Abstract
Background
Yaws is an important cause of chronic disfiguring ulcers in children in the tropics. WHO’s newly adopted strategy for yaws eradication employs a single round of mass azithromycin treatment followed by targeted treatment programs, and data from pilot studies have shown a short-term significant reduction of yaws. We assessed the long-term efficacy of the WHO strategy for yaws eradication.
Methods
We conducted a longitudinal study on a Papua New Guinea island (16092 population) in which yaws was endemic. In the initial study, the participants were followed for 12 months; in this extended follow-up study, clinical, serological, and PCR surveys were continued 6-monthly for 3·5 years; genotyping and travel history were used to identify importation events. Active yaws confirmed by a Treponema pallidum specific PCR was the primary-outcome indicator.
Findings
Mass azithromycin treatment (coverage rate of 84%) followed by targeted treatment programs reduced the prevalence of active yaws from 1·8% to a minimum of 0·1% at 18 months (difference from baseline, −1·7%; P<0·0001), but the infection began to re-emerge after 24 months with a significant increase to 0·4% at 42 months (difference from 18 months, 0·3%; P<0·0001). At each time point after baseline, > 70% of the total community burden of yaws was found in persons absent from the mass treatment or as new infections in non-travelling residents. At months 36 and 42, five cases of active yaws, all from the same village, demonstrated clinical failure following azithromycin treatment with PCR detected mutations in the 23S ribosomal RNA genes conferring resistance to azithromycin. A sustained decrease in the prevalence of high titre latent yaws in asymptomatic children aged 1–5 years from 13.7% to <1.5%, and of genetic diversity of yaws strains from 0·139 to <0·046 between 24 and 42 months indicated a reduction in transmission of infection.
Interpretation
The implementation of the WHO strategy did not, in the long-term, achieve elimination in a high-endemic community mainly due to persons who were absent at the time of mass treatment in whom yaws reactivated; repeated mass treatment may be necessary to eliminate yaws. For the first time, we report the emergence of azithromycin-resistant T. p. pertenue and spread within one village. Communities’ surveillance should be strengthened to detect any possible treatment failure and biological markers of resistance.
INTRODUCTION
The World Health Organization (WHO) has already begun implementing a program designed to eradicate yaws, an infectious disease caused by Treponema pallidum subsp. pertenue. The global burden of yaws is substantial, with over 89 million people living in yaws endemic areas and 100,000 annual reported cases of chronic ulcers or papilloma that are a major physical and psychological burden in young children.
The cornerstone of the WHO’s strategy is the mass administration of azithromycin aiming for a population coverage of >90%.1 The drug is well-tolerated and very effective against yaws.2,3 It is given as one supervised dose, so compliance is assured. Treatment of all members of a yaws-endemic community, irrespective of their clinical status, allows individuals harbouring the infection without any skin manifestation (latent infection) to be successfully exposed to curative doses of the treatment. Clearance of the pathogen responsible for yaws from individuals with active and latent infection, which constitute the infectious reservoir, has the potential to interrupt transmission.4 The WHO strategy calls for the use of PCR technology to confirm the diagnosis and to monitor the emergence of resistance to azithromycin after mass treatment.5
A second important element of the WHO strategy to increase the effectiveness of yaws eradication programmes is to follow mass drug administration (MDA) with active case detection surveys every 3- 6 months, consisting of blanket screening to identify and treat all active yaws cases and their contacts (often called total targeted treatment, TTT).6 This second element aims to achieve elimination by early detection of existing (e.g. missed MDA), recurrent (e.g. relapse of untreated latent infections), or newly introduced (e.g. crossing regional borders) active yaws cases. A third element is a strengthened health and community system for surveillance and management of patients who present to health care between surveys.6
We previously reported the effect of single-dose mass azithromycin treatment, with a coverage rate of 83·8%, on the prevalence of active and latent yaws 12 months after the intervention.7 MDA with azithromycin was associated with a nearly 90% reduction of serologically-confirmed active yaws from 2·4% to 0·3%. Single mass azithromycin treatment has also shown short-term efficacy in other clinical trials in Ghana (Aziz A, personal communication) and Solomon Islands;8,9 however, the long-term efficacy of the WHO strategy has not yet been determined.
We now report the results of 42 months of follow-up in our study communities to assess the long-term effect of the WHO strategy to eradicate yaws.
METHODS
Study setting and participants
Between May, 2013 and October, 2016, we conducted a longitudinal study of the population of Lihir Island, New Ireland Province, Papua New Guinea (PNG). The characteristics of the area have been described in detail elsewhere.7 The climate is tropical with two distinct seasons: rainy and dry seasons. All villages of Lihir Island had a high prevalence of active yaws before MDA (range 0·5 to 3·8%).10
We have previously reported the results of the first 12 months of follow up after MDA, where the primary objective was to estimate the prevalence of clinically suspected yaws with serologic confirmation of treponemal infection.7 All residents of Lihir have been followed during the extended phase of this study for an additional 30 months (months 12–42). Serologic methods do not result in identification of all cases of active yaws because very early infection, while highly infectious, may be seronegative. On the other hand, participants with latent yaws may present with skin ulcers caused by other bacteria (e.g. Haemophilus ducreyi) resulting in false positive diagnoses of active yaws.11–13
Consequently, throughout our study we incorporated molecular diagnostics as our case definition of active yaws. PCR testing of ulcers allowed us to more clearly delineate the effect of the intervention on participants with true active yaws (lesion PCR positive for T. pallidum) and on those participants with latent yaws and a different cause of the current skin infection (i.e. positive serology for treponemal infection but lesion PCR negative for T. pallidum).
After the initial MDA campaign,7 we conducted 6 monthly TTT surveys in accordance with the standards advocated by WHO. Prior to each survey, population sensitization was undertaken to inform village authorities of the program. Villages were visited by a mobile team of health workers that first screened the village schools to examine children, and then conducted house-to-house screening. All subjects with skin ulcers and their contacts (household, frequent family friends, schoolmates and playmates) were treated with directly observed single-dose azithromycin (30 mg/Kg) procured by WHO from Medopharm, India. To simplify the treatment in the field, dosing charts were used to guide the participants’ age-based dose. Individuals were observed for 30 minutes following treatment; if vomiting occurred within this time period, the child was re-treated. Treatment was provided without cost to subjects. Clinical follow-up exams were conducted 2 weeks after any treatment to identify potential treatment failures. All field workers and clinical and laboratory staff involved in the follow-up of study participants remained blinded to previous individual- and village-specific results.
