Abstract
Background
Socioeconomic status (SES) disparities in colorectal cancer screening are persistent. Lower education and income are both associated with lower screening rates. Both cognitive (e.g. perceived barriers) and affective (e.g. disgust, fear) decision-making constructs are known determinants of colorectal cancer screening behavior. This study tests the hypothesis that SES may be related to decision-making constructs and that this SES-decision-making construct relation may contribute to explaining the SES-screening behavior disparity.
Method
1841 African American participants, ages 50 and older, completed surveys assessing perceived benefits and barriers to screening, self-efficacy, positive and negative affective associations with colonoscopy, fear of colonoscopy, colorectal cancer knowledge, past screening behavior, and demographics including education and income. Both univariable and multivariable relations of SES to decision-making constructs were examined, as were univariable and multivariable models of the indirect effect of SES on screening via decision-making constructs.
Results
Socioeconomic status was related to both screening compliance and the decision-making constructs. Bootstrap modelling of the indirect effect showed that the total effect of the SES-screening behavior relation included an indirect effect via social-cognitive decision-making constructs.
Conclusion
These findings suggest that cognitive and affective decision-making constructs account for at least some of the SES disparities in colorectal cancer screening behavior. As such, more research is needed to explore the intra-individual-level influences of disparities in colorectal cancer screening. In addition, work is needed to develop effective intervention approaches to address the relation of SES to decision-making constructs.
Keywords: Socioeconomic factors, colonoscopy/utilization, guideline adherence, early detection of cancer/utilization, African American
Socioeconomic status (SES) is associated with colorectal cancer morbidity and mortality. Relative to those with higher SES, individuals with low SES have higher incidence (Doubeni, Laiyemo, Reed, Field, & Fletcher, 2009), are at a later, more advanced stage at diagnosis (Halpern, Pavluck, Ko, & Ward, 2009; Mandelblatt, Andrews, Kao, Wallace, & Kerner, 1996), and have higher mortality rates (Enewold, Horner, Shriver, & Zhu, 2014). The later stage at diagnosis and higher mortality rates may both be due to adherence to colorectal cancer screening recommendations. Lower education and lower income have both been associated with lower adherence to colorectal cancer screening recommendations (Doubeni et al., 2009; Ioannou, Chapko, & Dominitz, 2003; Klabunde et al., 2011; Steele, Rim, Joseph, King, & Seeff, 2013).
Many explanations for SES disparities in screening focus on environmental, structural, and policy factors (Centers for Disease Control and Prevention, 2008; Doubeni et al., 2009). However, undergoing screening is ultimately a behavior (albeit a complex behavior requiring cooperation with other individuals) engaged in by an individual person who makes a decision to be screened (or not to be screened) and then enacts that decision successfully. As such, to the extent that there is a relation between SES and screening compliance, it is important to understand the person side of the person-environment structure of causes for screening behavior.
In this paper, we report analyses examining the relation of SES to known judgment and decision-making factors and whether such relations might account for the underlying association between SES and colonoscopy screening behavior. By a substantial margin, most US individuals who are screened are screened using colonoscopy (Klabunde et al., 2011; Klabunde, King, White, & Plescia, 2013; Sauer, Siegel, Jemal, & Fedewa, 2017).
In this community-based study, African American adults age 50+ reported their education and income, responded to questions assessing a number of social cognitive determinants of cancer screening behavior, and reported on their previous colorectal cancer screening behavior. Using these data, we examined whether there was a relation between SES and the social cognitive screening predictors. We then examined whether the social cognitive predictors were involved in an indirect effect relation between SES and screening uptake.
Socioeconomic Status, Decision Making, and Behavior
A range of cognitive decision making factors are known determinants of screening behavior (Kiviniemi, Bennett, Zaiter, & Marshall, 2011) and are targets for effective screening interventions (Rawl et al., 2012). A common set of constructs, including perceived benefits/barriers, self-efficacy, social norms, and perceived behavioral control are included in many health decision making models (Weinstein, 1993). Cancer/cancer screening knowledge also relates to screening behavior (Crookes, Njoku, Rodriguez, Mendez, & Jandorf, 2014). In addition to cognitive factors, affective factors including colonoscopy-related fear (Sly, Edwards, Shelton, & Jandorf, 2013), embarrassment (Consedine, Ladwig, Reddig, & Broadbent, 2011), and general negative affective associations (Kiviniemi, Jandorf, & Erwin, 2014) are associated with lower rates of colonoscopy screening.
We hypothesize that the SES – colonoscopy relation might involve indirect effect pathways via these decision-making determinants. There are three converging lines of argument that support this hypothesis. First, we know that low SES is associated with multiple health problems and risk factors for these problems (Link & Phelan, 1995). There is evidence that these differences based on SES are greater for health outcomes, which, like screening behavior, are under individual control (Masters, Link, & Phelan, 2015). One of the several pathways through which SES ultimately affects health outcomes is through education’s effect on ability to understand and act upon health communications (Link & Phelan, 1995; Smith et al., 2012; Viswanath et al., 2006; Viswanath & Finnegan, 1996). Lower educational achievement is associated with lower health literacy (Rudd, 2007), and health literacy in turn has been shown to be related to differences in beliefs about screening benefits and barriers (Arnold et al., 2012), comprehension of screening educational materials (Smith et al., 2012), and to screening uptake (Kobayashi, Wardle, & von Wagner, 2014; von Wagner, Semmler, Good, & Wardle, 2009).
Second, there are a number of plausible ways in which SES might shift individuals’ beliefs about cancer and screening. First, some health decision-making constructs are plausibly directly related to education and health-issue knowledge. For example, one’s perceived risk for cancer, a known determinant of screening behavior (Kiviniemi et al., 2011), is dependent on one’s knowledge of risk factors and, beliefs about the relation of risk factors to likelihood of disease. Moreover, lower SES is associated with poorer knowledge about screening procedures (King-Marshall et al., 2016). In addition, SES may affect perceptions of barriers to screening and efficacy for screening since factors such as cost, insurance coverage, and ability to take time off work can serve as both real and perceived barriers to screening uptake (James et al., 2008).
Finally, SES may shift decision making in ways that impact both risk perception and perception of screening benefits. Individuals low in SES tend to focus on shorter time horizons in making decisions (i.e., are more focused on immediate benefits and costs (Haushofer & Fehr, 2014)) and less on future consequences of actions (Whitaker et al., 2011). This is often associated with challenges of immediate life issues and day-to-day practicalities that interfere with individuals’ abilities to consider and plan for future events (Shah, Mullainathan, and Shafir (2012). Given that both cancer risk and the primary benefits of screening are future-oriented whereas the barriers to and costs of screening are more immediate, low SES individuals may be predisposed to selectively focus on those factors associated with lower screening compliance.
