CASE PRESENTATION
Chief complaint
Anorexia, weight loss, periorbital edema, abdominal distension
History of the present illness
A previously healthy 2-year-old Guatemalan female with an undiagnosed chronic illness characterized by fever and rash had presented with anorexia, weight loss, periorbital edema, abdominal pain and distention. A chest radiograph documented cardiomegaly. An echocardiogram demonstrated a pericardial effusion, dilated right atrium, right ventricle, and main pulmonary artery as well as diminished right ventricular systolic function and pulmonary hypertension. Right ventricular systolic pressure was estimated at 90 mmHg. Computed tomography of her chest was performed. There was no evidence of interstitial lung disease but changes included dilated pulmonary arteries consistent with the presence of pulmonary hypertension as well as acute thrombosis of the superior vena cava and left brachiocephalic vein. These findings suggested thromboembolism as the etiology of pulmonary hypertension. A ventilation/perfusion lung scan also suggested differential lung perfusion. She was placed on systemic anticoagulation transiently. The presence of antiphospholipid antibodies was documented as described below. Rheumatology consultation was requested for consideration of a possible autoimmune/inflammatory etiology.
The past medical, social, and family history
She had been born at term following a cesarean delivery due to pregnancy induced hypertension. She had received her two month vaccines without complications.
At 4 months of age she was evaluated for erythema and distal swelling of a finger and a toe. There was no history of fever or trauma. Basic laboratory evaluation was completed (as depicted in Table 1) as well as magnetic resonance (MR) imaging (as depicted in Figure 1). Anti-nuclear antibody and anti-double stranded DNA antibody serologies were negative. A blood culture was also negative. A presumptive diagnosis of cellulitis was made. Distal swelling resolved with antibiotic administration.
Table 1.
Hematology and chemistry test results for patient on first evaluation at 2 years of age
| Test | Value | Normal Range | |
|---|---|---|---|
| Complete Blood Cell Count | |||
| White blood cell count (k/uL) | 5.5 | 3.9–13.7 | |
| Hemoglobin (g/dL) | 14.3 | 10.2–15.4 | |
| Hematocrit (%) | 46.7 | 32.1–40.9 | |
| Platelet (k/uL) | 151 | 150–450 | |
| Chemistry | |||
| Glucose (mg/dL) | 70 | 65–110 | |
| Sodium (mmol/L) | 139 | 132–143 | |
| Potassium (mmol/L) | 4.6 | 3.2–5.7 | |
| Chloride (mmol/L) | 111 | 98–116 | |
| Blood Urea Nitrogen (mg/dL) | 11 | 5–27 | |
| Creatinine (mg/dL) | 0.45 | 0.3–1.00 | |
| Total protein (g/dL) | 7.2 | 5.2–7.4 | |
| Albumin (g/dL) | 2.6 | 3.1–4.8 | |
| Aspartate Transaminase (U/L) | 60 | 18–63 | |
| Alanine Transaminase (U/L) | 17 | 10–32 | |
| Erythrocyte Sedimentation Rate (mm/hr) | 18 | 0–10 | |
| C-Reactive Protein (mg/dL) | 0.79 | 0–0.75 | |
| Other | |||
| C3 (mg/dL) | 109 | 90–180 | |
| C4 (mg/dL) | 9 | 16–47 | |
| Anti-cardiolipin IgA (units/mL) | 22 | <10 | |
| Anti-phosphatidylserine IgG (units/mL) | 17 | <11 | |
| Anti-phosphatidylserine IgM (units/mL) | 66 | <25 | |
| Anti-β2 glycoprotein 1 IgG (units/mL) | 24 | <21 | |
| Anti-β2 glycoprotein 1 IgA (units/mL) | 22 | <21 | |
Figure 1.
Magnetic resonance imaging (T1 weighted) of left hand demonstrating diffuse soft tissue edema.