All participants, or their parents, provided oral informed consent for screening. In addition, we obtained written informed consent from parents or guardians, as well as verbal agreement of the children with clinically suspected yaws before enrolment in ulcer aetiology studies and serological surveys. The protocol was approved by the National Medical Research Advisory Committee of the PNG Ministry of Health (MRAC n° 12·36).
Procedures
Clinical surveys were undertaken in the entire resident population present at the time of the visits for assessment of clinical signs and symptoms of active yaws at study months 18, 24, 30, 36, and 42. We used tally sheets to record the number of people examined at TTT surveys and a standardized form to record data of patients with suspected lesions. Specimens were collected from the largest lesion of all subjects with ulcer(s) or papilloma(s) using dacron swabs that were vigorously rotated over a 1 cm2 area and then placed into a tube containing transport medium as previously described.7 At the baseline and 6-month surveys we forwarded a subset of 90 and 84 randomly selected specimens, respectively, to the University of Washington laboratory (Seattle, WA, USA) for PCR testing; the number of active yaws cases in the entire population was estimated using the proportion of PCR-positive specimens among the subset of PCR-tested lesions multiplied by the total number of detected lesions. In 12-month to 42-month surveys, we forwarded for testing all specimens collected, therefore we obtained a direct measurement of the number of yaws cases in the entire population.
Laboratory methods used to confirm yaws and to detect macrolide-resistance mutations have been previously described.11,14 In short, three T. pallidum gene targets, tp0548, tpN47 (tp0574), and a pertenue-specific region of the tprL (tp1031) gene were PCR amplified to detect the presence of T. pallidum DNA and to confirm the subspecies; previously described restriction fragment length polymorphism methods were used to detect A2058G14 and A2059G15 point mutations in both copies of the 23S ribosomal RNA genes. We conducted strain genotyping by sequencing a panel of three molecular markers (tp0548, tp0136 and tp0326)16 to determine the genetic diversity of T. p. pertenue infections and to identify importation events. Significant reductions in transmission intensity are required to reduce the diversity of bacterial populations; hence diversity was used as a marker for transmission. We used PCR targeting the 16S rRNA gene to identify H. ducreyi.17 Participants with lesions underwent serological testing using both qualitative Rapid Plasma Reagin (RPR) and T. pallidum haemagglutination assay (TPHA); specimens which tested dually positive were then reflexed to the quantitative RPR.
Demographic and epidemiologic data were systematically collected for every case of ulcer detected. Compliance with yaws MDA therapy was assessed by self-reported data and verified using the MDA treatment register books. Travel history was assessed by self-reported travel out of Lihir Island to a yaws endemic area in the preceding 6 months, regardless of compliance to MDA. History of inmigration was assessed by self-reported migration to Lihir Island in the preceding 6 months and verified by non-appearance at the previous year census, regardless of compliance to MDA.
Serological surveys to detect latent yaws in a subgroup of asymptomatic children 1–15 years were conducted 18, 24, 30, 36, and 42 months after MDA. We randomly selected six villages, and all children within the age group were recruited for inclusion. The random sample was regenerated at each survey therefore different villages may have been selected for testing in different rounds. Venous blood samples were collected from assenting children for TPHA and qualitative and quantitative RPR testing.
Definitions and statistical methods
The primary outcome indicator to assess the infection levels was the prevalence of participants with active yaws lesions confirmed by PCR (regardless of their serology result) which was assessed by examining everyone in the population. Secondary outcome indicators included prevalence of serology-positive participants with an ulcer (regardless of PCR result). To control for potential confounders of infection persistence we looked at the proportion of yaws cases that had missed MDA therapy, the proportion of yaws cases that originated from travel vs. local residual source according to travel history and genotyping results, and the proportion of yaws samples with genetic mutations associated with macrolide resistance at each time point. Secondary outcome indicators that were used to assess onward transmission of infection were the prevalence of latent yaws with high titre seroreactivity (RPR ≥1:16) in a subset of children aged 1–5 years in randomly selected villages, and the mean genetic diversity of T. p. pertenue isolates from active yaws lesions.
The prevalence ratio was estimated for comparison of active yaws at seven time-points using a log-binomial regression model. The model accounted for the uncertainty in the estimate of number of active yaws cases at baseline. We estimated the adjusted prevalence ratio of high titre latent yaws using the cluster option in the models to account for the variability between clusters selected for serosurveys. Analyses were done using Stata version 13·1 (StataCorp LP, College Station, Texas, USA). The mean evolutionary diversity of T. p. pertenue isolates at each round was estimated by calculating the number of base substitutions per site for each round using the Kimura 2 parameter model in MEGA version 7;18 significant differences among years were determined using One Way ANOVA.
We initially calculated that a sample size of 1000 children would be needed at 24–42 months to estimate the prevalence of high titre latent yaws with a precision of 0·83%, at a two-sided significance level of 5% in a finite population of 6600 children 1–15 years. We assumed that the prevalence of latent yaws at 24–42 months would be 2%. However, we adjusted the sample size to reduce survey fatigue by the survey team and to minimize venipuncture of children; the revised calculations indicated that 500 children were enough to estimate prevalence with a precision of 1·18%.
The study is registered with www.clinicaltrials.gov (number NCT01955252).
RESULTS
The study population lives in the 28 villages of Lihir Island; in small subsistence farming communities with a mean (SD) population of 575 (225) persons per village. At baseline 16,092 people lived in the area, and a total of 13,490 individuals (83·8%) received single-dose azithromycin (or benzathine benzylpenicillin if azithromycin was contraindicated). Total population size remained fairly stable throughout the study, and a mean (SD) proportion of 79·0% (8·2) of the population was examined at each survey (Table 1). Some individuals could not be reached at scheduled visit times (e.g. children were absent from school, adults were working in the fields, families had moved away on temporary or permanent basis).