There are two previous studies that have shown patterns consistent with our hypotheses. Miles and colleagues (Miles, Rainbow, & von Wagner, 2011) reported that cancer fatalism was higher among lower SES individuals and mediated the relation between SES and engagement in fecal occult blood testing (FOBT). The pattern of relations is consistent with our hypotheses, but cancer fatalism is not typically thought of as a direct input to decision making. Lo et al. (2015) examined SES-FOBT relations and showed that two decision making constructs, barriers and norms, were part of indirect effects accounting for SES-FOBT relations. However, given that only two constructs were examined, this leaves the broader question of the relation of known judgment and decision-making factors unexplored. In addition, colonoscopy is used substantially more frequently than FOBT in the US (Sauer et al., 2017; Shapiro et al., 2012). Thus, it is important to examine these effects for colonoscopy screening.
Current Study
Given the known relation between SES and screening compliance, the known impact of a variety of judgment and decision making factors on screening compliance, and the plausible arguments for why there might be a relation between SES and many of these factors, examining the degree to which SES is related to judgment and decision making factors and whether and how the relation accounts for the known SES-behavior relation is critically important. This paper examines these two overarching factors. First, we examine the extent to which SES is associated with known influences on judgment and decision-making concerning colorectal cancer. Second, we explore to what degree such relations lead to indirect effects accounting for the relation between SES and screening uptake.
Methods
Participants
Participants in the analyses reported here are 2015 African American adults who took part in a larger randomized trial of colorectal cancer screening interventions. Participants were recruited in partnership with faith-based and other community-based organizations in the New York City and Buffalo, NY metropolitan areas. Community partners provided guidance on all aspects of study development. Inclusion criteria required self-identification as African American or Black. The analysis reported here includes participants ages 50 and older and for whom data were available (n=1841) or could be successfully imputed (n = 174) for education and income (total sample size for the randomized trial N=2453; under 50 n=438). Imputation of the education (for 7.7% of the sample who had missing data) and income (for 26.1% of the sample) data was accomplished using the Markov Chain Monte Carlo algorithm implemented in the SAS procedure MI. Inclusion versus exclusion of participants with imputed data did not change any of the reported results. Given this, all reported analyses are based on the imputed data sample.
Procedure
The larger RCT from which these analyses are drawn tests intervention strategies for increasing African American’s colorectal cancer screening rates. All data reported in this study are from baseline measures collected before delivery of the interventions. Data collection took place at community sites (e.g., churches, community centers) from February 2014 to May 2016. Participants consenting to accrual in the study completed both baseline paper and pencil questionnaires and responded to measures embedded in PowerPoint slides via audience response system (ARS) remote keypads (Jandorf et al., 2008; Sudarsan, Jandorf, & Erwin, 2011). For the ARS questionnaire, participants were each given a wireless device and indicated answers by clicking a button on the device based on response options projected on a screen. Store gift cards were given to each consented individual as a participation incentive; at the NYC sites, participants also received round-trip public transportation fare. All study procedures were IRB approved by Roswell Park Cancer Institute and the Icahn School of Medicine at Mount Sinai.
Measures
Perceived Benefits and Barriers
Participants responded to items assessing perceived benefits and barriers to colonoscopy uptake (Rawl et al., 2001). In the present study, respondents indicated level of agreement with 5 benefits (e.g. “A colonoscopy will decrease my chances of dying from colorectal cancer”) and 10 barriers (e.g. “The cost would keep me from having a colonoscopy”) using a 5 point scale with endpoints of 1=not at all and 5=extremely. The means of the benefit items and barrier items, respectively, were used to create summary measures. Original internal consistencies for benefits and barriers separately were reported at or above α= 0.70 (Champion, 1995). In the present study, both summary measures had high reliability (benefits α=0.76; barriers α=0.80).
Affective associations
Participants completed a modified version of a measure of affective attitude components (Crites, Fabrigar, & Petty, 1994). Published evidence for reliability of the original measures was consistent across attitude objects and strong (all αs for the original measures equal to or greater than 0.86). Modifications were made to separate positive and negative affective states as construct validity is higher in unipolar affect scales (Kiviniemi, 2017) and to remove items previously shown to not relate to screening uptake (Kiviniemi et al., 2014). Affective associations were assessed by asking respondents, “When you consider having a colonoscopy, how do you feel?” for each of 4 positive (e.g. “Do you feel relaxed?), and 5 negative affective states (“Do you feel sad?”). Respondents answered on a 5-point scale with endpoints 1=not at all and 5=extremely. Reliability for the positive and negative affective association scales was strong (positive α=0.90; negative α=0.85).
Self-efficacy
Self-efficacy was assessed using a 7-item measure (Champion, Skinner, & Menon, 2005; Vernon, Myers, & Tilley, 1997). Respondents reported degree of agreement with each item (e.g. “I can make an appointment for my colonoscopy”) using a 5-point scale with endpoints: 1=strongly disagree and 5=strongly agree. The mean of the items served as the measure of self-efficacy. Original published evidence for scale reliability was strong (α=0.82) as was reliability in the current study (α=0.93).
Fear of colonoscopy
Fear of colonoscopy was assessed using 6-items measuring fear associated with a variety of aspects of the colonoscopy process (prep, procedure, results; e.g. “How fearful are you of the procedure being painful?” and “How fearful are you of the colonoscopy preparation procedure (e.g. laxatives)?” (Manne et al., 2009)). Previous work has found high reliability for this measure in both screened and unscreened samples (α=0.85). Response options were on a 5-point scale with endpoints 1=not at all and 5= extremely. Reliability for this measure was strong in the present study (α=0.86).
Colorectal cancer knowledge
Participants answered 8 items assessing knowledge of colorectal cancer and colorectal cancer screening (Jandorf et al., 2013). Response options included “true”, “false” and “don’t know” (e.g. “If colorectal cancer is found at an early stage, the chances of being cured are very good”, “I would feel it if I had a growth in my colon”). The number of correctly answered items served as the measure of knowledge.
Socioeconomic Status
Both education and income were used as indices of SES. To measure educational attainment, participants were asked “What is the highest level of education you completed?” and were given the following response options: less than 8 years, 8 through 11 years, 12 years or completed high school, post high school training other than college, some college, college graduate, postgraduate. Household income was determined by asking “What is the estimated total income for your household for the past year, before taxes, from all sources?” Participants were given the following response options: less than $10,000, $10,000 to $14,999, $15,000 to $19,999, $20,000 to $24,999, $25,000 to $29,999, $30,000 to $39,999, $40,000 to $49,999, more than $50,000, I’d rather not answer. Income categories reflected sample proportions from a pilot study that recruited from similar community-based organizations. Note that the range of income per category differs across the scale (scale point ranges were set based on income distributions reported in our previous work with similar populations). For that reason, although the significance testing for the relation between income and screening is valid, caution should be used in interpreting the change per unit income based on the results.
