Abstract
Several lines of evidence support the link between maternal inflammation during pregnancy and increased likelihood of neurodevelopmental and psychiatric disorders in offspring. This longitudinal study seeks to advance understanding regarding implications of systemic maternal inflammation during pregnancy, indexed by plasma IL-6 concentrations, for large-scale brain system development and emerging executive function (EF) skills in offspring. Maternal IL-6 was assessed during pregnancy, functional MRI acquired in neonates, and working memory (an important component of EF) examined at 2-years-of-age. Functional connectivity within and between multiple neonatal brain networks can be modeled to estimate maternal IL-6 concentrations during pregnancy. Brain regions heavily weighted in these models overlap significantly with those supporting working memory in a large meta-analysis. Maternal IL-6 also directly accounts for a portion of the variance of working memory at two-years-of-age. Findings highlight the association of maternal inflammation during pregnancy with the developing functional architecture of the brain and emerging EF.
Keywords: Inflammation, Resting State, Functional Connectivity, Neonates, Brain, Working Memory
Introduction
Epidemiological evidence and work in animal models supports a strong correspondence between maternal inflammation during pregnancy and an increased likelihood of multiple psychiatric disorders in affected offspring including autism (ASD), schizophrenia (SCHZ), attention-deficit hyperactivity disorder (ADHD) and major depression (MDD)1–5. Until relatively recently, inflammation was thought to arise purely from infection or injury; however, it is now well documented that environmental, psychosocial, and general physical health factors (e.g. obesity, famine, diet, low socioeconomic status, poverty, physical and/or mental stress) can elicit alterations in the immune system leading to heightened inflammation4,5. The developing fetus receives cues about the extra-uterine environment via stress-sensitive aspects of maternal placental fetal (MPF) biology, including inflammatory processes, known to play a role in the intergenerational transmission of environmental risk factors6. Maternal inflammation during gestation has been linked to adverse outcomes during childhood and an elevated risk for psychopathology4,7. Maternal inflammatory processes during pregnancy are therefore of significant interest as a potential common mediator of a wide range of prenatal conditions associated with poor neurodevelopmental outcomes.
Inflammatory Markers (Cytokines)
Cytokines (inflammatory markers) and their receptors are expressed throughout the fetal brain and play a role in typical neurodevelopmental processes involved in cell survival, proliferation and differentiation, axonal growth and synaptogenesis8,9. Variations in cytokine concentrations therefore have strong potential to alter neurodevelopmental trajectories. One pro-inflammatory cytokine in particular, Interleukin-6 (IL-6), has been indicated as a mediating factor in processes leading from maternal inflammation to alterations in fetal brain development and subsequent risk for psychopathology emerging later in life10,11. The precise mechanisms linking maternal IL-6 concentrations with various neurodevelopmental disorders have not been fully established. However, research in animal models4,12,13 provides strong evidence demonstrating that IL-6 is indeed critical for relaying the effects of maternal inflammation to the developing fetus, which can then lead to altered social and cognitive behaviors in affected offspring14.
In the current work, IL-6 is likely best conceptualized as an indicator of overall maternal systemic inflammation with potential to influence placental and fetal inflammatory processes and subsequently fetal brain development in concert with other important inflammatory mediators. Research suggests higher systemic levels of inflammatory markers, including IL-6, may lead to cognitive and behavioral deficits in affected offspring by altering the formation of synapses and affecting synaptic function4,15. Disruption in normative synaptic signaling and transmission has been shown to alter the balance of neurotransmitters and the number of excitatory versus inhibitory connections16 in the developing brain - potentially setting the stage for a diverse range of adverse developmental outcomes. Given neuroinflammation appears to play a common role across multiple neuropsychiatric and neurological disorders, and inflammatory markers such as IL-6 are expressed throughout the brain, it appears cytokines have the potential to affect normative growth processes at every stage of fetal brain development. As such, it is unlikely that downstream of effects of maternal inflammation are restricted to a single brain region or canonical circuit, but are more likely to be broad.
Neuroimaging & Network Neuroscience
The relationship between maternal inflammation and fetal brain development has largely focused on animal models due to various methodological limitations. While this work is of critical importance, particularly for testing causal models and mechanisms of action, more studies evaluating associations between maternal inflammation and neurodevelopment in human offspring are needed to understand the relevance of this work for human health. Non-invasive neuroimaging methodologies are critical toward this end. Importantly, non-invasive functional neuroimaging allows investigators to examine large-scale distributed systems across the brain as they form and are modified during development17–22. Considering the ubiquitous role of IL-6 and other inflammatory processes in the CNS, such an approach is needed to identify and characterize effects on brain development. As a complex system, the brain exhibits systematic properties which are conserved across biological and non-biological systems alike23,24. One such feature is the degree to which the brain is organized into modular subsystems (i.e., communities or networks) that integrate to support complex behavior and cognition. Several studies have now shown that when communication within or between these systems is disrupted, deficits in cognitive performance, atypical behaviors and pervasive neurodevelopmental disorders can ensue25.
Executive Functioning
Executive function (EF) is a broad term, which describes a set of cognitive processes that support goal directed behavior. In adults and children, EF has been shown to rely on large scale distributed brain systems, such as those examined in the present study. Working memory, specifically, is a resource-limited executive function that relates to the ability to temporarily hold items in mind for manipulation. It is a core component of EF that can be reliably measured beginning at 2-years of age26. At these early ages, working memory performance serves as a foundation for later emerging academic skills, social skills, and theory of mind26,27. It is also relevant for long-term clinical outcomes, with deficits apparent across psychiatric disorders linked to inflammation during pregnancy, including ADHD, Autism, and Schizophrenia28. We therefore hypothesized that heightened maternal inflammation during pregnancy would have implications not only for large scale functional brain systems in the neonatal period, but also for subsequent emerging working memory skills. We examine working memory as a starting point for understanding the implications of maternal inflammation during pregnancy and associated alterations in early brain system development for subsequent core cognitive competencies. However, in line with our understanding that inflammation has potential to broadly affect developing neural systems, we conceptualize working memory as only one of several aspects of EF which may be altered in association with heightened maternal inflammation during pregnancy.
Purpose
In the current report we utilize resting-state functional connectivity MRI (rs-fcMRI), network-based analytics, and multivariate machine-learning methodologies to investigate associations between inflammation during pregnancy (indexed via maternal IL-6 concentrations in early, mid and late gestation) and newborn functional brain network topology. We posit, that if maternal inflammation during pregnancy is highly relevant for fetal development of large scale multivariate brain systems, then it should be possible to infer (i.e. estimate) levels of maternal inflammation based on large-scale brain connectivity patterns soon after birth. Furthermore, if maternal IL-6 concentrations during pregnancy are relevant for future working memory performance, and IL-6 related alterations in neonatal functional connectivity underlies this association then: A) the brain regions that most strongly contribute to the models ability to estimate IL-6 (i.e. are more heavily weighted) are likely to overlap with brain systems known to be involved with working memory, and B) maternal IL-6 levels in our sample should relate to future working memory itself. Thus, we assessed the multivariate relationship between newborn functional brain connectivity within and between previously identified large-scale brain networks, and maternal IL-6 levels concentrations during pregnancy. Further, we examined the correspondence of features identified within these multivariate brain models to a meta-analysis of the working memory literature in a large number of studies utilizing Neurosynth29. Last, we tested the association between serial measurements of IL-6 throughout pregnancy and working memory at 2-years of age. The results provide strong evidence linking maternal inflammation during pregnancy with newborn brain organization and future EF.
