Abstract
Over the last decade, a new understanding of tumor-immune system interplay has been ushered in, lead in large part by the discovery of immune checkpoints mediated through B7-CD28 family interactions. Therapeutic blockade of the PD-L1 immune checkpoint pathway has already shown great success as a cancer immunotherapy for advanced urothelial carcinoma, leading to durable clinical remissions in an otherwise incurable disease. There are newly described members of the B7-CD28 family including B7-H3, B7x, and HHLA2. These ligands are thought to play an essential role in suppressing T-cell response, leading to immune tolerance of tumors. This feature makes them attractive targets for novel immunotherapy treatment paradigms. Here, we review the literature of current strategies and future directions of immune checkpoint blockade therapy for bladder cancer.
1. Historical perspective of immunotherapy for bladder cancer
The first suggestion of interplay between tumor biology and host immunity was in the 19th century when William Coley observed that infections were associated with tumor regression [1]. This antineoplastic effect of concomitant infection was again observed by Raymond Pearl at Johns Hopkins in 1929. He performed autopsies on patients who died of tuberculosis and noticed that there was a surprisingly low rate of underlying malignancy in these patients [2]. Alvaro Morales was the first to introduce immunotherapy to bladder cancer via intravesical treatment with bacillus Calmette-Guerin (BCG). BCG is a live attenuated strain of Mycobacterium bovis [3] that is reconstituted in solution and instilled into the bladder following transurethral resection (TUR) of non–muscle-invasive bladder tumors. Morales originally treated 9 patients with intravesical BCG and reported a significant reduction in bladder tumor recurrence [4]. This was the foundation for subsequent randomized controlled trials comparing patients undergoing TUR followed by adjuvant BCG to patients undergoing TUR alone [5, 6]. These studies convincingly demonstrated that BCG lowers the risk of tumor recurrence and may delay tumor progression. Dr Morales established the currently used regimen of 6 weekly bladder instillations. Although the precise mechanism of action of BCG immunotherapy remains the subject of continued investigation, it is believed to function by activating both the innate and adaptive immune systems [7]. Intravesical BCG has been validated by multiple randomized controlled trials as a superior therapy to intravesical chemotherapy including mitomycin and epirubicin [8–11]. Since its introduction nearly 4 decades ago, BCG is still considered to be one of the most successful immunotherapy agents for any solid malignancy [12]. It would not be until the 21st century that our understanding of the relationship between cancer and immunity would make a significant leap forward by the discovery of new tumor-immune evasion pathways via immune checkpoints.
2. Overview of immune checkpoint receptors and pathways
Immune evasion is considered to be one of the hallmarks of cancer and is an essential step in the evolution of a tumor [13]. Tumor-immune evasion is achieved through multiple mechanisms: (1) selective evolution of tumors with down-regulated expression of neoantigens; (2) decrease or loss of expression of class I MHC molecules; (3) resistance to T-cell–mediated cytolytic killing; (4) presence of immune suppressing cells—regulatory T cells (T regs), myeloid-derived suppressor cells (MDSCs), and secretion of immunosuppressive cytokines in the tumor microenvironment; and (5) expression of coinhibitory ligands and induction of T-cell exhaustion/anergy [14]. Regulation of T-cell activation requires 2 signals: (1) engagement of the T-cell receptor (TCR) through recognition of a peptide MHC complex, and (2) presence of a second (costimulatory or coinhibitory) signal delivered by the interaction of the family of CD28 receptors and B7 ligands [15]. If the second signal delivered is a costimulatory signal, then T-cell activation takes place leading to cytolysis of the cell, whereas if it is a coinhibitory signal, this results in T-cell exhaustion. The CD28 family of receptors are CD28 (costimulatory), CTLA-4 (coinhibitory), ICOS (costimulatory), PD-1 (coinhibitory) and TMIGD2 (unclear function), whereas the B7 family of ligands are B7-1, B7-2, PD-L1, PD-L2, B7-H3, B7x, and HHLA2. This pathway is essential for regulating the T-cell response, and tumors can induce T-cell suppression by expressing B7 coinhibitory ligands on the surface of tumor cells or by stimulating their expression on antigen presenting cells (APCs). The more extensively studied pathways are the CTLA-4/B7-1/B7-2 pathway and the PD-1/PD-L1/PD-L2 pathway. The characterization of these pathways has led to many important therapeutic advances. In this section, we discuss these pathways and review the relevant clinical trials.
2.1. CTLA-4/B7-1/B7-2 pathway
CD28 is expressed constitutively on naïve and activated T cells, whereas CTLA-4 is constitutively expressed only on T regs [16]. After an antigenic stimulus, CD28 interacts with the B7-1/B7-2 ligands on the APC and colocalizes with the TCR (Fig. 1). This results in phosphorylation of TCR-dependent kinases and also activates a distinct signaling program including increased production of IL-2 and high expression of the IL-2 receptor, which is needed for clonal expansion of naïve T cells [17]. Activation of the T cell through the CD28/B7-1/B7-2 pathway also results in movement of CTLA-4 from the intracellular compartment to the cell surface. As CTLA-4 has a higher binding affinity for B7-1/B7-2 than CD28, this results in suppression of costimulation, and the amount of CTLA-4 that translocates to the cell surface is proportional to the strength of the antigenic stimulus. CTLA-4 then recruits phosphatases such as SH-2 that decrease CD28 and TCR-dependent signaling of the T cell via dephosphorylation of TCR and other upstream signaling molecules. This balance between CD28 and CTLA-4 signaling is essential in controlling the T-cell immune response. Most normal tissues do not express B7-1 or B7-2, and thus cannot activate naïve T cells. In the absence of costimulation, activation of TCR by antigenic stimulus alone will result in anergy or functional inactivation of these cells. The physiological function of this pathway is the maintenance of self-tolerance; however, when tumor cells present an antigen in the absence of costimulatory molecules, this also leads to anergy and can contribute to immune evasion.
Fig. 1.
