 25°C. To the suspension was added 1 mL of 0.5% (w/v) dextran sulfate. After incubation for 5 min at room temperature, 2 mL of 25% (v/v) Ficoll was added at a final concentration of 10% (w/v). The suspension was overlaid onto a Ficoll step gradient (1 mL each of 5%, 3%, 1.5%, and 0% [w/v] Ficoll). The interface portion between 1.5% and 0% (w/v) solutions was collected as intact vacuoles.
cDNA Construction and Screening
Total RNA was isolated from growing taproots of radish for construction of a cDNA library. Taproots were taken from 3-month-old plants and immediately frozen in liquid nitrogen, and then RNA was extracted by the phenol/SDS extraction method. RNA and DNA were precipitated by cold ethanol and resuspended in Tris-EDTA buffer (10 mm Tris-HCl, pH 8.0, and 1 mm EDTA). The RNA was precipitated with 4 m LiCl. The mRNA fraction was isolated with oligo(dT)-latex, and converted into cDNA using reverse transcriptase and an oligo-dT adapter primer [5′-CGGGATCCACTAGTTCTAGAGCGG+d(T)17 -3′]. Specific cDNA was then directly amplified by PCR using a degenerated oligonucleotide primer based on an N-terminal amino acid sequence of RVCaB [5′-GGAATTCCGCTACCG-CTGA(TC)GT(TCAG)GA(AG) CA-3′]. Amplified DNA fragments (950 bp) were purified and ligated into the EcoRI and BamHI sites of pBluescript SK(+) plasmid vector for transformation of Escherichia coli DH5α. The DNA sequence was determined from single-strand plasmid DNAs by the dideoxy chain-termination method with a Thermo sequenase cycle sequencing kit (Amersham Pharmacia Biotech). The full-length cDNA sequence was determined using 3′-RACE and 5′-RACE methods. The sequences were aligned using the DNASIS program (Hitachi Software Engineering, Tokyo).
Production of Recombinant Protein in E. coli
To produce a recombinant protein in E. coli, the cDNA of the radish RVCaB was ligated into the EcoRI/NotI site of pET23(b) expression vector (Novagen, Madison, WI). The recombinant plasmid was introduced into E. coli BL21(DE3) (Novagen), and production of the recombinant protein was induced by the addition of 1 mm isopropyl thio-β-d-galactoside to the culture medium at 37°C. A cell extract prepared from the transformed E. coli was subjected to SDS-PAGE and stained with Stains-all.
ACKNOWLEDGMENTS
We acknowledge the assistance of Dr. Hitoshi Mori in the analysis of amino acid sequences, Dr. Hanayo Ueoka-Nakanishi in determining the Ca2+ transport activity, Shinobu Suga for the preparation of radish RNA fraction and the antibody to radish plasma membrane aquaporin (PAQ1), and Ayako Takeda in the initial research of RVCaB. We also wish to thank Dr. Mikio Nishimura for the generous gift of anti-BiP antibody and Dr. Heven Sze for critical reading of the manuscript.
Footnotes
This work was supported in part by Grants-in-Aid for Scientific Research (nos. 11163212 and 10219203 to M.M.) from the Ministry of Education, Science, Sports and Culture of Japan.
LITERATURE CITED
- Battey NH, James NC, Greemland AJ. cDNA isolation and gene expression of the maize annexins p33 and p35. Plant Physiol. 1996;112:1391–1396. doi: 10.1104/pp.112.3.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford S, Rea PA, Sanders D. Voltage sensitivity of H+/Ca2+ antiport in higher plant tonoplast suggests a role in vacuolar calcium accumulation. J Biol Chem. 1990;265:9617–9620. [PubMed] [Google Scholar]
- Caday CG, Steiner RF. The interaction of calmodulin with the carbocyanine dye (Stains-all) J Biol Chem. 1985;260:5985–5990. [PubMed] [Google Scholar]
- Calvert CM, Gant SJ, Bowles DJ. Tomato annexins p34 and p35 bind to F-actin and display nucleotide phosphodiesterase activity inhibited by phospholipid binding. Plant Cell. 1996;8:333–342. doi: 10.1105/tpc.8.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KP, MacLennan DH, Jorgensen AO. Staining of the Ca2+-binding proteins, calsequestrin, calmodulin, troponin C, and S-100, with the cationic carbocyanine dye “Stains-all.”. J Biol Chem. 1983;258:11267–11273. [PubMed] [Google Scholar]
