Abstract
Aims
This study examined whether the association between hemoglobin A1c (HbA1c) and short-term clinical outcomes is moderated by CAD severity.
Methods
We studied 17,394 US Veterans with type 2 diabetes who underwent elective cardiac catheterization between 2005 and 2013. CAD severity was categorized as obstructive, non-obstructive, or no CAD. Using multivariable Cox proportional hazards regression, we assessed associations between time-varying HbA1c and two-year all-cause mortality and non-fatal MI, with an interaction term between HbA1c and CAD severity.
Results
61%, 22%, and 17% of participants had obstructive, non-obstructive, and no CAD, respectively. CAD severity modified the relationship between HbA1c and each outcome (interaction p-value 0.0005 for mortality and <0.0001 for MI). Low HbA1c (<42 mmol/mol) was associated with increased mortality, relative to HbA1c of 48–52 mmol/mol, in individuals with obstructive CAD (HR 1.52 [1.17, 1.97]) and non-obstructive CAD (HR 2.61 [1.61, 4.23]), but not in those with no CAD (HR 0.91 [0.46, 1.79]). In contrast, higher HbA1c levels (≥53 mmol/mol) were associated with increased MI risk only in individuals with obstructive CAD.
Conclusions
The associations between HbA1c and mortality and MI were moderated by CAD severity. Measures of cardiovascular disease severity may inform optimal individualized diabetes management.
Keywords: Coronary artery disease, glycemic control, interaction
1. Introduction
The global burden of type 2 diabetes mellitus is increasing rapidly and now affects nearly 10% of adults in the United States.1, 2 Although diabetes is recognized as a significant risk factor for cardiovascular disease,3, 4 optimal glycemic control strategies that minimize diabetes complications without excessive risk of treatment-related harm remain uncertain. The major diabetes and cardiovascular disease professional society guidelines recommend a personalized approach to glycemic control that takes into account an individual’s comorbidities, diabetes complications, and life expectancy.5–7 However, few studies offer direct evidence to support strategies to rationally individualize diabetes management.
Coronary artery disease (CAD) is a common comorbidity among patients with diabetes with a prevalence of approximately 65%,8 and may significantly impact safe and effective glycemic control. Indeed, randomized trials have shown that tight glycemic control in patients with diabetes can provide modest long-term cardiovascular benefits, but at the cost of a higher risk of short-term mortality and severe hypoglycemia.9–17 However, these trials have typically pooled together those with and without CAD, making it difficult to understand how CAD impacts optimal HbA1c goals.9–14, 16 Recent work using data from the Veterans Affairs (VA) Cardiac Catheterization Lab Clinical Assessment, Reporting, and Tracking Program (CART) has demonstrated a gradient of short-term risk of mortality and myocardial infarction (MI) across levels of CAD severity such that risks of both one-year MI and one-year mortality increase progressively from no CAD to non-obstructive CAD to obstructive CAD.18 Given the strong association between glycemic control and risk of incident cardiovascular disease,3, 4 evidence that specifically integrates cardiovascular disease risk into glycemic control strategies is needed.
Whether the association between HbA1c and clinical outcomes is uniform across individuals with varying cardiovascular risk is unknown. Accordingly, the aim of this study was to investigate whether the presence and severity of CAD modifies the association between HbA1c and short-term mortality or MI in a cohort of Veterans with diabetes undergoing coronary angiography. Given the frequency with which diabetes and CAD co-occur, determining if CAD severity can inform specific, individualized glycemic control goals could meaningfully inform future clinical trials and optimal clinical care, and evidence indicating short-term benefits or harms could motivate timely changes to diabetes management approaches.
2. Subjects, Materials, and Methods
2.1 Study cohort
Data for this study were derived from the VA CART Program, which is a national program used at all VA cardiac catheterization laboratories to track clinical quality.18–20 CART patient and procedural data can be merged with longitudinal patient data from the VA electronic health record (EHR), including hospitalizations, clinic visits, pharmacy data, laboratory testing, procedure codes, diagnosis codes, and vital status, as well as with VA claims data for hospitalizations at non-VA facilities paid for by the VA to capture care provided to veterans outside the VA.
We included all Veterans with type 2 diabetes who underwent elective coronary angiography in the VA between October 2005 and September 2013. We identified diabetes patients as those with at least one ICD-9-CM type 2 diabetes code for inpatient hospitalization or at least two ICD-9-CM type 2 diabetes codes for two separate outpatient visits occurring within 24 months prior to their index cardiac catheterization.21 We then included Veterans whose indication for elective cardiac catheterization was chest pain, stable angina, a positive functional study, or ischemic heart disease.
We excluded patients with secondary causes of diabetes, which have unique ICD9-CM diagnosis codes, and those who were newly diagnosed with diabetes within one day of index catheterization as we were interested in glycemic control in individuals with pre-existing diabetes. We also excluded individuals receiving angiography for acute coronary syndrome (ACS) and patients with a prior MI or coronary revascularization. We were primarily interested in how CAD severity, as opposed to prior MI or other major cardiovascular conditions, moderated the association of HbA1c and mortality and MI; each of these conditions are independently associated with mortality or with MI and including patients with any of these conditions could confound analyses of the association between HbA1c and outcomes. Patients with fewer than 6 months of health records in the VA system prior to the index catheterization were also excluded. Finally, we excluded patients without a baseline HbA1c measurement, defined as an HbA1c measured within 6 months before or 24 hours after the index catheterization, whichever was closest to the catheterization date if there were multiple measurements. For patients with multiple angiographies within the VA, we defined the first catheterization recorded during the study period as the index catheterization. The local VA Research and Development Committee and the Colorado Multiple Institutional Review Board provided approval for this study.