Table 1.
Prevalence of skin ulcers and active yaws
| Time post MDA |
No. of persons in the census |
No. of persons examined |
All Clinically Suspected Lesions | Active Yaws Lesions* | Clinically Suspected Lesions with Positive Serologic Findings |
|||
|---|---|---|---|---|---|---|---|---|
| No. of cases (%) |
Prevalence ratio (95%CI) †‡ |
No. of cases (%) |
Prevalence ratio (95%CI) †‡ |
No. of cases (%) |
Prevalence ratio (95%CI)†‡ |
|||
| Baseline | 16,092 | 13,490 (84%) | 690 (5·1) | 1 | 238 (1·8) § | 1 | 323 (2·4) | 1 |
| 6 mo. | 16,092 | 13,166 (82%) | 121 (0·9) | 0·18 (0·15; 0·22) | 59 (0·4) ¶ | 0·25 (0·19; 0·34) | 44 (0·3) | 0·14 (0·10; 0·19) |
| 12 mo. | 17,339 | 13,204 (76%) | 114 (0·9) | 0·17 (0·14; 0·21) | 19 (0·1) ∥ | 0·08 (0·05; 0·13) | 34 (0·3) | 0·11 (0·08; 0·15) |
| 18 mo. | 17,339 | 15,977 (92%) | 88 (0·6) | 0·11 ( 0·09; 0·13) | 17 (0·1) ∥ | 0·06 (0·04; 0·10) | 38 (0·2) | 0·10 (0·07; 0·14) |
| 24 mo. | 17,555 | 11,792 (67%) | 68 (0·6) | 0·11 (0·09; 0·14) | 13 (0·1) ∥ | 0·06 (0·04; 0·11) | 24 (0·2) | 0·09 (0·06; 0·13) |
| 30 mo. | 17,555 | 14,935 (85%) | 120 (0·8) | 0·16 (0·13; 0·19) | 31 (0·2) ∥ | 0·12 (0·08; 0·17) | 52 (0·3) | 0·15 (0·11; 0·19) |
| 36 mo. | 18,836 | 14,765 (78%) | 107 (0·7) | 0·14 ( 0·12; 0.17) | 36 (0·2) ∥ | 0·14 (0·10; 0·20) | 53 (0·4) | 0·15 (0·11; 0·20) |
| 42 mo. | 18,836 | 13,601 (72%) | 107 (0·8) | 0·15 ( 0·13; 0.19) | 51 (0·4) ∥ | 0·21 (0·16; 0·29) | 63 (0·5) | 0·19 (0·15; 0·25) |
Active yaws refers to the estimated number of participants with lesional PCR positive results in either tpN47 (tp0574) or tp0548, and in which the pertenue subspecies was confirmed by TprL PCR amplicon size.
The prevalence ratio was calculated by means of the log-binomial model. The baseline prevalence is the reference value.
P<0.0001 for the significance of the model overall.
At baseline, a random sample of 90 (out of 690) clinically suspected yaws lesions were tested by PCR; the number of active yaws cases in the entire population were estimated using the proportion of PCR-positive specimens among the subset of PCR-tested lesions (34·4% [31/90]) multiplied by the total number of clinically suspected yaws lesions detected (n 690).
At 6 months, the same approach as taken for baseline was used to estimate the number of active yaws cases. The proportion of PCR-positive specimens (48·8% [41/84]) was multiplied by the total number of clinically suspected yaws lesions detected (n 121).
In 12-month to 42-month surveys, we tested all clinically suspicious lesions by PCR; therefore we obtained a direct measurement of the number of active yaws cases in the entire population.
Baseline, 6, and 12 month data were previously published (Ref. 7) and are included here for comparison to later time points.
Changes in the prevalence of active disease
The overall prevalence of active yaws fell from an estimated 1·8% before MDA to a minimum of 0·1% at 18 months (difference from baseline, −1·7%; 95%CI, −1·9 to −1·4; P<0·0001), but began to reemerge after 24 months (Table 1). The prevalence increased to 0·4% at 42 months (difference from 18 months, 0·3%; 95%CI, 0·1 to 0·4; P<0·0001), with a major rise from 36 to 42 months. Similarly, rates of participants with clinically suspected yaws lesions and positive serologic findings fell initially, but there was evidence of increasing prevalence rate by month 30 (Table 1). We noted a significant increase from a minimum of 0·2% at 24 months to 0·5% at 42 months (difference, 0·3%; 95CI, 0·1 to 0·4; P<0·0001).
Incident infection in subgroups of the population
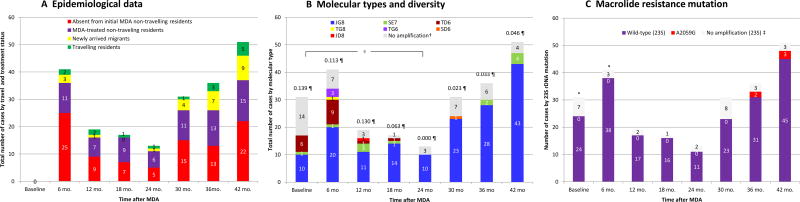
Overall, 239 participants were T. pallidum PCR positive throughout the study, including 31 at baseline and 208 in total in the post-MDA period. At each survey after MDA, from 36% to 61% of the community burden of newly identified active yaws were accounted for by non-travelling residents who had been absent at initial mass treatment visit (Figure-1A). Non-travelling residents who were present at MDA ranged from 27% to 53%, and migrants and residents who had travelled to a yaws endemic area after MDA represented <30% of cases at each time point.
Figure 1. Characteristics of PCR-confirmed active yaws by epidemiological history, molecular type, and macrolide resistance mutation.
PCR-confirmed active yaws refers to samples with positive results in either tpN47 (tp0574) or tp0548, and in which the pertenue subspecies was confirmed by TprL PCR amplicon size.
