Abstract
Objective
Abdominal aortic aneurysm (AAA) has high mortality rate when ruptured, but currently there is no proven pharmacological therapy for AAA due to our poor understanding of its pathogenesis. The current study explored a novel role of smooth muscle cell Arnt-like protein-1 (BMAL1), a transcription factor known to regulate circadian rhythm, in AAA development.
Approach and Results
Smooth muscle cell-selective deletion of BMAL1 potently protected mice from AAA induced by 1) mineralocorticoid receptor (MR) agonist deoxycorticosterone acetate (DOCA) or aldosterone (Aldo) when provided with high salt, and 2) angiotensin II (Ang II) infusion in hypercholesterolemia mice. Aortic BMAL1 was upregulated by DOCA-salt, and deletion of BMAL1 in smooth muscle cells selectively upregulated tissue inhibitor of metalloproteinase 4 (TIMP4), suppressed DOCA-salt-induced matrix metalloproteinase (MMP) activation and elastin breakages. Moreover, BMAL1 bound to the Timp4 promoter and suppressed Timp4 transcription.
Conclusions
These results reveal an important, but previously unexplored, role of smooth muscle cell BMAL1 in AAA. Moreover, these results identify TIMP4 as a novel target of BMAL1 which may mediate the AAA protective effect of smooth muscle cell BMAL1 deletion.
Keywords: abdominal aortic aneurysm, clock gene BMAL1, vascular smooth muscle, tissue inhibitor of metalloproteinase
Subject codes: Aneurysm, Mechanisms
INTRODUCTION
AAA is defined as a localized abnormal dilation of the abdominal aorta that exceeds the normal diameter by greater than 50% and is the most common form of aortic aneurysm. AAA affects 4% to 8% men over the age of 60 and accounts for approximately 2% of all deaths in Western countries.1 Patients harboring an AAA are often asymptomatic until the aneurysm ruptures, which results in severe morbidity and a high incidence of mortality. In particular, there is no drug of demonstrated efficacy for this devastating disease, and open or endovascular surgery is the only options for patients with AAA.2 Thus, there is an urgent clinical need to decipher molecular mechanisms to identify therapeutic targets to develop effective drugs to treat AAA.
We reported recently that administration of MR agonists, DOCA or Aldo, in combination with high salt to wild-type (WT) C57BL/6 male mice potently induced AAA formation and rupture in an age-dependent manner.3 These findings suggest high salt intake and high aldosterone are two factors for promotion of AAA. Some human studies support this notion:4, 5 An analysis of drug modulation of AAA growth through 25 years of surveillance in 1,269 patients demonstrated a strong association between MR blockers and slowed AAA progression4 and reported high-salt intake was associated with increased prevalence of AAA and larger aortic diameter in older men.5 However, the mechanism that underlies DOCA or Aldo plus salt-induced AAA is largely unknown.
Brain and muscle ARNT-like (BMAL1; also known as MOP3 in human or Arnt3 in mouse), a basic helix-loop-helix transcription factor and an obligatory core clock gene, is essential for normal circadian rhythmicity in physiology and behavior.6 Global deletion of BMAL1 causes immediate and complete loss of circadian rhythmicity in mice.7 Interestingly, aortic dissection and rupture exhibit circadian variability,8 suggesting a potential involvement of the clock gene in AAA. Moreover, accumulating evidence suggests that, in addition to regulating circadian rhythm, BMAL1 is involved in vascular pathologies,9 including flow-dependent vascular remodeling,10 aortic vascular stiffness,11 transplant arteriosclerosis,12 and increased superoxide and endothelial nitric oxide (NO) synthase uncoupling in the aorta.13 However, whether BMAL1 is indeed involved in AAA has not yet been investigated.
Vascular smooth muscle cells (VSMC) play a critical role in three aortic aneurysm hallmark processes: degradation of elastin and extracellular matrix protein, loss of medium layer smooth muscle cells, and intense inflammatory cell infiltration.14, 15 Smooth muscle cell (SMC) myosin and actin mutations have been linked to thoracic aortic aneurysm development in humans.16 To experimentally test the role of SMC BMAL1 in aortic aneurysm, we selectively deleted SMC BMAL1 and determined its effect on DOCA and salt (DOCA-salt) - or Aldo and salt (Aldo-salt)-induced AAA as well as Ang II-induced AAA in hypercholesterolemia mice. Here we report our surprising finding that selective deletion of SMC BMAL1 provides very effective protection from aortic aneurysm in two different AAA mouse models. Moreover, such protection was associated with selective up-regulation of tissue inhibitor of metalloproteinases 4 (TIMP4), raising the possibility that TIMP4 contributes to the protection.
MATERIALS AND METHODS
Animals
Ten-week-, 4-month- or 8- to 10-month-old male C57BL/6, SM-Bmal1−/− and WT littermates (Bmal1flox/flox) were used for this study. SM-Bmal1−/− mice were generated by crossing female Bmal1flox/flox mice17 (JAX stock #007668) with male smooth muscle-specific SM22α-Cre knockin mice18 (JAX stock # 006878). The genotyping methods and characterization of the SM-Bmal1−/− mice were described previously.19 10-week-old male C57BL/6 mice were purchased from the Jackson Laboratory (JAX stock 000664). All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Kentucky.
General experimental protocols
The procedures for the deoxycorticosterone acetate (DOCA)-salt and aldosterone (Aldo)-salt AAA mouse models and for the angiotensin II (Ang II)-infusion-induced AAA in hypercholesterolemia mice were described previously. 3, 20
Ultrasound imaging
The procedures for using a high-resolution ultrasound imaging system (Vevo 2100, Visualsonics) to measure maximal intraluminal diameter of abdominal aortas were described previously.3 Briefly, mice were laid supine on a heated table, warmed ultrasound transmission gel was placed on the abdomen. Cine loops of 300 frames were acquired throughout the renal region of the abdominal aorta (short axis) and used to determine the maximal diameters of the abdominal aorta in the suprarenal region. “Portal Triad” (hepatic artery, hepatic vein, and bile duct) was used as anatomic markers to assess transverse images in the same location in each mouse.
Blood pressure measurement
The procedures for using a non-invasive tail-cuff method (Coda 8; Kent Scientific Corp.) to measure blood pressure were described previously3. Briefly, blood pressure was measured before and two-weeks after DOCA-salt or three-weeks after Aldo-salt administration. Blood pressure was measured at the same time of day, five days a week on a 37°C heated stage and the data collected during the last three days were used for analysis. Each day, mice were subjected to ten preliminary and ten recorded measurements, and a minimum of five measurements on each mouse was required for inclusion of data.
Definition and quantification of Aortic Aneurysms
The definition of DOCA-salt-, Aldo-salt-, PCSK9.AAV/ Diet /Ang II-induced AAA and TAA were defined as having at least a 50% increase in the maximal intraluminal and external diameters compared with the same region of the aorta in the control mice. The maximal intraluminal diameters of suprarenal abdominal aortas were quantified in vivo by ultrasound imaging. The maximal external diameters of descending thoracic aortas and abdominal aortas were quantified ex vivo under a microscope.
Plasma Sodium Measurement
Plasma sodium and potassium concentrations were measured by the VetScan i-STAT1 handheld analyzer (Abaxis) with I-STAT E3+ Cartridge (Cat# 600-9004-25, Abaxis) according to the manufacturer’s instructions.
In Situ Zymography and gel Zymography
The procedures for in situ zymography were described previously.3 For gel zymography, the whole aortas were isolated from SM-Bmal1−/− mice and WT littermates administered with DOCA-salt, dissected and incubated in a Krebs buffer for 24 hours at 37°C. The medium containing MMPs was collected, and proteins were resolved in an SDS-PAGE containing 2% gelatin (Cat# G1890, Sigma-Aldrich). The gelatin zymogram gel was stained with GelCode Blue Safe Protein (Cat# 1860983, ThermoFisher) and scanned for visualization of MMPs as described.21
Real-time PCR
The procedures for real-time PCR were described previously.3, 19, 22–25 Briefly, aortic arch, descending aorta, suprarenal aorta, and infrarenal aorta, as well as whole aortas, were placed in Invitrogen™ RNAlater™ Stabilization Solution (Cat# AM7021, Fisher). After removing the adventitia and endothelium, total RNA was extracted using an RNeasy mini-kit (Cat# 74104, Qiagen) by following the manufacturer’s instructions.
