Abstract
Background
Primary aldosteronism is recognized as a severe form of “renin-independent aldosteronism” that results in excessive mineralocorticoid receptor (MR) activation.
Objective
To investigate whether there is a spectrum of subclinical renin-independent aldosteronism among normotensives that increases risk for hypertension.
Design
Cohort study.
Setting
National community-based study.
Participants
850 untreated normotensive participants in the Multi-Ethnic Study of Atherosclerosis with measurements of serum aldosterone, plasma renin activity (PRA).
Measurements
Longitudinal analyses investigated whether aldosterone concentrations, in the context of physiologic PRA phenotypes (suppressed: ≤0.50; indeterminate: 0.51–0.99; unsuppressed: ≥1.0 μg/L/h), associated with incident hypertension, defined as SBP≥140, DBP≥90 mmHg, or initiation of anti-hypertensive medications. Cross-sectional analyses investigated associations of aldosterone with MR activity, assessed via serum potassium and urinary fractional excretion of potassium.
Results
A suppressed renin phenotype was associated with a higher rate of incident hypertension when compared to other PRA phenotypes (85.4 [73.4, 99.3] vs. 53.3 [42.8, 66.4] vs. 54.5 [41.8, 71.0] cases per 1000 person-years of follow-up). With renin suppression, higher aldosterone concentrations were independently associated with an increased risk for incident hypertension; whereas no association between aldosterone and hypertension was observed when renin was not suppressed. Higher aldosterone concentrations were associated with lower serum potassium and higher urinary excretion of potassium, but only when renin was suppressed.
Limitations
Measurements of sodium and potassium occurred several years before renin and aldosterone.
Conclusions
Suppression of renin, and higher aldosterone concentrations in the context of this renin suppression, associated with an increased risk for developing hypertension and possibly also with increased MR activity. These findings suggest a clinically-relevant spectrum of subclinical primary aldosteronism (renin-independent aldosteronism) in normotension.
Funding
National Institutes of Health
INTRODUCTION
With an estimated prevalence of five to 25% among patients with hypertension, primary aldosteronism (PA) is the most common and modifiable form of secondary hypertension(1–7). The disorder is characterized by autonomous secretion of aldosterone, independent of renin, which results in excessive activation of the mineralocorticoid receptor (MR). Excessive stimulation of the renal and extra-renal MR in PA has been associated with hypertension and cardiovascular disease, independent of blood pressure(8–14), highlighting the important role of MR antagonists in mitigating the systemic sequelae of renin-independent aldosteronism.
Although PA has classically been typified as a clinical phenotype of severe hypertension and/or hypokalemia caused by adrenal neoplasia, recent evidence points to another potentially prevalent cause of autonomous aldosterone secretion by abnormal cell clusters within morphologically normal adrenal glands: aldosterone producing cell clusters (APCCs) (15–18).
Further, recent physiology studies have challenged the notion that PA is a categorical disease by demonstrating that there is a continuous spectrum of renin-independent aldosteronism in normotension, ranging from subtle to overtly autonomous(19). In this regard, the overt PA that we currently recognize in severe hypertension(20) may represent only the “tip of the iceberg” in the spectrum of renin-independent aldosteronism and excessive MR activation. Recognizing a potentially milder and expanded continuum of renin-independent aldosteronism, that originates in normotension and associates with inappropriate MR activation, may increase the opportunity to mitigate MR-mediated cardiovascular disease at an earlier stage.
Herein, we conducted a longitudinal cohort study that employed physiologic phenotypes of autonomous aldosterone secretion and MR activity. We investigated untreated normotensive participants enrolled in the Multi-Ethnic Study of Atherosclerosis to test the hypothesis that higher serum aldosterone levels in the context of renin suppression (renin-independent aldosterone secretion) would increase the risk for developing hypertension, when compared with normotensive participants without renin suppression. Further, we investigated whether inappropriate MR activity corresponded with these renin and aldosterone phenotypes.
METHODS
Study Population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multicenter cohort study of 6814 community-dwelling adults aged 45–84 years, established to study subclinical cardiovascular disease risk and its progression(21). Participants without evidence of clinical cardiovascular disease were recruited between August 2000 and July 2002 from six U.S. study sites and evaluated at serial examinations every two to three years over approximately ten years duration through December of 2011, when examination 5 was completed(22). All participants provided informed consent, and the study was approved by institutional review boards at all participating sites.
A random subset of 1960 participants had measurements of serum aldosterone and plasma renin activity (PRA) at either examination 2 (9/2002–2/2004) or 3 (3/2004–9/2005) as previously described(23). For the current study, we included only those participants with available aldosterone and PRA assessments, and who were normotensive (systolic blood pressure [SBP]<140 mmHg and diastolic blood pressure [DBP]<90 mmHg) and did not use any anti-hypertensive medications(24) at the time of serum aldosterone and PRA measurement (N=850) (Supplementary Figure 1).
Assessment of Aldosterone Levels in the Context of Renin Activity
Aldosterone was measured by competition-based radioimmunoassay (Diasorin, Stillwater, MN) (intra-assay coefficients of variation (CVs) between 6.30% and 8.87%). PRA was measured by radioimmunoassay (Diasorin) (inter-assay CV between 6.89% and 18.38%)(23). Both aldosterone and PRA were measured in duplicate and averaged.
We created a priori phenotypic categories to reflect renin physiology based on commonly observed and accepted thresholds to reflect hypothesized renin-angiotensin-aldosterone system and mineralocorticoid receptor (MR) activation physiology. Participants with a PRA ≤ 0.50 μg/L/hr (n=392) were classified as having a “suppressed renin phenotype” that could potentially reflect a state of suppressed renin-angiotensin-aldosterone activity, or inappropriate renin-independent aldosterone secretion and MR activation, depending on the corresponding aldosterone levels. Participants with a PRA ≥ 1.0 μg/L/h) (n=187) were classified as having an “unsuppressed renin phenotype” or a state of potentially appropriate MR activation in the setting of physiologic renin-dependent aldosterone secretion. Participants with PRA between 0.51–0.99 μg/L/h (n=271) were classified to have an “indeterminate renin phenotype.”
Assessment of Incident Hypertension
Blood pressure was measured in triplicate using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, Florida) after 5 minutes of rest in a seated position, as described previously(23). The last two of three measurements of systolic and diastolic blood pressure were averaged for the analysis. Antihypertensive medication use was determined by medication inventory: at each study examination, participants brought in all medications used in the preceding two weeks, and a member of the study staff transcribed each medication’s name, dose, and frequency from its container. Participants were then queried about their adherence to each medication over the preceding two-week interval.
Demographic and Laboratory Characterization of the Study Participants
At each study visit, participants completed self-administered questionnaires and underwent standardized interviews to evaluate demographics, medical history, medication use, and substance use, as well as measurement of body mass index (BMI) by trained study staff(25). Fasting venous blood samples were obtained after 12-hours of overnight fast and at least 5 minutes of seated posture, and a random urine sample was collected. Specimens were immediately flash frozen and stored at −80C after processing and were then thawed for analysis(23). Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine measurements using the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI)(26). Measurements of serum and urinary sodium, potassium, creatinine, and albumin were performed as previously described(22) and were used to calculate the urinary fractional excretion of potassium and the predicted 24h urinary sodium excretion using the INTERSALT equation(27, 28). Serum and urinary concentrations of sodium and potassium were measured at examination 1 only.
