Abstract
Purpose
We examined changes in visual function, and ocular and retinal blood flow (RBF) among retinitis pigmentosa (RP) participants in a randomized controlled trial of electro-stimulation therapies.
Methods
Twenty-one RP participants were randomized (1:1:1) to transcorneal electrical stimulation (TES) at 6 weekly half hour sessions, electro-acupuncture or inactive laser acupuncture (sham control) at 10 half hour sessions over 2 weeks. ETDRS visual acuity, quick contrast sensitivity function, Goldmann visual fields, AdaptDx scotopic sensitivity, spectral flow and color Doppler imaging of the central retinal artery (CRA), and RBF in macular capillaries were measured twice pre-treatment, after 2 TES sessions, within a week and a month after intervention completion.
Results
We measured a significant improvement in retrobulbar CRA mean flow velocity for both the TES (p=0.038) and electro-acupuncture groups (p=0.001) on average after 2 weeks of treatment when compared to sham controls. TES and electro-acupuncture subjects had significant 55% and 34% greater increases, respectively, in RBF in the macular vessels when compared to sham controls (p<0.001)(p=0.008) within a week of completing six TES sessions or a month after electro-acupuncture. There was a significant difference in the proportion of eyes that had improved visual function when comparing the three intervention groups (p=0.038): four of seven TES subjects (57%), two of seven electro-acupuncture subjects (29%), and none of the seven control subjects (0%) had a significant visual improvement outside of typical test-retest variability at two consecutive post-treatment visits.
Conclusion
Increased blood flow following electro-stimulation therapies is an objective, physiological change that occurred in addition to visual function improvements in some RP patients.
Keywords: retinitis pigmentosa, acupuncture, transcorneal electrical stimulation, retinal blood flow
INTRODUCTION
At the present time, there are no proven therapeutic options to improve or halt the slowly progressive visual loss that occurs in patients with retinitis pigmentosa (RP). Electro-acupuncture and transcorneal electrical stimulation (TES) are minimally invasive therapies that are readily available. Previous basic science studies of electro-stimulation therapies involving in vitro or animal models of RP have indicated that electro-acupuncture (Pagani et al. 2006) or TES (Morimoto et al. 2007)(Morimoto et al. 2012)(Hanif et al. 2016)(Rahmani et al. 2013) may have the potential to slow disease progression by preserving retinal function and thickness. To date, a few human studies or reports of electro-acupuncture without a randomized control group (Dabov et al. 1985)(Kiser & Dagnelie 2008)(Wong & Ching 1980)(Bittner et al. 2014)(Blechschmidt et al. 2016) and small-scale and/or relatively short-term (i.e., up to a year) randomized controlled trials (RCTs) of TES (Schatz et al. 2011)(Robles-Camarillo et al. 2013)(Schatz et al. 2017) involving RP patients have provided preliminary support that these interventions may help to improve various aspects of visual function. The potential for a beneficial effect following acupuncture is further supported by research that has demonstrated activation of visual cortical areas (Li et al. 2003)(Li et al. 2010)(Siedentopf et al. 2002) in response to stimulation of vision-related acupoints in normals.
Previous basic science studies indicate that electro-stimulation most likely does not have a direct effect on photoreceptor cell survival or function, but rather may exert an indirect effect on photoreceptors through its modulation of retinal microglia and secretion of several retinal neurotrophic factors and nerve growth factors from Müller cells, as well as changes in the microenvironment, such as downregulation of cytokines responsible for regulating immune responses (i.e., interleukins), or reductions in gene expression levels for proteins associated with inflammation or apoptosis.(Zhou et al. 2012)(Tao et al. 2016)(Sehic et al. 2016) Previous in vitro studies suggest that electro-stimulation of the retina inhibits microglial activation and their secretion of proinflammatory and toxic cytokines (Schmid et al. 2009)(Zhou et al. 2012); however, it is also possible that the microglia response in vivo is due to its sensitivity to microenvironment changes rather than to direct effects of electro-stimulation. It has been proposed that the neuroprotection mediated by electrostimulation occurs via several counteracting trophic factors that mediate microglial activation and suppression to create homeostatic balance and a nurturing microenvironment suitable for the rescue of apoptotic photoreceptor cells.
Presently, no clinical studies have confirmed these proposed hypotheses for the mechanisms that may be involved with visual and retinal preservation in RP patients following electro-acupuncture or TES. This is largely due to the inability to non-invasively measure these factors directly in human subjects. However, it is possible to measure blood flow to the eye and within retinal vessels, which could serve as a surrogate endpoint or biomarker of other retinal changes. Evidence of improved blood flow following electro-stimulation therapies has been documented in studies involving healthy subjects without RP. Immediately following needling of vision-related acupoints, normal subjects developed a significant increase in blood flow velocity in the ophthalmic artery, (Litscher 2002) and decreased vascular resistance in the central retinal artery (CRA) and posterior ciliary arteries.(Takayama et al. 2012) Electro-acupuncture increases blood fluidity by decreasing platelet aggregation in the systemic vascular system of normal subjects, and may involve an endogenous adrenergic mechanism.(Ishikawa et al. 2012) Healthy individuals without RP who received a single session of TES experienced an increase in chorioretinal blood flow increased in the macula, as well as midway between the optic nerve and macula, that was sustained for at least 24–40 hours.(Kurimoto et al. 2010) TES administered to cats without retinal degenerative disease produced reflectance changes (i.e., intrinsic signals) at the optic disc and retinal vessels in a manner that suggests that TES primarily activates retinal ganglion cells first and blood flow is recruited afterwards.(Mihashi et al. 2011)(Morimoto et al. 2014) Thus, a parsimonious hypothesis for electro-acupuncture or TES induced mechanisms may be related to changes in ocular and retinal blood flow, which could help serve as an indicator of physiological changes that occur in response to these interventions.
The goal of the current study was to determine if evidence exists to support the hypotheses that ocular and retinal blood flow in RP can improve after treatment with electro-acupuncture or TES in a small-scale randomized, double-masked, placebo controlled trial. Within the context of this single phase, 3-arm trial, we were interested in comparing changes in hemodynamics and visual function for these two electro-stimulation therapies to those for sham intervention across RP patients with a wide range of vision loss.
METHODS
Institutional Review Board approval was obtained from the Nova Southeastern University (NSU) and this research followed the tenets of the Declaration of Helsinki. Written informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study. The work presented here is HIPAA compliant.
Subjects
Trial participants included 21 adults with a confirmed diagnosis of RP. Their mean age was 44 years (SD: 12 years; range: 25–70 years), and nine were women (43%). There were ten Caucasians, six Hispanic/Latinos, three African Americans, and two Asians. One third of the subjects (n=7) were recruited through the clinical optometric practices (i.e., The Eye Care Institute and Lighthouse of Broward) affiliated with NSU in Fort Lauderdale, Florida, USA. The remaining 14 subjects received diagnoses of RP from eye care providers outside these practices and self-referred after learning of the trial through online listings (e.g., clinicaltrials.gov NCT02086890). Exclusion criteria were vision loss due to ocular diseases other than RP, previous electro-stimulation therapy for RP, non-English speaking, history of excessive bleeding, implanted cardiac pacemaker, pregnancy, or steroidal systemic medication. No participants had changes in their prescription medications or over the counter supplements during the study.
Study Design
The study coordinator (SK) received the randomization allocation for each individual after completion of baseline testing from a co-investigator (GD) at another institution who had no prior contact or information regarding the participants, and generated the assignment schedule prior to the start of the trial using random permuted blocks that were unknown to other co-investigators. Throughout the trial, all subjects remained in the treatment group to which they were initially randomized. Subjects were masked to treatment status (i.e., real or sham) since they were informed that they may be randomized to one of the six following alternatives: TES with or without active electro-stimulation, electro-acupuncture with needles applied to vision-related acupoints or random locations on the body, or laser acupuncture with an active or inactive (i.e., red light only) laser. In fact, the trial only administered three possible alternatives: TES with active electro-stimulation (n=7), electro-acupuncture applied to vision-related acupoints (n=7), or laser acupuncture with an inactive (i.e., red light only) laser (n=7).