For cultured smooth muscle cells, BMAL1-deficient and WT aortic vascular smooth muscle cells were isolated from SM-Bmal1−/− mice and WT littermates as described previously.19, 23–25 Cells were grown on a modified DMEM medium (Cat# D2902, Sigma-Aldrich; adding 1.85 g of NaHCO3 and 25 mM HEPES (PH7.4) to 1 litter DMEM to produce 133.8 mM NaCl final concentration) containing 10% (v/v) FBS until 70% confluence. Cells were then incubated with FBS-free medium overnight and stimulated with or without Aldo (10 nM) and high salt (by adding additional 10 mM NaCl) for 24 hours. Total RNA was extracted with RNAzol RT (Cat# RN190, Molecular Research Center) following the manufacturer’s protocol.
The cDNA was synthesized by M-MLV reverse transcriptase (Cat# 28025013, Invitrogen) using random hexamers. The primers used for amplification of mouse MR, Timp1, Timp2, Timp3 and Timp4 mRNAs were described in Supplemental Table I. The primers used for amplification of mouse Bmal1, Per1, Rev-erbA, Cry1, and 36B4 were described previously.19, 26 All PCR primers were designed by Primer3Plus and were chosen only if they either crossed introns or flanked introns to eliminate potential genomic DNA contamination. The real-time PCR reactions were performed using a GoTaq G2 Flexi DNA polymerase with SYBR green (Cat# M7801, Promega) in a BIO-RAD CFX96 Real-Time System. Two controls, one with no template and one with no reverse transcription, were included in each real-time PCR run to ensure the specificity of real-time PCR. Importantly, real-time PCR dissociation curve analysis was performed for all genes, and only the data that passed real-time PCR dissociation curve analysis were taken. All mRNA expressions were normalized to the 36B4 rRNA and quantified by a standard curve method.
Histological and Immunohistological Staining
The procedures for histological and immunohistological staining were described previously.3, 22
Tissue chromatin immunoprecipitation (ChIP) assay
The procedures for tissue ChIP assay were described previously19 and in supplemental materials.
Cloning mouse Timp4 promoter
A mouse Bacterial Artificial Chromosome clone containing the mouse Timp4 promoter was purchased from Invitrogen. A 2-kb of the Timp4 promoter was amplified by PCR using primers as described in Supplemental Table I. The cloned Timp4 promoter was verified by DNA sequencing (ACGT, Inc) and subcloned into a pGL3 basic vector (Cat# E1751, Promega) at KpnI and XhoI sites.
Timp4 promoter assay
The procedures for luciferase promoter assay were described previously.19, 25 In particular, BMAL1-deficient and WT aortic vascular smooth muscle cells were isolated from SM-Bmal1−/− and WT littermate as described previously.19, 23–25 Cells were grown on a DMEM medium containing 10% (v/v) FBS until 70% confluence. Cells were then incubated with FBS-free medium overnight and were co-transfected with a pGl3-Timp4-promoter firefly luciferase construct and a pRL-SV40 renilla luciferase control vector (Cat# E2231, Promega) using Lipofectamine-Plus reagent (Cat# 15338100, Invitrogen). The Timp4 luciferase promoter activity was assayed by a modified dual luciferase enzyme assay as described.19, 25
Enzyme-linked immunosorbent assay (ELISA) of TIMP4 protein
Protein was extracted by radioimmunoprecipitation assay buffer (RIPA buffer) from snap-frozen aortas from 10-month-old male SM-bmal1−/− mice and WT littermates administered with DOCA-salt for three weeks. The protein concentration was determined by a Pierce™ BCA™ Protein Assay kit (Cat# PI23227, Fisher). The equal amount of protein was used to determine the TIMP4 protein level in the aorta extracts by a Mouse TIMP-4 DuoSet ELISA kit (Cat# DY7667-05, R&D Systems) following the manufacturer’s instruction.
Production and injection of PCSK9.AAV
The production and injection of AAV into mice were described previously.20 Briefly, AAVs (serotype 8) were produced by the Viral Vector Core at the University of Pennsylvania. These AAVs contained inserts expressing mouse PCSK9D377Y mutation (equivalent to human PCSK9D374Y gain-of-function mutation (referred to “PCSK9.AAV” in this manuscript). PCSK9.AAV was diluted in sterile PBS (2 x 1011 AAVs in 200 μl) and was injected intraperitoneally (one time).
Statistical analysis
All data were expressed as mean ± S.E.M. Continuous data were assessed both visually and statistically for normality. Non-normally distributed data were log-transformed and re-analyzed for normality. Data were analyzed by unpaired Student t-tests for two groups, by one-way ANOVA for one group with multiple time point, by two-way repeated measures ANOVA on time for longitudinal data with between-group factors of mouse genotype, or by two-way ANOVA with between-group factors of mouse genotype, treatment, or aorta region. When difference existed between groups, a Bonferroni’s post-hoc analysis was performed. For comparison of the incidence of TAA or AAA, Chi-Square analysis was performed. The P < 0.05 was considered significant. The P ≥ 0.05 was considered non-significant (ns).
RESULTS
Deletion of BMAL1 in SMCs potently protected mice from DOCA or Aldo plus salt-induced AAA
We recently developed a novel SMC BMAL1 knockout mouse model (SM-Bmal1−/−) and demonstrated that SMC BMAL1 exerted a critical role in the normal amplitude and time-of-day variation in vasoconstriction and blood pressure circadian rhythm.19 To define a role of SMC BMAL1 in AAA, we investigated Aldo-salt-induced AAA in 4-month-old SM-Bmal1−/− mice and WT littermates. Ultrasound quantification of the intraluminal diameter of the abdominal aorta illustrated that Aldo-salt induced a time-dependent aortic dilation in WT mice, and this time-dependent aortic dilation was largely suppressed in SM-Bmal1−/− mice (Figure 1A). Similarly, an increase in the external diameter of the abdominal aorta by Aldo-salt was also found in WT mice but not in SM-Bmal1−/− mice (Figure 1B). Noticeably, 5 out of 14 WT mice (36% incidence) developed AAA and only 1 out of 14 WT mice (7% incidence) also developed TAA, but none of SM-Bmal1−/− mice developed either AAA or TAA (Figure 1C and 1G (left panel)).
Figure 1. Deletion of BMAL1 in SMCs protects mice from Aldo- or DOCA-salt-induced aortic aneurysms.
4-month-old SM-Bmal1−/− mice (n=13) and WT littermates (n=14) were administered with Aldo-salt for 4 weeks (A through C). 8-month-old male SM-Bmal1−/− mice (n=16) and WT littermates (n=16) were administered with DOCA-salt for 3 weeks (D through F). A and D, Quantifications of maximal intraluminal diameters of abdominal aorta by ultrasound. B and E, Quantifications of maximal external diameters of the abdominal and thoracic aorta by microscopy. C and F, Incidences of AAA, TAA, and aortic aneurysm rupture. G, Representative pictures of the aorta with or without AAAs. Two-way repeated measure ANOVA followed by Bonferroni’s post-hoc analysis was used for statistics in (A and D). Unpaired t-test was used for statistics in (B and E). Chi-Square was used for comparing AAA incidence in (C and F). *, P<0.05; ***, P<0.001. ns, not statistically significant.
