Abstract
Purpose
We report the first-in-human phase Ia study to our knowledge (ClinicalTrials.gov identifier: NCT01219699) identifying the maximum tolerated dose and assessing safety and preliminary efficacy of single-agent alpelisib (BYL719), an oral phosphatidylinositol 3-kinase α (PI3Kα)–selective inhibitor.
Patients and Methods
In the dose-escalation phase, patients with PIK3CA-altered advanced solid tumors received once-daily or twice-daily oral alpelisib on a continuous schedule. In the dose-expansion phase, patients with PIK3CA-altered solid tumors and PIK3CA-wild-type, estrogen receptor–positive/human epidermal growth factor receptor 2–negative breast cancer received alpelisib 400 mg once daily.
Results
One hundred thirty-four patients received treatment. Alpelisib maximum tolerated doses were established as 400 mg once daily and 150 mg twice daily. Nine patients (13.2%) in the dose-escalation phase had dose-limiting toxicities of hyperglycemia (n = 6), nausea (n = 2), and both hyperglycemia and hypophosphatemia (n = 1). Frequent all-grade, treatment-related adverse events included hyperglycemia (51.5%), nausea (50.0%), decreased appetite (41.8%), diarrhea (40.3%), and vomiting (31.3%). Alpelisib was rapidly absorbed; half-life was 7.6 hours at 400 mg once daily with minimal accumulation. Objective tumor responses were observed at doses ≥ 270 mg once daily; overall response rate was 6.0% (n = 8; one patient with endometrial cancer had a complete response, and seven patients with cervical, breast, endometrial, colon, and rectal cancers had partial responses). Stable disease was achieved in 70 (52.2%) patients and was maintained > 24 weeks in 13 (9.7%) patients; disease control rate (complete and partial responses and stable disease) was 58.2%. In patients with estrogen receptor–positive/human epidermal growth factor receptor 2–negative breast cancer, median progression-free survival was 5.5 months. Frequently mutated genes (≥ 10% tumors) included TP53 (51.3%), APC (23.7%), KRAS (22.4%), ARID1A (13.2%), and FBXW7 (10.5%).
Conclusion
Alpelisib demonstrated a tolerable safety profile and encouraging preliminary activity in patients with PIK3CA-altered solid tumors, supporting the rationale for selective PI3Kα inhibition in combination with other agents for the treatment of PIK3CA-mutant tumors.
INTRODUCTION
The phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) pathway is frequently dysregulated in cancer, often because of activating mutations or amplification of PIK3CA (encoding the catalytic p110α subunit of PI3K).1 PIK3CA mutations are among the most frequent alterations in solid tumors, identified in 42% to 55% of endometrial,2 42% of cervical,3 27% to 36% of breast,4,5 18% of colorectal,6 13% of head and neck,7 and 12% of ovarian cancers.8
Targeting the PI3K/mTOR pathway may be particularly effective in cancers that signal heavily through PI3Kα, such as those harboring PIK3CA alterations.9-12 The clinical development of pan-PI3K and dual PI3K/mTOR inhibitors has been limited by off-target toxicities (including GI toxicities, hepatotoxicity, and mood alterations) and modest clinical activity.13-15 Isoform-specific inhibitors could permit administration at higher, pharmacologically active doses, with fewer off-target effects.12,16
Alpelisib (BYL719; Novartis Pharma AG, Basel, Switzerland) is an oral, selective inhibitor of p110α, with half-maximum inhibitory concentrations (in vitro biochemical assay) for p110α, β, γ, and δ of 4.6, 1,156, 250, and 290 nM, respectively.12 Alpelisib has demonstrated antitumor activity in multiple cancer cell lines and tumor xenograft models, particularly those harboring PIK3CA mutations or amplifications,12,17 highlighting the potential for enhanced clinical activity in patients with PIK3CA-altered tumors. We report final results from a first-in-human dose-escalation and dose-expansion study, to our knowledge, of single-agent alpelisib in patients with PIK3CA-altered advanced solid tumors and PIK3CA-altered and PIK3CA-wild-type estrogen receptor (ER)–positive/human epidermal growth factor receptor 2 (HER2)–negative breast cancer.
PATIENTS AND METHODS
Patient Population
This study (ClinicalTrials.gov identifier: NCT01219699) enrolled patients with tumors harboring PIK3CA mutation and/or amplification, assessed locally or centrally. To explore whether tumors harboring PIK3CA alterations were more sensitive to alpelisib than PIK3CA-wild-type tumors, the dose-expansion phase also included patients with PIK3CA-wild-type, ER-positive/HER2-negative, locally advanced or metastatic breast cancer.
Patients age ≥ 18 years and with Eastern Cooperative Oncology Group performance status ≤ 2, with histologically confirmed, unresectable, advanced solid tumors, who had progression of disease per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 on their last line of therapy within 3 months of screening, or for whom no standard anticancer therapy existed, were eligible. Patients had at least one measurable or nonmeasurable lesion per RECIST v1.0 and fasting plasma glucose (FPG) < 140 mg/dL (7.8 mmol/L). Key exclusion criteria included diabetes mellitus (treated and/or symptomatic, or with FPG ≥ 140 mg/dL [7.8 mmol/L]), a history of gestational diabetes mellitus, or documented steroid-induced diabetes mellitus; and failure to benefit from a prior PI3K, AKT (Protein Kinase B), or mTOR inhibitor.
The study was approved by ethics committees and institutional review boards of participating institutions and appropriate regulatory authorities; all patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and guidelines for Good Clinical Practice, as defined by the International Conference on Harmonization.
Study Design
This was a phase Ia, multicenter, open-label, dose-escalation study of single-agent alpelisib with a dose-expansion arm at the maximum tolerated dose (MTD). The primary objective was to determine the MTD or recommended phase II dose of oral alpelisib administered in once-daily and twice-daily regimens. Secondary objectives included assessment of safety and tolerability, pharmacokinetics (PK), and preliminary efficacy. Exploratory objectives included characterization of pharmacodynamic activity and biomarkers of response to alpelisib.
