Abstract
Alcohol use disorder (AUD) is a chronic, relapsing disease characterized by maladaptive patterns of alcohol drinking and seeking. Though sex differences exist in the etiology of AUD, much remains to be elucidated concerning the mechanisms underlying sex-related vulnerability to developing excessive alcohol-motivated behavior. While a large body of evidence points to an important role of circulating gonadal hormones in mediating cocaine reinforcement, findings are less consistent with respect to ethanol. Critically, the effects of gonadal hormones on the reinstatement of ethanol seeking, a model of “craving”-like behavior that reveals pronounced sex differences, has not yet been examined. Thus, the goal of the present experiment was to directly compare manipulations of gonadal hormones in male and female rats on ethanol-motivated behavior. Rats received sham or gonadectomy surgery with or without hormone replacement prior to and throughout three weeks of operant ethanol self-administration to determine the effects of chronically high or low gonadal hormone levels on ethanol drinking. Hormone treatment ceased during extinction training, and the effects of an acute injection of either testosterone (in males) or estradiol (in females) on cue+yohimbine-induced reinstatement of ethanol seeking was determined. Separate groups of gonadally- intact female rats went through similar training, but the effects of either the antiestrogen, fulvestrant, the selective estrogen receptor modulator, clomiphene, or the estrogen receptor β antagonist, PHTPP, on the reinstatement of ethanol seeking were determined. Chronic estradiol replacement produced significant increases in ethanol drinking in female rats, while chronic testosterone significantly decreased ethanol drinking in male rats. Gonadectomy alone only produced modest shifts in drinking towards the opposite-sex pattern, and did not eliminate the robust sex differences that persisted regardless of hormone manipulations. Neither prior chronic nor acute hormone manipulations altered cue+yohimbine-induced reinstatement of ethanol seeking, though blockade of estrogen receptors tended to reduce reinstatement in gonadally- intact females. Overall, our findings indicate that gonadal hormones at least partially mediate, but do not totally account for the sex differences evident in ethanol self-administration, and circulating gonadal hormones have little effect on the reinstatement of ethanol seeking. These results provide a foundation for future studies examining the neuronal mechanisms underlying sex differences in ethanol drinking and seeking.
Keywords: Ethanol, Yohimbine, Reinstatement, Craving, Estrogen, Testosterone
1. Introduction
Alcohol use disorder (AUD) is a chronic, relapsing disease characterized by maladaptive patterns of alcohol drinking and seeking. Though AUD has historically been reported to be anywhere from one-third to three times more prevalent in men than in women [1, 2], more recent epidemiological data points to a narrowing in this gender gap, with younger cohorts of women, but not men, demonstrating increased problem drinking compared to previous cohorts [3, 4]. Further, women tend to show a more rapid transition from problem drinking to dependence, a phenomenon referred to as “telescoping” [5, 6], indicating that identification of factors that promote the escalation of alcohol drinking and seeking in females are needed. Indeed, evidence suggests that negative emotional states [7] and exposure to stress [8, 9] promote alcohol craving to a greater degree in women than in men, indicating that stress and anxiety play a significant role in female sensitivity to alcohol reinforcement. However, much remains to be elucidated concerning the mechanisms underlying sex differences in the vulnerability of developing excessive alcohol-motivated behavior.
One clear difference between males and females is the dominant gonadal hormone exerting effects on brain function and behavior: testosterone in males, and estrogen and progesterone in females. These hormones can exert organizational (permanent changes in brain organization at discrete developmental periods) and activational (transient changes that happen throughout life) effects that lead to sexual dimorphism in several behaviors, including alcohol seeking. Clinical [10, 11] and preclinical studies [12–14] demonstrate that adult-typical sex differences in alcohol drinking do not emerge until late adolescence, suggesting that the pubertal gonadal hormone surge is likely responsible for male- and female-typical drinking patterns, though whether these effects are organizational, activational, or both in nature are not well understood [15, 16]. The majority of research into the role of gonadal hormones in modulating sex-dependent behavior focuses on cycling ovarian hormones in adult females, though testosterone levels do fluctuate in males as well [17, 18]. These studies examining variations in ovarian hormone levels within the normal range have shown that the reinforcing effects of cocaine are highest in the late follicular (preovulatory) phase in women, when estradiol is at its peak, and are lowest in the mid-luteal (premenstrual) phase, when progesterone is at its peak (though estradiol levels are elevated here as well) [19–22]. However, the alcohol literature is less clear, with studies showing increased, decreased, or unchanged levels of alcohol drinking as a function of phase of the menstrual cycle [23, 24]. Similar results are evident in the preclinical drug abuse literature, with findings of increased cocaine taking in female rodents (relative to males) being mediated by estradiol [25, 26], though these effects are less consistent for alcohol. For example, in studies using gonadally- intact, freely-cycling female rats, estrous cycle did not change overall daily ethanol intake [27, 28], though patterns of drinking were altered during proestrus (when estradiol levels are highest) [27] and when estrous cycles were synchronized [28].
Another approach to investigating the mechanisms by which gonadal hormones influence behavior is to remove the gonads, with or without subsequent hormone replacement. Using ovariectomized (OVX) rats, estradiol has been shown to dose-dependently increase operant ethanol self-administration [29]; however, another study failed to detect an effect of OVX on limited access homecage drinking [30]. In contrast, the same investigators showed that castration (CAST) in males significantly increased ethanol intake [31], and testosterone replacement “rescued” (i.e., reduced) intake to levels evident in gonadally-intact males [32]. However, in a study that directly compared the effects of gonadectomy (GDX) in males and females on operant ethanol self-administration, no effects of gonadal hormones were found for either sex [33]. Thus, though there is evidence showing that estradiol promotes drinking in females and testosterone reduces drinking in males, a systematic comparison between males and females that have had surgical and/or pharmacological manipulation of circulating gonadal hormone levels is warranted to clearly elucidate their role in the sex differences evident in alcohol drinking.
Surprisingly, to our knowledge, no studies investigating the role of gonadal hormones on the motivation to seek ethanol in reinstatement models of “craving” have previously been undertaken. Craving is a significant impediment to maintaining abstinence from alcohol and other drugs, and the reinstatement model is a method for investigating factors that promote drug-seeking behavior in the absence of drug reinforcement [34, 35]. We have recently shown that female rats demonstrate significantly greater levels of reinstatement of alcohol seeking in response to cues previously associated with alcohol and to the pharmacological stressor, yohimbine, than males; further, this effect was enhanced in females when these two stimuli were given in combination [36]. Our results are consistent with those in the cocaine field [37]; importantly, Feltenstein & See further found that enhanced cue+yohimbine-induced reinstatement of cocaine seeking was particularly evident in females in proestrus. This echoes other studies finding estradiol-mediated increases in cocaine seeking [38] that specifically implicate the estrogen receptor β (ERβ) [39]. However, few, if any studies have tested the effects of testosterone in reinstatement of drug/alcohol seeking in males. Further, none have examined how modulation of gonadal hormone systems impacts alcohol “craving”-like behavior.
The goal of the present study was to determine the role of circulating gonadal hormones in sex-typical patterns of ethanol-motivated behavior in male and female rats. We predicted that reduction of estradiol levels in females by ovariectomy would decrease ethanol drinking and seeking, and that this effect would be rescued by E2 replacement. Conversely, we predicted that reduction in testosterone levels in males by castration would increase ethanol drinking and seeking, and that this effect would be rescued by T replacement. Further, we predicted that acute modulation of estrogen receptor signaling would block alcohol cue+yohimbine- induced reinstatement of alcohol seeking in gonadally intact, freely-cycling female rats.
2. Methods
2.1. Subjects
Adult (aged 67–68 days upon arrival) male and female Sprague-Dawley Rats (Harlan, Frederick, MD) were pair-housed and maintained on a 12:12 light:dark cycle in a temperature- and humidity-controlled room. All behavioral testing was conducted at the beginning of the dark cycle. Rats were given ad libitum access to water and food throughout the experiment except where noted. All procedures were conducted in accordance with the policies set forth by University of Pittsburgh Institutional Animal Care and Use Committee and the National Institutes of Health Guidelines on the Care and Use of Laboratory Animals.