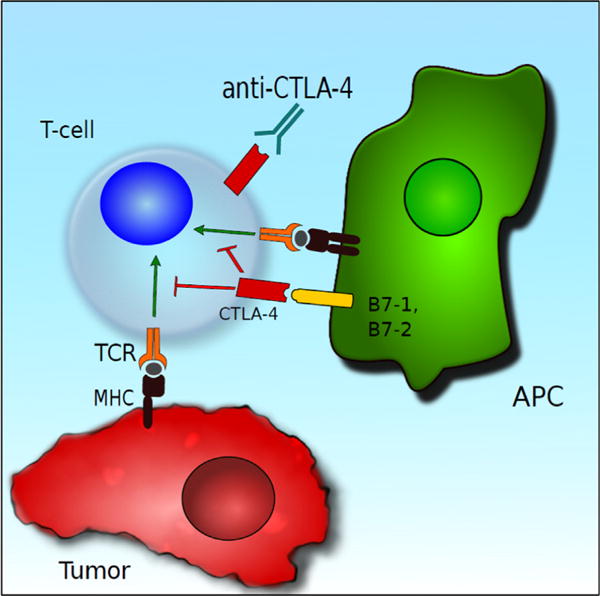
Antigen presenting cells (APC) present tumor cell antigens to T cells alongside costimulation via B7-1 and B7-2; however, when these ligands bind CTLA-4, this leads to T-cell coinhibition. CTLA-4 can be blocked with anti-CTLA-4 antibodies, leading to enhanced antitumor immunity. (Color version of the figure available online.)
2.2. PD-1/PD-L1/PD-L2 pathway
PD-L1 is widely expressed in a variety of tissues including vascular endothelium and APCs, whereas PD-L2 is predominantly expressed in immune cells such as macrophages and dendritic cells. IFN-g stimulates PD-L1 expression, and IL-4 induces PD-L2 expression. PD-1 is not constitutively expressed on naïve T cells but can be up-regulated on T cells, B cells, and other myeloid cells [18]. Such wide expression of PD-L1 suggests that it is important for tolerance. After binding to PD-L1 or PD-L2, the PD-1 receptor similar to the CD28 receptor colocalizes with the TCR and inhibits the phosphorylation of CD3-E and Zap-70, resulting in blockage of TCR-generated antigenic signals (Fig. 2). In addition, phosphorylation of intracellular immunoreceptor tyrosine-based inhibitory motif of PD-1 leads to SHP-2 activation, which in turn inhibits the PI3K/Akt pathway activated by CD28 signaling. Similar to CTLA-4, PD-1 activation can block TCR and CD28 signaling, but their mechanisms of actions are different. In addition, PD-1 expression plays an important role in chronic viral infections and cancers. Persistent antigenic stimulation leads to a T-cell exhaustion phenotype characterized by high levels of coinhibitory receptors such as PD-1 on CD8+ T cells. T cells with an exhaustion phenotype have poor proliferative, cytokine producing, and cytolytic capabilities. Such an exhaustion phenotype can be found in chronic viral infections and malignancies, and reversal of the exhaustion phenotype results in improved control of infections and disease. PD-L1 expression has been shown to be prognostic in several cancers including melanoma, colon, cervical, renal cell cancer, and breast and ovarian cancer [19]. Response to PD-1/PD-L1 therapy depends on the mutational landscape of malignancies with malignancies having higher mutational burden demonstrating better responses [20].
Fig. 2.
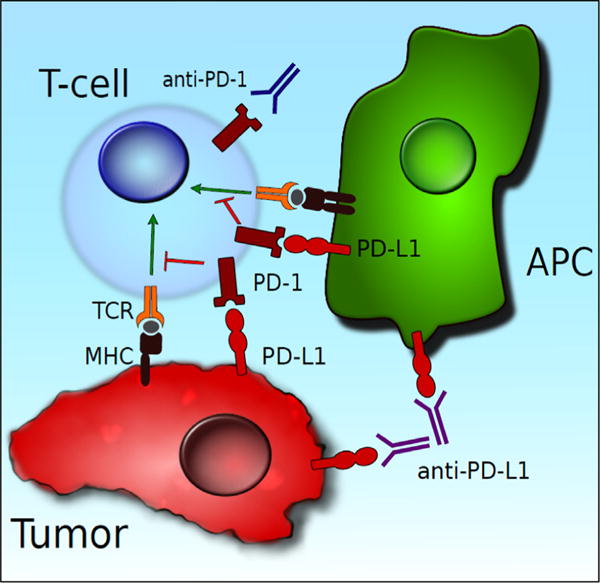
PD-L1 can be expressed on both APCs and tumor cells. PD-L1 binds to PD-1 on the T-cell surface, leading to coinhibition of T-cell activity. The PD-1/PD-L1 pathway can be blocked with anti-PD-1/PD-L1 antibodies, leading to immune-mediated tumor destruction. (Color version of the figure available online.)
3. Bladder tumors as immunogenic targets
Bladder cancers express high levels of PD-L1 [21]. Inman et al. demonstrated PD-L1 expression in bladder tumors of all stages, with particularly high levels in advanced stage tumors (30%) and tumors with carcinoma in situ (CIS) (45%). Furthermore, PD-L1 expression was abundant in tumors refractory to BCG treatment, suggesting that PD-L1 may play a role in shielding the tumor from immune-mediated tumor destruction. Boorjian et al. [22] observed that increased PD-L1 expression was associated with advanced urothelial cancer and independently predicted all-cause mortality.
Urothelial carcinoma genomes harbor a large number of somatic mutations. In a recent comprehensive genomic analysis by Lawrence et al. [23], a high frequency of somatic mutations was identified in exome sequences of human bladder tumors. Of the solid tumors evaluated to date, lung cancer, melanoma, and bladder cancer are characterized by the highest observed levels of mutational burden. Mutated cellular transcripts are processed into tumor neoantigens that are presented on the surface of APCs. This can lead to enhanced host immune recognition, a critical first step in generating a robust antitumor response.
4. Clinical trials of checkpoint inhibitors in urothelial carcinoma
Until 2016, there had been no new systemic treatments for advanced urothelial carcinoma approved by the FDA in 3 decades [12]. Recent clinical trials have demonstrated considerable therapeutic benefit of anti-CTLA-4 and anti-PD-1/PD-L1 agents in metastatic melanoma [24–26], leading to durable clinical remissions in an otherwise incurable disease.