Statistical Analysis
Our analytical approach was two-fold (Supplementary Figure 1). We first conducted a longitudinal analysis to investigate the hypothesis that the suppressed renin phenotype was enriched for subclinical renin-independent aldosteronism and an increased risk for incident hypertension. We subsequently conducted a cross-sectional analysis to investigate the hypothesis that the suppressed renin phenotype was enriched for increased MR activity.
We used multivariable discrete Cox proportional hazards models (proc PHREG, ties=discrete, SAS v9.4) to evaluate the association between serum aldosterone and incident hypertension by renin phenotypes and report the results as hazard ratios(29). To calculate risk differences for renin and aldosterone phenotypes, we computed standardized marginal risk differences using weighted adjusted discrete hazard models(30). Assessment of incident hypertension events occurred only at each follow-up examination and was defined as the development of SBP≥140 mmHg or DBP≥90 mmHg or the initiation of any anti-hypertensive medication at a follow-up visit. Participants who did not develop hypertension were censored at the time of their final follow-up examination. Covariates included baseline age, sex, race, BMI, LDL cholesterol, fasting blood glucose, smoking, alcohol, medication use, physical activity, educational attainment, income, and insurance status. Exploratory models further included SBP, eGFR, and proteinuria. We tested the interaction between serum aldosterone and phenotypes of renin and incident hypertension in adjusted interaction models using multiplicative terms in the regression models with Wald tests to evaluate significance.
Multivariable linear regression models were used to assess associations between serum aldosterone and biomarkers of MR activity in each renin phenotype, adjusting for sodium balance using serum sodium and predicted 24h urinary sodium excretion in addition to other potential confounding factors. Adjusted interaction models were used to assess whether serum aldosterone and renin phenotypes associated serum and urinary potassium.
Analyses were conducted using SAS v9.4 (SAS Institute, NC, USA) and Stata v15 (Statacorp, Texas, USA). A P < 0.05 was considered to be statistically significant for all analyses.
RESULTS
Participant characteristics
Baseline characteristics of the study population at the time of serum aldosterone and PRA measurements are shown in Table 1. Forty-six percent (392/850) of participants displayed a suppressed renin phenotype. When compared to participants with higher renin activity, participants with a suppressed renin phenotype were older, more enriched with women and African-Americans, and had higher systolic blood pressure. Notably, although these participants had the lowest serum aldosterone concentrations, they had markedly elevated aldosterone-to-renin ratios (ARR>750 pmol/L per μg/L/h, or >30 ng/dL per ng/mL/h in conventional units), since they also had the lowest PRA(18) (Table 1).
Table 1.
Characteristics of study participants in the longitudinal analysis.
| Plasma Renin Activity (μg/L/h) | ||||||
|---|---|---|---|---|---|---|
| ≤ 0.50 | 0.51 – 0.99 | ≥ 1.0 | ||||
|
| ||||||
| Suppressed Renin Phenotype (N=392) | Indeterminate Renin Phenotype (N=271) | Unsuppressed Renin Phenotype (N=187) | ||||
|
| ||||||
| Serum Aldosterone | Serum Aldosterone | Serum Aldosterone | ||||
| <Median | ≥Median | <Median | ≥Median | <Median | ≥Median | |
| N | 247 | 145 | 135 | 136 | 44 | 143 |
| Age (y) | 63.5 (9.3) | 63.0 (9.2) | 61.0 (9.0) | 59.8 (7.7) | 59.3 (9.1) | 59.8 (9.3) |
| Female sex (n/%) | 128 (51.8) | 79 (54.5) | 64 (47.4) | 60 (44.1) | 17 (38.6) | 45 (31.5) |
| Race/ethnicity (n/%) | ||||||
| White | 104 (42.1) | 61 (42.1) | 63 (46.7) | 62 (45.6) | 15 (34.1) | 67 (46.9) |
| Hispanic | 51 (20.7) | 33 (22.8) | 45 (33.3) | 37 (27.2) | 18 (40.9) | 50 (35.0) |
| African American | 54 (21.9) | 24 (16.6) | 11 (8.2) | 10 (7.4) | 3 (6.8) | 5 (3.5) |
| Chinese American | 38 (15.4) | 27 (18.6) | 16 (11.9) | 27 (19.9) | 8 (18.2) | 21 (14.7) |
| BMI (kg/m2) | 26.9 (4.9) | 27.1 (4.9) | 27.8 (4.9) | 27.1 (5.2) | 27.2 (5.4) | 26.7 (4.7) |
| Fasting Blood Glucose (mmol/L) | 5.14 (1.48) | 5.19 (0.95) | 5.26 (1.19) | 5.50 (1.88) | 5.33 (1.5) | 5.41 (1.9) |
| Systolic Blood Pressure (mmHg) | 116.1 (13.3) | 116.8 (13.6) | 111.0 (12.9) | 111.1 (12.5) | 108.0 (11.2) | 111.7 (14.5) |
| Diastolic Blood Pressure (mmHg) | 68.3 (8.5) | 68.3 (8.7) | 65.9 (9.1) | 68.3 (8.1) | 67.9 (8.2) | 68.5 (8.2) |
| eGFR (mL/min/1.73 m2) | 83.8 (15.0) | 81.0 (15.1) | 84.6 (14.4) | 83.2 (13.4) | 85.2 (14.8) | 84.5 (15.7) |
| Urine albumin to creatinine ratio (n/%) | ||||||
| Normal (<3.5 mg/mmol) | 237 (96.0) | 137 (94.5) | 131 (97.0) | 129 (94.9) | 39 (88.6) | 135 (94.4) |
| Microalbuminuria (3.5–35 mg/mmol) | 10 (4.1) | 8 (5.5) | 4 (3.0) | 6 (4.4) | 4 (9.1) | 6 (4.2) |
| Macroalbuminuria (≥35 mg/mmol) | 0 (0) | 0 (0) | 0 (0) | 1 (0.7) | 1 (2.3) | 2 (1.4) |
| LDL Cholesterol (mmol/L) | 29.8 (7.6) | 30.0 (8.6) | 30.8 (8.5) | 29.8 (7.3) | 30.2 (6.0) | 30.6 (7.6) |
| Physical Activity (METS* min/week) | 1406.1 (1957.3) | 1454.5 (1773.8) | 1296.5 (1668.1) | 1487.6 (1932.15) | 1308.5 (2437.9) | 1572.8 (1849.9) |
| Smoking status (n/%) | ||||||
| Never smoker | 113 (46.5) | 85 (59.0) | 53 (39.3) | 69 (50.7) | 26 (59.1) | 56 (39.4) |
| Former | 95 (39.1) | 44 (30.6) | 63 (46.7) | 45 (33.1) | 10 (22.7) | 64 (45.1) |
| Current | 35 (14.4) | 15 (10.4) | 19 (14.1) | 22 (16.2) | 8 (18.2) | 22 (15.5) |
| Current Alcohol Use (n/%) | 132 (53.7) | 77 (53.1) | 72 (53.3) | 80 (58.8) | 18 (40.9) | 95 (66.4) |
| Oral Estrogen Use (n/%) | 16 (6.5) | 10 (6.9) | 7 (5.2) | 8 (5.9) | 1 (2.3) | 11 (7.7) |