Screening Behavior
Participants were asked if they had ever had a colonoscopy (yes, no; adapted from Vernon et al., 2004).
Demographics
In addition to education and income, participants reported age, gender, insurance status, health care provider status, marital status, and employment status.
Analysis Strategy
SPSS version 23 was used for preliminary and univariable assessment of the indirect effects. MPlus version 8 was used for structural equation modelling for multivariable modeling of the indirect effects. We first used logistic regression to examine univariable relations with both education and income used as indices of SES and analyzed separately as predictors of colonoscopy uptake. Each affective and cognitive decision making construct was also analyzed separately as a predictor of screening behavior using logistic regression. The relationship between SES and affective and cognitive decision making constructs was examined by linear regression. Indirect effects models were estimated using bootstrap sampling for modeling the indirect effects of decision making constructs on the relationship between SES and screening behavior using the PROCESS macro for SPSS (A. Hayes, 2012; A. F. Hayes, 2013; MacKinnon, 2008). Each social cognitive variable was modeled as a possible source of an indirect effect for both the education to colonoscopy relation and the income to colonoscopy relation (14 total models).
We then tested an omnibus multivariable model with a SES latent variable (based on education and income), latent constructs for each decision-making factor, and an observed variable for screening behavior. Given the observed, dichotomous outcome variable, a weighted least squares with robust standard errors (WLSMV) estimator was used. Overall model fit was assessed with comparative fit index (CFI, (Bentler, 1990)) and root mean square error of approximation (RMSEA, (Steiger, 1990)). Error variances between one of the indicators for knowledge and one of indicators for benefits were allowed to co-vary based on modification indices. To examine the indirect effect hypotheses, indirect pathways between SES and colonoscopy behavior were estimated using the “model indirect” command in Mplus and bootstrapping with 5000 randomly generated samples.
Results
Participant demographics
Demographic characteristics of participants are reported in Table 1. As can be seen in the table, study participants had education and income levels across the full range of each measure. Eighty percent of the sample was unemployed and over two thirds were women. Additionally, while a high proportion of this sample earned less than $50,000 annual household income, over 90 percent of participants had access to care (97.4 percent had some form of health insurance and 93.5% had a primary care provider). Having access to care was associated with having had a colonoscopy (health insurance: OR=2.67, p<0.01; primary care provider: OR=3.29, p<0.001).
Table 1.
Characteristics of sample (n=1841)
| Demographic Variable | Percentage of sample, % or Mean (SD) |
|---|---|
|
| |
| Age | 66.5 (9.90) |
|
| |
| Gender | |
| Male | 22.5 |
| Female | 77.5 |
|
| |
| Marital Status | |
| Married/partnered | 21.1 |
| Single | 78.9 |
|
| |
| Education level | |
| Less than 8 years | 4.2 |
| 8 through 11 years | 13.4 |
| High school graduate | 30.9 |
| Post high school training | 9.1 |
| Some college | 21.2 |
| College graduate | 14.8 |
| Post graduate | 6.4 |
|
| |
| Income Level | |
| <$10,000 | 24.1 |
| $10,000–$14,999 | 19.0 |
| $15,000–$19,999 | 13.5 |
| $20,000–$24,999 | 12.2 |
| $25,000–$29,999 | 6.5 |
| $30,000–$39,999 | 8.6 |
| $40,000–$49,999 | 6.6 |
| >$50,000 | 9.6 |
|
| |
| Insurance Status | |
| Uninsured | 2.6 |
| Insured | 97.4 |
|
| |
| Have a health care provider | |
| No | 6.5 |
| Yes | 93.5 |
|
| |
| Employment status | |
| Unemployed | 80.0 |
| Employed | 20.0 |
|
| |
| Recruitment site | |
| NYC | 48.8 |
| Buffalo | 51.2 |
|
| |
| Ever had a colonoscopy | |
| Yes | 77.9 |
| No | 22.1 |
Demographic differences across sites were assessed. Participants recruited in New York City were slightly older, more likely to be college graduates, had higher annual household income, were more likely to have a primary care provider, and were more likely to be employed than Western New York participants. Analyses controlling for site did not change any reported results.
Participants that had been previously screened reported the following reasons for prior colonoscopy: screening as part of a routine exam or check-up (n=1010), screening due to a symptom or health problem (n=171), follow-up screening from an earlier test (n=94), increased risk due to family history (n=91), and some participants reported previous colonoscopy but were not sure of the reason for the test (n=187).
Relationship of SES to past screening behavior
Higher levels of education were positively associated with greater screening uptake; OR = 1.08, p < 0.05, 95% CI [1.01, 1.16]. Greater income was also associated with greater screening uptake; OR = 1.19, p < 0.001, 95% CI [1.13, 1.26].
Relationship of SES to decision-making constructs
The relation between education/income and decision making constructs is presented in Table 2. As can be seen in the table, with the exception of income and positive affective associations, both higher education and income levels were significantly associated with each construct. Higher levels of both were associated with perceiving more benefits to screening, greater self-efficacy to get screened and greater knowledge about colorectal cancer and colorectal cancer screening. Moreover, participants with greater education and income were less likely to report barriers to screening, negative affective associations with screening, and fear of colonoscopy. Higher education also predicted less positive affective associations with screening.
Table 2.
Relation of SES to cognitive and affective decision-making constructs and to behavior (n=1841)
| SES Metric | Behavior | ||
|---|---|---|---|
| Construct | Education | Income | OR |
| Benefits | β = 0.11 [0.09,0.14]*** | β = 0.08 [0.06,0.09]*** | 1.23 [1.06,1.41]** |
| Barriers | β = −0.21 [−0.24, −0.19]*** | β = −0.24[−0.25, −0.22]*** | 0.42 [0.35,0.49]*** |
| Self Efficacy | β = 0.19 [0.17,0.22]*** | β = 0.21 [0.19.0.23]*** | 1.28 [1.16,1.42]*** |
| Positive Affective Associations | β = −0.06 [−0.10, −0.03]* | β = −0.02 [−0.05, 0.002] | 0.85 [0.74,0.97]* |
| Negative Affective Associations | β = −0.10 [−0.12, −0.07]*** | β = −0.17[−0.19, −0.16]*** | 1.68 [1.48,1.91]*** |
| Fear of Colonoscopy | β = −0.15 [−0.17, −0.12]*** | β = −0.16[−0.18, −0.14]*** | 1.20 [1.13,1.27]*** |
| Knowledge | β = 0.13 [0.09,0.17]*** | β = 0.11 [0.08, 0.13]*** | 0.49 [0.43,0.56]*** |
p < 0.05,
p < 0.001
Relationship of decision-making constructs to screening behavior
All cognitive and affective decision making constructs were significantly related to colonoscopy screening behavior in the expected directions (see Table 2). Higher perceived benefits, positive affective associations, self-efficacy, and knowledge were all associated with greater engagement in screening behavior. Conversely, participants with higher perceived barriers, negative affective associations, and fear of colonoscopy were less likely to have been previously screened for colorectal cancer.
