Abstract
Enteropathogenic Escherichia coli (EPEC) is a foodborne pathogen that uses a type III secretion system to translocate effector molecules into host intestinal epithelial cells (IEC) subverting several host cell processes and signaling cascades. Interestingly, EPEC infection induces only modest intestinal inflammation in the host. The homologous EPEC effector proteins, NleH1 and NleH2, suppress the NF-κB pathway and apoptosis in vitro. Increased apoptosis and activation of NF-κB and MAP kinases (MAPK) contribute to the pathogenesis of inflammatory bowel diseases (IBD). The aim of this study was to determine if NleH1 and NleH2 also block MAPK pathways in vitro and in vivo and to compare the effects of these bacterial proteins on a murine model of colitis. Cultured IECs were infected with various strains of EPEC expressing NleH1 and NleH2, or not, and the activation of ERK1/2 and p38 was determined. In addition, the impact of infection with various strains of EPEC on murine DSS colitis was assessed by change in body weight, colon length, histology, and survival. Activation of apoptosis and MAPK signaling were also evaluated. Our data show that NleH1, but not NleH2, suppresses ERK1/2 and p38 activation in vitro. Interestingly, NleH1 affords significantly greater protection against and hastens recovery from DSS-induced colitis compared to NleH2. Strikingly, colitis-associated mortality was abolished by infection with EPEC strains expressing NleH1. Interestingly, in vivo NleH1 suppresses activation of ERK1/2 and p38 and blocks apoptosis independent of the kinase domain that inhibits NF-κB. In contrast, NleH2 suppresses only caspase-3 and p38, but not ERK1/2. We conclude that NleH1 affords greater protection against and improves recovery from DSS colitis compared to NleH2 due to its ability to suppress ERK1/2 in addition to NF-κB, p38, and apoptosis. These findings warrant further investigation of anti-inflammatory bacterial proteins as novel treatments for IBD.
INTRODUCTION
Enteropathogenic Escherichia coli (EPEC) is a foodborne pathogen causing diarrhea especially in children of developing countries. The virulence of EPEC depends on the translocation of effector proteins into host intestinal epithelial cells via a type III secretion system (T3SS) (1). EPEC infection induces an inflammatory response through host recognition of conserved pathogen associated molecular patterns (PAMPs) including flagellin, LPS, and CpG DNA which trigger innate receptors including Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain protein (NOD)-like receptors (2). These interactions elicit an inflammatory response in host cells in part by activating the mitogen activation protein kinase (MAPK) and nuclear factor-κB (NF-κB) signaling pathways. MAPK signaling cascades consist of three sequentially activated protein kinases ending at the MAPK groups, ERK, p38, and JNK, which convert extracellular signals into a wide range of cellular processes, including cell proliferation and differentiation, inflammation, and cell death (3). MAPKs mediate the activation of many different transcription factors, including but not limited to NF-κB, STAT1/3, c-Myc, and SMAD3/4, which regulate pro-inflammatory gene expression of cytokines (IL-1α, IL-1β, TNF, IL-6, etc.) and chemokines (IL-8 and CCL2, etc.) (4).
The complexity of the crosstalk between the MAPK and NF-κB makes it difficult to determine the specific roles for each pathway in causing disease. However, it has been demonstrated that constitutive activation of NF-κB does not cause destructive intestinal inflammation unless accompanied by MAPK activation (5). Crohn’s disease and ulcerative colitis are inflammatory bowel diseases (IBD) of which the etiology and predisposition have been linked to polymorphisms in both NOD2 and TLR4 receptors (6, 7). These alterations upregulate and activate both the MAPK and NF-κB pathways causing intestinal inflammation and dysbiosis of the intestinal microbiota (7). DSS administered to mice is used as a model for IBD as it induces colitis. Although the exact mechanisms by which DSS causes inflammation are unclear, it activates both MAPK (8–12) and NF-κB (13) pathways leading to pro-inflammatory cytokine and chemokine production (14, 15).
Interestingly, EPEC infection induces minimal intestinal inflammation, especially compared to other enteric pathogens such as Shigella, Yersenia, and Salmonella (16, 17). Part of this suppression is due to the arsenal of EPEC anti-inflammatory effector proteins including but not limited to the non-LEE encoded NleB, NleC, NleD, NleE, and the homologs NleH1 and NleH2 (NleH1/H2) (18). Most studies regarding NleH1/H2 have utilized in vitro models. NleH1 and NleH2 suppress inflammation in part by inhibiting apoptosis (19) and suppressing NF-κB when IκκB is overexpressed (20). NleH1 and NleH2 contain kinase domains on which NF-κB suppression, but not anti-apoptotic activity, is dependent (19, 21, 22). Limited in vivo studies of NleH1/H2 have focused on the colonization advantage afforded by NleH1/H2 during EPEC infection of mice (20). In addition, serum KC (the mouse equivalent of human IL-8) levels are higher in mice infected with EPEC lacking nleH1/H2 than those infected with wild-type EPEC (20), indicating that NleH1/H2 dampen pro-inflammatory cytokine expression and host inflammation.
NleH1/H2 are homologous to OspG, the Shigella flexneri effector kinase protein known to downregulate NF-κB activity by interacting with a host ubiquitin-conjugating enzyme (23–25). Similar to the activity of OspG, Salmonella AvrA and Yersinia YopJ, two proteins secreted by T3SSs, inhibit NF-κB activity via deubiquitination (26, 27). In addition, AvrA and YopJ also suppress MAPK pathways through their acetyl transferase activity (17, 28). The C-terminal kinase domains of NleH1 and NleH2 are involved in suppressing NF-κB through deubiquitination and other mechanisms (20–22). However, little is known about the activity of the N-terminal domains of NleH1 and NleH2. Similar to AvrA and YopJ, there may be other NleH1 and NleH2 domains or activities involved in suppression of inflammation after infection. Therefore we hypothesized that EPEC NleH1 and NleH2 also block MAPK pathways thus increasing their anti-inflammatory activities. This study compares the impact of these translocated EPEC homologues on host inflammation triggered by infectious or chemical stimuli and assesses their effect on p38 and ERK1/2 MAPK pathways.
MATERIALS AND METHODS
Materials and reagents
Bacterial strains and plasmids are described in Table S1 in the supplemental material. Antibiotics were purchased from Sigma-Aldrich: chloramphenicol (25 µg/mL), kanamycin (50 µg/mL), and ampicillin (200 µg/mL). Antibodies and protease/phosphatase inhibitors are described (see Table S2 in the supplemental material).
Intestinal epithelial cell (IEC) culture
T84 cells were grown in low glucose Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 (Gibco) medium with 15 mM HEPES (US Biological), 10% newborn calf serum (Gibco) and 100 UI/mL penicillin and 100 µg/mL streptomycin (pen/strep). Caco-2 cells were grown in low glucose DMEM, 19.4 mM D-dextrose (Fisher), 20.8 mM HEPES, and pen/strep. Cells were grown at 37°C in 5% CO2, used 7–10 days post plating, and media replaced with bacterial growth medium 24h prior to infection. Immunofluorescence methods are as described in supplemental material.