Patients received oral alpelisib 30 to 450 mg once daily or 120 to 200 mg twice daily on a continuous schedule in 28-day cycles until disease progression, unacceptable toxicity, withdrawal of consent, or investigator’s decision. After MTD/recommended phase II dose determination for the once-daily schedule, an expansion arm was opened to further characterize safety and assess preliminary efficacy at this dose.
The MTD was defined as the highest dose of alpelisib not causing a dose-limiting toxicity (DLT) in > 33% of patients during the first treatment cycle. Dose escalation was guided by an adaptive Bayesian logistic regression model incorporating the escalation with overdose control (EWOC) principle.18 Under EWOC, the dose selected as the MTD must have a < 25% chance that the true DLT rate exceeds 33%, given the available DLT information. The study data (including DLTs during cycle 1 and other safety and PK data) were reviewed by the sponsor and trial investigators at each dose level. Intrapatient dose escalation was not permitted during the first four treatment cycles.
DLTs were defined as adverse events (AEs) or laboratory abnormalities possibly related to study treatment (and unrelated to the underlying disease) occurring during the first treatment cycle. DLTs included: grade ≥ 2 hyperglycemia (see safety and efficacy assessments for grading); grade ≥ 2 photosensitivity; specified grade ≥ 3 hematologic, renal, hepatic, metabolic, or subcutaneous AEs lasting for > 7 days; or any other grade ≥ 3 toxicity.
Safety and Efficacy Assessments
Routine clinical and laboratory assessments, including hematology and biochemistry, were conducted at baseline, weekly until cycle 2 day 28, and then every 2 weeks. Glucose monitoring and other safety assessments were performed at baseline and regularly throughout the study. AEs were assessed continuously according to Common Terminology Criteria for Adverse Events version 4.0; hyperglycemia grading was based on modified American Diabetes Association criteria: grade 0 (FPG < 140 mg/dL [< 7.8 mmol/L]); grade 1 (FPG 140 to 199 mg/dL [7.8 to 11.1 mmol/L]); grade 2 (FPG 200 to 249 mg/dL [11.2 to 13.8 mmol/L], confirmed with a repeat FPG measurement within 24 hours, and not resolving to grade 0 within 14 days despite intervention with antidiabetic medication such as glimepiride, glibenclamide, or metformin); grade 3 (FPG 250 to 399 mg/dL [13.9 to 22.2 mmol/L] confirmed within 24 hours); grade 4 (FPG ≥ 400 mg/dL [≥ 22.3 mmol/L], confirmed within 24 hours). Serious AEs were defined as any medical or life-threatening AE resulting in hospitalization or significant disability. Radiologic tumor response assessments were performed by computerized tomography or magnetic resonance imaging at screening, cycle 2 day 28, every 8 weeks thereafter, and at end of treatment. Overall response rate (ORR), clinical benefit rate (CBR), and disease control rate (DCR) will be determined. See Data Supplement for pharmacokinetic profiling.
Biomarker and Pharmacodynamic Assessments
PIK3CA status was assessed by next-generation sequencing (NGS) using archival or fresh biopsy tissue collected before study start (Data Supplement). Blood samples for evaluation of glucose metabolism markers (plasma glucose, insulin, and C-peptide) were collected at baseline; predose and 2 and 4 hours postdose on cycle 1 day 2, cycle 1 day 9, and cycle 2 day 2; and 4 to 6 hours postdose on cycle 2 day 28. Metabolic response was assessed in a subset of patients by [18F]-fluorodeoxyglucose positron emission tomography.
RESULTS
Patient Characteristics and Disposition
Between October 2010 and March 2014, 134 patients were enrolled across 11 sites. These included 76 patients in the dose-escalation phase and 51 patients in the dose-expansion phase with PIK3CA-altered advanced solid tumors (including two patients with unconfirmed PIK3CA mutation). There were seven patients with PIK3CA-wild-type tumors, including five patients with ER-positive/HER2-negative breast cancer (one in the dose-escalation phase and four in the dose-expansion phase). The most common primary cancer sites were breast (n = 36; 26.9%), colorectal (n = 35; 26.1%), and head and neck (n = 19; 14.2%; Table 1). At initial diagnosis, 86 patients (64.2%) had stage III or IV cancer, and all patients had received prior antineoplastic therapy.
Table 1.
Patient Characteristics at Baseline
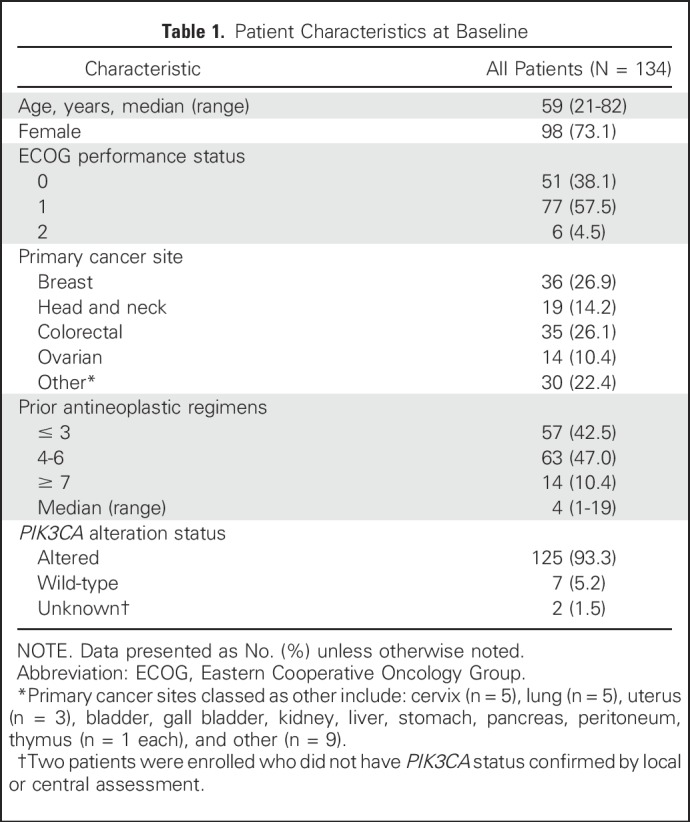
In total, 108 patients received alpelisib once daily at doses of 30 to 450 mg, including 65 patients at 400 mg once daily; and 26 patients received alpelisib twice daily at doses of 120 to 200 mg, including 15 patients at 150 mg twice daily. As of February 5, 2015, 130 (97.0%) patients had discontinued treatment: 105 (78.4%) because of disease progression, 18 (13.4%) because of AEs, three (2.2%) because of withdrawal of consent, three (2.2%) because of death (all considered unrelated to study treatment by the investigator), and one (0.7%) because of administrative problems.