Results
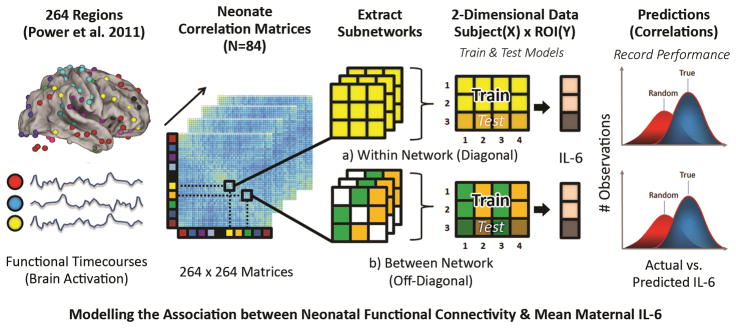
In order to assess the relationship between mean maternal IL-6 and newborn functional brain connectivity within and between systems, the current study harnessed a machine-learning approach along with random resampling to estimate generalized model performance. Machine-learning involves generating a multivariate model that reflects the underlying patterns of out-of-sample data. In the cognitive neuroscience literature it is often used with cross-validation or random resampling (as used in the current report and elsewhere30–32) and is well suited for modeling the high-dimensional nature of brain-connectivity data for the purposes of estimating (or predicting) a univariate outcome (e.g. IL-6) – even within an individual subject. Thus, the first aim in the current report was to determine whether enough information exists in newborn functional connectivity data at the systems level to estimate levels of maternal IL-6 during the prenatal period. Figure 1 provides an overview of the process.
Figure 1. Methods overview for combining rs-fcMRI, random resampling and PLSR.
The above diagram provides a step-by-step overview visually depicting the process of associating neonatal functional connectivity data with mean maternal IL-6. After standard preprocessing steps, for each individual neonate, functional timecourses representing regional activation for a given ROI are extracted and pairwise cross-correlation matrices are constructed for 264 regions as described in Power et al. 2011. From here, individual subnetworks are extracted; specifically, matrices are extracted for each of the 10 networks assessed within (a) and between (b) previously identified large-scale systems (i.e. DFM, VIS, etc.). Connections between ROIs for a given within or between network functional connectivity matrix are used as features to estimate mean maternal IL-6 using partial-least squares regression (PLSR). Using a repeated (k=4000) hold-out random resampling procedure, the data is randomly partitioned into training (80%) and test (20%) sets, and the resulting distribution of actual versus predicted IL-6 values is tested for significance against a null distribution (i.e. random chance).
Importantly, several potential confounding variables were tested prior to the analysis. Despite the tight window for which data were collected (see Methods), in order to ensure effects reported in the study were not due to differences in length of gestation or maturation, we show here that mean maternal IL-6 is not associated with: gestational age at birth (r = 0.073, p = .631), age at MRI scan (r = 0.193, p = .200), nor age at working memory assessment (24 months; r = 0.087, p = .563). Additionally, as maternal age may influence inflammatory processes and offspring neurodevelopmental outcomes, we examined the association between maternal age and IL-6 levels (r = 0.037, p = 0.870), and maternal age and infant working memory at two-years of age (r = 0.040, p = 0.800). The results suggest that these various factors are unlikely to serve as confounds in these analyses.
In order to examine associations between neonatal functional brain connectivity within and between previously identified networks and mean maternal IL-6 (see Methods), we first extract pairwise functional connections (correlations) within or between ROIs of ten common and previously defined functional brain networks: the Default Mode (DFM), Visual (VIS), Cingulo-opercular (CON), Sensorimotor (SSM), Salience (SAL), Frontoparietal (FP), Subcortical (SUB), Dorsal Attention (DAN), Ventral Attention (VAN) and Cerebellar (CER) systems33. Thus, a full cross-correlation matrix is created for every participant (264 x 264 x 84). Next, the connections for a given network (10 within; 45 between) across participants are used (Fig. 1)33 as input features to estimate maternal IL-6 concentrations using partial least-squares regression (PLSR). Examining connections by network increases interpretability of findings while also facilitating feature reduction. While these networks and their corresponding regions of interest are derived from work with adults, we posit that they are highly relevant for the organization of the newborn brain. Multiple studies have identified putative precursors of these networks in the neonatal period, and documented rapid development during infancy such that they resemble adult networks by two-years-of-age18,20,34.
As described further in the Methods section and elsewhere32, PLSR models are generated from a subset of participants (training set) and model parameters (beta-weights) are re-applied to data functional connectivity (FC) derived exclusively from a separate test set of participants (i.e., participants not used to construct the model) over many iterations. In order to assess performance of these models in the context of the main question, permutation testing is used whereby the process is repeated and the outcome (or response variable; i.e. IL-6) is shuffled (or permuted) on each round of random resampling 35. Finally, the resulting two distributions (true and random) of correlation values (index of model accuracy) are examined. An initial filtering of the strongest relationships for each within and between network model with maternal IL-6 is then conducted simply by highlighting those with p<0.001 using a Kolmogorov-Smirnov (KS) test corrected for multiple comparisons (See Table 1). However, because of the difficulty in interpreting p-values for random resampling or cross-validation tests in machine learning36,37, the primary outcome measure of interest for each model is the effect size (the amount of divergence between the true and random distributions) with larger effect sizes indicative of greater accuracy in estimating maternal IL-6 levels. Thus, only networks exceeding a small effect size (based on accepted criteria for small (0.2), medium (0.5), and large (0.8) effect sizes) were further examined. Here, to ensure the selection process is robust to the number of components used for a given model, the median effect size across a range of PLSR components is used (see Methods). Additionally, we examined the results using a nested leave-one-out cross-validation (LOOCV) procedure, an alternative method for evaluating predictive models, in order to provide further support for our findings. We refer the reader to the Methods section for further details regarding this approach (also see Supplementary Table 1).
Table 1. Partial least squares regression (PLSR) results.
Significant associations with mean maternal IL-6 in our sample of neonates (N=84) were identified via the cross-validated PLSR model for multiple large-scale functional systems. Within network and between networks findings were filtered based on: 1) statistical significance of the KS-test (with Bonferroni correction for multiple comparisons), and 2) median effect size (Cohen’s D) across all possible components (e.g. 1–20) exceeding a small effect size (> .2).
| Average Model Performance for
Optimal Component Model (4000 iterations) |
|||||||
|---|---|---|---|---|---|---|---|
| Mean Train | Mean Test | ||||||
|
| |||||||
| Networks | Cohen’s D (For Optimal Component Model) | Median Cohen’s D (Across All Components) | r | Std. Dev. | r | Std. Dev. | |
| SUB | DAN | 1.76 | 1.53 | 0.96 | 0.01 | 0.41 | 0.2 |
| SUB | CER | 1 | 0.5 | 0.77 | 0.02 | 0.25 | 0.22 |
| VIS | DAN | 0.86 | 0.5 | 0.56 | 0.04 | 0.2 | 0.21 |
| SAL | CON | 0.76 | 0.74 | 0.98 | 0 | 0.18 | 0.2 |
| SUB | VAN | 0.66 | 0.44 | 0.73 | 0.03 | 0.16 | 0.22 |
| DAN | FP | 0.61 | 0.58 | 0.91 | 0.01 | 0.14 | 0.21 |
| SAL | 0.45 | 0.39 | 0.97 | 0.01 | 0.11 | 0.23 | |
| VAN | CER | 0.42 | 0.32 | 0.33 | 0.04 | 0.1 | 0.23 |
All Models Significant (p<0.001) Based on Bonferroni Corrected KS-test
Estimating mean maternal IL-6 concentrations during pregnancy from newborn functional brain connectivity
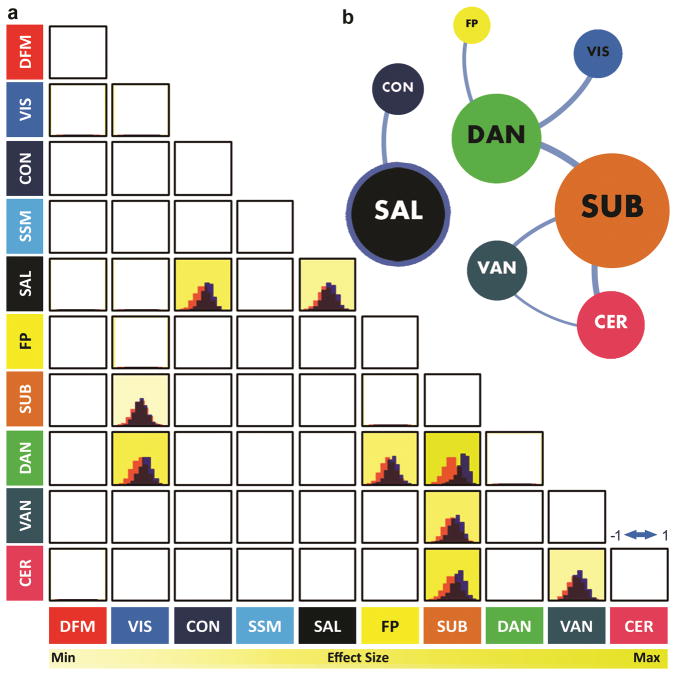
For this initial analysis maternal IL-6 was averaged over trimesters given the high degree of correlation between IL-6 concentrations across pregnancy (r = 0.553–0.684, p < .001; also see Methods). Significant associations between neonatal functional brain connectivity and mean maternal IL-6 concentrations during pregnancy were identified for multiple large-scale functional systems (Table 1; Figure 1). Of the 10 large-scale networks assessed, connectivity within 1 network (Salience, SAL) and between 7 network-by-network combinations passed the statistical significance and effect size filters, suggesting potential associations with mean maternal IL-6. Within and between network associations with maternal IL-6 concentrations during pregnancy were observed (number of observations) for the SUB (3), DAN (3), SAL (2), CER (2), VAN (2), VIS (1), CON (1), FP (1) networks as schematized in Figure 2 (also see findings for LOOCV in the Supplementary Material; Supplementary Figure 1, Supplementary Table 1). Connectivity between the SUB⬄DAN (d = 1.765), SUB⬄CER (d = 1.023), VIS⬄DAN (d = 0.869), and SAL⬄CON (d = 0.775) networks were most robustly associated with maternal IL-6 concentrations during pregnancy. A detailed summary of the results is provided in Table 1. Findings are listed by network and ranked according to their effect size. It is important to note that the number of connections used as input features for a given network model is unrelated to the effect sizes found for any of the models (i.e. effect sizes provided via Table 1; r = −0.0920, p = 0.510).
