Abstract
Cys proteinases play important roles in plant cell development and senescence. A cDNA, AsNODf32, obtained by differential screening of a nodule cDNA library of the leguminous plant Chinese milk vetch (Astragalus sinicus), represents a nodule-specific Cys proteinase similar to that reported for the actinorhizal Alnus glutinosa-Flankia symbiosis. A characteristic feature of this proteinase is the presence of a putative vacuolar targetting signal, LQDA, within its propeptide. Expression of the AsNODf32 gene, which was studied on northern blots and in situ hybridization, showed good correlation with the onset of nodule senescence. In situ hybridization studies revealed that AsNODf32 was expressed in senescent-infected tissue at the base of the nodule, as well as in interzone II-III of the infected nodules. In addition to degrading old nodule tissues and bacteroids, AsNODf32 protein may be required as a component of tissue remodeling during nodule development.
Chinese milk vetch (Astragalus sinicus) is one of the most popular legumes used as a green manure in Asian countries including Japan, China, and Korea. The small sized plant has a symbiotic relationship with the soil bacterium Mesorhizobium huakuii (Murooka et al., 1993), which forms nitrogen-fixing, indeterminate-type nodules (Chen et al., 1991). The interaction between Chinese milk vetch and M. huakuii has been studied in a test tube nodulation system (Murooka et al., 1993). The taxonomical characterization of the symbiotic bacterial strain M. huakuii subsp. rengei B3 isolated from Chinese milk vetch cv Japan has been well established (Nuswantara et al., 1999). Xu and Murooka (1995) isolated and characterized a megaplasmid (pRhYM) of approximately 420 kbp in size from this bacterial strain. Further, a novel acidic exopolysaccharide and cyclic (1–2) β-glucan produced by M. huakuii that may function as a determinant in the plant-bacteria association has been characterized (Hisamatsu et al., 1997). In contrast with this accumulated information on the bacteria, little is yet known about the plant factors involved in the Chinese milk vetch nodulation system. In addition to the information on various leguminous plants (Mylona et al., 1995; Long, 1996; Cohn et al., 1998), it is known that a special set of plant genes (nodulin genes) are also activated during nodulation in Chinese milk vetch. Fujie et al. (1998) isolated more than 100 nodule-specific or nodule-enhanced cDNA clones of Chinese milk vetch, and classified them into 11 groups. Based on the nucleotide and deduced amino acid sequence homologies, some of these were identified as homologs of early nodulins, including ENOD2, ENOD3/14, ENOD40, and leghemoglobin (Mylona et al., 1995). In addition to these, a novel nodulin gene, AsNODc22, encoding an 18-kD protein with unknown function was identified (Fujie et al., 1998). The AsNODc22 protein was shown by immunofluorescence microscopy to be located along the cell wall of bacteria-infected cells.
In this work another nodule-specific cDNA clone of Chinese milk vetch, AsNODf32, has been characterized to encode the gene for Cys proteinase. This gene showed striking homology with that found in the actinorhizal plant, Alnus glutinosa, having Frankia-induced nodules (Goetting-Minesky and Mullin, 1994). Cys proteinases have recently been shown to be induced in plant systems undergoing programmed cell death (PCD), such as tracheary element differentiation in Zinnia elegans (Minami and Fukuda, 1995; Ye and Varner, 1996), certain forms of cell aging (Drake et al., 1996), and cells in plants (soybean) under oxidative stresses (Solomon et al., 1999). Identification of the involvement of a specific Cys proteinase in the development of leguminous nodules would be very interesting.
RESULTS
Detection and Cloning of the AsNODf32 cDNA from Chinese Milk Vetch cv Japan
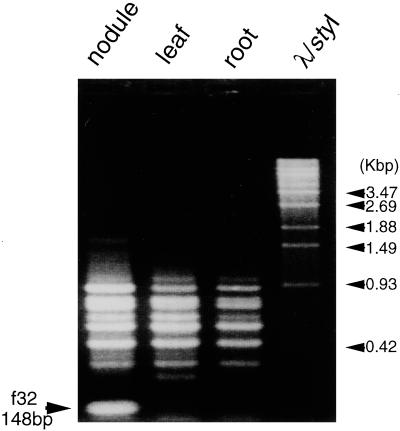
When products of the PCR amplification from RNAs of the Chinese milk vetch nodules with a combination of A21 and A22 primers (Bex) were compared with those from other tissues by differential display, a band of 148 bp in size was found to be specific to the nodules (Fig. 1). The fragment was cut out from the gel, cloned, and sequenced. The partial nucleotide sequence of this clone (pAsf32) showed significant homology with a 5′ region of the gene for Cys proteinases of various organisms in the databases. To obtain a full-length cDNA clone, a cDNA library prepared from Chinese milk vetch nodules (Fujie et al., 1998) was screened by plaque hybridization with pAsf32 DNA as the probe. Among 1.5 × 105 plaques, 10 positive clones were obtained, all of which contained a common 1.0- to 1.2-kbp insert. The nucleotide sequence determined for an approximately 1.2-kbp insert of one clone (AsNODf32) contained an open reading frame (ORF) of 343 amino acids (DDBJ accession no. AB040454). When the deduced amino acid sequence of this ORF was compared with those in the databases, the homology extended throughout the entire region of Cys proteinases of the papain superfamily (Barrett, 1986). Based on these results we decided that the nodule-specific AsNODf32 cDNA of Chinese milk vetch contained a full-length ORF encoding Cys proteinase of the papain superfamily.