We also investigated whether deletion of BMAL1 in SMCs afforded protection from DOCA-salt-induced AAA in 4-month-old SM-Bmal1−/− mice and WT littermates. We observed a similar protective effect of deleting BMAL1 in SMCs on DOCA-salt-induced aortic aneurysms. In particular, SM-Bmal1−/− mice exhibited less increased external diameters of the abdominal and thoracic aorta (Supplemental Figure I A), decreased the incidence of AAA, TAA, and aortic rupture (Supplemental Figure I B and I C) compared to WT mice. Since the incidence and severity of AAA induced by DOCA-salt are age-dependent,3 we investigated whether deletion of BMAL1 in SMCs was also effective in protecting mice from DOCA-salt-induced AAA in 8-month-old mice. The result show again that the increases in intraluminal diameters of abdominal aorta (Figure 1D), external diameters of abdominal and thoracic aorta (Figure 1E), and the incidence of AAA, TAA, and aortic rupture (Figure 1F and Figure 1G (right panel)) were all abolished in SM-Bmal1−/− mice compared to that in WT littermates.
Deletion of BMAL1 in SMCs had minimal effect on renal sodium handling and blood pressure changing in response to DOCA or Aldo plus salt
To investigate mechanisms by which deletion of BMAL1 in SMCs protects mice from DOCA- or Aldo-salt-induced AAA, we first tested the possibility that BMAL1 deletion in SMCs might affect renal sodium handling as high salt intake is required for DOCA- or Aldo-induced AAA.3 In the absence of DOCA-salt, there were no differences in kidney weight, length, urine volume at light phase and dark phase, and plasma sodium concentration at Zeitgeber Time (ZT) 5 and ZT17 between SM-Bmal1−/− mice and WT littermates (Figure 2A through 2D). In the presence of DOCA-salt, plasma sodium concentrations were increased whereas the level of plasma potassium was decreased to a similar extent in SM-Bmal1−/− mice and WT littermates (Figure 2E and 2F), suggesting that deletion of BMAL1 in SMCs has minimal effect on basal and DOCA-salt-induced electrolyte handling in the kidney.
Figure 2. Deletion of BMAL1 in SMCs does not affect basal and Aldo- or DOCA-salt-induced renal sodium handling and blood pressure.
A through D, there was no difference in kidney weight (A, n=16–17), kidney length (B, n=16–17), urine volume (C, n=6–9), and plasma sodium (D, n=5–7) between 4-month-old SM-Bmal1−/− mice and WT littermates. L, light phase. D, Dark phase. ZT, zeitgeber time. E through I, 8-month-old (E, n=4–7; F, n=4–7; and I, n=13) and 4-month-old (G, n=13 and H, n=24) SM-Bmal1−/− mice and littermates were administered with Aldo-salt for 4 weeks (G) or DOCA-salt for 3 weeks (E, F, H, and I). The unpaired t-test was used for statistics in (A and B). Two-way ANOVA followed by Bonferroni’ post-hoc test was used for statistics in (C, D, E, F, G, H, and I). *, P<0.05; **. P<0.01; ***, P<0.001. ns, not statistically significant.
We also measured blood pressure by a tail-cuff method in SM-Bmal1−/− mice and WT littermates before and after DOCA- or Aldo-salt administration. Lower basal blood pressure was found in 4-month-old SM-Bmal1−/− mice than that in WT littermates (Figure 2G and 2H), which is consistent with our previous results measured by a telemetry method.19 However, regardless of their ages, both SM-Bmal1−/− mice and WT littermates increased their blood pressures to a similar extent in response to DOCA-salt or Aldo-salt (Figure 2G through 2I). Interestingly, there was no difference in basal blood pressure between 8-month-old SM-Bmal1−/− mice and WT littermates (Figure 2I).
Deletion of BMAL1 in SMCs had minimal effect on MR mRNA expression in abdominal aorta
We then tested the possibility that BMAL1 deletion in SMCs may decrease MR expression in aorta and therefore protect mice from DOCA- or Aldo-salt-induced AAA as MR is increased in aged aortas,27 SMC MR is involved in age-related hypertension,28 and MR is required for DOCA- or Aldo-salt-induced AAA.3
We determined aortic MR mRNA abundance in SM-Bmal1−/− mice and WT littermates before and after administration with DOCA-salt for 7 days. First, DOCA-salt appeared to suppress MR mRNA expression in WT mice: a significant inhibition was found in the aortic arch and descending aorta, and a trend towards downregulation was found in the suprarenal and infrarenal aorta (Supplemental Figure II A through II D). Second, deletion of BMAL1 in SMCs appeared to have minimal effect on MR mRNA transcript in the aorta before and after DOCA-salt administration except for in descending aorta where deletion of smooth muscle cell BMAL1 significantly decreased MR mRNA abundance.
Deletion of BMAL1 in SMCs abolished DOCA-salt-induced MMP activation and elastin breakage in abdominal aorta
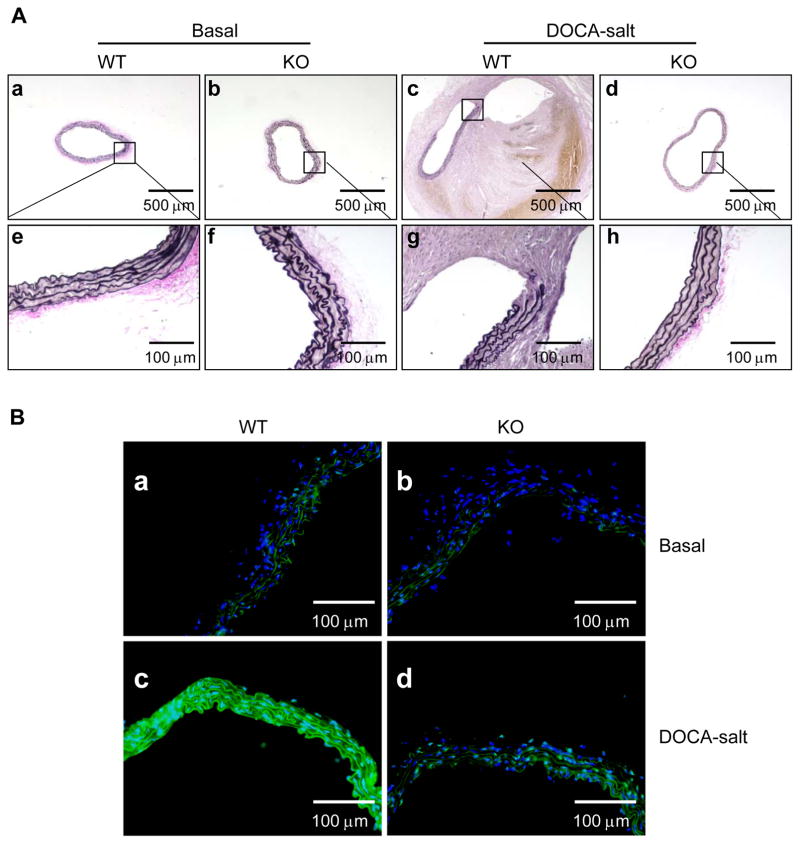
We investigated the effects of BMAL1 deletion in SMCs on DOCA-salt-induced elastin breakage as it is well-recognized for its pivotal causal role in aortic dilation and AAA formation and rupture.1, 2 Verhoeff’s Van Gieson (EVG) elastin staining (Figure 3A) and quantitative data (Supplemental Figure III) demonstrated that DOCA-salt-induced elastin breakage, which is consistent with our previous results,3 and deletion of BMAL1 in SMCs effectively prevented DOCA-salt-induced elastin breakage.
Figure 3. Deletion of BMAL1 in SMCs prevents DOCA-salt-induced elastic breakage and MMP activation.
A, Verhoeff’s Van Gieson (EVG) elastin staining of abdominal aortic cross-sections from 8-month-old SM-Bmal1−/− mice (b, d, f, and h) and WT littermates (a, c, e, and g) administrated with (c, d, g, and h) or without (a, b, e, and f) DOCA-salt for 4 weeks. B, In situ zymography detection of MMP activity in cryosections of abdominal aorta from 8-month-old SM-Bmal1−/− mice (b and d) and WT littermates (a and c) administered with (c and d) or without (a and b) DOCA-salt for 7 days. Nuclei were stain with DAPI (blue), elastin exhibited fiber-like autofluorescence (green), and MMP activity was detected by the fluorescence (green) between elastin fibers. Shown in the figure are representative images of three independent experiments.