Bacterial growth and infection of host cells
Bacterial cultures, grown overnight in Luria-Bertani (LB) broth with appropriate antibiotics, were diluted (1:33) in serum-free and antibiotic-free DMEM/Ham’s F12/ (1:1) supplemented with 14m M NaHCO3 (Fisher), 10 mM HEPES (pH 7.4), and 0.5% mannose (Fisher), and grown at 37°C to mid-log growth phase. For complemented strains, media was supplemented with 25 µM IPTG. Bacterial cultures were centrifuged, resuspended in media, and added to 24-well plates with glass coverslips, Transwell permeable supports (Costar #3740), or 6-well plates at a multiplicity of infection of 100. Infected monolayers were incubated at 37°C in 5% CO2 for indicated times.
Preparation of protein extracts and immunoblot analysis
Infected monolayers were washed with PBS, resuspended in lysis buffer (65.8 mM Tris-HCl, 2.1% SDS, 5% glycerol plus protease/phosphatase inhibitors), passed through a 25-gauge needle, and quantified. 80 µg of total protein was resolved on SDS-PAGE and immunoblot analysis was as described in supplemental material.
Murine infection and DSS treatment
Eight to ten-week-old male C57BL/6J mice were used (Jackson Laboratory Bar Harbor, ME, USA) and housed in a specific pathogen-free facility at the University of Illinois at Chicago (UIC) or Hines VA for 7–14 days with free access to food and water. Mice were infected with bacteria by oral gavage as previously described (29). Briefly, mice were pretreated with 5 g/mL streptomycin sulfate (Gibco) in drinking water for 24h, which was replaced with sterile water 24h prior to infection. Pelleted bacterial concentration was adjusted to ~2 × 109 cfu/200 µl in PBS. For complementation, 25 mM IPTG (Gold Biotechnology) was added to the drinking water in all groups of mice 18–24h prior to infection and maintained throughout the course of experiment. Mice were treated with 3% colitis grade DSS (MP Biomedicals, LLC) in drinking water for 6 days either before or after EPEC infection (20). All animal protocols were approved by UIC and Hines VA’s Animal Care and Use Committee.
Histological analysis and tissue immunofluorescence
Mouse intestines were processed as “Swiss rolls” and fixed in 10% phosphate-buffered formalin (24h). 5 µm sections of fixed paraffin-embedded tissues were stained with hematoxylin and eosin (H&E). For immunofluorescence studies, paraffin-embedded tissues were deparaffinized in xylene, rehydrated with ethanol and H2O washes, and antigen retrieval performed in Tris-EDTA plus Tween 20 buffer (pH 9). Tissue sections were blocked and incubated with primary and secondary antibodies in 5% normal goat serum in PBS/0.1% Tween 20/0.1% saponin. Images were taken with Leica DM 4000B confocal microscope (MetaMorph software) and processed using Adobe Photoshop and ImageJ software. For quantification, 5–10 images from at least 3 mice were assessed for relative intensity per area of IF staining in epithelial cells using Adobe Photoshop.
Statistical Analysis
All data are reported as mean ± SEM. For densitometry of immunoblots, data comparisons were made using non-parametric two-way ANOVA with Tukey post-tests. All other data comparisons were made using non-parametric one-way ANOVA and Tukey post-tests. Differences were considered significant when the p-value was ≤0.05. Data were graphed using GraphPad Prism software version 7 (La Jolla, CA).
RESULTS
NleH1 suppresses MAPK pathways in vitro
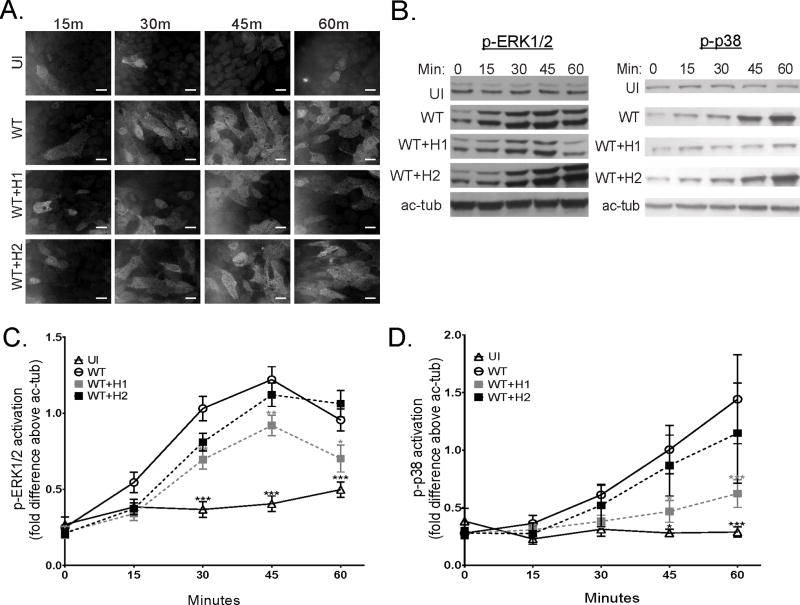
Although EPEC activates both ERK1/2 and p38 at early time points post-infection, it suppresses these pathways as infection progresses (30–33). Initial experiments were performed to determine if NleH1 or NleH2 plays a role in suppression of MAPK pathways following EPEC infection. T84 and Caco-2 IEC monolayers were infected with wild-type (wt) EPEC or wt EPEC overexpressing NleH1 (WT+H1) or NleH2 (WT+H2) and the levels of activation, measured as phosphorylated-ERK1/2 (p-ERK1/2) and phosphorylated-p38 (p-p38), were examined at the indicated time points by immunofluorescence and immunoblot analysis (Figure 1). EPEC infection initially activates ERK1/2 and p38, as reported previously, but activity diminishes by 60min post-infection. Overexpression of NleH1 significantly reduces ERK1/2 and p38 activation compared to wt EPEC (Figure 1a–d). In contrast, overexpression of NleH2 does not impact ERK1/2 or p38 compared to wt EPEC (Figure 1a–d). The levels of NleH1 and NleH2 expression were similar in both the bacterial lysates and in secreted supernatants of these strains (Supplementary Figure 1a–b).
Figure 1. NleH1 but not NleH2 dampens activation of ERK1/2 and p38 in cultured intestinal epithelial cells.
Cultured human intestinal epithelial cell monolayers were infected with various strains of EPEC for the indicated times (Min). T84 cells were immunostained for p-ERK1/2 (a) and total cell lysates analyzed for p-ERK1/2 in T84 cells and p-p38 in Caco-2 cells by immunoblot analysis (b). p-ERK1/2 and p-p38 levels quantified against acetylated-tubulin (ac-tub) using densitometry (c and d, respectively). Uninfected (UI), EPEC (WT), and WT complemented with inducible plasmid harboring nleH1 and nleH2 (WT+H1 and WT+H2, respectively). Representative blot of n=7 experiments. Statistical analysis was by 2-way ANOVA using Tukey post-hoc tests (*, p<0.05; **, p<0.01; ***, p<0.001; vs. WT of corresponding time point). Scale bars: 15 µm.
