Abstract
Matrix metalloproteinase (MMP)-21 and MMP-28, or epilysin, are overexpressed during the invasion and metastasis of solid tumors. The present study investigated MMP-21 and MMP-28 expression levels in human gastric cancer using tissue microarray (TMA) analysis, and determined their association with clinicopathological characteristics and patient prognosis. TMA blocks, including 436 cases of gastric cancer and 92 non-cancerous adjacent gastric tissues, were investigated using immunohistochemistry. Staining results were analyzed statistically in association with various clinicopathological characteristics and overall survival. The MMP-21 and MMP-28 positive detection rate was 31.9% (139/436) and 34.4% (150/436), respectively, in the gastric carcinoma tissue specimens. MMP-21 and MMP-28 expression levels were negative in the 92 normal gastric tissue samples. In patients with gastric cancer, positive expression of MMP-21 and MMP-28 was correlated with tumor diameter, depth of invasion, vessel invasion, lymph node and distant metastases and tumor-node-metastasis stage. The overall survival rate was significantly lower in MMP-21 and MMP-28-positive compared with negative patients. Cox multivariate analysis revealed that MMP-21 and MMP-28 levels were independent predictors of survival in patients with gastric cancer. These findings emphasize the importance of MMP-21 and MMP-28, which may serve as novel and independent prognostic markers for the invasion and metastasis of human gastric cancer.
Keywords: matrix metalloproteinase-21, matrix metalloproteinase-28, gastric cancer, tissue microarray, cancer progression, prognosis
Introduction
Gastric cancer is one of the most prevalent types of cancer globally, and is the second most frequent cause of cancer-associated mortality worldwide, with a particularly high incidence in East Asia, including China (1). Despite significant advances in treatment, including drugs and surgical technologies, the overall 5-year survival rate in China remains low (40%), as the majority of gastric cancer cases are diagnosed at stage III or IV with a high rate of lymph node metastasis (50–75%) (1). Therefore, it is of clinical significance to further elucidate the pathogenesis of gastric cancer, in addition to identifying novel prognostic markers and therapeutic strategies.
Human matrix metalloproteinases (MMPs) are a family of 23 structurally-associated enzymes that remodel and degrade the extracellular matrix (ECM). Based on substrate specificity, they are classified into collagenases (MMP-1, −8 and −13), gelatinases (MMP-2 and −9), stromelysins (MMP-7 and −26), membrane-type MMPs (MMP-14, −15, −16, −17, −24 and −25) and other MMPs (MMP-19, −20, −21, −23 and −28) (2,3). MMPs are able to act on non-ECM components to mediate the release and activation of soluble factors, including growth factors and cytokines, from the ECM; these enzymes are frequently overexpressed in various forms of human cancer and are associated with malignancy (2,3). The role of MMPs in cancer invasion and metastasis has been the subject of various studies due to their ECM-degrading capacity (4–10). In gastric cancer, previous studies have linked the overexpression of MMP-2, −21, −9, −3, −7, −28 and −13 with tumor aggressiveness (4–8). High expression levels of MMP-3, −7, −2, −9 and −21 have been suggested to be independent prognostic markers of poor overall survival in patients with gastric cancer (4–8). Additionally, MMP-7 and MMP-28 have been reported to promote gastric cancer cell invasion and migration (9,10). However, the association between the expression levels of MMP-21 and MMP-28 and pathological parameters has yet to be elucidated in gastric cancer.
In the current study, the protein expression levels of MMP-21 and MMP-28 in a large cohort of 436 gastric cancer cases were investigated, in order to examine their potential association with the clinical features and overall survival of patients with gastric cancer who had not received neo-adjuvant chemotherapy.
Materials and methods
Patients and tissue microarrays
The study protocol was approved by The Ethics Committee of Zhejiang Provincial People's Hospital (Hangzhou, China) and written informed consent was gained from all participants. A total of 436 formalin-fixed paraffin-embedded (FFPE) gastric carcinoma tissue specimens and 92 non-cancerous tissue specimens were retrospectively collected at the Department of Gastrointestinal Surgery of Zhejiang Provincial People's Hospital between 1 January 2003 and 31 December 2008. The patients with gastric cancer ranged in age from 17 to 91 years old (median, 60 years old) and had received no radiotherapy or chemotherapy prior to undergoing surgery. The histomorphology of all primary tumor specimens (obtained through surgical resection) was examined using hematoxylin and eosin staining at the Department of Pathology of Zhejiang Provincial People's Hospital. Clinical parameters, including gender, age, differentiation status, lymph node metastasis and tumor-node-metastasis (TNM) stage were obtained from the Department of Pathology's record system of Zhejiang Provincial People's Hospital and are presented in Table I. All cases were classified according to the World Health Organization (WHO) criteria for the pathological classification of tumors (11). During the follow-up period (≤5 years), overall survival was determined as the time of diagnosis to the date of mortality or the last follow-up. Follow-up information for all patients was updated every 3 months by telephone, visits and questionnaires. Mortality of the patients was verified with the family and by review of public records.
Table I.