Indirect effect relations
Table 3 shows results from univariable analyses exploring the involvement of each social cognitive decision-making construct in an indirect effect relation between SES and likelihood of having had a colonoscopy. As can be seen in the table, the relation of both education and income to screening involved an indirect effect via perceived benefits, barriers, self-efficacy, fear of colonoscopy, and colorectal cancer knowledge. An indirect effect via positive affective associations was found for the education-screening behavior relation, but not the income-screening behavior relation. The strength of association for the effects of the full set of mediators on behavior is Nagelkerke R2 = 0.20.
Table 3.
Indirect effects of cognitive and affective decision-making constructs on the relation between SES and screening behavior (N=1841)
| SES Metric | ||||
|---|---|---|---|---|
| Construct | Education | Income | ||
| Direct | Indirect | Direct | Indirect | |
| Benefits |
0.08* (0.006, 0.15) |
0.01* (0.003, 0.02) |
0.18* (0.12, 0.23) |
0.005* (0.0001, 0.01) |
| Barriers | −0.0003 (−0.08, 0.08) |
0.09* (0.06, 0.11) |
0.13* (0.07, 0.18) |
0.06* (0.05, 0.08) |
| Self-efficacy | 0.02 (−0.06, 0.09) |
0.05* (0.03, 0.07) |
0.15* (0.09, 0.20) |
0.04* (0.32, 0.05) |
| Positive Affect | 0.07 (−0.004, 0.15) |
−0.01* (−0.02, −0.002) |
0.20* (0.14, 0.25) |
−0.002 (−0.009, 0.004) |
| Negative Affect | 0.05 (−0.02, 0.13) |
0.01 (−0.02, 0.02) |
0.19* (0.13, 0.24) |
0.01 (−0.04, 0.01) |
| Fear | 0.04 (−0.04, 0.11) |
0.05* (0.03, 0.07) |
0.14* (0.09, 0.20) |
0.04* (0.03, 0.06) |
| Knowledge | 0.05 (−0.03, 0.12) |
0.04* (0.03, 0.06) |
0.17* (0.11, 0.22) |
0.02* (0.01, 0.03) |
95% CI is statistically significant
Multivariable relations: Structural Equation Model
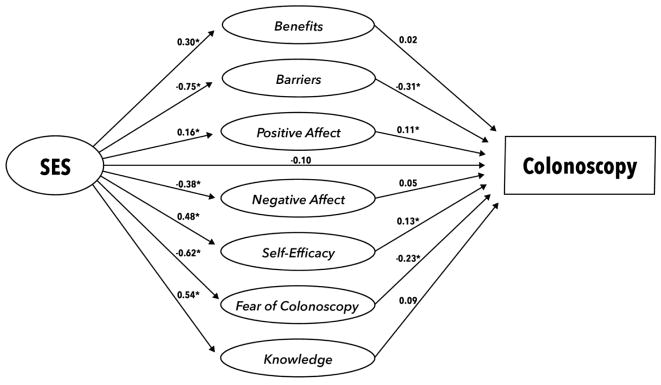
Full details of the measurement models can be obtained from the first author. Of particular relevance to the hypotheses here, both education and income had significant factor loadings on the common, SES latent variable (education loading = 0.35; income loading = 0.38). The structural model is depicted in Figure 1. Fit indices for the model indicated a well-fitting model: RMSEA = 0.03; CFI = 0.87. Results for the pathways specified in the structural model are presented in Table 4. Of particular note, SES was a significant predictor of all latent social cognitive variables, with higher SES associated with more perceived benefits, more positive affective associations with colonoscopy, greater self-efficacy to screen, more knowledge, fewer barriers to screening, less negative affective associations, and less colonoscopy fear.
Figure 1.
Final Structural Regression Model for the Relation Between SES and Colonoscopy Behavior
Table 4.
Direct Effects of SES on Constructs and Constructs on Behavior (N=1841)
| Constructs | SES →constructs | Constructs → behavior |
|---|---|---|
| B (95% CI) | B (95% CI) | |
| Benefits |
0.30*** (0.22, 0.38) |
0.02 (−0.06, 0.10) |
| Barriers | −0.75*** (−0.82, −0.68) |
−0.31* (−0.35, −0.18) |
| Self-efficacy |
0.48*** (0.41, 0.55) |
0.13* (0.05, 0.19) |
| Positive Affect |
0.16*** (0.09, 0.23) |
0.11** (0.04, 0.18) |
| Negative Affect | −0.38*** (−0.45, −0.30) |
0.05 (−0.01, 0.14) |
| Fear | −0.62*** (−0.69, −0.57) |
−0.23** (−0.29, −0.13) |
| Knowledge |
0.54*** (0.47, 0.60) |
0.09 (−0.02, 0.16) |
| SES | N/A | −0.10 (−0.89, 0.48) |
p<0.05,
p<0.01,
p<0.001
Examination of the relations of social cognitive constructs to screening behavior and of the indirect effect relations (indexed by the indirect effect of SES on behavior through a given social cognitive construct) showed that four of the constructs (barriers, self-efficacy, negative affect, and colonoscopy fear) were significantly related to behavior in the multivariable model (see Table 4). The indirect effects of SES on behavior through each decision-making construct is presented in Table 5. As can be seen in the table, an indirect effect was significant for all of the four constructs that were associated with behavior in the multivariable model.
Table 5.