The first phase I study of an anti-PD-L1 antibody (atezolizumab) in urothelial carcinoma was reported by Powles et al. [27]. Study participants had metastatic urothelial carcinoma who had failed prior systemic therapy. Sixty-seven patients received treatment and were stratified by their tumor PD-L1 expression status. Of these, 30/67 (45%) had high levels of PD-L1 expression and 35/67 (52%) had low levels of PD-L1 expression (2 patients had unknown expression). The objective response rate was 43% in PD-L1 high expression tumors and 11% in PD-L1 low expression tumors. Additionally, the study drug was tolerated remarkably well, with only 4% of the cohort experiencing grade 3 or greater toxicity. Immune-related adverse events (AEs) were the most common toxicities experienced. These preliminary data demonstrated a promising safety and efficacy profile for atezolizumab. In an effort to accelerate clinical trial expansion and increase patient access to the study drug, the FDA granted atezolizumab breakthrough designation in June 2014. The phase II study IMVigor 210 demonstrated an objective response rate of 18% in PD-L1+, platinum-pretreated tumors, and a grade 3/4 treatment-related AE rate of 16%, further supporting the safety and efficacy profile of atezolizumab for advanced urothelial carcinoma [28]. Based on these encouraging data, the FDA approved atezolizumab for treatment of metastatic or locally advanced bladder cancer in patients who have failed prior platinum-based chemotherapy in May 2016. More recently, the phase III study Keynote-045 demonstrated a survival benefit with anti-PD-1 antibody pembrolizumab vs. second-line chemotherapy in platinum refractory metastatic bladder cancer (10.3 vs. 7.4 mo, respectively) [29]. There were significantly less grade 3 to 5 AEs observed in the pembrolizumab arm compared to the chemotherapy arm (15% vs. 49%, respectively). The most common immune-related AEs of any grade associated with checkpoint inhibitor therapy is transaminitis, hyper/hypothyroidism, pneumonitis, and colitis [30].
Multiple additional checkpoint inhibitors targeting PD-1/ PD-L1 have been evaluated and have demonstrated activity in urothelial carcinoma (Table 1). Based on these encouraging early results, multiple phase III trials are planned or are underway (Table 2). Additional clinical trials are currently exploring the use of immune checkpoint inhibitors in the perioperative setting, in combination with radiotherapy, and in non–muscle-invasive disease. There are also several clinical trials studying combined checkpoint blockade targeting both CTLA-4 and PD-1/PD-L1 for advanced urothelial carcinoma [31, 32]. Although there are no data published yet regarding outcomes for combination immunotherapy in urothelial carcinoma, combination therapy has led to clinical remission in metastatic melanoma, although there is significant cumulative toxicity associated with dual therapy [33]. Initial results exploring the combination of nivolumab (anti-PD-L1) with ipilimumab (anti-CTLA-4) from the phase I/II CheckMate 032 study was presented at the Society for Immunotherapy of Cancer meeting in November 2016. Among 103 patients with urothelial carcinoma treated with nivolumab 3 mg/kg and ipilimumab 1 mg/kg IV every 3 weeks for 4 cycles followed by nivolumab 3 mg/kg every 2 weeks, the overall response rate was 26%. Among the 26 patients treated with nivolumab 1 mg/kg and ipilimumab 3 mg/kg IV every 3 weeks for 4 cycles followed by nivolumab 3 mg/kg every 2 weeks, the response rate was 38.5%. In the 78 patients treated with single-agent nivolumab at 3 mg/kg IV every 2 weeks, the response rate was 25.6% [34]. Final results of CheckMate 032 are eagerly anticipated. A randomized phase III clinical trial (DANUBE) is underway comparing combination immunotherapy (anti-CTLA-4 + anti-PD-L1) vs. single-agent immunotherapy (anti-PD-L1) vs. standard combination, platinum-based chemotherapy in the first-line metastatic or locally advanced setting (NCT02516241). DANUBE will be the first reported phase III trial evaluating combination immunotherapy in urothelial carcinoma.
Table 1.
Data reported from phase I to III clinical trials of immune checkpoint inhibitors in urothelial carcinoma
| NCT Identifier, Trial Name | Agent | Target | Phase | Population | Response, OR% (CR%) | OS (mo) | AEs | PD-L1 + definition | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| JAVELIN Solid Tumor, NCT01772004 | Avelumab | PD-L1 | Ib | Multiple tumor types, UC = 44. Metastatic, postplatinum |
18.2 (4.5), PD-L1 + : 50 | 12mo OS: 50.9% | Gr ≥ 3 trAE: 11 | PD-L1+ ≥5% IC | [85] |
| NCT02108652 IMvigor 210 | Atezolizumab | PD-L1 | II | C 1: first-line cisineligible (n = 119) | C 1: 24 (7) | C 1: 14.8 | Cohort 1: Gr 3-4 trAE: 15 |
PD-L1 IC0: <1% IC | C 1: [86] |
| C 2: Postplatinum (n = 311) | PD-L1+: 25 (6) C 2: 15 (5) PD-L1+: 18 (6) |
C 2: 7.9 | Gr 5 trAE: 1 Cohort 2: G3-4 trAE: 16 |
PD-L1 IC1: ≥1 ≤ 5% IC PD-L1 IC2/3: ≥5% IC |
C 2: [28] | ||||
| NCT01928394 | Nivolumab | PD-1 | I/II | Multiple tumor types (UC = 78) | 24.4 (6.4) | 9.7 | G3-4 trAE: 20.5 | PL-L1 + if expression ≥ 1% TC | [34, 87] |
| CheckMate 032 | Nivolumab + Ipiliumab (3/1 and 1/3) | CTLA-4 | Metastatic, postplatinum | G5 trAE: 2.6 | |||||
| N3/I1 (n = 104) | 26.0 | NR | 31.7 | ||||||
| N1/I3 (n = 26) | 38.5 | NR | 30.8 | ||||||
|
NCT02387996 CheckMate 275 |
Nivolumab | PD-1 | II | Postplatinum, metastatic or locally advanced UC (n = 265) | 19.6 (2) PD-L1 ≥ 5%: 28.4 PD-L1 ≥ 1%: 23.8 PD-L1-: 16.1 |
8.7 | G3-4 trAE: 18 G5 trAE: 1.1 |
PL-L1 + if expression ≥1% TC (also reported outcomes for PD-L1 ≥ 5%) | [88] |
| NCT01693562 | Durvalumab | PD-L1 | I/II | Multiple tumor types (UC = 61) Metastatic (57 > first line) |
31.0% PD-L1 + : 46.4 PD-L1-: 0 |
NR | G3 trAE: 4.9 G4-5 trAE: 0 |
PD-L1+ if ≥ 25% of TC or IC | [89] |
|
NCT01848834 KEYNOTE-012 |
Pembrolizumab | PD-1 | I | Multiple tumor types (UC = 33) Metastatic, 76% > first line |
27.6 (10.3) PD-L1 + : 29-33 PD-L1-: 0-9 |
12.7 | G3 trAE: 9.1 G4-5 tRAE: 0 |
PD-L1+ if staining in stroma or ≥ 1% TC | [90] |
|
NCT02335424 KEYNOTE-052 |
Pembrolizumab | PD-1 | II | First-line cisplatin-ineligible metastatic or locally advanced (n = 349) | 24.0 (6.0) PD-L1 ≥ 10%: 36.7 (13.3) PD-L1 ≥ 1%: 25.4 (6.3) |
NR | Gr ≥ 3 trAE: 16% | PD-L1 + if: ≥ 10% combined TC and IC (also reported response if ≥ 1%) | [91] |
|
NCT02256436 KEYNOTE-045 |
Pembrolizumab | PD-1 | III | Second-line postplatinum pembrolizumab (n = 270) vs. paclitaxel, docetaxel, or vinflunine (n = 272) | 21.1 (7.0) vs. 11.4 (3.3) | 10.3 vs. 7.4 (HR = 0.73, P = 0.002) | Gr ≥ 3 trAE: 15 vs. 49 | PD-L1 + if: ≥ 10% combined TC and IC | [29] |
BSC = best supportive care; C1 = cohort 1; C2 = cohort 2; Chemo = chemotherapy; Cis = cisplatin; CR = complete response; DFS = disease free survival; GC = gemcitabine þ cisplatin; GCa = gemcitabine þ carboplatin; MIBC = muscle-invasive bladder cancer; IC = immune cells; NR = not reported; OR = objective response; OS = overall survival; PC = placebo-controlled; PFS = progression free survival; Ref = reference; RPIII = randomized, phase III trial; SD = stable disease; TC = tumor cells; trAE = treatment-related adverse events; UC = urothelial carcinoma.