Clinicopathological characteristics of MMP-21- and MMP-28-positive patients with gastric cancer.
| Clinicopathological characteristics | No. of patients | MMP-21 positive, no. of patients (%) | P-value | MMP-28 positive, no. of patients (%) | P-value |
|---|---|---|---|---|---|
| Sex | 0.474 | 0.824 | |||
| Male | 311 | 96 (30.9) | 106 (34.1) | ||
| Female | 125 | 43 (34.4) | 44 (35.2) | ||
| Tumor diameter (cm) | <0.001 | <0.001 | |||
| <5 | 256 | 52 (20.3) | 62 (24.2) | ||
| ≥5 | 180 | 87 (48.3) | 88 (48.9) | ||
| Lauren type | 0.277 | 0.560 | |||
| Intestinal | 264 | 79 (29.9) | 88 (33.3) | ||
| Diffuse | 172 | 60 (34.9) | 62 (36.0) | ||
| Differentiation | 0.405 | 0.117 | |||
| Well | 13 | 3 (23.1) | 1 (7.7) | ||
| Moderate | 128 | 36 (28.1) | 42 (32.8) | ||
| Poor | 293 | 100 (34.1) | 107 (36.5) | ||
| Undifferentiated | 2 | 0 (0) | 0 (0) | ||
| Histology type | 0.196 | 0.512 | |||
| Papillary adenocarcinoma | 16 | 8 (50.0) | 5 (31.3) | ||
| Tubular adenocarcinoma | 326 | 96 (29.4) | 107 (32.8) | ||
| Mucinous adenocarcinoma | 29 | 10 (34.5) | 13 (44.8) | ||
| Signet-ring cell carcinoma | 65 | 25 (38.5) | 25 (38.5) | ||
| Infiltration depth | <0.001 | <0.001 | |||
| T1 | 57 | 2 (3.5) | 5 (8.8) | ||
| T2 | 109 | 21 (19.3) | 21 (19.3) | ||
| T3 | 244 | 100 (41.0) | 108 (44.3) | ||
| T4 | 26 | 16 (61.5) | 16 (61.5) | ||
| Lymph node metastasis | <0.001 | <0.001 | |||
| Negative | 166 | 18 (10.8) | 19 (11.4) | ||
| Positive | 270 | 121 (44.8) | 131 (48.5) | ||
| Vascular invasion | <0.001 | <0.001 | |||
| Negative | 183 | 22 (12.0) | 23 (12.6) | ||
| Positive | 253 | 117 (46.2) | 127 (50.2) | ||
| Distance metastasis | <0.001 | <0.001 | |||
| Negative | 375 | 99 (26.4) | 106 (28.3) | ||
| Positive | 61 | 40 (65.6) | 44 (72.1) | ||
| TNM stage | <0.001 | <0.001 | |||
| I | 90 | 5 (5.6) | 6 (6.7) | ||
| II | 104 | 14 (13.5) | 15 (14.4) | ||
| III | 173 | 72 (41.6) | 79 (45.7) | ||
| IV | 69 | 48 (69.6) | 50 (72.5) |
TNM, tumor-node-metastasis; MMP, matrix metalloproteinase.
Tissue microarray (TMA) blocks containing a total of 528 cases (436 cancer tissue samples and 92 non-cancerous tissue samples) were constructed by reviewing the core area of tumor region from the hematoxylin and eosin-stained slides by two independent pathologists and selecting one representative FFPE archival block for each case. Core tissue biopsies (2 mm in diameter) were obtained from individual FFPE blocks (donor blocks) and arranged in recipient paraffin blocks (TMA blocks) using a trephine.
Immunohistochemistry and staining evaluation
The protein expression levels of MMP-21 and MMP-28 in the normal gastric mucosa and in gastric carcinoma tissues were evaluated using immunohistochemistry. Tissue sections (4-µm-thick) were obtained from the TMAs, deparaffinized in xylene and hydrated using an ethanol-deionized water series (100, 95, 80 and 60% ethanol and water). The sections were submerged in EDTA antigenic retrieval buffer (0.1 M, pH 7.5) and microwaved 20 min for antigen retrieval, then followed by treatment with 3% H2O2 for 15 min to inhibit endogenous peroxidase activity. Tissue sections were blocked by incubation with 1% bovine serum albumin (cat no. B2064; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) to prevent nonspecific binding at room temperature for 20 min. Tissue sections were washed three times in phosphate buffered saline (PBS; 0.1 M, pH 7.5) three times and incubated with rabbit monoclonal antibodies against MMP-21 (cat no. ab52817) and MMP-28 (cat no. ab155507) at a dilution of 1:250 (both Abcam, Cambridge, USA) overnight at 4°C. Normal goat serum (cat no. 50062Z; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used as a negative control. The tissue sections were subsequently incubated with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:1,000 diluted in PBS; cat no. ZDR-5306; OriGene Technologies, Inc., Rockville, MD, USA) for 20 min at room temperature. Finally, 3,3′-diaminobenzidine was used to visualize the signal development and the tissue sections were counterstained with hematoxylin.