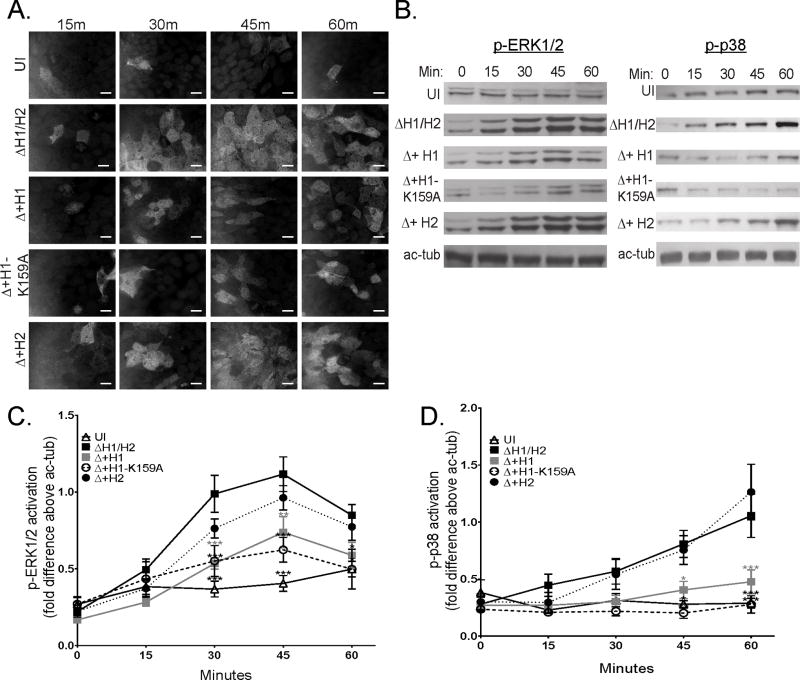
NleH1 suppresses the NF-κB pathway via its kinase domain at lysine 159 (21, 22). In order to determine if K159 is required for suppression of ERK1/2 or p38 activation, T84 and Caco-2 IEC monolayers were infected with wt EPEC, EPEC with both nleH1 and nleH2 deleted (ΔH1/H2), or ΔH1/H2 expressing NleH1 (Δ+H1), NleH1 mutated at K159 (Δ+H1-K159A) or NleH2 (Δ+H2), and p-ERK1/2 and p-p38 were assessed by immunofluorescence microscopy and immunoblot analysis. NleH1, but not NleH2, expressed in the double mutant strain suppressed both p-ERK1/2 and p-p38 (Figure 2a–d). In contrast to its effects on the NF-κB pathway, the suppressive activity of the NleH1-K159A mutant on ERK1/2 and p38 (Figure 2a–d) was as effective as that of wt NleH1 indicating that this site is not required for MAPK blockade. The levels of NleH1, NleH-K159A, and NleH2 expression are similar in both bacterial lysates and secreted supernatants (Supplementary Figure 1a–b).
Figure 2. NleH1-K159A mutant dampens activation of ERK1/2 and p38 in cultured intestinal epithelial cells.
Cultured human intestinal epithelial cell monolayers were infected with various strains of EPEC for indicated times (Min). T84 cells were immunostained for p-ERK1/2 (a) and total cell lysates analyzed for p-ERK1/2 in T84 cells and p-p38 in Caco-2 cells by immunoblot analysis (b). p-ERK1/2 and p-p38 levels quantified against acetylated-tubulin (ac-tub) using densitometry (c and d, respectively). Uninfected (UI), EPEC mutant lacking nleH1/H2 (ΔH1/H2), and ΔH1/H2 complemented with inducible plasmid harboring nleH1, nleH1-K159A, or nleH2 (Δ+H1, Δ+H1-K159A, and Δ+H2, respectively). Representative blot of n=7 experiments. Statistical analysis was by 2-way ANOVA using Tukey post-hoc tests (*, p<0.05; **, p<0.01; ***, p<0.001; vs. ΔH1/2 of corresponding time point). Scale bars: 15 µm.
To confirm the suppressive effects of NleH1 on MAPK pathways in cells grown on permeable supports, T84 monolayers were plated on Transwells and infected with various EPEC strains and processed for immunofluorescence microscopy. Overexpression of NleH1, in both the wt and double mutant backgrounds, and expression of NleH1-K159A displayed similar levels of p-ERK1/2 in cells grown on impermeable as compared to permeable supports (Figure 1a, 2a, and Supplementary Figure 2). Similarly, NleH2 failed to reduce p-ERK1/2 in T84 monolayers grown on permeable supports (Supplementary Figure 2).
Established EPEC infection attenuates DSS-induced murine colitis and increases survival
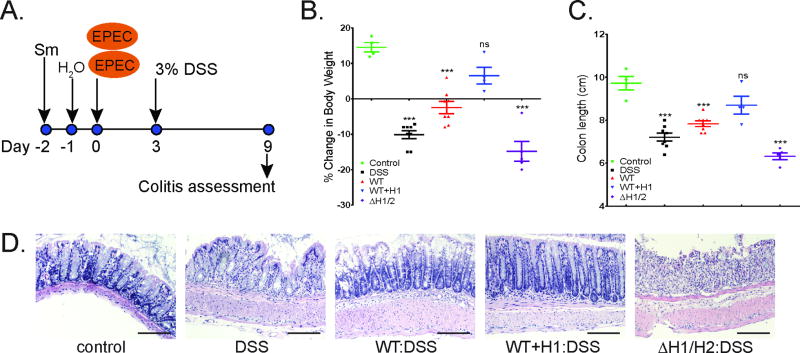
In view of the suppressive effects of NleH1 on MAPK pathways, the possibility that EPEC infection could ameliorate chemically-induced colitis was explored. The effect of EPEC infection on DSS-induced colitis in mice was determined. EPEC reaches peak colonization at 3 days post-infection (20, 34). Therefore, mice were infected with wt EPEC, WT+H1, or ΔH1/H2 for 3 days prior to administration of 3% DSS in drinking water for 6 days. Body weight, colon length, and histology were assessed at day 9 (Figure 3a). As expected, DSS caused a significant decrease in body weight compared to controls (Figure 3b) as well as colonic shortening (Figure 3c). Interestingly, established infection with wt EPEC protected mice from weight loss and colonic shortening (Figure 3b and c) associated with DSS colitis. Overexpression of NleH1 provided even further protection against weight loss and colonic shortening; these parameters were not significantly different from controls (Figure 3b and c). In stark contrast, infection with ΔH1/H2 exacerbated both weight loss and colonic shortening (Figure 3b and c). H&E staining of colonic tissues revealed that infection with wt EPEC reduced inflammation and improved epithelial integrity compared to DSS alone. Infection with wt EPEC overexpressing NleH1 restored colonic tissues to normal (Figure 3d). Consistent with exacerbated weight loss and colonic shortening by infection with ΔH1/H2, infection of mice with DSS colitis with the nleH1/nleH2 double mutant exacerbated both inflammation and epithelial destruction (Figure 3d). These data demonstrate that EPEC infection attenuates DSS-induced colitis in mice and that NleH1 greatly contributes to the protective effects.
