Abstract
Background
Low muscle mass and quality are associated with poor surgical outcomes. We evaluated CT measured psoas muscle density as a marker of muscle quality and physiologic reserve, and hypothesized that it would predict outcomes after enterocutaneous fistula repair (ECF).
Methods
We conducted a retrospective cohort study of patients 18 – 90 years old with ECF failing non-operative management requiring elective operative repair at Ohio State University from 2005 – 2016 that received a pre-operative abdomen/pelvis CT with intravenous contrast within 3 months of their operation. Psoas Hounsfield Unit average calculation (HUAC) were measured at the L3 level. 1 year leak rate, 90 day, 1 year, and 3 year mortality, complication risk, length of stay, dependent discharge, and 30 day readmission were compared to HUAC.
Results
100 patients met inclusion criteria. Patients were stratified into interquartile (IQR) ranges based on HUAC. The lowest HUAC IQR was our low muscle quality (LMQ) cutoff, and was associated with 1 year leak (OR 3.50, p < 0.01), 1 year (OR 2.95, p < 0.04) and 3 year mortality (OR 3.76, p < 0.01), complication risk (OR 14.61, p < 0.01), and dependent discharge (OR 4.07, p < 0.01) compared to non-LMQ patients.
Conclusions
Psoas muscle density is a significant predictor of poor outcomes in ECF repair. This readily available measure of physiologic reserve can identify patients with ECF on pre-operative evaluation that have significantly increased risk that may benefit from additional interventions and recovery time to mitigate risk before operative repair.
BACKGROUND
The frailty syndrome, which is the pathologic consequence of the decline in physiologic reserve, is associated with poor muscle mass, quality, and function, and leaves the body significantly more vulnerable to disease and injury(1, 2). Previous studies have shown that frailty, as measured by functional and mental status, past medical history, strength testing, and imaging morphometrics is strongly associated with worse outcomes during and after hospitalization, traumatic injury, and surgery(3–11). However, many of the methods to measure frailty rely on subjective clinical variables and assessments that can be time-consuming and reliant on the completeness of the medical record and patient effort(7–10).
Given the subjectivity of frailty assessment scores, several groups have utilized a multitude of imaging modalities to directly measure muscle quality and mass(3–6, 12–15). Of these, the computed tomography (CT) psoas muscle measurement at the L3 vertebral body level has been shown to predict poor outcomes, including liver transplantation, major vascular operations, and oncologic surgery(3–6). Recently, several studies have validated the CT psoas density, which measures the average Hounsfield Unit (HU) attenuation of the muscle, and therefore, captures information regarding the degree of fatty muscular infiltration, quality, and strength, which can be used to predict outcomes after elective gastrointestinal oncologic surgery(5, 6, 12).
Here we investigate whether CT measured psoas density as a marker of physiologic reserve, can predict outcomes in elective enterocutaneous fistula (ECF) repair. ECF represents a complex and challenging surgical problem fraught with peril. Unfortunately, the persistent loss of fluid and protein, electrolyte derangements, significant malnutrition, and persistent inflammatory state caused by the fistula often leave patients in a state of recursive physiologic decline, accounting for historically high rates of complications, mortality, and recurrence(16–19). In addition, patients often have a history of multiple previous abdominal operations, malignancy, and inflammatory bowel disease, increasing the risk of poor outcomes. Thus, evaluating pre-operative physiologic reserve would be a valuable tool in identifying patients at increased risk of poor outcomes, who may benefit from additional physical and nutritional regimens and further conservative management before definitive repair. The objective of this study was to evaluate CT based psoas muscle density as a prognostic marker for poor outcomes after ECF repair.