Immunohistochemical evaluation was performed independently by two pathologists who were blinded to the clinical data. The immunoreactivity levels of each case were estimated under a light microscope by assessing the average signal intensity (on a scale of 0–3) and the proportion of cells exhibiting positive staining (0, <5%; 1, 5–25%; 2, 26–50%; 3, 51–75%; 4, 76–100%). The intensity and proportion scores were then multiplied to obtain a composite score, with 0–3 defined as negative and 4–12 defined as positive, as described previously (12).
Statistical analysis
All statistical analyses were conducted using SPSS software (version 11.0; SPSS, Inc., Chicago, IL, USA). Categorical data were evaluated using χ2 or Fisher's exact tests. Survival curves were produced using the Kaplan-Meier estimator method and the log-rank test was used to analyze variation between curves. Multivariate analysis using the Cox proportional hazards regression model was performed to assess the prognostic value of MMP-21 and MMP-28 protein expression. Correlation coefficients between protein expression levels and clinicopathological findings were estimated using the Pearson's correlation coefficient method. The data was presented as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
Clinicopathological characteristics
The clinicopathological characteristics of the patients are presented in Table I. A total of 436 patients with gastric cancer (311 males, 125 females) were included in the present study. Based on the WHO classification criteria for gastric carcinoma, patient diagnoses included 16 cases of papillary adenocarcinoma, 326 cases of tubular adenocarcinoma, 29 cases of mucinous adenocarcinoma and 65 cases of signet ring cell carcinoma. Of these, 13 cases were well differentiated, 128 cases were moderately differentiated, 293 cases were poorly differentiated, and 2 cases were undifferentiated. According to the TNM staging criteria, 90 cases were TNM stage I, 104 were stage II, 173 were stage III and 69 were stage IV. In total, 253 cases presented with vessel invasion and 183 cases presented without vessel invasion; 270 cases exhibited lymph node metastasis and 166 cases were without lymph node metastasis; 61 cases had distant metastasis and 375 cases did not exhibit distant metastasis.
Association between MMP-21/MMP-28 expression and the clinicopathological characteristics of patients with gastric cancer
Tumor tissue specimens from a total of 436 patients with positive staining for MMP-21 and MMP-28 exhibited yellow-brown granules in the cytoplasm of gastric cancer cells, along with negative staining in normal gastric epithelial cells (Figs. 1 and 2). Positive staining that was detected in the gastric lamina propria cells was nonspecific and was therefore excluded from the staining evaluation. The MMP-21 and MMP-28 positive detection rates were 31.9% (139/436) and 34.4% (150/436), respectively (data not shown), in the gastric carcinoma tissue specimens. All normal gastric tissue samples were determined to be negative for MMP-21 and MMP-28.
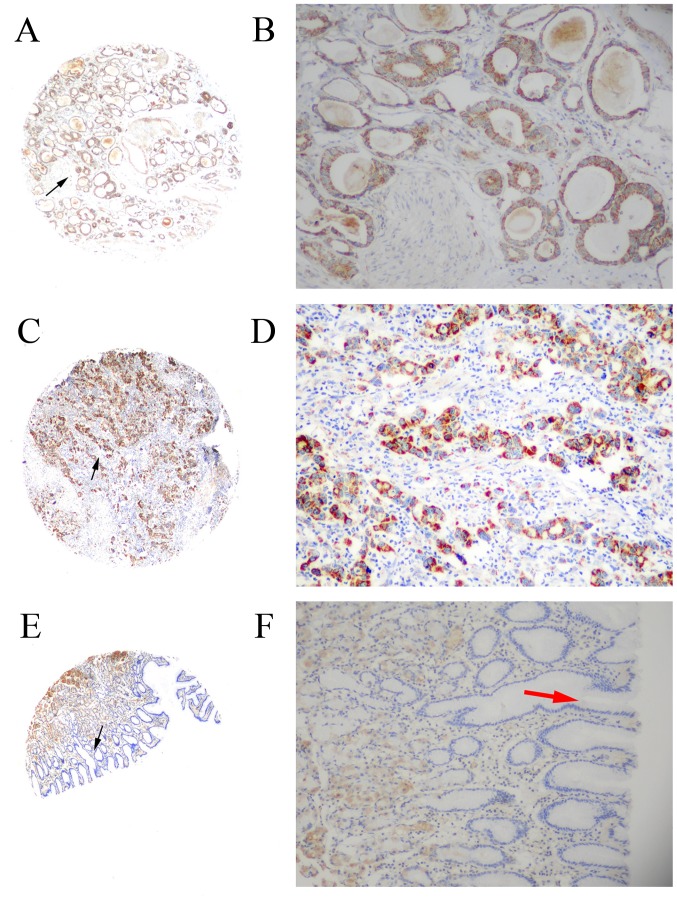
Figure 1.