Table 2.
Selected ongoing phase III trials of immune checkpoint inhibitors in urothelial carcinoma
| NCT Identifier Trial Name | Treatment arms | Design | Population | Notes | Status | Primary endpoint |
|---|---|---|---|---|---|---|
|
NCT02853305 KEYNOTE-361 |
(1) Pembrolizumab (2) Pembrolizumab + Chemo (3) Chemo |
RP III | (1) Metastatic (2) First line |
Chemo will be GC or GCa | Not yet open Accruing |
PFS OS |
|
NCT02632409 CheckMate 274 |
(1) Nivolumab (2) Placebo |
RP III PC |
Adjuvant | (1) Includes upper tract UC (2) PD-L1 status is not entry criteria |
DFS | |
|
NCT02603432 Javelin Bladder 100 |
(1) Avelumab + BSC (2) BSC |
RP III | (1) Metastatic (2) Maintenance |
Patients with SD or better after first-line chemo | Accruing | OS |
|
NCT02450331 IMvigor010 |
(1) Atezolizumab (2) Observation |
RP III | (1) MIBC Adjuvant (2) PD-L1 positive |
Does not include upper tract UC | Accruing | DFS |
|
NCT02807636 IMvigor130 |
(1) Atezolizumab + GCa (2) Placebo + GCa |
RP III PC |
(1) Metastatic (2) First line (3) Cisineligible |
Randomization is 2:1 | Accruing | OS PFS AEs |
|
NCT02302807 IMvigor211 |
(1) Atezolizumab (2) Chemotherapy |
RP III | (1) Metastatic (2) Postplatinum |
Investigator's choice in chemo arm: vinflunine, paclitaxel or docetaxel | Completed Accrual |
OS |
|
NCT02516241 DANUBE |
(1) Durvalumab + Tremelimumab(anti-CTLA-4 mAb) (2) Durvalumab (3) GC or GCa |
RP III | (1) Metastastic (2) First-line |
(1) PD-L1 status must be known but is not entry criteria (2) GC is cis-eligible. GCa if cisineligible |
Accruing | OS |
BSC = best supportive care; Chemo = chemotherapy; Cis = Cisplatin; DFS = disease free survival; GC = gemcitabine + cisplatin; GCa = gemcitabine + carboplatin; mAb = monoclonal antibody; MIBC = muscle-invasive bladder cancer; OS = overall survival; PC = placebo-controlled; PFS = progression free survival; RP III = randomized, phase III trial; SD = stable disease; UC = urothelial carcinoma.
5. New immune checkpoints B7-H3, B7x, and HHLA2
The evolution of the B7 family members can be divided into 3 groups based on amino acid similarity. B7-H3 [35], B7x [36–38], and HHLA2 [39] form the third group of molecules [36] (Fig. 3). These molecules are widely expressed in many urologic malignancies, and their expression is associated with a poor prognosis [22, 40]. Tumor cell surface overexpression of these ligands is thought to play an essential role in suppressing T-cell response, leading to immune tolerance of malignancies. This feature makes them attractive targets for novel immunotherapy strategies. In this section, we review the new immune checkpoint ligands B7-H3, B7x, and HHLA2.
Fig. 3.
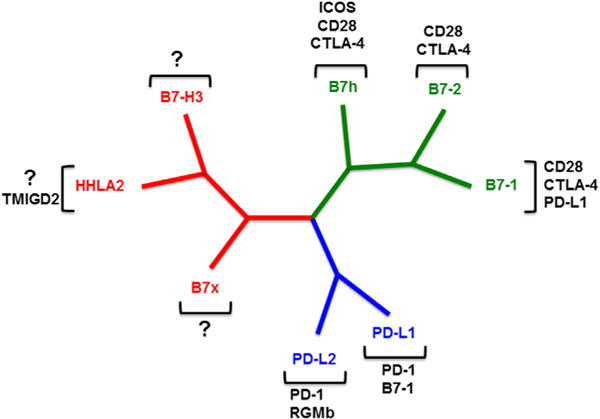
Phylogenetic tree of the human B7 and CD28 families. Group I (green) includes B7-1/B7-2/CD28/CTLA-4, and B7h/ICOS/CD28/CTLA-4. Group II (blue) consists of PD-L1/PD-L2/PD-1, PD-L1/B7-1, and PD-L2/RGMb. Group III (red) contains B7-H3, B7x, and HHLA2/TMIGD2. (Color version of the figure available online.)
5.1. B7-H3
B7-H3 (also known as CD276) is a ligand of the B7 family with a complex set of functions that is still being explored. Initial in vitro studies indicated that it stimulates proliferation, increases cytokine production of IFNγ [35], and promotes antitumor immunity [41]. Later studies demonstrated the opposite, however, showing that it inhibits proliferation, cytokine production, and T-cell immunity [42]. These apparently conflicting results may not be contradictory, but could instead entail multiple receptors (as in B7-1 with CD28 and CTLA-4), distinct functional isoforms, or posttranslational regulation. As of yet, the receptor for B7-H3 has not been identified.