*A random sample of 90 (out of 690) clinically suspected yaws cases at baseline and 84 (out of 121) at 6 months were tested by PCR; we provide data on the characteristics of lesions that were actually PCR-positive among the subset of PCR-tested lesions (31 at baseline and 41 at 6-months). In 12-month to 42-month surveys, we tested all clinically suspected lesions by PCR; therefore we provide data on the characteristics of all lesions detected for these time-points.
†Not all T. p. pertenue positive samples could be fully typed for all three typing targets.
§ P<0.0001 for the estimate of the mean evolutionary diversity of T. p. pertenue isolates at 24-month survey compared to baseline.
¶ Estimate of the mean evolutionary diversity of T. p. pertenue isolates at each round.
‡Not all T. p. pertenue positive samples could be amplified for 23S rRNA by PCR.
The aggregated molecular typing of strains causing incident infection has been reported in a paper detailing the development of the typing system.16 We here report (Figure-1B) the temporal variation of the molecular diversity over the 42-month study. At baseline, three molecular types were identified, with strain JG8 accounting for 58·8% of fully typable samples. Overtime, molecular type diversity was reduced to zero (only one genotype –JG8– was present) at 24 months which represented a reduction of the mean evolutionary diversity of T. p. pertenue from 0·139 to 0·000 (P<0·0001). Diversity remained at low levels <0.046 thereafter. After 24 months, strain JG8 caused all 76/76 new incident cases of yaws in non-travelling patients with typable samples, and 18/25 cases in travelling patients. The remaining 7/25 subjects who had a history of travel or migration were infected with genotypes SE7 or SD6, which supports that these cases were imported and not derived from a local source.
When we looked at the proportion of participants with a history of travel according to genotypes (Table 2), all TD6, TG6, and JD8, as well as 83·9% of JG8 specimens were seen in patients who had not travelled; all SD6 and TG8 strains, and 81·8% of SE7 occurred in patients who travelled or migrated.
Table 2.
Proportion of non-travelling vs travelling participants with yaws in the post-MDA period according to genotypes
| Genotypes of yaws strains | No Amplification (n=31) |
Total (n=208) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| JG8 (n=149) | SE7 (n=11) | TG8 (n=1) | TD6 (n=11) | TG6 (n=3) | SD6 (n=1) | JD8 (n=1) | |||
| Non-travelling resident* | 125 (83·9%) | 2 (18·2%) | 0 (0·0%) | 11 (100%) | 3 (100%) | 0 (0·0%) | 1 (100%) | 26 (83·9%) | 168 (80·8%) |
| Travelling or in-migrated participant† | 24 (16·1%) | 9 (81·8%) | 1 (100%) | 0 (0·0%) | 0 (0·0%) | 1 (100%) | 0 (0·0%) | 5 (16·1%) | 40 (19.2%) |
All non-travelling residents either absent or present at Mass Drug Administration.
Travel history was assessed by self-reported travel out of Lihir Island to a yaws endemic area in the preceding 6 months, regardless of compliance to MDA.
History of in-migration was assessed by self-reported migration to Lihir Island in the preceding 6 months and verified by non-appearance at the previous year census, regardless of compliance to MDA.
Proportion of PCR-confirmed active yaws with resistance mutations
Of 31 active yaws cases tested at baseline, the 23S rRNA gene could be amplified by PCR in 24 (77·4%); all had wild type 23S rRNA sequence at positions 2058 and 2059 (Figure-1C). Of 208 PCR-confirmed active yaws cases in the post-MDA period, the 23S rRNA gene could be amplified by PCR in 186 (89·4%), of which 181 (97·3%) had wild-type strains, but two cases at 36 months and three cases at 42 months revealed A2059G mutations associated with macrolide-resistance. The five samples had A2059G mutations in both of the 23S rRNA loci.
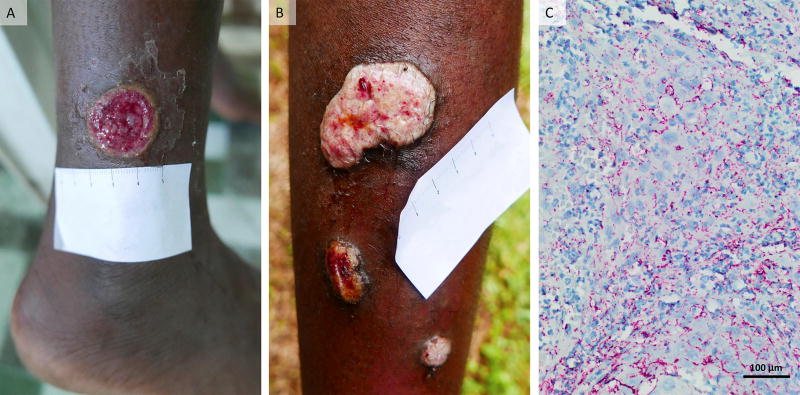
The index case was an 11-year old boy who was diagnosed with active yaws at the 30-month survey (lesional swab PCR-positive for T. p. pertenue with wild-type 23S rRNA) and treated with a full dose 1.5g of azithromycin without vomiting the medication (Figure-2A). The patient was seen 6 months later with recurrent papillomatous lesions (Figure-2B) and serological treatment failure. The skin biopsy of these lesions showed abundant spirochetes (Figure-2C) and T. p. pertenue containing the 23S rRNA A2059G macrolide resistance mutation was identified by PCR. The patient was treated with 2.4MUI of benzathine benzylpenicillin and showed clinical cure after 2 weeks and serological cure after 6 months.
Figure 2. Yaws lesions in a patient with treatment failure associated with macrolide-resistant Treponema pallidum subsp. pertenue.
(A) Primary lesion (red, moist 2·5 cm ulcer) on the left leg of an 11-year-old patient with yaws observed at the 30 months survey. Lesional swab PCR was positive for T. p. pertenue with wild-type 23S rRNA. (B) Secondary yaws papillomas (multiple nodules with yellow-colour granular surface) seen at 36 months survey. These lesions were PCR positive for T. p. pertenue with A2059G mutation in 23SrRNA. (C) Photomicrograph of skin biopsy of the larger papilloma lesion in Panel B with abundant spirochete organisms stained bright red by the Treponema pallidum immunohistochemical stain (×400 magnification).