2.2. Surgery
Male and female rats received either gonadectomy or sham surgery using isoflurane (2–3%) anesthesia under aseptic conditions ~1 week after arrival. For the castration (CAST) surgery, a 1–2 cm midline incision was made in the scrotum, followed by an incision in the vaginal tunic. Each testis and epididymis was exteriorized and clamped using hemostats and ligated with 3-0 silk sutures (Ethicon, Somerville, NJ), and then excised. The remaining tissue was rinsed with sterile saline and returned to the scrotal sac, which was then closed using 3-0 silk sutures. For the ovariectomy surgery (OVX), a 2–3 cm midline incision was first made in the abdominal skin, and subsequently in the muscle wall. Hemostats were used to localize oviducts, and the ovaries were gently externalized and separated from the surrounding tissue. The oviducts were then ligated with 3-0 silk sutures and the ovaries were excised. The remaining tissue was rinsed with sterile saline and returned to the abdominal cavity. The muscle wall was then sutured, and the skin was closed using wound clips (Reflex, Fine Science Tools, Foster City, CA). Sham surgery rats underwent the same procedure without the removal of the gonads. All rats received Rimadyl (Carpofen; Zoetis, Kalamazoo, MI) analgesic preoperatively and for 2 days postoperatively. Sutures or wound clips were removed 7–10 days after surgery.
2.3. Drugs
17-β estradiol (E2), testosterone propionate (T), fulvestrant, and 4-[2-phenyo-5,7 bis(trifluoromrthyl)pyrazolo(1,5- a)pyrimidin-3-yl]phenol (PHTPP) were dissolved in sesame oil. Clomiphene citrate was dissolved in sterile water with 1 drop of Tween-20 per 5 ml. The ethanol solution (EtOH; 10%v/v ethanol+0.1%w/v saccharin) was dissolved in tap water. Yohimbine HCl (YOH) was dissolved in sterile water. All drugs were obtained from Sigma (St. Louis, MO) except for PHTPP (Tocris, Minneapolis, MN). Drug doses were based on previous studies focused on the effects of yohimbine (1.25 mg/kg) [36, 43], estradiol (50 μg/kg) [38], testosterone (2 mg/kg) [44], clomiphene (5 mg/kg) [41], and PHTPP (50 μg/rat) [42] on drug- and anxiety-related behavior and neurochemistry, and the ability of fulvestrant (1–10 mg/kg) to reach the brain [40].
2.4. Apparatus
Operant alcohol self-administration, extinction, and reinstatement sessions were conducted in operant conditioning chambers housed in sound-attenuating cubicles (Med Associates, St. Albans, VT). Operant conditioning chambers were equipped with two retractable levers situated on either side of a magazine into which a dipper arm would deliver 0.05ml of the ethanol solution. Delivery of the reinforcer was accompanied by illumination of a cue light above the active lever and sounding of a 75 dB tone. During testing, the house light was illuminated and an exhaust fan was turned on to mask external noise. All operant sessions (except where noted) were 1 hour in length.
2.5. Procedures
2.5.1. Ethanol self-administration training and cue+yohimbine-induced reinstatement of ethanol seeking
A timeline of the experimental procedures is described in Fig. 1. During ethanol self-administration training, rats were first habituated to the ethanol solution by placing a bottle of 10% ethanol/0.1% saccharin in their home cage for 24 hours (watering system still available). The following day, rats received a 30-min magazine training session, during which ethanol was presented every 30 s. Rats responded on a fixed ratio 1 (FR1) schedule of reinforcement, where an active lever press produced 10-s presentation of the ethanol dipper and the tone+light cue. Inactive lever responses had no programmed consequences. Ethanol troughs were weighed before and after each session. Rats received a total of 20–21 self-administration sessions, and tail blood samples were then taken for BEC determination.
Fig. 1.
Timeline of experimental procedures in days. After acclimation, rats underwent gonadectomy (OVX, CAST), sham surgery (SHAM), or no surgery (INTACT). Following recovery, rats received hormone (estradiol [E2] in females, testosterone [T] in males) or vehicle injections for 5–7 days prior and throughout ethanol self-administration. During extinction (EXT) and reinstatement (REIN), chronic hormone treatments ceased. Rats were acutely treated with either E2, T, PHTPP, fulvestrant (FULV), or clomiphene (CLOM) prior to cue+yohimbine- induced reinstatement of ethanol seeking.
Following self-administration, instrumental lever extinction training began, in which responses had no programmed consequences. Rats were given 7–10 extinction sessions to meet criterion (≤25 active lever presses over two consecutive days) before reinstatement testing. During reinstatement, rats were injected with 1.25 mg/kg YOH 15 minutes prior to the reinstatement session, which consisted of response-contingent presentation of the light+tone cue previously associated with ethanol, and presence of the ethanol odor cue (ethanol-filled trough placed in chamber), but in the absence of ethanol reinforcement.
2.5.2. Effects of estrous cycle on EtOH drinking in gonadally-intact female rats
Two separate groups of gonadally-intact female rats were trained to self-administer ethanol and tested for reinstatement as described above. One group received a single injection of fulvestrant (10 mg/kg) or vehicle on the final day of ethanol self-administration. Rats were monitored for estrous cycle phase (see 2.6 below) throughout the final week of self-administration once drinking had stabilized.
2.5.3. Effects of acute estrogen receptor blockade on cue+-yohimbine-induced reinstatement of EtOH seeking in gonadally-intact female rats
Following self-administration (above), rats were tested for the effects of repeated, lower-dose fulvestrant (3 days × 1 mg/kg), acute, higher-dose fulvestrant (5 mg/kg), and clomiphene (5 mg/kg) versus their respective vehicles in three consecutive cue+yohimbine-induced reinstatement tests separated by 8–9 days. Assignment to the different treatment groups was balanced for initial self-administration performance as well as prior fulvestrant treatment. The other group received only subcutaneous oil injections during self-administration (as a control for the chronic hormone experiment below) and was tested for the effects of PHTPP (vehicle or 50 μg/rat) on cue+yohimbine-induced reinstatement of ethanol seeking. All drugs were given 1 hour prior to reinstatement testing.
2.5.4. Effects of chronic gonadal hormone manipulations on EtOH self-administration in male and female rats
Rats were allowed ~4–7 days to recover from surgery before hormone treatment began. Estradiol (50 μg/kg; OVX+E2), testosterone (2 mg/kg; CAST+T), or vehicle (OVX/CAST+VEH, SHAM, and INTACT) were injected subcutaneously in a ~0.1 ml volume daily for ~5–7 days prior to and throughout ethanol self-administration training. Injections occurred post-session to minimize the effects of injection stress on self-administration.
2.5.5. Effects of acute gonadal hormone manipulations on cue+yohimbine-induced reinstatement of EtOH seeking in male and female rats
During extinction, rats received no hormone or vehicle injections and were thus in their endogenous hormone state (i.e, all gonadectomized rats had low gonadal hormone levels, while sham and intact rats had normal gonadal hormone levels). OVX and SHAM females were treated with either 50 μg/kg E2 or vehicle, and CAST and SHAM males were treated with either 2 mg/kg T or vehicle, 30 minute prior to testing.
2.6. Estrous cycle determinations
To monitor circulating ovarian hormone levels, female rats received vaginal lavage immediately after behavioral testing periodically throughout the experiment: prior to self-administration (to confirm OVX/E2 treatment efficacy); during the last week of self-administration (to determine the effects of ethanol on cyclicity); during extinction (to confirm withdrawal of E2); and on reinstatement days. Estrous cycle determination procedures consisted of gently pipetting 150 μl saline into the vaginal canal and then onto a slide, which was coverslipped and visualized at 200x magnification under a light microscope that same day [45]. Male rats were handled in a similar manner to control for handling stress.
2.7. Statistics
Statistical analyses were conducted using IBM SPSS Statistics v21 (IBM Corporation, Armonk, NY). Ethanol intake, reinforcers earned, active lever presses, and body weight were subjected to mixed factorial or oneway ANOVAs (p < 0.05), followed by Bonferroni post-hoc comparisons where appropriate.