Standardized Indirect and Total Effects of the Relation between SES and Colonoscopy Behavior (N=1841)
| Construct | Indirect Effect |
|---|---|
| β (95% CI) | |
| Benefits | 0.01 (−0.02, 0.03) |
| Barriers |
0.20*** (0.13, 0.27) |
| Self-efficacy |
0.06** (0.02, 0.09) |
| Positive Affect |
0.02* (0.01, 0.04) |
| Negative Affect | −0.02 (−0.06, 0.004) |
| Fear |
0.13*** (0.08, 0.18) |
| Knowledge | 0.04 (−0.01, 0.09) |
| Total |
0.42*** (0.37, 0.47) |
p<0.05,
p<0.01,
p<0.001
Discussion
SES was associated with multiple decision-making factors, with individuals with lower SES tending to have standing on the constructs that would lead to lower engagement in screening behavior (e.g., fewer benefits, more fear). Moreover, the relation between SES and screening behavior was importantly influenced by indirect effects through these social cognitive decision-making constructs. For example, a portion of the difference in screening rates as a function of socio-economic status was explained by the relation between socioeconomic status and perceived benefits to screening and, in turn, the relation of perceived benefits to screening behavior.
Implications
These findings have at least two important implications for considering social determinants of health and SES-based disparities. First, much of the focus on SES screening disparities has centered on structural factors like insurance coverage, access to medical facilities, and feasibility of screening completion in the context of other life pressures. The findings that decision-making factors are related to SES and are responsible for an indirect effect portion of the SES-screening behavior relation suggest that a broader focus on both structural/environmental and person-level causes is needed. This broader focus should incorporate both examining SES differences in attitudes, beliefs, knowledge, and feelings about screening and how the structural/environmental factors might shape the decision-making context in ways that influence screening behavior.
Second, the findings provide a pathway to move from describing SES-based disparities in colonoscopy uptake to suggesting possible routes and mechanisms to address those disparities. Knowledge of the mechanisms through which SES differences are translated into differences in screening uptake indicates possible pathways for intervening to reduce the disparities. Whereas an individual’s education and income is difficult to modify through public health intervention, perceived benefits and barriers, positive and negative affective associations, self-efficacy, knowledge, and fear of colonoscopy are changeable intrapersonal determinants as evidenced in the cancer prevention literature (Daryani, Shojaeezadeh, Batebi, Charati, & Naghibi, 2016; Kiviniemi et al., 2013; Lee-Lin, Pedhiwala, Nguyen, & Menon, 2015; Rawl et al., 2012). Thus, these results present a possible direction for interventions with medically underserved populations. Interventions which address social-cognitive determinants in ways specific to the needs of lower SES populations (e.g., by attending carefully to issues of health literacy, addressing barriers and efficacy issues specific to limited resource populations) might effectively reduce the SES-screening behavior disparity that currently exists.
Von Wagner and colleagues suggest a framework to explain differential participation in cancer screening by socioeconomic status (von Wagner, Good, Whitaker, & Wardle, 2011). This framework posits that the interrelation of SES and psychosocial constructs as influences on cancer screening behavior is due to the effects of inhibiting information processing and affecting goal-setting and behavioral translation. Thus, factors that contribute to lower SES make cancer screening seem risky, difficult to complete, and skews the cost-benefit ratio for individuals. The current study adds to this framework by providing an example showing psychosocial beliefs and affective associations as part of indirect effects involved in the relation between SES and colonoscopy completion.
Possible Mechanisms of Action
What might account for this relation of SES to decision-making factors and screening uptake? First, SES is related to broader health knowledge. Health literacy, a known influence on knowledge, ability to process and make decisions about health outcomes, and health behavior (Committee on Health Literacy, 2004; US Department of Health and Human Services, 2000) is associated with SES, with lower SES associated with lower health literacy (Becerra, Becerra, Daus, & Martin, 2015; King-Marshall et al., 2016). Lower health literacy might lead directly to lower knowledge of things like potential benefits of behavioral action. This, in turn might lead to differences in risk perception and self-efficacy, in that lower knowledge about a health issue would affect risk perception accuracy and resources for self-efficacy. Moreover, patients with low health literacy may not have the skill set to critique sources of health information (Evans, Lewis, & Hudson, 2012). As such, lower health literacy patients may use information about colorectal cancer that is not evidence-based in the colorectal cancer screening decision-making process, which may contribute to higher fear of colonoscopy and cancer fatalism.
Second, SES is associated with access to health information. Aspects of patient provider communication, such as physician recommendation (Beydoun & Beydoun, 2008; Doubeni et al., 2010; Klabunde, Schenck, & Davis, 2006), time physician spends with a patient, and trust in a physician (or the medical system) (Ward, Coffey, Javanparast, Wilson, & Meyer, 2015) are important influencers of colorectal cancer screening uptake and may vary by SES (Carcaise-Edinboro & Bradley, 2008). Physicians may be more likely to discuss screening with higher SES patients (Popescu, Schrag, Ang, & Wong, 2016). Even if one is insured, lower SES may contribute to fewer preventive health appointments (and as a result, less face-to-face time with a primary care provider) as co-pays may be cost-prohibitive (Almufleh et al., 2015), transportation may be difficult, and/or time off from work may not match with physician office hours (Cheung, Wiler, Lowe, & Ginde, 2012). Additionally, community clinics or Federally Qualified Health Centers that accept walk-in patients may be overburdened and have less time to spend with patients (Geraghty, Franks, & Kravitz, 2007; Guerra et al., 2007). This is a different mechanism than the health literacy mechanism described above, but once the lower levels of knowledge are in place, the downstream implications for our findings are equivalent.
Finally, the differences in beliefs about screening might reflect differences in the relative salience of health risks and benefits of health behaviors relative to other priorities. It has long been posited that SES disparities in health might reflect, in part, the fact that there are competing demands for motivations and goal setting. SES might shift the relative perceived importance of and risk related to health concerns, specifically colorectal cancer, relative to other life pressures in ways that shift the decision-making dynamic (Pampel, Krueger, & Denney, 2010). Thus, the differences might reflect variation in the relative importance and salience of specific health risks and of the merits of engaging in different behavioral patterns.
Ross and Wu (1995) argue that, although income and occupation have effects, education is the central mechanism through which SES differences impact health. Education may relate to health through both a generalized sense of life control and through differences in social support. Both may account for the SES-decision making effects shown here. For example, a general sense of life control may translate into increased perceived behavioral control for successfully completing the colonoscopy procedure. Social support may provide barrier reduction as well as a route toward attitudes about and affective associations with screening (Heaney & Israel, 2002).