Dose Escalation and Dose-Limiting Toxicities
In the dose-escalation phase, nine of 68 evaluable patients experienced DLTs. At the highest dose (450 mg once daily), four of nine patients experienced DLTs of grade 3 nausea or grade 3 hyperglycemia (n = 2 each). No patients treated at ≤ 400 mg once daily experienced DLTs. At 200 mg twice daily, four of five patients had dose-limiting hyperglycemia (n = 1 grade 3; n = 3 grade 4). At 150 mg twice daily, one of 10 patients reported hyperglycemia and hypophosphatemia (both grade 3). The 400 mg once-daily and 150 mg once-daily dose levels met the requirements of the Bayesian logistic regression model–EWOC, having probabilities of 48.4% and 56.9%, respectively, that the true DLT rate was within the target toxicity range of 16% to 33% and probabilities of 4.5% and 16.0%, respectively, that the DLT rate exceeded 33%. Alpelisib single-agent MTDs were established as 400 mg once daily and 150 mg twice daily.
DLTs were monitored in the dose-expansion phase to support the MTD determined by dose escalation. In total, four of 58 evaluable patients receiving alpelisib 400 mg once daily experienced DLTs of grade 4 hyperglycemia (n = 2), grade 3 diarrhea (n = 1), and grade 3 nausea and grade 3 vomiting (n = 1).
Safety and Tolerability
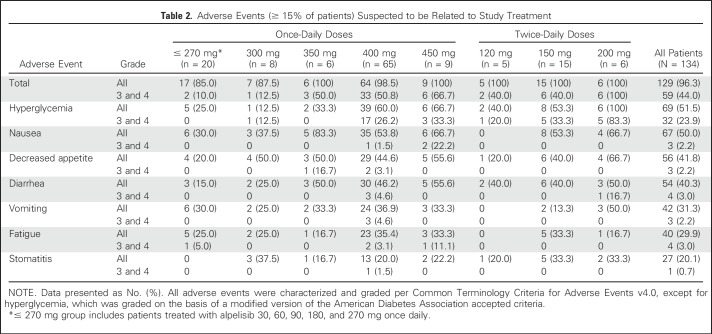
The most frequent treatment-related AE was hyperglycemia, an on-target effect of PI3K inhibition,19 reported in 69 (51.5%) patients across both dosing regimens (32 [23.9%] patients at grade 3 or 4; Table 2). Other treatment-related, all-grade AEs (≥ 15% of patients, any grade) were nausea (50.0%), skin toxicities (42.5%), decreased appetite (41.8%), diarrhea (40.3%), vomiting (31.3%), fatigue (29.9%), and stomatitis (20.1%). Overall, the frequency of AEs, including fatigue, GI toxicities, weight loss, and dyspnea, increased slightly with prolonged treatment. The rate of grade 3 or 4 hyperglycemia, rash, and liver toxicities was stable between cycle 1 and later cycles, and the AE profile was consistent throughout the study (Data Supplement).
Table 2.
Adverse Events (≥ 15% of patients) Suspected to be Related to Study Treatment
Hyperglycemia increased in a dose-dependent manner but was effectively managed, by dose interruption/reduction or by concomitant oral antidiabetic medication (metformin), and in some cases with the addition of insulin; only six (4.5%) patients permanently discontinued study treatment because of hyperglycemia. Treatment-related skin toxicities mainly comprised mild to moderate erythematous or maculopapular rash. Rash was dose dependent and typically occurred during the first 2 weeks of treatment. Skin toxicities were managed by concomitant antihistamines or corticosteroids; 12 (9.0%) patients required dose interruption/reduction because of skin toxicities, and one (0.7%) patient permanently discontinued treatment because of hypersensitivity.
In total, 51 (38.1%) patients had at least one dose reduction; most were in line with protocol-specified guidelines due to AEs. At the MTDs, dose reductions occurred in 27 of 65 (41.5%) patients starting at 400 mg once daily and nine of 15 (60.0%) patients starting at 150 mg twice daily. Dose interruptions were required by 78 (58.2%) patients, of which 63 (47.0%) were due to AEs; dose interruptions occurred in 40 of 65 (61.5%) patients at 400 mg once daily and 11 of 15 (73.3%) patients at 150 mg twice daily. Median exposure to alpelisib was 11.9 (0.4 to 145.4) weeks; 9.6 (1.0 to 145.4) weeks at 400 mg once daily and 13.0 (4.0 to 108.3) weeks at 150 mg twice daily.
Fourteen (10.4%) patients experienced treatment-related serious AEs; those reported in ≥ 4% of patients (regardless of study treatment relationship) were nausea, vomiting, abdominal pain, dyspnea, hyperglycemia, and pyrexia. None of the 13 (9.7%) on-treatment deaths were suspected to be treatment-related, and all were attributed to underlying disease progression (including one death due to hypoxia).