Figure 3. Infection with EPEC protects against DSS-induced colitis.
Schematic depicting experimental protocol of animal study as described in materials and methods (a). Mice were infected with: mock infection with PBS (DSS), EPEC (WT), WT complemented with inducible plasmid harboring nleH1 (WT+H1), or EPEC mutant lacking nleH1 and nleH2 (ΔH1/H2) and then given 3% DSS in drinking water. Control mice were not infected or given DSS. Percent change in body weight assessed at 9 days post-infection (dpi) from initial weight (day 0) (b). Colon length (cm) at 9dpi (c). H&E staining of mouse colonic tissue at 9 dpi; representative image of n=4–8. Scale bars: 100 µM (d).
EPEC infection enhances recovery of mice with pre-existing DSS-induced colitis
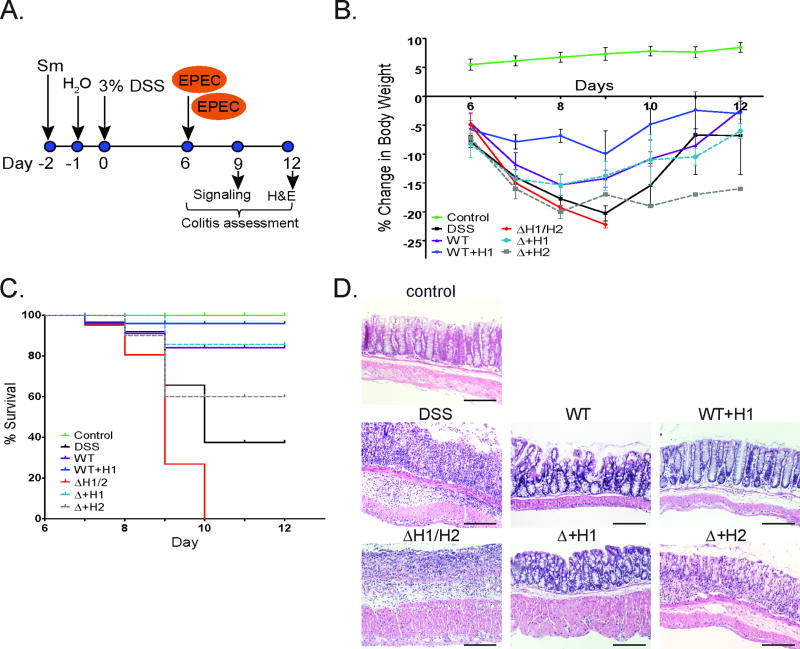
The data presented above demonstrate that pre-established EPEC infection provides protection of mice against DSS colitis. To explore the therapeutic potential of NleH1 in vivo, the impact of EPEC and NleH1 on established DSS colitis was determined. In view of the ability of NleH1, but not NleH2, to block ERK1/2 and p38 in vitro, mice were treated with 3% DSS for 6 days, switched to normal drinking water, then infected with wt EPEC, WT+H1, ΔH1/H2, or ΔH1/H2 expressing either NleH1 (Δ+H1) or NleH2 (Δ+H2) (Figure 4a). Body weight and survival were assessed over the subsequent 6 days (Figure 4b and c); colonic histology was assessed at the end of the experiment (Figure 4d).
Figure 4. EPEC infection enhances recovery from DSS-induced colitis.
Schematic depicting experimental design of recovery studies as described in materials and methods (a). Mice were given 3% DSS in drinking water and then infected with: mock infection with PBS (DSS), EPEC (WT), WT complemented with inducible plasmid harboring nleH1 (WT+H1), EPEC mutant lacking nleH1 and nleH2 (ΔH1/H2), and ΔH1/H2 complemented with inducible plasmid harboring nleH1 or nleH2 (Δ+H1 and Δ+H2, respectively). Control mice were not treated with DSS or infected. Percent change in body weight 6 days post-infection corresponding to 12 days post treatment (dpt) compared to initial weight (day 0) (b). Percent survival from days 6–12dpt (c). H&E staining of mouse colonic tissue at 12dpt; representative image of n=4–8. Scale bars: 100 µM (d).
All groups of treated mice exhibited the greatest decrease in body weight at 9 days post-DSS treatment (dpt) then began to regain weight, except those infected with ΔH1/H2 or Δ+H2 (Figure 4b). DSS-colitis mice infected with ΔH1/H2 suffered the most severe weight loss (−22.2±0.6%). Interestingly, DSS mice infected with Δ+H2 lost significantly more weight than any strain expressing NleH1 (WT, WT+H1, or Δ+H1) (Figure 4b) despite colonization levels shown to be similar to ΔH1/H2 expressing NleH1 (20).
Even more striking were the differences in survival rates (Figure 4c). While DSS-treated mice had a survival rate of 38%, this increased significantly in animals infected with wt EPEC (84%) and to control levels in mice infected with WT+H1 (96%). In stark contrast, 100% of DSS-treated mice infected with ΔH1/H2 either died or were sacrificed due to severe weight loss by day 10. Expression of NleH1 in the double mutant strain increased survival to 84% while expression of NleH2 increased survival to only 60%.
H&E staining of colonic tissues from mice treated with DSS alone showed marked inflammation and epithelial destruction as expected. Infection with wt EPEC significantly improved colonic architecture and those infected with WT+H1 were restored to normal (Figure 4d). In DSS-treated animals infected with ΔH1/H2, colonic architecture was completely replaced by extensive inflammation (Figure 4d). Expression of NleH1 in this background reduced inflammation and restored colonic architecture while expression of NleH2 afforded an intermediate phenotype (Figure 4d). These data demonstrate that infection with EPEC before or after the onset of DSS-induced colitis reduces the severity of colitis and increases survival. Although homologues, NleH1 provides significantly greater protection than NleH2.
Suppression of the apoptotic pathway in vivo by NleH1 and NleH2
DSS activates many signaling pathways causing severe colitis. Having observed that NleH1 affords greater protection and therapeutic effects against DSS colitis than NleH2, their effects on signaling pathways were investigated. Since mice exhibited the greatest weight loss at 9 days post-DSS treatment (Figure 4a and 4b), this time point was selected for examination via immunofluorescence staining and quantitation in colonic tissues.