Figure 1.
Detection of a nodule-specific DNA fragment by differential display. cDNAs synthesized along mRNAs isolated from either nodules, leaves, or roots of Chinese milk vetch were used as templates in PCR with a combination of A21 and A22 oligonucleotide primers (Bex). The PCR products were separated by agarose gel electrophoresis and compared with each other. Arrow indicates a 148-bp nodule-specific band (f32). Molecular markers are λ-DNA- digested with StyI.
Characterization of the AsNODf32 Gene
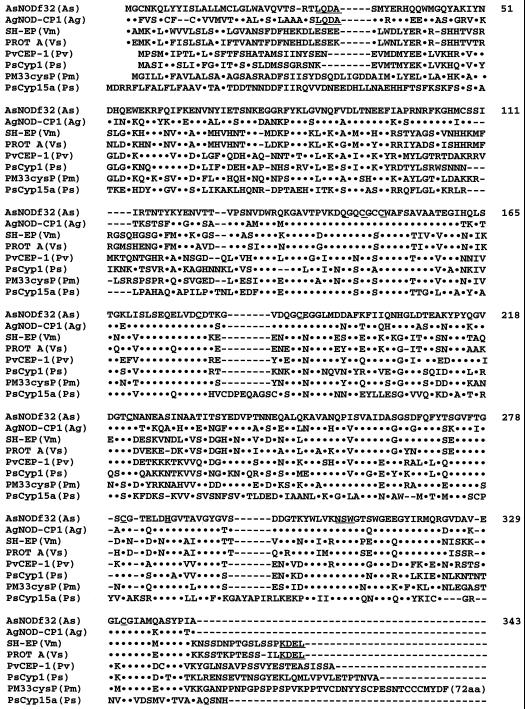
Figure 2 shows an alignment of the deduced amino acid sequence of AsNODf32 with those of Cys proteinases of the papain superfamily. Compared with these other Cys proteinases, amino acid residues (AsNODf32 numbering) involved in catalysis (C-149 and H-286), active-site formation (Q-143, N-307, S-308, and W-309), and disulfide bridges (C-146/C-189, C-180/C-222, and C-280/C-332) are maintained in the AsNODf32 sequence (Kamphius et al., 1985). In addition, the amino acids around the catalytic residues are very similar to those found in other Cys proteinases. The overall amino acid sequence of AsNODf32 showed the highest homology (69.4% identical) with that of AgNOD-CP1 from A. glutinosa, an actinorhizal plant with symbiotic, nitrogen-fixing actinomycete Frankia (Goetting-Minesky and Mullin, 1994). The majority of other plant Cys proteinases in the databases showed homology between 50.1% (PM33cysP, Pseudosuga menziesii; Tranbarger and Misra, 1996) and 34.4% (PsCyp15a, pea; Kardailsky and Brewin, 1996; Fig. 2); in comparison, this high value of homology found between a legume and a non-leguminous plant is very interesting. A characteristic feature of AsNODf32 Cys proteinase is the presence of a putative vacuole-targeting signal LQDA motif at the N-terminal region. This motif is also present in A. glutinosa enzyme, but is missing in Cys proteinases from leguminous plants such as vetch and Vigna mungo, which instead have the endoplasmic reticulum-targeting signal, KDEL (Denecke et al., 1992), at the C-terminal region as shown in Figure 2. Cys proteinases of the leguminous plants soybean (Kalinski et al., 1990), Phaseolus vulgaris (Sohlberg and Sussex, 1997), and pea (Kardailsky and Brewin, 1996) also do not possess the motifs. Therefore, AsNODf32 Cys proteinase seems to be functionally different from the leguminous Cys proteinases reported thus far.
Figure 2.
Alignment of the deduced amino acid sequence of AsNODf32 with those of Cys proteinases of the papain super family. Dots represent identical amino acid residues. Dashes represent blanks. Amino acid residues (AsNODf32 numbering) involved in catalysis (C-149 and H-286), active-site formation (Q-143, N-307, S-308, and W-309), and the disulfide bridges (C-146/C-189, C-180/C-222, and C-280/C-322) are underlined. A putative vacuole-targeting signal (LQDA) and the ER-targeting signal (KDEL) are also indicated by dotted underlines, respectively. As, Chinese milk vetch; Ag, A. glutinosa (Goetting-Minesky and Mullin, 1994); Pm, P. menziesii (Tranbarger and Misra, 1996); Ps, Pisum sativum (pea; Kardailsky and Brewin, 1996); Pv, P. vulgaris (Sohlberg and Sussex, 1997); Vm, Vigna mungo (Akasofu et al., 1989); Vs, vetch (Becker et al., 1997).