Pharmacokinetic and Pharmacodynamics Analyses
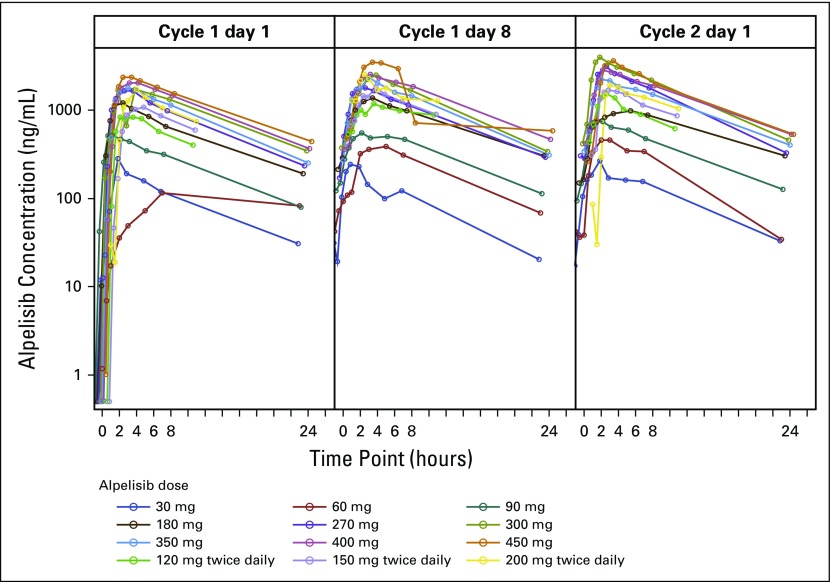
Alpelisib was rapidly absorbed, with median time to peak plasma concentrations on cycle 1 day 1 of approximately 2 hours at both MTDs. The median half-life of alpelisib was 7.6 (4.6 to 27.1) hours at 400 mg once daily. Alpelisib PK profiles and exposure were consistent on cycle 1 day 1, cycle 1 day 8, and cycle 2 day 1, suggesting minimal drug accumulation with repeated dosing (Fig 1; Data Supplement). Systemic exposure to alpelisib at both dosing schedules seemed to be dose-proportional within the range tested (Data Supplement). Interpatient variability at steady state for the once-daily dosing schedule was moderate, with mean coefficient of variation 17% to 43% for peak serum concentration and 16% to 41% for total exposure (area under the curve 0 to 24 hours), and was higher with twice-daily dosing, with mean coefficient of variation 37% to 54% for peak serum concentration and 26% to 40% for area under the curve 0 to 12 hours.
Fig 1.
Alpelisib geometric mean plasma concentration–time profiles.
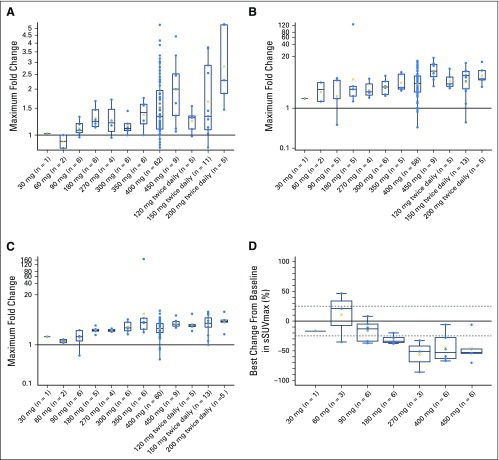
Plasma glucose, insulin, and C-peptide levels were monitored as pharmacodynamic markers of glucose regulation, because cellular glucose uptake is tightly regulated by PI3Kα.19 There was a dose-dependent increase in maximum fold change in FPG during cycle 1, starting from 180 mg once daily, with the most pronounced increase observed at doses higher than the MTDs (Fig 2A). Dose-limiting hyperglycemia was reported in seven patients. Similar increases were observed for insulin and C-peptide (Figs 2B and 2C). A decrease in glucose uptake measured by [18F]-fluorodeoxyglucose positron emission tomography was observed at doses ≥ 180 mg once daily (Fig 2D), with the first partial metabolic response occurring on cycle 1 day 24.
Fig 2.
Maximum fold increase from baseline in (A) fasting plasma glucose, (B) fasting serum insulin, and (C) fasting serum C-peptide during cycle 1, and (D) best percentage change from baseline in sum of maximum standardized uptake values (sSUVmax) measured by [18F]-fluorodeoxyglucose positron emission tomography. X represents the geometric mean value.
Clinical Activity and Biomarker Analysis
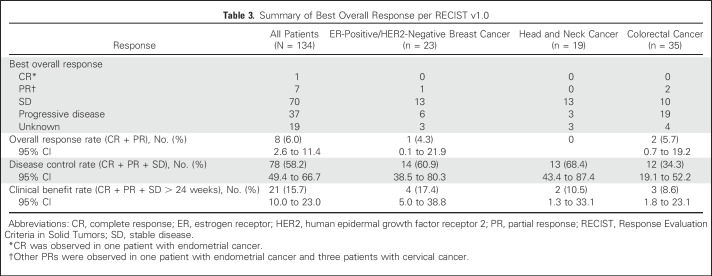
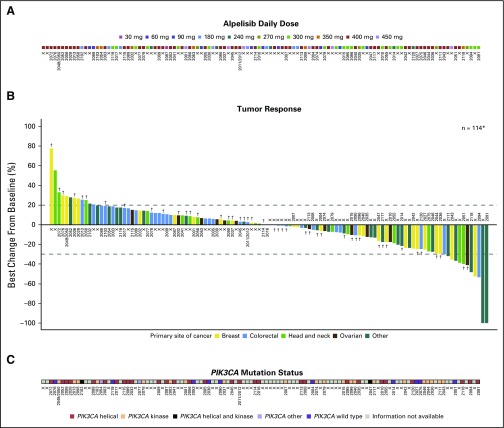
Best tumor response per RECIST v1.0 was available for 115 of 134 patients; 19 patients were not evaluable, mainly because of missing postbaseline assessments (for example, if patients discontinued before the first on-treatment scan or if they did not have measurable disease). Objective responses were observed after two to six treatment cycles at doses of ≥ 270 mg once daily, with signs of tumor growth suppression at doses ≥ 180 mg once daily. ORR was 6.0%. One patient with endometrial cancer achieved a complete response (CR) at 150 mg twice daily. Partial responses (PRs) were observed in seven patients: cervical (n = 3), breast, endometrial, colon, and rectal cancers (n = 1 each; Table 3). Stable disease (SD) was observed in more than half of patients (52%; n = 70); 13 patients (9.7%) had SD for > 24 weeks. No clinical benefit was observed in the four patients with PIK3CA-wild-type ER-positive/HER2-negative breast cancer in the dose-expansion arm.