2.2 CAD categories
CAD severity was defined based on standard definitions of flow-limiting stenosis in prior studies.18 Obstructive CAD was defined as any stenosis ≥ 50% in the left main coronary artery and/or ≥ 70% in any other coronary artery. Non-obstructive CAD was defined as a stenosis ≥ 20% but ≤ 50% in the left main coronary artery and/or ≥ 20% but ≤ 70% in any other coronary artery. No CAD was defined as the absence of stenosis exceeding 20% in any coronary artery.
2.3 Exposures
The primary exposure in this study was time-varying HbA1c over two years after index catheterization or until censoring. On average, participants had five HbA1c measurements over the follow-up period, with a median of 84 days between measurements (IQR 39 to 155 days). We specified models using both continuous HbA1c and HbA1c categories. Participants’ exposure category varied with time and was updated each time a participant had HbA1c measured during the post-catheterization follow-up period. That is, a participant’s HbA1c exposure level was based on the most recent HbA1c measurement and could change each time an HbA1c measurement was taken.
2.4 Outcomes
There were two primary outcomes: two-year all-cause mortality and two-year hospitalization for non-fatal MI. We focused on short-term outcomes for several reasons. First, we were interested in whether data regarding CAD severity acquired from coronary angiography could inform diabetes treatment targets, and more specifically motivate diabetes treatment modification during the clinical encounter at the time of angiography. Second, while the effects of uncontrolled glucose levels are believed to occur over a period of years, we were interested in exploring whether the associations between glycemia and outcomes are accelerated across levels of CAD severity. Third, the two-year follow-up period allowed for multiple HbA1c measurements for most patients, making the evaluation of time-varying HbA1c as the primary exposure feasible. Mortality was measured using VA vital status data, which comes from multiple VA and non-VA data sources including the VA Beneficiary Identification Records Locator Subsystem (BIRLS) Death File, VA Medicare Vital Status File, and the Social Security Administration Death Master File. MI was defined by a primary diagnosis ICD-9-CM code of 410.xx in VA inpatient and claims data for hospitalizations at non-VA facilities paid for by the VA.
We also performed secondary analyses examining cause of death using CART data linked with the National Death Index22 to determine whether the HbA1c categories were associated with either cardiovascular or non-cardiovascular causes of death. For these analyses, our cohort was limited to individuals who underwent catheterization prior to 12/31/2011, as this was the last date for which cause of death was available in our linked data. Cardiovascular mortality was defined as ICD-10 diagnostic codes listed as cause of death in National Death Index data beginning with “I”, excluding I00.xx through I09.xx (rheumatic heart diseases), I33.xx (infective endocarditis), I78.xx (disease of capillaries), I80.xx through I89.xx (diseases of the veins, lymphatic vessels, and lymph nodes). Non-cardiovascular mortality was defined as all other ICD-10 codes listed as cause of death in National Death Index data.
2.5 Statistical Analyses
Patient level demographics, cardiovascular risk factors, co-morbidities, angiography-related factors, CAD-related medications, baseline diabetes medications, diabetes duration, and creatinine clearance based on the Modification of Diet in Renal Disease (MDRD) equation were collected and compared across baseline HbA1c categories. We used chi-square tests to compare categorical data and Mann-Whitney Wilcoxon nonparametric tests for continuous or ordinal data.
We calculated and compared associations between HbA1c and two-year outcomes within each category of CAD severity using time-varying HbA1c over the follow-up period. In order to identify reasonable HbA1c cut points and allow for the non-linearity of the relationships, we first examined continuous HbA1c fit with a restricted cubic spline function. The knots used in the spline function were placed at HbA1c values of 15 mmol/mol, 42 mmol/mol, 58 mmol/mol, 75 mmol/mol, and 102 mmol/mol. This preliminary analysis yielded the following HbA1c categories for the remainder of our analyses: <42 mmol/mol, ≥42 to <48 mmol/mol, ≥48 to <53 mmol/mol, ≥53 to <64 mmol/mol, ≥64 to <75 mmol/mol, and ≥75 mmol/mol. For all analyses, we designated HbA1c ≥48 to <53 mmol/mol as the reference category as it represents the lowest range of values above the diagnostic threshold for diabetes. In addition, while <42 mmol/mol may reflect normoglycemia, this level of glycemic control is not typically recommended by the American Diabetes Association or the European Association for the Study of Diabetes. We fit 6-level categorical HbA1c models using multivariable Cox proportional hazards regression adjusted for covariates selected from prior studies,18 including demographics (age, sex, race), cardiovascular risk factors (hypertension, hyperlipidemia, Framingham risk score, smoking status, body mass index), CAD severity, comorbidities (congestive heart failure [CHF], chronic obstructive pulmonary disease [COPD], post-traumatic stress disorder [PTSD], peripheral artery disease, dialysis, depression), angiography indication (chest pain, stable angina, ischemic heart disease, positive functional study), post-angiography revascularization (none, percutaneous coronary intervention, coronary artery bypass graft), repeat catheterization during follow-up, diabetes duration, diabetes medications at baseline (no medications, non-insulin medications only, insulin), creatinine clearance, and cardioprotective medication adherence (statin, beta-blocker, and angiotensin converting enzyme inhibitor). Cardioprotective medication adherence was measured as proportion of days covered (PDC), dichotomized at a threshold of PDC ≥ 0.8 for each of the three medication classes as described previously.23 Creatinine clearance and medication adherence were modeled as time-varying covariates.
To assess differential associations between HbA1c and outcomes for patients in different CAD categories, we included an interaction term between HbA1c and CAD severity. To account for clustering by site, we used the robust estimator of the covariance matrix in Cox models.24
2.6 Sensitivity Analyses
We conducted three sensitivity analyses to address potential sources of bias. First, glycemic control of individuals with diabetes could vary greatly at the end-of-life, either due to simplification of treatment and permissiveness towards an increasing HbA1c, or due to declining HbA1c from worsening nutrition status or progression of a terminal illness. Thus, in the analysis of time-varying HbA1c, we excluded HbA1c measurements within 30 days of the date of death for patients who died during the follow-up period. Second, to account for all-cause mortality as a competing risk in the analysis of MI outcomes, we conducted competing risk analyses for MI using cumulative incidence function (CIF) methods.25, 26 Finally, we repeated the primary analyses using a reference HbA1c category that was reflective of typical clinical practice in the United States, ≥42 mmol/mol to <53 mmol/mol.