Figure 2. Within and between functional network associations with mean maternal IL-6.
In panel a) the distribution of correlations between actual and estimated mean maternal IL-6 values in our sample of neonates (N=84) obtained via PLSR with randomized holdouts (4000 iterations; blue) is shown for each within (diagonal) and between (off diagonal) network model that passed statistical threshold (see Methods). The corresponding null distribution for each model is shown in peach. Brighter highlighted cells denote stronger results according to effect size, the primary outcome of interest, indicative of the strength of the model in accurately estimating IL-6 (see Table 1 for actual statistics; Figure 4). In panel b) a network schematic depicting significant associations within and between large-scale functional networks and mean maternal IL-6 is visualized using the Gephi network visualization software. Circles (or nodes) represent individual networks and are scaled according their overall degree of association with mean maternal IL-6 (number of associations passing criteria for statistical significance and effect size). Nodes with thick borders represent significant within network associations with mean maternal IL-6. Line width between nodes represents the relative effect size of between network models. Note: The graph is undirected and used for illustrative purposes and does not represent graph theoretical relationships between communities.
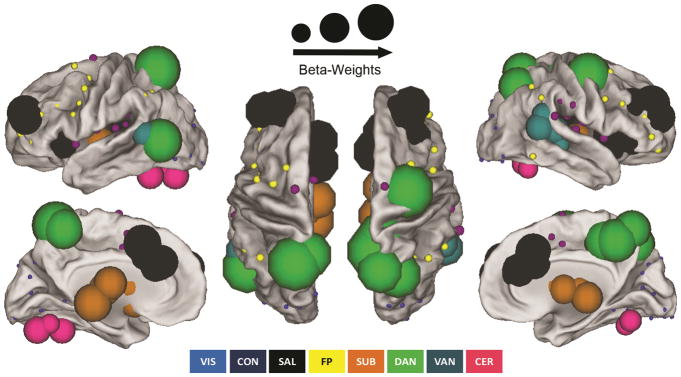
Features of neonatal functional brain connectivity most strongly associated with maternal IL-6 concentrations during pregnancy
Features of neonatal functional brain connectivity most strongly associated with maternal IL-6 concentrations during pregnancy are summarized in Figure 3. Here, absolute beta-weights for a given ROI are summed across all filtered within and between network models as described in the previous section and are reflected as the diameter of the node. ROIs are scaled proportionally. This measurement is a modified version of the graph theoretical metric node strength38; thus nodes with large diameters have connections with strong influences to a given model’s ability to estimate maternal IL-6 concentrations during pregnancy, and nodes with small diameters do not. As observed in Figure 3, regions in the SAL, DAN, and SUB appear to dominate the landscape. Similar findings were observed when using LOOCV (see Supplementary Figure 1, Supplementary Table 1). Within the supplemental materials we provide a table with the unscaled absolute beta-weights summed for each ROI (i.e. node strength) for models estimating maternal IL-6 (Supplementary Table 2).
Figure 3. Predictive features (ROIs) within and between networks associated with mean maternal IL-6.
Predictive features representing individual brain regions for a given network associated with mean maternal IL-6 are visualized on a standardized brain surface using Caret 5 software. ROIs are scaled proportionally; node (circle) sizes are determined by the overall degree of importance of a region in estimating IL-6 (beta-weights). ROIs for networks significantly associated with maternal IL-6 (see Table 1 for statistics) include the SAL (black), DAN (green), SUB (orange), VAN (turquoise), CER (pink), CON (purple), FP (yellow), and VIS (blue) networks.
Brain regions associated with working memory overlap with features more heavily weighted in models that estimate IL-6
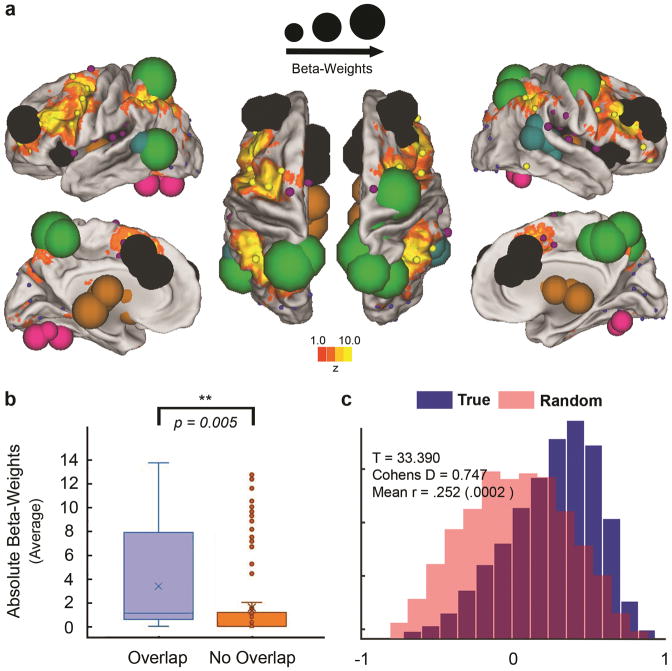
As noted in the introduction we posited that if these newborn multivariate brain models (i.e. weighted predictions derived from neonatal brain connectivity) estimating maternal IL-6 are relevant for future working memory, we should be able to validate the models by testing their correspondence to regions known to be involved with working memory. To do this we began with a meta-analysis of 901 fMRI working memory studies to identify the regions most tightly associated with working memory. This meta-analysis was conducted with the Neurosynth software29. A reverse-inference mask was generated that included all voxels in the brain that corresponded to our search term “working memory”. We did not include any additional thresholding to avoid any potential biases. Regions from our analysis were then split into those that fell inside the working memory mask (54 regions), and those that fell outside the mask (210 regions; see Figure 4 panel b). A simple comparison of the node strengths outlined above (detailed in Table 1, also Supplementary Table 2) showed that regions within the working memory mask are significantly more strongly associated with maternal IL-6 concentrations during pregnancy compared to those nodes outside of the mask (also See Supplementary Figure 1). While not arguing for specificity, this result suggests that brain regions supporting working memory may be particularly associated with higher levels of maternal IL-6 during pregnancy.