We then investigated the effect of BMAL1 deletion in SMCs on DOCA-salt-induced MMP activation as MMPs, in particular, MMP2 and MMP9, have been demonstrated to be critical for elastin breakage in AAA in animal models.29 We used in situ zymography to determine MMP activity in tissue sections of abdominal aortas from SM-Bmal1−/− mice and WT littermates administered with DOCA-salt for 7 days. In the absence of DOCA-salt, minimal MMP activity was detected in aortas from both SM-Bmal1−/− mice and WT littermates. In the presence of DOCA and salt, however, a pronounced MMP activity was detected in aortas from WT littermates, which was consistent with our previous results,3 but not in SM-Bmal1−/− mice (Figure 3B). This result indicates that deletion of BMAL1 in SMCs prevents mice from DOCA-salt-induced elastin breakage is associated with inhibition of MMP activity.
BMAL1 deletion in SMCs increased TIMP4 expression in abdominal aorta
The protective effect of BMAL1 deletion in SMCs on AAA may be attributable to inhibiting/downregulating MMP2/MMP9 and/or to activating/upregulating aortic TIMPs. To investigate which mechanisms operate in SM-Bmal1−/− mice, we determined aortic MMP2 and MMP9 activities in SM-Bmal1−/− mice and WT littermates administered with DOCA-salt for 7 days by gel zymography, a simple but sensitive method to detect MMP under a condition at which MMPs are dissociated from bound TIMPs.21 Consistent with the result of in situ zymography (Figure 3B), DOCA-salt significantly increased proMMP2, MMP2, proMMP9, and MMP9 activities in WT mice (Supplemental Figure IV). Surprisingly, in sharp contrast to the result of in situ zymography, deletion of BMAL1 in SMCs had minimal effect on DOCA-salt-induced proMMP2, MMP2, proMMP9, and MMP9 expression. In fact, under basal condition, a trend towards an increase in proMMP2, MMP2, and proMMP9 activities was found in SM-Bmal1−/− mice relative to that in WT mice.
Since in gel zymography detects MMP activity under a condition that all MMPs, including proMMPs, are activated by SDS, the activity of MMPs detected by in-gel zymography is proportional to the abundance of MMP protein in samples regardless of whether they are active or inactive.21 In contrast, in situ zymography is only to detect active MMPs. Thus, the different effects of deleting BMAL1 in SMCs on DOCA-salt-induced MMP activities between using gel zymography and using in situ zymography suggest that the activity of MMPs, but not the level of MMPs, is suppressed by the deletion of BMAL1 in SMCs. We, therefore, investigated the possibility that BMAL1 regulates TIMPs, which are the major endogenous cellular determinants of MMP activities.30, 31
Four TIMPs (TIMP1, TIMP2, TIMP3, and TIMP4) have been identified and characterized in mammals, and all four TIMPs can inhibit active forms of all MMPs with distinct affinity and potency.30, 31 To explore which TIMP is expressed in the aorta, responds to DOCA-salt and is regulated by SMC BMAL1, we determined the mRNA abundance of all four Timps in aortas isolated from SM-Bmal1−/− mice and WT littermates before and 7 days after DOCA-salt administration. While all four Timp mRNA were present in mouse aorta, no significant differences were detected in Timp1 and Timp2 mRNA abundance before and after DOCA-salt administration or between SM-Bmal1−/− mice and WT littermates (Figure 4A and 4B). In contrast, Timp3 mRNA was increased by DOCA-salt in both WT and SM-Bmal1−/− mice, but deletion of BMAL1 in SMCs suppressed such DOCA-salt-induced Timp3 up-regulation (Figure 4C). These results suggest that Timp3 may play a compensatory role in DOCA-salt-induced AAA and unlikely accounts for the protective effect of deletion of BMAL1 in SMCs on AAA.
Figure 4. Deletion of Bmal1 in SMCs selectively increases TIMP4 expression in mouse aorta.
A through D, Timp1 (A, n=5–6), Timp2 (B, n=5–6) Timp3 (C, n=5–6) and Timp4 (D, n=4–6) mRNA abundance in aortas from 8-month-old SM-Bmal1/− mice and WT littermates administrated with or without DOCA-salt for 7 days. E, Timp4 mRNA expression in aortas from 8-month-old SM-Bmal1−/− mice and WT littermates administrated with or without DOCA-salt for 21 days (n=3–4). F, TIMP4 protein concentration was determined by ELISA in medium released from aortas of 8-month-old SM-Bmal1−/− mice and WT littermates administrated with or without DOCA-salt for 7 days (n=3–4). G, Representative immunostaining of TIMP4 in paraffin-embedded abdominal aortic cross-sections from 8-month-old SM-Bmal1−/− mice and WT littermates administered with or without DOCA-salt for 21 days (n=3). Two-way ANOVA followed by Bonferroni’s post-hoc analysis was used for statistical analysis. *, P<0.05; **, P<0.01; ***, P<0.001. ns, not statistically significant.
Interestingly, Timp4 mRNA was upregulated in aortas from SM-Bmal1−/− mice compared to WT littermates, and such up-regulation was more obvious and significant at 7 days after DOCA-salt administration (Figure 4D). A similar and more dramatic result was also observed in aortas from SM-Bmal1−/− mice compared to that in WT littermates at 21 days after DOCA-salt administration (Figure 4E), suggesting that Timp4 mRNA upregulation in SM-Bmal1−/− mice was sustained. To investigate whether the Timp4 mRNA up-regulation leads to increased protein, we also determined TIMP4 protein expression in aortas from SM-Bmal1−/− mice and WT littermates administered with DOCA-salt for 21 days. Two distinct approaches were taken. First, TIMP4 protein in the isolated aortas was measured by ELISA. In agreement with Timp4 mRNA increase, the TIMP4 protein was upregulated in aortas from SM-Bmal1−/− mice relative to that in WT littermates before and after DOCA-salt-administration (Figure 4F). Second, isolated abdominal aortas were subjected to TIMP4 immunohistological staining. Again, a significant higher TIMP4 protein abundance was found in SM-Bmal1−/− mice than that in WT littermates under basal and DOCA-salt conditions (Figure 4G). Of note, the increase in TIMP4 protein was mainly observed in the medium SMC layer of the vessel wall from SM-Bmal1−/− mice relative to that in WT littermates, indicating a spatial correlation between SMC BMAL1 deletion and TIMP4 upregulation.
Since DOCA-salt causes infiltration of a large number of inflammatory cells into the aorta,3 the results from the in vivo experiments (Figure 4A through 4F) reflect Timp transcripts in all cells that were present in the aorta, including inflammatory cells, endothelial cells, SMCs, and fibroblasts. To determine the contribution of resident vascular cells relative to infiltrated inflammatory cells to the observed alterations in Timp transcripts, we determined mRNA abundance of the four Timps in an aortic organ culture under basal condition and after addition of Aldo (10 nM) and high salt (additional 10 mM NaCl) for 24 h. To further determine the contribution of SMCs to the observed alterations in Timp transcripts, we also determined mRNA abundance of all four Timps in cultured primary aortic VSMCs under a similar condition.
In agreement with the in vivo results (Figure 4D and 4E), Timp4 mRNA was significantly up-regulated by deletion of BMAL1 in SMCs in aortic organ culture (Supplemental Figure V D) and primary VSMC culture (Supplemental Figure VI D) under basal and Aldo-salt conditions although the extent of up-regulation seems to vary among in vivo (in mice), ex vivo (aortic organ culture), and in vitro (in VSMC cell culture). Interestingly, in disagreement with the results in vivo (Figure 4A and 4C) and ex vivo (Supplemental Figure V A and V C), Timp1 and Timp3 transcripts were significantly upregulated in BMAL1-deficient VSMCs (Supplemental Figure VI A and 6C). The variations in Timp1 and Timp3 regulations may be attributed to different types and/or numbers of cells (e.g., inflammatory cells, EC, SMCs, and fibroblasts) and/or the presence of extracellular matrix. Nonetheless, these data clearly demonstrate that TIMP4 is up-regulated at the mRNA levels by deletion of BMAL1 in SMCs.