As the primary question of interest in this study was the interaction between HbA1c category and severity of CAD on our two co-primary outcomes, we adjusted for multiple comparisons by applying a significance threshold of p<0.025 (0.05/2) for the interaction terms using the Wald chi-square test. All analyses were conducted in SAS 9.4 (SAS Inc., Cary, NC) and R (version 3.1, R Foundation for Statistical Computing, Vienna, Austria). All statistical code is available upon request.
3. Results
3.1 Study participant characteristics
There were 17,394 individuals included in our sample. Study participants had a mean age of 63.7 years, and were predominantly male and white (Table 1). Mean HbA1c at baseline was 58 mmol/mol, but individual values demonstrated significant dispersion: 13% of individuals had a baseline HbA1c less than 42 mmol/mol, and 13% had a baseline HbA1c of 75 mmol/mol or higher. Higher HbA1c at baseline was associated with younger age, longer diabetes duration, more HbA1c measurements during follow-up, and higher likelihood of being on at least one diabetes medication or on insulin. Several comorbidities, including CHF, COPD, CKD, dialysis use, depression, and PTSD, were more prevalent in individuals with baseline HbA1c <42 mmol/mol, compared to individuals with baseline HbA1c near the mean (≥48 to <53 and ≥53 to <64 mmol/mol; Supplemental Table 1). Since time-varying HbA1c was the primary exposure in this study, we examined HbA1c values over the course of follow-up for individuals in each baseline HbA1c category. The mean HbA1c during follow-up for the entire study cohort was unchanged from baseline (58 mmol/mol ± 13), and only the individuals in the highest HbA1c category at baseline (HbA1c ≥ 75 mmol/mol) exhibited a substantial change in mean glycemic control during the follow-up period, from 90 mmol/mol ± 12 to 77 mmol/mol ± 13 (Table 1). 61% of participants had obstructive CAD on index catheterization; 22% had non-obstructive CAD, and 17% had no CAD (Table 1). Supplemental Table S1 shows the distribution of all study covariates across the baseline HbA1c categories.
Table 1.
Study population characteristics.
| Overall n=17394 |
HbA1c < 42 mmol/mol n=2294 |
42 ≤ HbA1c < 48 mmol/mol n=2628 |
48 ≤ HbA1c < 53 mmol/mol n=3180 |
53 ≤ HbA1c < 64 mmol/mol n=4533 |
64 ≤ HbA1c < 75 mmol/mol n=2423 |
HbA1c ≥ 75 mmol/mol n=2336 |
p-value | |
|---|---|---|---|---|---|---|---|---|
| Age, mean years (SD) | 63.7 (8.1) | 64.0 (7.9) | 64.5 (8.2) | 64.7 (8.1) | 64.2 (7.8) | 63.1 (7.9) | 60.4 (8.1) | <.0001 |
| Male, n (%) | 16742 (96.3) | 2202 (96.0) | 2518 (95.8) | 3068 (96.5) | 4385 (96.7) | 2342 (96.7) | 2227 (95.3) | 0.04 |
| Race, n (%) | <.0001 | |||||||
| White | 12950 (74.5) | 1749 (76.2) | 2035 (77.4) | 2368 (74.5) | 3424 (75.5) | 1763 (72.8) | 1611 (69.0) | |
| Black | 3121 (17.9) | 391 (17.0) | 397 (15.1) | 572 (18.0) | 757 (16.7) | 454 (18.7) | 550 (23.5) | |
| Asian | 996 (5.7) | 110 (4.8) | 151 (5.7) | 187 (5.9) | 272 (6.0) | 152 (6.3) | 124 (5.3) | |
| Pacific Islander | 186 (1.1) | 24 (1.0) | 27 (1.0) | 36 (1.1) | 38 (0.8) | 33 (1.4) | 28 (1.2) | |
| Native American | 141 (0.8) | 20 (0.9) | 18 (0.7) | 17 (0.5) | 42 (0.9) | 21 (0.9) | 23 (1.0) | |
| CAD severity, n (%) | 0.0007 | |||||||
| No CAD | 2919 (16.8) | 365 (15.9) | 489 (18.6) | 575 (18.1) | 723 (15.9) | 363 (15.0) | 404 (17.3) | |
| Non-obstructive CAD | 3874 (22.3) | 473 (20.6) | 584 (22.2) | 749 (23.6) | 1016 (22.4) | 544 (22.5) | 508 (21.7) | |
| Obstructive CAD | 10601 (60.9) | 1456 (63.5) | 1555 (59.2) | 1856 (58.4) | 2794 (61.6) | 1516 (62.6) | 1424 (61.0) | |
|
| ||||||||
| Baseline HbA1c, mean mmol/mol (SD) | 58 (12) | 38 (2) | 44 (1) | 50 (1) | 57 (2) | 68 (2) | 90 (12) | <.0001 |
