Abstract
Objective
The adaptive response to vascular injury is the formation of functional collateral vessels to maintain organ integrity. Many of the clinical complications associated with sickle cell disease (SCD) can be attributed to repeated bouts of vascular insufficiency, yet the detailed mechanisms of collateral vessel formation following injury are largely unknown in SCD. Here, we characterize post-ischemic neovascularization in SCD and the role of neutrophils in the production of reactive oxygen species (ROS).
Approach and Results
We induced hind limb ischemia (HLI) by ligation of the femoral artery in Townes sickle cell (SS) mice compared to wild type (AA) mice. Perfusion recovery, ascertained using LASER Doppler perfusion imaging showed significant diminution in collateral vessel formation in SS mice following HLI (76 ±13 % AA vs 34±10 % in SS by day 28, p < 0.001 n=8 per group). The incidence of amputation (25% vs 5%) and foot necrosis (80% vs 15%) following HLI were significantly increased in the SS mice. Motor function recovery evaluation by the running wheel assay was also impaired in SS mice (36% vs 97% at 28 days post HLI, p < 0.001). This phenotype was associated with persistent and excessive production of ROS by neutrophils. Importantly, neutrophil depletion or treatment with the anti-oxidant N-acetylcysteine reduced oxidative stress and improved functional collateral formation in the SS mice.
Conclusions
Our data suggest dysfunctional collateral vessel formation in SS mice after vascular injury, and provides a mechanistic basis for the multiple vascular complications of SCD.
Keywords: sickle cell, collateral vessel formation, ischemia, neutrophil, hydrogen peroxide
Subject codes: Vascular Disease, Ischemia, oxidant stress
INTRODUCTION
Sickle cell disease (SCD) is the most common hemoglobinopathy worldwide.1 A hallmark of the disease is repeated bouts of ischemia secondary to polymerizing sickle red blood cells.2 This leads to vaso-occlusion and microvessel injury3, 4 as well as large vessel injury.5–7 Consequently, the vast array of vascular complications in SCD, including strokes, retinopathy, sudden cardiac death, recalcitrant leg ulcers, kidney failure and other organ dysfunction are likely multi-factorial and complex.2, 8, 9 A normal physiologic response to vascular injury is the development of collateral vessels in order to preserve end organ function. That these patients suffer from multiple vascular complications may suggest impairment in collateral vessel formation after ischemic injury. It is currently unknown if collateral vessel formation is impaired in SCD.
The formation of collateral vessels is an incompletely understood, multi-factorial process that involves the interplay of diverse cell types (immune cells, endothelial cells) and coordinated regulation of cytokines, chemokines and other cellular and angiogenic factors.10, 11 Reactive oxidative species (ROS), particularly hydrogen peroxide (H2O2) and superoxide radicals (O2·−) have emerged as critical mediator of collateral vessel formation. Specifically, we and others have shown that regulation of ROS is necessary to regulate the appropriate microenvironmental cues for effective collateral vessel formation. Either low or excessive production impairs collateralization.12–14
Neutrophils, first responder cells after vascular injury, are a major source of ROS.15 Furthermore, neutrophils may synthesize other chemokines and cytokines such as metalloproteinases that participate in collateral vessel formation.16 In addition, they may interact with endothelium to cause direct injury. However, because these cells have short half-lives, they are thought to play negligent role in collateral vessel formation. However, as we show in this study, prolonged and persistent neutrophil infiltration is a striking characteristic of the ischemic response in SCD. Given that neutrophils propagate inflammation and oxidative stress in SCD,17 we sought to examine their role in collateral vessel formation. We now show significant impairment in collateralization after hind limb ischemia in a mouse humanized with the sickle hemoglobin gene. This was associated with excessive H2O2 and O2·−, and a prominent role of neutrophils as a chief source of persistent ROS. Depletion of neutrophils and pharmacologic reduction of oxidative stress improved collateral vessel formation in this mouse model of SCD. Our data, for the first time to our knowledge, reveals impaired post-ischemic neovascularization as a critical pathophysiologic underpinning of the multiple vascular complications in SCD and implicates neutrophils as a potential target to improve this process.
MATERIALS AND METHODS
Animals
The humanized Townes sickle cell (SS) mice18 were an original generous gift of Dr Townes (University of Alabama, Birmingham). The Townes mice, hα/hα::βA/βS, hα/hα::-383 γ-βA/-1400 γ-βS (Jax # 013071) are on a mixed C57BL/6:129 background, and colonies were established, maintained and bred in-house at the Emory University Department of Animal Resources. Female wild type mice (genotype AA) and littermate sickle cell mice (genotype SS) between 8 and 12 weeks old were used for the described experiments. The animals were housed and cared for in agreement with guidelines approved by the Emory University Institutional Animal Care and Use Committee.
Hind Limb Ischemia
Mice were anesthetized with 2% isoflurane in a chamber and then anesthetized with 2% isoflurane through a nose cone. A unilateral incision was made over the left medial thigh of the mouse. The superficial femoral artery and vein were ligated with 6-0 silk caudally to the branching deep femoral artery. A second ligation was performed just proximal to the branching of the tibial arteries, and the length of the artery and vein was excised between the two ligation points. The skin was closed with monofilament suture. The animals received buprenorphine (0.1 mg/kg, subcutaneously) for analgesia and were allowed to recovered on a heated pad.
LASER Doppler Perfusion Imaging
LASER Doppler perfusion imaging (LDPI) was performed as described previously.14, 19 Briefly, mice were anesthetized by inhalation of 2% isoflurane and scanned with the LDPI system (PIM II Laser Doppler Perfusion Imager; Perimed). By defining equivalent regions of interest on the perfusion heat map generated by the instrument and calculating the mean perfusion (in arbitrary perfusion units) in both the proximal and distal leg regions, perfusion in the ischemic leg (IL) was normalized to flow in the non-ischemic leg (NIL).