Genomic Hybridization and Copy Number of the AsNODf32 Gene
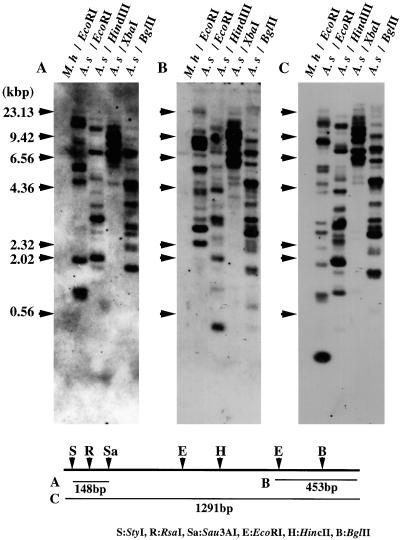
When the genomic DNA of Chinese milk vetch was digested with several restriction enzymes and hybridized with a full-length cDNA of AsNODf32 as the probe, many hybridizing bands appeared (Fig. 3C). Based on the banding patterns, especially that with XbaI, we assumed a multigene family consisting of five to seven members on the plant genome. Some of these bands disappeared in hybridization when a 148-bp 5′-terminal portion in pAsf32 was used as the probe (Fig. 3A), and others were missing when a 453-bp portion of the 3′-terminal region was used (Fig. 3B). Since the AsNODf32 protein seems to consist of composite domains (Kamphius et al., 1985), these results may suggest some structural variations in the N- and/or C-terminal regions of the protein. Otherwise, some may represent pseudogenes or truncated genes that are not expressed. None of the probes hybridized with the genomic DNA of the bacterial symbiont, M. huakuii, confirming the plant origin of the gene.
Figure 3.
Southern-blot analysis of Chinese milk vetch (A.s) and M. huakuii (M.h) genomic DNAs. Restriction enzymes used to digest DNA are as indicated. Probes used were a 148-bp N-terminal fragment (A), a 453-bp C-terminal fragment (B), and an entire region (1,291 bp, C) of AsNODf322 cDNA. Sequences used to generate probes A, B, and C are illustrated in the bottom section, relative to a restriction map of AsNODf32 cDNA. Arrows indicate the position of lambda DNA fragments digested with HindIII.
Tissue-Specific Expression of the AsNODf32 Gene
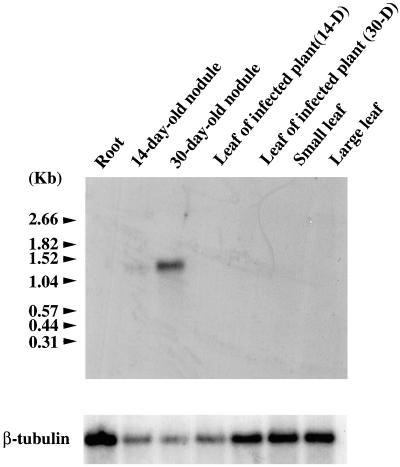
AsNODf32 cDNA was obtained as a nodule-specific clone by differential screening as described above. The AgNOD-CP1 gene was also shown to be expressed in the root nodules of the actinorhizal plant A. glutinosa. Therefore, we became interested in determining the detailed expression profile of the AsNODf32 gene during nodulation. When total RNA isolated from various tissues of Chinese milk vetch was subjected to northern-blot analysis, a characteristic tissue-specific and nodulation stage-specific expression of the AsNODf32 gene was revealed (Fig. 4). The gene expression was first detected in young root nodules 14 d after bacterial infection, and the transcripts accumulated to a fives-times higher level in 30-d-old root nodules. The size of the transcript (approximately 1.3 knt) was in good accordance with that expected from the gene sequence. Whether the plant was infected or not, roots nor leaves showed any discernible signal of the gene expression. According to Nap and Bisseling (1990), AsNODf32 can be designated as a late nodulin. Some Cys proteinase genes of leguminous plants, including vetch (pSK19; Becker et al., 1997), V. mungo (SH-EP; Akasofu et al., 1989), and P. vulgaris (PvCEP-1; Kardailsky and Brewin, 1996), were shown to be expressed in germinating seeds. Northern-blot analysis of total RNA isolated from Chinese milk vetch seeds 5, 7, and 10 d after imbibition with the probe of AsNODf32 DNA, respectively, showed no discernible hybridization signals (data not shown). Therefore, the AsNODf32 expression pattern was quite different from that of these leguminous Cys proteinase genes.
Figure 4.
Northern-blot analysis to detect AsNODf32 transcripts. Total RNA isolated from various tissues of Chinese milk vetch was hybridized with AsNODf32 cDNA as a probe. Arrows indicate positions of RNA size markers (Boehringer Mannheim, Basel). For control, the same filter was reprobed with the gene for β-tubulin (bottom).