Figure 4. Relationship between maternal IL-6, neonatal functional connectivity & working memory.
In panel a) predictive features are overlaid on top of voxelwise, meta-analysis maps related to working memory in our sample of neonates with assessment data (N=46) generated via Neurosynth.org. Neurosynth meta-analysis maps for working memory are comprised of results reported from 901 fMRI studies (reverse-inference; corrected for multiple comparisons using a false discovery rate (FDR) criterion of .01 as previously described29). In panel b) we show the combined sum of the beta weights of a given region (i.e. node strength) for those regions (N=54) within (overlapping) the meta-analysis working memory mask, and those regions (N=210) outside (non-overlapping) the mask. On average, regions within the working memory mask are more predictive of IL-6 as indicated by an independent two-tailed t-test assuming unequal variances (t(70)=2.90, p=.005). In the boxplot, the x indicates the mean value, horizontal lines within the box represent the medians; box limits indicate the 25th and 75th percentiles, whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles, outliers are represented by dots. In panel c) we show directly that using all three gestational time points for IL-6, we can also predict future working memory performance (d=0.747) at two years of age in these same infants (N=46) using PLSR.
Although it would not be feasible to exhaustively test the Neurosynth generated mask for all other behavioral and cognitive domains in relation to the multivariate brain models, we examined several other domains (including language and negative emotionality). Within the supplementary material we show how the meta-analytic brain masks for these domains relate to the IL-6 associated brain maps (Supplementary Materials; Supplementary Figures 1 and 2). None of these additional domains had a meta-analytic brain mask that overlapped significantly with the nodes identified in the IL-6 models. Though we do not argue that our findings are truly specific to working memory, these data provide examples that may illustrate how the results are not necessarily related to all behaviors
IL-6 measurements across each trimester directly predict working memory performance at 2-years of age
If indeed the newborn brain models that estimate IL-6 are related to future working memory performance, as suggested by the meta-analysis above, then maternal IL-6 concentrations during pregnancy would be expected to show an association with offspring working memory performance. Therefore, we examined working memory performance in 46 children in the current study who completed the Spin the Pots task27 at 2-years-of-age. Here, a PLSR model is generated using maternal IL-6 concentrations collected within each trimester as features used to predict working memory performance at 2 years age (Fig. 4). Given the high degree of correlation between these predictors (noted above), one component was selected to estimate working memory. Results from this model show a strong relationship when compared to a null distribution (Fig 4 panel c; p<0.001, d = 0.747). On average, the 3rd trimester carried the strongest weight (absolute beta-weights from the PLSR model) predicting the working memory outcome (1st trimester = 0.268; 2nd trimester = 0.3897; 3rd trimester = 0.5392). The univariate relationship between mean maternal IL-6 and working memory at 2 years shows a negative correlation (r = −0.314; p = .03), which establishes that increased systemic immune activation is associated with decreased working memory performance at 2 years.
As a follow-up to these analyses, and to further explore the specificity of these findings to working memory, we also examined negative emotionality as a separate developmental domain at the same timepoints for 63 of the 84 infants who had scores on this measure58. In this case, maternal IL-6 did not relate to offspring negative emotionality at 24 months (r=.003, p=0.983). In the absence of this relationship, we tested whether newborn connectivity can predict negative emotionality directly. We did this following the same steps described in the primary analyses predicting IL-6. We identified a subset of systems that were predictive of negative emotionality; however, predictive patterns, while showing some overlap, were mostly distinct with maps predicting IL-6. Unlike the strong relationship in the original report, the overlap of regions predictive of negative emotionality with working memory regions from the meta-analysis are statistically non-significant (t(96)=1.856, p=0.415; Supplementary Figure 3). Again, although this is not an argument for true specificity of the original findings, we believe these analyses provide another example that illustrates how our findings are not related to all behaviors.
Discussion
In the current report, we highlight associations between maternal IL-6 concentrations during pregnancy (as an index of maternal inflammation) and offspring functional brain networks shortly after birth. We show that based on these connectivity patterns within and between large scale neural systems in the newborn brain, mean maternal IL-6 concentrations can be estimated using machine-learning. This “estimation” capacity provides an empirical link (currently missing in humans) between prenatal exposure to inflammatory cytokines, such as IL-6, and patterns of newborn functional brain connectivity across the brain. In addition, we validate and show the potential implications of this relationship by highlighting the correspondence of brain regions estimating maternal IL-6 to regions in the brain tightly linked to working memory capacity throughout the lifespan. Last, to confirm the implications of maternal inflammation for working memory performance, we show, directly, that maternal IL-6 concentrations are significantly associated with working memory performance at 2-years-of-age.
Between & within-network neonatal functional connectivity is associated with maternal IL-6
The associations observed between mean maternal IL-6 and newborn functional connectivity are widespread and involve networks and regions important for supporting normative social, emotional and cognitive development. Many of these systems also have relevance for various neuropsychiatric disorders. Specifically, the subcortical (SUB), salience (SAL) and dorsal attention (DAN) systems were strongly associated with the estimation of maternal Il-6 concentrations during pregnancy. Aberrant connectivity between these networks are implicated in multiple neuropsychiatric disorders including ADHD, schizophrenia, and autism25.
Within Network
Within network connectivity for one neonatal brain system was able to estimate maternal IL-6 concentrations during pregnancy moderately: the salience (SAL) system. The SAL has long been identified as being involved with detection of salient, or biologically meaningful information, and interacting with other brain systems to result in orienting attention22,39–41. In addition, the SAL has repeatedly surfaced in the literature as being atypical across many psychopathologies25. Interestingly, in a recent review, DiMartino and colleagues (2014) point out that system-wide changes observed in functional topology can be influenced by alterations observed in a single network42. Due to the role of the SAL in detecting and attuning to relevant environmental stimuli, and prior work highlighting its role in engaging other cortical networks involved in executive function, it is highly plausible that individual differences in the SAL could relate to altered functional topology throughout the brain, both concurrently, and over the course of development39,40. This possibility is bolstered by findings that the insula, considered a core part of the SAL system, is a particularly highly interconnected brain region beginning in early infancy18.
Between Network
Overall, connectivity between higher-order systems (e.g. DAN, VAN, SAL) and lower-order (e.g. SUB) networks were robustly associated with mean maternal IL-6 (see Figure 2 and Table 1, and Supplemental Materials).
These results are interesting in light of prior research suggesting that individual differences in connectivity between subcortical regions and regions situated within the DAN in neonates are relevant for subsequent emotional and cognitive development during infancy.43 Development of and interactions between the SAL and early attention systems (e.g. DAN,VAN) have also been proposed to be important for emerging effortful (or executive) control of attention.44,45 . Emerging effortful control of attention involves the capacity to re-orient from irrelevant to relevant stimuli prior to the establishment of executive control of motor output – a process that is believed to support executive functioning later on in childhood, such as task-switching and impulse control. Refinement of executive control involves an increase in communication between the DAN and later developing FP system20,46. Our findings suggest that maternal inflammation during pregnancy is associated with the coordinated functioning between these systems as it emerges during the neonatal period.
The relative degree of integration versus segregation of functional networks over the course of development in relation to signals in the prenatal environment (such as maternal inflammation) is an important topic for future research. In addition to the early development of modular (integrated) networks, communication between systems has been shown to evolve over the first year of life in an independent and non-linear fashion18, which may further signify the importance of our current findings. It will be important in future work to identify how the trajectories of development in these systems may be modulated by prenatal exposure to inflammation. Last, it will be important to test the relationship of maternal inflammation and newborn functional connectivity signals in animal models where strict experimental controls can be applied, and causal inferences can be made47,48.
Newborn functional connectivity is associated with regions known to be involved with future working memory and maternal IL-6 levels directly predict working memory performance
As noted in the introduction, working memory is a core component of executive functioning which relates to emerging theory of mind, social skills, academic performance, and future mental health outcomes (including ADHD, ASD, and Schizophrenia)27,28. Such disorders (ADHD, ASD, and Schizophrenia) have previously been linked to maternal inflammation during pregnancy and share common deficits across a range of executive functions, including working memory performance28, which may precede the traditional age at diagnosis. While the current findings in no way suggest inflammation is the “cause” of these disorders, they do point to an association between at least one component dimension (i.e., atypical executive functioning/working memory) that spans across each of these diagnostic domains.