Histological Assessment of Vessel Density
Histological analysis was performed on both the ischemic and non-ischemic limbs at indicated days post hind limb ischemia surgery. Mice were euthanized and the tissue was perfused with saline followed by 10% buffered formalin for fixation. The bone was then demineralized in a formic acid-based solution (Cal-Ex II; Fisher Scientific) for 48 h before processing and paraffin embedding. 5- μm-thick sections were prepared for staining. Enzyme treatment was performed in 2 μg/ml proteinase K (Biolabs; Cat # P8107S) prior to incubation with primary antibodies. Sections were stained with Ly6G antibody (abcam Cat # ab25377), smooth muscle α-actin antibody (Sigma Cat # A2547) followed by incubation with Streptavadin QDot 655 (Invitrogen; Cat # Q10121MP).
In Vivo Staining by Tail Vein Injection of Lectin for Confocal Imaging
100 μl of 1mg/ml fluorescein isothiocyanate (FITC)-conjugated Griffonia simplicifolia Lectin I (VectorLab; Cat # FL-1201) was injected intravenously and allowed to circulate for 10 min before mice were euthanized and hind limb muscle were harvested. Tissues were fixed in 10% buffered formalin at 4 degrees, incubated in 30% sucrose for 24 hours, embedded in OCT, snap frozen, and cryosectioned (50-μm-thick sections). Sections were mounted with Vectashield with DAPI (VectorLab; Cat # H-1200). Perfused vessels were identified as FITC-I-labeled lumenal structures on confocal laser scanning microscope and reported as vessels/mm2.ImageJ software (NIH) was used to count the number of vessels for analysis.
Vascular Leakage Assay
The integrity of collateral vessels formed after hind limb ischemia was assessed by a modified Miles assay.20 Twenty-eight days after HLI, mice underwent intravenous injection of 100 μl of 1% cell-impermeable Evans Blue dye (Sigma-Aldrich, St. Louis, MO) via the tail vein. After 40 min, the animals were euthanized and hind limbs were isolated and incubated in formamide (Sigma, Cat # 11814320001) for 3 days to extract the dye, and the OD was determined at 620 nm.
Running Wheel Study
The measurement of voluntary running endurance were performed as a physiologic test of ischemic limb functional recovery. On postoperative day 21 or 28, each mouse was individually housed in a cage with a running wheel attached to a sensor that counted the number of revolutions made by the wheel during a 7-day period. The distance in meters was recorded both daily and cumulatively for 7 days.
H2O2 Measurement
Extracellular H2O2 produced from non-ischemic and ischemic tibialis anterior muscles was measured by Amplex red assay (Invitrogen, Carlsbad, CA Cat # A22188), according to manufacturer’s instructions as described previously.14 H2O2 production was normalized to tissue wet weight. For intracellular ROS, single cell suspensions were obtained by digesting hind limbs in collagenase-dispase solution. Cells were then incubated with 5 μM of 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA, Invitrogen Cat # C6827) at 37°C for 15 minutes, before analysis by flow cytometry. Neutrophils were identified with surface marker staining with anti-Gr-1 antibody (Biolegend, Cat # 108411).
Tissue Superoxide Measurement
Superoxide production by ischemic muscles were measured using Hydro-Cy5 probe (ROSstar 650, LI-COR Biosciences, Cat # 926-30000). Following digestion of ischemic tissue as previously described, equal number of cell lysate were seeded in triplicates in a 96-well plate, and fresh Hydro-Cy5 probes were prepared at 25 μM and incubated with samples for 20 minutes at room temperature in the dark, before fluorescence was read at emission/excitation 635/660 nm. Control wells were cell lysates without probe.
Neutrophil Depletion
Following hind limb ischemia, neutrophils were depleted by intraperitoneal injection of 100 μg of monoclonal anti-mouse Ly6G antibody (clone 1A8, BioXcell, Cat # BP0075-1) once every 2 days until the end of the experiment. Control mice were injected with IgG2a antibody (BioXcell). Depletion of neutrophils was confirmed by flow cytometry and by histological evaluation of ischemic limbs.
Antioxidant therapy
Sickle cell mice were treated with 0.163 g/L N-acetyl cysteine (Sigma-Aldrich, Cat # A7250) in their drinking water. Fresh drinking water was made every two days.
5/6 Nephrectomy
Animals underwent 5/6 nephrectomy or sham surgery under 2% isoflurane anesthesia and buprenorphine (0.1 mg/kg, subcutaneously) for analgesia. Briefly, the left kidney was exposed, and the upper and lower poles were tied with a polyglycolic acid suture line. The peritoneum and skin were then sutured, and the animals were returned to their individual cages. A week later, complete right nephrectomy was performed. Mice recovered completely and hematocrit was monitored to ensure anemia before HLI was performed.
Iron-Deficiency Anemia
To induce anemia, a cohort of AA mice were fed iron-deficient diet (~3 ppm Fe, TD.80396, Teklad Diets) compared to standard chow (48ppm Fe) for at least 6 weeks prior to HLI and throughout their time course after surgery. Mice on the iron-deficient diet underwent additional weekly submandibular bleed (250–300 μl) to ensure severe anemia. Complete blood counts were measured by a Hemavet 1500 blood analyzer (CDC Technologies, Oxford, CT).
Statistical Analysis
All reported results are expressed as mean ± SEM. Graphpad Prism (Graphpad Software, La Jolla, Ca) was used for statistical analysis. Blood flow recovery in the ischemic hind limb was compared between the two groups by two-way repeated measures ANOVA, followed by Bonferroni post host analysis. Comparison between groups was analyzed by unpaired Student 2-tailed t test or ANOVA for experiments with more than 2 subgroups followed by Bonferroni post hoc analysis. For categorical data, Pearson’s chi-square analysis was performed. A P value of <0.05 was considered statistically significant.