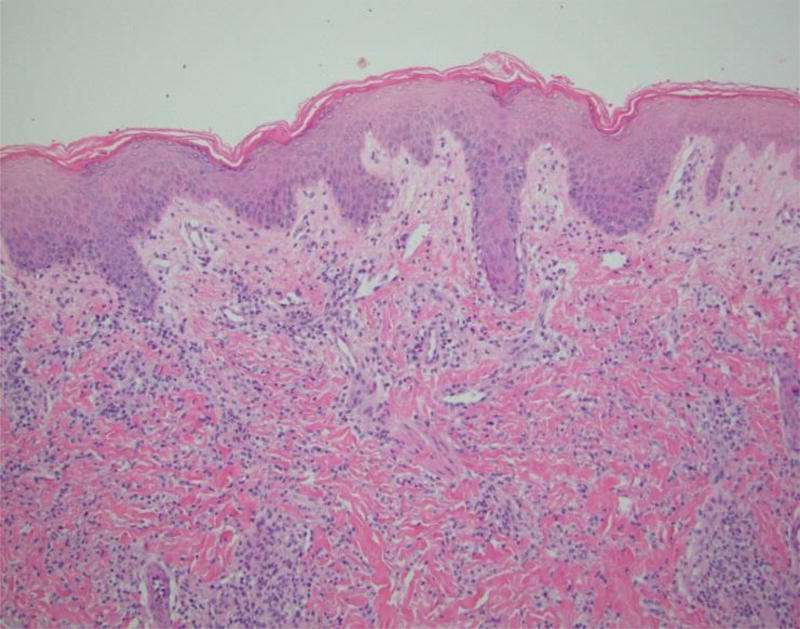
At 7 months of age she returned with fever, irritability, and erythematous nodular lesions that involved her face, trunk, abdomen, and extremities. The lesions would appear and then resolve without intervention every 1–2 weeks. A skin biopsy (as depicted below in Figure 2) was completed which demonstrated a deep dermal interstitial neutrophilic and histiocytic infiltrate suggestive of neutrophilic dermatosis/Sweet syndrome. The biopsy was of insufficient depth to evaluate the subcutaneous fat for neutrophilic panniculitis. A bone marrow examination documented a normocellular bone marrow with trilineage hematopoiesis and a normal cytogenetic evaluation. Corticosteroids were provided with apparent clinical resolution. Despite attempts at weaning her corticosteroids off, chronic corticosteroid treatment was required to treat the inflammatory condition. She continued to suffer from recurrent rash and subcutaneous edema suggestive of neutrophilic dermatosis.
Figure 2.
Histopathology of lesional skin in patient - H&E staining. Dense perivascular and interstitial mononuclear dermal infiltrate – 1st panel. Areas of karyorrhexis are seen in lesional skin – 2nd panel.
Histopathology of lesional skin in patient - H&E staining. Dense perivascular and interstitial mononuclear dermal infiltrate – 1st panel. Areas of karyorrhexis are seen in lesional skin – 2nd panel.
She lived with her mother and father as well as her maternal grandmother and aunt. Her family history was notable for consanguinity with parents being first degree cousins. She had two healthy biological siblings. The family is of Guatemalan ethnicity. There is no pertinent family medical history of autoimmune disorders or rheumatologic disorders.
Her medications included corticosteroids at 1 mg/kg/day in combination with omeprazole to prevent gastric irritation. Her pulmonary hypertension medications included amlodipine, captopril, remodulin, lasix, sildenafil, bosentan, and intermittent milrinone infusions. She also remained on aspirin secondary to her history of thrombosis and the presence of antiphospholipid antibodies.
Physical examination
The patient’s vital signs were normal; she appeared to be comfortable and in no acute distress. A central catheter was in place. Growth parameters were significant for severe linear growth retardation. Facial exam was abnormal with an appearance suggestive of lipodystrophy affecting the temporal areas as well as below the zygomatic bone. Her eye, ear, nose, and throat exam were unremarkable. No cervical adenopathy was present. Her lungs were clear to auscultation. Her cardiac exam demonstrated a regular rate and rhythm with no murmurs, rubs, or gallops. Her abdomen appeared protuberant without hepatosplenomegaly. The musculoskeletal exam was significant for absence of synovitis, muscle pain, edema or other extremity changes including joint contractures. No pitting of her nails was noted, and nail bed capillaroscopy was normal. Her skin exam demonstrated grouped patches of an elevated annular rash as well as erythroderma on her upper arms and torso which was non-tender to palpation. No evidence of scarring was present.
Laboratory evaluation
The results of the patient’s initial laboratory evaluation at 2 years of age are depicted in Table 1. At the time of her diagnosis with pulmonary hypertension at 2 years of age, her anti-nuclear antibodies were slightly elevated at 8 IU/mL (normal < 7.5) and she tested positive for anti-double strand DNA antibodies (40 IU/mL; normal <5). Additional laboratory testing included negative extractable nuclear antigen serologies such as anti-SM antibodies, anti-RNP antibodies, anti-SSA and anti-SSB antibodies. Studies for a lupus anticoagulant were negative.