METHODS
Data source and patient characteristics
We searched for patients by Current Procedural Terminology (CPT) codes for intestinal fistula repair 44625, 44626, 44640, 44650 from January 1st, 2005 to December 31st, 2015 performed at our tertiary academic medical center. Further in-depth chart review was conducted confirming surgical ECF repair comprising of laparotomy, en-bloc overlying skin with bowel resection and anastomosis that had a CT scan abdomen/pelvis with venous contrast within three months before operative repair. Exclusion criteria included patients requiring emergent operation and those who died from metastatic progression. For each patient record, detailed clinical variables were collected including age, sex, body mass index (BMI) at time of operation, number of comorbidities, number of medications at time of operation, , pre-operative albumin and pre-albumin taken within 48 hours before operation, pre-op parenteral nutrition (PN) as defined by any preoperative PN use before operation, history of inflammatory bowel disease (IBD) and history of malignancy, and past medical history variables making up the modified frailty index (MFI)(8, 9) The MFI score is a method derived from the Canadian Study of Health and Aging Clinical Frailty Scale. The MFI is an abbreviated version made up of eleven binominal variables; the presence of diabetes, congestive heart failure, hypertension, stroke, history of stroke with residual neurologic deficit, dependent functional status, myocardial infarction, peripheral vascular disease, chronic obstructive pulmonary disease/pneumonia, recent coronary artery bypass or percutaneous coronary intervention, and presence of delirium or altered sensorium(9). To date, this measure has been found to be a strong predictor of complications and mortality in multiple different operations(8, 9).
ECF and surgery characteristics included date of fistula occurrence, date of ECF repair surgery, number of fistula tracts, and small or large bowel fistula location. Records indicating high vs low output fistula were not sufficiently kept to measure this characteristic. Outcome data collected included 1 year leak rate including either post-operative intra-abdominal abscess and recurrent ECF, 90 day, 1 year, and 3 year mortality (confirmed using the Social Security Death Index), risk of complication, discharge to a dependent facility (long-term acute care hospital or skilled nursing facility), length of stay and 30 day re-admission. All patients in our cohort had previous abdominal operations.
CT image analysis and psoas muscle quality calculation
The right and left psoas muscles were traced by the same researcher at the L3 level with PACS (Picture Archiving and Communications System) EasyViz® imaging software (Karos Health, Waterloo ON). Psoas density was measured as the Hounsfield Unit Average Calculation (HUAC) and was calculated by summing the product of the right psoas Hounsfield Unit density (RPHU) and right psoas area (RPA) with that of the left psoas Hounsfield Unit (LPHU) and left psoas area (LPA), then dividing by the sum of RPA and LPA: [(RPHU x RPA) + (LPHU x LPA)]/[(RPA + LPA)](5, 6). CV% between the attenuation of the RPA and LPA was reasonably low (7.1%) indicating fair precision of this measurement at the L3 level. Patients were stratified based on the interquartile range (IQR) for our measures of muscle quality into 4 groups where IQR1 corresponds to the lowest 25th percentile of values to IQR4 which corresponds to the highest 25th percentile of values. We defined our low muscle quality (LMQ) cutoff at IQR1 corresponding to the 25th percentile value (HUAC ≤ 32.6 HU).
Statistical Analysis
The majority of all data was collected. Missing data were excluded from analysis. Variables were compared using Student’s t-test, Fischer’s Exact test, χ2 test, and Pearson correlation. Multivariate analysis was performed using multiple linear regression, multiple logistic regression and ordered logistic regression. All statistics were performed with STATA version 14.1 (StataCorp College Station TX). Kaplan-Meier curve was generated using STATA. Statistical significance was defined by α = 0.05. The study was approved by the Ohio State University Institutional Review Board.
RESULTS
Baseline patient characteristics
Patients were stratified into interquartile ranges based on HUAC (Table 1). Our muscle density cutoff for LMQ was defined as the 25th percentile value (IQR1) of HUAC (LMQ by HUAC ≤ 32.6 HU). Complications were categorized by a set list (Table 1). 100 patients met criteria over our 10 year study period. Patient characteristics (Table 2) include mean age 56.5 years, 54% female, BMI 27.8 kg/m2, number of comorbidities 5.0, number of medications 9.5, MFI 1.1, IBD 14%, cancer history 12%, pre-operative PN use 63%, pre-operative albumin 2.8 g/dL, pre-op pre-albumin 15.3 mg/dL, number of months waited from fistula occurrence to operative repair 7.3 months, and number of months from CT scan to operative repair. ECF characteristics of our cohort were 21% entero-atmospheric fistula (EAF), number of fistula tracts 1.4, and location of fistula in small bowel 92%.