RESULTS
Impaired Collateral Vessel formation after Hind Limb Ischemia
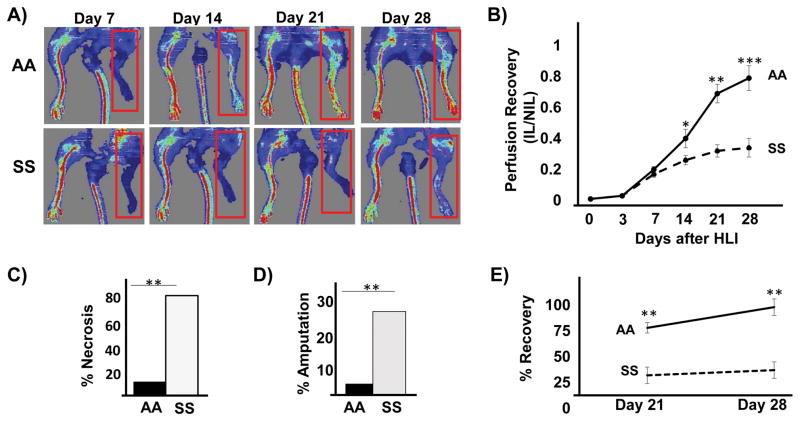
To investigate collateral vessel formation in SS mice, we used the hind limb ischemia (HLI) model. All mice survived after induction of unilateral HLI and appeared healthy during the follow-up period. Perfusion was quantified using LASER Doppler Imaging (LDPI). There were no differences in limb perfusion between AA and SS mice at baseline (Supplemental Figure 1). Following HLI, the perfusion measurements in SS and AA mice were similar at the earliest time points (day 0 through day 7), suggesting equivalent extent of vascular injury (Figure 1B). However, whereas AA mice showed continued perfusion recovery over time, SS mice demonstrated significant impairment in perfusion recovery (Figure 1A and 1B). For instance, at day 28 post HLI, perfusion recovery was 76 ± 13 % in AA, compared to 34 ± 10 % in SS mice, p < 0.001. In addition, the incidence of digit necrosis after HLI exceeded 80% in SS mice, compared to 15% in the AA mice, p < 0.01 (Figure 1C). Furthermore, the incidence of limb amputation was 25% in the SS mice, compared to 5% in AA mice, p < 0.01 (Figure 1D). Spontaneous functional muscle recovery was analyzed by distance covered on a computerized running wheel attached to mouse cages. In this assay (Figure 1E), spontaneous muscle activity recovery was impaired in SS mice, while AA mice recovered nearly full muscle function by 28 days (38.4% vs 96.8%). Altogether, these data demonstrate impaired functional collateral vessel formation in SS mice after ischemic injury.
Figure 1.
Impaired perfusion in sickle cell (SS) mice after hind limb ischemia. Ischemia was induced in SS and wild type (AA) mice using the HLI procedure. LPDI was used to noninvasively measure the perfusion recovery of the limb. (A) Representative LDPI showing perfusion recovery in mice at days 7, 14, 21 and 28 after HLI. Ischemic limbs are highlighted by red box. (B) The perfusion ratio between the ischemic (IL) and non-ischemic leg (NIL) on post-op days 0,3, 7, 14, 21 and 28. N = 10 per group. Number of mice that showed toe necrosis (C) and limb amputation. (D) 28 days after HLI. N = 20 mice per group, Pearson’s chi-square test. (E) Functional muscle recovery at day 21 and day 28. Data represents percent recovery from baseline after 21 and 28 days from HLI. N= 6–8 mice per study. *p<0.05, **p<0.01, ***p<0.001.
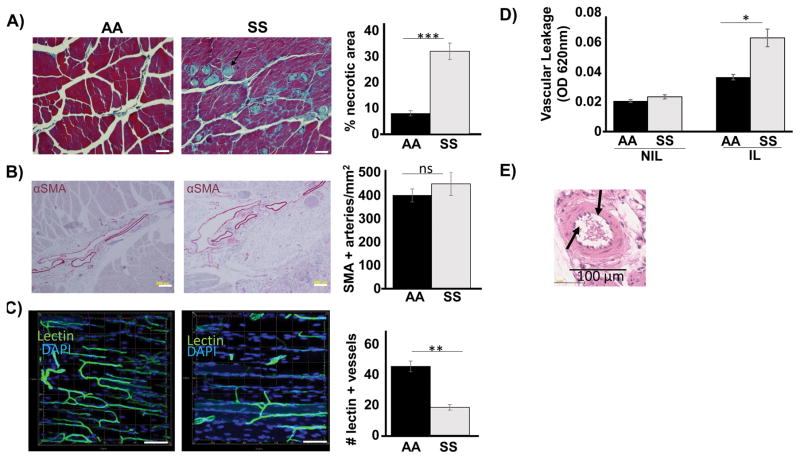
To further characterize the response to ischemic injury, we performed histological and immunohistochemical analyses. Histology at 28 days revealed markedly increased areas of tissue necrosis, loss of muscle mass, and persistent inflammation in the SS ischemic limb. In contrast, AA ischemic limbs demonstrated muscle mass integrity, resolved necrosis and inflammation (Figure 2A). At 28 days, there was no difference between the genotypes in terms of quantity of smooth muscle-positive vessels as evaluated by alpha smooth muscle actin (αSMA) staining (Figure 2B). However, immunofluorescence analysis from in vivo FITC-lectin perfused mice revealed that SS mice had a morphologically disorganized, non-contiguous and fewer lectin-positive vasculature (a representative Z-stack confocal imaging is shown in Figure 2C). Furthermore, in vivo injection of membrane-impermeable Evan’s blue dye, a common approach to evaluate vessel integrity disproportionally accumulated in the ischemic hind limb of the SS mice (Figure 2D), suggesting dysfunctional, leaky vessels. We also performed additional experiments by H&E analysis of non-perfusion fixed ischemic tissues to access small vessel patency. A representative data is shown in Figure 2E. This analysis showed that SS small vasculatures (capillaries, venules, arterioles) were not occluded by sickling red cells. Thus, the poor collateral vessel formation in the SS cannot be fully explained by acute vessel occlusion by local sickle crises.