Table 3.
Summary of Best Overall Response per RECIST v1.0
Across all tumor types, the CBR (CR + PR + SD > 24 weeks) was 15.7%, and the DCR (CR + PR + SD) was 58.2%. DCR was highest in patients with ER-positive/HER2-negative breast cancer (14 of 23 [60.9%] patients), head and neck cancer (13 of 19 [68.4%]), and cervical cancer (five of five [100.0%]). CBR for patients with ER-positive/HER2-negative breast cancer was 17.4% (four of 23 patients). DCR and CBR in patients with colorectal cancer, where two of 35 patients had PR, was 34.3% and 8.6%, respectively.
Of the 114 patients with tumor volume assessments, 55 (48.2%) patients achieved some degree of tumor shrinkage (Fig 3). Signs of increased activity were detected in patients with advanced breast cancer, with 15 of 27 (55.6%) achieving tumor shrinkage, mostly among those with PIK3CA-mutant ER-positive/HER2-negative disease, and two achieving tumor shrinkage of 25.0% and 23.5% (Data Supplement). Similarly, tumor shrinkage was observed in patients with ovarian (six of 14 [42.9%]) and head and neck tumors (seven of 17 [41.2%]). Among 22 patients with ER-positive/HER2-negative advanced breast cancer treated at ≥ 270 mg once daily, median progression-free survival was 5.5 months (95% CI, 3.0 to 7.0).
Fig 3.
Best percentage change in sum of longest diameters according to (A) study treatment dose, (B) primary site of cancer and metformin treatment, and (C) centrally obtained next-generation sequencing results. Dummy sample numbers for each patient correspond with the next-generation sequencing results in the Data Supplement. Of 12 patients with ≥ 30% reduction in tumor size, complete response (demonstrated with two or more scans taken at least 1 month apart) was observed in one patient with endometrial cancer. However, overall response was lower because some patients experienced rapid disease progression. A partial response was observed in one patient with breast cancer, two patients with colorectal cancer, and four patients with other cancers (one patient with endometrial cancer and three patients with cervical cancer). Stable disease was observed in one patient with ovarian cancer, two patients with head and neck cancer, and one patient with breast cancer. (*) Patients with no postbaseline assessment for target lesions or patients with only nontarget lesions were excluded. (†) Patients receiving metformin for hyperglycemia.
In addition to PIK3CA mutation status being measured locally during screening, tumor samples from 76 patients were also analyzed centrally by NGS (Data Supplement); PIK3CA mutations were confirmed in 64 of 76 (84.2%) tumors. Frequently mutated genes (≥ 10% of tumors) were TP53 (51.3%), APC (23.7%), KRAS (22.4%), ARID1A (13.2%), and FBXW7 (10.5%). PTEN mutations (which may contribute to PI3K inhibitor resistance)20 were detected in five patients, including three whose disease progressed during the first two treatment cycles. Concomitant KRAS mutations were detected in 13 of 17 (76.5%) colorectal tumors, but none in breast tumors (Data Supplement). Because of the heterogeneous patient population, a robust analysis of NGS results and tumor response was not possible.
DISCUSSION
We report a first-in-human study, to our knowledge, evaluating the safety, MTD, and preliminary efficacy of single-agent alpelisib, a potent and highly specific p110α inhibitor, in patients with PIK3CA-altered tumors. Alpelisib demonstrated a favorable safety profile up to the MTDs of 400 mg once daily and 150 mg twice daily. At equivalent total daily doses (400 mg once daily and 200 mg twice daily), the once-daily regimen was better tolerated. Overall, single-agent alpelisib demonstrated a wide therapeutic window, with significant pharmacodynamic effects and disease stabilization at ≥ 180 mg once daily, and objective activity at ≥ 270 mg once daily, both well below the MTD of 400 mg once daily. On the basis of improved tolerability and similar efficacy compared with 400 mg once daily, 300 mg once daily was selected as the dose in the phase III trial of fulvestrant with or without alpelisib (SOLAR-1; ClinicalTrials.gov identifier: NCT02437318).
Hyperglycemia was the most frequent DLT and was an anticipated on-target effect related to the role of PI3Kα in insulin signaling and glucose homeostasis; it was managed per guidance provided by Busaidy et al19,21 for this class of inhibitors. Indeed, serial monitoring of plasma glucose, insulin, and C-peptide levels revealed a dose-dependent increase in all three markers. Of 134 patients, only two permanently discontinued treatment during cycle 1 because of hyperglycemia (both grade 4), with a further four patients discontinuing during later cycles, reflecting effective management by dose interruptions and concomitant antidiabetic medications. Pretreatment patient characteristics and metabolic factors associated with hyperglycemia are under investigation. Other frequent AEs (including GI toxicities, fatigue, and rash) were effectively managed by standard supportive care and concomitant antihistamines or corticosteroids. Eight patients have received treatment of at least 1 year, demonstrating the long-term tolerability of alpelisib.
This study provides evidence of clinical activity of alpelisib in a subset of PIK3CA-altered tumors. The ORR in ER-positive/HER2-negative breast cancer was 4.3%, and these patients experienced the highest CBR (17.4%) and a high DCR (60.9%) compared with other cancer types. Although alpelisib single-agent activity was moderate in this group of patients, the combination of alpelisib with letrozole in patients with ER-positive/HER2-negative breast cancer resulted in an ORR of 19% and CBR of 35%22; CBR was higher in PIK3CA-altered versus PIK3CA-wild-type tumors (44% v 20%, respectively) in this study. In addition, translational research indicates the potential mechanistic synergy of combining PI3K inhibitors and endocrine therapies, particularly for patients with activating mutations of PIK3CA.23,24 Taken together, these findings highlight potential treatment benefits of alpelisib, either as a single agent or in combination with endocrine therapy, in PIK3CA-altered ER-positive/HER2-negative breast cancer. Moreover, modest single-agent activity is not uncommon in early studies of targeted agents in a heterogeneous patient population with multiple prior lines of therapy.25 Furthermore, PIK3CA mutation status at enrollment was determined locally in archival tissue, which may not provide an accurate assessment of tumor molecular status because of the spatiotemporal heterogeneity of PIK3CA mutations in tumors.26-28 Indeed, PIK3CA alterations were confirmed centrally by NGS in only 84.2% of locally positive samples, suggesting tumor heterogeneity or differences in sequencing methods. Interestingly, exploratory analyses of BELLE-2 (a phase III study of fulvestrant with or without the pan-PI3K inhibitor buparlisib) revealed a predictive value for PIK3CA status in circulating tumor DNA collected immediately before treatment start.29 The ongoing SOLAR-1 trial includes biomarker analyses to evaluate circulating tumor DNA PIK3CA status as a tool to identify patients who might derive increased benefit from alpelisib combination treatment.