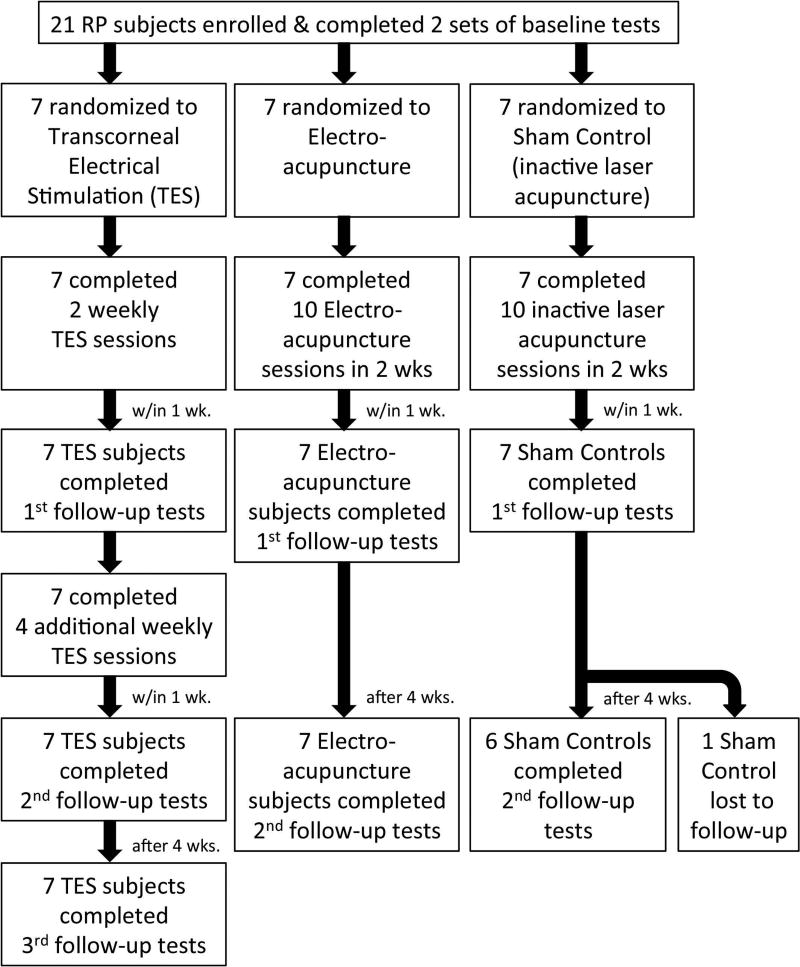
All vision tests were administered by a single examiner (AKB) who was masked to treatment allocation. All outcome measures involving ocular or retinal blood flow (RBF) or psychophysical vision measures were collected four or five times: at two baseline visits pre-intervention (mean intervisit time= 12.7 days, range 1–42 days); after completing two of six TES sessions; within one week of completing all six weekly TES sessions or all ten acupuncture sessions within 2 weeks (either electro-acupuncture or sham, inactive laser acupuncture); and one month after treatment completion. Figure 1 includes a chart to show the flow of participants, randomization to each group, interventions, timing, and study visits at which the baseline and follow-up outcome measures were obtained. Only one subject, who was in the sham control group, was lost to follow up at the last visit since she was unable to take time off from work to travel to the study site. Optical coherence tomography (OCT) and electrophysiology were performed once pre-intervention and once at the one-month post-intervention follow-up. Each visit lasted approximately 5–6 hours. Subjects were offered a lunch voucher to take a break about 2–4 hours after the start of the visit and after the ultrasound measures of ocular blood flow. At each visit, tests were obtained in the same order and time of day to minimize diurnal or other fluctuations. Data collection occurred from September 2014 through June 2015.
Figure 1.
A chart of the flow of participants, randomization to each group, interventions, timing, and study visits at which the baseline and follow-up outcome measures were obtained.
Interventions
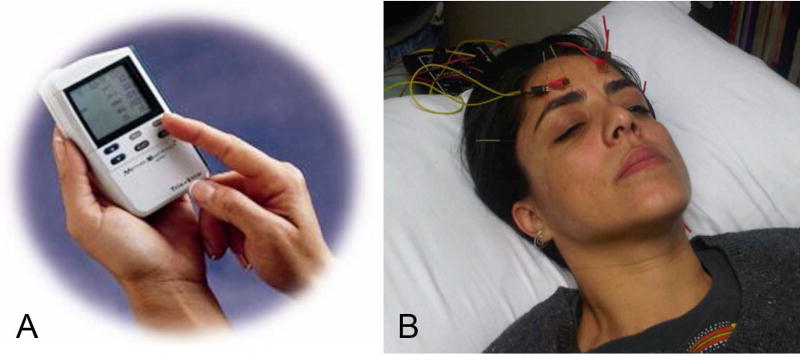
For TES, a single use, sterile DTL plus electrode was placed on the surface of each eye with corneal anesthetic drops (Proparacaine) and gold-cup ground electrode on the temple, as in a previous trial.(Schatz et al. 2011) TES was administered to both eyes by an optometrist (KS) using an FDA approved, commercially available neurostimulator (TrioStim; Mettler Electronics), off-label, as shown in figure 2A. The microcurrent settling of this instrument was set to deliver rectangular biphasic current pulses (5-ms positive, directly followed by 5-ms negative) with amplitudes up to 750µA (the instrument’s maximum level) at a frequency of 20 Hz, for 30 minutes during six weekly sessions. At the first session, we followed a previously published (Schatz et al. 2011) alternative forced choice procedure to assess each subject’s mean electrical phosphene threshold (EPT) in a dark room, which we intended to use to determine individual stimulation strength (i.e., 150% of their individual EPT). However, only one eye in one of the seven TES subjects had an EPT ≤500µA and was stimulated at 700µA, whereas the other six TES subjects did not have a measurable EPT ≤500µA in either eye and received stimulation at the maximum level of 750µA, which was maintained at a constant level at all six sessions. The absence of an EPT in most subjects was likely due to advanced or severe retinal degeneration since thresholds are elevated in RP compared to normals, and correlated with visual field loss in RP (Morimoto et al. 2006). Previous studies reported RP patients with logMAR VA 0.02–0.9 had a mean EPT of 371 µA ± 223(Naycheva et al. 2012) and those with VA worse than 2.0 logMAR had a mean EPT of 640 µA ± 101(Morimoto et al. 2006).
Figure 2.
Panel A shows the TrioStim microcurrent stimulator unit used for TES. Panel B displays the electrodes attached to the acupuncture needles above the eyes during electro-acupuncture.
A previously established electro-acupuncture protocol (Bittner et al. 2014) was administered by a licensed acupuncturist (NM) at ten sessions each lasting 30 minutes within a two week period. In addition to needled acupoints throughout the body, the leads of an electro-acupuncture device [Transcutaneous electrical nerve stimulator (TENS) unit; Lhasa OMS Inc.] were attached to four of the acupuncture points (two per session) located on the forehead and below the eyes, as shown in figure 2B. The sham control group received inactive laser (i.e., red light only) applied with a modified laser acupuncture device (RJ Laser; Waldkirch, Germany) to random locations on the body (non-acupoints) by the study acupuncturist (NM) according to the same treatment schedule, duration and practice location as the electro-acupuncture group.