Immunohistochemical analysis of MMP-21 protein expression levels in gastric cancer tissue and non-cancerous human gastric mucosa using tissue microarray analysis. (A) MMP-21 staining in moderately differentiated adenocarcinoma (magnification, ×40). (B) Magnified image (magnification, ×200) of the area indicated by black arrow in the previous image. Immunostaining of MMP-21 produced yellow-brown granules, primarily in the cytoplasm. (C) MMP-21 staining in poorly differentiated adenocarcinoma (magnification, ×40). (D) Magnified image (magnification, ×200) of the area indicated by black arrow in the previous image. Immunostaining of MMP-21 produced yellow-brown granules, primarily in the cytoplasm. (E) MMP-21 staining in non-cancerous human gastric mucosa (magnification, 40×). Negative staining was observed in normal gastric epithelial cells and a positive signal, considered as nonspecific staining, was detected in gastric basal cells. (F) Magnified image (magnification, ×200) of the area indicated by black arrow in the previous image. The red arrow indicates negative staining in normal gastric epithelial cells. MMP, matrix metalloproteinase.
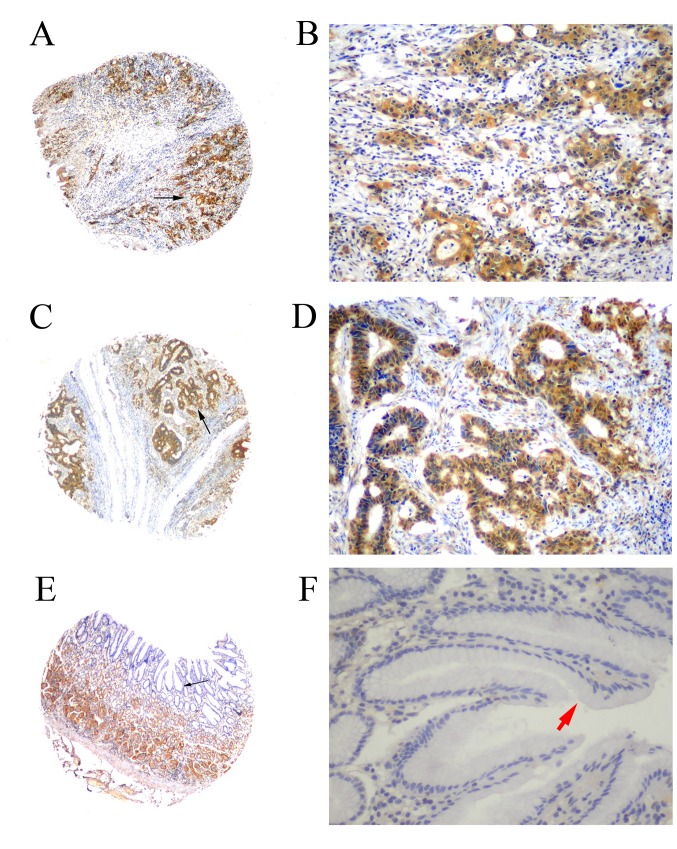
Figure 2.
Immunohistochemical analysis of MMP-28 expression in gastric cancer tissue and non-cancerous human gastric mucosa using tissue microarray analysis. (A) MMP-28 staining in poorly differentiated adenocarcinoma (magnification, ×40). (B) Magnified image (magnification, ×200) of the area indicated by black arrow in the previous image. Immunostaining of MMP-28 produced yellow-brown granules, primarily in the cytoplasm. (C) MMP-28 staining in moderately differentiated adenocarcinoma (magnification, ×40). (D) Magnified image (magnification, ×200) of the area indicated by black arrow in the previous image. Immunostaining of MMP-28 produced yellow-brown granules, primarily in the cytoplasm. (E) MMP-28 staining in non-cancerous human gastric mucosa (magnification, ×40). Negative staining was observed in normal gastric epithelial cells and a positive signal, considered as nonspecific staining, was detected in gastric basal cells. (F) Magnified image (magnification, ×200) of the area indicated by black arrow in the previous image. The red arrow indicates negative staining in the cytoplasm of normal gastric epithelial cells. MMP, matrix metalloproteinase.
The positive staining of MMP-21 and MMP-28 was significantly correlated with tumor diameter, depth of invasion, vessel invasion, lymph node and distant metastasis and TNM stage (P<0.05; Table I). MMP-21 and MMP-28 staining did not correlate significantly with gender, Lauren type (11), differentiation or histology type (P>0.05; Table I).
Results for the association between MMP-21/MMP-28 expression and the individual clinicopathological characteristics of patients with gastric cancer are presented in Table I. The rate of positive MMP-21 staining in patients with lymph node metastasis (44.8%; 121/270) was significantly higher compared with those without lymph node metastasis (10.8%; 18/166) (P<0.05). The MMP-21 expression rate in patients with distant metastasis (65.6%; 40/61) was also significantly higher, compared with those without distant metastasis (26.4%; 99/375) (P<0.05). Patients with stage III or IV gastric cancer exhibited significantly higher MMP-21 positivity (41.6 and 69.6%, respectively) compared with those with stage I or II gastric cancer (5.6 and 13.5%, respectively) (P<0.05). Similarly, patients with T3 and T4 tumors exhibited a significantly higher level of MMP-21 expression (41 and 61.5%, respectively), compared with those with T1 and T2 stage tumors (3.5 and 19.3%, respectively) (P<0.05).