| HbA1c during follow-up, mean mmol/mol (SD) | 58 (13) | 42 (5) | 48 (10) | 53 (15) | 60 (7) | 66 (9) | 77 (13) | <.0001 |
| Diabetes duration (years), mean (SD) | 4.1 (2.5) | 3.6 (2.4) | 3.5 (2.4) | 3.8 (2.5) | 4.4 (2.5) | 4.6 (2.5) | 4.5 (2.5) | <.0001 |
| Number of HbA1c measurements, mean (SD) | 5.1 (2.5) | 4.5 (2.4) | 4.5 (2.1) | 4.7 (2.2) | 5.3 (2.5) | 5.7 (2.6) | 5.9 (2.9) | <.0001 |
| Diabetes medications, n (%) | <.0001 | |||||||
| None | 3369 (19.4) | 706 (30.8) | 771 (29.3) | 601 (18.9) | 638 (14.1) | 357 (14.7) | 296 (12.7) | |
| Orals only | 12497 (71.8) | 1470 (64.1) | 1732 (65.9) | 2399 (75.4) | 3491 (77.0) | 1742 (71.9) | 1663 (71.2) | |
| Insulin | 1528 (8.8) | 118 (5.1) | 125 (4.8) | 180 (5.7) | 404 (8.9) | 324 (13.4) | 377 (16.1) | |
HbA1c=glycosylated hemoglobin; CAD=coronary artery disease; SD=standard deviation
3.2 Associations between HbA1c and mortality
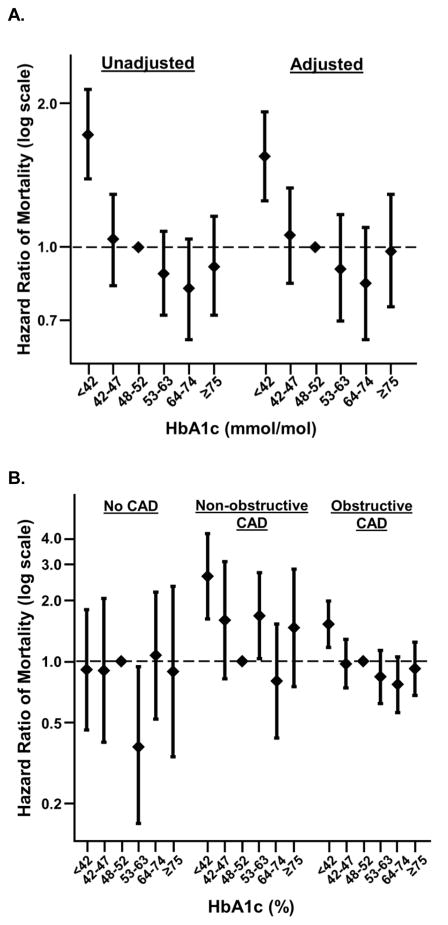
The crude mortality rates varied across baseline HbA1c categories from 1.77% to 3.66% in individuals with no CAD, from 3.4% to 7.6% in those with non-obstructive CAD, and from 6.22% to 9.73% in those with obstructive CAD (Supplemental Table S2). Individuals with an HbA1c <42 mmol/mol had a significantly higher risk of mortality in both unadjusted and adjusted models compared to individuals with an HbA1c ≥48 to <53 mmol/mol, whereas other HbA1c categories were not significantly associated with mortality (Figure 1a, Supplemental Table S3). However, the association between HbA1c and mortality varied according to CAD severity (interaction p-value=0.0005). Compared to an HbA1c ≥48 to <53 mmol/mol, HbA1c below 42 mmol/mol was associated with higher risk of mortality in individuals who had obstructive CAD (HR 1.52 [1.17, 1.97]) and non-obstructive CAD (HR 2.61 [1.61, 4.23]) but not in those with no CAD (HR 0.91 [0.46, 1.79]; Figure 1b, Supplemental Table S3).
Figure 1. Relationship between HbA1c level and mortality, overall and stratified by CAD.
HbA1c below 42 mmol/mol is associated with increased risk of short-term mortality, specifically among individuals with obstructive and non-obstructive CAD. (A) Unadjusted and adjusted associations between HbA1c categories and mortality in the entire study population. (B) Adjusted associations between HbA1c categories and mortality in participants with no CAD, non-obstructive CAD, and obstructive CAD. The pattern of association between HbA1c and mortality varied across categories of CAD severity (p-value for interaction = 0.0005).
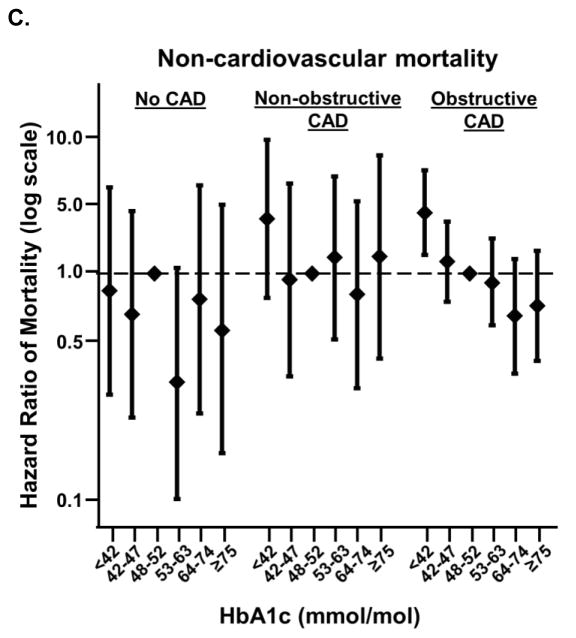
Next, we examined cause-specific mortality in a subset of our study cohort who had cause-of-death available through the National Death Index. The crude event rates of non-cardiovascular mortality exceeded those for cardiovascular mortality in nearly all combinations of CAD severity and baseline HbA1c category (Supplemental Table S4). Among non-cardiovascular mortality, diabetes and cancer were the most frequently occurring primary causes of death for the study participants (Supplemental Table S5). As in the full study population, compared to an HbA1c of ≥48 to <53 mmol/mol, HbA1c <42 mmol/mol was associated with a higher risk of mortality in individuals with obstructive CAD and non-obstructive CAD but not in those with no CAD in this subgroup (Figure 2a). However, the relationship appeared to be driven primarily by a higher risk of non-cardiovascular mortality among obstructive CAD patients with low HbA1c only (Figures 2b, c).
Figure 2. Relationship between HbA1c level and all-cause, cardiovascular, or non-cardiovascular mortality, stratified by CAD.