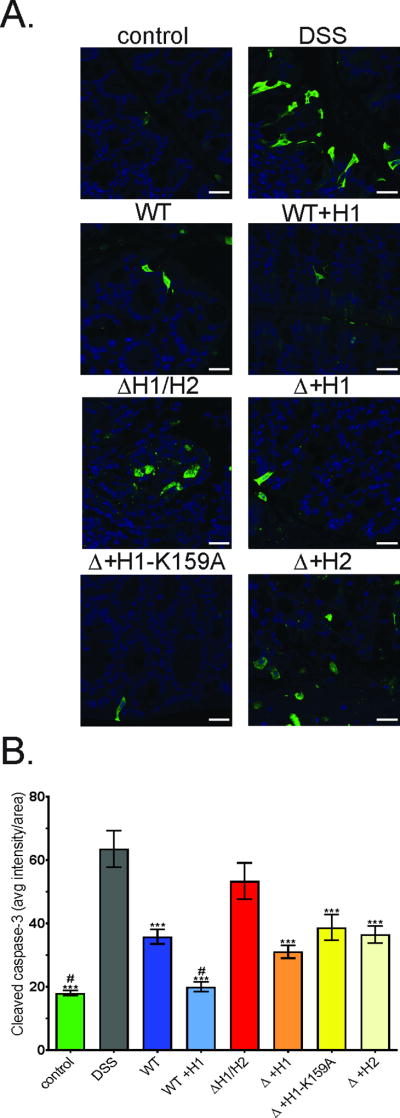
NleH1 and NleH2 have been demonstrated to have anti-apoptotic activity in vitro by binding BI-1 thus blocking procaspase-3 cleavage (19). To determine if NleH1 and NleH2 exhibit differential anti-apoptotic activity in vivo, mice with acute colitis were infected with various EPEC strains, depicted in Figure 4a, and cleaved caspase-3 levels were assessed in colonic tissues. As expected, cleaved caspase-3 was increased in colonic tissues from mice with DSS colitis (Figure 5a and b). Infection of these mice with wt EPEC reduced cleaved caspase-3, and WT+H1 further suppressed to control levels. In contrast, infection with ΔH1/H2 increased cleaved caspase-3 to levels associated with DSS treatment alone (Figure 5a and b). Expression of NleH1, NleH1-K159A, or NleH2 in the double mutant background reduced cleaved caspase-3 comparably (Figure 5a and b). These in vivo data support previously reported in vitro data demonstrating that NleH1 and NleH2 are equally capable of preventing apoptosis and that suppression is independent of the known kinase/NF-κB blocking domain.
Figure 5. EPEC suppresses apoptosis associated with DSS-induced colitis.
Mice were treated with DSS then infected with various EPEC strains as described in figure 4A plus an additional group infected with ΔH1/H2 complemented with inducible plasmid harboring nleH1-K159A (Δ+H1-K159A). Distal colonic tissues were immunostained (a) and quantitated (b) at 9dpt for cleaved caspase-3. Representative image and analysis of n>20 high-powered fields from n=4 mice. Statistical analysis was by ANOVA using Tukey post-hoc tests (*, p<0.05; ***, p<0.001; vs. DSS alone). Scale bars: 25 µm.
NleH1 and NleH2 differentially suppress MAPK pathways in vivo
Having demonstrated that NleH1 and mutant NleH1-K159A, but not NleH2, suppress both p38 and ERK1/2 pathways in vitro, the effect of these anti-inflammatory EPEC effector homologues were studied in vivo using the DSS colitis/EPEC infection model (Figure 4a).
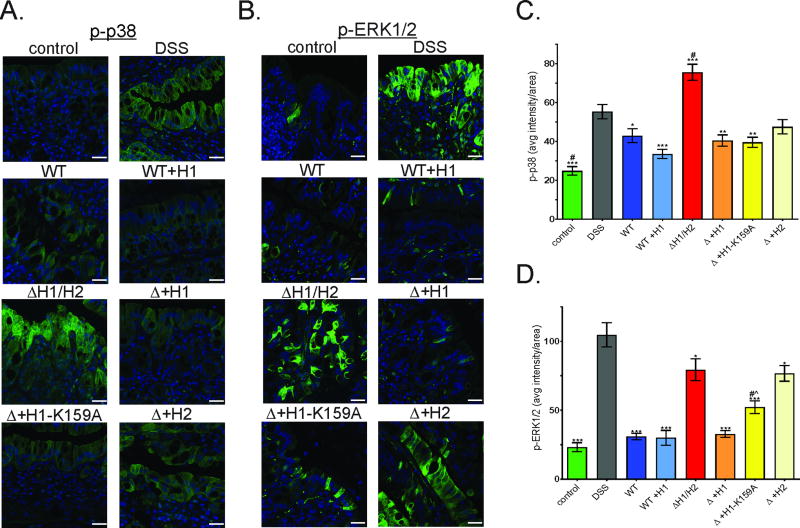
p38 is activated in DSS colitis (8, 9) and as expected mice treated with DSS had significantly increased levels of p-p38 in colonic epithelial cells compared to controls. Infection of these mice with either wt EPEC or WT+H1 blocked p38 activation (Figure 6a and c). Interestingly, infection with ΔH1/H2 significantly increased p-p38 beyond the levels seen in response to DSS alone (Figure 6a and c). In contrast, expression of NleH1, NleH1-K159A, or NleH2 in the double mutant background was equally effective in reducing p38 activated by DSS (Figure 6a and c). These findings are similar to the in vitro data in that NleH1 suppression of p38 is not dependent on the kinase domain. However, in contrast to in vitro data, NleH2 did attenuate p38 activation in vivo.
Figure 6. Differential suppression of p38 and ERK1/2 by NleH1 and NleH2 in DSS-induced colitis.
Mice were treated with DSS then infected with various EPEC strains as described in figure 4A plus a group infected with Δ+H1-K159A. Distal colonic sections were immunostained and quantitated at 9dpt for p-p38 (a and c) and p-ERK1/2 (b and d). Representative image and analysis of n>20 high power fields from n=4 mice. Statistical analysis was by ANOVA using Tukey post-hoc tests (*, p<0.05; ***, p<0.001; vs. DSS alone. #, p<0.01; vs. WT, WT+H1 or Δ+H1 and ^, p<0.05; vs. Δ+H2). Scale bars: 25 µm.
ERK1/2 activation occurs in macrophages (10) and the muscularis propria (8) during murine DSS colitis, however, little is known about IEC activation (12). Here we demonstrate that DSS induces ERK1/2 activation in IECs as shown in Figure 6b and d. Infection of DSS-colitis mice with wt EPEC or EPEC overexpressing NleH1 reduced p-ERK1/2 to near control levels. Infection with ΔH1/H2 had only a modest impact on ERK1/2 activation while expression of NleH1, but not NleH2, in the double mutant background restored the suppressive phenotype (Figure 6b and d). Interestingly, expression of NleH1-K159A provided an intermediate level of ERK1/2 suppression. These data demonstrate that NleH1, but not NleH2, suppresses ERK1/2 activation in vivo. In contrast to in vitro data, the NleH1 kinase domain may play a role in this phenotype.
DISCUSSION
The hallmark of inflammatory bowel diseases is the marked increase in signaling pathways leading to host inflammation. Several studies have reported that bacterial effector proteins alter these signaling pathways and reduce cytokine production (35). However, this is the first study to compare the ability of two known anti-inflammatory EPEC homologues, NleH1 and NleH2, to suppress key signaling pathways and improve established colitis. NleH1 and NleH2 are 89% similar and 83% homologous at the amino acid level and contain an intrinsic disordered domain in the N’terminus and a C-terminal atypical kinase module (36, 37). Their closest homologue is the Shigella effector kinase OspG, which suppresses NF-κB activation (25). However, OspG only contains the homologous C-terminal kinase domain and an N-terminal secretion signal (23). Therefore, it is hypothesized that the functional differences between OspG, NleH1, and NleH2 result from sequence variations in their N-terminal domains (37–39).