In addition, the MMP-21 detection rate was 48.3% (87/180) in gastric carcinoma specimens of a tumor diameter ≥5 cm, which was significantly higher compared with specimens of a tumor diameter <5 cm (20.3%; 51/256) (P<0.05). The MMP-28 positive expression rate was significantly higher in gastric carcinoma specimens of tumor diameter ≥5 cm (48.9%; 88/180) compared with specimens of tumor diameter <5 cm (24.2%; 62/256) (P<0.05). The frequency of MMP-28-positive tissue samples from patients with lymph node metastasis (48.5%; 131/270) was significantly increased compared with tissue specimens without lymph node metastasis (12.6%; 23/183) (P<0.001). The detection rate of MMP-28 was 50.2% (127/253) and 72.1% (44/61) in tissue specimens with vascular invasion and distant metastasis, respectively, which was significantly higher compared with tissue specimens without vascular invasion (12.6%; 23/183) or distant metastasis (28.3%; 106/375) (both P<0.05). MMP-28 was detected in 6.7% (6/90) of TNM stage I tissues and 14.4% (15/104) of stage II tissue samples, which was significantly lower compared with stage III (45.7%; 79/173) and IV (72.5%; 50/69) tissue samples (both P<0.001). MMP-28-positivity was significantly positively correlated with the infiltration depth of gastric cancer and the MMP-28 positive rate was gradually increased with T stage (T1, 8.8%; T2, 19.3%; T3, 44.3%; T4, 61.5%; P<0.001). There was no significant association between MMP-21 and MMP-28 expression levels and other clinicopathological parameters. Cox multivariate analysis revealed that Lauren classification (P=0.028), TNM stage (P=0.002), MMP-21 (P<0.001) and MMP-28 (P=0.001) expression levels were significant independent prognostic factors for GC survival (Table II). These results indicate that MMP-21 and MMP-28 are independent predictors of survival in patients with gastric cancer.
Table II.
Multivariate Cox's proportional hazard analysis of overall survival with different clinicopathological characteristics.
| 95% confidence interval | |||||||
|---|---|---|---|---|---|---|---|
| Clinicopathological characteristic | Lower bound | Upper bound | Wald value | B-value | Standard error | Odds ratio | P-value |
| Lauren classification | 1.048 | 2.304 | 4.800 | 0.441 | 0.201 | 1.550 | 0.028a |
| TNM stage | 1.248 | 2.671 | 9.631 | 0.602 | 0.194 | 1.826 | 0.002a |
| MMP-21 | 1.796 | 4.057 | 22.823 | 0.993 | 0.208 | 2.700 | <0.001a |
| MMP-28 | 1.294 | 2.778 | 10.790 | 0.640 | 0.195 | 1.896 | 0.001a |
aP<0.05. MMP, matrix metalloproteinase; TNM, tumor node metastasis.
MMP-21 and MMP-28 overexpression is associated with poor prognosis
In total, 260 gastric cancer cases were negative for MMP-21 and MMP-28 expression, whereas 113 gastric cancer cases exhibited positive expression for MMP-21 and MMP-28 simultaneously, with a significant correlation between positive MMP-21 and MMP-28 expression (r=0.675; P<0.001) (data not shown).
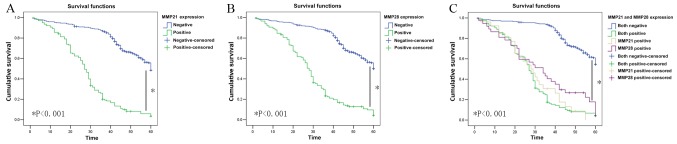
In the present cohort of patients (n=436), the mean survival time in MMP-21-positive patients was significantly shorter compared with that of MMP-21-negative patients (27.95±1.19 vs. 50.42±0.86 months; P<0.001; data not shown) and the 5-year survival rate of MMP-21-positive patients (7.9%; 11/139) was significantly lower compared with MMP-21-negative patients (56.2%; 167/297) (P<0.001; Fig. 3A). The mean survival time of MMP-28-positive patients was also significantly shorter compared with that of MMP-28-negative patients (29.06±1.27 vs. 50.73±0.85 months; P<0.001; data not shown) and the 5-year survival rate of MMP-28-positive patients (9.3%; 14/150) was significantly lower, compared with that of MMP-28-negative patients (57.3%; 164/286) (P<0.001; Fig. 3B). In the present cohort of patients (n=436), the mean survival time of MMP-28- and MMP-21-positive patients was significantly shorter compared with that of MMP-21- and MMP-28-negative patients (27.65±1.31 vs. 52.87±0.77 months; P<0.001; data not shown). The 5-year survival time and rate of MMP-28- and MMP-21-positive patients was significantly lower compared with that of MMP-28- and MMP-21-negative patients (27.66±1.31 vs. 52.88±0.77 months, P<0.001; 8% vs. 62.3%, P<0.001, respectively; Fig. 3C).
Figure 3.