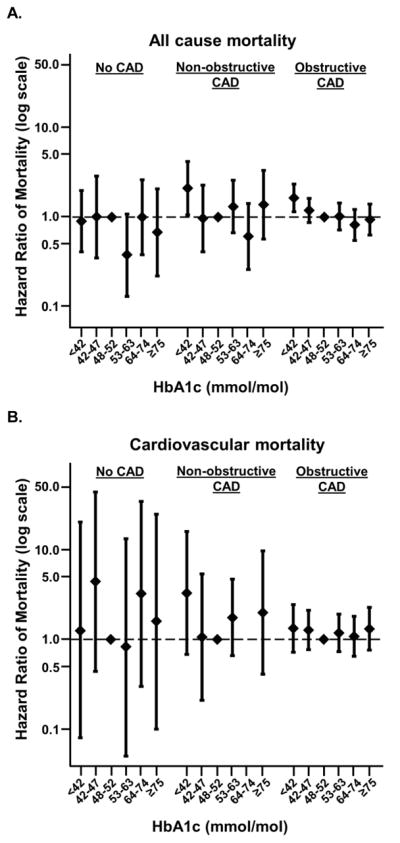
HbA1c below 42 mmol/mol is associated with increased risk of non-cardiovascular but not cardiovascular mortality, specifically among individuals with obstructive CAD. Adjusted associations between HbA1c categories and all-cause mortality (A), cardiovascular mortality (B), and non-cardiovascular mortality (C) in participants with obstructive CAD, non-obstructive CAD, and no CAD. Data limited to those participants who underwent cardiac catheterization prior to 12/31/2011, through which data linked to National Death Index were available. There were too few cardiovascular mortality events to accurately model the association in those with non-obstructive CAD and 64 ≤ HbA1c < 75 mmol/mol.
3.3 Associations between HbA1c and MI
In contrast to mortality, increasing HbA1c categories were associated with higher MI risk in the overall population. The crude rates of MI varied across baseline HbA1c categories from 0% to 0.38% in individuals with no CAD, from 0.59% to 1.53% in those with non-obstructive CAD, and from 2.28% to 3.99% in those with obstructive CAD (Supplemental Table S2). Compared to a reference HbA1c of ≥48 to <53 mmol/mol, HbA1c ≥53 to <64 mmol/mol and HbA1c ≥75 mmol/mol were associated with a significantly higher risk of MI (Figure 3a, Supplemental Table S6). Here again, the association between HbA1c and MI varied according to CAD severity (interaction p-value<0.0001). Increasing HbA1c levels were associated with higher risk of two-year MI among individuals with obstructive CAD (HR 1.51 [1.01, 2.26] for HbA1c ≥53 to <64 mmol/mol, HR 1.55 [1.05, 2.29] for HbA1c ≥64 to <75 mmol/mol, and HR 1.77 [1.11, 2.81] for HbA1c ≥75 mmol/mol, all relative to HbA1c ≥48 to <53 mmol/mol; Figure 3b, Supplemental Table S6). Among individuals with non-obstructive or no CAD, however, HbA1c levels were not consistently associated with risk of MI.
Figure 3. Relationship between HbA1c level and MI, overall and stratified by CAD.
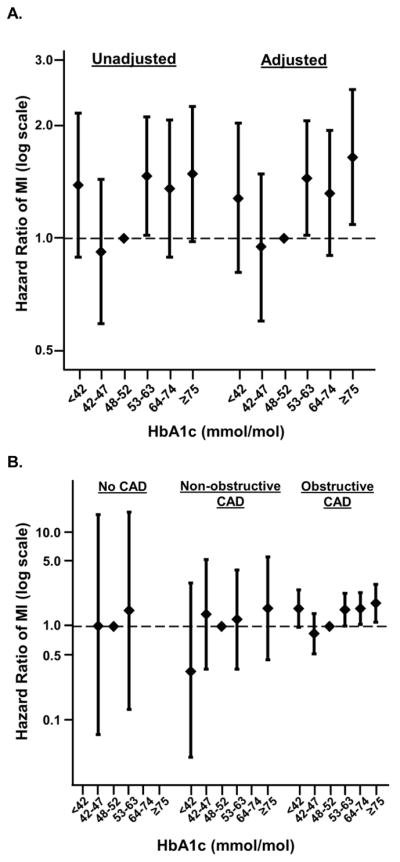
Increasing HbA1c is associated with increased risk of short-term myocardial infarction (MI), specifically among individuals with obstructive CAD. (A) Unadjusted and adjusted associations between HbA1c categories and MI in the entire study population. (B) Adjusted associations between HbA1c categories and MI in participants with no CAD, non-obstructive CAD, and obstructive CAD. The pattern of association between HbA1c and MI varied significantly across categories of CAD severity (p-value for interaction < 0.0001). There were too few MI events to accurately model the association in several HbA1c categories among those with non-obstructive (64 ≤ HbA1c < 75 mmol/mol) and no CAD (HbA1c < 42 mmol/mol, 64 ≤ HbA1c < 75 mmol/mol, and HbA1c ≥ 75 mmol/mol).
3.4 Sensitivity analyses
We examined whether declining HbA1c in terminally ill individuals contributed a possible “end of life” bias that affected our primary results. After excluding HbA1c values within 30 days of death, HbA1c <42 mmol/mol remained associated with a higher risk of mortality in individuals with obstructive and non-obstructive CAD but not in those without CAD (Supplemental Figure S1). Similarly, the examination of non-fatal MI could be biased by competing risk due to mortality. However, our competing risks analysis also did not alter our primary findings of the associations between HbA1c and MI (Supplemental Figure S2).