Figure 2.
Histologic analysis of ischemic limb in SS and AA mice 28 days after HLI. (A) Representative trichrome staining showed significant loss of muscle mass, tissue necrosis (blue staining) and excessive inflammation in SS mice compared to AA mice. Scale bar =40 μm. (B) Representative αSMA staining showed no difference between SS and AA. Scale bar = 200μm. (C) Representative Z-stack confocal microscopy of FITC-lectin perfused vessels showing significantly decreased functional vessel formation. Scale bar = 100 μm. Number of lectin-perfused vessel per mm2 is indicated. (D) Mice were injected with Evan’s blue dye, and spectrophotometric absorbance of harvested ischemic (IL) and non-ischemic (NIL) limbs was read at 620nm to evaluate vascular leakage. (E) Representative H&E of non-perfused blood vessel showing patency in the SS. N = 4–6 mice per genotype per indicated study. Data are plotted as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001
Townes SS mice are severely anemic (Supplemental Table 1)21. In certain clinical and experimental contexts, anemia may impact collateral vessel formation.22–24 To determine the influence, if any, of anemia on the impaired collateral vessel formation in the SS mice, we induced severe anemia in AA mice by performing 5/6 nephrectomy. The hemoglobin of these mice was 9.2 ± 0.3 g/dl at the time of hind limb ischemia (Supplemental Table 1). AA mice that underwent 5/6 nephrectomy showed no impairment in collateral vessel formation as measured by perfusion recovery with LDPI (Supplemental Figure 2A). A second approach, using iron-deficient diet with weekly phlebotomy, was employed to induce anemia. Long-term iron-deficient diet with weekly phlebotomy significantly reduced hemoglobin (11.3 ± 0.2 g/dl) compared to mice fed standard diet (14.5 ± 0.4 g/dl, p < 0.01). These anemic mice also showed equivalent collateral vessel formation compared to non-phlebotomized mice fed the standard chow (Supplemental Figure 2B). These sets of experiments demonstrate that anemia independently does not play a significant role in the poor perfusion recovery and functional outcomes in the SS mice after HLI.
Increased Reactive Oxygen Species in the Ischemic Limb of Sickle Cell Mice
To determine the mechanisms contributing to the impaired collateral vessel formation, we focused on the role of reactive oxygen species (ROS), specifically hydrogen peroxide (H2O2) and superoxide (O2·−). We and others have shown the critical role of these molecules in collateral vessel formation.12, 14 Specifically, an appropriate amount of H2O2 is necessary to orchestrate multiple of the mechanisms required for collateral vessel formation, but excessive H2O2 may be detrimental. We first measured H2O2 levels in hind limb of SS mice and repeatedly found significantly increased amounts of H2O2 at 3, 5 and 7 days after HLI (Figure 3A). Of note, there was no difference in H2O2 production in the non-ischemic limbs of the AA and SS mice (data not shown). This data suggest that overproduction of H2O2 may contribute to the impaired post-ischemic neovascularization in the sickle mice. Similarly, we found significantly elevated levels of O2·− in the ischemic limbs of the SS mice, compared to the AA mice (Figure 3B).
Figure 3.
Excessive ROS production in ischemic SS limbs. (A) The Amplex red assay was used to measure hydrogen peroxide (H2O2) levels in ischemic hind limbs (IL) of SS and AA mice. H2O2 measurements were normalized to tissue wet weight. H2O2 was increased in the IL in the SS mice at 3, 5, and 7 days with a peak at day 5. N = 5 mice per time point. (B) The hydro-Cy5 dye was used to measure superoxide levels in the IL of SS and AA mice (n=5 per group) 3 days after ischemia. Cell homogenate from ischemic AA and SS mice were incubated with hydro-Cy5 dye, and fluorescence of the microplate was read at em/ex 635/660 nm. Bars are means ± SEM. *P<0.05
Sickle Cell Neutrophils Produce elevated ROS
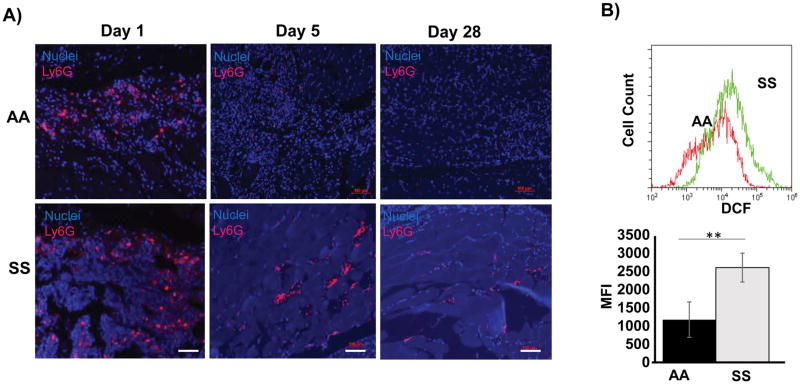
To identify the cellular source of ROS, we assessed the hind limb ischemic tissues by histology and immunohistochemistry. The excessive inflammation and necrosis in the SS hind limbs prompted evaluation of neutrophils. Interestingly, we noted persistent neutrophils in the ischemic limbs of the SS mice beyond day 3, and up to 28 days after HLI (Figure 4A). Because neutrophils are an important source of ROS and have been previously described to be highly inflammatory in SS disease,17, 25 we used flow cytometry to measure intracellular H2O2 in the neutrophils in the context of HLI using the DCFDA probe. We found a 2.5 fold increased H2O2 production in SS neutrophils compared to AA neutrophils in the ischemic tissue (Figure 4C). Thus both the total number of infiltrating neutrophils and the production of H2O2 by these neutrophils were increased in the SS mice.