Our analysis of the overall genomic landscape reveals the frequent presence of multiple concomitant oncogenic alterations in addition to PIK3CA. Preclinical studies in ovarian cancer have shown that both PIK3CA mutation and loss of phosphatase and tensin homolog (PTEN) are required to drive tumor growth.30 Moreover, clinical studies suggest that KRAS mutations might confer resistance to PI3K/mTOR pathway inhibitors in PIK3CA-mutant tumors.31 Interestingly, we found that breast tumors exhibited fewer alterations with an absence of KRAS mutations and more promising clinical activity. Meanwhile, concomitant KRAS mutations were detected in > 76.5% of colorectal tumors, which points toward a potential mechanism of resistance to PI3K inhibitors, illustrated by the lesser clinical benefit in these patients. Even in the absence of concomitant alterations at treatment initiation, PIK3CA-mutant breast tumors may develop resistance to PI3Kα inhibition via increased dependency on PI3Kβ,32 upregulation of ER pathway signaling23 or acquisition of a variety of alterations leading to PTEN loss.20 In the future, sustained disease control might be achieved through further molecular characterization to identify PIK3CA-dependent tumors and avoid those with concomitant KRAS or PTEN alterations, or by combinatorial approaches, such as addition of alpelisib to endocrine therapies. Although the sample size was small, patients with PIK3CA helical domain mutations (E545K or E542K), unusual kinase mutations (eg T1052K), or PTEN loss had partial or complete response, whereas no responses were observed in patients with kinase mutations on H1047. However, differential activity of alpelisib in helical versus kinase mutants cannot be accurately determined in such a heterogeneous set of samples, because the analysis is heavily confounded by an unequal number of patients in each disease type, cell lineage effects, and/or concomitant mutations.
In summary, this first-in-human study, to our knowledge, demonstrates that alpelisib has a favorable safety profile with predictable PK characteristics. Encouraging signs of antitumor activity were observed in patients with PIK3CA-mutant, ER-positive/HER2-negative breast cancer and other PIK3CA-altered advanced solid tumors. MTD in this phase Ia study is determined on the basis of the tolerability profile of this agent during the first cycle of treatment, using widely accepted safety boundaries for single-agent dose-escalation studies. Since we observed single-agent activity at ≥ 270 mg, a subsequent phase Ib study tested both 300-mg and 400-mg doses in combination with fulvestrant, with special attention to long-term tolerability of this agent in patients with breast cancer.33 This phase Ib study was used to inform the dose for subsequent phase III trials. Ongoing studies are evaluating alpelisib in combination with endocrine therapy and other targeted anticancer agents in a range of tumor types.
ACKNOWLEDGMENT
We thank the participating patients, their families, coinvestigators, and research coordinators, without whom this study could not have been completed. The study was funded by Novartis Pharmaceuticals Corporation and designed by the sponsor in collaboration with the trial investigators who treated patients and collected the data. We thank Stefan De Buck for contributions to the study design and conduct. We also thank Lellean JeBailey and Rose Brannon for their support with the NGS-related outputs. Christina Coughlin and Alan Huang were employees of Novartis Pharmaceuticals Corporation at the time of study conduct and manuscript development. Data analysis and interpretation were performed by the sponsor and trial investigators. Financial support for medical editorial assistance was provided by the sponsor. We thank Sara Shaw (Articulate Science) for medical editorial assistance with this manuscript. All authors contributed at each stage of manuscript development and approved the final version for submission; the authors did not receive payment for writing the manuscript. The corresponding author had full access to the study data and had final responsibility for the decision to submit for publication.
Footnotes
Supported by Novartis Pharmaceuticals Corporation.
Presented in part at San Antonio Breast Cancer Symposium, San Antonio, Texas, December 4-8, 2012; American Association for Cancer Research, Washington, DC, April 6-10, 2013; American Society of Clinical Oncology, Chicago, IL, May 31-June 4, 2013; San Antonio Breast Cancer Symposium, San Antonio, TX, December 10-14, 2013; and European Society for Medical Oncology, Madrid, Spain, September 26-30, 2014.
Clinical trial information: NCT01219699.