3. Results
3.1. Effects of estrous cycle on EtOH drinking in gonadally-intact female rats
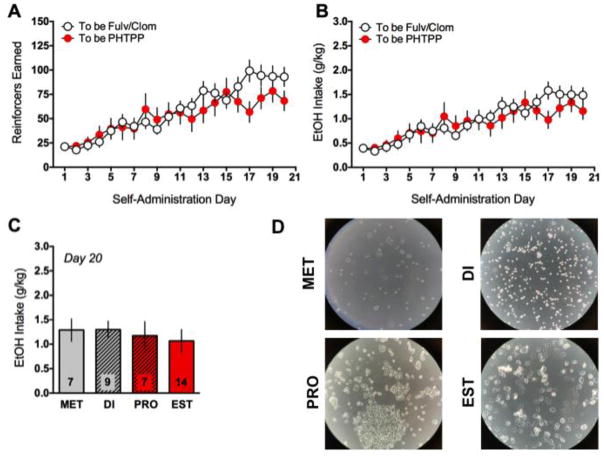
In one group of gonadally intact female rats (“To be Fulv/Clom”, n=24), repeated-measures ANOVAs showed significant differences in the number of reinforcers earned [F(20,460)=28.63, p<0.001; Fig. 2A] and ethanol intake [F(20,460)=24.52, p<0.001; Fig. 2B] across the self-administration period, with a general pattern of a steady increase in ethanol-motivated behavior during the second week of self-administration that stabilized during the last week. These rats were then treated with either fulvestrant or vehicle during a final self-administration day, but this treatment was without any significant effect (not shown). Note, rats in this group underwent mild food restriction during the first week of operant training, which was lessened during the second week, with rats being ad lib fed during the final week of self-administration per our previously published protocols [36]. A separate cohort of gonadally intact female rats (n=15, “To be PHTPP”) tested alongside a group of OVX and SHAM rats (and were thus not food restricted) similarly showed steady increases in the number of reinforcers earned [F(20,280)=7.99, p<0.001; Fig. 2A] and ethanol intake [F(20,280)=7.16, p<0.001; Fig. 2B] during the self-administration period. To determine whether estrous cycle phase affected ethanol drinking in intact female rats, all INTACT and SHAM rats (described below) were collapsed to increase power, and only rats that were decidedly in one of the four phases of the estrous cycle (i.e., were not transitioning between phases) were included in the analysis (n=37). A one-way ANOVA revealed no significant effect of estrous cycle phase on ethanol intake on the last day of self-administration (Fig. 2C). Representative pictograms of cytology are shown in Fig. 2D.
Fig. 2.
Ethanol self-administration in gonadally-intact female rats. Females that were later tested for the effects of fulvestrant (Fulv), clomiphene (Clom), or PHTPP showed typical acquisition of ethanol self-administration, increasing the amount of reinforcers earned (A) and ethanol intake (B) across the three weeks of training. Across all gonadally- intact rats (INTACT and SHAM), no significant differences as a function of phase of the estrous cycle were evident on the last day of ethanol self-administration (C). Representative pictographs of vaginal cytology are shown in (D).
3.2. Effects of acute estrogen receptor blockade on cue+-yohimbine-induced reinstatement of EtOH seeking in gonadally-intact female rats
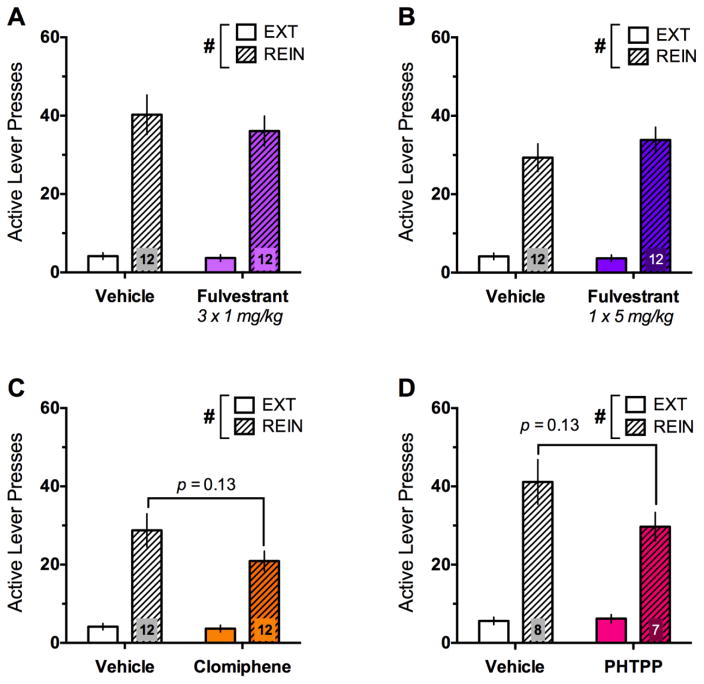
During reinstatement, rats were first tested for the effects of 3 consecutive days of the anti-estrogen fulvestrant (1 mg/kg vs. vehicle; n=12/group; Fig. 3A). Though rats showed significant increases in responding during reinstatement [F(1,22)=82.36, p<0.001], fulvestrant had no effect. Rats were subjected to at least two additional extinction sessions before being tested for the effects of a higher, single dose of fulvestrant (5 mg/kg vs. vehicle) on reinstatement (Fig. 3B). Once again, rats demonstrated significant reinstatement [F(1,22)=83.79, p<0.001], that was not modulated by fulvestrant. Finally, rats were once again subjected to at least two extinction sessions before being tested for the effects of the selective estrogen receptor modulator (SERM), clomiphene, on reinstatement (Fig. 3C). As before, rats demonstrated significant reinstatement [F(1,22)=52.21, p<0.001], but a trend for a day × treatment interaction [F(1,22)=2.61, p=0.12] emerged, with clomiphene-treated rats tending to show reduced reinstatement [t(22)=1.59, p=0.13]. ANCOVAs conducted for each reinstatement test that added “prior fulvestrant treatment” as a covariate did not show that prior treatment significantly altered subsequent reinstatement.
Fig. 3.
Effects of estrogen receptor blockade on cue+yohimbine- induced reinstatement of ethanol seeking in gonadally-intact female rats. Though significant increases in active lever presses during reinstatement (REIN) relative to extinction (EXT) were evident in all groups, no significant effects of chronic (A) or acute fulvestrant treatment (B), clomiphene (C) or PHTPP (D) were evident, though the effects of clomiphene and PHTPP approached significance. Sample sizes are shown within the bars of each graph. #p<0.001 (day effect)
Intact female rats tested alongside a group of OVX and SHAM rats were injected with the ERβ antagonist, PHTPP (50μg/rat vs. vehicle; n=7–8/group) prior to reinstatement (Fig. 3D). In a similar pattern as clomiphene, rats in these groups demonstrated significant reinstatement [F(1,13)=70.14, p<0.001], and a trend for a day × treatment interaction [F(1,13)=2.9, p=0.11] emerged, with PHTPP-treated rats tending to show reduced reinstatement [t(13)=1.63, p=0.13]. No differences in responding were evident when comparing freely-cycling rats in high (proestrus) or low (estrus, metestrus, diestrus) estradiol phases for any of the reinstatement tests.
3.3. Effects of chronic gonadal hormone manipulations on EtOH self-administration in male and female rats
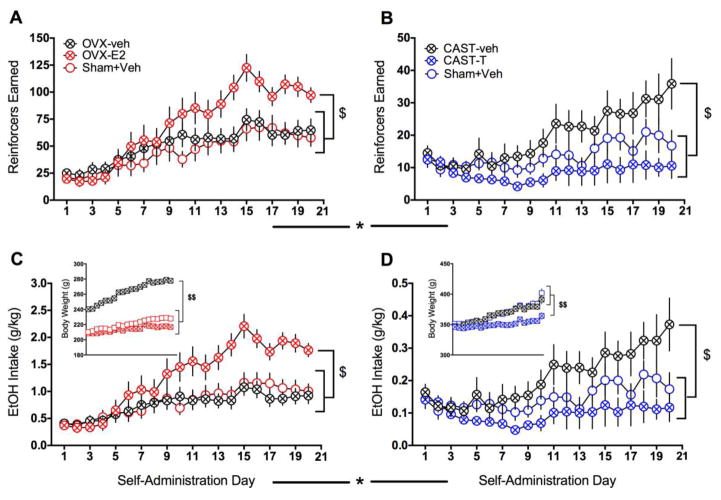
A mixed factorial ANOVA with self-administration day as the within-subjects factor and sex and treatment (GDX+hormone, GDX+VEH, and SHAM) as between-subjects factors revealed significant main effects of day [F(19,1748)=31.07, p<0.001], and sex [F(1,92)=61.4, p<0.001] and a day × sex interaction [F(19,1748)=16.52, p<0.001] for the number of reinforcers earned (Fig. 4A–B). Due to the very large effect size for the sex difference (partial η2=0.4), and the qualitatively different nature of the GDX and hormone manipulations in males versus females, subsequent analyses aimed at determining treatment effects were performed separately for each sex. In female rats (n=18–19/group; Fig. 4A), a significant increase in the number of reinforcers earned across the self-administration period was found [F(19,1007)=33.28, p<0.001]. However, a day × treatment interaction [F(38,1007)=3.33, p<0.001] revealed that the pattern of this increase differed as a function of treatment, a factor that approached significance (p=0.055). In general, the OVX+E2 group showed a steeper acquisition curve compared to the other groups, and pairwise t-tests indicated that this effect was sustained starting on day 13 (though OVX+E2 and SHAM groups differed on days 10–11 as well). Further, when only the last 5 days of self-administration were analyzed (at which point behavior had stabilized and effects of day were abolished), a treatment effect emerged [F(2,53)=6.94, p=0.002], with post-hoc tests indicating significantly increased number of reinforcers earned in the OVX+E2 group compared to all other groups (all ps<0.05). Similar analyses in males (n=14/group; Fig. 4B) also revealed a significant increase in the number of reinforcers earned across the self-administration period [F(19,741)=8.88, p<0.001] as well as a main effect of treatment [CAST+T, CAST+VEH, and SHAM; F(2,39)=3.65, p=0.035] and a day × treatment interaction [F(38,741)=2.33, p<0.001]. Post-hoc comparisons showed that CAST+VEH rats earned significantly more reinforcers than CAST+T rats (p=0.031), and pairwise t-tests showed that this difference emerged on day 8 of self-administration.