Localization of the AsNODf32 Gene Products by in Situ Hybridization
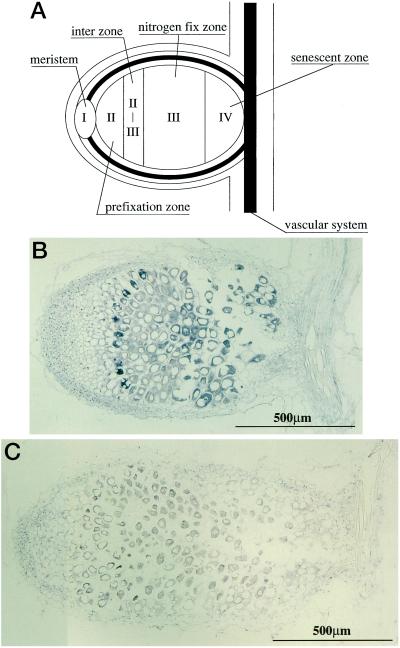
The characteristic pattern of the AsNODf32 gene expression prompted us to localize the expression area in the nodule tissues of Chinese milk vetch. In situ hybridization with sense and antisense digoxigenin-labeled probes was used to visualize the localized expression pattern on longitudinal sections of nodules as presented in Figure 5. An indeterminate type nodule of Chinese milk vetch consists of five distinct regions (Vasse et al., 1990; Mylona et al., 1995): I, nodule meristem; II, prefixation zone; II-III, interzone; III, nitrogen fixation zone; and IV, senescence zone (Fig. 5A). Strong signals were seen with the antisense probe in the cells of the senescence zone (IV; Fig. 5B). The transcript levels gradually decreased from the senescence zone (IV) to the interzone (II-III), where the expression increased again, forming one or two layers of cells with signals. Cells in regions I and II did not show any discernible signals. No such characteristic hybridization patterns could be detected with the sense probe as a control (Fig. 5C). These results indicated that the gene for AsNODf32 is expressed differentially in the nodule tissues, especially in senescent cells, reflecting its roles in the development, maintenance, and re-formation of the nodules.
Figure 5.
In situ hybridization analysis of the AsNODf32 gene expression. Median longitudinal sections of Chinese milk vetch nodules infected with M. huakuii (30 d post-infection) were hybridized with riboprobes containing digoxygenin. Hybridization signal was detected as a blue precipitate by staining with BCIP and NBT. A, Schematic representation of the five distinct regions (Vasse et al., 1990; Mylona et al., 1995) of an indeterminate type nodule of Chinese milk vetch. B, Nodule section hybridized with AsNODf32 antisense strand RNA probe. C, Nodule section hybridized with AsNODf32 sense strand RNA as the probe.
DISCUSSION
A nodule-specific cDNA clone obtained from Chinese milk vetch was found to encode Cys proteinase. According to the general amino acid sequence homology, as well as the conservation of amino acid residues involved in the proteolytic activity surrounding the active site and forming disulfide bridges, plant Cys proteinases are classified into the papain superfamily (Barrett, 1986). These proteinases play important roles in various aspects of plant development and senescence; in, for example, leaf abscission (Wittenbach et al., 1982), leaf senescence (Hensel et al., 1993), and ovary senescence (Vercher et al., 1989). Increased levels of Cys proteinase activity are also observed as a result of environmental stresses including dehydration (Guerrero et al., 1990), mechanical wounding (Linthorst et al., 1993; Lidgett et al., 1995), and exposure to low temperature (Schaffer and Fischer, 1988). The genes for Cys proteinases that have thus far been isolated and characterized from various plants and are summarized in Table I, where the genes are classified based on their structures, expression patterns, and supposed roles. Cys proteinases from the symbiotic associations are separated into the nodule-specific group and the group of others that are mainly involved in the degradation and recycling of storage proteins. This classification was supported by the fact that antibody against Cys proteinase SH-EP of V. mungo did not cross react with AsNODf32 protein (Y. Naito and T. Yamada, unpublished data). The AsNODf32 gene is the one most similar to the AgNOD-CP1 gene (Goetting-Minesky and Mullin, 1994) not only in size, deduced amino acid sequence, and, notably, the presence of a putative vacuole-targetting signal, LQDA, at the N-terminal region of the protein product (Fig. 2), but also in the specific expression in root nodules. This is not surprising because the evolutionary relatedness of actinorrhizal and legume root nodules is suggested (Gaultieri and Bisseling, 2000). The PsCyp1 product of pea, which is also expressed in nodules, showed considerable amino acid sequence homology with AsNODf32 (44.6% identical), but the motif LQDA is absent; instead, it contains a characteristic 29-amino acid extension at the C terminus (Fig. 2). At present, there is no information about the presence of a gene corresponding to AsNODf32 in other leguminous plants.
Table I.