See accompanying article on page 1339
AUTHOR CONTRIBUTIONS
Conception and design: Dejan Juric, Jordi Rodon, José Baselga
Provision of study materials or patients: Dejan Juric, Jordi Rodon, Josep Tabernero, Filip Janku, Howard A. Burris, Jan H.M. Schellens, Mark R. Middleton, Jordan Berlin, Martin Schuler, Marta Gil-Martin, Hope S. Rugo, Ruth Seggewiss-Bernhardt, José Baselga
Collection and assembly of data: Dejan Juric, Jordi Rodon, Josep Tabernero, Filip Janku, Howard A. Burris, Jan H.M. Schellens, Mark R. Middleton, Jordan Berlin, Martin Schuler, Marta Gil-Martin, Hope S. Rugo, Ruth Seggewiss-Bernhardt, José Baselga
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phosphatidylinositol 3-Kinase α–Selective Inhibition With Alpelisib (BYL719) in PIK3CA-Altered Solid Tumors: Results From the First-in-Human Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Dejan Juric
Consulting or Advisory Role: Novartis, EMD Serono, Eisai, Genentech
Jordi Rodon
Consulting or Advisory Role: Novartis, Eli Lilly, Orion Pharma, SERVIER, MSD, Peptomyc
Research Funding: Bayer, Novartis
Josep Tabernero
Consulting or Advisory Role: Bayer, Boehringer Ingelheim, Eli Lilly, MSD, Merck Serono, Novartis, Roche, Sanofi, Symphogen, Taiho Pharmaceutical, Genentech, Amgen, Celgene, Chugai, Pfizer, Takeda
Filip Janku
Stock or Other Ownership: Trovagene
Consulting or Advisory Role: Guardant Health, Novartis, Deciphera Pharmaceuticals, Trovagene, Foundation Medicine, Sequenom
Research Funding: Novartis (Inst), Piqur Therapeutics (Inst), BioMed Valley Discoveries (Inst), Deciphera (Inst), FujiFilm (Inst), Astellas Pharma (Inst), Agios Pharmaceuticals (Inst), Plexxikon (Inst), Symphogen (Inst), Trovagene (Inst), Biocartis (Inst), Foundation Medicine (Inst)
Howard A. Burris
Employment: HCA Healthcare/Sarah Cannon
Leadership: HCA Healthcare/Sarah Cannon
Stock and Other Ownership Interests: HCA Healthcare/Sarah Cannon
Consulting or Advisory Role: Mersana (Inst), AstraZeneca (Inst), FORMA Therapeutics (Inst), Janssen (Inst), Novartis (Inst), Roche/Genentech (Inst), TG Therapeutics (Inst), MedImmune (Inst), Bristol-Myers Squibb (Inst)
Research Funding: Roche/Genentech (Inst), Bristol-Myers Squibb (Inst), Incyte (Inst), Tarveda Therapeutics (Inst), Mersana Therapeutics (Inst), AstraZeneca (Inst), MedImmune (Inst), Macrogenics (Inst), Novartis (Inst), Boehringer Ingelheim (Inst), Eli Lilly (Inst), Seattle Genetics (Inst), AbbVie (Inst), Bayer (Inst), Celldex Therapeutics (Inst), Merck (Inst), Celgene (Inst), Agios Pharmaceuticals (Inst), Jounce Therapeutics (Inst), Moderna Therapeutics (Inst), CytomX Therapeutics (Inst), GlaxoSmithKline (Inst), Verastem (Inst), Tesaro (Inst), Immunocore (Inst), Takeda (Inst), Millennium (Inst), BioMed Valley Discoveries (Inst), Pfizer (Inst), PTC Therapeutics (Inst), TG Therapeutics (Inst), Loxo (Inst), Vertex (Inst), eFFECTOR Therapeutics (Inst), Janssen (Inst), Gilead Sciences (Inst), Valent Technologies (Inst), BioAtla (Inst), CicloMed (Inst), Harpoon Therapeutics (Inst), Jiangsu Hengrui Medicine (Inst), Revolution Medicines (Inst), Daiichi Sankyo (Inst), H3 Biomedicine (Inst), Neon Therapeutics (Inst), OncoMed (Inst), Regeneron (Inst), Sanofi (Inst)
Expert Testimony: Novartis
Jan H.M. Schellens
Employment: Modra Pharmaceuticals
Stock or Other Ownership: Modra Pharmaceuticals
Mark R. Middleton
Consulting or Advisory Role: Bristol-Myers Squibb, BiolineRx, RigonTEC, Merck, Novartis, Roche
Research Funding: GlaxoSmithKline (now Novartis) (Inst), AstraZeneca (Inst), Immunocore (Inst), Roche (Inst), Amgen (Inst), Millennium (Inst), Bristol-Meyers Squibb (Inst), Vertex (Inst), Merck (Inst), Pfizer (Inst), RigonTEC (Inst), Replimune (Inst), Array BioPharma (Inst), TC Biopharm (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Merck
Jordan Berlin
Consulting or Advisory Role: Celgene, Symphogen, Genentech, Vertex, EMD Serono, Aduro Biotech, Cornerstone Pharmaceuticals, ARMO BioSciences, Five Prime Therapeutics, Opsona Therapeutics, Exelixis
Research Funding: Genentech (Inst), OncoMed (Inst), Novartis (Inst), Immunomedics (Inst), AbbVie (Inst), Gilead Sciences (Inst), Merrimack Pharmaceuticals (Inst), Taiho Pharmaceutical (Inst), Five Prime Therapeutics (Inst), Loxo Oncology (Inst), Vertex (Inst), Bayer (Inst), Symphogen (Inst), Incyte (Inst), Pharmacyclics (Inst)
Travel, Accommodations, Expenses: Genentech, Celgene
Other Relationship: AstraZeneca
Martin Schuler
Honoraria: MSD, AbbVie, Alexion, Boehringer Ingelheim, Bristol-Meyers Squibb, Novartis
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Lilly, Novartis, Roche, MSD
Research Funding: Boehringer Ingelheim, Novartis, Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: University Duisburg-Essen
Marta Gil-Martin
No relationship to disclose
Hope S. Rugo
Research Funding: Plexxikon (Inst), Macrogenics (Inst), OBI Pharma (Inst), Eisai (Inst), Pfizer (Inst), Novartis (Inst), Eli Lilly (Inst), Genentech (Inst), Merck (Inst), GTx (Inst)
Travel, Accommodations, Expenses: Novartis, Genentech, OBI Pharma, Pfizer, Puma Biotechnology, Mylan, Merck, Lilly
Ruth Seggewiss-Bernhardt
Honoraria: Novartis, MSD, Bristol-Myers Squibb, AstraZeneca, Roche, Celgene, Amgen
Consulting or Advisory Role: MSD, Bristol-Myers Squibb, AstraZeneca
Expert Testimony: Novartis, MSD, Bristol-Myers Squibb, AstraZeneca
Travel, Accommodations, Expenses: Astellas Pharma, Roche Pharma AG, Ipsen, Bristol-Myers Squibb
Alan Huang
Employment: Novartis
Douglas Bootle
Employment: Novartis
David Demanse
Employment: Novartis
Lars Blumenstein
Employment: Novartis
Stock or Other Ownership: Novartis
Travel, Accommodations, Expenses: Novartis
Christina Coughlin
Employment: Immunocore, Novartis
Stock or Other Ownership: Immunocore
Cornelia Quadt
Employment: Novartis
Stock or Other Ownership: Novartis
José Baselga
Leadership: Infinity Pharmaceuticals, Varian Medical Systems, GRAIL
Stock or Other Ownership: PMV Pharma, Juno Therapeutics, Infinity Pharmaceuticals, GRAIL, Varian Medical Systems, Northern Biologics, Tango Therapeutics, Foghorn Therapeutics, Aura Biomedical, Apogen
Honoraria: PMV Pharma, Juno Therapeutics, Infinity Pharmaceuticals, Northern Biologics
Consulting or Advisory Role: Eli Lilly, Novartis
Research Funding: Roche/Genentech
Patents, Royalties, Other Intellectual Property: Combination therapy using PDK1 and PI3K inhibitors. Pending. Memorial Sloan Kettering (MSK) owned, listed as investigator. Use of phosphoinositide 3-kinase inhibitors for treatment of vascular malformations. Licensed. MSK owned, listed as investigator. Inhibition of KMT2D for the treatment of breast cancer. Pending. MSK owned, listed as investigator.