Table 1.
Ranges of Muscle Quality by HUAC
| IQR1 (Low Muscle Quality) | 15.5–32.6 HU |
| IQR2 | 32.7–40.9 HU |
| IQR3 | 41.0–48.8 HU |
| IQR4 | 48.9–58.6 HU |
HUAC; Hounsfield unit average calculation, IQR; interquartile range, HU; Hounsfield unit
Table 2.
Characteristics of Fistula Cohort by Low Muscle Quality
| Total Cohort (n=100) | LMQ Cohort (n=25) | LMQ Cohort (n=75) | p-value | |
|---|---|---|---|---|
| Patient Characteristics | ||||
| Age (years) | 56.5 (14.4) | 58.6 (11.8) | 55.8 (15.1) | 0.40 |
| Sex | 54.0% Female | 56.0% Female | 53.3% Female | 0.86 |
| BMI | 27.8 (9.9) | 33.2 (10.7) | 26.0 (8.8) | 0.002* |
| #Comorbidities | 5.0 (3.0) | 5.1 (2.2) | 5.0 (3.2) | 0.83 |
| #Medications | 9.5 (5.5) | 10.4 (5.0) | 9.1 (5.6) | 0.33 |
| MFI | 1.1 (1.3) | 1.8 (1.6) | 1.0 (1.1) | 0.01* |
| IBD | 14 (14.0%) | 3 (12.0%) | 11 (14.7%) | 0.52 |
| Cancer History | 12 (12.0%) | 3 (12.0%) | 9 (12.0%) | 0.62 |
| Pre-op TPN | 63 (63.0%) | 13 (52.0%) | 50 (66.7%) | 0.27 |
| Pre-op Albumin | 2.8 (0.7) | 2.6 (0.7) | 2.9 (0.6) | 0.11 |
| Pre-op Pre-Albumin | 15.3 (7.7) | 14.0 (8.0) | 15.9 (7.6) | 0.34 |
| #Months from Fistula | 7.3 (11.7) | 4.3 (3.6) | 8.0 (12.9) | 0.25 |
| #Months from CT Scan | 1.1 (0.9) | 0.9 (0.9) | 1.2 (0.9) | 0.10 |
| ECF Characteristics | ||||
| EAF vs ECF | 21 (21%) EAF | 6 (24%) EAF | 15 (20%) EAF | 0.67 |
| #Tracts | 1.4 (0.7) | 1.6 (1.1) | 1.3 (0.6) | 0.04* |
| Location | 92 (92%) SB | 21 (84%) SB | 71 (95%) SB | 0.09 |
LMQ; low muscle quality, BMI; body mass index, MFI; modified frailty index, IBD; inflammatory bowel disease, TPN; total parenteral nutrition, EAF; entero-atmospheric fistula, ECF; enterocutaneous fistula, SB; small bowel, values are means or total number in group, values in parenthesis are standard deviation, or percentage of total group,
marks significance α = 0.05 level
Univariate comparisons for collected variables are summarized in Table 2. Patients with LMQ had increased BMI 33.2 vs 26.0, p < 0.008) and increased MFI score (1.8 vs. 1.0, p = 0.01) compared to those without LMQ. Pre-operative albumin was decreased in the LMQ group, but was not statistically significant (2.6 vs 2.9. p = 0.11). There was no significant difference in age, sex, number of co-morbidities, or number of medications. There was no difference in the rate of IBD, cancer history, or days from CT scan to operation between groups. LMQ patients had a small but significantly higher number of fistula tracts (1.6 vs 1.3, p = 0.04).