Limitations
Some limitations must be considered when interpreting these analyses. First, it is important to note that the study design is cross-sectional. Thus, results must be interpreted as correlations between socioeconomic status, decision-making constructs, and prior screening behavior, and not evidence for causal relations between any of the predictors and outcomes. Second, colonoscopy screening behavior is based on self-report and thus is subject to the limitations and recall biases of self-report behavioral data. Third, the community/organization-based recruitment and delivery procedure used here may tend to attract participants with characteristics (e.g., community engagement) that differ from the population as a whole. On the other hand, for the purposes of examining the relation of SES to screening, participant demographics in this study include strong representation of less educated and lower income individuals. In addition, a higher proportion of this sample reported previous screening than one finds in population-representative estimates. That should be considered with respect to the decision-making models and their constructs. Fourth, education and income were used as proxies for SES. Although both are frequently used proxies, there are other measures (e.g., social class, perceived social standing) that could provide additional converging measures of SES. Finally, the income distribution for the participants in this study does not reflect the income distribution in the general population. According to 2014 census data for African American heads of household ages 45 and above, 14% had an annual household income less than $10,000 and 37% had an annual household income greater than $50,000. In this reported sample, 28% earned less than $10,000 and 13% earned greater than $50,000. However, with a population skewed toward lower income, it might be harder to achieve the relations reported. Thus, there is reason to believe that the population distribution in this sample does not erroneously strengthen findings reported in this analysis.
Conclusion
These results indicate that decision making constructs are involved in important indirect effects of the relation between SES and screening behavior. Addressing multiple social cognitive constructs in interventions targeted at lower SES populations may help reduce this disparity in screening. As future research continues to examine the influence of SES on colorectal cancer screening, further investigation of the interplay between affective and cognitive intrapersonal determinants may increase our understanding of colonoscopy avoidance in medically underserved populations.
Supplementary Material
Acknowledgments
This work was supported by National Cancer Institute grant R01CA171935 and was supported in part by Roswell Park Cancer Institute and National Cancer Institute grant 3P30CA01605. The authors kindly acknowledge the extensive support of the community members of New York City, the NYC Community Advisory Board, and the First Ladies of Western New York for their contributions to the science and data collection for this study. Special thanks to Ms. Veronica Meadows Ray, Bishop James Bowman and UB Medical Services for their contributions to the development of the Witness CARES video.
Footnotes
ClinicalTrials.gov Registration Number NCT02100254
Contributor Information
Marc T. Kiviniemi, University at Buffalo, SUNY
Lynne B. Klasko-Foster, University at Buffalo, SUNY
Deborah O. Erwin, Roswell Park Cancer Institute
Lina Jandorf, Icahn School of Medicine at Mount Sinai.
References
- Almufleh A, Gabriel T, Tokayer L, Comerford M, Alaqeel A, Kurlansky P. Role of community health outreach program “living for health” in improving access to federally qualified health centers in Miami-dade county, Florida: a cross-sectional study. BMC Health Serv Res. 2015;15:181. doi: 10.1186/s12913-015-0826-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold CL, Rademaker A, Bailey SC, Esparza JM, Reynolds C, Liu D, … Davis TC. Literacy barriers to colorectal cancer screening in community clinics. J Health Commun. 2012;17(Suppl 3):252–264. doi: 10.1080/10810730.2012.713441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra MB, Becerra BJ, Daus GP, Martin LR. Determinants of Low Health Literacy Among Asian-American and Pacific Islanders in California. J Racial Ethn Health Disparities. 2015;2(2):267–273. doi: 10.1007/s40615-015-0092-0. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative Fit Indexes in Structural Models. Psychological Bulletin. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes & Control. 2008;19(4):339–359. doi: 10.1007/s10552-007-9100-y. [DOI] [PubMed] [Google Scholar]
- Carcaise-Edinboro P, Bradley CJ. Influence of patient-provider communication on colorectal cancer screening. Medical Care. 2008;46(7):738–745. doi: 10.1097/MLR.0b013e318178935a. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Use of colorectal cancer tests--United States, 2002, 2004, and 2006. MMWR Morb Mortal Wkly Rep. 2008;57(10):253–258. [PubMed] [Google Scholar]
- Champion VL. Development of a Benefits and Barriers Scale for Mammography Utilization. Cancer Nursing. 1995;18(1):53–59. [PubMed] [Google Scholar]
- Champion VL, Skinner CS, Menon U. Development of a self-efficacy scale for mammography. Res Nurs Health. 2005;28(4):329–336. doi: 10.1002/nur.20088. [DOI] [PubMed] [Google Scholar]
- Cheung PT, Wiler JL, Lowe RA, Ginde AA. National study of barriers to timely primary care and emergency department utilization among Medicaid beneficiaries. Ann Emerg Med. 2012;60(1):4–10e12. doi: 10.1016/j.annemergmed.2012.01.035. [DOI] [PubMed] [Google Scholar]
- Committee on Health Literacy. Health Literacy: A Prescription to End Confusion. The National Academies Press; 2004. [PubMed] [Google Scholar]
- Consedine NS, Ladwig I, Reddig MK, Broadbent EA. The many faeces of colorectal cancer screening embarrassment: preliminary psychometric development and links to screening outcome. Br J Health Psychol. 2011;16(3):559–579. doi: 10.1348/135910710X530942. [DOI] [PubMed] [Google Scholar]
- Crites SL, Fabrigar LR, Petty RE. Measuring the affective and cognitive properties of attitudes: Conceptual and methodological issues. Personality and Social Psychology Bulletin. 1994;20(6):619–634. [Google Scholar]
- Crookes DM, Njoku O, Rodriguez MC, Mendez EI, Jandorf L. Promoting Colorectal Cancer Screening through Group Education in Community-Based Settings. Journal of Cancer Education. 2014;29(2):296–303. doi: 10.1007/s13187-013-0599-1. [DOI] [PubMed] [Google Scholar]
- Daryani S, Shojaeezadeh D, Batebi A, Charati JY, Naghibi A. The effect of education based on a health belief model in women’s practice with regard to the Pap smear test. Journal of Cancer Policy. 2016;8:51–56. doi: 10.1016/j.jcpo.2015.11.001. [DOI] [Google Scholar]
- Doubeni CA, Laiyemo AO, Reed G, Field TS, Fletcher RH. Socioeconomic and Racial Patterns of Colorectal Cancer Screening among Medicare Enrollees in 2000 to 2005. Cancer Epidemiology Biomarkers & Prevention. 2009;18(8):2170–2175. doi: 10.1158/1055-9965.EPI-09-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubeni CA, Laiyemo AO, Young AC, Klabunde CN, Reed G, Field TS, Fletcher RH. Primary care, economic barriers to health care, and use of colorectal cancer screening tests among Medicare enrollees over time. Ann Fam Med. 2010;8(4):299–307. doi: 10.1370/afm.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enewold L, Horner MJ, Shriver CD, Zhu K. Socioeconomic disparities in colorectal cancer mortality in the United States, 1990–2007. J Community Health. 2014;39(4):760–766. doi: 10.1007/s10900-014-9824-z. [DOI] [PubMed] [Google Scholar]
- Evans KR, Lewis MJ, Hudson SV. The role of health literacy on African American and Hispanic/Latino perspectives on cancer clinical trials. J Cancer Educ. 2012;27(2):299–305. doi: 10.1007/s13187-011-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty EM, Franks P, Kravitz RL. Primary care visit length, quality, and satisfaction for standardized patients with depression. J Gen Intern Med. 2007;22(12):1641–1647. doi: 10.1007/s11606-007-0371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra CE, Schwartz JS, Armstrong K, Brown JS, Halbert CH, Shea JA. Barriers of and facilitators to physician recommendation of colorectal cancer screening. J Gen Intern Med. 2007;22(12):1681–1688. doi: 10.1007/s11606-007-0396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern MT, Pavluck AL, Ko CY, Ward EM. Factors associated with colon cancer stage at diagnosis. Dig Dis Sci. 2009;54(12):2680–2693. doi: 10.1007/s10620-008-0669-0. [DOI] [PubMed] [Google Scholar]
- Haushofer J, Fehr E. On the psychology of poverty. science. 2014;344(6186):862–867. doi: 10.1126/science.1232491. [DOI] [PubMed] [Google Scholar]
- Hayes A. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. White paper. 2012 http://www.afhayes.com/public/process2012.pdf.