Using a meta-analysis29, we showed that brain regions most strongly associated with estimating maternal IL-6 also strongly overlap with regions in the brain known to be important for working memory performance. The implication of this finding is that fluctuations in maternal IL-6 levels may be relevant for offspring working memory later in life due to associations with early emerging variation in functional brain systems. The follow-up analysis directly showed that maternal IL-6 concentrations in our sample are predictive of, and negatively correlated with, actual working memory performance in the same children 2-years later. Importantly, these findings do not indicate an isolated association between maternal inflammation during pregnancy and offspring working memory performance; it is likely that maternal inflammation is also relevant for other cognitive domains. Indeed, it is largely known that working memory covaries with other executive functions including inhibition, task control, and impulse control, amongst others49. The brain systems capable of estimating maternal IL-6 levels in this sample (e.g. CON, SAL, DAN, and FP) also span a host of other higher order cognitive functions. Therefore, we assume that while our findings clearly suggest an association between prenatal inflammation and individual differences in working memory performance, the effects of prenatal inflammation are unlikely to be specific and direct causality cannot be inferred. Future translational work that leverages both animal and human models with this regard will be of high importance to elucidate these issues further.
Another consideration of these findings, is that maternal IL-6 acts in concert with other aspects of maternal-placental-fetal biology with potential to influence brain development and subsequent working memory. We anticipate that the amount of variance explained with regard to future working memory or other executive functions will be greatly increased by including in our model other aspects of maternal biology during pregnancy, including endocrine, metabolic and additional inflammatory markers, which also have potential to act as mediating pathways for the influence of diverse prenatal conditions on the developing fetal brain14. Incorporating indicators of the quality of the postnatal environment, such as socioeconomic status, availability of nutrition, and responsive caregiving, would also likely enhance our capacity to predict working memory and other EF outcomes, and will be an important direction for future research. Animal models will continue to be highly informative and allow for teasing apart the individual and combined influence of various conditions and biological pathways on the developing brain. While beyond the scope of the current report, such work on both of these fronts is already underway.
Limitations
The networks used to evaluate the relationship between mean maternal IL-6 and neonatal functional connectivity in the present study were originally obtained in adults, but have now been well documented across studies, age cohorts and imaging modalities33. Specifically, work by Gao and colleagues has previously reported the modular architecture of the infant brain is dominated by early developing primary sensory systems, followed by the emergence of default-mode and dorsal-attention systems18. Lin et al., using the same regions utilized in the current paper, have also shown that while the networks are not fully integrated at these early stages of development, their component parts are formed early on 17,18,20,50. As outlined within the introduction of the current manuscript, this approach allows us to examine well-established and validated networks of interest and how they relate to complex behavior at 2 years of age. Nonetheless, future work utilizing robust network definitions defined during the neonatal period will be of great interest. It should also be noted that, by necessity, assessment of connectivity in the neonatal brain is conducted during sleep. While the multivariate models estimating maternal IL-6 are relatively strong and accurate during this state, more work attempting to clarify awake versus sleep patterns in infants is warranted19,20.
Our approach to identifying correspondence between the features identified in the multivariate brain models and brain regions involved in working memory based on the Neurosynth meta-analysis does not indicate that these features are uniquely involved in working memory versus other cognitive abilities. Working memory itself is a well-studied phenomenon, and we chose it because it is a core component of EF that can be reliably measured beginning at 2-years of age26. While our findings highlight sensitivity of maternal IL-6 and associated brain features at birth to future working memory, this finding should not be taken to imply specificity. Maternal inflammation and brain features associated at birth are likely to influence in some respect other cognitive domains, as well.
With regard to the sample, the mother-infant dyads recruited and analyzed in the current study are not representative of high-risk populations and future investigations within such samples are warranted. However, we feel analyzing maternal IL-6 within a normative range, as opposed to more extreme ends of the scale (i.e. infection and/or neuro-trauma), is a particular strength of the study. This approach highlights the associations of even modest variation in IL-6 with neonatal functional connectivity and later EF. While maternal age, gestational age and age at scan were not correlated with our variables of interest (i.e. maternal IL-6 concentrations and working memory at two years of age) future work assessing the factors contributing to elevated maternal inflammation, and interactions between pre- and post-natal factors are warranted.
Conclusion
Research to date, largely conducted in animal models, has shown associations between prenatal exposure to maternal inflammation and atypical offspring neurodevelopment and behavior. Here using machine-learning and resting-state functional MRI in a sample of 84 neonates, we show variations in maternal IL-6 concentrations (across the course of pregnancy) are associated with individual differences in functional brain networks in the neonatal period and relate to future working memory performance. These results support and extend prior work examining prenatal IL-6 administration in animal models, and studies at the molecular level, which highlight the role of inflammatory processes in typical and atypical neurodevelopment. Importantly, by examining brain function shortly after birth, we increase the capacity to distinguish between the influences of prenatal (such as maternal inflammation during pregnancy) versus postnatal environmental factors on functional brain development. Undoubtedly, pre- and postnatal environmental conditions have the potential to interactively affect brain developmental trajectories. Thus, future work aims to characterize how pre- and postnatal factors (biological, psychosocial, environmental, etc.) interact to influence later brain and cognitive trajectories. Ultimately, such an understanding can help elucidate the complex interplay between biological transmission of risk for poor neurodevelopmental outcomes, and may inform early intervention efforts aimed at reducing the impact of prenatal adversity on offspring brain development and subsequent developmental outcomes.
Methods
Participants
Neonates included in the study (N=84; M=25.45 days, SD=12.09 days; 50% Female) are part of an ongoing longitudinal study for which mothers (N=84; M=28.48 years, SD=5.15 years) were recruited during the first trimester of pregnancy. Exclusionary criteria for mothers were as follows: maternal use of psychotropic medication during pregnancy; maternal use of corticosteroids during pregnancy; maternal alcohol or drug use during pregnancy; and known congenital, genetic, or neurologic disorder of the fetus (e.g., Down syndrome, fragile X). Exclusionary criteria for infants were birth before 34 weeks gestation, and evidence of a congenital, genetic or neurologic disorder. Our final study population of 84 mother/infant dyads came from a total of 152 mothers who were originally recruited for the study. Twenty-one mothers opted out of the MRI/fMRI scan after birth. Of the remaining 131 that were attempted, 24 were deemed unsuccessful, as no data were obtained, and 1 participant was not utilized because of maternal use of corticosteroids during pregnancy (which was discovered prior to application of the initial exclusionary criteria). The remaining 22 participants not utilized either did not have a successful resting state functional MRI scan acquisition, or had insufficient amounts of resting-state data (see below for more details). All procedures were approved by the Institutional Review Board at the University of California, Irvine in compliance with ethical regulations and standards. All participants provided written informed consent. Participants with behavioral data did not differ from full sample with regard to demographic variables. These details have been provided previously43. All neonates with usable MRI data, and maternal IL-6 measurements were used in the current study. Simulation models incorporating effect sizes from studies on maternal stress biology at University of California Irvine, and data regarding variation in the neonatal brain51 were used to determine the original sample size for study. While no formal statistical analysis was done to predetermine sample-size for this specific analysis, our sample is similar in size to prior infant functional brain imaging work52,53, and to our knowledge, the largest infant longitudinal sample to date that also includes maternal prenatal immune response data54. As this was one normative sample without any specific sample manipulations, no participant randomization was conducted during sample collection.
Maternal IL-6 Collection & Assessment
Collection of maternal blood samples for measurement of IL-6 occurred in early, mid and late pregnancy. Mean gestational age in weeks at each collection was 12.7(1.71)), 20.5 (1.39)), 30.4 (1.33)) for each time point respectively. To determine concentrations of IL-6 concentrations, peripheral blood was collected in serum tubes (BD Vacutainer). Serum samples were allowed to clot for 30 min on room temperature and were centrifuged at 4 °C at 1500 x g. Serum was then separated and stored at −80 C. Serum IL-6 levels were determined using a commercial high sensitive ELISA (eBioscience) with a sensitivity of 0,03 pg/ml according to the manufacturer’s instructions. The intra- and inter-assay coefficients of variability for IL-6 measurements were 10% and 14% respectively. Measurements for the imaging portion of the analysis were averaged across trimesters given IL-6 concentrations at each time point were highly correlated (r= 0.553–0.684, p < .001).