Figure 6. BMAL1 binds to the TIMP4 promoter and suppresses its transcription.
A, Schematic diagram of 2 kb mouse TIMP4 promoter showing the positions of the seven putative E-boxes and the five regions on the TIMP4 promoters amplified by ChIP PCR primers. E1–E7: E-box1-7; F4/R4-F7/R7: 4 sets of forward and reverse ChIP PCR primers. B, Representative aortic tissue ChIP PCR shows that BMAL1 binds to the TIMP4 promoter at E-box 2, 3, 4, 6, and 7, but not E-box 5. C, TIMP4 promoter activity relative to pGL3-basic vector in WT mouse aortic VSMCs (n=4–5). D, TIMP4 promoter activity in aortic VSMCs from SM-Bmal1−/− mice and WT littermates (n=5). E and F, BMAL1 mRNA (E) and protein (F) level in aortic VSMCs from SM-Bmal1−/− mice and WT littermates (n=3). G, Timp4 mRNA level in aortic VSMCs from SM-Bmal1−/− mice and WT littermates (n=3). The unpaired t-test was used for statistics in (C, D, E, and G). *, P<0.05; **, P<0.01; ***, P<0.001.
BMAL1 was upregulated in aorta by DOCA-salt
It is clear that SMC BMAL1 is critically involved in DOCA- or Aldo-salt-induced MMP activation, elastin breakage, and AAA formation, which probably involves TIMP4. However, it is unclear how SMC BMAL1 is involved. To address this mechanistic issue, we first investigated whether BMAL1 mRNA is increased in older mice compared to younger mice thus potentially contribute to the increased susceptibility of older mice to DOCA-salt-induced AAA. We determined and compared the BMAL1 mRNA abundance in aortas from 4-month- vs. 10-month-old WT C57BL/6J mice. Surprisingly, we found a slight but significant decrease in BMAL1 mRNA in the aortas from 10-month-old mice compared to those from 4-month-old mice (0.089 ± 0.014 vs. 0.133 ± 0.013, n=5–6, P<0.05). We then investigated whether BMAL1 mRNA was increased by DOCA-salt in aorta from 8-month-old C57BL/6J mice. Using a specific BMAL1 antibody that we recently developed,19 we found that BMAL1 protein was upregulated in aorta 21 days after DOCA-salt administration (Figure 5A). To investigate whether DOCA-salt-induced BMAL1 up-regulation precedes AAA, we determined BMAL1 mRNA abundance in aorta 7 days after DOCA-salt administration and found that BMAL1 mRNA abundance was increased by 6-fold in aortas from mice administered with DOCA-salt compared to that in control mice (Figure 5B).
Figure 5. BMAL1 is upregulated in mouse aorta by DOCA-salt administration.
A, Representative immunohistological analysis of BMAL1 in paraffin-embedded aortic cross-sections from 8-month-old C57BL/6J mice administrated with DOCA-salt for 21 days (c and d) compared to that in the basal level (a and b). B, Bmal1 mRNA level in the aorta from 8-month-old C57BL/6J mice administrated with DOCA-salt for 7 days (n=5–6). Unpaired student t-test was used for statistical analysis. ***, P<0.001.
TIMP4 was identified as a new target of BMAL1 in aorta
To identify the molecular mechanism by which deletion of BMAL1 in SMCs upregulates TIMP4 mRNA and protein, we tested the possibility that BMAL1 binds directly to Timp4 gene promoter and suppresses its transcription.
First, we analyzed the mouse Timp4 promoter DNA sequence and identified several canonical E-boxes (CANNTG, where N can be any nucleotide) that are potential binding sites for BMAL1 (Figure 6A). To determine whether BMAL1 binds to these putative E-boxes, we performed chromatin immunoprecipitation (ChIP) assays in aortas from WT mice. The results showed that BMAL1 bound to the Timp4 promoter at E-box 2 through E-box 7 except for E-box 5 (Figure 6B).
Second, to investigate whether binding of BMAL1 to the Timp4 promoter regulated its activity, we cloned a 2-kb mouse Timp4 promoter, inserted it into a luciferase reporter vector (pGl3-Timp4P-luc), and transfected the pGl3-Timp4P-luc construct into aortic VSMCs isolated from SM-Bmal1−/− mice and WT littermate. In WT cells, the Timp4 promoter exhibited a 6-fold increase in luciferase activity over the pGL3 luciferase vector (Figure 6C). In contrast, when transfected into in BMAL1-deficient cells, the Timp4 promoter activity was further increased by 9-fold over that in WT cells (Figure 6D), suggesting that Timp4 transcription is suppressed by BMAL1 in WT cells. BMAL1-deficient cells were verified by quantification of BMAL1 mRNA (Figure 6E) and protein (Figure 6F) expression levels.
Third, to investigate whether the observed BMAL1-mediated Timp4 transcriptional suppression translated to its mRNA suppression, we determined Timp4 mRNA expression in WT and BMAL1-deficient cells. We found that Timp4 mRNA was 27-fold higher in BMAL1 deficient cells than in WT cells (Figure 6G), suggesting that BMAL1 suppresses Timp4 promoter activity and mRNA expression in cultured VSMCs.
BMAL1 was implicated in DOCA-salt-induced apoptosis and neutrophil infiltration in aorta
BMAL1 has been shown to regulate apoptosis and inflammation in atherosclerosis and its complications.32 Since apoptosis and inflammation also play pivotal roles in aortic aneurysm,1 we explored whether deletion of BMAL1 in smooth muscle cells, in addition to upregulating TIMP4 and inhibiting MMP activity, is also involved in DOCA-salt-induced apoptosis and neutrophil infiltration in mouse aorta.
Aortas were isolated from 10-month-old male SM-Bmal1−/− mice and WT littermates 10 days after DOCA-salt administration. Apoptotic cells were detected by a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) kit. In accordance with our previous report,3 DOCA-salt-induced TUNEL positive cells in aorta from WT littermates (Supplemental Figure VII, a vs. c), but not in aorta from SM-Bmal1−/− mice (Supplemental Figure VII, b vs. d), indicating that BMAL1 deletion in SMCs protects mice from apoptosis. Consistent with this interpretation, we also found that immunostaining of cleaved active caspase 3, an index of activation of caspase 3, was induced by DOCA-salt in aorta from WT littermates (Supplemental Figure VII, e vs. g), but such caspase 3 activation was abolished in aorta from SM-Bmal1−/− mice (Supplemental Figure VII, f vs. h). In addition, we also investigated whether deletion of BMAL1 in SMCs affects DOCA-salt-induced aortic cell proliferation. Interestingly, deletion of BMAL1 in SMCs had minimal effect on DOCA-salt-induced proliferating cell nuclear antigen (PCNA)-positive cells (Supplemental Figure VII i through VII l).
To define a potential role of SMC BMAL1 in DOCA-salt-induced vascular inflammation, we determined neutrophil infiltration by immunohistological analysis of aortic cross-sections with a rabbit anti-mouse polymorphonuclear leukocyte (PMN) antibody. We found that 10 days after DOCA-salt administration, PMN-positive cells were significantly increased only in aortas from the WT littermates, but not SM-Bmal1−/− mice (Supplemental Figure VIII a through VIII d). In contrast, deletion of BMAL1 in SMCs had minimal effect on SMC-specific protein caldesmon expression under basal and DOCA-salt conditions (Supplemental Figure VIII e through VIII h).
Taken together, these results suggest that inhibiting apoptosis, and neutrophil infiltration can also contribute to the protective effects of BMAL1 deletion in SMCs on AAA.