Outcome Measures
Retrobulbar ocular hemodynamics in the CRA were measured with color Doppler imaging and spectral flow Doppler using the GE Logiq 7 according to previously published guidelines (Stalmans et al. 2011) and procedures.(Kayser et al. 2017) by trained sonographers (DM, PV, JH) who were masked to the subjects’ intervention group. Mean peak systolic velocity (PSV) and end diastolic velocity (EDV) were used to calculate mean flow velocity (MFV) using the equation: MFV=((PSV)+(2×EDV))/3.(Naqvi et al. 2013)
RBF velocity was assessed in macular capillaries using instrumentation developed by our co-investigator (JR), which included a retinal imager and RBF velocimeter. The optical design, image registration, calibration, and results in human subjects have been previously described in detail.(Lemaillet et al. 2010)(Duncan et al. 2010)(Ibrahim et al. 2015) This approach relies on tracking the non-uniform distribution of red blood cells within the capillary, and is capable of dealing with very low signal-to-noise ratios. Vessel diameter and blood velocity were used to calculate mean flow velocity [MFV =((((Diameter/2)2)*π)/1000)*velocity] as a measure of RBF. Reliable measures were not obtainable in RP subjects who were very photophobic or had advanced RP with nystagmus or poor fixation; therefore, we analyzed RBF data from 9 eyes in 5 TES subjects, 10 eyes in 5 electro-acupuncture subjects and 11 eyes in 6 sham controls.
Best-corrected visual acuity (VA) was measured in each eye using the Early Treatment of Diabetic Retinopathy Study (ETDRS; Lighthouse International, New York, NY) three-chart series at three meters, which was modified to one meter for severely reduced acuities if fewer than 10 letters were initially identified. Contrast sensitivity (CS) was measured with the Pelli-Robson chart binocularly at one meter (Metropia, Ltd., Essex, UK), followed by the quick Contrast Sensitivity Function (qCSF) letter identification test (Adaptive Sensory Technology; San Diego, CA) with 25 trials for each eye at four meters, or at two meters for subjects with severely reduced VA worse than 0.7 logMAR, tested in each eye, as well as binocularly with and without a 4% transmission U23 NoIR filter to simulate low luminance. Goldman visual field (GVF) kinetic perimetry was obtained in each eye using V4e and III4e test targets according to previously published methodology, (Bittner et al. 2011) which was later digitized to calculate the total seeing log retinal area.(Barry et al. 2016) Mean scotopic sensitivity within the first 5–6 minutes of dark-adaption after a 76% initial bleach flash was assessed at 5° from fixation in each eye with the AdaptDx (Maculogix; Hummelstown, PA). For 18 of our subjects (86%), their responses during AdaptDx scotopic sensitivity testing were only cone-mediated (i.e., no measurable rod intercept; <2 log units sensitivity). Multifocal electroretinography (mfERG) was obtained in each eye with Burian-Allen electrodes and the Veris system (Electro-Diagnostic Imaging, Inc.; Milpitas, CA) using an array of 103 hexagons that stimulated the central 40–50 degrees of the retina. OCT using the Cirrus 4000 (Zeiss; San Diego, CA) was obtained to detect any presence of cystoid changes within the foveal region.
Data analysis
Primary outcome measures included blood flow in the CRA and RBF in the macular vessels, while the secondary outcomes included the visual function measures. Normal distribution of variables was confirmed with Shapiro-Wilk analysis. PSV was not normally-distributed at the second follow-up visit and therefore we performed a transformation of this variable (cube power) at that time point to create a normal distribution for the analysis. We used the reciprocal of the changes in MFV for the RBF measures and mfERG ratios, as indicated by Box-Cox analysis to obtain a normal distribution for those variables. Changes from mean baseline for CRA blood flow (i.e., PSV, EDV and MFV) or RBF (i.e., MFV) in the TES or electro-acupuncture groups compared to sham controls at each follow-up visit were adjusted for age (Asejczyk-Widlicka et al. 2015) and gender, (Yanagida et al. 2015) and analyzed using multilevel linear regression models (i.e., random-effects maximum likelihood estimation) to account for correlations between a subject’s eyes (with data from both eyes included), and p<0.05 was defined as statistically significant, using Stata/IC version 13.1 (Stata Corp., College Station, TX). This was an exploratory trial that was not specifically planned to be powered with an adequate sample size to demonstrate significant changes in blood flow or visual function; therefore, it was not appropriate to adjust for multiple comparisons (e.g., Bonferroni).
A subgroup analysis of 11 eyes in nine of our trial participants was completed to determine mean coefficients of variation (CoV) for baseline RBF measures, which was 16% on average within a single vessel location in an image analyzed by two examiners. The mean CoV was 17% for RBF within a single artery or vein location in two within-visit images from 11 eyes of ten subjects analyzed by one examiner. We determined the proportion of RBF measurement locations that had significant changes from baseline that we defined as ≥20%.
Box-Cox analysis indicated that it was not possible to use power transformation to obtain normally distributed data for VA and GVF; therefore, we stratified those data into categorical variables for analysis on the basis of changes outside of test-retest variability. We calculated 95% coefficients of repeatability (CR.95) for the baseline between-visit visual function tests collected in our trial participants, and also considered previously published CR.95s in RP for VA, CS, and GVF, displayed in table 1. along with the CR.95 values that we used to determine significant changes in our trial participants. In order for changes in our trial participants to be considered significant, we required that the difference at both (i.e., two consecutive) post-treatment follow-up visits compared to mean baseline would need to exceed the CR.95.
Table 1.
Test-retest variability (CR.95) of visual function tests in RP subjects from previously published studies, for the two baseline visits in our trial, and the values we used to consider changes as significant (i.e., outside the CR.95), as well as the number of subjects in our trial who had a significant improvement beyond this defined amount of test-retest variability at both follow-up visits.
| Previously Published | Present Trial Baseline | Considered Significant | Present Trial Improved | |
|---|---|---|---|---|
| ETDRS VA | 0.14–0.23 logMAR(Kiser et al. 2005) | 0.15 logMAR | ≥0.20 log units | 2 TES |
| Pelli-Robson CS | ~0.30 logCS(Kiser et al. 2005)(monocular) | 0.21 logCS(binocular) | ≥0.25 log units | none |
| GVF V4e log retinal area | 33%(Bittner et al. 2011)(within-visit) | 65%(between-visit) | >65% | 2 TES |
| GVF III4e log retinal area | 24%(Bittner et al. 2011)(within-visit) | 109%(between-visit) | >109% | 1 TES |
| cone-mediated AdaptDx | Not previously reported | 0.5 log units | 0.5 log units | 1 Electro-acupuncture |
Changes from mean baseline for Pelli-Robson and qCSF measures in the TES or electro-acupuncture groups compared to sham controls were analyzed using multilevel linear regression models (i.e., mixed-effects maximum likelihood estimation) using a treatment-by-time interaction to model how treatment differences change over time across the two follow-up visits. However, due to floor effects for the qCSF test in six of the trial participants, we also analyzed those results using receiver operator characteristic (ROC) curve analysis to evaluate changes within-subject by describing the probability that the area under the log contrast sensitivity function (AULCSF) across spatial frequencies 1–18 cpd was better for the mean of the two post-treatment tests (i.e., within a week and after a month) than the mean of the two baseline tests. We utilized the qCSF test’s Bayesian method to generate full distributions of the confidence intervals for the mean AULCSF at pre- and post-treatment. An ROC value of 50% indicates no change (i.e., complete overlap of the two distributions), while a maximum ROC value of 100% indicates no overlap of the two distributions. We utilized an ROC value ≥97.5% (for the separation of the distributions) to indicate a significant improvement in the qCSF test.