Finally, we repeated the primary analyses after combining the HbA1c ≥42 to <47 mmol/mol and ≥48 to <53 mmol/mol categories into a single category of ≥42 to <53 mmol/mol that is more typical of clinical care in the United States. The pattern of association between HbA1c category and mortality were unchanged; HbA1c <42 mmol/mol was associated with increased risk of two-year all-cause mortality relative to HbA1c ≥42 and <53 mmol/mol in individuals with non-obstructive CAD (HR 2.03 [1.41, 2.93]) and obstructive CAD (HR 1.48 [1.19, 1.84]), but not in those with no CAD (HR 0.98 [0.49, 1.98]; Supplemental Table S7). When the HbA1c reference category was expanded to include ≥42 to <53 mmol/mol, the pattern of association between HbA1c and MI changed in that HbA1c <42 mmol/mol was associated with increased MI risk in individuals with obstructive CAD (HR 1.56 [1.03, 2.37]; Supplemental Table S8). Otherwise, the pattern of association between HbA1c category and MI was unchanged; HbA1c categories ≥53 mmol/mol were associated with increased two-year MI risk relative to HbA1c ≥42 and <53 mmol/mol only in individuals with obstructive CAD (Supplemental Table S8).
4. Discussion
In this study, we aimed to determine if the presence and severity of CAD moderated the association between HbA1c and short-term clinical outcomes in a cohort of Veterans. We found that HbA1c was associated with both two-year mortality and non-fatal MI, and that the relationships between HbA1c and mortality and MI differed based on CAD severity. Low HbA1c (below 42 mmol/mol) was associated with short-term mortality in those with obstructive and non-obstructive CAD but not in those without CAD, a finding that was driven by non-cardiovascular mortality. High HbA1c (above 53 mmol/mol) was associated with short-term nonfatal MI only in individuals with obstructive CAD, highlighting the relatively narrow therapeutic window of HbA1c within which short-term clinical outcomes were optimized for individuals with obstructive CAD in our study population. Furthermore, by examining outcomes over a shorter follow-up period than in prior studies, our results suggest that active, individualized diabetes management incorporating CAD severity might have meaningful short-term clinical impacts in the significant subgroup of diabetic patients who have CAD.8
Our findings support the emerging body of literature suggesting that HbA1c below 42 mmol/mol is associated with adverse clinical outcomes, specifically mortality, and extends this work by determining that this relationship is particularly powerful in individuals with CAD. The professional societies governing diabetes care in the United States and Europe have advocated for individualization of diabetes treatment.5–7 Indeed, treatment guidelines suggest that the presence of vascular complications of diabetes, including CAD, should motivate less stringent glycemic control.7 However, randomized trials, on which these recommendations are based, and prior observational studies evaluating HbA1c targets and potential diabetes overtreatment did not differentiate between individuals with varying CAD severity.9–11, 13, 14, 16, 27, 28 Similarly, studies that have demonstrated the risks associated with overtreatment and hypoglycemia, including increasing cardiovascular events and mortality, did not discriminate individuals based on CAD severity.15–17, 29–37 There are several possible explanations for the finding that the association between low HbA1c and mortality was driven by non-cardiovascular mortality. First, while cardiovascular diseases were the most common causes of death, they accounted for a minority of all causes of mortality. Thus, we had greater power to detect an association of HbA1c with non-cardiovascular mortality than with cardiovascular mortality. Second, prior studies have suggested hypoglycemia and diabetes overtreatment are associated with increased risk of cancer mortality,38, 39 and cancer was the most common non-cardiovascular cause of death in our study. Third, diabetes itself was commonly documented as a primary cause of non-cardiovascular mortality. While the ability to examine hypoglycemia was limited in our data, increased mortality in individuals with low HbA1c could be at least partially explained by hypoglycemia. Furthermore, prior work suggests that individuals with pre-existing cardiovascular disease are more sensitive to hypoglycemia,30, 40 which would be consistent with our finding that low HbA1c was associated with increased mortality only in individuals with obstructive or non-obstructive CAD.
Despite recommendations to the contrary, a recent analysis of data from the National Health and Nutrition Examination Survey (NHANES) found that age, sex, comorbidities, diabetes duration, and self-reported measures of physical and mental health did not impact an individual’s target HbA1c as set by their treating clinician,41 highlighting current limitations in the implementation of individualized diabetes management. Similarly, we observed wide dispersion in HbA1c in our study, underscoring the heterogeneity of the diabetes patient population and the wide variation in diabetes management in real-world practice even among patients from an integrated national healthcare system with uniform practice guidelines and quality measures. Indeed, a majority of patients with obstructive CAD and an HbA1c below 42 mmol/mol remain on diabetes medications in our study, corroborating the findings of other studies of potential overtreatment of diabetes in individuals with indications for less intensive glycemic control.31, 32, 34–36
Specific evidence is needed to guide rational diabetes management based on measurable risk factors or comorbidities. In the absence of a clinical trial of glycemic control strategies stratified by CAD severity or other relevant clinical factors, observational comparative effectiveness studies may offer the best insight into approaches for individualization of diabetes treatment. Our study begins to create such an evidence base by demonstrating how personalized treatment goals might in part be informed by the presence or absence, and severity of CAD. Furthermore, the precarious balance between cardioprotective benefit and risk of harm across adjacent HbA1c categories highlights the limitations of targeting HbA1c as the primary goal of treatment. Rather, treating with specific classes of diabetes medications that confer cardiovascular benefits, while avoiding hazardously low HbA1c values may prove to be a safer alternative.42, 43 Future studies are needed to examine whether diabetes medication changes can impact mortality in individuals with low HbA1c and CAD, whether specific treatment regimens or trajectories of HbA1c lowering are associated with lower risk of short-term MI in individuals with high HbA1c and obstructive CAD, and whether noninvasive studies such as cardiac CT are as informative as angiography for providing CAD severity estimates that can inform diabetes treatment goals. In addition, only 54% of participants with obstructive CAD in our study underwent revascularization. While prior work has explored revascularization for stable CAD in patients with diabetes,44 future research examining revascularization strategies across HbA1c strata could inform individualization of CAD management in patients with diabetes. Finally, a clinical trial of glycemic control targets and diabetes medication choice that specifically addresses comorbidities and patient characteristics, including CAD severity, would be valuable for guiding personalized diabetes management.