Psoas density correlates with other markers of malnutrition and frailty
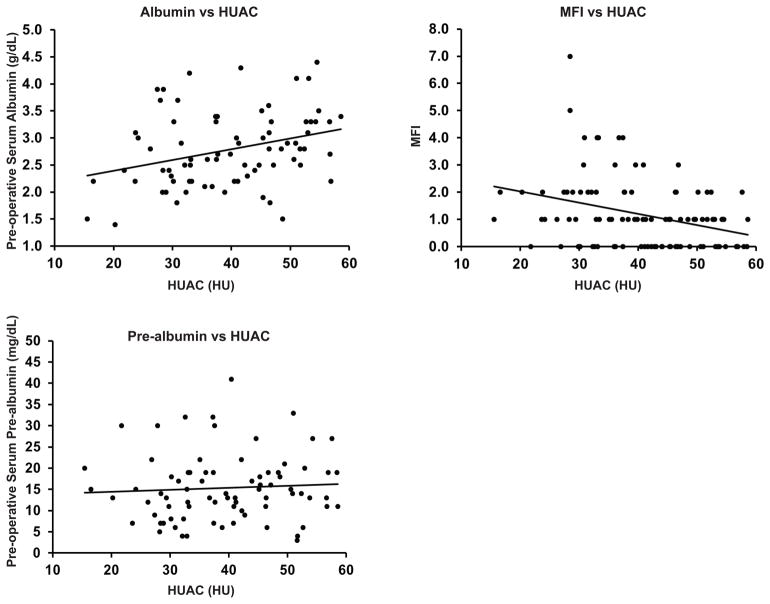
Given that patients with LMQ by HUAC had a significantly increased MFI score as well as decreased albumin, we examined whether there was a linear relationship between HUAC and these other markers of frailty and malnutrition. There was a significant positive correlation between HUAC and albumin (Figure 1a, r = 0.31, p = 0.006). There was a significant negative correlation between HUAC and MFI score (Figure 1b, r = −0.33, p < 0.001). Preoperative pre-albumin did not correlate with HUAC (Figure 1c, r = 0.06, p = 0.59). Multiple linear regression controlling for covariates with p ≤ 0.10 including BMI, # of tracts, small bowel location, and # of days from CT scan to operation continued to demonstrate a significant linear relationship. Every 1 g/dL increase in albumin increasing HUAC by 5.7 HU on average (p = 0.002). Every 1 unit increase in MFI decreased HUAC by an average of 2.2 HU (p = 0.03).
Figure 1. Psoas density correlates with serum albumin and MFI score.
HUAC of the psoas muscles correlate significantly with serum pre-operative albumin (1a, r = 0.31, p = 0.006) and MFI score (1b, r = −0.31, p < 0.001). Pre-operative pre-albumin did not correlate with HUAC (1c, r = 0.06, p = 0.59).
MFI; modified frailty index, HUAC; Housnfield unit average calculation, HU; Hounsfield unit.
Low muscle quality outperforms pre-operative serum albumin and MFI in predicting risk of poor outcome
Univariate outcomes comparisons are summarized in Table 3. Our cohort 1-year mortality rate (21.2%) was similar to outcomes reported at other centers. Patients with LMQ had multiple significantly poorer outcomes; with an increased 1 year leak rate resulting in ECF recurrence or intra-abdominal abscess (56.0% vs 26.7%, p < 0.008), 1 year, (36.0% vs 16.0%, p = 0.04) and 3 year mortality rate (57.1% vs 24.0%, p = 0.007). They also had higher complication rate, with nearly all LMQ patients suffering from at least one (96.2% vs 62.2%, p = 0.001), and dependent discharge to a long term acute care hospital or skilled nursing facility (50% vs 19.7%, p = 0.004). Inpatient length of stay and 30 day readmission rate were not significantly different between groups.
Table 3.