B7-H3 mRNA is widely transcribed in somatic tissues; however, its protein is only found on certain cell types. It is constitutively expressed at low levels on nonlymphoid cells such as fibroblasts [43] and osteoblasts [44]. It is not constitutively expressed on immune cells, but can be induced on T cells, natural killer cells, dendritic cells, and monocytes [35]. This limited expression pattern suggests posttranscriptional regulation, which is at least partly due to RNA interference mediated by microRNA such as miR-29 [45]. Although B7-H3 expression is limited in normal tissues, it is found on many cancer types and in tumor vasculature [46]. As with the early in vitro experiments, some studies of human pancreatic [47] and gastric [48] cancer specimens seemed to indicate that B7-H3 is associated with improved patient survival. Conversely, other analyses of pancreatic [49], colorectal [50], breast [51], lung [52], liver [53], kidney [54] and bladder cancers show that it is associated with a greater risk of progression and worse prognosis. In urothelial cancer of the bladder, preoperative bacillus Calmette-Guerin treatment is associated with B7-H3 overexpression [22]. Subsequent studies on urothelial cell tumors confirmed that B7-H3 overexpression is present in bladder tumors across all pathologic stages [22, 55] and is likely a consequence of increased B7-H3 mRNA expression in urothelial carcinoma cells [56].
Therapeutics targeting B7-H3 are being actively explored. The anti-B7-H3 monoclonal antibody Enoblituzumab (MGA271) was shown to reduce growth of renal cell and bladder carcinoma xenografts in mice [57]. It is currently being tested as a monotherapy in a phase I dose-escalation study in patients with refractory cancers (NCT01391143), and interim results show that it is well tolerated and has antitumor activity [58]. It is also being evaluated in combination with ipilimumab (anti-CTLA-4 mAb) or pembrolizumab (anti-PD-1 mAb) in safety studies of refractory cancers (trial NCT02381314 and NCI201501495). The murine mAb 8H9 was found to target an unknown antigen on many solid tumors, and only recently has this antigen been discovered to be B7-H3 [59]. Radioimmunoconjugated 8H9 with cytotoxic I131 is currently in phase I trials for central nervous system and peritoneal tumors (NCT01099644 and NCT01502917), and other groups are testing 8H9 with other toxic conjugates [60] in murine models. Targeting B7-H3 in combination with other treatments has synergistic effects in animal models, as seen in pancreatic tumor grafts treated with gemcitabine [61] and lymphoma xenografts with idarubicin and cytarabine [62]. Thus, targeting B7-H3 has been effective in preclinical models, and depending on the results of ongoing clinical trials, may be a viable treatment option for bladder cancer in the future.
5.2. B7x
B7x (also known as B7-H4, VTCN1, or B7S1) is a B7 family member discovered in 2003 that is shown to inhibit T-cell–mediated immunity. This coinhibitory ligand inhibits T-cell proliferation, reduces cytokine production [36], and induces cell-cycle arrest [37] in vitro. Further, it promotes the development of immunosuppressive immune cells such as regulatory T cells [63], tumor-associated macrophages [64], and MDSCs [65], which in turn inhibit T-cell function. B7x also has a role in the innate immune system: In a Listeria model of infection, it inhibited the proliferation of neutrophil progenitors [66]. The receptor for B7x has not yet been identified, though in vitro binding assays indicate that it must be distinct from known members of the B7 family [36] and is found on T cells [36] and MDSCs [65].
The B7x gene is widely transcribed, and its mRNA can be detected in tissues and cell types across the body [36]. Conversely, protein expression of B7x is fairly limited and found on tissues of lung [67]; pancreas [68]; breast, gynecological tract, and placenta [67], suggesting posttranscriptional regulation similar to B7-H3. Despite its restricted expression on healthy somatic tissues, B7x is frequently expressed in many cancer types including those of the breast, lung [52], colon [69], ovary [70], endometrium [71], kidney [72], pancreas [73], prostate [40], and bladder. B7x expression in renal cell carcinoma is associated with a greater cancer progression and decreased overall survival [72]. Likewise, in prostate cancer, strong intensity of staining with immunohistochemistry for B7x is associated with greater cancer spread, recurrence, and risk of death [40]. In urothelial carcinoma, B7x overexpression is associated with increased TNM stage, pathological grade, and poorer outcomes [74]. Serum B7x is also elevated in patients with urothelial carcinoma, suggesting that it may be useful as a diagnostic marker [75].
Considering the immunosuppressive effects of B7x and its expression profile in human cancers, it is a prime target for immunotherapy. Some strategies to target B7x include monoclonal antibodies, single-chain fragment variables (scFVs, that is, recombinant antibodies with single binding sites), and chimeric antigen receptor (CAR) T cells. Anti-B7x monoclonal antibodies reduce lung metastases of B7x-expressing colon cancer cells in a mouse experimental metastasis model [76]. Similarly, anti-B7x scFVs reduced the growth of ovarian tumor xenografts [77]. B7x-targeted CAR T-cell therapy proved to be effective in eliminating xenografts of B7x-expressing ovarian tumors, but also caused lethal, delayed toxicity 6 to 8 weeks postengraftment [78]. Thus, for the CAR T-cell strategy to be clinically feasible, a suicide-gene or comparable “off” switch would need to be incorporated to prevent long-term adverse effects. Taken together, B7x shows promise as an anticancer target, but further research is needed to refine the treatment strategies.
5.3. HHLA2
The newest member of the B7 family, HHLA2 (also known as B7-H5 or B7-H7) is unique among B7 family molecules in that it is found in humans but not in murine organisms. Within the B7 family, it shares the greatest amino acid similarity to B7-H3 and B7x [39]. In vitro experiments with human immune cells indicate that it inhibits T-cell function by reducing proliferation and cytokine release [39], although it was also reported that it can act as a costimulatory molecule to promote T-cell function [79]. Like B7-H3, HHLA2 may also have multiple receptors or functional isoforms that mediate distinct effects. Based on the consensus of in vitro and clinical data, HHLA2 has a largely inhibitory role on the immune system.
In humans, it can be found constitutively expressed on monocytes and can be induced on B cells [39]. It has limited expression in somatic tissues, but it can be found in the placenta, gut, kidney, and breast [80]. A receptor for HHLA2 is TMIGD2 (CD28H or IGPR-1), a protein present on endothelial cells believed to have a role in angiogenesis [81]. TMIGD2 mRNA is broadly transcribed in somatic tissues [81], and the protein can be found on resting T cells as well as monocytes, dendritic cells, and B cells but not on activated T cells [79], despite recombinant HHLA2 protein binding activated T cells in vitro. This suggests that there is a yet undiscovered receptor for HHLA2 that is responsible for its inhibitory effects.