| NSAID Use (n/%) | 36 (14.6) | 15 (10.3) | 19 (14.1) | 19 (14.0) | 6 (13.6) | 11 (7.7) |
| Level of Education (n/%) | ||||||
| None to Grade 11 | 37 (15.0) | 25 (17.2) | 14 (10.4) | 22 (16.2) | 9 (20.5) | 29 (20.4) |
| High School to some College | 75 (30.4) | 45 (31.0) | 40 (29.6) | 42 (30.9) | 14 (31.8) | 39 (27.5) |
| Associate’s, Bachelor’s, or Professional degree | 135 (54.7) | 75 (51.7) | 81 (60) | 72 (52.9) | 21 (47.7) | 74 (52.1) |
| Annual Income, (n/%) | ||||||
| <$30,000 | 79 (32.0) | 53 (36.6) | 32 (23.7) | 44 (32.4) | 17 (38.6) | 46 (32.2) |
| $30,000 – $75,000 | 111 (44.9) | 44 (30.3) | 72 (53.3) | 55 (40.4) | 15 (34.1) | 54 (37.8) |
| >$75,000 | 57 (23.1) | 48 (33.1) | 31 (23.0) | 37 (27.2) | 12 (27.3) | 43 (30.1) |
| No health insurance (n/%) | 17 (6.9) | 11 (7.6) | 9 (6.7) | 10 (7.4) | 4 (9.1) | 10 (7.0) |
| Aldosterone (pmol/L)* | 241.3 (66.9) 246.7 [192.4, 295.1] |
475.6 (102.8) 449.9 [401.9, 527.5] |
252.1 (71.0) 266.3 [195.6, 313.9] |
503.2 (129.6) 468.1 [406.9, 559.5] |
271.0 (64.6) 288.2 [225.2, 326.4] |
584.1 (225.3) 521.5 [448.8, 642.4] |
| Plasma Renin Activity (μg/L/h)* | 0.28 (0.12) 0.29 [0.19, 0.37] |
0.28 (0.13) 0.29 [0.17, 0.40] |
0.69 (0.13) 0.66 [0.57, 0.78] |
0.75 (0.13) 0.86 [0.65, 0.92] |
1.81 (1.02) 1.39 [1.17, 1.87] |
1.76 (0.90) 1.55 [1.17, 1.93] |
| Aldosterone-to-Renin Ratio (ARR)* (pmol/L per μg/L/h) | 1672 (4784) 849 [636, 1300] |
3589 (8616) 1603 [1154, 3053] |
384 (142) 373 [272, 491] |
697 (223) 644 [542, 804] |
181 (73) 197 [136, 235] |
371 (145) 355 [273, 448] |
| Proportion with Serum Aldosterone and Plasma Renin Activity Measured at Examination 2 (n/%) | 93 (37.7) | 48 (33.1) | 47 (34.8) | 54 (39.7) | 17 (38.6) | 55 (38.5) |
| Proportion with Serum Aldosterone and Plasma Renin Activity Measured at Examination 3 (n/%) | 154 (62.4) | 97 (66.9) | 88 (65.2) | 82 (60.3) | 27 (61.4) | 88 (61.5) |
Baseline characteristics are presented corresponding to the examination when serum aldosterone and plasma renin activity were measured, either at examination 2 or examination 3. Characteristics are stratified by phenotypes of renin and serum aldosterone < median (Q1 & Q2) (<349.7 pmol/L) or ≥ median (Q3 & Q4) (≥349.7 pmol/L).
BMI: Body Mass Index; eGFR: Estimated Glomerular Filtration Rate.
Values shown as Mean (Standard Deviation) unless otherwise shown.
Serum aldosterone, plasma renin activity, and ARR shown as both Mean (SD) and Median [Interquartile Range].
Renin Phenotype and Incident Hypertension
Participants with a suppressed renin phenotype had the highest incidence rate of hypertension when compared to the indeterminate and unsuppressed renin phenotypes (85.4 [73.4, 99.3] vs. 53.3 [42.8, 66.4] vs. 54.5 [41.8, 71.0] cases per 1000 person-years of follow-up), and a significantly higher risk for incident hypertension when compared to the unsuppressed renin phenotype (adjusted hazard ratio=1.68 [1.16, 2.44], adjusted risk difference = 48.1 [15.8, 80.4] events per 1,000 person-years) (Table 2). Additional exploratory analyses including adjustments for baseline blood pressure and kidney function – factors that may be considered confounders, but are also considered to be in the causal pathway as they are known to be negatively impacted by renin-independent aldosteronism(14, 31, 32) – attenuated the effect estimates and significance, but did not meaningfully alter the direction of the findings (Table 2).
Table 2.
Renin Phenotype and the multivariable adjusted risk for incident hypertension.
| Plasma Renin Activity (μg/L/h) | |||
|---|---|---|---|
| ≤ 0.50 | 0.51–0.99 | ≥ 1.0 | |
| Suppressed Renin Phenotype | Indeterminate Renin Phenotype | Unsuppressed Renin Phenotype | |
|
| |||
| Eligible Participants | 392 | 271 | 187 |
| Total Number of Incident Hypertension Events | 168 | 80 | 55 |
| Person-Years at Risk | 1969 | 1501 | 1013 |
| Unadjusted Incidence Rate of Hypertension (Events per 1,000 Person-Years) | 85.4 (73.4, 99.3) | 53.3 (42.8, 66.4) | 54.5 (41.8, 71.0) |
| Unadjusted Risk Difference (Events per 1,000 Person-Years) | 30.1 (11.5, 50.1) | −1.2 (−19.7, 17.3) | Ref |
| Unadjusted Relative Risk | 1.74 (1.24, 2.44) | 0.98 (0.67, 1.43) | Ref |
| Model 1 Risk Difference (Events per 1,000 Person-Years)* | 38.8 (8.6, 69.0) | −4.4 (−38.6, 29.8) | Ref |
| Model 1 Relative Risk* | 1.59 (1.12, 2.26) | 0.98 (0.67, 1.44) | Ref |
| Model 2 Risk Difference (Events per 1,000 Person-Years)† | 48.1 (15.8, 80.4) | −7.1 (−44.3, 30.1) | Ref |
| Model 2 Relative Risk† | 1.68 (1.16, 2.44) | 0.94 (0.63, 1.40) | Ref |
| Exploratory Model Risk Difference (Events per 1,000 Person-Years)‡ | 28.4 (−4.4, 61.2) | −1.0 (−46.9, 26.1) | Ref |
| Exploratory Model Relative Risk‡ | 1.27 (0.85, 1.88) | 0.93 (0.61, 1.41) | Ref |
Relative risks are hazard ratios from Cox models representing risk for incident hypertension compared to the unsuppressed renin phenotype.
Multivariable Model 1: Adjusted for baseline age, sex, race/ethnicity (Caucasian-, Chinese-, African-, Hispanic-American).
Multivariable Model 2: Adjusted for Model 1 plus baseline: BMI, cigarette smoking status (never former, current), weekly physical activity (MET*min/week), alcohol use (Y/N), education level (no school to grade 11, high school to some college, Associate’s degree or Bachelor’s degree or other professional degree), annual income (<$30,000, $30,000–75,000, >$75,000), health insurance status (Y/N), oral estrogen use (Y/N), nonsteroidal anti-inflammatory medication use (Y/N), fasting blood glucose, low-density lipoprotein cholesterol.
Exploratory Model: Adjudted for Model 2 plus baseline: systolic blood pressure, estimated glomerular filtration rate, and urinary albumin-to-creatinine ratio.