The other four cases of antibiotic-resistant infection, diagnosed at 36 months (one) and 42 months (three), were 9 to 14 year old boys who lived in the same village as the index case (related to or friends of the index case); they had not travelled outside the village. All of them reported no oral antibiotic-treatment other than that received during MDA. All presented with worsening skin lesions 2-weeks after azithromycin treatment and were subsequently treated with benzathine benzylpenicillin. All strains with A2059G mutations were molecular type JG8, the most common type on Lihir Island.
Changes in the prevalence of latent yaws
The prevalence of high titre latent yaws in children aged 1–5 years fell from 13·7% before MDA to 0·9% at 12 months (P=0·0005)7 and remained <1.5% at each time point thereafter. No subject 1–5 years of age had high titre latent yaws infection at 30 or 42 months (Table 3). Decreases in this index were also observed in the older age group of 6–15 years. The decrease from baseline to 12 months was significant (P<0·0001). 7 At 18, 24, 36, and 42 months, the prevalence of high titre latent yaws infection remained significantly lower that it had been before mass treatment, though in each instance it was not significantly below that recorded for the previous round.
Table 3.
Prevalence of latent yaws in subgroups determined by age
| Time (month) |
Children aged 1 – 5 years | Children aged 6 – 15 years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of children tested |
All Cases of Latent Yaws* | High-titre Latent Yaws† | No. of children tested |
All Cases of Latent Yaws* | High-titre Latent Yaws† | |||||
| no. of children (%) |
Adjusted prevalence ratio (95%CI)ठ|
no. of children (%) |
Adjusted prevalence ratio (95%CI)ঠ|
no. of children (%) |
Adjusted prevalence ratio (95%CI)‡ ∥∥∥∥ |
no. of children (%) |
Adjusted prevalence ratio (95%CI)‡**∥ |
|||
| Baseline | 117 | 26 (22·2) | 1 | 16 (13·7) | 1 | 874 | 299 (34·2) | 1 | 165 (18·9) | 1 |
| 6 mo | 77 | 10 (13·0) | 0·58 (0·20; 1·74) | 6 (7·8) | 0·57 (0·15; 2·19) | 797 | 251 (31·5) | 0·92 (0·55; 1·53) | 117 (14·7) | 0·78 (0·40; 1·53) |
| 12 mo | 114 | 6 (5·3) | 0·24 (0·09; 0·63) | 1 (0·9) | 0·06 (0·01; 0·48) | 796 | 143 (18·0) | 0·53 (0·35; 0·79) | 58 (7·3) | 0·39 (0·19; 0·77) |
| 18 mo | 81 | 9 (11·1) | 0·50 (0·22; 1·12) | 1 (1·2) | 0·09 (0·02; 0·52) | 462 | 129 (27·9) | 0·82 (0·55; 1·22) | 50 (10·8) | 0·57 (0·34; 0·98) |
| 24 mo | 69 | 6 (8·7) | 0·39 (0·17; 0·88) | 1 (1·4) | 0·11 (0·01; 0·75) | 445 | 113 (25·4) | 0·74 (0·48; 1·15) | 24 (5·4) | 0·29 (0·16; 0·52) |
| 30 mo | 65 | 4 (6·2) | 0·28 (0·10; 0·79) | 0 (0·0) | -- | 416 | 136 (32·7) | 0·96 (0·62; 1·48) | 33 (7·9) | 0·42 (0·18; 0·97) |
| 36 mo | 66 | 3 (4·5) | 0·20 (0·05; 0·80) | 1 (1·5) | 0·11 (0·02; 0·78) | 470 | 130 (27·7) | 0·81 (0·47; 1·40) | 22 (4·7) | 0·25 (0·08; 0·81) |
| 42mo. | 68 | 6 (8·8) | 0·40 (0·17; 0·94) | 0 (0·0) | -- | 422 | 84 (19·9) | 0·58 (0·37; 0·91) | 28 (6·6) | 0·35 (0·20; 0·62) |
The analysis included all seropositive children with a reactive TPHA and RPR titre of at least 1:2.
The analysis included children with a reactive TPHA and RPR titre of at least 1:16.
The adjusted prevalence ratio was calculated with the use of the cluster option of a log-binomial regression model. The baseline prevalence is the reference value.
P = 0·0165 for the significance of the model overall.
P = 0·0014 for the significance of the model overall
P = 0·0024 for the significance of the model overall
P = 0·0002 for the significance of the model overall.
Baseline, 6 and 12 month data were previously published (Ref. 7) and are included here for comparison to later time points
Proportion of ulcers due to T. p. pertenue and H. ducreyi
The pre-treatment and 12-month post-treatment PCR analyses of lesions swabs to detect T. p. pertenue and H. ducreyi DNA have been previously described.7 The proportion of subjects that had detectable T. p. pertenue DNA (either alone or co-infection with H. ducreyi) was lower at 12, 18, and 24 months than it had been at baseline (P<0·050 for each comparison), but by 30 months it increased to a similar proportion as baseline (Extended data Table 1), despite the fact that the overall prevalence of lesions remained much lower than baseline. The proportion of ulcers due to H. ducreyi remained relatively stable during the 3·5 years of follow up.
DISCUSSION
A single mass administration of antibiotics followed by targeted treatment of symptomatic cases and their contacts caused a transient sharp decline on infection levels and transmission, but did not eliminate yaws in a highly endemic island community. This is the largest study to date that evaluates a yaws eradication program using oral antibiotics and with a long, 42-month, follow-up. The initial impact of a single-dose treatment with oral azithromycin was a large reduction in prevalence of active yaws, similar to the findings subsequently described for Ghana and Solomon Islands.8–9 The maximum impact of the intervention in this study was observed by the end of the second year, but thereafter the overall prevalence of yaws increased with numerous children presenting active yaws lesions in all villages. When we performed molecular typing of T. p. pertenue samples from Lihir, we found a great reduction in genetic diversity of the circulating strains over time, which reached a minimum at 24 months when only one genotype was present. A reduction in genetic diversity and in seroreactivity of children 1–5 years of age indicated an overall reduction in transmission, and hence the susceptibility of the bacteria population to the MDA intervention.