MRI Data acquisition
Neuroimaging data was collected during a tight window at approximately 4 weeks-of-age (M=3.79 weeks, SD=1.84) during natural sleep on a TIM Trio, Siemens Medical System 3.0T scanner. Neonates were swaddled and fitted with ear protection to reduce scanner noise. Waking and respiration were monitored. High resolution T2- (TR=3200 ms, echo time=255 ms, resolution=1×1×1 mm, 4.18 mins) and T1-weighted scans (MP-RAGE TR=2400 ms, inversion time=1200 ms, echo time=3.16 ms, flip angle=8°, resolution=1×1×1 mm, 6.18 mins) were collected. Functional images for resting state functional connectivity MRI (rs-fcMRI) were obtained using a gradient-echo, echoplanar imaging (EPI) sequence sensitive to blood oxygen level-dependent (BOLD) contrast (TR=2000 ms, TE=30 ms, FOV=220x220x160mm, flip angle = 77°). Full brain coverage was obtained with 32 ascending-interleaved 4 mm axial slices with a 1 mm skip. Steady-state magnetization was assumed after 4 frames (8s). Functional data was acquired in a single scan consisting of 195 volumes for all but eight participants whose scans consisted of 150 volumes during the initial phase of the study.
MRI and fMRI data preprocessing
Brain images were separated from the rest of the head tissue with the Brain Extraction Tool from the FMRIB Software Library (FSL)55, and an additional refinement with an in-house technique (labeled refine mask) to improve brain masks as necessary. This technique utilizes the mask generated from co-registered functional data back-registered to the anatomical image to ensure accurate results. Functional images were preprocessed to reduce artifacts utilizing tools from FSL and the 4dfp Suite of Image Processing Programs (ftp://ftp.imaging.wustl.edu/pub/raichlab/4dfptools/)38,43. These steps included: (i) removal of a central spike caused by MR signal offset, (ii) correction of odd versus even slice intensity differences attributable to interleaved acquisition without gaps, (iii) realignment, and (iv) intensity normalization to a whole brain mode value of 1000. Atlas transformation of the functional data was computed for each individual via the high-resolution T2 scan. The transformation involved calculation of a single matrix for each individual to facilitate registration both to a standard infant template (0- to 2-month age range; MRI Study of Normal Brain Development)56, and to the Talairach coordinate system57 (by aligning the infant template to a custom atlas-transformed target template [711-2B] using a series of affine transforms. Each run was then resampled in atlas space, combining realignment and atlas transformation in one interpolation. All subsequent operations were performed on the atlas-transformed volumetric time series.
rs-fcMRI preprocessing
Additional preprocessing steps were employed to reduce spurious variance stemming from non-neuronal activity22,38,43. Steps included: 1) regression of six parameters (head re-alignment estimates) obtained by rigid body head motion correction, 2) regression of the whole brain signal38,58,59, 3) regression of ventricular signal averaged from ventricular regions-of-interest (ROI), 4) regression of white matter signal averaged from white matter ROI, 5) regression of first order derivative terms for whole brain, ventricular, and white matter signals (to account for variance between regressors), and 6) temporal bandpass filtering (0.009 Hz < f < 0.08 Hz)22,38,55. As described in the steps above, nuisance regression was applied prior to bandpass filtering to circumvent the potential for reintroducing unfiltered noise (i.e. previously filtered frequencies) back into the data60.
Motion
Additional steps were taken to examine movement of a given frame relative to the previous frame, known as framewise displacement (FD)55. We used a volume censoring approach, removing volumes associated with greater than .3 mm FD (and 1 preceding and 2 following volumes to account for temporal blurring)55. Scans with less than 4 minutes of data remaining after volume censoring (N=10) were not included in analyses. Additionally, 6 functional scans were either not successfully acquired (N=4) or excluded for poor quality after visual inspection (N=2), resulting in our final sample size of N=84. For the remaining infants, scan length was approximately 5 minutes (M=5.33, S=0.072). For remaining volumes, mean FD was approximated (M=0.083, S=0.02). No association was found between the number of frames remaining (r2 = 0.0018, r2-adj = −0.0103, p = .698), nor remaining mean FD (r2 = 0.0001, r2-adj = −0.0120, p = .910) with gestational age (GA). The same is true for mean IL-6 (frames remaining r2 = 0.0297, r2-adj = 0.0178, p = .117; remaining mean FD r2 = 0.0076, r2-adj = −0.0045, p = .492) and working memory (frames remaining r2 = 0.0150, r2-adj = −0.007, p = .418; remaining mean FD r2 = 0.0451, r2-adj = −0.0233, p = .157).
Partial Least Squares Regression (PLSR)
We chose to use PLSR to assess associations between neonatal functional brain connectivity and variations in mean maternal IL-6 due to the high-dimensional feature space (number predictors). PLSR is a multivariate technique similar to Principle Components Analysis (PCA) that models a response by reducing a large set of correlated features into orthogonal (uncorrelated) components. However, PLSR takes the outcome variable of interest (y; maternal IL-6) into consideration by limiting the relationship (amount of covariance) between the predictor variables (x) and maximizing covariance (prediction) between x and y via singular-value decomposition (SVD)61.
Applying PLSR to within and between connectivity matrices
Here, (x) represents an n-by-m two-dimensional input matrix where n is the number of participants (rows) and m is the number of connections (columns) within a given functional matrix (within or between network). (y) is a 1-dimensional vector containing our outcome measure of interest (mean maternal IL6) for each participant. We used cross-validation to identify the optimal number of components used to estimate mean maternal IL-6 in our sample of 84 neonates. Cross-validation is an iterative process whereby a sample dataset is randomly partitioned into training sets used (exclusively) to build the models and independent test sets used to assess a model’s robustness, prevent overfitting, and increase generalizability to unseen data. This approach identifies a given number of components capable of providing the best overall fit while simultaneously reducing the mean-squared error (MSE) and explaining the greatest variance. In order to avoid selection bias, maximize sample-size and generalizability within our dataset in the absence of a true validation set, we used 10-fold cross-validation to estimate an optimal number of components to use per network model. With that said, to be sure that our findings were robust to this model selection step, we ran a subsequent analysis without component selection. In this case, we run the predictions across a large number of components (e.g., 1–20), and take the median effect size of those predictions. Thus, the procedure is agnostic and does not require the original component selection step. Findings from both procedures are shown in Table 1. Due to the nature of the analysis, data collection and analytics were not performed blind to the conditions of the experiments.
Random Resampling
Using a fixed number of components, or across a range of components as identified in the previous step for a given network model, a holdout procedure is used to generate a distribution of correlations between true and estimated mean maternal IL-6. Specifically, here participants are pseudo-randomly partitioned using a 20% holdout procedure resulting in 80% training (68) and 20% test (16) sets. This process is repeated over a large number of iterations (k=4000) in order to reduce sampling bias. The distribution of correlations (fit) between true and estimated mean maternal IL-6 is then tested for robustness against a null distribution (i.e. random chance). In order to achieve this, a process identical to that described above is repeated, however, on each iteration the outcome variable (y) is randomly permuted (i.e. shuffled) and new PLSR models are generated. Networks are then initially filtered to those most strongly related to IL-6 by simply choosing those networks with a p-value < 0.001 using a Kolmogorov-Smirnov test, and whose effect size is small (0.2), medium (0.5), or large (0.8). In addition to our random resampling procedures, a Leave-one-out-cross validation procedure was also used to confirm the overall nature of our findings (Supplementary Materials; Supplemental Figure 1).