- Carroll AD, Moyen C, Van Kesteren P, Tooke F, Battey NH, Brownlee C. Ca2+, annexins, and GTP modulate exocytosis from maize root cap protoplasts. Plant Cell. 1998;10:1267–1276. doi: 10.1105/tpc.10.8.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Hayes PM, Mulrooney DM, Pan A. Identification and characterization of cDNA clones encoding plant calreticulin in barley. Plant Cell. 1994;6:835–843. doi: 10.1105/tpc.6.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan SJ, Hastings C, Winfrey R., Jr Cloning and characterization of the calreticulin gene from Ricinus communis L. Plant Mol Biol. 1997;34:897–911. doi: 10.1023/a:1005822327479. [DOI] [PubMed] [Google Scholar]
- Crofts AJ, Denecke J. Calreticulin and calnexin in plants. Trends Plant Sci. 1998;3:396–399. [Google Scholar]
- Denecke J, Carlsson LE, Vidal S, Hoglund AS, Ek B, van Zeijl MJ, Sinjorgo KMC, Palva ET. The tobacco homolog of mammalian calreticulin is present in protein complexes in vivo. Plant Cell. 1995;7:391–406. doi: 10.1105/tpc.7.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresselhaus T, Hagel C, Lrz H, Kranz E. Isolation of a full-length cDNA encoding calreticulin from a PCR library of in vitro zygotes of maize. Plant Mol Biol. 1996;31:23–34. doi: 10.1007/BF00020603. [DOI] [PubMed] [Google Scholar]
- Gidrol X, Sabelli PA, Fern YS, Kush AK. Annexin-like protein from Arabidopsis thaliana rescues delta oxyR mutant of Escherichia coli from H2O2 stress. Proc Natl Acad Sci USA. 1996;93:11268–11273. doi: 10.1073/pnas.93.20.11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AM, Wesson C, Trumble WR. Calreticulin is the major Ca2+ storage protein in the endoplasmic reticulum of the pea plants (Pisum sativum) Biochem Biophys Res Commun. 1995;211:54–59. doi: 10.1006/bbrc.1995.1777. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Nakayama S, Dretsinger RH. Classification and evolution of EF-hand proteins. Biometals. 1998;11:277–295. doi: 10.1023/a:1009282307967. [DOI] [PubMed] [Google Scholar]
- Kovacs I, Ayaydin F, Oberschall A, Ipacs I, Bottka S, Pongor S, Dudits D, Toth EC. Immunolocalization of a novel annexin-like protein encoded by a stress and abscisic acid responsive gene in alfalfa. Plant J. 1998;15:185–197. doi: 10.1046/j.1365-313x.1998.00194.x. [DOI] [PubMed] [Google Scholar]
- Krause K-H, Simmerman HKB, Jones LR, Campbell KP. Sequence similarity of calreticulin with a Ca2+-binding protein that co-purifies with an Ins(1,4,5)P3-sensitive Ca2+ store in HL-60 cells. Biochem J. 1990;270:545–548. doi: 10.1042/bj2700545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretsinger RH. EF-hands embrace. Nat Struct Biol. 1997;4:514–516. doi: 10.1038/nsb0797-514. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lim EK, Roberts MR, Bowles DJ. Biochemical characterization of tomato annexin p35: independence of calcium binding and phosphatase activities. J Biol Chem. 1998;273:34920–34925. doi: 10.1074/jbc.273.52.34920. [DOI] [PubMed] [Google Scholar]
- Mackrill JJ. Protein-protein interactions in intracellular Ca2+-release channel function. Biochem J. 1999;337:345–361. [PMC free article] [PubMed] [Google Scholar]
- Maeshima M. Oligomeric structure of H+-translocating inorganic pyrophosphatase of plant vacuole. Biochem Biophys Res Commun. 1990;168:1157–1162. doi: 10.1016/0006-291x(90)91150-q. [DOI] [PubMed] [Google Scholar]
- Maeshima M. Characterization of the major integral protein of vacuolar membranes. Plant Physiol. 1992;98:1248–1254. doi: 10.1104/pp.98.4.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima M, Nakanishi Y, Matsuura-Endo C, Tanaka Y. Proton pumps of the vacuolar membrane in growing plant cells. J Plant Res. 1996;109:119–125. [Google Scholar]
- Maeshima M, Yoshida S. Purification and properties of vacuolar membrane proton-translocating inorganic pyrophosphatase from mung bean. J Biol Chem. 1989;264:20068–20073. [PubMed] [Google Scholar]
- Maruyama K, Mikawa T, Ebashi S. Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J Biochem. 1984;95:511–519. doi: 10.1093/oxfordjournals.jbchem.a134633. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Neuhaus JM. Cis-element of targeting to the vacuole. J Exp Bot. 1999;50:165–174. [Google Scholar]