Kaplan-Meier 5-year survival curves for patients with gastric tumors based on their MMP-21 and MMP-28 expression status. (A) Survival curve for MMP-21-positive and MMP-21-negative patients with gastric cancer. The 5-year survival rate of MMP-21-positive patients was significantly lower compared with that of MMP-21-negative patients. (B) Survival curve for MMP-28-positive and MMP-28-negative patients with gastric cancer. The 5-year survival rate of MMP-28-positive patients was significantly lower compared with that of MMP-28-negative patients. (C) Survival curve for patients with gastric cancer that were positive or negative for MMP-21 and MMP-28. The 5-year survival rate of MMP-28- and MMP-21-positive patients was significantly lower compared with that of MMP-28- and MMP-21 negative patients. MMP, matrix metalloproteinase.
Discussion
Surgical resection is the most effective treatment for advanced and metastatic gastric cancer in ~60% of patients; however, ~40% of cases exhibit recurrence following curative surgery (1). Thus, predicting patient prognosis is a challenge in the management of gastric cancer and there is a requirement for sensitive novel prognostic markers.
Several MMPs have been reported to serve a role in inflammation, mucosal wound healing and cancer progression (2). MMP-28 and MMP-21 are the most recently cloned human MMPs, and are important in cancer progression (4,10). MMP-21 is a 57 kDa proprotein convertase-activated MMP, and is a newly identified member of the MMP family (13). It is encoded by a gene containing only seven exons, whereas other MMP genes comprise ten exons (10,13). MMP-21 is widely expressed in a variety of human malignancies, including Merkel cell carcinoma, pancreatic adenocarcinoma, colon cancer, breast cancer, squamous cell carcinoma and hepatocellular carcinoma (13–20). MMP-28 is a 59 kDa protein, containing a signal sequence, propeptide, zinc-binding catalytic domain and haemopexin-like C-terminal domain (21). Within the propeptide is a furin activation sequence, as MMP-28 is activated by furin (21,22). The highest expression levels of MMP-28 mRNA are observed in the basal and suprabasal keratinocytes of the skin, the testis and the lung (23). MMP-28 is also expressed in several forms of carcinoma, and is associated with proliferative cells in epithelial wound healing (23). In oral squamous cell carcinoma and esophageal carcinoma cells, downregulation of MMP-28 expression leads to a reduction in anchorage-independent growth (23,24). In lung cancer, a previous study has suggested that MMP-28 induces epithelial-mesenchymal transition (EMT) via the activation of transforming growth factor β (25).
Numerous transcription factors, including specificity protein (sp)1, transcription factor 4, paired box protein, notch, retinoic acid receptor, mothers against decapentaplegic homolog 3 and activator protein 1, have been reported to bind to the promoter region of the MMP-21 gene (26). The MMP-28 promoter region contains a conserved GT-box that binds to sp1 and sp3, and is essential for the expression of this gene (27,28). GT-boxes and the transcription factors described above are required for the expression of numerous genes involved in the regulation of cell growth, cell cycle progression and cancer progression (28). Unlike other MMPs, which are primarily expressed in vivo by stromal cells, the expression of MMP-21 and MMP-28 is primarily restricted to epithelial and tumor cells, suggesting that MMP-21 and MMP-28 may regulate a wide range of cellular functions during cancer progression (10,28). Therefore, the present study investigated the expression levels and prognostic value of MMP-21 and MMP-28 in gastric cancer.
The results of the present study demonstrated significantly elevated protein expression levels of MMP-21 and MMP-28 in a cohort of 436 patients with gastric cancer, with a 31.9 and 34.4% positive detection rate, respectively. MMP-21 and MMP-28 expression was significantly associated with the depth of tumor invasion, lymph node metastasis, vascular invasion and distant metastasis, suggesting that the role of MMP-21 and MMP-28 in the breakdown of the ECM is important for the invasion and metastasis of gastric cancer. The expression of MMP-21 and MMP-28 was observed to significantly increase from stage I to stage IV gastric cancer, further indicating a role for these enzymes in gastric cancer progression. However, neither type of MMP was associated with gender, age, Lauren type, histological type or tumor differentiation status. Additionally, Kaplan-Meier survival analysis revealed that patients with higher levels of MMP-21 and MMP-28 expression had a poorer overall survival. However, the underlying factors, including the upstream or downstream genes or which pathways involve MMP21 and MMP28 remains unclear. It may be that MMP-21 and MMP-28 are associated with ECM-degrading capacity and EMT induction by regulating the expression of a number of different transcription factors, which may degrade or denature collagens, including type IV, V, VII, IX and X collagens.
The current study identified that MMP-21 and MMP-28 expression levels were significantly correlated with poorer patient outcomes and are independent prognostic factors for gastric cancer. The expression of these enzymes may be used as a prognostic marker in these patients, in addition to the TNM staging system, in order to enable the identification of patients with a high risk of disease recurrence or metastasis, who are candidates for aggressive adjuvant chemotherapy.