RESULTS
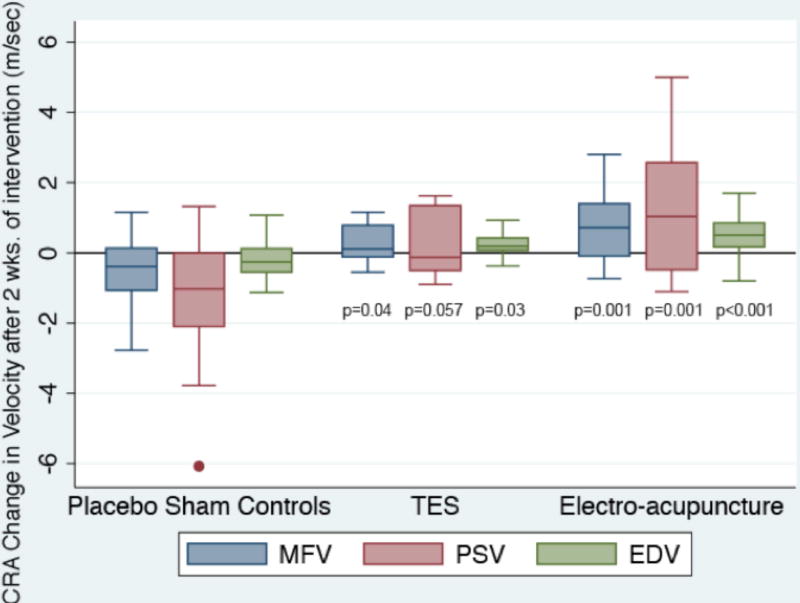
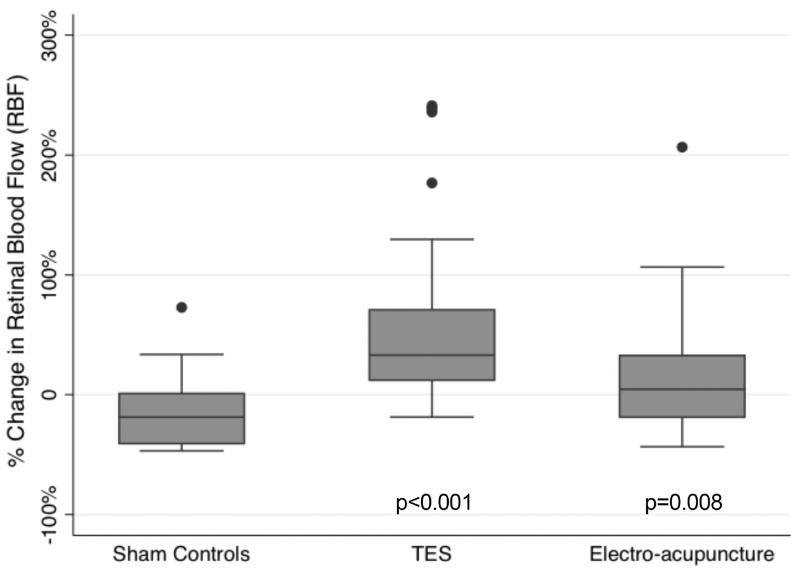
Data for the mean baseline outcome measures of blood flow and visual function are listed in table 2. Figure 3 and table 2 show the significant improvements in the retrobulbar CRA blood flow on average after two TES sessions for PSV, EDV and MFV, as well as within one week of electro-acupuncture for PSV, EDV and MFV when compared to sham controls. These changes in CRA blood flow were no longer significantly different from the sham control group after completing six TES sessions (all p≥0.61) and a month after completing electro-acupuncture (all p≥0.38). Figure 4 and table 2 display the significant RBF improvements in MFV measured in the TES and electro-acupuncture subjects compared to the sham controls within a week after completing all six TES sessions and a month after completing electro-acupuncture. Changes in RBF for the TES and electro-acupuncture groups were not significantly different from the controls when assessed earlier at the first post-treatment visit (i.e., after just two TES sessions or within a week of completing ten electro-acupuncture sessions) (p=0.32 and p=0.25, respectively).
Table 2.
Data for the mean, SD and range of baseline values across all participants for the blood flow and visual function measures. Also listed are the magnitude of change (Δ) and significance levels [95% confidence intervals (CI) and p-values] for the blood flow measures post-TES and post-electro-acupuncture (post-EA) when compared to sham controls.
| Baseline mean (SD) | Baseline range | post-TES Δ | TES: 95% CI | TES: p-value | post-EA Δ | EA: 95% CI | EA: p-value | |
|---|---|---|---|---|---|---|---|---|
| CRA: MFV | 3.58 cm/sec (0.73) | 2.43 to 5.81 | 0.69 | 0.04, 1.34 | p=0.038 | 1.27 | 0.54, 1.99 | p=0.001 |
| CRA: PSV | 6.12 cm/sec (1.52) | 3.93 to 11.48 | 1.26 | −0.04, 2.56 | p=0.057 | 2.38 | 0.94, 3.82 | p=0.001 |
| CRA: EDV | 2.31 cm/sec (0.41) | 1.58 to 3.37 | 0.4 | 0.05, 0.76 | p=0.026 | 0.71 | 0.31, 1.10 | p<0.001 |
| RBF: MFV | 13.56 mm/sec (9.4) | 3.3 to 75.82 | 55% | 32%, 78% | p<0.001 | 34% | 9%, 58% | p=0.008 |
| RBF: Diameter | 54.19µm (11.86) | 28.40 to 88.42 | ||||||
| RBF: Velocity | 5.41 mm/sec (1.74) | 2.67 to 12.35 | ||||||
| ETDRS VA | 0.46 logMAR (0.47) | −0.09 to 1.56 | ||||||
| Pelli-Robson CS | 1.22 logCS (0.52) | 0.33 to 1.93 | ||||||
| qCSF AULCSF | 0.69 (0.59) | 0 to 1.93 | ||||||
| GVF: V4e | 1.71 log ret. area (0.72) | 0.29 to 2.93 | ||||||
| GVF: III4e | 1.18 log ret. area (0.78) | −0.54 to 2.74 |
Figure 3.
Box plot of changes from mean baseline for MFV, mean log PSV and mean EDV in the CRA of each eye according to intervention group after 2 TES sessions or within a week of completing the acupuncture intervention for the electro-acupuncture subjects or laser acupuncture (i.e., placebo sham controls). The bottom and top of the box are the 25th and 75th percentile (i.e., the upper and lower quartiles, respectively), and the band near the middle of the box is the 50th percentile (i.e., the median). The ends of the whiskers represent the lowest datum within 1.5 times the interquartile range of the lower quartile, and the highest datum still within 1.5 times the interquartile range of the upper quartile. Any data not included between the whiskers is plotted as an outlier indicated by a dot.
Figure 4.
Box plot of percent changes in MFV for RBF in the macular vessels according to intervention group within a week of completing six TES sessions and a month after completing electro-acupuncture compared to baseline. Dots: data not included between the whiskers and regarded as outliers.
After two TES sessions, 23% of the veins (i.e., 3 of 13) measured across all TES subjects developed significantly improved RBF ≥20%. Immediately post-TES (i.e., within a week of the sixth or last session), 63% of the vein locations (i.e., 10 of 16) had significantly improved RBF compared to baseline. Fewer arterioles were measurable since they are more attenuated. After two and six TES sessions, 67% (i.e., 4 of 6) and 60% (i.e., 3 of 5) of arteries measured, respectively, developed significantly improved RBF, which is roughly equal to the proportion of vein locations with improved RBF after six TES sessions. In sham controls, only 21% (i.e., 3 of 14) of vessel locations developed improved RBF ≥20% at the same follow-up visit. Nearly half (i.e., 46%) of vessel locations developed significantly improved RBF immediately after electro-acupuncture and 41% of the vessel locations (i.e., 7 of 17) had a significant improvement in RBF a month after completing electro-acupuncture.