Fistula Cohort Outcomes by LMQ
| Total Cohort (n=100) | LMQ Cohort (n=25) | Non-LMQ Cohort (n=75) | p-value | |
|---|---|---|---|---|
| 1-year Leak | 20 (46.4%) | 14 (56%) | 20 (26.7%) | 0.008* |
| Abscess | 11 (11.0%) | 5 (20.0%) | 6 (8.0%) | 0.10 |
| Fistula | 23 (23.0%) | 9 (36.0%) | 14 (18.7%) | 0.08 |
| 90-day Mortality | 11 (11.0%) | 4 (16.0%) | 7 (9.3%) | 0.36 |
| 1-year Mortality | 21 (21.2%) | 9 (36%) | 12 (16.2%) | 0.04* |
| 3-year Mortality | 24 (33.8%) | 12 (57.1%) | 12 (24.0%) | 0.007* |
| Length of Stay (days) | 19.9 (18.4) | 21 (15.0) | 19.5 (19.4) | 0.73 |
| 30-Day Complication | 70 (70.7%) | 24 (96.2%) | 46 (62.2%) | <0.001* |
| 30-Day Readmission | 30 (30%) | 10 (40%) | 20 (26.7%) | 0.21 |
| Dependent Discharge | 26 (27.4%) | 12 (50%) | 14 (19.7%) | 0.004* |
LMQ; low muscle quality, Values are means or total number in group, values in parenthesis are standard deviation, or percentage of total group,
marks significance α = 0.05 level
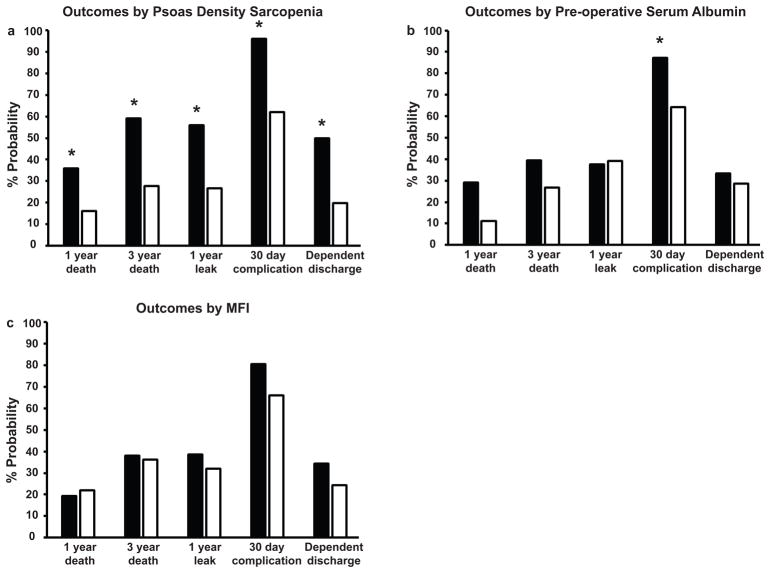
To determine if psoas density is associated with other markers of malnutrition and frailty, we compared our outcome measures against cut-off values of pre-operative serum albumin and MFI score. Patients with a serum albumin of 3.0 g/dL and greater were compared against those with a value of <3.0 g/dL. We chose this value given its strength in predicting ECF recurrence and mortality in other studies ((20, 21). Patients with an MFI score of 2 and greater were compared against those with an MFI score of < 2. As discussed above, LMQpatients had significantly increased risk of 1 year death, 3 year death, 1 year leak, 30 day complication, and dependent discharge (Figure 2a). Patients with pre-operative albumin < 3.0 g/dL had significant increased risk in complications only (Figure 2b, 87% vs 64%, p = 0.02), and had overall increased probability of 1 and 3 year mortality, although this was not significant. Patients with MFI scores of 2 did not have increased risk of poor outcomes.
Figure 2. Psoas density-based sarcopenia outperforms MFI and serum pre-operative albumin.
Psoas density-based sarcopenic patients (2a, black bars) had significantly worse 1 year death, 3 year death, 1 year leak 30 day complication, dependent discharge rates in comparison to non-sarcopenic patients (2a, white bars).
Patients with serum pre-operative albumin < 3.0 g/dL (2b, black bars) had significantly greater risk of 30 day complications compared to patients with values of 3.0 g/dL or greater (2b, white bars).