CASE SUMMARY
This is a 2-year-old Guatemalan female from a consanguineous union with failure to thrive, facial lipodystrophy, recurrent non-periodic fevers associated with neutrophilic dermatosis lesions and primary pulmonary hypertension with presumed recurrent thrombosis in the setting of modestly elevated anti-phospholipid antibodies.
DIFFERENTIAL DIAGNOSIS
Sweet syndrome
Sweet syndrome is a rare disorder in children as documented by the limited number (<100 cases) reported in the medical literature (1). The clinical features of Sweet syndrome include the development of tender or painful plaques on the face, hands, arms, or upper trunk (2). These eruptions are typically acute in onset and often occur following an antecedent upper respiratory tract infection (3). They are often associated with systemic features including leukocytosis, fever, arthralgias, and malaise. Histologic examination of the skin plaques demonstrates diffuse neutrophilic infiltrates in the dermis as was documented in our case. Fragmentation of leukocytes or so-called “karyorrhexis” is present without evidence of primary leukocytoclastic vasculitis. Sweet syndrome is often idiopathic although it may be associated with neoplastic disorders, infectious etiologies, pregnancy, and specific drugs (4–7). Sweet syndrome has been associated with autoimmune disorders, particularly systemic lupus erythematosis (SLE) (8). Clinical and laboratory findings were neither suggestive nor diagnostic of SLE; however, anti-dsDNA antibodies were detected. Moreover, Sweet syndrome has not been previously associated with pulmonary hypertension. Treatments of Sweet syndrome typically include systemic corticosteroids which lead to dramatic improvement although relapses are frequent (9–10). Colchicine may be effective as a maintenance or steroid-sparing agent (9–10). Other immunomodulatory agents have been utilized effectively (9–10).
Autoinflammatory diseases including the cryopyrin associated periodic syndromes (CAPS)
CAPS is caused by autosomal dominant mutations in NOD-like receptor family, pyrin domain containing 3 (NLRP3) that assembles an IL-1 activating inflammasome. Mutations in NLRP3 cause a spectrum of CAPS associated with systemic inflammation and recurrent fevers, neutrophilic urticaria, arthralgias, and elevations of acute phase reactants. Although many features of our case had overlap with CAPS (e.g., systemic inflammation, recurrent fevers, etc.) some clinical features (e.g., lipodystrophy) could not be explained by CAPS. Familial cold autoinflammatory syndrome is inherited in an autosomal dominant fashion and is associated with symptoms that are often triggered by cold (11–12). Muckle-Wells syndrome is also inherited in an autosomal dominant manner with similar symptoms of recurrent fever, urticaria, and arthralgias; however, renal amyloid deposition and deafness are also notable findings (11–13). Chronic Infantile neurological cutaneous and articular syndrome (CINCA) or Neonatal Onset Multisystem Inflammatory Disease (NOMID) is the most severe form with a perinatal onset of symptoms including recurrent fever, urticaria, and arthralgias (11–12, 14). Although all three severity phenotypes of CAPS can occur sporadically caused by de novo mutations, almost all cases of NOMID are caused by de novo mutations. In addition to the inflammatory features described above, NOMID patients have severe arthropathy and central nervous system involvement including aseptic meningitis and mental retardation. Progressive hearing loss starting in the first decade of life and progressive vision loss due to optic nerve atrophy are common.