The current study demonstrated that MMP-21 and MMP-28 expression levels were significantly associated with tumor invasion, metastasis and poor prognosis in patients with gastric cancer. Although prospective studies are required to further determine the prognostic value of MMP-21 and MMP-28 in malignant tumors, the results of the current study support their role as independent prognostic factors in gastric cancer. In addition, the results of the present study indicate that MMP-21 and MMP-28 are novel therapeutic targets for the treatment and prevention of gastric cancer metastasis.
Acknowledgements
The authors would like to thank ICU specialty nurse Yajuan Yu from Department of Intensive Care Units (ICU), Zhejiang Provincial Hospital of Tradition Chinese Medicine (TCM), Xiasha Campus for help and support our research.
Funding
The present study was supported by the Medicine and Health Research Foundation of Zhejiang Province (grant nos., 2014KYA160 and 2013KYB022), The National Natural Science Foundation of China (grant no., 81502090), Zhejiang Provincial Natural Science Foundation of China (grant no., LY14H160039).
Availability of data and materials
The datasets generated and analyzed in the present study are included in this published article.
Authors' contributions
XH and ZZ designed and wrote the paper, JZ and QP collected the samples and produced the TMA arrays, WY performed the IHC staining and YW analyzed the data.
Ethics and consent to participate
The study protocol was approved by The Ethics Committee of Zhejiang Provincial People's Hospital (Hangzhou, China) and the written informed consent was gained from all participants.
Consent for publication
All patients provided written informed consent for the publication of their data.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Yabusaki H, Nashimoto A, Matsuki A, Aizawa M. Significance of surgical treatment in multimodal therapy for stage IV highly advanced gastric cancer. Hepatogastroenterology. 2013;60:377–381. doi: 10.5754/hge12653. [DOI] [PubMed] [Google Scholar]
- 2.Curran S, Murray GI. Matrix metalloproteinases: Molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer. 2000;36:1621–1630. doi: 10.1016/S0959-8049(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 3.Stetler-Stevenson WG. Type IV collagenases in tumor invasion and metastasis. Cancer Metastasis Rev. 1990;9:289–303. doi: 10.1007/BF00049520. [DOI] [PubMed] [Google Scholar]
- 4.Wu T, Li Y, Lu J, Qiao Q, Bao G, Wang N, He X, Du X. Increased MMP-21 expression is associated with poor overall survival of patients with gastric cancer. Med Oncol. 2013;30:323. doi: 10.1007/s12032-012-0323-8. [DOI] [PubMed] [Google Scholar]
- 5.Ye Y, Zhou X, Li X, Tang Y, Sun Y, Fang J. Inhibition of epidermal growth factor receptor signaling prohibits metastasis of gastric cancer via downregulation of MMP7 and MMP13. Tumor Biol. 2014;35:10891–10896. doi: 10.1007/s13277-014-2383-1. [DOI] [PubMed] [Google Scholar]
- 6.Łukaszewicz-Zając M, Mroczko B, Guzińska-Ustymowicz K, Pryczynicz A, Gryko M, Kemona A, Kędra B, Szmitkowski M. Matrix metalloproteinase 2 (MMP-2) and their tissue inhibitor 2 (TIMP-2) in gastric cancer patients. Adv Med Sci. 2013;58:235–243. doi: 10.2478/ams-2013-0018. [DOI] [PubMed] [Google Scholar]
- 7.Liu HQ, Song S, Wang JH, Zhang SL. Expression of MMP-3 and TIMP-3 in gastric cancer tissue and its clinical significance. Oncol Lett. 2011;2:1319–1322. doi: 10.3892/ol.2011.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koskensalo S, Mrena J, Wiksten JP, Nordling S, Kokkola A, Hagström J, Haglund C. MMP-7 overexpression is an independent prognostic marker in gastric cancer. Tumor Biol. 2010;31:149–155. doi: 10.1007/s13277-010-0020-1. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Liu X, Jiao H, Peng L, Huo Z, Yang W, Shen Q, Li T, Liu Q. Prognostic and clinical significance of STAT3 and MMP9 in patients with gastric cancer: A meta-analysis of a Chinese cohort. Int J Clin Exp Med. 2015;8:546–557. [PMC free article] [PubMed] [Google Scholar]
- 10.Jian P, Yanfang T, Zhuan Z, Jian W, Xueming Z, Jian N. MMP28 (epilysin) as a novel promoter of invasion and metastasis in gastric cancer. BMC Cancer. 2011;11:200. doi: 10.1186/1471-2407-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3:251–261. doi: 10.3978/j.issn.2078-6891.2012.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao ZS, Wang YY, Chu YQ, Ye ZY, Tao HQ. SPARC is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2010;16:260–268. doi: 10.1158/1078-0432.CCR-09-1247. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, Li W, Chu D, Zheng J, Ji G, Li M, Zhang H, Wang W, Du J, Li J. Overexpression of matrix metalloproteinase-21 is associated with poor overall survival of patients with colorectal cancer. J Gastrointest Surg. 2011;15:1188–1194. doi: 10.1007/s11605-011-1519-5. [DOI] [PubMed] [Google Scholar]