Classification of cysteine proteinases of the papain super family
| Type and Characteristic Features | Predicted Roles | Examples | Reference |
|---|---|---|---|
| From leguminous plants | |||
| A Nodule specific with an LQDA motif | Protein turnover, remodeling of tissues, cell cycle component, defence response to microbial invasion | AsNODf32 (Chinese milk vetch)AgNOD-CP1 (A. glutinosa) | Goetting-Minesky and Mullin (1994) |
| B Nodule specific without an LQDA motif | Degradation of senescent nodule cells and bacteroids | PsCyp1 (pea) | Kardailsy and Brewin (1996) |
| C Expressed in oil bodies localized in vacuoles | Storage protein degradation for recycling, binds to sylingilide elicitor | P34 (soybean) | Kalinski et al. (1990) |
| D Expressed in cotyledons with a KDEL motif | Degradation of storage protein in seeds | pSK19 (vetch)SH-EP (V. mungo) | Becker et al. (1997),Akasofu et al. (1989) |
| E Expressed in cotyledons without a KDEL motif | Degradation of storage protein in seeds | PvCEP-1 (P. vulgaris) | Sohlberg and Sussex (1997) |
| F Expressed in all tissues | Unknown | PsCyp15a (pea) | Kardailsky and Brewin (1996) |
| From non-leguminous plants | |||
| G With a signal peptide for transportation to vacuole | Degradation of a specific protein responding to fungal infection, tolerance mechanism to suboptimal conditions, involved in leaf senescence, peptide degradation in ripe fruits, and degradation of storage proteins during germination | LCYP-2 (tomato)ZMSsel (maize)P21 (petunia)pARP152 (Prunus armeniaca)PM33cysP (P. menziesii) | Linthorst et al. (1993),Griffiths et al. (1997),Tournaire et al. (1996),Mbeguie-A-Mbeguie et al. (1997),Tranbarger and Misra (1996) |
| H With a signal peptide for transportation to ER | Programmed cell death in flower senescence and expressed in ovules during seed formation | SEN11 (Hemerocallis sp.)O141 (Phalaenopsis sp.) | Guerrero et al. (1998),Nadeau et al. (1996) |
For the AgNOD-CP1, four possible roles have been previously proposed, though without any supportive evidence, as follows: (a) A defense response to root invasion by microorganisms, (b) a component of tissue remodeling in root and nodule tissues, (c) a cell cycle component, and (d) an element of protein turnover (Goetting-Minesky and Mullin, 1994). In this work we observed the characteristic expression patterns of the AsNODf32 gene by northern-blot and in situ hybridizations and found that the transcripts accumulated in the older nodules where the interzone and the senescence zone were the major regions of mRNA accumulation (Figs. 4 and 5). The interzone or early symbiotic zone is a region located between the infection zone (II) and the nitrogen-fixing zone (III; Vasse et al., 1990). The invaded host cells of this region are predominantly filled with amyloplasts. The bacteroids stop elongation and display cytoplasmic heterogeneity in this region. Therefore, the AsNODf32 product may be involved in tissue remodeling in nodule tissues and bacteroids at this stage of development. On the other hand, the mRNA levels were much higher in older nodules (Fig. 4) where the senescence zone was largely expanded (Fig. 5). In the senescence zone, plant cells and bacteroids are degraded. Thus the data presented here provide a strong link between Cys proteinase gene (AsNODf32) expression and cell senescence in nodules of Chinese milk vetch.
In the nodules of annual legumes like Chinese milk vetch, symbiosome membranes of senescing nodules fuse to form vacuoles containing debris of digested bacteroids, suggesting recovery of nitrogenous compounds (Mellor, 1989; Roth and Stacey, 1989). AsNODf32 nor AgNOD-CP1 contains either of the C-terminal tetrapeptides, HDEL and KDEL, which serve as endoplasmic reticulum-retention signals in plants (Denecke et al., 1992). Instead, these genes have a tetrapeptide motif within the propetide (amino acids 30–33; Fig. 2), which may correspond to signals that have been associated with vacuolar targeting in plant vacuolar hemaglutinin (LQDA) and yeast vacuolar carboxypeptidase Y (LQRP) (Chrispeels, 1991). For future study it would be very interesting to elucidate whether the Cys proteinase represented by AsNODf32 is transported and sorted into vacuoles through the secretory system of root nodule cells.
MATERIALS AND METHODS
Plant Materials
The seedlings of Chinese milk vetch (Astragalus sinicus cv Japan, Takayama Seed, Kyoto) were germinated under axenic conditions as described previously (Cho et al., 1995). Four- to 7-d-old seedlings with roots were inoculated with M. huakuii subsp. rengei B3 and allowed to grow for 3 to 4 weeks in nitrogen-free medium as described by Murooka et al. (1993). Harvested nodules, roots, and leaves were frozen in liquid nitrogen and kept at −80°C until used for isolation of DNA, RNA, or proteins.
Isolation of DNA and RNA
DNA was isolated and purified from M. huakuii by the method of Saito and Miura (1963). Plant genomic DNA was isolated by the method of Liu et al. (1995). Total RNA was prepared from nodules and other plant tissues using the method of Shirzadegan et al. (1991). Poly(A)+ RNA was prepared from total RNA using an Oligotex-dT30 super kit (Takara Shuzo, Kyoto).
Differential Display
Nodule-specific cDNA fragments were discriminated by the differential display method (Liang and Pardee, 1992; Yoshida et al., 1994). For the synthesis of the first strand of cDNA for PCR amplifications, 2 to 3 pmol of oligo(dT)15-18 was annealed to 0.5 μg of poly(A)+ RNA and treated with 40 units of avian myeloblastosis virus reverse transcriptase (Takara Shuzo) in the buffer recommended by the manufacturer at 42°C for 1 h. After heating at 95°C for 5 min, the reaction mixture was deprived of the primers by filtrating through Suprec-02 (Takara Shuzo). Two microliters of the reaction mixture containing 2 to 3 ng cDNA was used as template for the PCR amplification with oligonucleotide primers. Amplification reactions contained 10 mm Tris [tris(hydroxymethyl)-aminomethane] HCl, pH 8.3, 50 mm KCl, 1.5 mm MgCl2, 200 μm dNTP, 100 pmol of oligonucleotides (A21 and A22, Bex, Tokyo), and 2.5 units of ExTaq DNA polymerase (Takara Shuzo) in a 50-μL total volume. Amplifications were performed for four cycles (at 92°C for 30 s; 25°C for 1 min; and 72°C for 3 min), and for 30 cycles (at 85°C for 5 s; 92°C for 25 s; 45°C for 1 min, and 72°C for 3 min), with a final extension for 5 min at 72°C. Products of the PCR amplification from RNAs of nodules and other tissues were compared by 2.0% (w/v) agarose gel electrophoresis. Bands that appeared for only nodule cDNAs were identified as nodule-specific transcripts.