Patients with greater MFI scores of 2 or greater (2c, black bars) had no significantly greater probabiloty of poor outcomes compared to those with MFI < 2 (2c, white bars).
MFI; modified frailty index, * marks p < 0.05.
Direct univariate comparison of LMQ vs albumin < 3.0 g/dL or MFI ≥ 2 quantitatively shows that LMQ outperforms these other markers of frailty (Table 3). Patients with LMQ for 1-year leak, 1 and 3 year mortality, 30 day complication rate, and dependent discharge had an increased likelihood ratio and significant value compared to albumin or MFI. To determine if muscle quality was an independent risk factor for poor outcomes, multiple logistic regression was performed comparing LMQ to 1 year leak rate, 1 year mortality, 3 year mortality, complication and dependent discharge, controlling for albumin < 3.0 g/dL and MFI ≥ 2, BMI, multiple fistula tracts, small bowel fistula location, and time of CT scan to operative repair (Table 3). No risk factors were associated with 1 year leak rate after adjustment, however LMQ had the strongest trend toward independent effect (OR = 2.92, p = 0.09) as compared to MFI (OR = 0.93, p = 0.91) and albumin (OR = 0.80, p = 0.70). LMQ had a significant increased likelihood of 1 year (OR = 7.79, p = 0.01) and 3 year death (OR = 22.37, p < 0.01) while MFI and albumin were not significant. LMQ trended toward increased likelihood of 30 day complication (OR = 7.12, p = 0.10). Albumin < 3.0 g/dL did significantly increase 30 day complication likelihood (OR = 5.73, p = 0.02). Lastly, only LMQ was significantly associated with dependent discharge (OR = 3.54, p = 0.04).
Progressively lower muscle attenuation increases probability of poor outcome
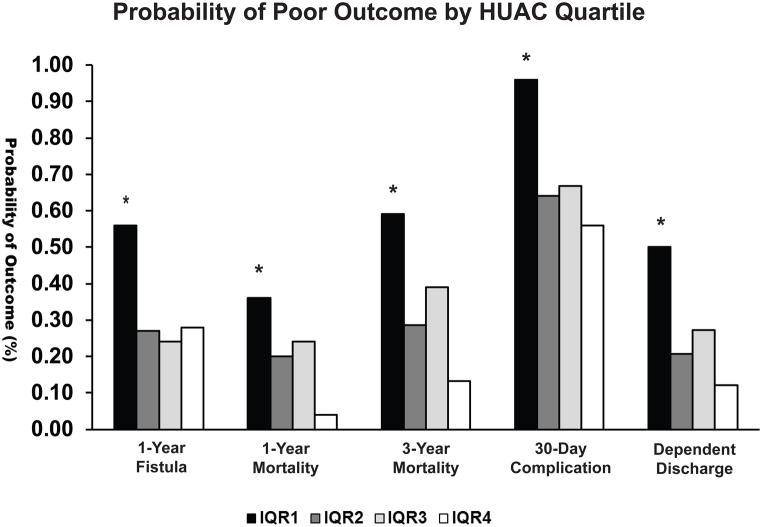
To determine if decreasing muscle attenuation worsened poor outcomes in a dose-dependent fashion, we compared the different quartiles (Table 1) of muscle attenuation vs significant measures of poor outcome (Figure 3). Patients with LMQ represented by IQR1, had significantly worse probability in 1 year leak rate, 1 year mortality, 3 year mortality, 30 day complication rate, and dependent discharge. This effect appeared to be dose-dependent, as patients with the highest muscle attenuation values (IQR4), had overall, the lowest associated risk of poor outcome compared to the other quartile groups.
Figure 3. Decreasing muscle attenuation is associated with increasing risk of poor outcomes.
LMQ patients (IQR1) had a worse risk of 1-year fistula recurrence (p<0.01), 1-year mortality (p=0.04), 3-year mortality (p = 0.01), 30-day complication rate (p = 0.01) and dependent discharge (p < 0.01) compared to patients in other quartiles. This effect appears to be attenuation dependent as patients with the highest interquartile range values (IQR4) representing the best muscle quality had overall, lower risk of fistula recurrence, 1-year mortality, 3-year mortality, 30-day complication risk and dependent discharge.