Deletion of BMAL1 in SMCs potently protected hypercholesterolemia mice from Ang II-induced AAA
To test whether the protective effect of SMC BMAL1 deletion on AAA is only effective in the specific Aldo- or DOCA-salt-induced AAA model, we investigated whether SM-Bmal1−/− mice are also protected from Ang II-induced AAA in hypercholesterolemia condition. We recently reported that injection of normocholesterolemic C57BL/6J mice with an adeno-associated virus (AAV) expressing a gain-of-function mutation of mouse protein convertase subtilisin/kexin type 9 (PCSK9D377Y) increased Ang II-induced AAA.20 To induce hypercholesterolemia, 4-month-old male SM-Bmal1−/− mice and WT littermates were i.p. injected with AAV-PCSK9D377 and fed a Western diet. In agreement with our previous report,20 plasma cholesterol concentration was drastically elevated in WT littermates 3 weeks after AAV-PCSK9D377 injection and Western diet feeding, which maintained high concentrations throughout the subsequent 8-weeks of the experiments (Figure 7A). Importantly, there was no difference in the plasma cholesterol concentrations between SM-Bmal1−/− mice and WT littermates, suggesting that deletion of BMAL1 in SMCs did not affect cholesterol homeostasis.
Figure 7. Deletion of BMAL1 in SMCs attenuates Ang II-induced aortic aneurysms in hypercholesterolemia mice.
Four-month-old SM-Bmal1-KO mice and WT littermates were i.p. injected with PCSK9.AAV and started a Western diet at the same time. Three weeks later, blood cholesterol levels were determined, and 26 (out of 27) mice which had serum cholesterol > 250 mg/dl were infused with Ang II for 8 weeks. A, Plasma cholesterol concentrations of SM-Bmal1-KO mice and WT littermates before (the 1st group), 3 weeks after PCSK9.AAV injection and Western diet feeding (the 2nd group), and 8 weeks after PCSK9.AAV injection, Western diet feeding, and Ang II infusion (the 3rd group). B, Quantifications of the maximal intraluminal of abdominal aorta from SM-Bmal1-KO mice and WT littermates before and during 8 weeks of Western diet feeding and Ang II infusion. C, Quantifications of the maximal external diameters of abdominal and thoracic aorta from SM-Bmal1-KO mice and WT littermates 8 weeks after PCSK9.AAV injection, Western diet feeding, and Ang II infusion. The mice with aneurysmal rupture were excluded. D, Incidences of AAA, TAA, and aortic aneurysm rupture. E, Systolic blood pressure of SM-Bmal1-KO mice and WT littermates before and 3 weeks after PCSK9.AAV injection, Western diet feeding, and Ang II infusion. Two-way ANOVA followed by Bonferroni’s post-hoc analysis was used for statistical analysis in (A, n=6–14 and E, n=12–15). Two-way repeated measure ANOVA followed by Bonferroni’s post-hoc analysis was used for statistics in (B). Unpaired t-test was used for statistics in (C). Chi-Square was used for comparing AAA incidence in (D). *, P<0.05; **, p<0.01, ***, P<0.00. ns, not statistically significant.
To define a role of SMC BMAL1 in Ang II-induced AAA in hypercholesterolemia mice, 26 (out of 27) mice that had cholesterol greater than 250 mg/dl were infused with Ang II for 8 weeks, which induced dilation of the abdominal aorta in WT littermates in a time-dependent manner (Figure 7B). Importantly, such abdominal aorta dilations were significantly attenuated in SM-Bmal1−/− mice. To test the possibility that AAA may occur at a later stage in SM-Bmal1−/− mice, we prolonged the Ang II infusion interval from 4-weeks to 8-weeks by replacing the osmotic minipump after 4-weeks. The results show that maximal intraluminal abdominal aorta diameters were continually increased in a time-dependent manner in WT littermates, but not in SM-Bmal1−/− mice (Figure 7B). Infusion of WT littermates with Ang II also caused a large increase in maximal external diameter of the abdominal and thoracic aortas (Figure 7C), 100% AAA, 62% TAA, and 27% aneurysmal rupture (Figure 7D and Supplemental Figure IX), which were either abolished or significantly diminished in SM-Bmal1−/− mice. The protection from AAA provided by BMAL1 deletion was unlikely attributable to its effect on blood pressure as Ang II infusion increased blood pressure to similar extents in SM-Bmal1−/− mice and WT littermate (Figure 7E).
Taken together, these results indicate that BMAL1 deletion from SMCs provides similar robust protection from AAA in divergent mouse models.
DISCUSSION
The current study is the first to report an unexpected pronounced protection of mice from aortic aneurysms induced by both DOCA- or Aldo-salt and Ang II infusion in hypercholesterolemia mice by deletion of BMAL1 in SMCs. The protection is associated with inhibition of elastin breakage, upregulation of TIMP4 mRNA and protein, and inhibition of apoptosis and neutrophil infiltration in mouse aortas.
BMAL1 has been implicated in several vascular pathologies; however, whether BMAL1 is involved in aortic aneurysm formation has not been previously investigated. Using an SMC-specific BMAL1 knockout mouse model, we recently demonstrated that SMC BMAL1 is essential for normal amplitude as well as time-of-day variations in vascular smooth muscle contraction, and normal blood pressure circadian rhythm.19 Moreover, BMAL1 has been shown to be altered in aortas from diabetes and obesity 33–35 and in cultured VSMCs isolated from human atherosclerotic tissue.36 The current study provides the first direct evidence that SMC BMAL1 is essential in MR agonist, DOCA or Aldo, plus salt-induced AAA as well as Ang II-induced AAA in hypercholesterolemia mice.
Global deletion of BMAL1 in mice caused impaired vascular structural remodeling in response to blood flow reduction,10 increased vascular expression and activity of MMP2 and 9,11 and increased vascular superoxide and endothelial NO synthase uncoupling,13, 37 suggesting detrimental vascular effects of global BMAL1 deletion. However, global BMAL1 knockout mice were used in these studies; the observed vascular phenotypes, therefore, may result from the loss of vascular BMAL1 or alternatively from the systemic disruptions caused by deleting BMAL1 from multiple systems. A subsequent elegant study demonstrated that an aortic graft from BMAL1−/− mice transplanted into littermate WT mice developed robust arteriosclerotic disease with up-regulation of T-cell receptors, macrophages, and infiltrating cells,12 suggesting that vascular BMAL1 deletion alone has detrimental effects. In this context, it was surprising that the current study found that, in contrast to this transplant model, deletion of BMAL1 in SMCs provides effective protection from both DOCA- or Aldo-salt and Ang II-induced aortic aneurysm. While the precise mechanisms underlying such discrepancy remain unclear, we speculate that it may be due to cell-specific consequences of BMAL1 deletion. Detrimental effects of deleting BMAL1 from endothelial cells or adventitial fibroblast cells may be dominant and mask beneficial effects of the smooth muscle cell BMAL1 deletion in the aorta transplant experiment.12 A recent study found that inducible global BMAL1 deletion protected mice from atherosclerosis, suggesting significant embryonic development effects of BMAL1 may be involved as well.38
One of novel findings from the current study is that deletion of BMAL1 in SMCs selectively up-regulated TIMP4, which likely mediates, at least in part, inhibiting MMP and decreasing of elastin breakage. MMP activity is critical for the pivotal elastin breakage step in the aorta dilation process during aneurysm development. TIMP is one determinant of the final MMP activity by forming tight 1:1 complexes with activated MMPs, inhibiting their enzymatic activity. Therefore, TIMPs may play an important role in aortic aneurysm. Indeed, loss of TIMP1 or TIMP3 enhanced abdominal aortic aneurysm in response to elastase39 or Ang II infusion.40 Local overexpression of TIMP1 prevents aortic aneurysm in orthotopically graft rat aneurysm model.41 These results suggest a protective role of TIMPs in aortic aneurysm. However, a precise role of TIMPs in the aortic aneurysm pathogenesis can be animal model specific and TIMP isoform-specific as targeted deletion of TIMP2 results in attenuation, rather than enhancement, of CaCl2 induced aneurysm development.42
To the best of our knowledge, a role of TIMP4 in aortic aneurysm has not been reported. Moreover, the role of TIMPs in the DOCA-or Aldo-salt induced aortic aneurysm model has not been reported. The current study provided several novel insights into these two important issues. First, the mRNA of Timp4, among the four TIMPs, is selectively up-regulated in aorta from SM-Bmal1−/− mice under basal and 7 days and 21 days after DOCA-salt administration. Second, such in vivo Timp4 mRNA upregulation can be manifested by ex vivo aortic organ culture and in vitro VSMC culture, suggesting that increased Timp4 mRNA abundance occurs mainly in aortic VSMC. Consistent with this possibility, a drastic TIMP4 protein upregulation occurred in the medial aortic layer and the culture medium of aortic organ culture. Third, we identified TIMP4 as a novel target of BMAL1 in smooth muscle cells. While the four members of the TIMP family inhibit secreted MMPs with roughly comparable potencies,43, 44 individual TIMPs differ markedly in their pro-MMP interaction, gene regulatory mechanisms, and biochemical properties.31 Timp4 promoter lacks a classical TATA-box sequence and contains relatively few identifiable transcription–factor-binding consensus motifs.45 Vascular TIMP4 is up-regulated after balloon injury.46 A recent study reported that Timp4 is a direct transcriptional target of PPARγ in vascular smooth muscle cells.47 In along with these studies, the current study demonstrated that BMAL1 bound to the Timp4 promoter via E-boxes, and deletion of BMAL1 in SMCs resulted in increased Timp4 promoter activity and mRNA expression, indicating that BMAL1 binds and suppresses Timp4 transcription in this cell type.