Construction of a cDNA Library and Screening of Nodule-Specific Clones
A cDNA library was constructed using a Time-Saver cDNA synthesis kit (Pharmacia Biotech, Uppsala) with reverse-transcribed poly(A)+ RNA from 3-week-old nodules ligated to a lambda-gt10 vector and a Giga-pack Gold packaging kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. Oligo(dT) primers and EcoRI/NotI adapters were used in the construction of the library. Specific DNA fragments obtained from differential display were labeled with fluorescein (Gene Images kit, Amersham International, Buckinghamshire, UK) to be used as probes for screening. The phage DNA of the positive clones was cut with EcoRI, and the insert was cloned into the EcoRI site of pBluescript II SK+ vector (Stratagene). Both strands of the positive cDNA clone (AsNODf32) were sequenced using a Cy5 Automated Sequencing kit and an ALFred automated DNA sequencer (Pharmacia Biotech).
Genomic Southern Blotting
Genomic DNAs of Chinese milk vetch and M. huakuii were digested with restriction endonucleases, separated by agarose gel electrophresis (Sambrook et al., 1989), and blotted onto a Hybond-N membrane (Amersham International). A fluorescein isothiocianate-labeled probe of AsNODf32 DNA was prepared with a Gene Image kit (Amersham International) and hybridized to the blotted membranes at 60°C according to the manufacturer's instructions. The hybridized membrane was washed with 0.2× SSC (20× SSC is 1.5 m NaCl and 0.5 m trisodium citrate, pH 7.2) and 0.1% (w/v) SDS at 60°C. The hybridization signals were detected by exposing them onto film (RX-U, Fuji Film, Tokyo).
Northern Blotting
The total RNAs (20 μg) isolated from the nodules, roots, and leaves were size-separated on a 1% (w/v) agarose gel in 10 mm phosphate buffer, pH 7.0, and transferred to Hybond-N nylon filters. A 32P-labeled probe of AsNODf32 DNA was prepared using a Megaprime DNA labeling system (Amersham International) per the manufacturer's instructions. The membrane was hybridized with the probe in 50% (w/v) formamide, 5× SSC, 50 mm sodium phosphate (pH 7.0), 0.1% (w/v) SDS, 50 μg/mL salmon sperm DNA, and 1× Denhardt's at 45°C for 12 h. The blots were washed at high stringency and exposed to x-ray film under an intensifying screen at −80°C. The filter was reprobed with a cDNA clone of β-tubulin as a control, as described elsewhere (Fujie et al., 1998).
In Situ Hybridization
Digoxigenin-labeled riboprobes were synthesized from linearized plasmids using T7 or T3 promoters of the pBluescript II SK+ vector. Labeling was performed essentially as recommended by the supplier (Boehringer Mannheim). Nodules were fixed in 4% (w/v) formaldehyde and 0.1% (w/v) glutaraldehyde, dehydrated in a graded ethanol series, embedded in paraffin, and sectioned following standard protocols (De Block and Debrouwer, 1993). Slides with sections were hybridized with riboprobes at 42°C for 16 h and the signal was visualized as alkaline phosphatase activity using bromochloroindophenol and nitrobluetetrazolium as substrates.
ACKNOWLEDGMENTS
We thank Hiromichi Morikawa and Misa Takahashi (Graduate School of Science, Hiroshima University) and Hiroshi Kouchi (National Institute of Agricultural Resources) for helpful discussions and Tatsushi Okamura for providing antibody against SH-EP.
LITERATURE CITED
- Akasofu H, Yamaguchi D, Mistuhashi W, Minamikawa T. Nucleotide sequence of cDNA for sulfhydryl-endopeptidase (SH-EP) from cotyledons of germinating Vigna mungoseeds. Nucleic Acids Res. 1989;17:6733. doi: 10.1093/nar/17.16.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AJ. Cysteine proteinases. In: Dalling MJ, editor. Plant Proteolytic Enzymes. Vol. 1. Boca Raton, FL: CRC Press; 1986. pp. 1–16. [Google Scholar]
- Becker C, Senyuk VJ, Shutov AD, Nong VH, Fischer J, Horstmann C, Muentz K. Proteinase A, a storage-globulin-degrading endopeptidase of vetch (Vicia sativaL.) seeds is not involved in early steps of storage-protein mobilization. Eur J Biochem. 1997;248:304–312. doi: 10.1111/j.1432-1033.1997.00304.x. [DOI] [PubMed] [Google Scholar]
- Chen WX, Li GS, Qi YL, Wang ET, Wang HL, Yuan HL, Li L. Rhizobium huakuii sp. Nov. isolated from the root nodules of Astragalus sinicus. Int J Syst Bacteriol. 1991;41:275–280. [Google Scholar]
- Cho HJ, Xu Y, Murooka Y. Formation of adventitious shoots and plant regeneration by culture of cotyledon segment in Astragalus sinicus(Chinese milk vetch) Plant Tissue Culture Lett. 1995;12:87–90. [Google Scholar]
- Chrispeels MJ. Sorting of proteins in the secretory system. Annu Rev Plant Physiol Mol Biol. 1991;42:21–53. [Google Scholar]
- Cohn J, Day RB, Stacey G. Legume nodule organogenesis. Trends Plant Sci. 1998;3:100–110. [Google Scholar]