Predictive Features
As schematized in Figure 1, for each participant, a functional connectivity matrix is generated from a set of 264 ROIs which belong to larger network or communities33. Of these previously identified networks, we assess 10 commonly cited and well-validated functional brain networks including the Default Mode (DFM), Visual (VIS), Cingulo-opercular (CON), Sensorimotor (SSM), Salience (SAL), Frontoparietal (FP), Subcortical (SUB), Dorsal Attention (DAN), Ventral Attention (VAN) and Cerebellar (CER) systems. From the larger FC matrices comprising all 264 ROIs and networks, within and between subnetwork matrices of interest are extracted, and the unique connections between ROI pairs are used as features (x) in the PLSR models used to estimate mean maternal IL-6. The beta weights obtained, signifying the importance of a particular connection between ROIs in the model, were ranked and summed by their absolute values across tests (consensus features). ROIs were then plotted on a standardized brain surface using Caret 5 software (University of Washington, St. Louis) and scaled proportionally by their absolute beta weights.
Infant working memory performance
Spin-the-pots27, is a visuospatial, multi-location search task designed to probe working memory in toddlers and young children. Pots are arranged on a spinning tray (“lazy susan”) and participants are asked to place stickers inside 6 of 8 pots. Participants must try to remember which pots have stickers in them, and choose one after each time the tray is spun. Scoring is calculated by taking the total number of possible trials (16) minus the number of errors (turns taken to recover the stickers unsuccessfully). Of the 84 neonates with resting state functional connectivity data, to date 46 (M=24.66 mo, SD=0.73 mo; 48% Female) have been assessed with this measure at two years of age. Using the same procedure as in the primary analyses (i.e. PLSR paired with random resampling), associations between maternal IL-6 concentrations in early, mid and late pregnancy and infant working memory performance were tested. Further, a traditional regression analysis was used to assess the direction (positive or negative) of the relationship between mean maternal IL-6 and working memory in our sample of neonates.
Infant negative emotionality
The revised parent-report measure of infant temperament, the Infant Behavior Questionnaire (IBQ) was used to assess infant negative emotionality62.
Nested Leave-One-Out Cross-Validation (LOOCV)
We repeated the validation process described above using a leave-one out approach. Here, the number of folds is equal to the number of participants (N=84). Within each fold, a test participant (N=1) is held-out and predictive models are constructed using a nested LOOCV with the training data of remaining participants (N=83). Again, in order to test against a null-distribution, this process is repeated a number of times. Here, because of the computing load of generating random bootstrap models with nested LOOCV, we only ran 100 permutations to test against. Importantly, the networks we focus on in the manuscript (SUB, DAN, SAL) tend to remain the best performing models compared to random chance. In addition, the nodes most strongly related to IL-6 using the LOOCV procedure continue to overlap more strongly with regions activated in the working memory meta-analysis using Neurosynth (Supplemental Figure 1).
Of note, leave-one out cross-validation approaches tend to produce less reliable estimates of model performance, and is one reason we chose the random resampling procedure noted above. There are several limitations to the LOOCV with respect to generalizability. For example, early simulation studies37,63 have evaluated a range of methods for model validation. These reports found an interesting relationship between the number of folds (2-fold to LOOCV were tested) and the variability of the predictive error. As the validation procedure uses increasingly more data (i.e. becomes more LOOCV), the models themselves become more stable because the structure of the training data is increasingly similar across the models. However, this is not necessarily a positive result because there is very little ability to improve a poorly generalizable model (i.e., it comes at a cost to the testing data, where the testing error becomes highly variable across the folds). As a result, the predictive estimate of model performance: the mean accuracy across all folds which is an estimate of the generalization error, becomes more variable as well. In other words, the generalizability of the performance of the model (i.e. whether this model will work with new training data) is more difficult to assess with a LOOCV procedure, as it exhibits a large pessimistic bias. This particular behavior is now well documented in the literature and discussed to some degree in the imaging field here (http://www.russpoldrack.org/2012/12/the-perils-of-leave-one-out.html). Again, this is one reason why we chose our current approach. Nonetheless, our general findings in the main manuscript replicate with this cross-validation procedure as well.
Code availability
Partial least-squares regression is available as a standalone function within the Matlab (The MathWorks, Inc., Natick, Massachusetts, United States) software package. Custom Matlab code used within the manuscript for all analyses is available from the corresponding author upon reasonable request.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Life Sciences Reporting Summary
Further information on experimental design is available in the Life Sciences Reporting Summary.
Supplementary Material
Acknowledgments
We greatly acknowledge the assistance of Eric R. Earl at OHSU for pipeline preparation and assistance. This work was funded by National Institutes of Mental Health (Grants R01 MH091351 C.B, and R01 MH091351 C.B. D.A.F) and was supported by the National Institutes of Health (Grants R01 MH096773 and K99/R00 MH091238 to D.A.F.), Oregon Clinical and Translational Research Institute (D.A.F), NIMH K99 MH111805 (AG), the Gates Foundation (D.A.F, R.N., A.G., C.B.), The Destafano Innovation Fund (D.A.F.), and the National Library of Medicine Postdoctoral Fellowship (E.F.).
Footnotes
Author Contributions
M. D. Rudolph, A. M. Graham and D. A. Fair drafted the manuscript and designed analyses. M. D. Rudolph managed data and performed data analysis under supervision of D. A. Fair., and A. M. Graham. C. Buss designed the study and provided valuable insight regarding developmental models incorporating prenatal health factors. C. Buss and J. Rasmussen collected and disseminated all raw behavioral and imaging data. All authors provided critical revisions and have approved the final version of the manuscript for submission.
Competing Financial Interests
No other competing financial interests are reported.
References
- 1.Buka SL. Maternal Infections and Subsequent Psychosis Among Offspring. Archives of General Psychiatry. 2001;58:1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- 2.Patterson PH. Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behavioural Brain Research. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Hava G, Vered L, Yael M, Mordechai H, Mahoud H. Alterations in behavior in adult offspring mice following maternal inflammation during pregnancy. Developmental Psychobiology. 2006;48:162–168. doi: 10.1002/dev.20116. [DOI] [PubMed] [Google Scholar]
- 4.Estes ML, McAllister AK. Maternal immune activation: Implications for neuropsychiatric disorders. Science. 2016;353:772–777. doi: 10.1126/science.aag3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knuesel I, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nature Reviews Neurology. 2014;10:643–660. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- 6.Buss C, et al. Intergenerational Transmission of Maternal Childhood Maltreatment Exposure: Implications for Fetal Brain Development. Journal of the American Academy of Child and Adolescent Psychiatry. 2017;56:373–382. doi: 10.1016/j.jaac.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monk C, Spicer J, Champagne FA. Linking prenatal maternal adversity to developmental outcomes in infants: The role of epigenetic pathways. Development and Psychopathology. 2012;24:1361–1376. doi: 10.1017/S0954579412000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deverman BE, Patterson PH. Cytokines and CNS Development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Boulanger LM. Immune Proteins in Brain Development and Synaptic Plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Kohli S, et al. Self-Reported Cognitive Impairment in Patients With Cancer. Journal of Oncology Practice. 2007;3:54–59. doi: 10.1200/JOP.0722001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buss C, Entringer S, Wadhwa PD. Fetal Programming of Brain Development: Intrauterine Stress and Susceptibility to Psychopathology. Science Signaling. 2012;5:pt7–pt7. doi: 10.1126/scisignal.2003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith SEP, Li J, Garbett K, Mirnics K, Patterson PH. Maternal Immune Activation Alters Fetal Brain Development through Interleukin-6. Journal of Neuroscience. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu WL, Hsiao EY, Yan Z, Mazmanian SK, Patterson PH. The placental interleukin-6 signaling controls fetal brain development and behavior. Brain, Behavior, and Immunity. 2017;62:11–23. doi: 10.1016/j.bbi.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham AM, et al. Maternal Systemic Interleukin-6 During Pregnancy Is Associated With Newborn Amygdala Phenotypes and Subsequent Behavior at 2 Years of Age. Biological Psychiatry. 2017 doi: 10.1016/j.biopsych.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buss C, Entringer S, Swanson JM, Wadhwa PD. The Role of Stress in Brain Development: The Gestational Environment’s Long-Term Effects on the Brain. Cerebrum. 2012;2012:1–16. [PMC free article] [PubMed] [Google Scholar]