There was a statistically significant difference in the proportion of eyes that improved when comparing the three intervention groups (p=0.038): Four of seven TES subjects (57%), two of seven electro-acupuncture subjects (29%), and none of the seven control subjects (0%) had a significant improvement in VA, qCSF, GVF and/or AdaptDx scotopic sensitivity outside of typical test-retest variability at both (i.e., two consecutive) follow-up visits; their results are presented in table 3. The sham control subject who was lost to follow-up at the second follow-up did not have any significant improvements in the visual function tests at the first follow-up visit.
Table 3.
Data for participants who had a significant improvement in visual function [i.e., change (Δ) outside of test-retest variability listed in table 1 or qCSF ROC value ≥97.5%] at both post-treatment (post-tx) assessments following TES or electro-acupuncture (EA). Also listed are these participants’ characteristics, such as age in years, self-reported onset age of initial symptoms of nyctalopia or night vision loss in years, self-reported onset age of initial indications of visual field (VF) loss in years, female (F) or male (M) gender, and presumed genetic mutation inheritance type based on family history of RP (i.e., AD: autosomal dominant, AR: autosomal recessive).
| ID | Age | Onset nyctalopia | Onset VF loss | Gender | Race | Genetic | Intervention | Eye(s) | Test | Baseline | Δ Post-tx 1 | Δ Post-tx 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 44 | 13 | 8 | F | Caucasian | AD | TES | OS | logMAR VA | 1.53 | 0.41 | 1.01 |
| 2 | 47 | 16 | 24 | M | Black | AR | TES | OS | GVF: V4e | 0.71 | 0.31 (104%) | 0.33 (114%) |
| 2 | TES | OS | logMAR VA | 1.14 | 0.27 | 0.41 | ||||||
| 2 | TES | OS | qCSF AULCSF | 0. 02 | 0.12 (97.5% ROC) | 0.13 | ||||||
| 3 | 34 | birth | birth | F | Asian Indian | AR | TES | OD | qCSF AULCSF | 0. 02 | 0.16 (100% ROC) | 0.16 |
| 3 | TES | OS | qCSF AULCSF | 0. 01 | 0.09 (99.9% ROC) | 0.08 | ||||||
| 3 | TES | OU | qCSF AULCSF | 0. 03 | 0.08 (100% ROC) | 0.13 | ||||||
| 4 | 40 | 4 | 25 | M | Hispanic | AR | TES | OD | GVF: V4e | 0.54 | 0.26 (82%) | 0.40 (151%) |
| 4 | TES | OD | GVF: III4e | −0.54 | 0.58 (276%) | 0.56 (259%) | ||||||
| 5 | 46 | birth | 8 | M | Caucasian | AR | EA | OD | AdaptDx nasal | 0.25 | 1.16 log units | 1.00 |
| 5 | EA | OD | AdaptDx sup. | 0. 56 | 0.89 log units | 1.03 | ||||||
| 6 | 58 | birth | 16 | M | Hispanic | AR | EA | OS | qCSF AULCSF | 0. 68 | 0.21 (98.5% ROC) | 0.17 |
| 6 | EA | OU | qCSF AULCSF | 0. 67 | 0.26 (99.5% ROC) | 0.21 | ||||||
| 6 | EA | OU | qCSF filter | 0.36 | 0.24 (99.5% ROC) | 0.16 |
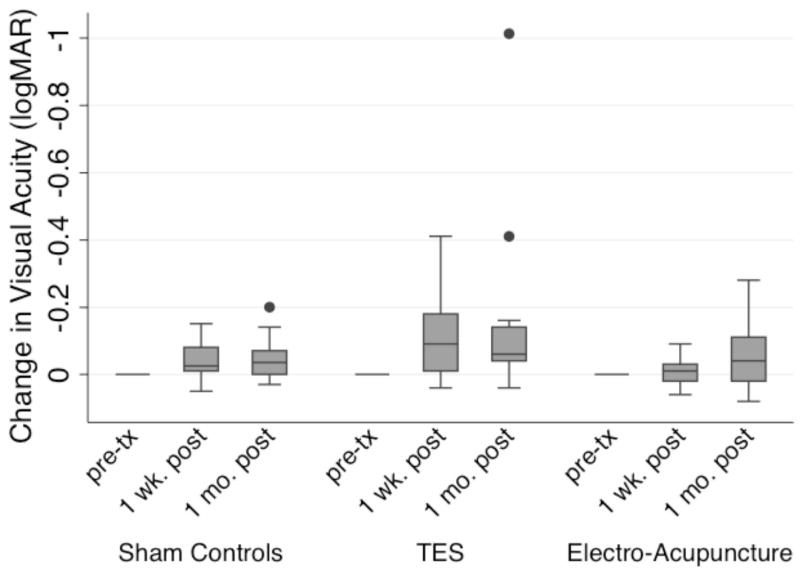
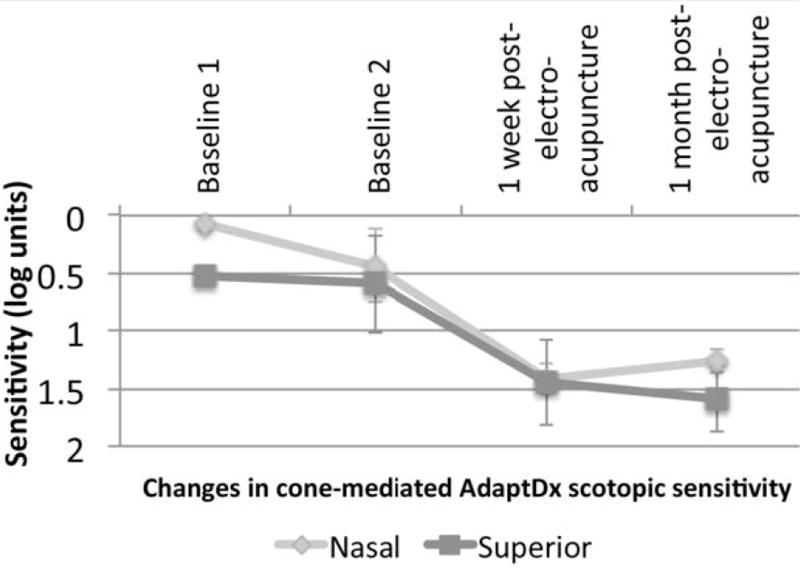
Figure 5 shows the changes in VA at each follow-up visit post-treatment compared to mean baseline for each of the three intervention groups. Figure 6 displays the changes in GVF with the III4e test target at each follow-up visit post-treatment compared to mean baseline for each of the three intervention groups. The significant changes in VA or GVF among those who received TES occurred in the eye with worse vision at baseline. All sham control subjects had ROC values <90% for all of the qCSF AULCSF measures. Figure 7 and table 3 show the changes in the mean AULCSF pre- versus post-treatment for the TES and electro-acupuncture subjects with significant changes for the ROC analysis. Figure 8 and table 3 display the changes across visits for AdaptDx sensitivity in the better eye at baseline for an electro-acupuncture subject. No subjects had a significant change outside of typical variability for Pelli-Robson CS testing at both follow-ups. No subjects had a significant visual function loss for any of the study outcomes at both follow-up visits that was outside of baseline test-retest variability. On average, neither the TES subjects nor the electro-acupuncture subjects had significantly different binocular Pelli-Robson CS or qCSF AULCSF changes from baseline over time compared to the sham controls (all p≥0.48).
Figure 5.
Box plot of changes in logMAR VA according to intervention group within a week and a month after completing each intervention compared to baseline. Dots: data not included between the whiskers and regarded as outliers.