- De Block M, Debrouwer D. RNA-RNA in situ hybridization using digoxigenin-labeled probes: the use of high-molecular-weight polyvinyl-alcohol in the alkaline-phosphatase indoxyl-nitroblue tetrazolium reaction. Anal Biochem. 1993;215:86–89. doi: 10.1006/abio.1993.1558. [DOI] [PubMed] [Google Scholar]
- Denecke J, De Rycke R, Botterman J. Plant and mammalian sorting signals for protein retention in the endoplasmic reticulum contain a conserved epitope. EMBO J. 1992;11:2345–2355. doi: 10.1002/j.1460-2075.1992.tb05294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake R, John I, Farrell A, Cooper W, Schuch W, Grierson D. Isolation and analysis of cDNAs encoding tomato cysteine proteases expressed during leaf senescence. Plant Mol Biol. 1996;30:755–767. doi: 10.1007/BF00019009. [DOI] [PubMed] [Google Scholar]
- Fujie M, Nakanishi Y, Murooka Y, Yamada T. AsNODc22, a novel nodulin gene of Astragalus sinicus, encodes a protein that localizes along the cell wall of bacteria-induced cells in a nodule. Plant Cell Physiol. 1998;39:846–852. doi: 10.1093/oxfordjournals.pcp.a029443. [DOI] [PubMed] [Google Scholar]
- Gaultieri T, Bisseling T. The evolution of nodulation. Plant Mol Biol. 2000;42:181–194. [PubMed] [Google Scholar]
- Goetting-Minesky P, Mullin BC. Differential gene expression in an actinorhizal symbiosis: evidence for a nodule-specific cysteine proteinase. Proc Natl Acad Sci USA. 1994;91:9891–9895. doi: 10.1073/pnas.91.21.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths CM, Hosken SE, Oliver D, Chojecki AJS, Thomas H. Sequencing, expression pattern and RFLP mapping of a senescence-enhanced cDNA from Zea mayswith high homology to oryzain gamma and aleurin. Plant Mol Biol. 1997;34:815–821. doi: 10.1023/a:1005896713830. [DOI] [PubMed] [Google Scholar]
- Guerrero C, de la Calle M, Reid MS, Valpuesta V. Analysis of the expression of two thiolprotease genes from daylily (Hemerocallisspp.) during flower senescence. Plant Mol Biol. 1998;36:565–571. doi: 10.1023/a:1005952005739. [DOI] [PubMed] [Google Scholar]
- Guerrero FD, Jones JT, Mullet JE. Turgor-responsive gene transcription and RNA levels increase rapidly when pea shoots are wilted: sequence and expression of three inducible genes. Plant Mol Biol. 1990;15:11–26. doi: 10.1007/BF00017720. [DOI] [PubMed] [Google Scholar]
- Hensel LL, Grbic CSV, Baumgarten DA, Bleecker AB. Developmental and age-related processes that influence the longevity and senescence of photosynthetic tissues in Arabidopsis. Plant Cell. 1993;5:553–564. doi: 10.1105/tpc.5.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamatsu M, Nomura S, Shutsrirung A, Obata H, Teranishi K, Yamada T, Nuswantara S, Yamashita M, Murooka Y. Structural characterization of a new acidic exopolysaccharide and cyclic (1–2) β-glucan produced by Rhizobium huakuii forming nodules on Astragalus sinicus. J Ferment Bioeng. 1997;83:315–320. [Google Scholar]
- Kalinski A, Weisemann JM, Matthews BF, Herman EM. Molecular cloning of a protein associated with soybean seed oilbodies that is similar to thiol proteases of the papain family. J Biol Chem. 1990;265:13843–13848. [PubMed] [Google Scholar]
- Kamphius IG, Drench J, Baker EN. Thiol proteases: comparative studies based on the amino acid sequence information for cathepsin B and H, and stem bromelain. J Mol Biol. 1985;182:317–329. doi: 10.1016/0022-2836(85)90348-1. [DOI] [PubMed] [Google Scholar]
- Kardailsky IV, Brewin NJ. Expression of cysteine protease genes in pea nodule development and senescence. Mol Plant-Microbe Interact. 1996;9:689–695. doi: 10.1094/mpmi-9-0689. [DOI] [PubMed] [Google Scholar]
- Liang P, Pardee AB. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Lidgett AJ, Moran M, Wong KA, Furze J, Rhodes MJ, Hamill JD. Isolation and expression pattern of a cDNA encoding a cathepsin B-like protease from Nicotiana rustica. Plant Mol Biol. 1995;29:379–384. doi: 10.1007/BF00043660. [DOI] [PubMed] [Google Scholar]
- Linthorst HJM, van der Does C, Van Can JA, Bol JF. Nucleotide sequence of a cDNA clone encoding tomato (Lycopersicon esculentum) cysteine proteinase. Plant Physiol. 1993;101:705–706. doi: 10.1104/pp.101.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YG, Mitsukawa N, Oosumi I, Whittier RF. Efficient isolation and mapping of Arabidopis thalianaT-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 1995;8:457–463. doi: 10.1046/j.1365-313x.1995.08030457.x. [DOI] [PubMed] [Google Scholar]
- Long SR. Rhizobiumsymbiosis: Nod factors in perspective. Plant Cell. 1996;8:1885–1898. doi: 10.1105/tpc.8.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbeguie-A-Mbeguie D, Gomez RM, Fils-Lycaon B. Sequence of AFTP-1, a cysteine proteinase from apricot fruit: gene expression during fruit ripening. Plant Physiol. 1997;115:1730. [Google Scholar]