The majority of NleH1 and NleH2 studies have focused on mechanisms of host protein interactions affecting the NF-κB pathway and revealed that NleH1 and NleH2 have different effects in vitro. NleH1 suppresses NF-κB activation following EPEC infection and TNF stimulation whereas NleH2 was shown to slightly increase NF-κB activity (22). However, under conditions where IκB kinase is overexpressed in cells, both NleH1 and NleH2 suppress NF-κB activation by inhibiting ubiquitination of phospho-IκBα (20, 22). In addition, NleH2 has been reported to interact with ubiquitin-fold modifier-conjugating enzyme 1 (UFC1) further supporting a possible role in modulating TNF-induced IκBα degradation (40). NleH1 binds to and phosphorylates CRKL, an adaptor protein involved in tyrosine kinase signal transduction pathways (41). The interaction of NleH1 and CRKL is believed to facilitate NleH1 binding to and prevent phosphorylation of RPS3, thus excluding RPS3 and NF-κB from the nucleus. Both NleH1 and NleH2 bind RPS3 through their N-terminal domain, however despite binding to RPS3, NleH2 does not inhibit NF-κB nuclear translocation (21). In addition, when NleH1 was mutated to resemble NleH2, NF-κB suppression was ablated (21, 22). These studies underscore the functional differences of NleH1 and NleH2 despite their nearly identical homology.
The limited in vivo studies of NleH1 and NleH2 have focused on the colonization effects these proteins afford. However, the results vary depending on the host and the attaching and effacing organism (EPEC, EHEC, or Citrobacter rodentium)(19, 20, 22, 42). Although we previously determined that EPEC lacking NleH1 and NleH2 have decreased colonization and persistence levels compared to wild-type, ΔnleH1/H2 still colonizes at a high level (~107)(20). In fact, ΔnleH1/H2 induces more inflammation determined by higher serum KC levels and H&E staining of intestinal tissues showing earlier and increased inflammatory changes compared to animals infected with wild-type (20). These findings indicate that the nleH1/H2 double mutant reaches a sufficient level of colonization to induce an enhanced inflammatory response. An additional study with C. rodentium supports the notion that the primary functions of NleH1 and NleH2 are anti-inflammatory in nature and that these effects may enhance colonization but are not required (42).
Despite their apparent anti-inflammatory effects, little is known about the impact of NleH1 and NleH2 on inflammation in vivo or the mechanisms involved. In this study we show that EPEC has anti-inflammatory effects in mice with DSS-induced colitis. When EPEC was introduced either pre- or post-DSS treatment, colitis was attenuated as evidenced by the preservation of colonic length and body weight, restoration of colonic tissue integrity, and most importantly, increased survival. These protective effects are dependent on NleH1 and to a lesser extent NleH2, and led us to compare their potential to decrease intestinal inflammation in vivo and the underlying mechanisms.
In addition to the known effects of NleH1 and NleH2 on the NF-κB pathway, NleH1 and NleH2 bind Bax inhibitor-1 (BI-1) in vitro (19). NleH1 binding to BI-1 is not dependent on kinase domain activity and functionally blocks caspase-3 activation preventing apoptosis (19). We determined that both NleH1 and NleH2 reduce cleaved caspase-3 in colonic epithelial cells of mice with DSS-induced colitis. Similar to in vitro data, prevention of apoptosis in vivo is not dependent on NleH1-K159 in the kinase domain, suggesting a mechanism independent of NF-κB suppression. Prevention of apoptosis likely contributes to the intermediate protection of colitis offered by NleH2. However, the enhanced anti-inflammatory capacity of NleH1 suggests it impacts additional signaling pathways.
Recent studies have demonstrated that persistent NF-κB activation alone is not sufficient to cause severe inflammation and damage to intestinal epithelia unless accompanied by MAPK activation, likely due to their coordinated enhancement of key inflammatory cytokines (4, 5). The DSS-induced colitis mouse model mimics IBD in that NF-κB (13) and MAPK pathways (8, 9) are activated and apoptosis is induced increasing cytokines and chemokines (14, 15, 43). Increased expression and activation of both p38 and ERK1/2 have been implicated in the pathogenesis of IBD (7). The cellular regulation, mechanisms, and functional roles of downstream nuclear targets of MAPK signaling in IBD have been reviewed (7, 44). In addition, both p38 and ERK inhibitors are efficient modulators of inflammation in animal and cell models (4). Furthermore, pre-clinical and clinical studies have shown beneficial effects of MAPK inhibitors in treatment of IBD (4, 44). Therefore, we examined the role of EPEC NleH1 and NleH2 in suppressing the MAPK pathways in DSS-induced colitis in mice.
Our data show that NleH1, but not NleH2, suppresses both p38 and ERK1/2 phosphorylation in vitro. However, in vivo both NleH1 and NleH2 suppress DSS-induced activation of p38 and likely have an additive effect indicated by the increase in p-p38 levels in DSS-treated:ΔH1/H2 infected mice compared to DSS treatment alone. The differing in vitro and in vivo effects on p38 may be due to the different stimuli used to trigger inflammation. Two other EPEC effectors, NleD and NleC, have been shown to cleave and block phosphorylation of p38, respectively, after late stages of in vitro EPEC infection (31, 32). However, NleH1 and NleH2 likely have the greatest contribution in suppressing p38 after DSS-induced colitis as indicated by the stark increase in p-p38 levels after infection with the nleH1/H2 double mutant compared to DSS treatment alone. The NleH1 kinase domain is not required for p38 suppression indicating the involvement of a protein domain unrelated to the NF-κB pathway. A common domain either in the N-terminal disordered region or the C-terminal PDZ-binding motif of NleH1 and NleH2 is likely responsible for p38 suppression. PDZ-domain-containing scaffold proteins regulate multiple biological processes including the organization and targeting of signaling complexes at specific cellular compartments (45). NHERF1 and NHERF2 are PDZ-domain containing proteins (46); NleH1 binds NHERF2 (47). Interestingly, NHERF proteins have been implicated in linking G-coupled receptors and Na+/H+ exchangers to MAPK signaling pathways (48). Therefore, it is plausible that the binding of NleH1/H2 to NHERF proteins may affect MAPK activation and the associated downstream signaling consequences.