HHLA2 is overexpressed in many cancers, including lung, breast, kidney, prostate, and bladder [80]. The prognostic significance of HHLA2 overexpression has been identified for triple-negative breast carcinoma, non–small cell lung carcinoma, and osteosarcoma. In triple-negative breast cancer, HHLA2 overexpression is associated with lymph node metastasis and higher stage [80]. Similarly, EGFR mutational status and high tumor-infiltrating lymphocyte density are associated with HHLA2 expression in non–small cell lung carcinoma [82]. In osteosarcoma, HHLA2 expression is associated with advanced disease and almost universally found in metastatic specimens, and confers a poorer prognosis [83]. HHLA2 is also overexpressed in over half of high-grade urothelial tumors [84]. Taken together, these data suggest that HHLA2 may be an important therapeutic target for cancer immunotherapy, but further work is needed to elucidate its value as a diagnostic or prognostic tool, and to develop therapeutics that can target it.
6. Summary
The understanding of the regulation of T-cell activation and function along with the discovery of immune checkpoints with respect to the CD28 and B7 family has led to major advances scientifically and therapeutically in cancer. Clinical trials of agents targeting the PD-L1 checkpoint pathway have demonstrated durable clinical remissions in patients with otherwise untreatable advanced bladder cancer. Future studies should aim to further characterize the role of the newest B7 family molecules B7-H3, B7x, and HHLA2 in the mechanisms of bladder tumor-immune evasion pathways. This will surely lead to the development of new therapeutic strategies to enhance immune-mediated tumor destruction.
References
- 1.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. Clin Orthop Relat Res. 1991;262:3–11. [PubMed] [Google Scholar]
- 2.Pearl R. Cancer and tuberculosis. Am J Hyg. 1929;9:97. [Google Scholar]
- 3.Kavoussi LR, Brown EJ, Ritchey JK, Ratliff TL. Fibronectin-mediated Calmette-Guerin bacillus attachment to murine bladder mucosa. Requirement for the expression of an antitumor response. J Clin Invest. 1990;85:62–7. doi: 10.1172/JCI114434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–3. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 5.Pinsky CM, Camacho FJ, Kerr D, Geller NL, Klein FA, Herr HA, et al. Intravesical administration of bacillus Calmette-Guerin in patients with recurrent superficial carcinoma of the urinary bladder: report of a prospective, randomized trial. Cancer Treat Rep. 1985;69:47–53. [PubMed] [Google Scholar]
- 6.Lamm DL, Thor DE, Harris SC, Reyna JA, Stogdill VD, Radwin HM. Bacillus Calmette-Guerin immunotherapy of superficial bladder cancer. J Urol. 1980;124:38–40. doi: 10.1016/s0022-5347(17)55282-9. [DOI] [PubMed] [Google Scholar]
- 7.Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer—a current perspective. Nat Rev Urol. 2014;11:153–62. doi: 10.1038/nrurol.2014.15. [DOI] [PubMed] [Google Scholar]
- 8.Lamm DL, Blumenstein BA, David Crawford E, Crissman JD, Lowe BA, Smith JA, Jr, et al. Randomized intergroup comparison of bacillus Calmette-Guerin immunotherapy and mitomycin C chemotherapy prophylaxis in superficial transitional cell carcinoma of the bladder a southwest oncology group study. Urol Oncol. 1995;1:119–26. doi: 10.1016/1078-1439(95)00041-f. [DOI] [PubMed] [Google Scholar]
- 9.Malmstrom PU, Wijkstrom H, Lundholm C, Wester K, Busch C, Norlen BJ. 5-year followup of a randomized prospective study comparing mitomycin C and bacillus Calmette-Guerin in patients with superficial bladder carcinoma. Swedish-Norwegian Bladder Cancer Study Group J Urol. 1999;161:1124–7. [PubMed] [Google Scholar]
- 10.Jarvinen R, Kaasinen E, Sankila A, Rintala E, FinnBladder G. Long-term efficacy of maintenance bacillus Calmette-Guerin versus maintenance mitomycin C instillation therapy in frequently recurrent TaT1 tumours without carcinoma in situ: a subgroup analysis of the prospective, randomised FinnBladder I study with a 20-year followup. Eur Urol. 2009;56:260–5. doi: 10.1016/j.eururo.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Duchek M, Johansson R, Jahnson S, Mestad O, Hellstrom P, Hellsten S, et al. Bacillus Calmette-Guerin is superior to a combination of epirubicin and interferon-alpha2b in the intravesical treatment of patients with stage T1 urinary bladder cancer. A prospective, randomized, Nordic study. Eur Urol. 2010;57:25–31. doi: 10.1016/j.eururo.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y, Enting D, Rudman S, Chowdhury S. Immunotherapy for urothelial cancer: from BCG to checkpoint inhibitors and beyond. Expert Rev Anticancer Ther. 2015;15:509–23. doi: 10.1586/14737140.2015.1015419. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg Robert A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Töpfer K, Kempe S, Müller N, Schmitz M, Bachmann M, Cartellieri M, et al. Tumor evasion from T Cell surveillance. J Biomed Biotechnol. 2011;2011:19. doi: 10.1155/2011/918471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bretscher PA. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci U S A. 1999;96:185–90. doi: 10.1073/pnas.96.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zang X. B7 and CD28 families. In: Mackay I, Rose N, Diamond B, Davidson A, editors. Encyclopedia of medical immunology. Springer; New York: 2014. pp. 174–81. [Google Scholar]
- 17.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chinai JM, Janakiram M, Chen F, Chen W, Kaplan M, Zang X. New immunotherapies targeting the PD-1 pathway. Trends Pharmacol Sci. 2015;36:587–95. doi: 10.1016/j.tips.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science (New York, NY) 1970;169:1042–9. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 20.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science (New York, NY) 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- 22.Boorjian SA, Sheinin Y, Crispen PL, Farmer SA, Lohse CM, Kuntz SM, et al. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res. 2008;14:4800–8. doi: 10.1158/1078-0432.CCR-08-0731. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010. 363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–62. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–26. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473–86. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 31.A Phase 1/2, Open-label study of nivolumab monotherapy or nivolumab combined with ipilimumab in subjects with advanced or metastatic solid tumors.
- 32.A Phase 1 Study of (Cabozantinib) Plus Nivolumab (CaboNivo) alone or in combination with ipilimumab (CaboNivoIpi) in patients with advanced/metastatic urothelial carcinoma and other genitourinary tumors.