Figure 4.
Neutrophils in SS mice play a prominent role in ROS production. (A) Representative histology showing presence of neutrophils (pink) in ischemic legs of SS mice at day 1, day 5 and day 28. In contrast, no neutrophils were detected in hind limb of AA mice beyond day 5. Scale bar = 100 μm. (B) Single cell suspension obtained from ischemic legs of SS and AA mice were cultured with DCFDA. Flow cytometry was used to assess DCF fluorescence. Representative plot of three independent experiments is shown. Median Fluorescence intensity maps showed SS neutrophils produced significantly increased levels of H2O2. **P<0.01, n=5. Bars are means ± SEM.
Neutrophil Depletion Improves Collateral Vessel formation
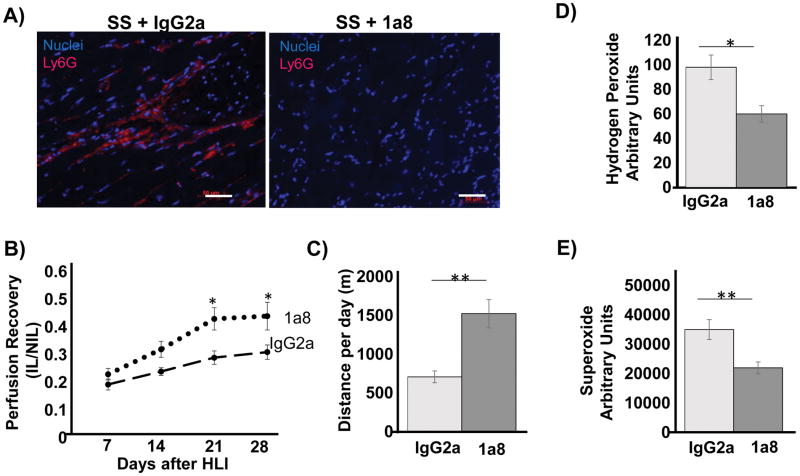
We reasoned that if neutrophils contributed to excessive ROS, then elimination of neutrophils would reduce ROS levels and potentially improve collateral vessel formation in SS mice. To test this, we used the monoclonal anti-Ly6G antibody to deplete neutrophils in the SS mice. Control SS mice were treated with IgG2a. Injection every two days successfully depleted neutrophils in the SS mice (Figure 5A). Neutrophil depletion significantly improved collateral vessel formation in the SS mice, beginning day 14 through 28, as shown in Figure 5B. Functional muscle recovery (Figure 5C) significantly improved in SS mice treated with anti-LyG antibody (620m per day, vs 1540m per day, p < 0.01) with an insignificant trend towards improvement in toe necrosis and limb amputation after neutrophil depletion (data not shown). Importantly, neutrophil depletion was associated with significant reduction in both H2O2 and O2·− in the ischemic limb of the SS mice (Figure 5C and 5D). Not surprisingly, depletion of neutrophils in AA mice did not significantly impact collateral vessel formation (Supplemental Figure 3).
Figure 5.
Neutrophil depletion reduces ROS and improves collateral formation in SS mice. (A) Representative histology showing near complete depletion of neutrophils using 100 μg of anti-Ly6G antibody. Control SS mice were treated with IgG2a. Scale bar = 50 μm. (B) LDPI showed significantly improved perfusion in SS mice after neutrophil depletion. N= 7 mice per group. (C) 28 days post HLI, functional muscle recovery was evaluated by the running wheel assay, showing improved recovery in 1a8-treated SS mice. N = 7 mice per group. (D) The Amplex red assay was used to measure H2O2 levels in ischemic legs (IL) of neutrophil-depleted (1a8) and undepleted (IgG2a) SS mice at day 28. N = 7 mice per group. (E) The Hydro-Cy5 probe was used to measure superoxide in the IL of neutrophil-depleted and control SS mice. Bars are mean ± S.E.M. *p<0.05, **p<0.01.