- Napier RM, Trueman S, Henderson J, Bouce JM, Hawes C, Fricker MD. Purification, sequencing and functions of calreticulin from maize. J Exp Bot. 1995;46:1603–1613. [Google Scholar]
- Proust J, Houlne G, Schantz ML, Shen WH, Schantz R. Regulation of biosynthesis and cellular localization of Sp32 annexins in tobacco BY2 cells. Plant Mol Biol. 1999;39:361–372. doi: 10.1023/a:1006199814795. [DOI] [PubMed] [Google Scholar]
- Rosenfeld J, Capdevielle J, Guillemot JC, Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem. 1992;203:173–179. doi: 10.1016/0003-2697(92)90061-b. [DOI] [PubMed] [Google Scholar]
- Saalbach G, Jung R, Kunze G, Saalbach I, Adler K, Münts K. Different legumin protein domains act as vacuolar targeting signals. Plant Cell. 1991;3:695–708. doi: 10.1105/tpc.3.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Brownlee C, Harper JF. Communicating with calcium. Plant Cell. 1999;11:691–706. doi: 10.1105/tpc.11.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DF, Randall SK. A vacuole-associated annexin protein, VCaB42, correlates with the expression of tobacco cells. Plant Physiol. 1997;115:753–761. doi: 10.1104/pp.115.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker KS, Sze H. Calcium transport into the vacuole of oat roots. Characterization of H+/Ca2+ exchange activity. J Biol Chem. 1986;261:12172–12178. [PubMed] [Google Scholar]
- Sharma Y, Balasubramanian D. Stains-all is a dye that probes the conformational features of calcium binding proteins. In: Heizmann CW, editor. Novel Calcium-Binding Proteins. Berlin: Springer-Verlag; 1991. pp. 51–61. [Google Scholar]
- Sharma Y, Rao CM, Rao SC, Krishna AG, Somasundaram T, Balasubramanian D. Binding site conformation dictates the color of the dye stains-all: a study of the binding of this dye to the eye lens proteins crystallins. J Biol Chem. 1989;264:20923–20927. [PubMed] [Google Scholar]
- Shin H, Brown RM., Jr GTPase activity and biochemical characterization of a recombinant cotton fiber annexin. Plant Physiol. 1999;119:925–934. doi: 10.1104/pp.119.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga S, Imagawa S, Maeshima M (2000) Specificity of the accumulation of mRNAs and proteins of the plasma membrane and tonoplast aquaporins in radish organs. Planta (in press) [DOI] [PubMed]
- Sze H, Li X, Palmgren MG. Energization of plant cell membranes by H+-pumping ATPases: regulation and biosynthesis. Plant Cell. 1999;11:677–690. doi: 10.1105/tpc.11.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H, Liang F, Hwang I, Curran AC, Harper JF. Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:433–462. doi: 10.1146/annurev.arplant.51.1.433. [DOI] [PubMed] [Google Scholar]
- Ueoka-Nakanishi H, Nakanishi Y, Tanaka Y, Maeshima M. Properties and molecular cloning of Ca2+/H+ antiporter in the vacuolar membrane of mung bean. Eur J Biochem. 1999;262:417–425. doi: 10.1046/j.1432-1327.1999.00377.x. [DOI] [PubMed] [Google Scholar]
- Ueoka-Nakanishi H, Tsuchiya T, Sasaki M, Nakanishi Y, Cunningham KW, Maeshima M. Functional expression of mung bean Ca2+/H+ antiporter in yeast and its intracellular localization in the hypocotyls and tobacco cells. Eur J Biochem. 2000;267:3090–3098. doi: 10.1046/j.1432-1033.2000.01343.x. [DOI] [PubMed] [Google Scholar]
- von Schaewen A, Chrispeels MJ. Identification of vacuolar sorting information in phytohemagglutinin, an unprocessed vacuolar protein. J Exp Bot. 1993;44:339–342. [Google Scholar]
- Vorum H, Liu X, Madsen P, Rasmussen HH, Honore B. Molecular cloning of a cDNA encoding human calumenin, expression in Escherichia coli and analysis of its Ca2+-binding activity. Biochim Biophys Acta. 1998;1386:121–131. doi: 10.1016/s0167-4838(98)00089-2. [DOI] [PubMed] [Google Scholar]
- Yoshihisa T, Anraku Y. A novel pathway of import of α-mannosidase, a marker enzyme of vacuolar membrane, in Saccharomyces cerevisiae. J Biol Chem. 1990;265:22418–22425. [PubMed] [Google Scholar]