Aldosterone, Renin, and Incident Hypertension
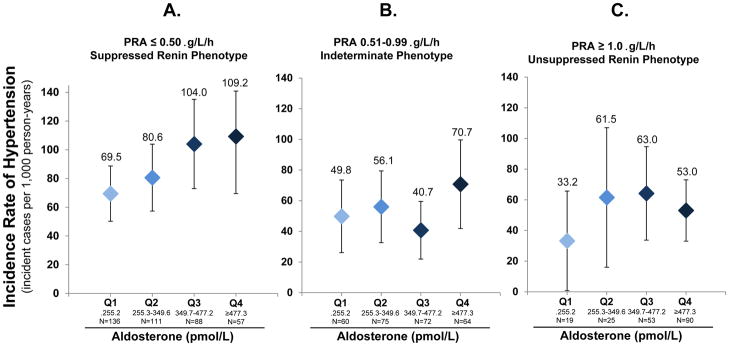
Higher aldosterone levels were signficantly associated with a higher risk for developing hypertension, but only in the context of a suppressed renin phenotype (Table 3). Effect estimates suggest an 18% (3–36%) higher risk for incident hypertension per 100 pmol/L of aldosterone when PRA was ≤ 0.50 μg/L/h (Table 3). The same trends were also seen when aldosterone was analyzed as a categorical variable (quartiles); there was an evident relationship between higher aldosterone quartiles and incident hypertension when renin was suppressed, but this was not apparent when renin was not suppressed (Figure 1 and Supplementary Table 1).
Table 3.
Aldosterone (continuous) and the multivariable adjusted relative risk for incident hypertension by renin phenotype.
| Plasma Renin Activity (μg/L/h) | ||||
|---|---|---|---|---|
| ≤ 0.50 | 0.51–0.99 | ≥ 1.0 | P-interaction | |
| Suppressed Renin Phenotype | Indeterminate Renin Phenotype | Unsuppressed Renin Phenotype | ||
|
| ||||
| Eligible Participants | 392 | 271 | 187 | |
| Total Number of Incident Hypertension Events | 168 | 80 | 55 | |
| Person-Years at Risk | 1969 | 1501 | 1013 | |
| Unadjusted | 1.16 (1.03, 1.32) | 1.09 (0.94, 1.26) | 1.06 (0.94, 1.19) | 0.57 |
| Multivariable Model 1* | 1.18 (1.04, 1.34) | 1.12 (0.96, 1.30) | 1.07 (0.94, 1.21) | 0.59 |
| Multivariable Model 2† | 1.18 (1.03, 1.36) | 1.12 (0.96, 1.32) | 1.07 (0.93, 1.23) | 0.69 |
| Exploratory Model‡ | 1.15 (0.99, 1.35) | 1.13 (0.94, 1.35) | 1.04 (0.89, 1.21) | 0.36 |
Units of hazard ratios are risk for incident hypertension per 100 pmol/L of serum aldosterone
Multivariable Model 1: Adjusted for baseline age, sex, race/ethnicity (Caucasian-, Chinese-, African-, Hispanic-American).
Multivariable Model 2: Adjusted for Model 1 plus baseline: BMI, cigarette smoking status (never former, current), weekly physical activity (MET*min/week), alcohol use (Y/N), education level (no school to grade 11, high school to some college, Associate’s degree or Bachelor’s degree or other professional degree), annual income (<$30,000, $30,000–75,000, >$75,000), health insurance status (Y/N), oral estrogen use (Y/N), nonsteroidal anti-inflammatory medication use (Y/N), fasting blood glucose, low-density lipoprotein cholesterol.
Exploratory Model: Adjusted for Model 2 plus baseline: systolic blood pressure, estimated glomerular filtration rate, and urinary albumin-to-creatinine ratio.
Figure 1. Renin-independent aldosteronism and the incidence rate of hypertension.
Figures depict the unadjusted incidence rate of hypertension (number of incident cases per 1,000 person-years at risk) by phenotypes of renin and quartiles of aldosterone. Conversion of plasma renin activity (PRA) from SI to conventional units: 1 μg/L/h = 1 ng/mL/h. Conversion of aldosterone from SI to conventional units: 1 pmol/L=0.036 ng/dL. Quartiles of aldosterone in conventional units are: Q1 (<9.23 ng/dL), Q2 (9.23–12.73 ng/dL), Q3 (12.74–17.32 ng/dL), Q4 (≥17.32 ng/dL). A) Participants with a suppressed renin phenotype (PRA ≤ 0.50 μg/L/h); B) Participants with an indeterminate renin phenotype (PRA 0.51–0.99 μg/L/h); C) Participants with an unsuppressed renin phenotype (PRA ≥ 1.0 μg/L/h).
In an exploratory analysis restricted to participants who had their final longitudinal blood pressure assessment without concurrent use of anti-hypertensive medications (i.e. including only untreated normotensive and hypertensive participants, and excluding those initiated on anti-hypertensives), higher aldosterone levels were associated with a greater increase in systolic blood pressure, independent of baseline blood pressure and other confounders, but only when renin was suppressed (Supplemental Table 2).
Mineralocorticoid Receptor Activity
We examined biomarkers of MR activity (serum potassium and urinary fractional excretion of potassium) to investigate whether the risk for developing hypertension might be MR mediated. Characteristics of the study population at examination 1, when biomarkers of MR activity were available, were similar to those at examinations 2 and 3, when aldosterone and PRA were measured (Supplementary Table 3). There were no notable differences in the mean serum sodium or potassium, predicted 24h urinary sodium balance (approximately 4 grams of sodium per day), or urinary fractional excretion of potassium across renin phenotypes (Supplementary Table 3). Despite this similarity in group means, participants with a suppressed renin phenotype displayed a significant association between higher serum aldosterone and lower serum potassium concentrations within the normal range (4.0–5.5 mmol/L) and higher urinary fractional excretion of potassium (Table 4). In contrast, no relationship between serum aldosterone levels and serum or urinary potassium was observed in higher renin phenotypes, where MR activation was hypothesized to be more physiologic and appropriate (Table 4).
Table 4.
Cross-sectional relationship between serum aldosterone levels and indicators of mineralocorticoid receptor activity by categories of renin.
| Serum Potassium | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Plasma Renin Activity (μg/L/h) | Mean Serum Potassium (mmol/L) | Mean Serum Aldosterone (pmol/L) | Unadjusted | Adjusted* | P-Interaction*† | ||
|
| |||||||
| Change in serum potassium (mmol/L) per 100 pmol/L of serum aldosterone | P | Change in serum potassium (mmol/L) per 100 pmol/L of serum aldosterone | P | ||||
| ≤ 0.50 | 4.36 (0.32) | 328.0 (139.8) | −0.033 | 0.007 | −0.034 | 0.002 | 0.012 |
| 0.51–0.99 | 4.35 (0.32) | 378.1 (163.4) | 0.010 | 0.42 | 0.002 | 0.86 | |
| ≥ 1.0 | 4.33 (0.29) | 510.4 (239.7) | −0.004 | 0.66 | 0.006 | 0.56 | |
|
| |||||||
| Urinary Fractional Excretion of Potassium (FeK) | |||||||
|
| |||||||
| Plasma Renin Activity (μg/L/h) | Mean Fractional Excretion of Potassium (%) | Mean Serum Aldosterone (pmol/L) | Unadjusted | Adjusted* | P-Interaction*† | ||
|
| |||||||
| Change in FeK (%) per 100 pmol/L of serum aldosterone | P | Change in FeK (%) per 100 pmol/L of serum aldosterone | P | ||||
| ≤ 0.50 | 11.9 (5.3) | 328.0 (139.8) | 0.421 | 0.033 | 0.501 | 0.008 | 0.041 |
| 0.51–0.99 | 11.0 (4.9) | 378.1 (163.4) | 0.191 | 0.49 | 0.068 | 0.73 | |
| ≥ 1.0 | 11.5 (4.8) | 510.4 (239.7) | −0.097 | 0.55 | 0.010 | 0.95 | |
Tables show the relationship between serum aldosterone levels and serum potassium concentrations (top) and urinary fractional excretion of potassium (FeK) (bottom) by renin phenotype.