Proteasome Associated Autoinflammatory syndromes (PRAAS)/Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperatures (CANDLE)
PRAAS/CANDLE is inherited in an autosomal recessive fashion. It is associated with recessive loss-of-function mutations in the PSBM8 gene or digenic mutations in two proteasome components that results in an inflammatory disease by upregulating and perpetuating Type I interferon dysregulation that is manifest by the chronic presence of an IFN response gene signature in the blood (15–16). Several names have been given to patients with PRAAS including Nakajo-Nishimura syndrome (NNS), and Japanese Autoinflammatory syndrome with Lipodystrophy (JASL) to describe the disease in Japan (17–18, 19) and panniculitis induced lipodystrophy (JMP) syndrome (20–21) or CANDLE syndrome (16, 22) in the United States and Spain. However, the identification of the genetic causes revealed that the descriptions are disease manifestations of the same disease now referred to as PRAAS or PRAAS/CANDLE. Patients present with recurrent fevers, nodular erythema, and often with arthritis. The presence of autoantibodies, anemia, elevations in acute phase reactants, and hypergammaglobulinemia are common but more variable, depending on disease severity (17–18, 19). Disease manifestations of chronic disease that have resulted in organ damage include joint contractures and muscle atrophy. Most of these features can be seen in childhood in patients with severe disease (20). Many patients develop hepatosteatosis in childhood. Fevers are mainly low-grade and may not be a prominent feature of the disease. Erythematous skin lesions are frequently seen and growth failure is common. Patients can present with lymphocytic meningitis; and neurological changes including seizures and neurocognitive deficits have been described. It is important to point out that the neutrophils in PRAAS/CANDLE are immature and the infiltrate is in areas (i.e.subcutaneuos fat) where Sweet syndrome rashes do not appear. Unfortunately, our biopsy was not adequate to enough to discern the presence of immature neutrophillic infiltrates in areas such as subcutaneous fat.
DIAGNOSIS
Given the phenotypic overlap of her clinical presentation with CANDLE/PRAAS syndrome, targeted sequencing of the PSMB8 gene was preformed which documented homozygous missense PSMB8 mutations c.224C>T substituting threonine with methionine at position 75 (T75M) in the PSMB8 gene, which is a founder mutation in Hispanic patients.
DISCUSSION
CANDLE/PRAAS syndrome is a autoinflammatory disorder with clinical features including recurrent fever, violaceous periorbital changes, panniculitis induced lipodystrophy, arthralgias, cytopenias, myositis, dyslipidemia, systemic hypertension, severe growth retardation, elevated acute phase reactants, and a spectrum of other findings (16,22–23). A founder mutation (G201V) in Japanese patients has also been described (19,24). Homozygous loss of function mutations in the proteasome subunit beta type-8 (PSMB8) gene are disease-causing. In our case, astute clinical evaluation coupled with targeted sequencing supported the assignment of a accurate diagnosis. Although targeted sequencing allows for the accurate analysis of specific genes or regions of interest a variety of next generation sequencing approaches exist including targeted sequencing panels and exome analysis. Next generation sequencing approaches are being employed widely in the diagnostic evaluation of patients with complex disorders including autoinflammatory disorders (25).
Proteasomes are large protein complexes located in the cytoplasm and nucleus that function to degrade intracellular proteins (26). They are subject to activation and inhibition by a complex network of regulatory proteins that serve to regulate the proteolytic capacity of the proteasome (26). Disorders affecting the immunoproteasome have been described under different pseudonymes (17–18). Defective proteasome function is hypothesized to lead to accumulation of damaged proteins, resulting in increased cellular stress, and increased interferon signaling (15, 27). Although the role of inflammation in pulmonary hypertension is established (28), a mechanistic understanding of the role of interferon dysregulation in endothelial-cell dysfunction and its role in the development of pulmonary vascular pathology is unknown. A causative role of increased IFN signaling in CANDLE has been assessed in the context of a compassionate use study using Janus kinases (JAK) inhibition (NCT 01724580), with preliminary encouraging results (29).
To our knowledge, this report is the first description of pulmonary hypertension in a patient with CANDLE syndrome, although a second patient has been identified at the National Institutes of Health (NIH), who did not have thromboembolic disease (personal communication). Pulmonary hypertension has been described in another presumed IFN-mediated autoinflammatory disorder, STING-associated vasculopathy with onset in infancy (SAVI) in the context of interstitial lung disease (30), which however is absent in our patient. In CANDLE syndrome, loss of function in the immunoproteasome leads to dysregulated interferon signaling which may have downstream effects on endothelial cell function or may affect coagulation homeostasis. These derangements may impact vascular remodeling leading to the subsequent development of pulmonary hypertension. Greater understanding of this process in IFN mediated autoinflammatory disorders may shed light on mechanisms that lead to pulmonary hypertension not only in autoinflammatory diseases but also in systemic autoimmune diseases.