In contrast to suppression of p38, only NleH1 suppresses ERK1/2 activation in DSS-treated mice. This suppression is partially dependent on the kinase domain. The kinase domain is also required for CRKL phosphorylation, RPS3 binding, and NF-κB suppression (21, 22, 41). Interestingly, the signaling protein CRKL activates the Rap1-B-Raf-MEK-ERK pathway (49, 50) and NleH1 interaction with CRKL may disrupt this signaling cascade. In addition, CRKL binds IκB kinase-β (IKKβ) and is thought to localize NleH1 to the IKKβ complex enabling NF-κB suppression (41). However, IKKβ activation also causes ERK1/2 phosphorylation(4); thus, the NleH1-CRKL-IKKβ complex may also play a role in suppression of ERK1/2. In addition to the kinase domain, there is likely a unique NleH1 domain that differs from NleH2 contributing to ERK1/2 suppression. Both NleH1 and NleH2 autophosphorylate homologous serine and threonine residues predominantly in their N’terminus (37). Interestingly, NleH1 lacks an NleH2 autophosphorylation site in a region of non-homology that is not involved in NF-κB attenuation (21) indicating a potential NleH1 domain that is kinase-independent. Last, there may be other EPEC mechanisms involved in ERK suppression (33) as the nleH1/H2 double mutant reduced ERK levels below DSS alone, however, the degree of attenuation was modest compared to those afforded by NleH1. Even more significant, infection of mice with DSS colitis with the double mutant significantly enhanced mortality. No other EPEC effectors have been reported to suppress the ERK1/2 signaling pathway.
Based on these data, we conclude that NleH1 attenuates intestinal inflammation by suppressing several key signaling cascades, including p38 and ERK1/2, and by reducing apoptosis, whereas NleH2 only blocks p38 and apoptosis. Our data support that the ability of NleH1 to block ERK1/2 is responsible for its enhanced anti-inflammatory activity over NleH2. Suppression of the ERK1/2 pathway is a novel function of NleH1 and further implicates NleH1 as a multi-functional anti-inflammatory protein. The striking reversal of colitis and increased survivability in mice with DSS colitis warrants further investigation into the mechanisms of action of bacterial anti-inflammatory molecules, such as NleH1. Data generated from such studies may provide novel strategies for treating or controlling intestinal inflammatory disorders.
Supplementary Material
Acknowledgments
We thank members of Hecht lab for providing invaluable comments. This research was supported by National Institutes of Health grant (DK097043 to GH) and the Department of Veterans Affairs (BX000785 and BX002687 to GH).
Footnotes
Supplementary information is available at Laboratory Investigation's website.
DISCLOSURES
There are no conflict of interests to report for any of the contributing authors.
References
- 1.Dean P, Kenny B. The effector repertoire of enteropathogenic E. coli: ganging up on the host cell. Curr. Opin. Microbiol. 2009;12:101–109. doi: 10.1016/j.mib.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Morrison DK. MAP kinase pathways. Cold Spring Harb Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaestel M, Kotlyarov A, Kracht M. Targeting innate immunity protein kinase signalling in inflammation. Nat. Rev. Drug Discov. 2009;8:480–499. doi: 10.1038/nrd2829. [DOI] [PubMed] [Google Scholar]
- 5.Guma M, Stepniak D, Shaked H, et al. Constitutive intestinal NF-kappaB does not trigger destructive inflammation unless accompanied by MAPK activation. J. Exp. Med. 2011;208:1889–1900. doi: 10.1084/jem.20110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quetglas EG, Mujagic Z, Wigge S, et al. Update on pathogenesis and predictors of response of therapeutic strategies used in inflammatory bowel disease. World J. Gastroenterol. 2015;21:12519–12543. doi: 10.3748/wjg.v21.i44.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broom OJ, Widjaya B, Troelsen J, et al. Mitogen activated protein kinases: a role in inflammatory bowel disease? Clin. Exp. Immunol. 2009;158:272–280. doi: 10.1111/j.1365-2249.2009.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ihara E, Beck PL, Chappellaz M, et al. Mitogen-activated protein kinase pathways contribute to hypercontractility and increased Ca2+ sensitization in murine experimental colitis. Mol. Pharmacol. 2009;75:1031–1041. doi: 10.1124/mol.108.049858. [DOI] [PubMed] [Google Scholar]
- 9.Li YY, Yuece B, Cao HM, et al. Inhibition of p38/Mk2 signaling pathway improves the anti-inflammatory effect of WIN55 on mouse experimental colitis. Lab. Invest. 2013;93:322–333. doi: 10.1038/labinvest.2012.177. [DOI] [PubMed] [Google Scholar]
- 10.Kwon KH, Ohigashi H, Murakami A. Dextran sulfate sodium enhances interleukin-1 beta release via activation of p38 MAPK and ERK1/2 pathways in murine peritoneal macrophages. Life Sci. 2007;81:362–371. doi: 10.1016/j.lfs.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Samak G, Chaudhry KK, Gangwar R, et al. Calcium/Ask1/MKK7/JNK2/c-Src signalling cascade mediates disruption of intestinal epithelial tight junctions by dextran sulfate sodium. Biochem. J. 2015;465:503–515. doi: 10.1042/BJ20140450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miampamba M, Larson G, Lai C, et al. The ACG Annual Scientific Meeting and Postgraduate Course. Orlando, FL: Oct 3–8, 2008. RDEA110, a potent and highly selective MEK1/2 inhibitor is beneficial in dextran sulfate sodium (DSS)-induced chronic colits in mice. [Google Scholar]
- 13.Marrero JA, Matkowskyj KA, Yung K, et al. Dextran sulfate sodium-induced murine colitis activates NF-kappaB and increases galanin-1 receptor expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;278:G797–804. doi: 10.1152/ajpgi.2000.278.5.G797. [DOI] [PubMed] [Google Scholar]
- 14.Alex P, Zachos NC, Nguyen T, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm. Bowel Dis. 2009;15:341–352. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perse M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J. Biomed. Biotechnol. 2012;2012:718617. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma R, Tesfay S, Tomson FL, et al. Balance of bacterial pro- and anti-inflammatory mediators dictates net effect of enteropathogenic Escherichia coli on intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G685–94. doi: 10.1152/ajpgi.00404.2005. [DOI] [PubMed] [Google Scholar]
- 17.Reis RS, Horn F. Enteropathogenic Escherichia coli, Samonella, Shigella and Yersinia: cellular aspects of host-bacteria interactions in enteric diseases. Gut Pathog. 2010;2 doi: 10.1186/1757-4749-2-8. 8-4749-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong AR, Pearson JS, Bright MD, et al. Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements. Mol. Microbiol. 2011;80:1420–1438. doi: 10.1111/j.1365-2958.2011.07661.x. [DOI] [PubMed] [Google Scholar]