Figure 6.
Box plot of changes in GVF log retinal area with the III4e test target according to intervention group within a week and a month of completing each intervention compared to baseline. Dots: data not included between the whiskers and regarded as outliers.
Figure 7.
Mean CSFs at baseline in blue and post-treatment in red for two TES subjects and one electro-acupuncture subject with high ROC values indicating significantly improved CSFs post-treatment.
Figure 8.
Changes in mean cone-mediated scotopic sensitivity at nasal and superior locations tested with the AdaptDx in one eye of an electro-acupuncture subject.
The mfERG P1 response amplitude for the single central hexagon in ring 1 subtending approximately 2.4 degrees was only measurable (i.e., ≥3.5 nV/deg2) in eight participants [i.e., one who received TES, two who received electro-acupuncture, and five sham controls], whereas the other subjects did not have a measurable response. When comparing the mfERG P1 amplitude ratio from post-treatment (i.e., within one week of the last treatment session) to baseline, we found a mean difference in ratio of 1.63 across both eyes for one of the TES subjects (ID #2 in table 3, who also had significant improvements in VA, qCSF and GVF) when compared to the five sham controls with a measurable mfERG response for ring 1 (p=0.01). For this TES subject, the mfERG P1 response amplitudes for ring 1 in the worse seeing eye at baseline and post-treatment were 3.5 nV/deg2 and 12.5 nV/deg2, respectively. We found no significant difference in the mfERG P1 amplitude ratio post-treatment from baseline for the two electro-acupuncture subjects compared to the five sham controls (p=0.77).
There were no adverse events among the study participants, and no evidence of corneal superficial punctate keratopathy or conjunctival irritation post-TES administration. Inadvertently, four TES subjects, each during only one of the six sessions, received a small, brief electrical shock when there was a loss of connection with the ground electrode on the temple (n=3), and in one instance during which the DTL electrode lost contact with the cornea and was reapplied. Other than discomfort at the moment of the shock, there were no other related issues. Cystoid changes within the foveal region at baseline were detected with OCT in only three eyes of two subjects who were randomized to the sham control group. We found no gross changes in the appearance or magnitude of these cystoid changes during the study period; thus they did not appear to influence changes in central visual function.
DISCUSSION
The present RCT provides evidence of a plausible mechanism (i.e., enhanced blood flow to and within the retinal vessels) that may be associated with or collateral to visual function improvements following electroacupuncture or TES. In addition, findings of several previous basic science studies support the hypothesis that TES might induce a beneficial, neuroprotective effect by creating a nurturing microenvironment for the retina via several other possible mechanisms: neurotrophic, anti-apoptotic, anti-glutamate, and anti-inflammatory, (Sehic et al. 2016) which may influence visual function improvements. In the present RCT, we measured MFV in the CRA and RBF in the macular capillaries as objective outcomes to help support the improvements noted with subjective visual function test results. Not only do these objective measures of blood flow indicate that physiological changes occur following electrostimulation therapies in a diverse group of RP patients, but they are especially valuable since other objective measures (e.g., mfERG) are essentially extinguished and non-measurable in the majority of RP patients.
Our trial of electrostimulation therapies provides evidence that blood flow to the retrobulbar aspect of the eye is recruited first, followed by enhanced blood flow in the macular arterioles and venules. Perhaps this occurred due to blood flow autoregulation processes in the CRA, which are independent of blood flow regulation in the macular capillaries that is influenced by the metabolic needs of the retina.(Kornfield & Newman 2014) Then visual improvements developed in some patients during the timeframe of the blood flow improvements and in the weeks to follow. Thus there appears to be a tendency for a time lag for VA and VF improvements post-TES, which were greater at one month post-treatment than within a week of completing six sessions; this outcome is in agreement with the previous finding of slightly delayed VF improvements post-TES measured by another group.(Schatz et al. 2011)
The current results of improved blood flow to the optic nerve after two TES sessions and subsequently improved blood flow in retinal vessels after six TES sessions is supported by previous studies of TES in cats, (Mihashi et al. 2011)(Morimoto et al. 2014) in which intrinsic signals of stimulation occurred first at the optic disc, suggesting an increase in blood flow or volume, followed by slightly delayed effects that subsequently reached the retinal arteries, and lastly the retinal veins. The slow increase in RBF in our RCT and the sustained chorioretinal blood flow increase found in a previous study of a single session of TES administered to normals without RP (Kurimoto et al. 2010) suggests that such changes are likely mediated by molecular changes in the ocular or retinal tissues rather than neural mediation. Previous studies of TES have found upregulation of IGF-1 (insulin growth factor-1) in activated retinal Müller cells, which may induce vasodilation and therefore has been hypothesized to be responsible for improved RBF following TES.(Kurimoto et al. 2010)
The finding of improved RBF for a majority but not all locations at which RBF was measured is not unexpected given that regional variations in RBF and oxygenation may occur in RP, as is the case in normals and in diabetic retinopathy, (Jørgensen & Bek 2016) and increased RBF is correlated with changes in oxygenation.(Palkovits et al. 2014) The safety of TES is supported by lack of adverse events and of significant reductions in RBF outside of typical test-retest variability post-intervention. If the RBF measures were just fluctuating across visits, one would find a similar number of decreases and increases in RBF post-TES, which was not documented in our trial.
Although we had anticipated that subjects with significantly larger improvements in RBF might be more likely to develop improvements in visual function after either treatment, we did not find support for this hypothesis; RBF was significantly improved on average across all subjects who received electrostimulation therapy, regardless of whether they subsequently developed a measurable significant visual improvement. For the participants who did not have a measurable improvement in vision during this short trial, several basic science research studies suggest that it may still be possible for these therapies to reduce the progression rate of RP long-term, (Sehic et al. 2016) which will need to be confirmed in future RCTs. A limitation of the present RCT and most clinical trials involving people with RP was that the progression rate of RP for each participant was not formally quantified since they were not evaluated longitudinally and systematically using consistent visual function measures prior to joining the study. Therefore, it is currently unknown whether the efficacy of electrostimulation therapies might be related to RP progression rate, which could be elucidated in a future RCT of longitudinally administered treatment to one eye with the fellow eye as a sham control.
In the RCS rat model of RP, TES prolonged photoreceptor survival and retinal function, (Morimoto et al. 2007) which was also found when TES was used to treat a rhodopsin transgenic rabbit model.(Morimoto et al. 2012) In transgenic rats with a P23H-1 rhodopsin mutation, whole-eye electrical stimulation preserved ganglion cells, inner retinal function and VA in one study, (Hanif et al. 2016) while TES preserved photoreceptor function in another study involving rats with the same P23H-1 mutation.(Rahmani et al. 2013) These effects in animal models with different mutation types or stimulation levels support the finding that TES may not be equally effective for all RP mutations. Furthermore, another study of TES in rats that underwent optic nerve crush found a subgroup of ‘responder animals’ that exhibited long-term benefits; however, reasons to account for the responder effects were not elucidated.(Henrich-Noack et al. 2013) This group hypothesized that perhaps TES might induce greater survival benefits for neuronal axons in animals with milder lesions.