- 33.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma P. Efficacy and safety of nivolumab plus ipilimumab in metastatic urothelial carcinoma: first results from the Phase I/II CheckMate 032 Study. Poster 449. 31st Annual Meeting, Society for Immunotherapy of Cancer. 2016 [Google Scholar]
- 35.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–74. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 36.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A. 2003;100:10388–92. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–61. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 38.Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18:863–73. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 39.Zhao R, Chinai JM, Buhl S, Scandiuzzi L, Ray A, Jeon H, et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci U S A. 2013;110(24):9879–84. doi: 10.1073/pnas.1303524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A. 2007;104:19458–63. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun X, Vale M, Leung E, Kanwar JR, Gupta R, Krissansen GW. Mouse B7-H3 induces antitumor immunity. Gene Ther. 2003;10:1728–34. doi: 10.1038/sj.gt.3302070. [DOI] [PubMed] [Google Scholar]
- 42.Prasad DV, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, et al. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173:2500–6. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 43.Tran CN, Thacker SG, Louie DM, Oliver J, White PT, Endres JL, et al. Interactions of T cells with fibroblast-like synoviocytes: role of the B7 family costimulatory ligand B7-H3. J Immunol. 2008;180:2989–98. doi: 10.4049/jimmunol.180.5.2989. [DOI] [PubMed] [Google Scholar]
- 44.Suh WK, Wang SX, Jheon AH, Moreno L, Yoshinaga SK, Ganss B, et al. The immune regulatory protein B7-H3 promotes osteoblast differentiation and bone mineralization. Proc Natl Acad Sci U S A. 2004;101:12969–73. doi: 10.1073/pnas.0405259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu H, Cheung IY, Guo HF, Cheung NK. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69:6275–81. doi: 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zang X, Sullivan PS, Soslow RA, Waitz R, Reuter VE, Wilton A, et al. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol. 2010;23:1104–12. doi: 10.1038/modpathol.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loos M, Hedderich DM, Ottenhausen M, Giese NA, Laschinger M, Esposito I, et al. Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer. 2009;9:463. doi: 10.1186/1471-2407-9-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu KF, et al. Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J Gastroenterol. 2006;12:457–459. doi: 10.3748/wjg.v12.i3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamato I, Sho M, Nomi T, Akahori T, Shimada K, Hotta K, et al. Clinical importance of B7-H3 expression in human pancreatic cancer. Br J Cancer. 2009;101:1709–16. doi: 10.1038/sj.bjc.6605375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun J, Chen LJ, Zhang GB, Jiang JT, Zhu M, Tan Y, et al. Clinical significance and regulation of the costimulatory molecule B7-H3 in human colorectal carcinoma. Cancer Immunol Immunother. 2010;59:1163–1171. doi: 10.1007/s00262-010-0841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arigami T, Narita N, Mizuno R, Nguyen L, Ye X, Chung A, et al. B7-h3 ligand expression by primary breast cancer and associated with regional nodal metastasis. Ann Surg. 2010;252:1044–51. doi: 10.1097/SLA.0b013e3181f1939d. [DOI] [PubMed] [Google Scholar]
- 52.Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, et al. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53:143–51. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 53.Sun TW, Gao Q, Qiu SJ, Zhou J, Wang XY, Yi Y, et al. B7-H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer Immunol Immunother. 2012;61:2171–82. doi: 10.1007/s00262-012-1278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crispen PL, Sheinin Y, Roth TJ, Lohse CM, Kuntz SM, Frigola X, et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14:5150–7. doi: 10.1158/1078-0432.CCR-08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xylinas E, Robinson BD, Kluth LA, Volkmer BG, Hautmann R, Kufer R, et al. Association of T-cell co-regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Eur J Surg Oncol. 2014;40:121–7. doi: 10.1016/j.ejso.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 56.Wu D, Zhang Z, Pan H, Fan Y, Qu P, Zhou J. Upregulation of the B7/ CD28 family member B7-H3 in bladder cancer. Oncol Lett. 2015;9:1420–4. doi: 10.3892/ol.2014.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loo D, Alderson RF, Chen FZ, Huang L, Zhang W, Gorlatov S, et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res. 2012;18:3834–45. doi: 10.1158/1078-0432.CCR-12-0715. [DOI] [PubMed] [Google Scholar]
- 58.Powderly J, Cote G, Flaherty K, Szmulewitz RZ, Ribas A, Weber J, et al. Interim results of an ongoing Phase I, dose escalation study of MGA271 (Fc-optimized humanized anti-B7-H3 monoclonal antibody) in patients with refractory B7-H3-expressing neoplasms or neoplasms whose vasculature expresses B7-H3. J Immunother Cancer. 2015;3(Suppl. 2):O8. [Google Scholar]
- 59.Ahmed M, Cheng M, Zhao Q, Goldgur Y, Cheal SM, Guo HF, et al. Humanized affinity-matured monoclonal antibody 8H9 has potent antitumor activity and binds to FG loop of tumor antigen B7-H3. J Biol Chem. 2015;290:30018–29. doi: 10.1074/jbc.M115.679852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luther N, Cheung NK, Souliopoulos EP, Karampelas I, Bassiri D, Edgar MA, et al. Interstitial infusion of glioma-targeted recombinant immunotoxin 8H9scFv-PE38. Mol Cancer Ther. 2010;9:1039–46. doi: 10.1158/1535-7163.MCT-09-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao X, Zhang GB, Gan WJ, Xiong F, Li Z, Zhao H, et al. Silencing of B7-H3 increases gemcitabine sensitivity by promoting apoptosis in pancreatic carcinoma. Oncol Lett. 2013;5:805–12. doi: 10.3892/ol.2013.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang W, Wang J, Wang Y, Dong F, Zhu M, Wan W, et al. B7-H3 silencing by RNAi inhibits tumor progression and enhances chemosensitivity in U937 cells. Oncol Targets Ther. 2015;8:1721–33. doi: 10.2147/OTT.S85272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Podojil JR, Liu LN, Marshall SA, Chiang MY, Goings GE, Chen L, et al. B7-H4Ig inhibits mouse and human T-cell function and treats EAE via IL-10/Treg-dependent mechanisms. J Autoimmun. 2013;44:71–81. doi: 10.1016/j.jaut.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kryczek I, Wei S, Zou L, Zhu G, Mottram P, Xu H, et al. Cutting edge: induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J Immunol. 2006;177:40–4. doi: 10.4049/jimmunol.177.1.40. [DOI] [PubMed] [Google Scholar]