Fig. 4.
Effects of chronic gonadal hormone manipulations on ethanol self-administration. OVX females treated with E2 (estradiol) earned significantly more reinforcers than those receiving vehicle or SHAM surgery (A). In contrast, CAST males treated with vehicle earned significantly more reinforcers than those receiving T (testosterone) or SHAM surgery (B). Similar findings were evident for ethanol intake in female (C) and male (D) rats. OVX alone significantly increased body weight in females (C, inset), while T treatment significantly decreased body weight in males (D, inset). Across all measures, significant and pronounced sex differences were seen (note different scales of the y-axes). $p<0.05 (treatment effect over the last 5 days of self-administration); $$p<0.001 (overall treatment effect); *p<0.001 (sex effect)
Finally, as it was of interest to determine if gonadectomy was sufficient to abolish the sex difference evident in ethanol reinforcement, planned comparisons between OVX females and CAST males treated with vehicle showed persistent main effects of sex [F(1,30)=11.1, p<0.001], in addition to main effects of day [F(19,570)=10.42, p<0.001] and a day × sex interaction [F(19,570)=2.14, p=0.003]; (Fig. 4A–B). Overall, these results indicate that females acquired self-administration more rapidly than males and self-administered more ethanol than males regardless of hormone status.
An initial mixed factorial ANOVA revealed significant main effects of day [F(19,1748)=25.95, p<0.001], and sex [F(1,92)=80.86, p<0.001] and day × sex [F(19,1748)=17.98, p<0.001], day × treatment [F(19,1748)=2.48, p<0.001], and sex × treatment [F(2,92)=5.05, p<0.001] interactions for ethanol intake (Fig. 4C–D), with a very large sex effect (partial η2=0.47). Subsequent analyses of ethanol intake conducted separately for each sex echoed the findings for reinforcers earned, with both females [F(19,1007)=30.03, p<0.001]; (Fig. 4C) and males [F(19,741)=7.48, p<0.001]; (Fig. 4D) increasing their intake across the self-administration period. However, unlike reinforcers earned, intake in females showed a significant day × treatment interaction [F(38,1007)=4.34, p<0.001] and now a main effect of treatment [F(2,53)=5.16, p=0.009], with post-hoc comparisons showing an overall increase in amount of ethanol consumed in the OVX+E2 group compared to the OVX+VEH and SHAM groups (ps=0.03). Larger treatment effects in the amount of ethanol consumed (in g/kg) versus the number of reinforcers earned in females was driven by significant weight differences as a function of treatment [F(2,53)=148.76, p<0.001], with OVX+VEH females weighing more (all ps <0.001) and OVX+E2 females weighing less (all ps <0.03) than all other groups (Fig. 4C, inset). In males, a significant day × treatment interaction [F(19,741)=2.02, p<0.001] and a main effect of treatment [F(2,39)=3.26, p=0.049] were found for ethanol intake. As with reinforcers earned, post-hoc comparisons showed that CAST+VEH males drank significantly more than CAST+T males (p=0.045). However, in contrast to the females where weight differences magnified group differences in drinking, lower weights in CAST+T males [F(2,39)=6.84, p=0.003] compared to the other groups (ps<0.011) had less of an impact on their already low intake (Fig. 4D, inset).
With respect to ethanol intake, though trough weights were analyzed, these measures overestimated intake at low levels of responding (likely due to evaporation of the solution), which had a greater impact in the lower-drinking males. Therefore, to better estimate and equate intake, the number of reinforcers earned in the session was multiplied by the volume of the dipper cup (0.05 ml) to calculate the total grams of ethanol consumed by body weight. The method for calculating intake did not alter whether between-group differences were significant, but did change the total calculated ethanol intake.
3.4. Effects of acute gonadal hormone manipulations on cue+yohimbine-induced reinstatement of EtOH seeking in gonadectomized male and female rats
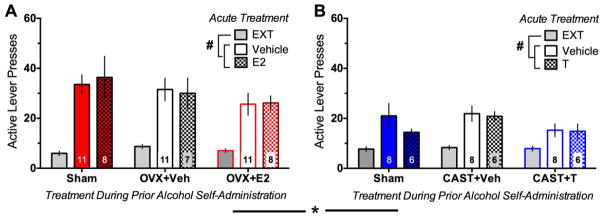
An initial 2×2 factorial ANOVA with day (average of the last two days of extinction vs. reinstatement) as the within-subjects factor and sex as the between subjects factor revealed significant main effects of sex [F(1,111)=18, p<0.001], day [F(1,111)=207.8, p<0.001], and a day × sex interaction [F(1,111)=33.33, p<0.001] for number of active lever presses (Fig. 5A–B). Importantly, these sex differences were not evident during the average of the final two days of extinction (used to determine extinction criterion), indicating that males and females extinguished similarly. In contrast, a follow-up independent-samples t-test showed robust sex differences [t(111)=5.11, p<0.001; Hedge’s g=0.99] in responding during reinstatement. Thus, as was done for self-administration, reinstatement data were analyzed separately by sex. In females (n=7–11/group; Fig. 5A), though responses were significantly increased from extinction to reinstatement [F(1,50)=145.13, p<0.001], neither prior hormonal manipulations (OVX+E2, OVX+VEH, SHAM) nor acute hormone treatment (E2 or vehicle) affected responding. Similarly, responses in males (n=6–8/group; Fig. 5B) were significantly increased from extinction to reinstatement [F(1,36)=61.8, p<0.001], but neither prior hormonal manipulations (CAST+T, CAST+VEH, SHAM) nor acute hormone treatment (T or vehicle) affected responding. Note, pronounced order effects were evident when reinstatement tests were initially given in an ABA (vehicle, hormone, vehicle) within-subjects design, and as such only the first reinstatement test was used, with acute hormone treatment as a between-subjects factor.
Fig. 5.
Effects of acute gonadal hormone manipulations on ethanol self-administration. Though significant increases in active lever presses during reinstatement (REIN) relative to extinction (EXT) were evident in all groups, no significant effects of either prior chronic nor acute manipulation of estradiol in females (A) or testosterone in males (B) were evident. Significant and sex differences were evident for responding on the day of reinstatement. Sample sizes are shown within the bars of each graph. #p<0.001 (day effect); *p<0.001 (sex effect on reinstatement day only)
3.5. Estrous cycle determinations: general patterns
To confirm the efficacy of the chronic hormone manipulations and to monitor estrous cycles in gonadally intact female rats, vaginal cytology was evaluated. OVX produced cell populations that were either very sparse or were consistent with metestrus or diestrus. Chronic E2 replacement very reliably produced an abundance of cornified epithelial cells consistent with estrus [45]. The majority of females in the E2 replacement group demonstrated cytology consistent with their vehicle-treated OVX counterparts by the end of extinction training after hormone treatment cessation. Only between ~30–50% of SHAM and INTACT rats showed normal 4–5 day cycles (proestrus → estrus → metestrus → diestrus) during operant training.
4. Discussion
Overall, these results demonstrate that circulating gonadal hormones at least partially mediate the sex differences evident in ethanol self-administration, but have little effect on the reinstatement of ethanol seeking. Chronic estradiol replacement produced significant increases in ethanol drinking in female rats, while chronic testosterone replacement virtually abolished ethanol drinking in male rats. Postpubertal gonadectomy alone only produced moderate shifts in drinking towards the opposite-sex pattern, and did not eliminate the robust sex differences that persisted regardless of hormone manipulations. Neither prior chronic nor acute hormone manipulations altered cue+yohimbine-induced reinstatement of ethanol seeking, though blockade of estrogen receptors tended to reduce reinstatement in gonadally- intact females. These results indicate that though gonadal hormones can have modest activational effects on ethanol drinking, the robust sex differences observed are primarily due to permanent factors, such as sex chromosomes and/or the organizational effects of gonadal hormones.