Travel, Accommodations, Expenses: Roche/Genentech, Daiichi, Bristol-Myers Squibb
REFERENCES
- 1.Shaw RJ, Cantley LC: Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441:424-430, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research Network. Kandoth C, Schultz N, et al. : Integrated genomic characterization of endometrial carcinoma. Nature 497:67-73, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network, Albert Einstein College of Medicine, Analytical Biological Services. et al. : Integrated genomic and molecular characterization of cervical cancer. Nature 543:378-384, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Network : Comprehensive molecular portraits of human breast tumours. Nature 490:61-70, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerji S, Cibulskis K, Rangel-Escareno C, et al. : Sequence analysis of mutations and translocations across breast cancer subtypes. Nature 486:405-409, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Network : Comprehensive molecular characterization of human colon and rectal cancer. Nature 487:330-337, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lui VW, Hedberg ML, Li H, et al. : Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov 3:761-769, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine DA, Bogomolniy F, Yee CJ, et al. : Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res 11:2875-2878, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Engelman JA, Chen L, Tan X, et al. : Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med 14:1351-1356, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Nicolantonio F, Arena S, Tabernero J, et al. : Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J Clin Invest 120:2858-2866, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janku F, Tsimberidou AM, Garrido-Laguna I, et al. : PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol Cancer Ther 10:558-565, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fritsch C, Huang A, Chatenay-Rivauday C, et al. : Characterization of the novel and specific PI3Kα inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol Cancer Ther 13:1117-1129, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Rodon J, Braña I, Siu LL, et al. : Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Invest New Drugs 32:670-681, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Sarker D, Ang JE, Baird R, et al. : First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin Cancer Res 21:77-86, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro GI, Rodon J, Bedell C, et al. : Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245408 (XL147), an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. Clin Cancer Res 20:233-245, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Dienstmann R, Rodon J, Serra V, et al. : Picking the point of inhibition: A comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol Cancer Ther 13:1021-1031, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Elkabets M, Vora S, Juric D, et al. : mTORC1 inhibition is required for sensitivity to PI3K p110α inhibitors in PIK3CA-mutant breast cancer. Sci Transl Med 5:196ra99, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuenschwander B, Branson M, Gsponer T: Critical aspects of the Bayesian approach to phase I cancer trials. Stat Med 27:2420-2439, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Engelman JA, Luo J, Cantley LC: The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7:606-619, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Juric D, Castel P, Griffith M, et al. : Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature 518:240-244, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busaidy NL, Farooki A, Dowlati A, et al. : Management of metabolic effects associated with anticancer agents targeting the PI3K-Akt-mTOR pathway. J Clin Oncol 30:2919-2928, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer IA, Abramson VG, Formisano L, et al. : A Phase Ib study of alpelisib (BYL719), a PI3Kα-specific inhibitor, with letrozole in ER+/HER2- metastatic breast cancer. Clin Cancer Res 23:26-34, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosch A, Li Z, Bergamaschi A, et al. : PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med 7:283ra51, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toska E, Osmanbeyoglu HU, Castel P, et al. : PI3K pathway regulates ER-dependent transcription in breast cancer through the epigenetic regulator KMT2D. Science 355:1324-1330, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baselga J, Tripathy D, Mendelsohn J, et al. : Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol 14:737-744, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Angulo AM, Ferrer-Lozano J, Stemke-Hale K, et al. : PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol Cancer Ther 10:1093-1101, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Keymeulen A, Lee MY, Ousset M, et al. : Reactivation of multipotency by oncogenic PIK3CA induces breast tumour heterogeneity. Nature 525:119-123, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Koren S, Reavie L, Couto JP, et al. : PIK3CA(H1047R) induces multipotency and multi-lineage mammary tumours. Nature 525:114-118, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Baselga J, Im SA, Iwata H, et al. : Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 18:904-916, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinross KM, Montgomery KG, Kleinschmidt M, et al. : An activating Pik3ca mutation coupled with Pten loss is sufficient to initiate ovarian tumorigenesis in mice. J Clin Invest 122:553-557, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janku F, Hong DS, Fu S, et al. : Assessing PIK3CA and PTEN in early-phase trials with PI3K/AKT/mTOR inhibitors. Cell Reports 6:377-387, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa C, Ebi H, Martini M, et al. : Measurement of PIP3 levels reveals an unexpected role for p110β in early adaptive responses to p110α-specific inhibitors in luminal breast cancer. Cancer Cell 27:97-108, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Janku F, Juric D, Cortés J, et al: Phase I study of PI3Kα inhibitor alpelisib (BYL719) plus fulvestrant in patients with PIK3CA-altered and PIK3CA-wild-type, ER+/HER2–, locally advanced or metastatic breast cancer. Cancer Res 75:PD5-5, 2015 (abstr PD5-5) [Google Scholar]