It appears that there may be differences in which aspects of visual function are affected by either TES or electro-acupuncture, with improvements in scotopic visual function or CS, rather than VA or VF, more likely among RP subjects who received electro-acupuncture, which was also found in our previous case-series study.(Bittner et al. 2014) On the other hand, a recent study of acupuncture in 14 patients with inherited retinal degeneration found significant improvements in visual acuity, contrast vision and temporal GVF radius;(Blechschmidt et al. 2016) perhaps different acupuncture protocols may affect different aspects of visual function, or the heterogeneity of RP and/or other patient factors affect responses across individuals. Our RCT found central visual improvements in VA and CS following TES, which is supported by a study of TES in mice with an induced form of RP that showed greater preservation of the central than of the peripheral photoreceptors.(Tao et al. 2016) Another previous small-scale RCT of TES in RP patients indicated significant preservation of VA but did not measure VFs, (Robles-Camarillo et al. 2013) while another group reported a significant improvement in peripheral VF area but not VA;(Schatz et al. 2011) therefore, a larger scale RCT conducted over several years including several aspects of visual function and genotyping is needed to elucidate the effects since RP is a heterogeneous disease. The present trial attempted to reduce the risk that chance could have accounted for a significant improvement outside typical test variability for visual function by utilizing a strict criteria of improvement at two consecutive follow-up visits; however, appropriately powered larger scale trials are still needed before these interventions may be recommended clinically.
Multiple pathways are critical to help maintain visual and retinal function in RP, including control of RBF, tissue oxygenation, and metabolic support. Future longitudinal trials should evaluate the efficacy of retreatments with TES for maintaining RBF and oxygenation, as well as the possibility to reduce the rate of visual function loss in RP when applied longer-term. Clinical studies should also work to optimize the dose (i.e., duration, frequency, stimulation level) for electro-stimulation therapies for RP. As it is hypothesized that these therapies may create a more nurturing and supportive retinal microenvironment, perhaps they may also serve as an adjunctive approach to potentiate the effects of other emerging treatments for RP, such as retinal progenitor cells, which would also require future studies.
Acknowledgments
Grant funding for this trial was provided by NIH R21 award EY023720 to AKB.
The authors wish to thank Dr. Albert David Woods for administering TES to some of the study participants and assisting with the mfERGs, Dr. Andy Rosenfarb for providing training in the administration of the electro-acupuncture protocol to our study acupuncturist (NM), Dr. Luis Lesmes for assisting with the ROC analysis for the qCSF data, Drs. Pradeep Ramulu and Adrienne Scott for serving on the data safety and monitoring board, Mariana Ferraz for assisting with some of the visual function testing, Tracey Topacio for helping with the data entry and analysis for the AdaptDx scotopic sensitivities, Brennan Nelson for his assistance with some of the data entry for the CRA blood flow measures, and Robert De Jong for sharing his expertise related to the measurement and acquisition of CDI of the CRA using ultrasonography, which was valuable in developing a methodological protocol and training our study’s sonographers.
Footnotes
The study team has no potential conflicts of interest to disclose, including financial interests, activities, relationships, and affiliations.
References
- Asejczyk-Widlicka M, Krzyzanowska-Berkowska P, Sander BP, Iskander DR. Age-Related Changes in Ocular Blood Velocity in Suspects with Glaucomatous Optic Disc Appearance. Comparison with Healthy Subjects and Glaucoma Patients. PLoS ONE. 2015;10(7):e0134357. doi: 10.1371/journal.pone.0134357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MP, Bittner AK, Yang L, Marcus R, Iftikhar MH, Dagnelie G. Variability and Errors of Manually Digitized Goldmann Visual Fields. Optom Vis Sci. 2016;93(7):720–30. doi: 10.1097/OPX.0000000000000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner AK, Gould JM, Rosenfarb A, Rozanski C, Dagnelie G. A Pilot Study of an Acupuncture Protocol to Improve Visual Function in Retinitis Pigmentosa Patients. Clin Exp Optom. 2014;97(3):240–7. doi: 10.1111/cxo.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner AK, Iftikhar MH, Dagnelie G. Test-retest, within-visit variability of Goldmann visual fields in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011;52(11):8042–6. doi: 10.1167/iovs.11-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechschmidt T, Krumsiek M, Todorova MG. Improvement in Visual Function in Patients with Inherited Diseases of the Retina Following Acupuncture Treatment. Klin Monbl Augenheilkd. 2016;233(4):416–23. doi: 10.1055/s-0041-111819. [DOI] [PubMed] [Google Scholar]
- Dabov S, Goutoranov G, Ivanova R, Petkova N. Clinical application of acupuncture in ophthalmology. Acupunct Electrother Res. 1985;10(1–2):79–93. doi: 10.3727/036012985816714577. [DOI] [PubMed] [Google Scholar]
- Duncan DD, Lemaillet P, Ibrahim M, Nguyen QD, Hiller M, Ramella-Roman J. Absolute blood velocity measured with a modified fundus camera. J Biomed Opt. 2010;15(5):056014. doi: 10.1117/1.3494565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchsjäger-Mayrl G, Polak K, Luksch A, et al. Retinal blood flow and systemic blood pressure in healthy young subjects. Graefe's Arch Clin Exp Ophthalmol. 2001;239(9):673–677. doi: 10.1007/s004170100333. [DOI] [PubMed] [Google Scholar]
- Hanif AM, Kim MK, Thomas JG, et al. Whole-eye electrical stimulation therapy preserves visual function and structure in P23H-1 rats. Exp Eye Res. 2016;149:75–83. doi: 10.1016/j.exer.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich-Noack P, Voigt N, Prilloff S, Fedorov A, Sabel BA. Transcorneal electrical stimulation alters morphology and survival of retinal ganglion cells after optic nerve damage. Neurosci Lett. 2013;543:1–6. doi: 10.1016/j.neulet.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Ibrahim MA, Annam RE, Sepah YJ, et al. Assessment of oxygen saturation in retinal vessels of normal subjects and diabetic patients with and without retinopathy using Flow Oximetry System. Quant Imaging Med Surg. 2015;5(1):86–96. doi: 10.3978/j.issn.2223-4292.2014.11.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, Suga H, Fukushima M, et al. Blood fluidity enhancement by electrical acupuncture stimulation is related to an adrenergic mechanism. J Acupunct Meridian Stud. 2012;5(1):21–8. doi: 10.1016/j.jams.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Jørgensen CM, Bek T. Lack of differences in the regional variation of oxygen saturation in larger retinal vessels in diabetic maculopathy and proliferative diabetic retinopathy. Br J Ophthalmol. 2017;101(6):752–757. doi: 10.1136/bjophthalmol-2016-308894. [DOI] [PubMed] [Google Scholar]
- Kayser S, Vargas P, Mendelsohn D, Han J, Bi H, Benavente A, Bittner AK. Reduced Central Retinal Artery Blood Flow is Related to Impaired Central Visual Function in Retinitis Pigmentosa Patients. Curr Eye Res. 2017 doi: 10.1080/02713683.2017.1338350. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser AK, Dagnelie G. Reported Effects of Non-traditional Treatments and Complementary and Alternative Medicine (CAM) by Retinitis Pigmentosa (RP) Patients. Clin Exp Optom. 2008;91(2):166–176. doi: 10.1111/j.1444-0938.2007.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser AK, Mladenovich D, Eshraghi F, Bourdeau D, Dagnelie G. Reliability and Consistency of Visual Acuity and Contrast Sensitivity Measures in Advanced Eye Disease. Optom Vis Sci. 2005;82:946–954. doi: 10.1097/01.opx.0000187863.12609.7b. [DOI] [PubMed] [Google Scholar]