Despite the presence of sex differences in a number of diseases, including psychiatric disorders, females have historically been understudied. Not until the early 1990s was the inclusion of women as subjects in clinical research mandated by the National Institutes of Health [46], and only very recently (2015) was consideration of sex as a biological variable in preclinical studies also mandated [47]. A recent review emphasizing this research gap showed that roughly half of studies in neuroscience, pharmacology, and endocrinology used only males, and the latter two fields directly compared across sex in only about 20% of studies, though behavioral studies were more sex-balanced [48]. One reason for this bias against using females is due to the variability attributed to circulating gonadal hormones. However, a recent meta-analysis showed that female rats are not more variable than male rats in a number of neuroscience-related measures [49]. Similarly, we do not see greater variability in our female rats compared to the males, nor are freely-cycling females more variable than OVX females. This is not to suggest, however, that circulating gonadal hormones have no overall effect on behavioral or neurochemical measures, or that significant sex differences do not exist, only that these groups are not more variable.
A small number of studies have determined the effects of estrous cycle in alcohol-motivated behavior. In freely-cycling female rodents, no overall effect of estrous cycle phase on daily home cage drinking [27] or limited-access operant ethanol self-administration [28] were evident. However, microstructural analysis of drinking patterns revealed that females in proestrus (when estradiol levels are high) showed greater drinking bout frequency, but lower bout sizes compared to the other phases, and also maintained consistently elevated lick patterns across the dark cycle, rather than peaking during the light-to-dark transition [27]. These findings have implications particularly for short-term, limited-access paradigms. However, rats in the present study were tested at the initiation of the dark cycle, potentially minimizing this cross-estrous phase variability. Interestingly, only ~30–50% of intact and SHAM females were observed to have normal cycles. This is not surprising given evidence that chronic ethanol exposure disrupts estrous cycles in rodents [50, 51] and menstrual cycles in nonhuman primates [52] and women [53], though the level of ethanol exposure in these studies was much higher than those evident in the present study. Despite this lack of normal cycling, when all SHAM and INTACT females in the present experiments were compared as a function of estrous cycle phase on the last day of self-administration, no effects on ethanol drinking were evident. Thus, taken together, the present and previous studies show that fluctuating gonadal hormone levels in freely-cycling female rats do not significantly alter ethanol intake.
To explicitly study the effects of chronically high or low levels of gonadal hormones, a number of studies have examined the effects of gonadectomy with or without hormone replacement on ethanol drinking. A logical prediction would be that testicular hormones are responsible for reduced drinking in males, and ovarian hormones are responsible for increased drinking in females. Postpubertal gonadectomy, which seeks to eliminate the activational effects of gonadal hormones, has been shown to slightly reduce [33], increase [31, 32], or have no effect [54] on drinking in males; these same studies showed either slight trends (though confounded by several factors) for reductions in [33, 54] or no effect [31] of drinking in females. Still other studies in females have shown OVX to significantly reduce ethanol intake [27, 29, 55, 56]. Similar findings are evident when animals are gonadectomized prepubertally, with increased drinking in CAST males [31, 32, 57], and either decreased [57] or unaffected [31] drinking in OVX females, indicating that prepubertal organizational effects of hormones are likely responsible for these outcomes. Though there are slight differences in methodology (e.g., ethanol concentration(s) used, sweetening/sucrose- fading, duration of ethanol access), overall, these studies utilizing 2-bottle choice procedures indicate that removal of gonadal hormones either shifts drinking levels to the opposite-sex pattern, or does not significantly affect drinking. The present findings are aligned with this general consensus, in that while GDX did not produce consumption patterns that significantly differed from SHAM rats, clear reductions in drinking in OVX females and increases in CAST males were evident using an operant self-administration procedure. When hormones are replaced, both testosterone [32] and dihydrotestosterone (DHT), an androgen resulting from the catalytic conversion of testosterone by 5α-reductase [54], reduces drinking in CAST males, though estradiol replacement either reduces [54] or dose-dependently increases [29, 55] intake in OVX females. Again, in general, these studies indicate that the dominant gonadal hormone is responsible for sex-specific drinking patterns, and our findings of significantly increased drinking as a function of chronic E2 replacement in OVX females and decreased drinking as a function of chronic T replacement in CAST males are consistent with this notion. Importantly, while we found general hormone dose-dependent alterations in ethanol self-administration, removal of hormones by gonadectomy did not eliminate the robust sex differences evident in ethanol self-administration. Taken together, the present and previous research indicate an important role for the activational effects of these hormones within each sex, the overall differences observed between the sexes are likely due to the organizational effects of hormones in masculinizing or feminizing the brain, especially during gestation and the early postnatal period [58, 59]. Thus, studies targeting the long-term behavioral consequences of hormone manipulations during these critical neurodevelopmental periods can shed more light on the ontogeny of sex differences in drug-motivated behavior.
While gonadal hormone effects were evident for ethanol self-administration, neither prior chronic nor acute gonadal hormone manipulation significantly altered cue+yohimbine-induced reinstatement of ethanol seeking. No studies to date have examined the role of gonadal hormones in the reinstatement of ethanol seeking; however, ovarian hormones have been implicated in enhancement of reinstatement of cocaine [37, 39, 60, 61] and cannabinoid [62], but not methamphetamine [63, 64] seeking in females compared to males. One potential difference between the present and several of the previous studies is the use of a pharmacological stressor in conjunction with alcohol cues as opposed to drug priming or drug cues alone to provoke reinstatement. This combined stimulus approach was utilized in the present study as individuals attempting to maintain abstinence are often confronted with multiple factors (e.g., drug cues and stress) that can increase craving [65], and has been shown to augment reinstatement of ethanol seeking over cues or yohimbine alone [66], especially in female rats [36]. Both estradiol [67–69] and testosterone [70, 71] have been shown to have anxiolytic effects, which could potentially minimize the efficacy of the anxiogenic drug, yohimbine [72, 73], to elicit alcohol seeking. However, the anxiolytic effects of estradiol are mediated via ERβ, while ERα is suggested to mediate anxiogenic effects [74, 75]; thus, actions at ERα may have modulated the ability of estradiol to dampen the effects of yohimbine. This suggestion is consistent with our findings that the ERβ antagonist PHTPP tended to reduce reinstatement in gonadally- intact female rats, though ERβ has also been implicated in cocaine prime-induced reinstatement [39]; thus, the degree to which ERβ is mediating anxiety-related or drug-motivated reinstatement is unclear. However, despite its anxiolytic effects, estradiol has been shown to increase glucocorticoid levels [68, 76, 77], consistent with our previous findings that both yohimbine- induced increases in corticosterone and basal estradiol levels were correlated with the magnitude of reinstatement of ethanol seeking [36]. In contrast, testosterone has been shown to decrease glucocorticoid levels [76, 78], which may relate to the slight reduction in reinstatement in testosterone-treated SHAM male rats in the present study. Finally, the degree to which gonadal hormones alter cue-induced reinstatement of ethanol seeking is unknown from the results of the present study, though others have indicated a lack of an effect of estradiol on cue-induced cocaine seeking [37, 60]. Taken together, though there are indications that under certain conditions gonadal hormone manipulations have modest effects on the reinstatement of ethanol seeking, the robust sex differences evident in this behavior do not appear to be mediated by circulating gonadal hormones.
There are a number of factors to consider with respect to the interpretation of the results of the present experiment and in informing future studies. First, gonadectomy with testosterone replacement in males and estradiol replacement in females only accounts for a portion of the variance attributed to circulating gonadal hormones on ethanol-motivated behavior. For example, gonadectomy does not completely eliminate circulating hormones, as there are extragonadal sources of testosterone like the adrenal glands [79], and estradiol from the adrenals, adipose tissue, and brain (among other sites), and aromatization from testosterone [80, 81]. Relatedly, testosterone treatment in males could also increase estradiol levels depending on aromatase activity; thus, future studies could use flutamide, an aromatase inhibitor, to control for these effects. In addition, few, if any studies have examined cross-sex hormone replacement (e.g., testosterone in females and estradiol in males) in drug-motivated behavior, and it would be of potential interest to determine how masculinization or feminization of females and males, respectively, alter ethanol-motivated behavior. Another caveat is that ovariectomy minimizes progesterone levels as well, but this hormone was not replaced in the current study. However, since the goal of the present investigation was to explore the mechanism underlying increased drinking in females relative to males, we focused on estradiol, as this ovarian hormone promotes drug seeking, while progesterone tends to inhibit drug seeking [82, 83]. Seemingly only one study has examined the effects of progesterone on ethanol drinking in OVX female rats, which showed no effects [54], though a number of studies have focused on the progesterone metabolite, allopregnenalone, and its effects as a positive allosteric modulator of GABAA receptors in altering response to ethanol [84–86]. Finally, though in general females show more rapid alcohol pharmacokinetics, gonadal hormones do not appear to be responsible for this effect, as hormonal status does not significantly alter blood ethanol concentrations in rats [87] or humans [88, 89], suggesting that our hormone-related effects are not due to ethanol metatoblism.