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- Heaney CA, Israel BA. Social networks and social support. In: Glanz K, Rimer BK, Lewis FM, editors. Health Behavior and Health Education. 3. San Francisco, CA: Jossey-Bass; 2002. pp. 185–208. [Google Scholar]
- Ioannou GN, Chapko MK, Dominitz JA. Predictors of colorectal cancer screening participation in the United States. American Journal of Gastroenterology. 2003;98(9):2082–2091. doi: 10.1016/s0002-9270(03)00423-4. [DOI] [PubMed] [Google Scholar]
- James AS, Hall S, Greiner KA, Buckles D, Born WK, Ahluwalia JS. The impact of socioeconomic status on perceived barriers to colorectal cancer testing. Am J Health Promot. 2008;23(2):97–100. doi: 10.4278/ajhp.07041938. [DOI] [PubMed] [Google Scholar]
- Jandorf L, Braschi C, Ernstoff E, Wong CR, Thelemaque L, Winkel G, … Itzkowitz SH. Culturally targeted patient navigation for increasing african americans’ adherence to screening colonoscopy: a randomized clinical trial. Cancer epidemiology, biomarkers & prevention. 2013;22(9):1577–1587. doi: 10.1158/1055-9965.epi-12-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandorf L, Bursac Z, Pulley L, Trevino M, Castillo A, Erwin DO. Breast and cervical cancer screening among Latinas attending culturally specific educational programs. Prog Community Health Partnersh. 2008;2(3):195–204. doi: 10.1353/cpr.0.0034. [DOI] [PubMed] [Google Scholar]
- King-Marshall EC, Mueller N, Dailey A, Barnett TE, George TJ, Jr, Sultan S, Curbow B. “It is just another test they want to do”: Patient and caregiver understanding of the colonoscopy procedure. Patient Educ Couns. 2016;99(4):651–658. doi: 10.1016/j.pec.2015.10.021. [DOI] [PubMed] [Google Scholar]
- Kiviniemi MT. Structure and content of affective associations with health behaviors: Is the behavior ‘good OR bad’ or ‘good AND bad’. Psychology & Health. 2017 doi: 10.1080/08870446.2017.1314476. [DOI] [PubMed] [Google Scholar]
- Kiviniemi MT, Bennett A, Zaiter M, Marshall JR. Individual-level factors in colorectal cancer screening: a review of the literature on the relation of individual-level health behavior constructs and screening behavior. Psycho-Oncology. 2011;20(10):1023–1033. doi: 10.1002/pon.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi MT, Jandorf L, Erwin DO. Disgusted, embarrassed, afraid: Affective associations relate to uptake of colonoscopy screening in an urban, African American population. Annals of Behavioral Medicine. 2014;48:112–119. doi: 10.1007/s12160-013-9580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi MT, Saad-Harfouche FG, Ciupak GL, Davis W, Moysich K, Hargrave NC, … Erwin DO. Pilot intervention outcomes of an educational program for biospecimen research participation. Journal of cancer education. 2013;28(1):52–59. doi: 10.1007/s13187-012-0434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in Colorectal Cancer Test Use among Vulnerable Populations in the United States. Cancer Epidemiology Biomarkers & Prevention. 2011;20(8):1611–1621. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde CN, King JB, White A, Plescia M. Vital signs: colorectal cancer screening test use--United States, 2012. Morb Mortal Wkly Rep. 2013;62(44):881–888. [PMC free article] [PubMed] [Google Scholar]
- Klabunde CN, Schenck AP, Davis WW. Barriers to colorectal cancer screening among Medicare consumers. Am J Prev Med. 2006;30(4):313–319. doi: 10.1016/j.amepre.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Kobayashi LC, Wardle J, von Wagner C. Limited health literacy is a barrier to colorectal cancer screening in England: evidence from the English Longitudinal Study of Ageing. Prev Med. 2014;61:100–105. doi: 10.1016/j.ypmed.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Lin F, Pedhiwala N, Nguyen T, Menon U. Breast Health Intervention Effects on Knowledge and Beliefs Over Time Among Chinese American Immigrants-a Randomized Controlled Study. Journal of Cancer Education. 2015;30(3):482–489. doi: 10.1007/s13187-014-0727-6. [DOI] [PubMed] [Google Scholar]
- Link BG, Phelan J. Social conditions as fundamental causes of disease. Journal of Health and Social Behavior. 1995;(Spec No):80–94. [PubMed] [Google Scholar]
- Lo SH, Waller J, Vrinten C, Kobayashi L, von Wagner C. Social Cognitive Mediators of Sociodemographic Differences in Colorectal Cancer Screening Uptake. Biomed Res Int. 2015;2015:165074. doi: 10.1155/2015/165074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP. Introduction to Statistical Mediation Analysis. New York: Erlbaum; 2008. [Google Scholar]
- Mandelblatt J, Andrews H, Kao R, Wallace R, Kerner J. The late-stage diagnosis of colorectal cancer: demographic and socioeconomic factors. Am J Public Health. 1996;86(12):1794–1797. doi: 10.2105/ajph.86.12.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne SL, Coups EJ, Markowitz A, Meropol NJ, Haller D, Jacobsen PB, … Winkel G. A randomized trial of generic versus tailored interventions to increase colorectal cancer screening among intermediate risk siblings. Ann Behav Med. 2009;37(2):207–217. doi: 10.1007/s12160-009-9103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters RK, Link BG, Phelan JC. Trends in education gradients of ‘preventable’ mortality: a test of fundamental cause theory. Soc Sci Med. 2015;127:19–28. doi: 10.1016/j.socscimed.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles A, Rainbow S, von Wagner C. Cancer fatalism and poor self-rated health mediate the association between socioeconomic status and uptake of colorectal cancer screening in England. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2132–2140. doi: 10.1158/1055-9965.EPI-11-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampel FC, Krueger PM, Denney JT. Socioeconomic Disparities in Health Behaviors. Annual review of sociology. 2010;36:349–370. doi: 10.1146/annurev.soc.012809.102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu I, Schrag D, Ang A, Wong M. Racial/Ethnic and Socioeconomic Differences in Colorectal and Breast Cancer Treatment Quality: The Role of Physician-level Variations in Care. Med Care. 2016;54(8):780–788. doi: 10.1097/MLR.0000000000000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawl SM, Champion V, Menon U, Loehrer PJ, Vance GH, Skinner CS. Validation of scales to measure benefits of and barriers to colorectal cancer screening. Journal of Psychosocial Oncology. 2001;19(3):47–63. [Google Scholar]