IQR1; 1st–25th percentile, IQR2; 26th – 50th percentile, IQR3; 51st – 75th percentile, IQR4; 76th – 100th percentile, LMQ; Low muscle quality, HUAC; Hounsfield Unit average calculation, * marks significance below α = 0.05.
To quantify risk by degree of muscle attenuation, we compared the likelihood ratios of each interquartile, comparing them to the highest muscle attenuation group (IQR4), representing patients with the best muscle quality, and therefore, best physical and nutritional state before surgery (Table 4). Ordered logistic regression, adjusting for BMI, albumin < 3.0 g/dL and MFI ≥ 2, multiple fistula tracts, small bowel fistula location, and time of CT scan to operative repair found that LMQ patients had significantly worse 1 year mortality (OR 25.78, p = 0.02), 3 year mortality (OR 10.19, p = 0.02), 30-day complication rate (OR 15.52, p = 0.02), and dependent discharge (6.03, p = 0.02) vs IQR4. IQR 2 and 3 overall trended towards worse outcomes after multivariate adjustment, however these values were not significant.
Table 4.
Univariate and Multivariate Predictor Comparisons of Poor Outcomes after ECF Repair
| Univariate Likelihood Ratio | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| LMQ (OR) | 95% CI | p-value | Albumin < 3.0 g/dl (OR) | 95% CI | p-value | MFI ≥ 2 (OR) | 95%CI | p-value | |
| 1-year Leak | 3.50 | 1.36–8.97 | <0.01* | 0.90 | 0.35–2.33 | 0.82 | 1.34 | 0.56–3.26 | 0.51 |
| 1-year Mortality | 2.95 | 1.06–8.22 | 0.04* | 3.33 | 0.87–12.84 | 0.08 | 0.86 | 0.30–2.49 | 0.79 |
| 3-year Mortality | 3.76 | 1.33–10.60 | 0.01* | 1.80 | 0.49–6.58 | 0.38 | 1.08 | 0.38–3.04 | 0.89 |
| 30-Day Complication | 14.61 | 1.87–114.02 | 0.01* | 3.89 | 1.23–12.31 | 0.02* | 2.13 | 0.77–5.92 | 0.15 |
| Dependent Discharge | 4.07 | 1.51–10.97 | <0.01* | 1.21 | 0.43–3.37 | 0.72 | 1.64 | 0.64–4.25 | 0.31 |
| Multivariate Likelihood Ratio† | |||||||||
| LMQ (OR) | 95% CI | p-value | Albumin < 3.0 g/dl (OR) | 95% CI | p-value | MFI ≥ 2 (OR) | 95%CI | p-value | |
| 1-year Leak | 2.92 | 0.85–10.02 | 0.09 | 0.80 | 0.26–2.46 | 0.70 | 0.93 | 0.27–3.26 | 0.91 |
| 1-year Mortality | 7.79 | 1.57–38.57 | 0.01* | 2.36 | 0.46–12.02 | 0.30 | 0.15 | 0.02–1.13 | 0.07 |
| 3-year Mortality | 22.37 | 3.07–162.95 | <0.01* | 1.22 | 0.20–7.61 | 0.83 | 0.16 | 0.02–1.27 | 0.08 |
| 30-Day Complication | 7.12 | 0.69–73.57 | 0.10 | 5.73 | 1.40–23.44 | 0.02* | 1.11 | 0.21–5.96 | 0.13 |
| Dependent Discharge | 3.54 | 1.05–11.95 | 0.04* | 1.28 | 0.0.39–4.26 | 0.69 | 0.53 | 0.13–2.08 | 0.36 |