Adjusted for factors at examination 1: age, sex, race/ethnicity (Caucasian-, Chinese-, African-, Hispanic-American), BMI, education level (no school to grade 11, high school to some college, Associate’s degree or Bachelor’s degree or other professional degree), fasting blood glucose, systolic blood pressure, serum sodium, predicted 24h urine sodium excretion based on INTERSALT equation, estimated glomerular filtration rate.
Interaction between plasma renin activity phenotypes and aldosterone as a continuous variable, and adjusted for all aforementioned covariates.
DISCUSSION
Herein, we report evidence from a large and ethnically diverse cohort of untreated normotensives, demonstrating that higher serum aldosterone concentrations in the setting of suppressed renin activity (i.e. renin-independent aldosteronism) are associated with increased risk for incident hypertension despite overall lower absolute aldosterone concentrations. These findings underscore the concept that the risk for aldosterone-mediated hypertension is not dictated by the absolute level of serum aldosterone, but rather to aldosterone autonomy from its dominant regulators: renin and angiotensin II. Importantly, we observed that higher aldosterone levels were associated with lower serum potassium, and with higher urinary fractional excretion of potassium, but only when renin was suppressed. Collectively, these findings indicate an expanded spectrum of clinically relevant renin-independent aldosteronism, and possibly MR activation, that increases the risk for hypertension in a normotensive population that is never suspected to have autonomous aldosterone secretion or PA.
The results of this physiology-based longitudinal study challenge the long-standing dogma on the arbitrary definitions of PA and on the pathogenesis and treatment of hypertension. First, these results support the notion that renin-independent aldosterone secretion need not be a pathologic phenotype reserved only for severe cases of PA; rather, it may be a common phenotype that is associated with subtle and inappropriate MR activity and that can even exist among normotensives(19, 33). Second, a substantial proportion of hypertension may not be “essential” (or idiopathic); rather, the current findings suggest that MR-mediated hypertension may be a common pathogenic mechanism. Since MR antagonists are widely available, this potentially large subset of MR-mediated hypertension may be amenable to targeted therapy. Third, if the latter is implicated, then treatment guidelines for new-onset hypertension may be improved by using a renin-phenotype guided approach that more liberally incorporates MR antagonists in an individualized manner, as has been successfully demonstrated in resistant hypertension(34–37). Ultimately however, these findings and challenges to existing dogma stem from an observational design; therefore, these implications may serve as a foundation for future intervention studies that employ MR antagonists.
The current findings extend and build upon prior studies. Our cross-sectional physiology studies have demonstrated that renin phenotype can predict the degree of autonomous aldosterone secretion and MR activation(38). We previously demonstrated that healthy normotensives with a suppressed renin phenotype exhibit a continuum of non-suppressible aldosterone secretion that was associated with greater urinary potassium excretion, indicating a spectrum of renin-independent aldosterone secretion and corresponding MR activation in normotension(19). A prior longitudinal study of mostly Caucasian normotensives in the Framingham Heart Study reported that higher serum aldosterone levels associated with an increased risk for developing hypertension over four years(39); however, renin phenotype and biomarkers of MR activity (such as potassium regulation) were not available to implicate the mechanism for the outcome. These prior cross-sectional and longitudinal findings suggested that excessive aldosterone in normotension may be an independent risk factor for hypertension, but also raised questions as to how “clinically relevant” autonomous aldosterone secretion should be assessed. The most specific characterization of PA, and less severe autonomous and renin-independent aldosteronism, involves the demonstration of the triad of: 1) renin suppression; 2) inappropriate secretion of aldosterone relative to renin and sodium status; and 3) excessive MR activation. In this regard, our current prospective study provides a unique and fairly comprehensive characterization of clinically relevant renin-independent aldosteronism in that it included measures of both renin activity and serum aldosterone, measures of potassium and sodium homeostasis to assess MR activity, a long duration of follow-up, and an ethnically diverse population that permitted assessments suggesting that normotensive African-Americans and women may be disproportionately enriched with subclinical renin-independent aldosteronism. Taken together, the current results frame the spectrum of renin-independent aldosteronism in normotension, and the pathogenesis of MR-mediated hypertension, with greater clarity and scope.
It is important to note that our study design was observational and community-based and therefore did not include interventions to control dietary factors that influence renin and aldosterone. Day-to-day variability in dietary sodium and potassium intake could have influenced our renin and aldosterone results, an issue that is further compounded by the fact that sodium and potassium phenotyping occurred years before the renin and aldosterone phenotyping. However, the sodium and potassium balances were comparable across renin phenotypes (Supplemental Table 3), and large cohort studies have shown intra-individual correlation and reasonable reproducibility in these metrics over 1–3 years of follow-up(40, 41). More importantly, the aforementioned limitation does not degrade the validity of our observational findings which are supported by physiologic principles: 1) the decoupling of elevated aldosterone levels and suppression of renin that associated with the highest risk for incident hypertension (Figure 1A) cannot be explained by dietary sodium balance, rather can only be explained as an autonomous or renin-independent aldosteronism; 2) conversely, renin-dependent aldosterone secretion (Figure 1C) cannot be ascribed to autonomous aldosterone secretion since renin was not suppressed, and was therefore presumptively physiologic. Further, the suppressed renin phenotype (PRA ≤ 0.50 μg/L/h) was probably comprised of a combination of: participants with “normal” physiology who displayed an appropriately suppressed renin and aldosterone while on a relatively high sodium intake; participants who had autonomous aldosteronism despite renin suppression and high sodium intake (renin-independent aldosterone secretion); and possibly also participants with suppressed renin and aldosterone due to non-aldosterone MR ligands that were not directly assessed in the current study(42, 43). Thus, this heterogeneous mixture of normal (physiologic) and abnormal (pathophysiologic) phenotypes when PRA was ≤ 0.50 μg/L/h should have favored the null hypothesis by decreasing the enrichment and detectability of autonomous aldosteronism. The fact that we observed our main findings despite this heterogeneity should provide increased confidence that the renin-independent aldosteronism that is associated with incident hypertension can be detected in the “real world” setting despite ad libitum dietary sodium intake.