- Rawl SM, Skinner CS, Perkins SM, Springston J, Wang HL, Russell KM, … Champion VL. Computer-delivered tailored intervention improves colon cancer screening knowledge and health beliefs of African-Americans. Health Education Research. 2012;27(5):868–885. doi: 10.1093/her/cys094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CE, Wu CL. The Links Between Education And Health. American Sociological Review. 1995;60(5):719–745. doi: 10.2307/2096319. [DOI] [Google Scholar]
- Rudd RE. Health literacy skills of U.S. adults. Am J Health Behav. 2007;31(Suppl 1):S8–18. doi: 10.5555/ajhb.2007.31.supp.S8. [DOI] [PubMed] [Google Scholar]
- Sauer AG, Siegel RL, Jemal A, Fedewa SA. Updated review of prevalence of major risk factors and use of screening tests for cancer in the United States. Cancer Epidemiology and Prevention Biomarkers. 2017 doi: 10.1158/1055-9965.EPI-15-0134. cebp. 0219.2017. [DOI] [PubMed] [Google Scholar]
- Shah AK, Mullainathan S, Shafir E. Some Consequences of Having Too Little. Science. 2012;338(6107):682–685. doi: 10.1126/science.1222426. [DOI] [PubMed] [Google Scholar]
- Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC, White A. Patterns of Colorectal Cancer Test Use, Including CT Colonography, in the 2010 National Health Interview Survey. Cancer epidemiology, biomarkers & prevention. 2012;21(6):895–904. doi: 10.1158/1055-9965.EPI-12-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly JR, Edwards T, Shelton RC, Jandorf L. Identifying Barriers to Colonoscopy Screening for Nonadherent African American Participants in a Patient Navigation Intervention. Health Education & Behavior. 2013;40(4):449–457. doi: 10.1177/1090198112459514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SG, von Wagner C, McGregor LM, Curtis LM, Wilson EA, Serper M, Wolf MS. The influence of health literacy on comprehension of a colonoscopy preparation information leaflet. Diseases of the colon and rectum. 2012;55(10):1074. doi: 10.1097/DCR.0b013e31826359ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CB, Rim SH, Joseph DA, King JB, Seeff LC. Colorectal Cancer Incidence and Screening - United States, 2008 and 2010. Morbidity and Mortality Weekly Report. 2013;62(3):53–60. [PubMed] [Google Scholar]
- Steiger JH. Structural Model Evaluation and Modification: An Interval Estimation Approach. Multivariate Behav Res. 1990;25(2):173–180. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- Sudarsan NR, Jandorf L, Erwin DO. Multi-site implementation of health education programs for Latinas. Journal of Community Health. 2011;36(2):193–203. doi: 10.1007/s10900-010-9297-7. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Healthy People 2010: Understanding and Improving Health. Washington, DC: US Government Printing Office; 2000. [Google Scholar]
- Vernon SW, Meissner H, Klabunde C, Rimer BK, Ahnen DJ, Bastani R, … Zapka J. Measures for ascertaining use of colorectal cancer screening in behavioral, health services, and epidemiologic research. Cancer Epidemiology, Biomarkers, and Prevention. 2004;13(6):898–905. [PubMed] [Google Scholar]
- Vernon SW, Myers RE, Tilley BC. Development and validation of an instrument to measure factors related to colorectal cancer screening adherence. Cancer Epidemiology, Biomarkers, and Prevention. 1997;6(10):825–832. [PubMed] [Google Scholar]
- Viswanath K, Breen N, Meissner H, Moser RP, Hesse B, Steele WR, Rakowski W. Cancer Knowledge and Disparities in the Information Age. Journal of Health Communication. 2006;11:1–17. doi: 10.1080/10810730600637426. [DOI] [PubMed] [Google Scholar]
- Viswanath K, Finnegan JR., Jr The Knowledge Gap Hypothesis: Twenty-Five Years Later. Communication Yearbook. 1996;19:187–227. [Google Scholar]
- von Wagner C, Good A, Whitaker KL, Wardle J. Psychosocial determinants of socioeconomic inequalities in cancer screening participation: a conceptual framework. Epidemiologic reviews. 2011;33:135–147. doi: 10.1093/epirev/mxq018. [DOI] [PubMed] [Google Scholar]
- von Wagner C, Semmler C, Good A, Wardle J. Health literacy and self-efficacy for participating in colorectal cancer screening: The role of information processing. Patient Education and Counseling. 2009;75(3):352–357. doi: 10.1016/j.pec.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Ward PR, Coffey C, Javanparast S, Wilson C, Meyer SB. Institutional (mis)trust in colorectal cancer screening: a qualitative study with Greek, Iranian, Anglo-Australian and Indigenous groups. Health Expect. 2015;18(6):2915–2927. doi: 10.1111/hex.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein ND. Testing four competing theories of health-protective behavior. Health Psychology. 1993;12(4):324–333. doi: 10.1037//0278-6133.12.4.324. [DOI] [PubMed] [Google Scholar]
- Whitaker KL, Good A, Miles A, Robb K, Wardle J, von Wagner C. Socioeconomic inequalities in colorectal cancer screening uptake: does time perspective play a role? Health Psychol. 2011;30(6):702–709. doi: 10.1037/a0023941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.