Notably, our patient had radiographic evidence of thrombosis and antiphospholipid antibodies which were present at low titers. The degree to which this contributed to the development of pulmonary hypertension remains unknown as the other patient with pulmonary hypertension had no evidence of thromboembolic disease nor the presence of autoantibodies. The presence of antiphospholipid antibodies in specific autoimmune disorders is associated with increased risk for the development of pulmonary hypertension. Hypothesized mechanism include thrombosis and remodeling of vascular endothelium (30). This suggests a potential effect of proteasome dysfunction on primary endothelial dysfunction likely aggravated in the context of additional risk factors such as the development of anticardiolipin antibodies which are known to disrupt endothelial function and lead to platelet activation (32).
THE PATIENT’S COURSE
From 2 years of age until 6 years of age she continued to experience exacerbations in her nodular erythematous rash with attempts to taper the corticosteroid daily dose as well as intermittent fevers and extremity pain. Periorbital edema and lipoatrophy of her face and extremities had developed. Occasional exacerbations in her pulmonary hypertension have been managed with the aforementioned vasoactive medications. At 6 years of age, our patient continued to remain corticosteroid dependent (0.6 mg/kg/day) despite a trial of cyclosporine and the introduction of colchicine. She had developed evidence of corticosteroid toxicity including growth failure and cataracts.
Given the encouraging results observed in CANDLE patients on treatment with the JAK inhibitor baricitinib, (29, 33), and concern for hemodynamic instability made participation in the compassionate use study at the NIH impossible, we initiated treatment at 7 years of age with the commercially available JAK inhibitor, tofacitininb, that has been approved by the FDA for the treatment of adult patients with rheumatoid arthritis. Tofacitinib is an oral small molecule inhibitor of the JAK1 and JAK3 kinases (34) and its use in CANDLE patients has not been reported. A dose of 2.5 mg by mouth twice a day using a liquid suspension prepared by dissolving a half of a tablet was chosen based on upon dosing utilized in an ongoing study focused on the use of tofacitinib in the treatment of pediatric polyarticular juvenile idiopathic arthritis (NCT 02592434). After 9 months of therapy with tofacitinib no apparent adverse effects were noted and there were no significant intercurrent infections. No elevation of her low-density lipoprotein level, transaminases, or creatinine had been observed and there was no neutropenia. Although limited by few data points we noted a decreasing trend in her ESR and CRP as depicted in Table 2. Despite clinical improvement (e.g. resolution of her rash, fever and arthritis) on weaning doses of steroids (from 0.6 mg/kg/day to 0.4 mg/kg/day), she had no improvement in the extent of pulmonary hypertension, suggesting that much of her pulmonary vascular pathology was irreversible. Unfortunately, she died suddenly and emergent investigations did not reveal a cause of the sudden event. Post-mortem evaluation was not completed. The potential of oral JAK inhibitors to alter the course of pulmonary hypertension in CANDLE patients particularly when initiated early in their life remains to be investigated. Moreover, additional evaluation regarding differential efficacy of various JAK inhibitors and clinical phenotypes of CANDLE/PRAAS is unknown and warrants further investigation.
Table 2.
ESR and CRP Value Pre-Tofacitinib and Post-Tofacitinib
| Pre-Tofacitinib | 2 months Post |
7 months Post |
10 months Post |
Normal | |
|---|---|---|---|---|---|
| Marker | |||||
| ESR (mm/hr) | 85 | 70 | 101 | NA | 0–22 |
| CRP (mg/L) | 108 | 22 | 26.5 | 13.3 | 0–10 |
FINAL DIAGNOSIS
CANDLE/PRAAS syndrome
Acknowledgments
This research was supported by the Intramural Research Program of the NIH and NIAID.
Contributor Information
David Buchbinder, Division of Hematology, CHOC Children’s Hospital, 1201 W. La Veta Ave., Orange, CA 92868, Work phone 714 / 509 – 8459, Fax 714 / 509 – 8771, dbuchbinder@choc.org.
Gina A. Montealegre Sanchez, NIH Translational Autoinflammatory Disease Studies/NIAID, Bldg.10, room 11C436, 10 Center Drive, Work phone 301 / 761-7747, gina.montealegre@nih.gov.
Raphaela Goldbach-Mansky, NIH Translational Autoinflammatory Disease Studies/NIAID, Bldg.10, room 11C205, 10 Center Drive, Bethesda, MD 20892, Work phone 301 / 761-7553, goldbacr@mail.nih.gov.
Hermine Brunner, Division of Rheumatology, Cincinnati Children’s Hospital, 3333 Burnet Avenue, Cincinnati, Ohio 45229-3026, Work phone 513 / 636-6320, hermine.brunner@cchmc.org.