- 65.Jeon H, Ohaegbulam KC, Abadi YM, Zang X. B7x and myeloid-derived suppressor cells in the tumor microenvironment: a tale of two cities. Oncoimmunology. 2013;2:e24744. doi: 10.4161/onci.24744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu G, Augustine MM, Azuma T, Luo L, Yao S, Anand S, et al. B7-H4-deficient mice display augmented neutrophil-mediated innate immunity. Blood. 2009;113:1759–67. doi: 10.1182/blood-2008-01-133223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tringler B, Zhuo S, Pilkington G, Torkko KC, Singh M, Lucia MS, et al. B7-h4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res. 2005;11:1842–8. doi: 10.1158/1078-0432.CCR-04-1658. [DOI] [PubMed] [Google Scholar]
- 68.Lee JS, Scandiuzzi L, Ray A, Wei J, Hofmeyer KA, Abadi YM, et al. B7x in the periphery abrogates pancreas-specific damage mediated by self-reactive CD8 T cells. J Immunol. 2012;189:4165–74. doi: 10.4049/jimmunol.1201241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao LW, Li C, Zhang RL, Xue HG, Zhang FX, Zhang F, et al. B7-H1 and B7-H4 expression in colorectal carcinoma: correlation with tumor FOXP3(+) regulatory T-cell infiltration. Acta Histochem. 2014;116:1163–8. doi: 10.1016/j.acthis.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Simon I, Katsaros D, Rigault de la Longrais I, Massobrio M, Scorilas A, Kim NW, et al. B7-H4 is over-expressed in early-stage ovarian cancer and is independent of CA125 expression. Gynecol Oncol. 2007;106:334–41. doi: 10.1016/j.ygyno.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 71.Vanderstraeten A, Luyten C, Verbist G, Tuyaerts S, Amant F. Mapping the immunosuppressive environment in uterine tumors: implications for immunotherapy. Cancer Immunol Immunother. 2014;63:545–57. doi: 10.1007/s00262-014-1537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM, et al. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A. 2006;103:10391–6. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qian Y, Sang Y, Wang FX, Hong B, Wang Q, Zhou X, et al. Prognostic significance of B7-H4 expression in matched primary pancreatic cancer and liver metastases. Oncotarget. 2016;7:72242–9. doi: 10.18632/oncotarget.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu WH, Chen YY, Zhu SX, Li YN, Xu YP, Wu XJ, et al. B7-H4 expression in bladder urothelial carcinoma and immune escape mechanisms. Oncol Lett. 2014;8:2527–34. doi: 10.3892/ol.2014.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fan M, Zhuang Q, Chen Y, Ding T, Yao H, Chen L, et al. B7-H4 expression is correlated with tumor progression and clinical outcome in urothelial cell carcinoma. Int J Clin Exp Pathol. 2014;7:6768–75. [PMC free article] [PubMed] [Google Scholar]
- 76.Jeon H, Vigdorovich V, Garrett-Thomson SC, Janakiram M, Ramagopal UA, Abadi YM, et al. Structure and cancer immunotherapy of the B7 family member B7x. Cell Rep. 2014;9:1089–98. doi: 10.1016/j.celrep.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dangaj D, Lanitis E, Zhao A, Joshi S, Cheng Y, Sandaltzopoulos R, et al. Novel recombinant human b7-h4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses. Cancer Res. 2013;73:4820–9. doi: 10.1158/0008-5472.CAN-12-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith JB, Lanitis E, Dangaj D, Buza E, Poussin M, Stashwick C, et al. Tumor regression and delayed onset toxicity following B7-H4 CAR T cell therapy. Mol Ther. 2016;24:1987–99. doi: 10.1038/mt.2016.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu Y, Yao S, Iliopoulou BP, Han X, Augustine MM, Xu H, et al. B7-H5 costimulates human T cells via CD28H. Nat Commun. 2013;4:2043. doi: 10.1038/ncomms3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Janakiram M, Chinai JM, Fineberg S, Fiser A, Montagna C, Medavarapu R, et al. Expression, clinical significance, and receptor identification of the newest B7 family member HHLA2 protein. Clin Cancer Res. 2015;21:2359–66. doi: 10.1158/1078-0432.CCR-14-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rahimi N, Rezazadeh K, Mahoney JE, Hartsough E, Meyer RD. Identification of IGPR-1 as a novel adhesion molecule involved in angiogenesis. Mol Biol Cell. 2012;23:1646–56. doi: 10.1091/mbc.E11-11-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cheng H, Janakiram M, Borczuk A, Lin J, Qiu W, Liu H, et al. HHLA2, a new immune checkpoint member of the B7 family, is widely expressed in human lung cancer and associated with EGFR mutational status. Clin Cancer Res. 2017;23:825–32. doi: 10.1158/1078-0432.CCR-15-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koirala P, Roth ME, Gill J, Chinai JM, Ewart MR, Piperdi S, et al. HHLA2, a member of the B7 family, is expressed in human osteosarcoma and is associated with metastases and worse survival. Sci Rep. 2016;6:31154. doi: 10.1038/srep31154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sankin A, Gartrell B, Cumberbatch K, Stern MP, Huang H, Schoenberg M, et al. New targets for immune checkpoint inhibition in urothelial carcinoma. American Urological Association Annual Meeting; San Diego, CA. 2016. [Google Scholar]
- 85.Apolo AB, Infante JR, Hamid O, Patel MR, Wang D, Kelly K, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with metastatic urothelial carcinoma from the JAVELIN solid tumor phase 1b trial: analysis of safety, clinical activity, and PD-L1 expression. J Clin Oncol. 2016;(Suppl):34. abstract 4514. [Google Scholar]
- 86.Balar AV, Galsky MD, Loriot Y, Dawson NA, Necchi A, Srinivas S, et al. Atezolizumab (atezo) as first-line (1L) therapy in cisplatin-ineligible locally advanced/metastatic urothelial carcinoma (mUC): primary analysis of IMvigor210 cohort 1. J Clin Oncol. 2016;(suppl):34. abstract LBA4500. [Google Scholar]
- 87.Sharma P, Bono P, Kim JW, Spiliopoulou P, Calvo E, Pillai RN, et al. Efficacy and safety of nivolumab monotherapy in metastatic urothelial cancer (mUC): Results from the phase I/II CheckMate 032 study. J Clin Oncol. 2016;(suppl):34. abstract 4501. [Google Scholar]
- 88.Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312–22. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 89.Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34:3119–25. doi: 10.1200/JCO.2016.67.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Plimack ER, Bellmunt J, Gupta S, Berger R, Montgomery RB, Heath K, et al. Pembrolizumab (MK-3475) for advanced urothelial cancer: Updated results and biomarker analysis from KEYNOTE-012. J Clin Oncol. 2015;(suppl):33. abstract 4502. [Google Scholar]
- 91.Balar A, Bellmunt J, O’Donnell PH, Castellano D, Grivas P, Vuky J, et al. Pembrolizumab (pembro) as first-line therapy for advanced/ unresectable or metastatic urothelial cancer: preliminary results from the phase 2 KEYNOTE-052 study. European Society for Medical Oncology Annual Meeting. 2016 [Google Scholar]