Our observations that SMC BMAL1 is also involved in DOCA-salt-induced apoptosis, caspase 3 activation, and neutrophil infiltration in aortas, which is consistent with the scenario that multiple mechanisms, in addition to targeting TIMP4, mediate the protection from AAA by SMC BMAL1 deletion. However, further studies are required to fully address these potentially important mechanistic issues.
Supplementary Material
HIGHLIGHTS.
Deletion of clock protein BMAL1 from smooth muscle protects mice from DOCA-salt- and Ang II-induced aortic aneurysms
DOCA-salt administration in mice upregulates the mRNA and protein of tissue inhibitor of metalloproteinase 4 and BMAL1 in aorta
BMAL1 binds to the promoter of tissue inhibitor of metalloproteinase 4 and suppresses its transcription activity
Acknowledgments
We thank Mrs. Ming Zhang for her excellent technical assistance in breeding mice.
Sources of Funding: This work was supported by NIH Grants HL125228 and HL106843 (to M.C.G. and Z.G.), VA Merit Award I01 BX002141 (to Z.G.), NIH Kirschstein-NRSA predoctoral fellowship F31HL123315 (to J.L.), and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM10352.
Non-standard Abbreviations and Acronyms
- AAA
Abdominal Aortic Aneurysm
- AAV
Adeno-associated virus
- Aldo
Aldosterone
- Aldo-salt
Aldo and salt
- Ang II
Angiotensin II
- BMAL1
Brain and Muscle ARNT-Like 1
- ChIP
Chromatin immunoprecipitation
- DOCA
Deoxycorticosterone Acetate
- DOCA-salt
DOCA and salt
- MMP
Matrix Metalloproteinase
- MR
Mineralocorticoid Receptor
- PCNA
Proliferating cell nuclear antigen
- PCSK9
Protein convertase subtilisin/kexin type 9
- SMC
Smooth muscle cells
- TAA
Thoracic Aortic Aneurysm
- TIMP4
Tissue Inhibitor of Metalloproteinase4
- VSMC
Vascular smooth muscle cells
- ZT
Zeitgeber Time
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
Footnotes
Disclosures: None
References
- 1.Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365:1577–89. doi: 10.1016/S0140-6736(05)66459-8. [DOI] [PubMed] [Google Scholar]
- 2.Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26:2605–13. doi: 10.1161/01.ATV.0000245819.32762.cb. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Xie Z, Daugherty A, Cassis LA, Pearson KJ, Gong MC, Guo Z. Mineralocorticoid receptor agonists induce mouse aortic aneurysm formation and rupture in the presence of high salt. Arterioscler Thromb Vasc Biol. 2013;33:1568–79. doi: 10.1161/ATVBAHA.112.300820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson A, Cooper JA, Fabricius M, Humphries SE, Ashton HA, Hafez H. An analysis of drug modulation of abdominal aortic aneurysm growth through 25 years of surveillance. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2010;52:55–61e2. doi: 10.1016/j.jvs.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Golledge J, Hankey GJ, Yeap BB, Almeida OP, Flicker L, Norman PE. Reported high salt intake is associated with increased prevalence of abdominal aortic aneurysm and larger aortic diameter in older men. PLoS One. 2014;9:e102578. doi: 10.1371/journal.pone.0102578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–42. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–17. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta RH, Manfredini R, Hassan F, Sechtem U, Bossone E, Oh JK, Cooper JV, Smith DE, Portaluppi F, Penn M, Hutchison S, Nienaber CA, Isselbacher EM, Eagle KA International Registry of Acute Aortic Dissection I. Chronobiological patterns of acute aortic dissection. Circulation. 2002;106:1110–5. doi: 10.1161/01.cir.0000027568.39540.4b. [DOI] [PubMed] [Google Scholar]
- 9.Reilly DF, Westgate EJ, FitzGerald GA. Peripheral circadian clocks in the vasculature. Arterioscler Thromb Vasc Biol. 2007;27:1694–705. doi: 10.1161/ATVBAHA.107.144923. [DOI] [PubMed] [Google Scholar]
- 10.Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119:1510–7. doi: 10.1161/CIRCULATIONAHA.108.827477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anea CB, Ali MI, Osmond JM, Sullivan JC, Stepp DW, Merloiu AM, Rudic RD. Matrix metalloproteinase 2 and 9 dysfunction underlie vascular stiffness in circadian clock mutant mice. Arterioscler Thromb Vasc Biol. 2010;30:2535–43. doi: 10.1161/ATVBAHA.110.214379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng B, Anea CB, Yao L, Chen F, Patel V, Merloiu A, Pati P, Caldwell RW, Fulton DJ, Rudic RD. Tissue-intrinsic dysfunction of circadian clock confers transplant arteriosclerosis. Proc Natl Acad Sci U S A. 2011;108:17147–52. doi: 10.1073/pnas.1112998108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anea CB, Cheng B, Sharma S, Kumar S, Caldwell RW, Yao L, Ali MI, Merloiu AM, Stepp DW, Black SM, Fulton DJ, Rudic RD. Increased superoxide and endothelial NO synthase uncoupling in blood vessels of Bmal1-knockout mice. Circ Res. 2012;111:1157–65. doi: 10.1161/CIRCRESAHA.111.261750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curci JA. Digging in the “soil” of the aorta to understand the growth of abdominal aortic aneurysms. Vascular. 2009;17(Suppl 1):S21–9. doi: 10.2310/6670.2008.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, Thompson RW. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997;150:993–1007. [PMC free article] [PubMed] [Google Scholar]
- 16.Humphrey JD, Schwartz MA, Tellides G, Milewicz DM. Role of mechanotransduction in vascular biology: focus on thoracic aortic aneurysms and dissections. Circ Res. 2015;116:1448–61. doi: 10.1161/CIRCRESAHA.114.304936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Storch KF, Paz C, Signorovitch J, Raviola E, Pawlyk B, Li T, Weitz CJ. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007;130:730–41. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Zhong W, Cui T, Yang M, Hu X, Xu K, Xie C, Xue C, Gibbons GH, Liu C, Li L, Chen YE. Generation of an adult smooth muscle cell-targeted Cre recombinase mouse model. Arterioscler Thromb Vasc Biol. 2006;26:e23–4. doi: 10.1161/01.ATV.0000202661.61837.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie Z, Su W, Liu S, Zhao G, Esser K, Schroder EA, Lefta M, Stauss HM, Guo Z, Gong MC. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J Clin Invest. 2015;125:324–36. doi: 10.1172/JCI76881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu H, Howatt DA, Balakrishnan A, Graham MJ, Mullick AE, Daugherty A. Hypercholesterolemia Induced by a PCSK9 Gain-of-Function Mutation Augments Angiotensin II-Induced Abdominal Aortic Aneurysms in C57BL/6 Mice-Brief Report. Arterioscler Thromb Vasc Biol. 2016;36:1753–7. doi: 10.1161/ATVBAHA.116.307613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toth M, Sohail A, Fridman R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol Biol. 2012;878:121–35. doi: 10.1007/978-1-61779-854-2_8. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Xie Z, Zhao Q, Pang H, Turk J, Calderon L, Su W, Zhao G, Xu H, Gong MC, Guo Z. Smooth muscle-specific expression of calcium-independent phospholipase A2beta (iPLA2beta) participates in the initiation and early progression of vascular inflammation and neointima formation. J Biol Chem. 2012;287:24739–53. doi: 10.1074/jbc.M112.340216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie Z, Gong MC, Su W, Turk J, Guo Z. Group VIA phospholipase A2 (iPLA2beta) participates in angiotensin II-induced transcriptional up-regulation of regulator of g-protein signaling-2 in vascular smooth muscle cells. The Journal of biological chemistry. 2007;282:25278–89. doi: 10.1074/jbc.M611206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie Z, Gong MC, Su W, Xie D, Turk J, Guo Z. Role of calcium-independent phospholipase A2beta in high glucose-induced activation of RhoA, Rho kinase, and CPI-17 in cultured vascular smooth muscle cells and vascular smooth muscle hypercontractility in diabetic animals. The Journal of biological chemistry. 2010;285:8628–38. doi: 10.1074/jbc.M109.057711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie Z, Liu D, Liu S, Calderon L, Zhao G, Turk J, Guo Z. Identification of a cAMP-response element in the RGS2 promoter as a Key cis-regulatory element for RGS2 transcriptional regulation by angiotensin II in cultured VSMC. The Journal of biological chemistry. 2011 doi: 10.1074/jbc.M111.265462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su W, Xie Z, Guo Z, Duncan MJ, Lutshumba J, Gong MC. Altered Clock Gene Expression and Vascular Smooth Muscle Diurnal Contractile Variations in Type 2 Diabetic db/db Mice. American journal of physiology Heart and circulatory physiology. 2011 doi: 10.1152/ajpheart.00825.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krug AW, Allenhofer L, Monticone R, Spinetti G, Gekle M, Wang M, Lakatta EG. Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathways. Hypertension. 55:1476–83. doi: 10.1161/HYPERTENSIONAHA.109.148783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nature medicine. 2012;18:1429–33. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–32. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–27. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 31.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steffens S, Winter C, Schloss MJ, Hidalgo A, Weber C, Soehnlein O. Circadian Control of Inflammatory Processes in Atherosclerosis and Its Complications. Arterioscler Thromb Vasc Biol. 2017;37:1022–1028. doi: 10.1161/ATVBAHA.117.309374. [DOI] [PubMed] [Google Scholar]
- 33.Su W, Guo Z, Randall DC, Cassis L, Brown DR, Gong MC. Hypertension and disrupted blood pressure circadian rhythm in Type 2 diabetic db/db mice. Am J Physiol Heart Circ Physiol. 2008;295:H1634–41. doi: 10.1152/ajpheart.00257.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nernpermpisooth N, Qiu S, Mintz JD, Suvitayavat W, Thirawarapan S, Rudic DR, Fulton DJ, Stepp DW. Obesity alters the peripheral circadian clock in the aorta and microcirculation. Microcirculation. 2015;22:257–66. doi: 10.1111/micc.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S, Ding Y, Zhang Z, Wang H, Liu C. Hyperlipidaemia impairs the circadian clock and physiological homeostasis of vascular smooth muscle cells via the suppression of Smarcd1. The Journal of pathology. 2014;233:159–69. doi: 10.1002/path.4338. [DOI] [PubMed] [Google Scholar]
- 36.Lin C, Tang X, Zhu Z, Liao X, Zhao R, Fu W, Chen B, Jiang J, Qian R, Guo D. The rhythmic expression of clock genes attenuated in human plaque-derived vascular smooth muscle cells. Lipids Health Dis. 2014;13:14. doi: 10.1186/1476-511X-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anea CB, Zhang M, Chen F, Ali MI, Hart CM, Stepp DW, Kovalenkov YO, Merloiu AM, Pati P, Fulton D, Rudic RD. Circadian clock control of Nox4 and reactive oxygen species in the vasculature. PLoS One. 2013;8:e78626. doi: 10.1371/journal.pone.0078626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang G, Chen L, Grant GR, Paschos G, Song WL, Musiek ES, Lee V, McLoughlin SC, Grosser T, Cotsarelis G, FitzGerald GA. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Science translational medicine. 2016;8:324ra16. doi: 10.1126/scitranslmed.aad3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eskandari MK, Vijungco JD, Flores A, Borensztajn J, Shively V, Pearce WH. Enhanced abdominal aortic aneurysm in TIMP-1-deficient mice. J Surg Res. 2005;123:289–93. doi: 10.1016/j.jss.2004.07.247. [DOI] [PubMed] [Google Scholar]
- 40.Basu R, Fan D, Kandalam V, Lee J, Das SK, Wang X, Baldwin TA, Oudit GY, Kassiri Z. Loss of Timp3 gene leads to abdominal aortic aneurysm formation in response to angiotensin II. The Journal of biological chemistry. 2012;287:44083–96. doi: 10.1074/jbc.M112.425652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allaire E, Forough R, Clowes M, Starcher B, Clowes AW. Local overexpression of TIMP-1 prevents aortic aneurysm degeneration and rupture in a rat model. J Clin Invest. 1998;102:1413–20. doi: 10.1172/JCI2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong W, Knispel R, Mactaggart J, Baxter BT. Effects of tissue inhibitor of metalloproteinase 2 deficiency on aneurysm formation. J Vasc Surg. 2006;44:1061–6. doi: 10.1016/j.jvs.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 43.Apte SS, Olsen BR, Murphy G. The gene structure of tissue inhibitor of metalloproteinases (TIMP)-3 and its inhibitory activities define the distinct TIMP gene family. The Journal of biological chemistry. 1995;270:14313–8. doi: 10.1074/jbc.270.24.14313. [DOI] [PubMed] [Google Scholar]
- 44.Greene J, Wang M, Liu YE, Raymond LA, Rosen C, Shi YE. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. The Journal of biological chemistry. 1996;271:30375–80. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- 45.Young DA, Phillips BW, Lundy C, Nuttall RK, Hogan A, Schultz GA, Leco KJ, Clark IM, Edwards DR. Identification of an initiator-like element essential for the expression of the tissue inhibitor of metalloproteinases-4 (Timp-4) gene. Biochem J. 2002;364:89–99. doi: 10.1042/bj3640089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dollery CM, McEwan JR, Wang M, Sang QA, Liu YE, Shi YE. TIMP-4 is regulated by vascular injury in rats. Ann N Y Acad Sci. 1999;878:740–1. doi: 10.1111/j.1749-6632.1999.tb07777.x. [DOI] [PubMed] [Google Scholar]
- 47.Ketsawatsomkron P, Keen HL, Davis DR, Lu KT, Stump M, De Silva TM, Hilzendeger AM, Grobe JL, Faraci FM, Sigmund CD. Protective Role for Tissue Inhibitor of Metalloproteinase-4, a Novel Peroxisome Proliferator-Activated Receptor-gamma Target Gene, in Smooth Muscle in Deoxycorticosterone Acetate-Salt Hypertension. Hypertension. 2016;67:214–22. doi: 10.1161/HYPERTENSIONAHA.115.06391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.