The relapse of untreated latent infections was the most important factor that hindered elimination efforts in this community, along with, to a lesser extent, the reintroduction of yaws through imported cases. Almost half of subjects with new infections at follow up surveys had not been present for MDA. Despite the fact that we had not evaluated baseline serostatus for these children (because they were absent at the baseline exam and MDA), given the high rates of seropositivity in other children at baseline, it is likely that the active yaws episode was due to relapsed latent yaws. This finding is significant, because it highlights the importance of achieving high initial treatment coverage of all persons to be sure of treating latent cases. Results of studies done in the 1950s indicate that because of the tendency of latent yaws to relapse early in the course of untreated infection, it is critical to treat latent infections as part of eradication efforts.19–21 In addition, results of a mathematical model predict that >65% coverage of latent cases is required during each TTT program to achieve eradication;22 however, TTT, which focuses only on cases and contacts, is unlikely to detect this proportion of latently infected individuals. Given the high coverage requirements for latent cases, and the relatively high fixed-costs of reaching endemic communities when compared to the relatively low costs of generic azithromycin, it may be preferable to conduct multiple rounds of MDA prior to the switch to TTT. Determining the optimal number of MDA rounds to achieve eradication, the best intervals between rounds, and suitable interventions to prevent repeated non-attendance at MDA will require careful examination.
Importantly, we report the first documented macrolide resistance in T. p. pertenue infections. Analysis of five clinical specimens, out of 208 samples tested during the post-MDA period of 3·5 years, demonstrated a T. p. pertenue strain carrying the 23S rRNA A2059G point mutation. We speculate that the first case likely had a de novo drug resistance mutation; antibiotic pressure has been associated with selection of mutants in syphilis studies.23 There was a direct epidemiological relationship among all macrolide-resistant yaws patients suggesting that all had been infected with a single macrolide-resistant strain resulting from direct transmission by the index case; hence there was local spread of the resistant clone. The reason for this emergence is not entirely clear, as azithromycin was used in these communities (after MDA) only for targeted treatment of yaws and to treat urethritis and genital ulcer disease in adults. In Treponema pallidum subsp. pallidum, the syphilis agent, there has been a rapid increase in prevalence of macrolide resistant strains, up to 64- 100% in some developed countries.24–28 It is unclear, however, whether this increase in prevalence is due to de novo point mutations or spread of a resistant strain, or both. 14 In syphilis, macrolide-resistance mutations have been found in many different molecular strain types, consistent with de novo mutation. The frequency of emergence of primary resistance is proposed to be low because T. pallidum has two copies of the 23s rRNA gene and mutations in both operons seem to be required;29 indeed in all five mutant samples from our study, the same mutation A2059G was found in both copies of the 23S rRNA gene. However, Treponema denticola (a related spirochete) can have phenotypic resistance with a mutation in only a single allele.30 The role of dissemination of a single resistant strain in increased prevalence is unknown for syphilis, as network analyses have not been performed. For yaws, travel of individuals is limited in endemic countries, and the risk of spread from one country to another may be minimal. Emergence of macrolide-resistant strains, however, may cause local outbreaks.
The strengths of this study are the use of PCR to conclusively diagnose active yaws and the use of genotyping to differentiate between indigenous and imported cases. Also the clinical surveys involved the entire population of interest which is important to accurately measure a reduction of infection levels to zero because of the focal nature of the disease. A weakness of this study is the smaller than anticipated sample size in latent infection surveys. This may have reduced the power of our study to show definitively whether transmission of yaws had ceased (i.e. infection in children 1–5 years indicates recent infection). However, the finding of zero cases of high titre latent yaws in repeated surveys, together with results showing an overall reduced genetic diversity of strains causing active yaws, strongly indicates that transmission was largely interrupted.
The generalizability of our findings is subject to three factors. First, rural PNG might differ from other high-endemic zones in important environmental or cultural characteristics; however most yaws is found in rural tropical settings. Second, the impact of importation of infection could be larger in communities that are geographically contiguous to neighbouring areas where the disease is also endemic than in isolated island communities. Third, if the high coverage we achieved in MDA and subsequent TTT programs cannot be attained, as might be the case outside a research setting, re-emergence of disease could happen more rapidly.
Our findings have substantial implications for the scalability of yaws eradication programs internationally and support the following adaptations to the current WHO strategy. First, a considerable effort to achieve coverage rates >90% should be the goal in the first round of treatment. Second, there may be substantial benefit in distributing a second or third round of azithromycin at 6–12 months intervals. Third, efforts to eradicate yaws should aim to treat much broader geographical areas, especially in regions with substantial migration. Finally, there is the need for clinical and biological surveillance to immediately detect drug resistance through the strengthening of capacities of laboratory networks in endemic countries. Because the identified macrolide resistance mutations appear to cause no fitness disadvantage in T. pallidum, resistant strains would likely persist in communities even in the absence of antibiotic pressure. Therefore, communities where significant resistance is identified will need guidelines for clinical and operational management of macrolide resistant yaws with benzathine benzylpenicillin treatment to achieve cure and to avoid dissemination of resistant strains.
Extended Data
Extended data Table 1.