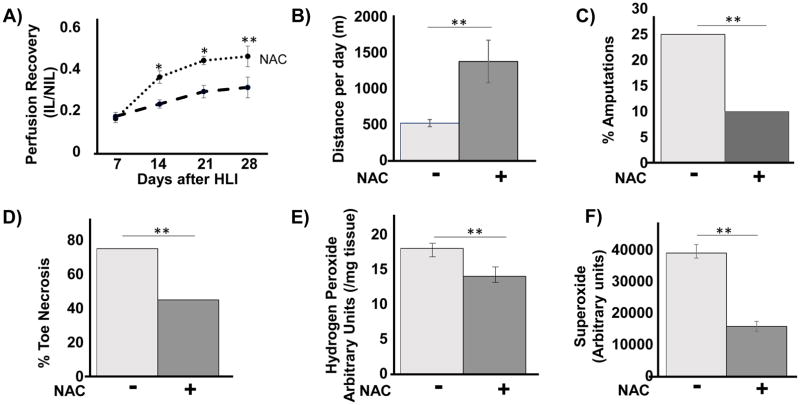
Reduced Oxidative Stress Improves Collateral Vessel Formation in SS mice
Excessive oxidant stress has been shown to inhibit collateral vessel formation in diabetes.26 To directly demonstrate that excessive ROS impairs collateral vessel formation in SS mice, we treated SS mice with the potent antioxidant N-acetylcysteine (NAC), starting 7 days prior to HLI. LDPI measurements and the running wheel experiments showed that treatment with NAC significantly improved perfusion recovery in the ischemic limb of SS mice after HLI. For example, at 3 weeks post HLI, SS mice showed 43 ± 3% recovery vs 29 ± 2% untreated, p< 0.05). This was associated with increased spontaneous motor functional recovery on the running wheel, 28 days after HLI (Figure 6B), improvement in extent of toe necrosis (76% vs 45%, p < 0.01), and improvement in the incidence of limb amputations after ischemia (25% vs 10%, p <0.01). Importantly, NAC treatment also significantly reduced the excessive H2O2 and O2·− production in the SS mice 3 days after HLI (Figure 6C & 6D). Thus, as in diabetes models, anti-oxidant therapy dampens excessive ROS production and improves collateral vessel formation in SS mice. NAC treatment in AA mice did not significantly impair collateral vessel formation (Supplemental Figure 4A, B).
Figure 6.
Treatment with antioxidant N-acetylcysteine (NAC) improves collateral vessel formation and motor function in ischemic limbs. A cohort of SS mice were treated with NAC in their drinking water. (A) LDPI showed improved perfusion in SS mice receiving NAC therapy. (B) Average distance run per day on computerized wheels 28 days after HLI improved when mice were treated with NAC before ischemia and during the course of recovery from HLI. N=5 mice per group. Incidence of amputation. (C) and toe necrosis (D) were significantly decreased in the SS mice treated with NAC (n= 10 mice per group), **P<0.01 by chi-square test. (E) The Amplex red assay used to measure H2O2 levels in ischemic legs of NAC-treated and untreated SS mice 3 days after HLI. N=7 mice per group. *P<0.05, **P<0.01). (F) Hydro-Cy5 probe was used to measure superoxide in the IL of SS treated with or without NAC. Bars are mean ± S.E.M. *p<0.05, **p<0.01, ***p<0.001.
DISCUSSION
Collateral formation in response to tissue ischemia is an important adaptive mechanism necessary for maintenance of end organ function. For sickle cell disease where repeated bouts of ischemic injury are common, this adaptive process is even more vital to protect from both small and large vessel complications. We hypothesized that sickle cell patients suffer numerous vascular complications, including strokes, increased morbidity from stroke, sudden cardiac death, recalcitrant leg ulcers, among others, because of potentially impaired collateralization following vessel injury. Indeed the development of the dysfunctional microvessels in Moyamoya syndrome,27 a common finding in SCD, provides additional evidence for likely impaired collateralization after vessel injury. However, to our knowledge, the process of collateral vessel formation after injury has not been previously described in a SCD mouse model. Using the hind limb ischemia model, we found significant impairment in functional collateral vessel formation in SS mice, resulting in an increased incidence of amputation, toe necrosis and impaired recovery of muscle function. While hind limb ischemia is a common model to investigate peripheral artery disease (PAD), an atypical clinical complication of SCD, this model is an ideal testbed to study collateral vessel formation because it allows for extensive functional, physiologic and anatomical characterization in vivo. Indeed, PAD may be uncommon in SCD in part because it is a disease of the elderly, whereas life expectancy among SCD patients is markedly lower than the general population.28, 29 Nonetheless, large vessel vasculopathy is an underappreciated potential complication of sickle cell disease,30 and our findings suggest that impaired functional collaterals may contribute to this pathophysiology.
Direct vaso-occlusion of small vessels lead to tissue ischemia. In our model, careful analysis of ischemic tissue did not reveal complete occlusion of vessels by sickling red blood cells. Thus, while we cannot rule this out, direct sickling apparently plays a minor role in our model. Rather, the excessive inflammatory response and reactive oxygen species (ROS) were the most striking feature in the SS mice. The role of ROS as mediators that orchestrate various aspects of collateralization is well described by our previous studies and by others. Specifically, a fine balance of ROS is necessary to sustain collateral formation after ischemia: both excessive levels as in diabetes and low levels as in NADPH-deficiency states are detrimental to functional collateral formation.12, 13, 26, 31 It has previously been shown that infiltrating inflammatory cells contribute ROS in the acute phase of HLI, followed by neovascular endothelial cells playing a more prominent role at later time points.32 However, the contribution of neutrophils in neovascularization has received minimal attention, in part because this important cellular source of ROS is often considered a transient first responder population. Only recently have neutrophils received greater attention as a potential modulator of diseases of chronic inflammation.33 Here, we show that in the SS mice, neutrophils play a detrimental role by contributing excessive amounts of ROS. Depletion of neutrophils with a monoclonal antibody and reduction of oxidative stress by NAC treatment both significantly improved collateral vessel formation in SS mice. Additional work is required to delineate the mechanisms of the enhanced inflammatory and oxidative potential of neutrophils from SS mice, as well as the perplexing finding of persistent neutrophils within the ischemic tissue days after vascular injury. Understanding these mechanisms might provide novel strategies to promote the resolution of inflammation in SCD. Interestingly, the dose of NAC did not significantly impact perfusion recovery in AA mice, although previous studies have demonstrated that NAC treatment may impair collateral vessel formation in non-diseased mice.13, 26 This discrepancy may be due to the dose used in our study, which may impact the optimal window of ROS levels necessary to promote collateralization. In addition, depletion of neutrophils did not significantly impact collateral vessel formation in the AA mice, a finding that is perhaps unsurprising, given that these cells are rapidly cleared in the ischemic limbs of the AA mice.