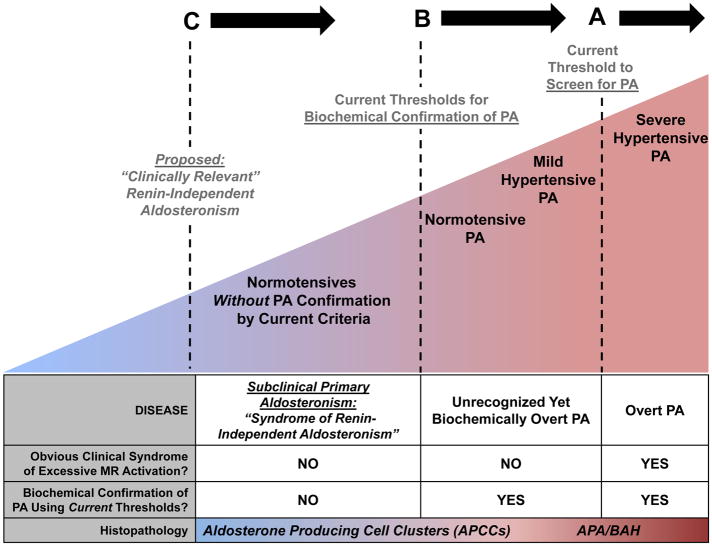
Based on these results, we propose an expanded continuum of renin-independent aldosteronism that is conceptualized in Figure 2. At its most severe, PA is characterized by an overt clinical phenotype of severe or resistant hypertension and/or hypokalemia (Figure 2A). This “Overt PA” is readily recognized by the highly sensitive ARR screen, and confirmed as PA using a recommended confirmatory test(20). The presumptive histopathology underlying Overt PA is typically an aldosterone producing adenoma or bilateral adrenocortical hyperplasia(20). Although the ARR is a highly sensitive tool for detecting PA in severe hypertensive and/or hypokalemic populations(20), multiple studies have now shown that when confirmatory testing for PA is applied to populations with no obvious clinical phenotype for PA or MR overactivation, a surprisingly high prevalence of PA is still detected (Figure 2B). Studies in mild hypertensives have shown that three to 20% of stage I hypertensives demonstrate overt biochemical confirmation for PA using salt suppression, fludrocortisone, or captopril suppression tests(44–46), and that the degree of renin suppression predicts the severity of PA(44). Even in cohorts that are entirely normotensive, 3–14% have been confirmed to have overt biochemical PA with a concomitant increased risk for developing hypertension(19, 33). Therefore, this extension of the renin-independent aldosteronism continuum is pertinent since PA screening is not recommended for normotensive or mildly hypertensive patients, yet the prevalence of “Unrecognized Yet Biochemically Overt PA” may be substantial(47) (Figure 2B).
Figure 2. The proposed spectrum of renin-independent aldosteronism.
A) The Endocrine Society Clinical Practice Guidelines recommend screening for primary aldosteronism (PA) using the aldosterone-to-renin ratio (ARR) in severe or resistant hypertension. This practice of screening for a very high ARR (>20–30 ng/dL per ng/dL/h in conventional units or >750–830 pmol/L per μg/L/h in SI units) is highly sensitive at detecting patients with severe hypertensive PA: patients with an obvious clinical syndrome of excessive mineralocorticoid receptor (MR) activation (hypertension and/or hypokalemia) who are confirmed to have biochemically “Overt PA,” and likely have an aldosterone-producing adenoma (APA) or bilateral adrenal hyperplasia (BAH) as the cause of their disease.
B) Using confirmatory testing thresholds recommended by The Endocrine Society (such as oral or intravenous sodium loading, flurdrocortisone suppression, or captopril challenge), it has been observed that a substantial portion of normotensives and mild hypertensives, populations for whom PA screening is not routinely recommended, have biochemically overt PA. These patients have “Unrecognized Yet Biochemically Overt PA.”
C) Even below the thresholds of what is currently considered biochemical confirmation of PA, there exists a continuum of renin-independent aldosteronism among healthy normotensives and mild hypertensives, in whom no obvious clinical syndrome of MR overactivation is apparent. These individuals have subtle evidence of renin-independent aldosteronism (renin suppression with inappropriately “normal” or high aldosterone levels) and higher risk for developing incident hypertension (as seen in the current study). This phenotype may best be described as “subclinical primary aldosteronism” or a “syndrome of clinically-relevant renin-independent aldosteronism.” The newly described and prevalent histopathology of aldosterone producing cell clusters (APCCs) may provide one explanation for this expanded continuum of subtle autonomous aldosteronism.
We now propose that the spectrum of clinically relevant renin-independent aldosteronism may extend even to individuals with no apparent clinical syndrome of excessive MR activation and who fall below the current thresholds used to confirm PA (Figure 2C). Participants in the current study were untreated, normotensive, and normokalemic, without known PA, and yet nearly half the study population displayed a phenotype of renin suppression wherein higher aldosterone levels within what is considered to be the “normal range” (mean ~300–335 pmol/L or ~11–12 ng/dL) were associated with an increased risk for developing hypertension and potential evidence for high MR activity. Although MESA participants did not undergo confirmatory tests to conclusively assert that all of our study participants definitely did not have occult normotensive PA, we have seen similar results before. Prior studies of untreated normotensives and hypertensives without PA have described a broad spectrum of aldosterone suppressibility with sodium loading(48, 49).
Why might a syndrome of renin-independent aldosteronism that imparts inappropriate MR activation and cardiovascular risk be so prevalent? The recent discovery of aldosterone producing cell clusters (APCCs) provides the most compelling evidence to account for this frequency of dysregulated aldosterone physiology (Figure 2C). APCCs are histopathological findings of large clusters of CYP11B2 (also known as aldosterone synthase) expression that invade the zona fasciculata, harbor pathogenic mutations known to increase aldosterone secretion, and are not suppressed in the face of volume expansion or aldosterone excess(15, 16). Importantly, APCCs have been found in >50% of morphologically normal adrenal glands (with no apparent tumor or hyperplasia) and increase in prevalence with older age(18); consistent with our findings that autonomous aldosterone secretion may be a common pathogenic contributor to incident hypertension.
Our observations that a large proportion of normotensives have clinically relevant renin-independent aldosteronism (or “subclinical” primary aldosteronism) raises the question as to whether MR antagonists may be a “targeted therapy” in new-onset hypertensives, or even pre-hypertensives, with a suppressed renin phenotype. It should be noted that most hypertension treatment guidelines do not emphasize the early use of MR antagonists in the treatment algorithm, nor include a phenotype-driven approach based on renin or aldosterone(50). However, prior studies suggest that this type of phenotype-driven approach may indeed be more important than currently considered. Intervention studies in severe and resistant hypertensives with a low-renin phenotype have shown that use of MR antagonists provides superior blood pressure control(34–36) and cardiovascular benefit(37). Further, the addition of MR antagonists in resistant hypertension appears to be most beneficial to participants with the lowest renin(36).
Limitations
This study has several important limitations. First, all of the baseline measures for the longitudinal analysis were obtained at either examination 2 or 3, depending on when PRA and aldosterone were measured; however, cross-sectional analyses to assess MR activity relied on serum and urinary measures of potassium that were obtained only at examination 1. Although this resulted in comparisons of aldosteronism phenotypes with measures of MR activity across examinations, we used these comparisons as a cross-sectional and mechanistic validation to our longitudinal findings, and in this regard, our two analyses were complimentary and consistent. It should be noted that all participants were normotensive and untreated at examination 2 or 3 (when aldosterone and PRA were measured), and therefore it is likely that these phenotypic characteristics were stable and representative of each participant’s renin and aldosterone phenotype(40, 41). Second, as discussed earlier, measures of renin and aldosterone were performed on ad libitum dietary sodium intake. Third, as described earlier, the physiology between renin and aldosterone is complex, heterogeneous, and dynamic across a large continuum, and therefore likely contributed to the fact that our simple interaction models were not statistically significant and that there were notable overlaps in confidence intervals between phenotypes and incident hypertension. The implications include that there are certainly other contributors to hypertension and that larger studies with greater power may provide more precise estimates for the risk for hypertension associated with renin and aldosterone phenotypes. Fourth, confirmatory testing for PA in this cohort to identify participants who may have had unrecognized overt biochemical PA despite being normotensive was not conducted(33); however, our overarching message of using renin to identify normotensives who potentially have clinically relevant renin-independent aldosteronism and may benefit from MR antagonists would not have changed. Finally, our data are observational in nature and could be limited by residual confounding rather than representing causal relationships.