Another consideration in the interpretation of the results of the present experiment is the timing of the hormone treatment. A chronic dosing regimen was implemented during ethanol self-administration to reduce the likelihood that estrogen [90, 91] or androgen receptor downregulation/trafficking changes [92, 93] in the weeks following gonadectomy would reduce the efficacy of an acute hormone treatment prior to reinstatement. However, chronic versus acute hormone treatment likely implicate different mechanisms. For example, the pronounced effects of chronic hormone treatment on ethanol drinking could be attributed to the genomic effects of estradiol and testosterone, as these hormones, coupled to their cognate receptors, act as transcription factors on the order of hours to days following exposure [94, 95]. In contrast, rapid (seconds to minutes), membrane-bound hormone receptor effects were recruited during reinstatement given the 30-minute pretreatment period [96, 97]. As such, it may be of interest to determine the effects of a prolonged pretreatment period or chronic dosing prior to reinstatement to uncover effects that may be mediated by downstream targets of estradiol or testosterone acting as transcription factors.
In addition, the dosing and selectivity of the hormone treatment are important considerations for future studies. For example, a relatively large dose of estradiol was used in the present experiment. Though this dose has been successfully used to promote reinstatement of cocaine seeking in OVX rats [38, 39], other studies have used much lower doses [29, 55]. However, supraphysiological doses of estradiol produced similar effects on ethanol drinking as physiological doses [29], indicating a ceiling effect on intake. The testosterone dose used is in the middle of a range of doses typically used in behavioral studies [44]. Thus, it would be of interest to further explore dose-dependent effects of gonadal hormones on ethanol drinking. To determine the effects of blockade of estrogen receptors, we used the antiestrogen fulvestrant, the SERM clomiphene, and the ERβ-selective antagonist, PHTPP. As fulvestrant and clomiphene have been primarily used to study cancer and reproductive functions, their ability to block ER in the brain should be considered. Though there is evidence that fulvestrant does not cross the blood-brain barrier, our dosing regimen was based on findings of detectable levels of fulvestrant in the brain, which were consistent with plasma levels one hour post-injection [40]. Similarly, though clomiphene and other SERMs can have either agonist or antagonist effects depending upon the tissue, ER antagonism has been evident in the hypothalamus and pituitary following clomiphene treatment [98, 99], suggesting that it was blocking the effects of estradiol in the brain. Further, systemic injection of clomiphene blocks ethanol-induced increases in dopamine efflux in the prefrontal cortex [41], demonstrating its effects on modulating reward system activity. Finally, based on the role of an ERβ agonist in promoting reinstatement of cocaine seeking in OVX females [39], PHTPP was predicted to block reinstatement of alcohol seeking in intact females. While our results are only at the trend level, higher doses may uncover mediation of reinstatement by ERβ. In addition, specific comparison between the contribution of ERβ and ERα in modulating ethanol-motivated behavior is warranted.
In addition to direct effects in modulating their cognate receptors, gonadal hormones can influence several other systems, and previous and present findings point to an interaction between organizational and activational effects of hormones. Though much remains to be determined regarding which systems are involved, identification of circuits that are differentially activated as a function of sex during drug seeking are of particular interest. For example, females show greater Fos expression, a marker of neural activity, in the nucleus accumbens and VTA in response to cocaine-related cues compared to males; however, Fos expression in the prelimbic prefrontal cortex, accumbens shell, and basolateral amygdala were correlated with the magnitude of cocaine cue-induced reinstatement regardless of sex [100]. Parallel studies in ethanol-seeking males and females would be crucial in identifying key regions to target in determining sex and sex hormone-related changes in ethanol-motivated behavior. Important to the present investigation, there is considerable overlap between gonadal and stress hormones, and pronounced sex differences in response to stress [101, 102]; thus, future studies will be aimed at determining the mechanisms underlying stress- and cue-related increased in ethanol-motivated behavior as a function of sex.
Finally, though several studies have focused on the role of gonadal sex on drug-motivated behavior, a role for genetic sex in differential drug-motivated behavior in males and females has been suggested [103, 104]. For example, using the four core genotype mice [105, 106], a strain that allows for the dissociation of chromosomal and gonadal sex, sex chromosome complement has been shown to underlie sex differences in habit formation to both food [107] and alcohol [108] reinforcers. Barker and colleagues also found that gonadal phenotype influenced sex differences in ethanol intake, though this was in a limited ethanol access paradigm as opposed to operant self-administration as used in the current study. Indeed, the genetic sex-driven difference in habit formation may be more related to the reinstatement test described here [108]. Thus, further use of a genetic animal model could be utilized in subsequent studies to disentangle the role of sex chromosomes and the organizational effects of gonadal hormones in ethanol-motivated behavior.
Taken together, our findings that gonadal hormones at least partially mediate, but do not totally account for the sex differences evident in ethanol self-administration, and circulating gonadal hormones have little effect on the reinstatement of ethanol seeking provide a foundation for future studies examining the neuronal mechanisms underlying sex differences in ethanol drinking and seeking.
Highlights.
Chronic estradiol treatment significantly increased ethanol drinking in female rats.
Chronic testosterone treatment virtually abolished ethanol drinking in male rats.
Gonadectomy alone did not eliminate the robust sex differences in ethanol-motivated behavior.
Neither chronic nor acute hormone manipulations altered reinstatement of ethanol seeking.
Acknowledgments
The authors wish to thank Dr. Marianne Seney, Dr. Erin Kirschmann, Jenna Parrish, and Bryan McElroy for their technical and advisory assistance in these projects. This research was supported by The Pennsylvania Department of Health, R01DA042029, R21AA025547 (all to MMT), and DSF Charitable Foundation 132RA03 (MLB).
Footnotes
Conflicts of Interest
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. J Stud Alcohol. 1997;58:464–73. doi: 10.15288/jsa.1997.58.464. [DOI] [PubMed] [Google Scholar]
- 2.Nelson CB, Heath AC, Kessler RC. Temporal progression of alcohol dependence symptoms in the U.S. household population: results from the National Comorbidity Survey. J Consult Clin Psychol. 1998;66:474–83. doi: 10.1037//0022-006x.66.3.474. [DOI] [PubMed] [Google Scholar]
- 3.Keyes KM, Li G, Hasin DS. Birth cohort effects and gender differences in alcohol epidemiology: a review and synthesis. Alcohol Clin Exp Res. 2011;35:2101–12. doi: 10.1111/j.1530-0277.2011.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson DA, Goldstein RB, Saha TD, Grant BF. Changes in alcohol consumption: United States, 2001–2002 to 2012–2013. Drug Alcohol Depend. 2015;148:56–61. doi: 10.1016/j.drugalcdep.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diehl A, Croissant B, Batra A, Mundle G, Nakovics H, Mann K. Alcoholism in women: is it different in onset and outcome compared to men? Eur Arch Psychiatry Clin Neurosci. 2007;257:344–51. doi: 10.1007/s00406-007-0737-z. [DOI] [PubMed] [Google Scholar]
- 6.Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. J Stud Alcohol. 1999;60:252–60. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- 7.King AC, Bernardy NC, Hauner K. Stressful events, personality, and mood disturbance: gender differences in alcoholics and problem drinkers. Addict Behav. 2003;28:171–87. doi: 10.1016/s0306-4603(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 8.Hartwell EE, Ray LA. Sex moderates stress reactivity in heavy drinkers. Addict Behav. 2013;38:2643–6. doi: 10.1016/j.addbeh.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver SB, Sirota AD. Alcohol cue reactivity and mood induction in male and female alcoholics. J Stud Alcohol. 1994;55:487–94. doi: 10.15288/jsa.1994.55.487. [DOI] [PubMed] [Google Scholar]
- 10.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2008. Bethesda, MD: National Institute on Drug Abuse; 2009. [Google Scholar]
- 11.Swendsen JD, Merikangas KR, Canino GJ, Kessler RC, Rubio-Stipec M, Angst J. The comorbidity of alcoholism with anxiety and depressive disorders in four geographic communities. Compr Psychiatry. 1998;39:176–84. doi: 10.1016/s0010-440x(98)90058-x. [DOI] [PubMed] [Google Scholar]