- Mellor RB. Bacteroids in the Rhizobium-legume symbiosis inhabit a plant internal lytic compartment: implications for other microbial endosymbioses. J Exp Bot. 1989;40:831–839. [Google Scholar]
- Minami A, Fukuda H. Transient and specific expression of a cysteine endopeptidase associated with autolysis during differentiation of Zinniamesophyll cells into tracheary elements. Plant Cell Physiol. 1995;36:1599–1606. [PubMed] [Google Scholar]
- Murooka Y, Xu Y, Sanada H, Araki M, Morinaga T, Yokata A. Formation of root nodules by Rhizobium huakuii biobar. rengei bv. ov. on Astragalus sinicuscv. Japan J Ferment Bioeng. 1993;76:39–44. [Google Scholar]
- Mylona P, Pawlowski K, Bisseling T. Symbiotic nitrogen fixation. Plant Cell. 1995;7:869–885. doi: 10.1105/tpc.7.7.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau JA, Zhang XS, Li J, O'Neill SD. Ovule development: identification of stage-specific and tissue-specific cDNAs. Plant Cell. 1996;8:213–239. doi: 10.1105/tpc.8.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nap J-P, Bisseling T. Developmental biology of a plant-prokaryote symbiosis: the legume root nodule. Science. 1990;250:948–954. doi: 10.1126/science.250.4983.948. [DOI] [PubMed] [Google Scholar]
- Nuswantara S, Fujie M, Yamada T, Malek W, Inaba M, Kaneko Y, Murooka Y. Phylogenetic position of Mesorhizobium huakuii subsp. rengei, a symbiont of Astragalus sinicuscv. Japan J Biosci Bioeng. 1999;87:49–55. doi: 10.1016/s1389-1723(99)80007-3. [DOI] [PubMed] [Google Scholar]
- Roth LE, Stacey G. Bacterium release into host cells of nitrogen-fixing soybean nodules: the symbiosome membrane comes from three sources. Eur J Cell Biol. 1989;46:12–23. [PubMed] [Google Scholar]
- Saito H, Miura K. Preparation of transforming deoxyribonucleic acid by phenol treatment. Biochim Biophys Acta. 1963;72:619–629. [PubMed] [Google Scholar]
- Sambrook J, Frisch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schaffer MA, Fischer RL. Analysis of mRNAs that accumulate in response to low temperature identifies a thiol protease gene in tomato. Plant Physiol. 1988;87:431–436. doi: 10.1104/pp.87.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirzadegan M, Christie P, Seemann JR. An efficient method for isolation of RNA from tissue cultured plant cells. Nucleic Acids Res. 1991;19:6055. doi: 10.1093/nar/19.21.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlberg L, Sussex IM. Nucleotide sequence of a cDNA encoding a Cys proteinase from germinating bean cotyledons (PGR 97-055) Plant Physiol. 1997;113:1463. [Google Scholar]
- Solomon M, Belenghi B, Delledonne M, Menachem E, Levine A. The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell. 1999;11:431–443. doi: 10.1105/tpc.11.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournaire C, Kushnir S, Baum G, Inze D, Teyssendier dela Serve B, Renaudin JP. A thiol protease and an anionic peroxidase are induced by lowering cytokinins during callus growth in Petunia. Plant Physiol. 1996;111:159–168. doi: 10.1104/pp.111.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranbarger TJ, Misra S. Structure and expression of a developmentally regulated cDNA encoding a cysteine protease (pseudotzain) from Douglas fir. Gene. 1996;172:221–226. doi: 10.1016/0378-1119(96)00161-8. [DOI] [PubMed] [Google Scholar]
- Vasse J, de Billy F, Camut S, Truchet G. Correlationbetween ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J Bacteriol. 1990;172:4295–4306. doi: 10.1128/jb.172.8.4295-4306.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercher Y, Carrasco P, Carbonell J. Biochemical and histochemical detection of endoproteolytic activities involved in ovary senescence or fruit development in Pisum sativum. Physiol Plant. 1989;76:405–411. [Google Scholar]
- Wittenbach VA, Lin W, Hebert R. Vacuolar localization of proteases and degradation of chloroplasts in mesophyll protoplasts from senescing primary wheat leaves. Plant Physiol. 1982;69:98–102. doi: 10.1104/pp.69.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Murooka Y. A large plasmid isolated from Rhizobium huakuii bv. renge that includes genes for both nodulation of Astragalus sinicuscv. Japan and nitrogen fixation. J Ferment Bioeng. 1995;80:276–279. [Google Scholar]
- Ye ZH, Varner JE. Induction of cysteine and serine proteases during xylogenesis in Zinniaelegans. Plant Mol Biol. 1996;30:1233–1246. doi: 10.1007/BF00019555. [DOI] [PubMed] [Google Scholar]
- Yoshida KT, Naito S, Takeda G. cDNA cloning of regeneration-specific genes in rice by differential screening of randomly amplified cDNAs using RAPD primers. Plant Cell Physiol. 1994;35:1003–1009. [PubMed] [Google Scholar]