Aetiology of skin ulcers prior to MDA of azithromycin and in subsequent rounds of targeted treatment *†
| Time (month) | Participants tested for PCR |
Treponema pallidum subsp. pertenue only detected |
Both T. p. pertenue and H. ducreyi detected |
Haemophilus ducreyi only detected |
Negative for T. pallidum and H. ducreyi |
|---|---|---|---|---|---|
| no. | no. of participants (%) | ||||
| Baseline | 90* | 19 (21·1) | 12 (13·3) | 42 (46·7) | 17 (18·9) |
| 6 mo. | 84* | 14 (16·7) | 27 (32·1) | 32 (38·1) | 11 (13·1) |
| 12 mo. | 114 | 12 (10·5) | 7 (6·1) | 53 (46·5) | 42 (36·8) |
| 18 mo. | 88 | 12 (13·6) | 5 (5·7) | 35 (39·8) | 36 (40·9) |
| 24 mo. | 68 | 6 (8·8) | 7 (10·3) | 28 (41·2) | 27 (39·7) |
| 30 mo. | 120 | 19 (15·8) | 12 (10·0) | 63 (52·5) | 26 (21·7) |
| 36 mo. | 107 | 24 (22·4) | 12 (11·2) | 49 (45·8) | 22 (20·6) |
| 42mo. | 107 | 37 (34·6) | 14 (13·1) | 30 (28·0) | 26 (24·3) |
Data of PCR-confirmed yaws cases for baseline and 6 months represent a random sample of 90 and 84 ulcers that were tested by PCR from a total of 690 and 121 participants with skin ulcers detected, respectively. Data from 12 months to 42 months represent all ulcers detected.
P<0.0001 by the chi-square test for the between-group comparison within each type of infection.
Panel 1. Research in context.
Evidence before this study
We searched PubMed on February 17, 2017 for studies published in English using the search terms “yaws”, “Treponema pallidum”, “mass treatment”, “azithromycin”, and “penicillin”. We searched for studies that assessed the efficacy of mass azithromycin treatment for yaws. Empirical data on the short-term impact of the WHO strategy became available after the start of this study; a single round of mass treatment in communities with high baseline infection rates in Ghana and Solomon Islands resulted in a significant decrease in prevalence of active and latent yaws at 12 and 18 months after treatment, respectively. The quality of the evidence was low, comprising primarily small sample size cross-sectional studies. Moreover, previous experience in the 1950s using mass treatment with penicillin and recent mathematical modelling suggested that, after an initial significant reduction, the disease may persist or rebound to pre-mass treatment levels.
Added value of this study
This is the first study to report the long-term efficacy of the modern WHO strategy for yaws eradication. We repeatedly examined a community of about 16,000 people and used a Treponema pallidum specific PCR to determine the prevalence of active disease over 42 months. Through this approach we were able to accurately measure the prevalence of active yaws and detect the appearance of macrolide-resistant strains. We used a novel genotyping method to determine temporal changes on molecular diversity of T. p. pertenue strains and to identify importation events.
Implications of all the available evidence
Active and latent yaws can be cured with single-dose azithromycin treatment and a single round of mass antibiotic treatment with coverage as high as 84% greatly reduces infection levels and transmission, but does not achieve complete and permanent reduction to zero new cases, with evidence of re-emergence after 24 months. We suggest that yaws eradication policies should be revised with consideration of expansion to repeated 6–12 monthly mass treatments for at least 2–3 rounds. The data also suggest that azithromycin resistance in T. p. pertenue has emerged as a result of the program implementation, which reveals the need for effective drug resistance monitoring as part of yaws eradication programs to prevent spread of antibiotic-resistant strains.
Acknowledgments
We thank the village chairmen, elders, and villagers of Lihir Island for their willingness to be involved in the study; our field teams for efforts with study implementation; and the National Department of Health of Papua New Guinea for guidance and oversight of the trial and continued cooperation, Sergi Gavilán for project management and general administrative support, and Laia Bertran for effective knowledge management, inter-institutional liaison and acquisition of funding.
This study was funded by ISDIN and Newcrest Mining Limited; the laboratory work was supported in part by the US Public Health Service National Institutes of Health grant R01AI42143 to SAL; WHO provided generic azithromycin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
OM, KA, QB and SAL conceived and designed the study with input from SVB and JW. OM, WH, AK, RP, HA, and CGB implemented the study, and gathered data and samples. SAL and CG were primarily responsible for microbiological and molecular studies. SAL designed microbiological laboratory techniques and supervised laboratory work at University of Washington. AEB conducted the diversity analyses. SS did the statistical analyses. OM and SAL wrote the first draft of the report. All authors contributed to revisions and approved the final version.
Conflicts of interest
No author declared a conflict of interest.
References
- 1.WHO. Eradication of yaws - the Morges Strategy. WHO Wkly Epidemiol Rec. 2012;87:189–94. [PubMed] [Google Scholar]
- 2.Mitjà O, Hays R, Ipai A, et al. Single-dose azithromycin versus benzathine benzylpenicillin for treatment of yaws in children in Papua New Guinea: an open-label, non-inferiority, randomised trial. Lancet. 2012;379:342–47. doi: 10.1016/S0140-6736(11)61624-3. [DOI] [PubMed] [Google Scholar]
- 3.Kwakye-Maclean C, Agana N, Gyapong J, et al. A single dose oral azithromycin versus intramuscular benzathine penicillin for the treatment of yaws- a randomized non inferiority trial in Ghana. PLoS Negl Trop Dis. 2017;11:e0005154. doi: 10.1371/journal.pntd.0005154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinman AR, Hopkins DR. Lessons from previous eradication programs. In: Dowdle WR, Hopkins DR, editors. The eradication of 460 infectious diseases: report of the Dahlen Workshop on the Eradication of Infectious Diseases. Clichester, UK: John Wiley & Sons; 1988. pp. 19–32. [Google Scholar]