- 19.Hemrajani C, Berger CN, Robinson KS, et al. NleH effectors interact with Bax inhibitor-1 to block apoptosis during enteropathogenic Escherichia coli infection. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3129–3134. doi: 10.1073/pnas.0911609106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royan SV, Jones RM, Koutsouris A, et al. Enteropathogenic E. coli non-LEE encoded effectors NleH1 and NleH2 attenuate NF-kappaB activation. Mol. Microbiol. 2010;78:1232–1245. doi: 10.1111/j.1365-2958.2010.07400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao X, Wan F, Mateo K, et al. Bacterial effector binding to ribosomal protein s3 subverts NF-kappaB function. PLoS Pathog. 2009;5:e1000708. doi: 10.1371/journal.ppat.1000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pham TH, Gao X, Tsai K, et al. Functional differences and interactions between the Escherichia coli type III secretion system effectors NleH1 and NleH2. Infect. Immun. 2012;80:2133–2140. doi: 10.1128/IAI.06358-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grishin AM, Condos TE, Barber KR, et al. Structural basis for the inhibition of host protein ubiquitination by Shigella effector kinase OspG. Structure. 2014;22:878–888. doi: 10.1016/j.str.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Halavaty AS, Anderson SM, Wawrzak Z, et al. Type III effector NleH2 from Escherichia coli O157:H7 str. Sakai features an atypical protein kinase domain. Biochemistry. 2014;53:2433–2435. doi: 10.1021/bi500016j. [DOI] [PubMed] [Google Scholar]
- 25.de Jong MF, Liu Z, Chen D, et al. Shigella flexneri suppresses NF-kappaB activation by inhibiting linear ubiquitin chain ligation. Nat. Microbiol. 2016;1:16084. doi: 10.1038/nmicrobiol.2016.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou H, Monack DM, Kayagaki N, et al. Yersinia virulence factor YopJ acts as a deubiquitinase to inhibit NF-kappa B activation. J. Exp. Med. 2005;202:1327–1332. doi: 10.1084/jem.20051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johannessen M, Askarian F, Sangvik M, et al. Bacterial interference with canonical NFkappaB signalling. Microbiology. 2013;159:2001–2013. doi: 10.1099/mic.0.069369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu H, Jones RM, Neish AS. The Salmonella effector AvrA mediates bacterial intracellular survival during infection in vivo. Cell. Microbiol. 2012;14:28–39. doi: 10.1111/j.1462-5822.2011.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhee KJ, Cheng H, Harris A, et al. Determination of spatial and temporal colonization of enteropathogenic E. coli and enterohemorrhagic E. coli in mice using bioluminescent in vivo imaging. Gut Microbes. 2011;2:34–41. doi: 10.4161/gmic.2.1.14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savkovic SD, Koutsouris A, Hecht G. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect. Immun. 1996;64:4480–4487. doi: 10.1128/iai.64.11.4480-4487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baruch K, Gur-Arie L, Nadler C, et al. Metalloprotease type III effectors that specifically cleave JNK and NF-kappaB. Embo J. 2011;30:221–231. doi: 10.1038/emboj.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sham HP, Shames SR, Croxen MA, et al. Attaching and effacing bacterial effector NleC suppresses epithelial inflammatory responses by inhibiting NF-kappaB and p38 mitogen-activated protein kinase activation. Infect. Immun. 2011;79:3552–3562. doi: 10.1128/IAI.05033-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruchaud-Sparagano MH, Maresca M, Kenny B. Enteropathogenic Escherichia coli (EPEC) inactivate innate immune responses prior to compromising epithelial barrier function. Cell. Microbiol. 2007;9:1909–1921. doi: 10.1111/j.1462-5822.2007.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen M, Rizvi J, Hecht G. Expression of enteropathogenic Escherichia coli map is significantly different than that of other type III secreted effectors in vivo. Infect. Immun. 2015;83:130–137. doi: 10.1128/IAI.02467-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhuang X, Chen Z, He C, et al. Modulation of host signaling in the inflammatory response by enteropathogenic Escherichia coli virulence proteins. Cell. Mol. Immunol. 2017;14:237–244. doi: 10.1038/cmi.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halavaty AS, Anderson SM, Wawrzak Z, et al. Type III effector NleH2 from Escherichia coli O157:H7 str. Sakai features an atypical protein kinase domain. Biochemistry. 2014;53:2433–2435. doi: 10.1021/bi500016j. [DOI] [PubMed] [Google Scholar]
- 37.Grishin AM, Cherney M, Anderson DH, et al. NleH defines a new family of bacterial effector kinases. Structure. 2014;22:250–259. doi: 10.1016/j.str.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 38.de Jong MF, Alto NM. Thinking outside the Osp(G)--kinase activation by E2-ubiquitin. Embo J. 2014;33:403–404. doi: 10.1002/embj.201387606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grishin AM, Beyrakhova KA, Cygler M. Structural insight into effector proteins of Gram-negative bacterial pathogens that modulate the phosphoproteome of their host. Protein Sci. 2015;24:604–620. doi: 10.1002/pro.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blasche S, Arens S, Ceol A, et al. The EHEC-host interactome reveals novel targets for the translocated intimin receptor. Sci. Rep. 2014;4:7531. doi: 10.1038/srep07531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pham TH, Gao X, Singh G, et al. Escherichia coli virulence protein NleH1 interaction with the v-Crk sarcoma virus CT10 oncogene-like protein (CRKL) governs NleH1 inhibition of the ribosomal protein S3 (RPS3)/nuclear factor kappaB (NF-kappaB) pathway. J. Biol. Chem. 2013;288:34567–34574. doi: 10.1074/jbc.M113.512376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feuerbacher LA, Hardwidge PR. Influence of NleH effector expression, host genetics, and inflammation on Citrobacter rodentium colonization of mice. Microbes Infect. 2014;16:429–433. doi: 10.1016/j.micinf.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.te Velde AA, de Kort F, Sterrenburg E, et al. Comparative analysis of colonic gene expression of three experimental colitis models mimicking inflammatory bowel disease. Inflamm. Bowel Dis. 2007;13:325–330. doi: 10.1002/ibd.20079. [DOI] [PubMed] [Google Scholar]
- 44.Coskun M, Olsen J, Seidelin JB, et al. MAP kinases in inflammatory bowel disease. Clin. Chim. Acta. 2011;412:513–520. doi: 10.1016/j.cca.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 45.Ye F, Zhang M. Structures and target recognition modes of PDZ domains: recurring themes and emerging pictures. Biochem. J. 2013;455:1–14. doi: 10.1042/BJ20130783. [DOI] [PubMed] [Google Scholar]
- 46.Donowitz M, Cha B, Zachos NC, et al. NHERF family and NHE3 regulation. J. Physiol. 2005;567:3–11. doi: 10.1113/jphysiol.2005.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinez E, Schroeder GN, Berger CN, et al. Binding to Na(+) /H(+) exchanger regulatory factor 2 (NHERF2) affects trafficking and function of the enteropathogenic Escherichia coli type III secretion system effectors Map, EspI and NleH. Cell. Microbiol. 2010;12:1718–1731. doi: 10.1111/j.1462-5822.2010.01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ardura JA, Friedman PA. Regulation of G protein-coupled receptor function by Na+/H+ exchange regulatory factors. Pharmacol. Rev. 2011;63:882–900. doi: 10.1124/pr.110.004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Birge RB, Kalodimos C, Inagaki F, et al. Crk and CrkL adaptor proteins: networks for physiological and pathological signaling. Cell. Commun. Signal. 2009;7 doi: 10.1186/1478-811X-7-13. 13-811X-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki M, Mimuro H, Suzuki T, et al. Interaction of CagA with Crk plays an important role in Helicobacter pylori-induced loss of gastric epithelial cell adhesion. J. Exp. Med. 2005;202:1235–1247. doi: 10.1084/jem.20051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.