CONCLUSIONS
In addition to recognizing the public health relevance of overt PA as a modifiable risk factor for cardiovascular disease, our results demonstrate that there exists a much broader and more subtle spectrum of renin-independent aldosteronism and MR activation that extends into healthy normotensives, a population in which PA is never suspected(20). In the context of a suppressed renin phenotype in normotension, even serum aldosterone levels that appear “low” or “normal” may be autonomous and result in inappropriate MR activation and a higher risk for developing hypertension. Our findings suggest autonomous (or primary) aldosterone secretion may be more common than currently recognized, and that it may play an important role in the pathogenesis of hypertension. Future studies using a renin phenotype-driven approach may efficiently identify new-onset or mild hypertensives, or even high-risk pre-hypertensives, with renin-independent aldosteronism that may preferentially benefit from MR antagonists.
Supplementary Material
Supplementary Figure 1. The figure depicts how the final study population was determined and which study examination variables were assessed for the longitudinal and cross-sectional analyses.
Supplementary Table 1. Aldosterone (quartiles) and the multivariable adjusted risks for incident hypertension by renin phenotype. Results are incident rates, risk differences, and relative risks for developing hypertension for each aldosterone quartile in the context of renin phenotype. Aldosterone quartiles compared to quartile 1 as the reference. Effect estimates are hazard ratios (HR) with 95% confidence intervals.
Supplementary Table 2: Serum Aldosterone in the Context of Renin Phenotypes and the Change in Systolic Blood Pressure.
Supplementary Table 3. Demographic and biochemical characteristics of participants for cross-sectional analyses. All presented characteristics are from examination 1, except for aldosterone, plasma renin activity, and the aldosterone-to-renin ratio, which represent the renin-angiotensin-aldosterone phenotype from either examination 2 or 3.
Acknowledgments
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. Research reported in this publication was also supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award numbers: R01 HL096875, N01 HC95159, N01 HC95166, N01 HC95169, R01 HL071739, R01 HL072403. AV was supported by the National Institutes of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under Award Number R01 DK107407, by the National Heart, Lung, and Blood Institute under award number K23 HL111771 and by Grant 2015085 from the Doris Duke Charitable Foundation. RB was supported by FONDECYT 1150437, 1150327, 1160836, 1160695 and CORFO 13CTI-21526-P1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health or other funding agencies.
Role of the Funding Sources: The funding sources had no role in the collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to submit for publication.
Authors’ Contributions: MALF contributed to data analysis, statistical review, and manuscript preparation. All other authors contributed to data analysis and interpretation, writing of the manuscript, and design of figures and tables.
Footnotes
This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to Annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
DECLARATIONS OF INTEREST: The authors have nothing to disclose.
References
- 1.Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40:892–6. doi: 10.1161/01.hyp.0000040261.30455.b6. [DOI] [PubMed] [Google Scholar]
- 2.Gordon RD, Stowasser M, Tunny TJ, Klemm SA, Rutherford JC. High incidence of primary aldosteronism in 199 patients referred with hypertension. Clin Exp Pharmacol Physiol. 1994;21:315–8. doi: 10.1111/j.1440-1681.1994.tb02519.x. [DOI] [PubMed] [Google Scholar]
- 3.Hannemann A, Bidlingmaier M, Friedrich N, Manolopoulou J, Spyroglou A, Volzke H, et al. Screening for primary aldosteronism in hypertensive subjects: results from two German epidemiological studies. Eur J Endocrinol. 2012;167:7–15. doi: 10.1530/EJE-11-1013. [DOI] [PubMed] [Google Scholar]
- 4.Loh KC, Koay ES, Khaw MC, Emmanuel SC, Young WF. Prevalence of primary aldosteronism among asian hypertensive patients in Singapore. Journal of Clinical Endocrinology and Metabolism. 2000;85:2854–9. doi: 10.1210/jcem.85.8.6752. [DOI] [PubMed] [Google Scholar]
- 5.Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. Journal of Clinical Endocrinology and Metabolism. 2004;89:1045–50. doi: 10.1210/jc.2003-031337. [DOI] [PubMed] [Google Scholar]
- 6.Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 7.Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, et al. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J Am Coll Cardiol. 2017;69(14):1811–20. doi: 10.1016/j.jacc.2017.01.052. [DOI] [PubMed] [Google Scholar]
- 8.Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Archives of internal medicine. 2008;168:80–5. doi: 10.1001/archinternmed.2007.33. [DOI] [PubMed] [Google Scholar]
- 9.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–8. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98:4826–33. doi: 10.1210/jc.2013-2805. [DOI] [PubMed] [Google Scholar]
- 11.Reincke M, Fischer E, Gerum S, Merkle K, Schulz S, Pallauf A, et al. Observational study mortality in treated primary aldosteronism: The German conn’s registry. Hypertension. 2012;60:618–24. doi: 10.1161/HYPERTENSIONAHA.112.197111. [DOI] [PubMed] [Google Scholar]
- 12.Rossi GP, Sechi LA, Giacchetti G, Ronconi V, Strazzullo P, Funder JW. Trends in Endocrinology and Metabolism. 2008. Primary aldosteronism: cardiovascular, renal and metabolic implications; pp. 88–90. [DOI] [PubMed] [Google Scholar]
- 13.Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62:331–6. doi: 10.1161/HYPERTENSIONAHA.113.01060. [DOI] [PubMed] [Google Scholar]
- 14.Sechi LA, Novello M, Lapenna R, Baroselli S, Nadalini E, Colussi GL, et al. Long-term renal outcomes in patients with primary aldosteronism. JAMA: the journal of the American Medical Association. 2006;295:2638–45. doi: 10.1001/jama.295.22.2638. [DOI] [PubMed] [Google Scholar]
- 15.Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, et al. Adrenocortical zonation in humans under normal and pathological conditions. Journal of Clinical Endocrinology and Metabolism. 2010;95:2296–305. doi: 10.1210/jc.2009-2010. [DOI] [PubMed] [Google Scholar]
- 16.Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proceedings of the National Academy of Sciences. 2015;112:E4591–E9. doi: 10.1073/pnas.1505529112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lalli E, Barhanin J, Zennaro M-C, Warth R. Local Control of Aldosterone Production and Primary Aldosteronism. Trends in Endocrinology & Metabolism. 2016;27:123–31. doi: 10.1016/j.tem.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Nanba K, Vaidya A, Williams GH, Zheng I, Else T, Rainey WE. Age-Related Autonomous Aldosteronism. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.117.028201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baudrand R, Guarda FJ, Fardella CE, Hundemer G, Brown J, Williams GH, et al. Continuum of Renin-Independent Aldosteronism in Normotension. Hypertension. 2017 doi: 10.1161/HYPERTENSIONAHA.116.08952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. The Journal of clinical endocrinology and metabolism. 2016;101:jc20154061. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee R, Zelnick L, Mukamal KJ, Nettleton JA, Kestenbaum BR, Siscovick DS, et al. Potassium measures and their associations with glucose and diabetes risk: The Multi-Ethnic Study of Atherosclerosis (MESA) PLoS ONE. 2016:11. doi: 10.1371/journal.pone.0157252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rifkin DE, Khaki AR, Jenny NS, McClelland RL, Budoff M, Watson K, et al. Association of renin and aldosterone with ethnicity and blood pressure: The multi-ethnic study of atherosclerosis. American Journal of Hypertension. 