- 12.Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–9. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- 13.Truxell EM, Molina JC, Spear NE. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol Clin Exp Res. 2007;31:755–65. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- 14.Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44:547–54. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romeo RD. Puberty: a period of both organizational and activational effects of steroid hormones on neurobehavioural development. J Neuroendocrinol. 2003;15:1185–92. doi: 10.1111/j.1365-2826.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- 16.Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–74. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Bartke A, Steele RE, Musto N, Caldwell BV. Fluctuations in plasma testosterone levels in adult male rats and mice. Endocrinology. 1973;92:1223–8. doi: 10.1210/endo-92-4-1223. [DOI] [PubMed] [Google Scholar]
- 18.Rowe PH, Lincoln GA, Racey PA, Lehane J, Stephenson MJ, Shenton JC, et al. Temporal variations of testosterone levels in the peripheral blood plasma of men. J Endocrinol. 1974;61:63–73. doi: 10.1677/joe.0.0610063. [DOI] [PubMed] [Google Scholar]
- 19.Evans SM, Foltin RW. Pharmacokinetics of repeated doses of intravenous cocaine across the menstrual cycle in rhesus monkeys. Pharmacol Biochem Behav. 2006;83:56–66. doi: 10.1016/j.pbb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–74. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- 21.Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- 22.Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–83. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- 23.Carroll HA, Lustyk MK, Larimer ME. The relationship between alcohol consumption and menstrual cycle: a review of the literature. Arch Womens Ment Health. 2015;18:773–81. doi: 10.1007/s00737-015-0568-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witt ED. Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol Teratol. 2007;29:81–95. doi: 10.1016/j.ntt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–38. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- 26.Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68:641–6. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 27.Ford MM, Eldridge JC, Samson HH. Microanalysis of ethanol self-administration: estrous cycle phase-related changes in consumption patterns. Alcohol Clin Exp Res. 2002;26:635–43. [PubMed] [Google Scholar]
- 28.Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF. Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res. 1998;22:1564–9. [PubMed] [Google Scholar]
- 29.Ford MM, Eldridge JC, Samson HH. Determination of an estradiol dose-response relationship in the modulation of ethanol intake. Alcohol Clin Exp Res. 2004;28:20–8. doi: 10.1097/01.ALC.0000108647.62718.5A. [DOI] [PubMed] [Google Scholar]
- 30.Vetter-O’Hagen CS, Spear LP. The effects of gonadectomy on sex- and age-typical responses to novelty and ethanol-induced social inhibition in adult male and female Sprague-Dawley rats. Behav Brain Res. 2011;227:224–32. doi: 10.1016/j.bbr.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vetter-O’Hagen CS, Spear LP. The effects of gonadectomy on age- and sex-typical patterns of ethanol consumption in Sprague-Dawley rats. Alcohol Clin Exp Res. 2011;35:2039–49. doi: 10.1111/j.1530-0277.2011.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vetter-O’Hagen CS, Sanders KW, Spear LP. Evidence for suppressant effects of testosterone on sex-typical ethanol intake in male Sprague-Dawley rats. Behav Brain Res. 2011;224:403–7. doi: 10.1016/j.bbr.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cailhol S, Mormede P. Sex and strain differences in ethanol drinking: effects of gonadectomy. Alcohol Clin Exp Res. 2001;25:594–9. [PubMed] [Google Scholar]
- 34.Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229:453–76. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertholomey ML, Nagarajan V, Torregrossa MM. Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology (Berl) 2016;233:2277–87. doi: 10.1007/s00213-016-4278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl) 2011;216:53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larson EB, Roth ME, Anker JJ, Carroll ME. Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol Biochem Behav. 2005;82:98–108. doi: 10.1016/j.pbb.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Larson EB, Carroll ME. Estrogen receptor beta, but not alpha, mediates estrogen’s effect on cocaine-induced reinstatement of extinguished cocaine-seeking behavior in ovariectomized female rats. Neuropsychopharmacology. 2007;32:1334–45. doi: 10.1038/sj.npp.1301249. [DOI] [PubMed] [Google Scholar]
- 40.Alfinito PD, Chen X, Atherton J, Cosmi S, Deecher DC. ICI 182,780 penetrates brain and hypothalamic tissue and has functional effects in the brain after systemic dosing. Endocrinology. 2008;149:5219–26. doi: 10.1210/en.2008-0532. [DOI] [PubMed] [Google Scholar]
- 41.Dazzi L, Seu E, Cherchi G, Barbieri PP, Matzeu A, Biggio G. Estrous cycle-dependent changes in basal and ethanol-induced activity of cortical dopaminergic neurons in the rat. Neuropsychopharmacology. 2007;32:892–901. doi: 10.1038/sj.npp.1301150. [DOI] [PubMed] [Google Scholar]
- 42.Santollo J, Katzenellenbogen BS, Katzenellenbogen JA, Eckel LA. Activation of ERalpha is necessary for estradiol’s anorexigenic effect in female rats. Horm Behav. 2010;58:872–7. doi: 10.1016/j.yhbeh.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertholomey ML, Verplaetse TL, Czachowski CL. Alterations in ethanol seeking and self-administration following yohimbine in selectively bred alcohol-preferring (P) and high alcohol drinking (HAD-2) rats. Behav Brain Res. 2013;238:252–8. doi: 10.1016/j.bbr.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen R, Osterhaus G, McKerchar T, Fowler SC. The role of exogenous testosterone in cocaine-induced behavioral sensitization and plasmalemmal or vesicular dopamine uptake in castrated rats. Neurosci Lett. 2003;351:161–4. doi: 10.1016/j.neulet.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 45.Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- 46.NIH; Services DoHaH, editor. NIH guidelines on the inclusion of women and minorities as subjects in clinical research. 1994. [Google Scholar]
- 47.NIH; Services DoHaH, editor. Consideration of Sex as a Biological Variable in NIH-funded Research. 2015. [Google Scholar]
- 48.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–72. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Becker JB, Koob GF. Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev. 2016;68:242–63. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchis R, Esquifino A, Guerri C. Chronic ethanol intake modifies estrous cyclicity and alters prolactin and LH levels. Pharmacol Biochem Behav. 1985;23:221–4. doi: 10.1016/0091-3057(85)90560-x. [DOI] [PubMed] [Google Scholar]
- 51.Eskay RL, Ryback RS, Goldman M, Majchrowicz E. Effect of chronic ethanol administration on plasma levels of LH and the estrous cycle in the female rat. Alcohol Clin Exp Res. 1981;5:204–6. doi: 10.1111/j.1530-0277.1981.tb04889.x. [DOI] [PubMed] [Google Scholar]
- 52.Mello NK, Bree MP, Mendelson JH, Ellingboe J, King NW, Sehgal P. Alcohol self-administration disrupts reproductive function in female macaque monkeys. Science. 1983;221:677–9. doi: 10.1126/science.6867739. [DOI] [PubMed] [Google Scholar]
- 53.Ryback RS. Chronic alcohol consumption and menstruation. JAMA. 1977;238:2143. [PubMed] [Google Scholar]
- 54.Almeida OF, Shoaib M, Deicke J, Fischer D, Darwish MH, Patchev VK. Gender differences in ethanol preference and ingestion in rats. The role of the gonadal steroid environment. J Clin Invest. 1998;101:2677–85. doi: 10.1172/JCI1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ford MM, Eldridge JC, Samson HH. Ethanol consumption in the female Long-Evans rat: a modulatory role of estradiol. Alcohol. 2002;26:103–13. doi: 10.1016/s0741-8329(01)00203-8. [DOI] [PubMed] [Google Scholar]
- 56.Forger NG, Morin LP. Reproductive state modulates ethanol intake in rats: effects of ovariectomy, ethanol concentration, estrous cycle and pregnancy. Pharmacol Biochem Behav. 1982;17:323–31. doi: 10.1016/0091-3057(82)90087-9. [DOI] [PubMed] [Google Scholar]