- Kornfield TE, Newman EA. Regulation of Blood Flow in the Retinal Trilaminar Vascular Network. J Neurosci. 2014;34(34):11504–13. doi: 10.1523/JNEUROSCI.1971-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto T, Oono S, Oku H, et al. Transcorneal electrical stimulation increases chorioretinal blood flow in normal human subjects. Clin Ophthalmol. 2010;4:1441–6. doi: 10.2147/OPTH.S14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaillet P, Duncan DD, Lompado A, Ibrahim M, Nguyen QD, Ramella-Roman JC. Retinal Spectral Imaging and Blood Flow Imaging. Journal of Innovative Optical Health Sciences. 2010;3(4):255–65. [Google Scholar]
- Li G, Cheung RT, Ma QY, Yang ES. Visual cortical activations on fMRI upon stimulation of the vision-implicated acupoints. Neuroreport. 2003;14(5):669–73. doi: 10.1097/00001756-200304150-00002. [DOI] [PubMed] [Google Scholar]
- Li L, Qin W, Bai L, Tian J. Exploring vision-related acupuncture point specificity with multivoxel pattern analysis. Magn Reson Imaging. 2010;28(3):380–7. doi: 10.1016/j.mri.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Litscher G. Computer-based quantification of traditional chinese-, ear- and Korean hand acupuncture: needle-induced changes of regional cerebral blood flow velocity. Neurol Res. 2002;24(4):377–80. doi: 10.1179/016164102101200177. [DOI] [PubMed] [Google Scholar]
- Mihashi T, Okawa Y, Miyoshi T, Kitaguchi Y, Hirohara Y, Fujikado T. Comparing retinal reflectance changes elicited by transcorneal electrical retinal stimulation with those of optic chiasma stimulation in cats. Jpn J Ophthalmol. 2011;55(1):49–56. doi: 10.1007/s10384-010-0906-x. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Fujikado T, Choi JS, et al. Transcorneal electrical stimulation promotes the survival of photoreceptors and preserves retinal function in royal college of surgeons rats. Invest Ophthalmol Vis Sci. 2007;48(10):4725–32. doi: 10.1167/iovs.06-1404. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Fukui T, Matsushita K, Okawa Y, Shimojyo H, Kusaka S, Tano Y, Fujikado T. Evaluation of residual retinal function by pupillary constrictions and phosphenes using transcorneal electrical stimulation in patients with retinal degeneration. Graefes Arch Clin Exp Ophthalmol. 2006;244(10):1283–92. doi: 10.1007/s00417-006-0260-3. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Kanda H, Kondo M, Terasaki H, Nishida K, Fujikado T. Transcorneal electrical stimulation promotes survival of photoreceptors and improves retinal function in rhodopsin P347L transgenic rabbits. Invest Ophthalmol Vis Sci. 2012;53(7):4254–61. doi: 10.1167/iovs.11-9067. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Kanda H, Miyoshi T, et al. Characteristics of retinal reflectance changes induced by transcorneal electrical stimulation in cat eyes. PLoS One. 2014;9(3):e92186. doi: 10.1371/journal.pone.0092186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi J, Yap KH, Ahmad G, Ghosh J. Transcranial Doppler Ultrasound: A Review of the Physical Principles and Major Applications in Critical Care. Int J Vasc Med. 2013:1–13. doi: 10.1155/2013/629378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naycheva L, Schatz A, Röck T, Willmann G, Messias A, Bartz-Schmidt KU, Zrenner E, Gekeler F. Phosphene thresholds elicited by transcorneal electrical stimulation in healthy subjects and patients with retinal diseases. Invest Ophthalmol Vis Sci. 2012;53(12):7440–8. doi: 10.1167/iovs.12-9612. [DOI] [PubMed] [Google Scholar]
- Pagani L, Manni L, Aloe L. Effects of electroacupuncture on retinal nerve growth factor and brain-derived neurotrophic factor expression in a rat model of retinitis pigmentosa. Brain Res. 2006;1092(1):198–206. doi: 10.1016/j.brainres.2006.03.074. [DOI] [PubMed] [Google Scholar]
- Palkovits S, Lasta M, Told R, et al. Retinal oxygen metabolism during normoxia and hyperoxia in healthy subjects. Invest Ophthalmol Vis Sci. 2014;55(8):4707–13. doi: 10.1167/iovs.14-14593. [DOI] [PubMed] [Google Scholar]
- Rahmani S, Bogdanowicz L, Thomas J, Hetling JR. Chronic delivery of low-level exogenous current preserves retinal function in pigmented P23H rat. Vision Res. 2013;76:105–13. doi: 10.1016/j.visres.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Robles-Camarillo D, Niño-de-Rivera L, López-Miranda J, Gil-Carrasco F, Quiroz-Mercado H. The effect of transcorneal electrical stimulation in visual acuity: Retinitis pigmentosa. J Biomedical Science and Engineering. 2013;6:1–7. [Google Scholar]
- Schatz A, Röck T, Naycheva L, et al. Transcorneal electrical stimulation for patients with retinitis pigmentosa: a prospective, randomized, sham-controlled exploratory study. Invest Ophthalmol Vis Sci. 2011;52(7):4485–96. doi: 10.1167/iovs.10-6932. [DOI] [PubMed] [Google Scholar]
- Schatz A, Pach J, Gosheva M, et al. Transcorneal Electrical Stimulation for Patients With Retinitis Pigmentosa: A Prospective, Randomized, Sham-Controlled Follow-up Study Over 1 Year. Invest Ophthalmol Vis Sci. 2017;58(1):257–69. doi: 10.1167/iovs.16-19906. [DOI] [PubMed] [Google Scholar]
- Schmid H, Herrmann T, Kohler K, Stett A. Neuroprotective effect of transretinal electrical stimulation on neurons in the inner nuclear layer of the degenerated retina. Brain Res Bull. 2009;79:15–25. doi: 10.1016/j.brainresbull.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Sehic A, Guo S, Cho KS, Corraya RM, Chen DF, Utheim TP. Electrical Stimulation as a Means for Improving Vision. Am J Pathol. 2016;186(11):2783–2797. doi: 10.1016/j.ajpath.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedentopf CM, Golaszewski SM, Mottaghy FM, Ruff CC, Felber S, Schlager A. Functional magnetic resonance imaging detects activation of the visual association cortex during laser acupuncture of the foot in humans. Neurosci Lett. 2002;327(1):53–6. doi: 10.1016/s0304-3940(02)00383-x. [DOI] [PubMed] [Google Scholar]
- Stalmans I, Vandewalle E, Anderson DR, et al. Use of colour Doppler imaging in ocular blood flow research. Acta Ophthalmol. 2011;89(8):e609–630. doi: 10.1111/j.1755-3768.2011.02178.x. [DOI] [PubMed] [Google Scholar]
- Takayama S, Watanabe M, Kusuyama H, et al. Evaluation of the effects of acupuncture on blood flow in humans with ultrasound color Doppler imaging. Evid Based Complement Alternat Med. 2012:513638. doi: 10.1155/2012/513638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Chen T, Liu ZY, et al. Topographic Quantification of the Transcorneal Electrical Stimulation (TES)-Induced Protective Effects on N-Methyl-N-Nitrosourea-Treated Retinas. Invest Ophthalmol Vis Sci. 2016;57(11):4614–24. doi: 10.1167/iovs.16-19305. [DOI] [PubMed] [Google Scholar]
- Wong S, Ching R. The use of acupuncture in ophthalmology. Am J Chin Med. 1980;8(1–2):104–53. doi: 10.1142/s0192415x80000098. [DOI] [PubMed] [Google Scholar]
- Yanagida K, Iwase T, Yamamoto K, et al. Sex-Related Differences in Ocular Blood Flow of Healthy Subjects Using Laser Speckle Flowgraphy. Invest Ophthalmol Vis Sci. 2015;56(8):4880. doi: 10.1167/iovs.15-16567. [DOI] [PubMed] [Google Scholar]
- Zhou WT, Ni YQ, Jin ZB, et al. Electrical stimulation ameliorates light-induced photoreceptor degeneration in vitro via suppressing the proinflammatory effect of microglia and enhancing the neurotrophic potential of Müller cells. Exp Neurol. 2012;238(2):192–208. doi: 10.1016/j.expneurol.2012.08.029. [DOI] [PubMed] [Google Scholar]