2014;27:801–10. doi: 10.1093/ajh/hpt276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The sixth report of the Joint National Committee on prevention, detection evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–46. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 25.Brown J, de Boer IH, Robinson-Cohen C, Siscovick DS, Kestenbaum B, Allison M, et al. Aldosterone, parathyroid hormone, and the use of renin-angiotensin-aldosterone system inhibitors: the multi-ethnic study of atherosclerosis. J Clin Endocrinol Metab. 2015;100:490–9. doi: 10.1210/jc.2014-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens La, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown IJ, Dyer AR, Chan Q, Cogswell ME, Ueshima H, Stamler J, et al. Estimating 24-hour urinary sodium excretion from casual urinary sodium concentrations in Western populations: the INTERSALT study. Am J Epidemiol. 2013;177:1180–92. doi: 10.1093/aje/kwt066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cogswell ME, Wang CY, Chen TC, Pfeiffer CM, Elliott P, Gillespie CD, et al. Validity of predictive equations for 24-H urinary sodium excretion in adults aged 18–39 y1–5. American Journal of Clinical Nutrition. 2013;98:1502–13. doi: 10.3945/ajcn.113.059436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allison PD. Survival Analysis Using SAS: A Practical Guide. 2. SAS Institute; 2010. [Google Scholar]
- 30.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45–9. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2014:CD007004. doi: 10.1002/14651858.CD007004.pub3. [DOI] [PubMed] [Google Scholar]
- 32.Lu Y, Ku E, Campese VM. Aldosterone in the pathogenesis of chronic kidney disease and proteinuria. Curr Hypertens Rep. 2010;12:303–6. doi: 10.1007/s11906-010-0116-4. [DOI] [PubMed] [Google Scholar]
- 33.Markou A, Pappa T, Kaltsas G, Gouli A, Mitsakis K, Tsounas P, et al. Evidence of primary aldosteronism in a predominantly female cohort of normotensive individuals: A very high odds ratio for progression into arterial hypertension. Journal of Clinical Endocrinology and Metabolism. 2013;98:1409–16. doi: 10.1210/jc.2012-3353. [DOI] [PubMed] [Google Scholar]
- 34.Hood SJ, Taylor KP, Ashby MJ, Brown MJ. The Spironolactone, Amiloride, Losartan, and Thiazide (SALT) double-blind crossover trial in patients with low-renin hypertension and elevated aldosterone-renin ratio. Circulation. 2007;116:268–75. doi: 10.1161/CIRCULATIONAHA.107.690396. [DOI] [PubMed] [Google Scholar]
- 35.Weinberger MH, White WB, Ruilope LM, MacDonald TM, Davidson RC, Roniker B, et al. Effects of eplerenone versus losartan in patients with low-renin hypertension. American Heart Journal. 2005;150:426–33. doi: 10.1016/j.ahj.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Williams B, Macdonald TM, Morant S, Webb DJ, Sever P, McInnes G, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): A randomised, double-blind, crossover trial. The Lancet. 2015;386:2059–68. doi: 10.1016/S0140-6736(15)00257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitt B, Reichek N, Willenbrock R, Zannad F, Phillips RA, Roniker B, et al. Effects of Eplerenone, Enalapril, and Eplerenone/Enalapril in Patients With Essential Hypertension and Left Ventricular Hypertrophy: The 4E-Left Ventricular Hypertrophy Study. Circulation. 2003;108:1831–8. doi: 10.1161/01.CIR.0000091405.00772.6E. [DOI] [PubMed] [Google Scholar]
- 38.Hundemer GL, Baudrand R, Brown JM, Curhan G, Williams GH, Vaidya A. Renin Phenotypes Characterize Vascular Disease, Autonomous Aldosteronism, and Mineralocorticoid Receptor Activity. J Clin Endocrinol Metab. 2017;102(6):1835–43. doi: 10.1210/jc.2016-3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasan RS, Evans JC, Larson MG, Wilson PWF, Meigs JB, Rifai N, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. The New England journal of medicine. 2004;351:33–41. doi: 10.1056/NEJMoa033263. [DOI] [PubMed] [Google Scholar]
- 40.Sun Q, Bertrand KA, Franke AA, Rosner B, Curhan GC, Willett WC. Reproducibility of urinary biomarkers in multiple 24-h urine samples. Am J Clin Nutr. 2017;105(1):159–68. doi: 10.3945/ajcn.116.139758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Espeland MA, Kumanyika S, Wilson AC, Reboussin DM, Easter L, Self M, et al. Statistical issues in analyzing 24-hour dietary recall and 24-hour urine collection data for sodium and potassium intakes. Am J Epidemiol. 2001;153(10):996–1006. doi: 10.1093/aje/153.10.996. [DOI] [PubMed] [Google Scholar]
- 42.Campino C, Martinez-Aguayo A, Baudrand R, Carvajal CA, Aglony M, Garcia H, et al. Age-related changes in 11beta-hydroxysteroid dehydrogenase type 2 activity in normotensive subjects. Am J Hypertens. 2013;26(4):481–7. doi: 10.1093/ajh/hps080. [DOI] [PubMed] [Google Scholar]
- 43.Adlin EV, Braitman LE, Vasan RS. Bimodal aldosterone distribution in low-renin hypertension. Am J Hypertens. 2013;26(9):1076–85. doi: 10.1093/ajh/hpt091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baudrand R, Guarda FJ, Torrey J, Williams G, Vaidya A. Dietary Sodium Restriction Increases the Risk of Misinterpreting Mild Cases of Primary Aldosteronism. J Clin Endocrinol Metab. 2016:jc20161963. doi: 10.1210/jc.2016-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito Y, Takeda R, Karashima S, Yamamoto Y, Yoneda T, Takeda Y. Prevalence of primary aldosteronism among prehypertensive and stage 1 hypertensive subjects. Hypertension research: official journal of the Japanese Society of Hypertension. 2011;34:98–102. doi: 10.1038/hr.2010.166. [DOI] [PubMed] [Google Scholar]
- 46.Mosso L, Carvajal C, González A, Barraza A, Avila F, Montero J, et al. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42:161–5. doi: 10.1161/01.HYP.0000079505.25750.11. [DOI] [PubMed] [Google Scholar]
- 47.Rossi GP. Does primary aldosteronism exist in normotensive and mildly hypertensive patients, and should we look for it? Hypertension research: official journal of the Japanese Society of Hypertension. 2011;34:43–6. doi: 10.1038/hr.2010.206. [DOI] [PubMed] [Google Scholar]
- 48.Brown JM, Underwood PC, Ferri C, Hopkins PN, Williams GH, Adler GK, et al. Aldosterone dysregulation with aging predicts renal vascular function and cardiovascular risk. Hypertension. 2014;63:1205–11. doi: 10.1161/HYPERTENSIONAHA.114.03231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaidya A, Underwood PC, Hopkins PN, Jeunemaitre X, Ferri C, Williams GH, et al. Abnormal aldosterone physiology and cardiometabolic risk factors. Hypertension. 2013;61:886–93. doi: 10.1161/HYPERTENSIONAHA.111.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults. JAMA. 2014;311:507. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. The figure depicts how the final study population was determined and which study examination variables were assessed for the longitudinal and cross-sectional analyses.
Supplementary Table 1. Aldosterone (quartiles) and the multivariable adjusted risks for incident hypertension by renin phenotype. Results are incident rates, risk differences, and relative risks for developing hypertension for each aldosterone quartile in the context of renin phenotype. Aldosterone quartiles compared to quartile 1 as the reference. Effect estimates are hazard ratios (HR) with 95% confidence intervals.
Supplementary Table 2: Serum Aldosterone in the Context of Renin Phenotypes and the Change in Systolic Blood Pressure.
Supplementary Table 3. Demographic and biochemical characteristics of participants for cross-sectional analyses. All presented characteristics are from examination 1, except for aldosterone, plasma renin activity, and the aldosterone-to-renin ratio, which represent the renin-angiotensin-aldosterone phenotype from either examination 2 or 3.