- 57.Sherrill LK, Koss WA, Foreman ES, Gulley JM. The effects of pre-pubertal gonadectomy and binge-like ethanol exposure during adolescence on ethanol drinking in adult male and female rats. Behav Brain Res. 2011;216:569–75. doi: 10.1016/j.bbr.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Konkle AT, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 2011;152:223–35. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lenz B, Muller CP, Stoessel C, Sperling W, Biermann T, Hillemacher T, et al. Sex hormone activity in alcohol addiction: integrating organizational and activational effects. Prog Neurobiol. 2012;96:136–63. doi: 10.1016/j.pneurobio.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 60.Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005;179:662–72. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- 61.Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, et al. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 2005;182:245–52. doi: 10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- 62.Fattore L, Spano MS, Altea S, Fadda P, Fratta W. Drug- and cue-induced reinstatement of cannabinoid-seeking behaviour in male and female rats: influence of ovarian hormones. Br J Pharmacol. 2010;160:724–35. doi: 10.1111/j.1476-5381.2010.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruda-Kucerova J, Amchova P, Babinska Z, Dusek L, Micale V, Sulcova A. Sex Differences in the Reinstatement of Methamphetamine Seeking after Forced Abstinence in Sprague-Dawley Rats. Front Psychiatry. 2015;6:91. doi: 10.3389/fpsyt.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cox BM, Young AB, See RE, Reichel CM. Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology. 2013;38:2343–53. doi: 10.1016/j.psyneuen.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- 66.Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–61. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Filova B, Malinova M, Babickova J, Tothova L, Ostatnikova D, Celec P, et al. Effects of testosterone and estradiol on anxiety and depressive-like behavior via a non-genomic pathway. Neurosci Bull. 2015;31:288–96. doi: 10.1007/s12264-014-1510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lunga P, Herbert J. 17Beta-oestradiol modulates glucocorticoid, neural and behavioural adaptations to repeated restraint stress in female rats. J Neuroendocrinol. 2004;16:776–85. doi: 10.1111/j.1365-2826.2004.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 2001;74:435–40. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 70.Bitran D, Kellogg CK, Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Horm Behav. 1993;27:568–83. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- 71.Hodosy J, Zelmanova D, Majzunova M, Filova B, Malinova M, Ostatnikova D, et al. The anxiolytic effect of testosterone in the rat is mediated via the androgen receptor. Pharmacol Biochem Behav. 2012;102:191–5. doi: 10.1016/j.pbb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 72.Mattila M, Seppala T, Mattila MJ. Anxiogenic effect of yohimbine in healthy subjects: comparison with caffeine and antagonism by clonidine and diazepam. Int Clin Psychopharmacol. 1988;3:215–29. doi: 10.1097/00004850-198807000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 74.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety- like behavior of wildtype, but not estrogen receptor beta knockout, mice. Behav Neurosci. 2008;122:974–81. doi: 10.1037/a0012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Borrow AP, Handa RJ. Estrogen Receptors Modulation of Anxiety-Like Behavior. Vitam Horm. 2017;103:27–52. doi: 10.1016/bs.vh.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kalil B, Leite CM, Carvalho-Lima M, Anselmo-Franci JA. Role of sex steroids in progesterone and corticosterone response to acute restraint stress in rats: sex differences. Stress. 2013;16:452–60. doi: 10.3109/10253890.2013.777832. [DOI] [PubMed] [Google Scholar]
- 77.Lo MJ, Wang PS. Relative and combined effects of estradiol and prolactin on corticosterone secretion in ovariectomized rats. Chin J Physiol. 2003;46:103–9. [PubMed] [Google Scholar]
- 78.Ajdzanovic V, Jaric I, Zivanovic J, Filipovic B, Ristic N, Miler M, et al. Testosterone application decreases the capacity for ACTH and corticosterone secretion in a rat model of the andropause. Acta Histochem. 2015;117:528–35. doi: 10.1016/j.acthis.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 79.Turcu A, Smith JM, Auchus R, Rainey WE. Adrenal androgens and androgen precursors-definition, synthesis, regulation and physiologic actions. Compr Physiol. 2014;4:1369–81. doi: 10.1002/cphy.c140006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barakat R, Oakley O, Kim H, Jin J, Ko CJ. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2016;49:488–96. doi: 10.5483/BMBRep.2016.49.9.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86:225–30. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 82.Feltenstein MW, Byrd EA, Henderson AR, See RE. Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology. 2009;34:343–52. doi: 10.1016/j.psyneuen.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol. 2007;15:472–80. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- 84.Finn DA, Beckley EH, Kaufman KR, Ford MM. Manipulation of GABAergic steroids: Sex differences in the effects on alcohol drinking- and withdrawal-related behaviors. Horm Behav. 2010;57:12–22. doi: 10.1016/j.yhbeh.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- 86.Follesa P, Biggio F, Talani G, Murru L, Serra M, Sanna E, et al. Neurosteroids, GABAA receptors, and ethanol dependence. Psychopharmacology (Berl) 2006;186:267–80. doi: 10.1007/s00213-005-0126-0. [DOI] [PubMed] [Google Scholar]
- 87.Ogilvie KM, Rivier C. Gender difference in hypothalamic-pituitary-adrenal axis response to alcohol in the rat: activational role of gonadal steroids. Brain Res. 1997;766:19–28. doi: 10.1016/s0006-8993(97)00525-8. [DOI] [PubMed] [Google Scholar]
- 88.Lammers SM, Mainzer DE, Breteler MH. Do alcohol pharmacokinetics in women vary due to the menstrual cycle? Addiction. 1995;90:23–30. doi: 10.1046/j.1360-0443.1995.901235.x. [DOI] [PubMed] [Google Scholar]
- 89.Marshall AW, Kingstone D, Boss M, Morgan MY. Ethanol elimination in males and females: relationship to menstrual cycle and body composition. Hepatology. 1983;3:701–6. doi: 10.1002/hep.1840030513. [DOI] [PubMed] [Google Scholar]
- 90.McGinnis MY, Krey LC, MacLusky NJ, McEwen BS. Steroid receptor levels in intact and ovariectomized estrogen-treated rats: an examination of quantitative, temporal and endocrine factors influencing the efficacy of an estradiol stimulus. Neuroendocrinology. 1981;33:158–65. doi: 10.1159/000123222. [DOI] [PubMed] [Google Scholar]
- 91.Rose’Meyer RB, Mellick AS, Garnham BG, Harrison GJ, Massa HM, Griffiths LR. The measurement of adenosine and estrogen receptor expression in rat brains following ovariectomy using quantitative PCR analysis. Brain Res Brain Res Protoc. 2003;11:9–18. doi: 10.1016/s1385-299x(02)00219-2. [DOI] [PubMed] [Google Scholar]
- 92.Moghadami S, Jahanshahi M, Sepehri H, Amini H. Gonadectomy reduces the density of androgen receptor-immunoreactive neurons in male rat’s hippocampus: testosterone replacement compensates it. Behav Brain Funct. 2016;12:5. doi: 10.1186/s12993-016-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wood RI, Newman SW. Intracellular partitioning of androgen receptor immunoreactivity in the brain of the male Syrian hamster: effects of castration and steroid replacement. J Neurobiol. 1993;24:925–38. doi: 10.1002/neu.480240706. [DOI] [PubMed] [Google Scholar]
- 94.Claessens F, Denayer S, Van Tilborgh N, Kerkhofs S, Helsen C, Haelens A. Diverse roles of androgen receptor (AR) domains in AR-mediated signaling. Nucl Recept Signal. 2008;6:e008. doi: 10.1621/nrs.06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–57. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 96.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav Brain Res. 2006;166:110–23. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 97.Pfaff DW, McEwen BS. Actions of estrogens and progestins on nerve cells. Science. 1983;219:808–14. doi: 10.1126/science.6297008. [DOI] [PubMed] [Google Scholar]
- 98.Igarashi M, Ibuki Y, Kubo H, Kamioka J, Yokota N, Ebara Y, et al. Mode and site of action of clomiphene. Am J Obstet Gynecol. 1967;97:120–3. doi: 10.1016/0002-9378(67)90604-7. [DOI] [PubMed] [Google Scholar]
- 99.Kahwanago I, Heinrichs WL, Herrmann WL. Estradiol “receptors” in hypothalamus and anterior pituitary gland: inhibition of estradiol binding by SH-group blocking agents and clomiphene citrate. Endocrinology. 1970;86:1319–26. doi: 10.1210/endo-86-6-1319. [DOI] [PubMed] [Google Scholar]
- 100.Zhou L, Pruitt C, Shin CB, Garcia AD, Zavala AR, See RE. Fos expression induced by cocaine-conditioned cues in male and female rats. Brain Struct Funct. 2014;219:1831–40. doi: 10.1007/s00429-013-0605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ter Horst GJ, Wichmann R, Gerrits M, Westenbroek C, Lin Y. Sex differences in stress responses: focus on ovarian hormones. Physiol Behav. 2009;97:239–49. doi: 10.1016/j.physbeh.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 103.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sanchis-Segura C, Becker JB. Why we should consider sex (and study sex differences) in addiction research. Addict Biol. 2016;21:995–1006. doi: 10.1111/adb.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, et al. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–14. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Sex chromosome complement regulates habit formation. Nat Neurosci. 2007;10:1398–400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- 108.Barker JM, Torregrossa MM, Arnold AP, Taylor JR. Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. J Neurosci. 2010;30:9140–4. doi: 10.1523/JNEUROSCI.0548-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]