Andrew I. Shulman, Division of Rheumatology, CHOC Children’s Hospital, 1201 W. La Veta Ave., Orange, CA 92868, Work phone 714 / 509 – 8617, ashulman@choc.org.
References
- 1.García-Romero MT, Ho N. Pediatric Sweet syndrome. A retrospective study. Int J Dermatol. 2015;54(5):518–22. doi: 10.1111/ijd.12372. [DOI] [PubMed] [Google Scholar]
- 2.Cohen PR. Sweet's syndrome--a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34, 1–28. doi: 10.1186/1750-1172-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos TB, Sales BC, Sigres M, Rosman F, Cerqueira AM. Sweet Syndrome in childhood. An Bras Dermatol. 2015;90(4):567–9. doi: 10.1590/abd1806-4841.20153247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raza S, Kirkland RS, Patel AA, Shortridge JR, Freter C. Insight into Sweet's syndrome and associated-malignancy: a review of the current literature. Int J Oncol. 2013;42(5):1516–22. doi: 10.3892/ijo.2013.1874. [DOI] [PubMed] [Google Scholar]
- 5.Fortna RR, Toporcer M, Elder DE, Junkins-Hopkins JM. A case of sweet syndrome with spleen and lymph node involvement preceded by parvovirus B19 infection, and a review of the literature on extracutaneous sweet syndrome. Am J Dermatopathol. 2010;32(6):621–7. doi: 10.1097/DAD.0b013e3181ce5933. [DOI] [PubMed] [Google Scholar]
- 6.Cohen PR. Pregnancy-associated Sweet's syndrome: world literature review. Obstet Gynecol Surv. 1993;48(8):584–7. doi: 10.1097/00006254-199308000-00027. [DOI] [PubMed] [Google Scholar]
- 7.Hoelt P, Fattouh K, Villani AP. Dermpath & Clinic: Drug-induced Sweet syndrome. Eur J Dermatol. 2016;26(6):641–642. doi: 10.1684/ejd.2016.2928. [DOI] [PubMed] [Google Scholar]
- 8.Miyauchi T, Nishie W, Sakata M, Osawa R, Noguchi A, Shimizu H. Sweet syndrome-like eruption with prominent dermal leukocytoclasis associated with systemic lupus erythematosus. J Dermatol. 2015;42(4):442–3. doi: 10.1111/1346-8138.12802. [DOI] [PubMed] [Google Scholar]
- 9.Maalouf D, Battistella M, Bouaziz JD. Neutrophilic dermatosis: disease mechanism and treatment. Curr Opin Hematol. 2015;22(1):23–9. doi: 10.1097/MOH.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 10.Cohen PR. Neutrophilic dermatoses: a review of current treatment options. Am J Clin Dermatol. 2009;10(5):301–12. doi: 10.2165/11310730-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, Kone-Paut I, Goldbach-Mansky R, Lachmann H, et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS) Ann Rheum Dis. 2017;76(6):942–947. doi: 10.1136/annrheumdis-2016-209686. [DOI] [PubMed] [Google Scholar]
- 12.Kuemmerle-Deschner JB. CAPS--pathogenesis, presentation and treatment of an autoinflammatory disease. Semin Immunopathol. 2015;37(4):377–85. doi: 10.1007/s00281-015-0491-7. [DOI] [PubMed] [Google Scholar]
- 13.Scarpioni R, Rigante D, Cantarini L, Ricardi M, Albertazzi V, Melfa L, et al. Renal involvement in secondary amyloidosis of Muckle-Wells syndrome: marked improvement of renal function and reduction of proteinuria after therapy with human anti-interleukin-1β monoclonal antibody canakinumab. Clin Rheumatol. 2015;34(7):1311–6. doi: 10.1007/s10067-013-2481-2. [DOI] [PubMed] [Google Scholar]
- 14.Goldbach-Mansky R. Current status of understanding the pathogenesis and management of patients with NOMID/CINCA. Curr Rheumatol Rep. 2011;13(2):123–31. doi: 10.1007/s11926-011-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brehm A, Liu Y, Sheikh A, Marrero B, Omoyinmi E, Zhou Q, et al. Additive loss-of-function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J Clin Invest. 2016;126(2):795. doi: 10.1172/JCI86020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Ramot Y, Torello A, Paller AS, Si N, Babay S, et al. Mutations in proteosome subunit β type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum. 2012;64(3):895–907. doi: 10.1002/art.33368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott A, Jacks J, Kessler M, Emanuel PD, Gao L. Proteasome-associated autoinflammatory syndromes: advances in pathogeneses, clinical presentations, diagnosis, and management. Int J Dermatol. 2015;54(2):121–9. doi: 10.1111/ijd.12695. [DOI] [PubMed] [Google Scholar]