- 5.Marks M, Mitjà O, Vestergaard LS, et al. Challenges and key research questions for yaws eradication. Lancet Infect Dis. 2015;15:1220–5. doi: 10.1016/S1473-3099(15)00136-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Summary report of a consultation on the eradication of yaws: 5–7 March 2012. Morges, Switzerland: 2012. [accessed April 25, 2017]. http://www.who.int/iris/handle/10665/75528. [Google Scholar]
- 7.Mitjà O, Houinei W, Moses P, et al. Mass treatment with single-dose azithromycin for yaws. N Eng J Med. 2015;372:703–10. doi: 10.1056/NEJMoa1408586. [DOI] [PubMed] [Google Scholar]
- 8.Ghinai R, El-Duah P, Chi KH, et al. A cross-sectional study of 'yaws' in districts of Ghana which have previously undertaken azithromycin mass drug administration for trachoma control. PLoS Negl Trop Dis. 2015;9:e0003496. doi: 10.1371/journal.pntd.0003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marks M, Sokana O, Nachamkin E, et al. Prevalence of active and latent yaws in the Solomon Islands 18 months after azithromycin mass drug administration for trachoma. PLoS Negl Trop Dis. 2016;10:e0004927. doi: 10.1371/journal.pntd.0004927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitjà O, Hays R, Ipai A, et al. Outcome predictors in treatment of yaws. Emerg Infect Dis. 2011;17:1083–5. doi: 10.3201/eid1706.101575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitjà O, Lukehart SA, Pokowas G, et al. Haemophilus ducreyi as a cause of skin ulcers in children from a yaws-endemic area of Papua New Guinea: a prospective cohort study. Lancet Global Health. 2014;2:e235–41. doi: 10.1016/S2214-109X(14)70019-1. [DOI] [PubMed] [Google Scholar]
- 12.Marks M, Chi K-H, Vahi V, et al. Haemophilus ducreyi associated with skin ulcers among children, Solomon Islands. Emerg Infect Dis. 2014;20:1705–7. doi: 10.3201/eid2010.140573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi KH, Danavall D, Taleo F, et al. Molecular differentiation of Treponema pallidum subspecies in skin ulceration clinically suspected as yaws in Vanuatu using real-time multiplex PCR and serological methods. Am J Trop Med Hyg. 2015;92:134–8. doi: 10.4269/ajtmh.14-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukehart SA, Godornes C, Molini BJ, Sonnett P, Hopkins S, Mulcahy, et al. Macrolide resistance in Treponema pallidum in the United States and Ireland. N Engl J Med. 2004;351:154–8. doi: 10.1056/NEJMoa040216. [DOI] [PubMed] [Google Scholar]
- 15.Matejková P, Flasarová M, Zákoucká H, et al. Macrolide treatment failure in a case of secondary syphilis: a novel A2059G mutation in the 23S rRNA gene of Treponema pallidum subsp. pallidum. J Med Microbiol. 2009;58:832–36. doi: 10.1099/jmm.0.007542-0. [DOI] [PubMed] [Google Scholar]
- 16.Godornes C, Giacani L, Barry AM, Mitjà O, Lukehart SA. Development of a multilocus sequence typing (MLST) scheme for Treponema pallidum subsp. pertenue: application to yaws in Lihir Island, Papua New Guinea. PLoS Negl Trop Dis. 2017 doi: 10.1371/journal.pntd.0006113. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orle KA, Gates CA, Martin DH, Body BA, Weiss JB. Simultaneous PCR detection of Haemophilus ducreyi, Treponema pallidum, and herpes simplex virus types 1 and 2 from genital ulcers. J Clin Microbiol. 1996;34:49–54. doi: 10.1128/jcm.34.1.49-54.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016;33:1870–4. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahra A. Yaws eradication campaign in Nsukka Division, Eastern Nigeria. Bull World Health Organ. 1956;15:911–35. [PMC free article] [PubMed] [Google Scholar]
- 20.Rein CR. Treatment of yaws in the Haitian peasant. J National Med Ass. 1949;41:60–65. [PMC free article] [PubMed] [Google Scholar]
- 21.Hackett CJ, Guthe T. Some important aspects of yaws eradication. Bull World Health Organ. 1956;15:869–96. [PMC free article] [PubMed] [Google Scholar]
- 22.Marks M, Mitja O, Fitzpatrhick Mathematical modeling of programmatic requirements for yaws eradication. Emerg Inf Dis. 2017;23(1):22–28. doi: 10.3201/eid2301.160487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marra CM, Colina AP, Godornes C, et al. Antibiotic selection may contribute to increases in macrolide-resistant Treponema pallidum. J Infect Dis. 2006;194:1771–3. doi: 10.1086/509512. [DOI] [PubMed] [Google Scholar]
- 24.Grillová L, Ptrošová H, Mikalová L, et al. Molecular typing of Treponema pallidum in the Czech Republic during 2011 to 2013: increased prevalence of identified genotypes and of isolates with macrolide resistance. J Clin Microbiol. 2014;52:3693–3700. doi: 10.1128/JCM.01292-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Hou J, Zheng R, et al. Two mutations associated with macrolide resistance in Treponema pallidum in Shandong, China. J Clin Microbiol. 2013;51:4270–1. doi: 10.1128/JCM.01261-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muldoon EG, Walsh A, Crowley B, Mulcahy F. Treponema pallidum azithromycin resistance in Dublin, Ireland. Sex Transm Dis. 2012;39:784–6. doi: 10.1097/OLQ.0b013e318269995f. [DOI] [PubMed] [Google Scholar]
- 27.Su JR, Pillay A, Hook EW, Ghanem KG, et al. on behalf of A2058G Prevalence Workgroup. Prevalence of the 23S rRNA A2058G point mutation and molecular subtypes in Treponema pallidum in the United States, 2007 to 2009. Sex Transm Dis. 2012;39:794–8. doi: 10.1097/OLQ.0b013e31826f36de. [DOI] [PubMed] [Google Scholar]
- 28.Grimes M, Sahi SK, Godornes BC, et al. Two mutations associated with macrolide resistance in Treponema pallidum: increasing prevalence and correlation with molecular strain type in Seattle, Washington. Sex Transm Dis. 2012;39:954–8. doi: 10.1097/OLQ.0b013e31826ae7a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Šmajs D, Paštěková L, Grillová L. Macrolide Resistance in the Syphilis Spirochete, Treponema pallidum ssp. pallidum: Can We Also Expect Macrolide-Resistant Yaws Strains? Am J Trop Med Hyg. 2015;93:678–83. doi: 10.4269/ajtmh.15-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SY, Ning Y, Fenno JC. 23S rRNA point mutation associated with erythromycin resistance in Treponema denticola. FEMS Microbiol Lett. 2002;207:39–42. doi: 10.1111/j.1574-6968.2002.tb11025.x. [DOI] [PubMed] [Google Scholar]