Our study has several limitations. First, as with all observational studies, our results are subject to bias from unmeasured confounding, and thus we cannot draw causal inferences. However, we were able to adjust for a significant number of potential demographic, patient, and treatment confounders that are associated with both glycemic control and outcomes. Second, we cannot exclude reverse causality as an explanation for the association between HbA1c below 42 mmol/mol and mortality, in spite of the sensitivity analysis in which we excluded HbA1c values within 30 days of death. We cannot infer causality due to the correlational nature of the data. That said, if low HbA1c were merely a marker of unmeasured advanced illness contributing to excess mortality in those individuals, we would not expect to detect the interaction with CAD severity that we observed. Furthermore, our study population (elective cardiac catheterization without known history of ischemic heart disease) was unlikely to include a significant number of individuals with advanced ischemic heart disease or congestive heart failure – individuals at risk for cardiac cachexia who might have both low HbA1c and differential risk of mortality related to CAD severity. Third, our cohort was based on individuals who underwent elective cardiac catheterization, which could represent a source of selection bias. However, the distribution of no, non-obstructive, and obstructive CAD in our cohort was similar to that observed for patients with diabetes in an independent national sample.45 In addition, we observed event rates of MI and mortality that are consistent with the general population of US patients with diabetes,46–48 as well as overall patterns of association between HbA1c and/or CAD with mortality that have been described in other population cohorts.18, 27, 28 Fourth, our study is predominantly male and mostly white race. While other studies of glycemic control have not observed substantial race or gender effects, our findings warrant replication in a more diverse cohort. Fifth, the cause-specific mortality analysis was limited by sample size, and analyses of MI were limited by infrequent outcome events, which may have limited our ability to detect clinically meaningful events. Finally, CAD severity categories were determined by the treating provider, rather than by a core lab, which could have introduced misclassification. That said, we have no a priori reason to believe that this impacted our results, and the data used in this study were well-suited to our goal of studying how clinical data in health records can contribute to diabetes control strategies.
In spite of these limitations, we found that the relationship between HbA1c and clinical outcomes was modified by CAD severity, with individuals with obstructive CAD seemingly most sensitive to the adverse effects of both low and high HbA1c over time. We conclude that CAD severity, when available, may help differentiate diabetes management strategies that minimize mortality and MI risks. Furthermore, our findings suggest that CAD severity may be an important variable to consider in the design of randomized trials focused on informing the individualization of HbA1c goals for patients with diabetes.
Supplementary Material
Acknowledgments
5.1 Author contributions: SR, WGL, PMH, MEP, SMB, and TMM conceived of and designed the study; WGL, PMH, MEP, SMB, and TMM contributed to data acquisition and availability; WGL, AEB, and MEP contributed to analysis plan refinement and performed all data analysis; SR, WGL, AEB, MEP, DS, KJ, TMM, LC, DM, CIV, SMB, and PMH contributed to interpretation of results. All authors contributed to drafting and critical revision of the manuscript, as well as final approval of the version submitted for review and publication.
5.2 Funding source: SR is supported by the University of Colorado School of Medicine Division of General Internal Medicine Small Grant Program, and American Heart Association Award 17MCPRP33670728. LC is supported by VA HSR&D IIR 14-048-3. CIV is supported by VA HSR&D research career scientist award RCS 14-443. KEJ is supported by NHLBI K23HL109177. TMM was the national director for the VA CART Program at the time that this study was performed.
5.3 Conflicts of Interest: None of the authors have conflicts of interest relevant to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. [Google Scholar]
- 3.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Annals of Internal Medicine. 2004;141:413–20. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- 4.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Annals of Internal Medicine. 2004;141:421–31. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. 6. Glycemic Targets. Diabetes Care. 2017;40:S48–S56. doi: 10.2337/dc17-S009. [DOI] [PubMed] [Google Scholar]
- 6.Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, de Boer IH, et al. Update on Prevention of Cardiovascular Disease in Adults With Type 2 Diabetes Mellitus in Light of Recent Evidence: A Scientific Statement From the American Heart Association and the American Diabetes Association. Diabetes Care. 2015;38:1777–803. doi: 10.2337/dci15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–9. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 8.Scognamiglio R, Negut C, Ramondo A, Tiengo A, Avogaro A. Detection of coronary artery disease in asymptomatic patients with type 2 diabetes mellitus. Journal of the American College of Cardiology. 2006;47:65–71. doi: 10.1016/j.jacc.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 10.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. The New England Journal of Medicine. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerstein HC, Miller ME, Ismail-Beigi F, Largay J, McDonald C, Lochnan HA, et al. Effects of intensive glycaemic control on ischaemic heart disease: analysis of data from the randomised, controlled ACCORD trial. Lancet. 2014;384:1936–41. doi: 10.1016/S0140-6736(14)60611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. The New England Journal of Medicine. 2015;372:2197–206. doi: 10.1056/NEJMoa1414266. [DOI] [PubMed] [Google Scholar]
- 13.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. The New England Journal of Medicine. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 14.Zoungas S, Chalmers J, Neal B, Billot L, Li Q, Hirakawa Y, et al. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. The New England Journal of Medicine. 2014;371:1392–406. doi: 10.1056/NEJMoa1407963. [DOI] [PubMed] [Google Scholar]
- 15.Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, et al. Severe hypoglycemia and risks of vascular events and death. The New England Journal of Medicine. 2010;363:1410–8. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 16.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassai B, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ. 2011;343:d4169. doi: 10.1136/bmj.d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013;347:f4533. doi: 10.1136/bmj.f4533. [DOI] [PubMed] [Google Scholar]
- 18.Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312:1754–63. doi: 10.1001/jama.2014.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrd JB, Vigen R, Plomondon ME, Rumsfeld JS, Box TL, Fihn S, et al. Data quality of an electronic health record tool to support VA cardiac catheterization laboratory quality improvement: the VA Clinical Assessment, Reporting, and Tracking System for Cath Labs (CART) program. American Heart Journal. 2013;165:434–40. doi: 10.1016/j.ahj.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Maddox TM, Plomondon ME, Petrich M, Tsai TT, Gethoffer H, Noonan G, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program) The American Journal of Cardiology. 2014;114:1750–7. doi: 10.1016/j.amjcard.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 21.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(Suppl 2):B10–21. doi: 10.2337/diacare.27.suppl_2.b10. [DOI] [PubMed] [Google Scholar]
- 22.Center for Excellence for Suicide Prevention. Joint Department of Veterans Affairs (VVA) and Department of Defense (DoD) Suicide Data Repository - National Death Index (NDI) http://vawww.virec.research.va.gov/Mortality/Overview.htm.