Our study does not address the potential contribution of ROS by other important cellular sources such as macrophages, smooth muscle cells and endothelial cells. Here, we focused on neutrophils given the striking histological finding of excessive neutrophil infiltration in ischemic sickle cell tissue and the well-documented observation of neutrophil activation in sickle cell disease.34, 35 In our experiments, antioxidant therapy improved collateral formation with significantly greater inhibition of ROS compared to neutrophil depletion alone, suggesting that other cell types, such as macrophages, contribute to ROS and impaired collateral formation in SS mice. Further work is required to identify the role of these cells, as they interact intimately with neutrophils in propagating inflammation in SCD and other inflammatory conditions. Indeed, neither neutrophil depletion nor NAC therapy improved collateral vessel formation to the level of AA mice. This clearly underscores the complex mechanisms involved in functional collateral formation after ischemia; other aspects of collateral formation may be differentially regulated in the SS compared to the AA. Additionally work is required is also required to delineate the mechanisms of the enhanced inflammatory and oxidative response of neutrophils from the SS mice, as well as the perplexing finding of persistent neutrophils within the ischemic tissues for several weeks after vascular injury. Understanding these mechanisms might provide novel strategies to promote the resolution of inflammation in SCD.
In summary, our study shows a previously unappreciated deficit in functional collateral vessel formation in SCD after vascular injury. Neutrophil-mediated excessive ROS production plays a critical role in this deficiency. In recent years, it has become apparent that small and large vessel complications of SCD, such as strokes, Moyamoya syndrome, cardiomyopathy and sudden cardiac deaths8, 27, 36 are not merely secondary to red cell biology, but also inflammatory pathophysiology. These vascular complications are likely to increase as the life expectancy of sickle cell patients improve with emerging therapies. Targeting neutrophils and oxidative stress may improve the neurovascular and cardiovascular consequences of SCD.
Supplementary Material
Highlights.
Sickle cell mice demonstrate impaired ability to form collateral vessels after ischemia.
This is secondary to excessive oxidative stress mediated by neutrophils.
Elimination of neutrophils and oxidative stress improved collateral formation.
Acknowledgments
The authors of this study would like to thank the Emory Pediatric Flow Cytometry Core for their technical support during this project.
Sources of Funding: This work was supported by NIH RO1 HL131414, NIH 2P01 HL095070 and a seed grant from the Children’s Heart Research and Outcomes Center of Children’s Healthcare of Atlanta and Emory University.
Non Standard Abbreviation
- SCD
Sickle cell disease
- SS
Sickle cell
- AA
Wild type
- HLI
Hind limb ischemia
- ROS
Reactive oxygen species
Footnotes
Disclosure: WR Taylor was a co-developer of hydrocyanine dyes and is eligible for royalties related to their sale.
References
- 1.Weatherall DJ, Clegg JB. Inherited haemoglobin disorders: an increasing global health problem. Bulletin of the World Health Organization. 2001;79:704–12. [PMC free article] [PubMed] [Google Scholar]
- 2.Malowany JI, Butany J. Pathology of sickle cell disease. Seminars in diagnostic pathology. 2012;29:49–55. doi: 10.1053/j.semdp.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Hyacinth HI, Sugihara CL, Spencer TL, Archer DR, Shih AY. Higher prevalence of spontaneous cerebral vasculopathy and cerebral infarcts in a mouse model of sickle cell disease. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17732275. 271678X17732275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. The Journal of clinical investigation. 2017;127:750–760. doi: 10.1172/JCI89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francis RB. Large-vessel occlusion in sickle cell disease: pathogenesis, clinical consequences, and therapeutic implications. Medical hypotheses. 1991;35:88–95. doi: 10.1016/0306-9877(91)90029-x. [DOI] [PubMed] [Google Scholar]
- 6.Chopdar A. Multiple major retinal vascular occlusions in sickle cell haemoglobin C disease. The British journal of ophthalmology. 1975;59:493–6. doi: 10.1136/bjo.59.9.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhury NA, DeBaun MR, Ponisio MR, Jordan LC, Rodeghier M, Pruthi S, McKinstry RC. Intracranial vasculopathy and infarct recurrence in children with sickle cell anaemia, silent cerebral infarcts and normal transcranial Doppler velocities. British journal of haematology. 2017 doi: 10.1111/bjh.14979. [DOI] [PMC free article] [PubMed]
- 8.Gladwin MT. Cardiovascular complications and risk of death in sickle-cell disease. Lancet. 2016;387:2565–74. doi: 10.1016/S0140-6736(16)00647-4. [DOI] [PubMed] [Google Scholar]
- 9.Kato GJ, McGowan V, Machado RF, Little JA, Taylor Jt, Morris CR, Nichols JS, Wang X, Poljakovic M, Morris SM, Jr, Gladwin MT. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107:2279–85. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopp HG, Ramos CA, Rafii S. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Current opinion in hematology. 2006;13:175–81. doi: 10.1097/01.moh.0000219664.26528.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirculation. 2003;10:83–97. doi: 10.1038/sj.mn.7800173. [DOI] [PubMed] [Google Scholar]