- 14.Suomela S, Koljonen V, Skoog T, Kukko H, Böhling T, Saarialho-Kere U. Expression of MMP-10, MMP-21, MMP-26, and MMP-28 in merkel cell carcinoma. Virchows Arch. 2009;455:495–503. doi: 10.1007/s00428-009-0856-1. [DOI] [PubMed] [Google Scholar]
- 15.Bister V, Skoog T, Virolainen S, Kiviluoto T, Puolakkainen P, Saarialho-Kere U. Increased expression of matrix metalloproteinases-21 and −26 and TIMP-4 in pancreatic adenocarcinoma. Mod Pathol. 2007;20:1128–1140. doi: 10.1038/modpathol.3800956. [DOI] [PubMed] [Google Scholar]
- 16.Shagisultanova EI, Novikova IA, Sidorenko YS, Marchenko GN, Strongin AY, Malkhosyan SR. The matrix metalloproteinase-21 gene 572C/T polymorphism and the risk of breast cancer. Anticancer Res. 2004;24:199–201. [PubMed] [Google Scholar]
- 17.Skoog T, Elomaa O, Pasonen-Seppänen SM, Forsberg S, Ahokas K, Jeskanen L, Pärssinen J, Tiala I, Rollman O, Lohi J, Saarialho-Kere U. Matrix metalloproteinase-21 expression is associated with keratinocyte differentiation and upregulated by retinoic acid in HaCaT cells. J Invest Dermatol. 2009;129:119–130. doi: 10.1038/jid.2008.206. [DOI] [PubMed] [Google Scholar]
- 18.Ahokas K, Lohi J, Illman SA, Llano E, Elomaa O, Impola U, Karjalainen-Lindsberg ML, Saarialho-Kere U. Matrix metalloproteinase-21 is expressed epithelially during development and in cancer and is up-regulated by transforming growth factor-β1 in keratinocytes. Lab Invest. 2003;83:1887–1899. doi: 10.1097/01.LAB.0000106721.86126.39. [DOI] [PubMed] [Google Scholar]
- 19.Ahokas K, Lohi J, Lohi H, Elomaa O, Karjalainen-Lindsberg ML, Kere J, Saarialho-Kere U. Matrix metalloproteinase-21, the human orthologue for XMMP, is expressed during fetal development and in cancer. Gene. 2002;301:31–41. doi: 10.1016/S0378-1119(02)01088-0. [DOI] [PubMed] [Google Scholar]
- 20.Boyd S, Virolainen S, Pärssinen J, Skoog T, van Hogerlinden M, Latonen L, Kyllönen L, Toftgard R, Saarialho-Kere U. MMP-10 (Stromelysin-2) and MMP-21 in human and murine squamous cell cancer. Exp Dermatol. 2009;18:1044–1052. doi: 10.1111/j.1600-0625.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 21.Pavlaki M, Zucker S, Dufour A, Calabrese N, Bahou W, Cao J. Furin functions as a nonproteolytic chaperone for matrix metalloproteinase-28: MMP-28 propeptide sequence requirement. Biochem Res Int. 2011;2011:630319. doi: 10.1155/2011/630319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodgers UR, Kevorkian L, Surridge AK, Waters JG, Swingler TE, Culley K, Illman S, Lohi J, Parker AE, Clark IM. Expression and function of matrix metalloproteinase (MMP)-28. Matrix Biol. 2009;28:263–272. doi: 10.1016/j.matbio.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchenko GN, Strongin AY. MMP-28, a new human matrix metalloproteinase with an unusual cysteine-switch sequence is widely expressed in tumors. Gene. 2001;265:87–93. doi: 10.1016/S0378-1119(01)00360-2. [DOI] [PubMed] [Google Scholar]
- 24.Saarialho-Kere U, Kerkelä E, Jahkola T, Suomela S, Keski-Oja J, Lohi J. Epilysin (MMP-28) expression is associated with cell proliferation during epithelial repair. J Invest Dermatol. 2002;119:14–21. doi: 10.1046/j.1523-1747.2002.01790.x. [DOI] [PubMed] [Google Scholar]
- 25.Illman SA, Lehti K, Keski-Oja J, Lohi J. Epilysin (MMP-28) induces TGF-beta mediated epithelial to mesenchymal transition in lung carcinoma cells. J Cell Sci. 2006;119:3856–3865. doi: 10.1242/jcs.03157. [DOI] [PubMed] [Google Scholar]
- 26.Marchenko GN, Marchenko ND, Strongin AY. The structure and regulation of the human and mouse matrix metalloproteinase-21 gene and protein. Biochem J. 2003;372:503–515. doi: 10.1042/bj20030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Illman SA, Keski-Oja J, Lohi J. Promoter characterization of the human and mouse epilysin (MMP-28) genes. Gene. 2001;275:185–194. doi: 10.1016/S0378-1119(01)00664-3. [DOI] [PubMed] [Google Scholar]
- 28.Swingler TE, Kevorkian L, Culley KL, Illman SA, Young DA, Parker AE, Lohi J, Clark IM. MMP28 gene expression is regulated by Sp1 transcription factor acetylation. Biochem J. 2010;427:391–400. doi: 10.1042/BJ20091798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed in the present study are included in this published article.