- 18.Almeida de Jesus A, Goldbach-Mansky R. Monogenic autoinflammatory diseases: concept and clinical manifestations. Clin Immunol. 2013;147(3):155–74. doi: 10.1016/j.clim.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arima K, Kinoshita A, Mishima H, Kanazawa N, Kaneko T, Mizushima T, et al. Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome. Proc Natl Acad Sci U S A. 2011;108(36):14914–9. doi: 10.1073/pnas.1106015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal AK, Xing C, DeMartino GN, Mizrachi D, Hernandez MD, Sousa AB, et al. PSMB8 encoding the β5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am J Hum Genet. 2010;87(6):866–72. doi: 10.1016/j.ajhg.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garg A, Hernandez MD, Sousa AB, Subramanyam L, Martinez de Villarreal L, dos Santos HG, et al. An autosomal recessive syndrome of joint contractures, muscular atrophy, microcytic anemia, and panniculitis-associated lipodystrophy. J Clin Endocrinol Metab. 2010;95(9):E58–63. doi: 10.1210/jc.2010-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torrelo A, Patel S, Colmenero I, Gurbindo D, Lendinez F, Hernandez A, et al. Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome. J Am Acad Dermatol. 2010;62(3):489–95. doi: 10.1016/j.jaad.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 23.Ramot Y, Czarnowicki T, Maly A, Navon-Elkan P, Zlotogorski A. Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature syndrome: a case report. Pediatr Dermatol. 2011;28(5):538–41. doi: 10.1111/j.1525-1470.2010.01163.x. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura A, Maekawa Y, Uehara H, Izumi K, Kawachi I, Nishizawa M, et al. A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. J Clin Invest. 2011;121(10):4150–60. doi: 10.1172/JCI58414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oda H, Kastner DL. Genomics, Biology, and Human Illness: Advances in the Monogenic Autoinflammatory Diseases. Rheum Dis Clin North Am. 2017;43(3):327–345. doi: 10.1016/j.rdc.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka K, Mizushima T, Saeki Y. The proteasome: molecular machinery and pathophysiological roles. Biol Chem. 2012;393(4):217–34. doi: 10.1515/hsz-2011-0285. [DOI] [PubMed] [Google Scholar]
- 27.Seifert U, Bialy LP, Ebstein F, Bech-Otschir D, Voigt A, Schroter F, et al. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell. 2010;142(4):613–24. doi: 10.1016/j.cell.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 28.George PM, Oliver E, Dorfmuller P, Dubois OD, Reed DM, Kirkby NS, et al. Evidence for the Involvement of Type I Interferon in Pulmonary Arterial Hypertension. Circ Res. 2014;114(4):677–688. doi: 10.1161/CIRCRESAHA.114.302221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montealegre G, Reinhardt A, Brogan P, Berkun Y, Zlotogorski A, Brown D, et al. Preliminary response to Janus kinase inhibition with baricitinib in chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperatures (CANDLE) Pediatr Rheumatol Online J. 2015;13(Suppl 1):O31. [Google Scholar]
- 30.Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Sanchez GAM, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371(6):507–18. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuily S, Domingues V, Suty-Selton C, Eschwège V, Bertoletti L, Chaouat A, et al. Antiphospholipid antibodies can identify lupus patients at risk of pulmonary hypertension: A systematic review and meta-analysis. Autoimmun Rev. 2017;16(6):576–586. doi: 10.1016/j.autrev.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Lim W. Antiphospholipid syndrome. ASH Education Book. 2013;1:675–80. doi: 10.1182/asheducation-2013.1.675. [DOI] [PubMed] [Google Scholar]
- 33.Jabbari A, Dai Z, Xing L, Cerise JE, Ramot Y, Berkun Y, et al. Reversal of Alopecia Areata Following Treatment With the JAK1/2 Inhibitor Baricitinib. EBioMedicine. 2015;2(4):351–5. doi: 10.1016/j.ebiom.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367(6):495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]