- 12.Urao N, Sudhahar V, Kim SJ, Chen GF, McKinney RD, Kojda G, Fukai T, Ushio-Fukai M. Critical role of endothelial hydrogen peroxide in post-ischemic neovascularization. PloS one. 2013;8:e57618. doi: 10.1371/journal.pone.0057618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodara R, Weiss D, Joseph G, Velasquez-Castano JC, Landazuri N, Han JW, Yoon YS, Taylor WR. Overexpression of catalase in myeloid cells causes impaired postischemic neovascularization. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2203–9. doi: 10.1161/ATVBAHA.111.233247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyle AN, Joseph G, Fan AE, Weiss D, Landazuri N, Taylor WR. Reactive oxygen species regulate osteopontin expression in a murine model of postischemic neovascularization. Arterioscler Thromb Vasc Biol. 2012;32:1383–91. doi: 10.1161/ATVBAHA.112.248922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxidants & redox signaling. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 16.Muhs BE, Gagne P, Plitas G, Shaw JP, Shamamian P. Experimental hindlimb ischemia leads to neutrophil-mediated increases in gastrocnemius MMP-2 and -9 activity: a potential mechanism for ischemia induced MMP activation. The Journal of surgical research. 2004;117:249–54. doi: 10.1016/j.jss.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Lum AF, Wun T, Staunton D, Simon SI. Inflammatory potential of neutrophils detected in sickle cell disease. American journal of hematology. 2004;76:126–33. doi: 10.1002/ajh.20059. [DOI] [PubMed] [Google Scholar]
- 18.Wu LC, Sun CW, Ryan TM, Pawlik KM, Ren J, Townes TM. Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood. 2006;108:1183–8. doi: 10.1182/blood-2006-02-004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–59. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radu M, Chernoff J. An in vivo assay to test blood vessel permeability. J Vis Exp. 2013:e50062. doi: 10.3791/50062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan TM, Ciavatta DJ, Townes TM. Knockout-transgenic mouse model of sickle cell disease. Science. 1997;278:873–6. doi: 10.1126/science.278.5339.873. [DOI] [PubMed] [Google Scholar]
- 22.Kawamura I, Takemura G, Kanamori H, Takeyama T, Kawaguchi T, Tsujimoto A, Goto K, Maruyama R, Watanabe T, Shiraki T, Aoyama T, Fujiwara T, Fujiwara H, Minatoguchi S. Repeated phlebotomy augments angiogenesis to improve blood flow in murine ischemic legs. American journal of physiology Heart and circulatory physiology. 2010;299:H372–8. doi: 10.1152/ajpheart.00035.2010. [DOI] [PubMed] [Google Scholar]
- 23.Rastmanesh R. Possibility of enhanced risk of retinal neovascularization in repeated blood donors: blood donation and retinal alteration. International journal of general medicine. 2011;4:647–56. doi: 10.2147/IJGM.S23206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luders F, Engelbertz C, Meyborg M, Freisinger E, Malyar NM, Zeller T, Reinecke H. Acute and chronic anemia and short- and long-term outcome of patients with peripheral arterial disease and critical limb ischemia. European journal of internal medicine. 2016;31:62–7. doi: 10.1016/j.ejim.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ, Burk RD, Kunisaki Y, Jang JE, Scheiermann C, Merad M, Frenette PS. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525:528–32. doi: 10.1038/nature15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebrahimian TG, Heymes C, You D, Blanc-Brude O, Mees B, Waeckel L, Duriez M, Vilar J, Brandes RP, Levy BI, Shah AM, Silvestre JS. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am J Pathol. 2006;169:719–28. doi: 10.2353/ajpath.2006.060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JY, Choi YH, Cheon JE, Paeng JC, Ryu HW, Kim KJ, Phi JH, Wang KC, Cho BK, Chae JH, Kim SK. Delayed posterior circulation insufficiency in pediatric moyamoya disease. J Neurol. 2014;261:2305–13. doi: 10.1007/s00415-014-7484-7. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. Jama. 2001;286:1317–24. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 29.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease. Life expectancy and risk factors for early death. The New England journal of medicine. 1994;330:1639–44. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 30.Guilliams KP, Fields ME, Ragan DK, Chen Y, Eldeniz C, Hulbert ML, Binkley MM, Rhodes JN, Shimony JS, McKinstry RC, Vo KD, An H, Lee JM, Ford AL. Large-Vessel Vasculopathy in Children With Sickle Cell Disease: A Magnetic Resonance Imaging Study of Infarct Topography and Focal Atrophy. Pediatr Neurol. 2017;69:49–57. doi: 10.1016/j.pediatrneurol.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haddad P, Dussault S, Groleau J, Turgeon J, Michaud SE, Menard C, Perez G, Maingrette F, Rivard A. Nox2-containing NADPH oxidase deficiency confers protection from hindlimb ischemia in conditions of increased oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1522–8. doi: 10.1161/ATVBAHA.109.191437. [DOI] [PubMed] [Google Scholar]
- 32.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–55. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 33.Soehnlein O, Steffens S, Hidalgo A, Weber C. Neutrophils as protagonists and targets in chronic inflammation. Nature reviews Immunology. 2017;17:248–261. doi: 10.1038/nri.2017.10. [DOI] [PubMed] [Google Scholar]
- 34.Kangne HK, Jijina FF, Italia YM, Jain DL, Nadkarni AH, Gupta M, Pradhan V, Mukesh RD, Ghosh KK, Colah RB. The Fc receptor polymorphisms and expression of neutrophil activation markers in patients with sickle cell disease from Western India. Biomed Res Int. 2013;2013:457656. doi: 10.1155/2013/457656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lard LR, Mul FP, de Haas M, Roos D, Duits AJ. Neutrophil activation in sickle cell disease. J Leukoc Biol. 1999;66:411–5. doi: 10.1002/jlb.66.3.411. [DOI] [PubMed] [Google Scholar]
- 36.Bakeer N, James J, Roy S, Wansapura J, Shanmukhappa SK, Lorenz JN, Osinska H, Backer K, Huby AC, Shrestha A, Niss O, Fleck R, Quinn CT, Taylor MD, Purevjav E, Aronow BJ, Towbin JA, Malik P. Sickle cell anemia mice develop a unique cardiomyopathy with restrictive physiology. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E5182–91. doi: 10.1073/pnas.1600311113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.