- 23.Ho PM, Lambert-Kerzner A, Carey EP, Fahdi IE, Bryson CL, Melnyk SD, et al. Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: a randomized clinical trial. JAMA Internal Medicine. 2014;174:186–93. doi: 10.1001/jamainternmed.2013.12944. [DOI] [PubMed] [Google Scholar]
- 24.Lin DY, Wei LJ. The Robust Inference for the Cox Proportional Hazards Model. Journal of the American Statistical Association. 1989;84:1074–1078. [Google Scholar]
- 25.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 26.Varadhan R, Weiss CO, Segal JB, Wu AW, Scharfstein D, Boyd C. Evaluating health outcomes in the presence of competing risks: a review of statistical methods and clinical applications. Medical Care. 2010;48:S96–105. doi: 10.1097/MLR.0b013e3181d99107. [DOI] [PubMed] [Google Scholar]
- 27.Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375:481–9. doi: 10.1016/S0140-6736(09)61969-3. [DOI] [PubMed] [Google Scholar]
- 28.Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care. 2011;34:1329–36. doi: 10.2337/dc10-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA Internal Medicine. 2014;174:251–8. doi: 10.1001/jamainternmed.2013.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leong A, Berkowitz SA, Triant VA, Porneala B, He W, Atlas SJ, et al. Hypoglycemia in Diabetes Mellitus as a Coronary Artery Disease Risk Factor in Patients at Elevated Vascular Risk. The Journal of Clinical Endocrinology and Metabolism. 2016;101:659–68. doi: 10.1210/jc.2015-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Internal Medicine. 2015;175:356–62. doi: 10.1001/jamainternmed.2014.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCoy RG, Lipska KJ, Yao X, Ross JS, Montori VM, Shah ND. Intensive Treatment and Severe Hypoglycemia Among Adults With Type 2 Diabetes. JAMA Internal Medicine. 2016;176:969–78. doi: 10.1001/jamainternmed.2016.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seaquist ER, Miller ME, Bonds DE, Feinglos M, Goff DC, Jr, Peterson K, et al. The impact of frequent and unrecognized hypoglycemia on mortality in the ACCORD study. Diabetes Care. 2012;35:409–14. doi: 10.2337/dc11-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sussman JB, Kerr EA, Saini SD, Holleman RG, Klamerus ML, Min LC, et al. Rates of Deintensification of Blood Pressure and Glycemic Medication Treatment Based on Levels of Control and Life Expectancy in Older Patients With Diabetes Mellitus. JAMA Internal Medicine. 2015;175:1942–9. doi: 10.1001/jamainternmed.2015.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorpe CT, Gellad WF, Good CB, Zhang S, Zhao X, Mor M, et al. Tight glycemic control and use of hypoglycemic medications in older veterans with type 2 diabetes and comorbid dementia. Diabetes Care. 2015;38:588–95. doi: 10.2337/dc14-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tseng CL, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Internal Medicine. 2014;174:259–68. doi: 10.1001/jamainternmed.2013.12963. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Campbell CR, Fonseca V, Shi L. Impact of hypoglycemia associated with antihyperglycemic medications on vascular risks in veterans with type 2 diabetes. Diabetes Care. 2012;35:1126–32. doi: 10.2337/dc11-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong AP, Chan JC. Hypoglycemia and Comorbidities in Type 2 Diabetes. Current Diabetes Reports. 2015;15:80. doi: 10.1007/s11892-015-0646-x. [DOI] [PubMed] [Google Scholar]
- 39.Lee AK, Warren B, Lee CJ, McEvoy JW, Matsushita K, Huang ES, et al. The Association of Severe Hypoglycemia With Incident Cardiovascular Events and Mortality in Adults With Type 2 Diabetes. Diabetes Care. 2017 doi: 10.2337/dc17-1669. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yun JS, Ko SH, Ko SH, Song KH, Yoo KD, Yoon KH, et al. Cardiovascular Disease Predicts Severe Hypoglycemia in Patients with Type 2 Diabetes. Diabetes & Metabolism Journal. 2015;39:498–506. doi: 10.4093/dmj.2015.39.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shahraz S, Pittas AG, Lundquist CM, Danaei G, Kent DM. Do Patient Characteristics Impact Decisions by Clinicians on Hemoglobin A1c Targets? Diabetes Care. 2016;39:e145–6. doi: 10.2337/dc16-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. The New England Journal of Medicine. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 43.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. The New England Journal of Medicine. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 44.BARI 2D Study Group. Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. The New England Journal of Medicine. 2009;360:2503–15. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, et al. Low diagnostic yield of elective coronary angiography. The New England Journal of Medicine. 2010;362:886–95. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gregg EW, Cheng YJ, Saydah S, Cowie C, Garfield S, Geiss L, et al. Trends in death rates among U.S. adults with and without diabetes between 1997 and 2006: findings from the National Health Interview Survey. Diabetes Care. 2012;35:1252–7. doi: 10.2337/dc11-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Annals of Internal Medicine. 2007;147:149–55. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
- 48.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, et al. Changes in diabetes-related complications in the United States, 1990–2010. The New England Journal of Medicine. 2014;370:1514–23. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.