- 16.Wei H, et al. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2012;1822:831–842. doi: 10.1016/j.bbadis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Smyser CD, Snyder AZ, Neil JJ. Functional connectivity MRI in infants: Exploration of the functional organization of the developing brain. NeuroImage. 2011;56:1437–1452. doi: 10.1016/j.neuroimage.2011.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao W, et al. Temporal and spatial evolution of brain network topology during the first two years of life. PLoS ONE. 2011;6:e25278. doi: 10.1371/journal.pone.0025278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham AM, Fair DA. Commentary: Developmental connectomics to advance our understanding of typical and atypical brain development - A commentary on Vértes and Bullmore (2015) Journal of Child Psychology and Psychiatry and Allied Disciplines. 2015;56:321–323. doi: 10.1111/jcpp.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grayson DS, Fair DA. Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. NeuroImage. 2017;160:15–31. doi: 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomason ME, et al. Cross-Hemispheric Functional Connectivity in the Human Fetal Brain. Science Translational Medicine. 2013;5:173ra24–173ra24. doi: 10.1126/scitranslmed.3004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fair DA, et al. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khambhati AN, Sizemore AE, Betzel RF, Bassett DS. Modelling And Interpreting Network Dynamics. bioRxiv. 2017:1–17. doi: 10.1101/124016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sporns O. Structure and function of complex brain networks. Dialogues in Clinical Neuroscience. 2013;15:247–262. doi: 10.31887/DCNS.2013.15.3/osporns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sporns O. Towards network substrates of brain disorders. Brain. 2014;137:2117–2118. doi: 10.1093/brain/awu148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck DM, Schaefer C, Pang K, Carlson SM. Executive function in preschool children: Test-retest reliability. Journal of Cognition and Development. 2011;12:169–193. doi: 10.1080/15248372.2011.563485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes C, Ensor R. Executive function and theory of mind: Predictive relations from ages 2 to 4. Developmental Psychology. 2007;43:1447–1459. doi: 10.1037/0012-1649.43.6.1447. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz E, Tost H, Meyer-Lindenberg A. Working memory genetics in schizophrenia and related disorders: An RDoC perspective. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics. 2016;171:121–131. doi: 10.1002/ajmg.b.32353. [DOI] [PubMed] [Google Scholar]
- 29.Yarkoni T, Poldrack R, Nichols T, Van Essen D, Wager T. NeuroSynth: a new platform for large-scale automated synthesis of human functional neuroimaging data. Frontiers in Neuroinformatics Conference Abstract: 4th INCF Congress of Neuroinformatics. 2011 doi: 10.3389/conf.fninf.2011.08.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pruett JR, et al. Accurate age classification of 6 and 12 month-old infants based on resting-state functional connectivity magnetic resonance imaging data. Developmental Cognitive Neuroscience. 2015;12:123–133. doi: 10.1016/j.dcn.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dosenbach NUF, et al. Prediction of Individual Brain Maturity Using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudolph MD, et al. At risk of being risky: The relationship between ‘brain age’ under emotional states and risk preference. Developmental Cognitive Neuroscience. 2017;24:93–106. doi: 10.1016/j.dcn.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Power JD, et al. Functional Network Organization of the Human Brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao W, Lin W, Grewen K, Gilmore JH. Functional Connectivity of the Infant Human Brain. The Neuroscientist. 2017;23:169–184. doi: 10.1177/1073858416635986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Combrisson E, Jerbi K. Exceeding chance level by chance: The caveat of theoretical chance levels in brain signal classification and statistical assessment of decoding accuracy. Journal of Neuroscience Methods. 2015;250:126–136. doi: 10.1016/j.jneumeth.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Goodman S. A Dirty Dozen: Twelve P-Value Misconceptions. Seminars in Hematology. 2008;45:135–140. doi: 10.1053/j.seminhematol.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Kohavi R. A Study of Cross-Validation and Bootstrap for Accuracy Estimation and Model Selection. 1995 [Google Scholar]
- 38.Fair DA, et al. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Frontiers in Systems Neuroscience. 2013;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seeley WW, et al. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menon V. Salience Network. Brain Mapping: An Encyclopedic Reference. 2015;2:597–611. [Google Scholar]
- 42.DiMartino A, et al. Unraveling the miswired connectome: A developmental perspective. Neuron. 2014;83:1335–1353. doi: 10.1016/j.neuron.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graham AM, et al. Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Developmental Cognitive Neuroscience. 2016;18:12–25. doi: 10.1016/j.dcn.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Posner MI. Imaging attention networks. NeuroImage. 2012;61:450–456. doi: 10.1016/j.neuroimage.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin S, Young CB, Supekar K, Uddin LQ, Menon V. Immature integration and segregation of emotion-related brain circuitry in young children. Proceedings of the National Academy of Sciences. 2012;109:7941–7946. doi: 10.1073/pnas.1120408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao W, Alcauter S, Smith JK, Gilmore JH, Lin W. Development of human brain cortical network architecture during infancy. Brain structure & function. 2015;220:1173–1186. doi: 10.1007/s00429-014-0710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stafford JM, et al. Large-scale topology and the default mode network in the mouse connectome. Proceedings of the National Academy of Sciences. 2014;111:18745–18750. doi: 10.1073/pnas.1404346111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miranda-Dominguez O, et al. Bridging the Gap between the Human and Macaque Connectome: A Quantitative Comparison of Global Interspecies Structure-Function Relationships and Network Topology. Journal of Neuroscience. 2014;34:5552–5563. doi: 10.1523/JNEUROSCI.4229-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diamond A. Executive Functions. Annual Review of Psychology. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li G, Lin W, Gilmore JH, Shen D. Spatial Patterns, Longitudinal Development, and Hemispheric Asymmetries of Cortical Thickness in Infants from Birth to 2 Years of Age. Journal of Neuroscience. 2015;35:9150–9162. doi: 10.1523/JNEUROSCI.4107-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knickmeyer RC, et al. A Structural MRI Study of Human Brain Development from Birth to 2 Years. Journal of Neuroscience. 2010;28:12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smyser CD, et al. Resting-State Network Complexity and Magnitude Are Reduced in Prematurely Born Infants. Cerebral Cortex. 2016;26:322–333. doi: 10.1093/cercor/bhu251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smyser CD, et al. Prediction of brain maturity in infants using machine-learning algorithms. NeuroImage. 2016;136:1–9. doi: 10.1016/j.neuroimage.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Short SJ, et al. Associations between white matter microstructure and infants’ working memory. NeuroImage. 2013;64:156–166. doi: 10.1016/j.neuroimage.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith SM, Bannister P, Beckmann C, Brady M. FSL: New tools for functional and structural brain image analysis. NeuroImage. 2001;2001 [Google Scholar]
- 56.Fonov V, Evans A, McKinstry R, Almli C, Collins D. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage. 2009;47:S102. [Google Scholar]
- 57.Talairach P, Tournoux J. Co-planar Stereotaxic Altas of the Human Brain. Georg Thieme Verlag; 1988. [Google Scholar]
- 58.Power JD, et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burgess GC, et al. Evaluation of Denoising Strategies to Address Motion-Correlated Artifacts in Resting-State Functional Magnetic Resonance Imaging Data from the Human Connectome Project. Brain Connectivity. 2016;6:669–680. doi: 10.1089/brain.2016.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: Spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. 2013;82:208–225. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abdi H, Williams LJ. Partial least squares methods: Partial least squares correlation and partial least square regression. Methods in Molecular Biology. 2013;930:549–579. doi: 10.1007/978-1-62703-059-5_23. [DOI] [PubMed] [Google Scholar]
- 62.Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior and Development. 2003;26(1):64–86. https://doi.org/10.1016/S0163-6383(02)00169-8. [Google Scholar]
- 63.Breiman L, Spector P. Submodel Selection and Evaluation in Regression. The X-Random Case. Source: International Statistical Review/Revue Internationale de Statistique. 1992;60:291–319. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.