Controlling for BMI, Time from CT Scan to Operative Repair, Multiple Tracts, Small Bowel Fistula Location, Low Muscle Quality, Albumin < 3.0, MFI ≥ 2 ECF; Enterocutaneous fistula, LMQ; Low muscle quality, OR; Odds ratio, CI, Confidence interval, MFI; modified frailty index
Low muscle quality patients have significantly poorer fistula-free survival
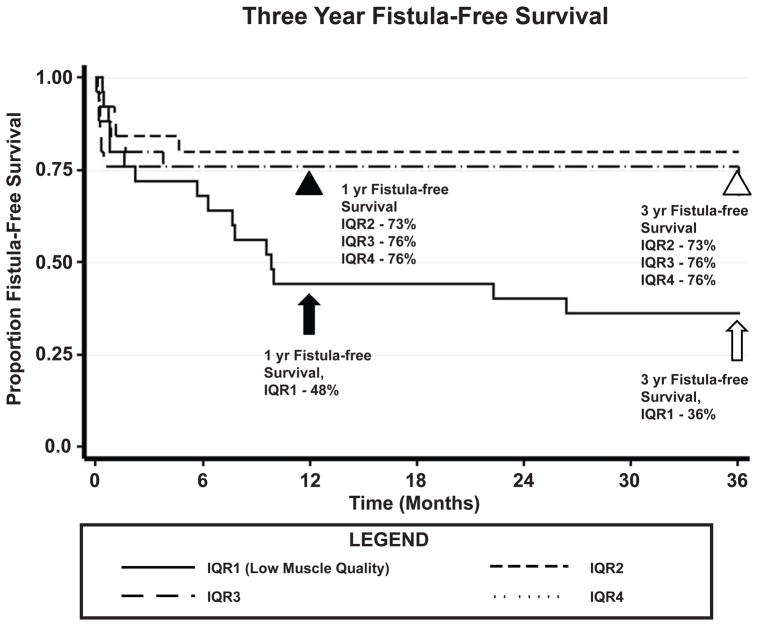
We plotted Kaplan-Meier survival curves by interquartile range against our composite outcome of fistula-free survival over three years (Figure 4). Fistula-free survival was defined as the absence of recurrent fistula or mortality after enterocutaneous fistula repair, representing the best clinical quality outcome, free from both mortality and morbidity related to enterocutaneous fistula. There is a significant difference in fistula-free survival at 1 year in LMQ patients vs. the other quartiles. Interestingly, non-LMQ patients that survive without fistula recurrence at 1 year (black arrowhead, event-free survival, IQR2 – 76%, IQR3 – 73%, IQR4 – 73%) overall continue to do well, with no deaths or recurrences in this group at 3 years (white arrowhead, event-free survival, IQR2 – 76%, IQR3 – 73%, IQR4 – 73%). In contrast, LMQ patients not only have decreased fistula-free survival at 1 year (black arrow, event-free survival 48%) but also continue to suffer from progressive fistula recurrence and mortality at 3 years (white arrow, event-free survival 36%).
Figure 4. Differences in fistula-free survival between low muscle quality and non-low muscle quality patients.
Sarcopenic patients had significantly worse fistula-free survival at 1 year (black arrow, event-free survival 48%) compared to non-sarcopenic patients (black arrowhead, event-free survival IQR2 73%, IQR3 76%, IQR4 76%). Over time, non-low muscle quality patients stabilize with no deaths or re-fistula events at 3 years (white arrowhead, event-free survival IQR2 73%, IQR3 76%, IQR4 76%). Low muscle density patients continue to do poorly with continued deaths and re-fistula events at 3 years (white arrow, event-free survival 36%). IQR1; interquartile range 1, IQR2: interquartile range 2, IQR3; interquartile range 3, IQR4, interquartile range 4.
DISCUSSION
Enterocutaneous fistula remains a formidable complication for the abdominal surgeon. The ongoing losses of gastrointestinal fluid, protein, and inflammation create a prolonged catabolic state, predisposing patients to further malnutrition and frailty, contributing to the high rates of morbidity, recurrence, and death(16, 17). Unfortunately, it is common for patients to undergo multiple operations due to recurrence, leading to years of debilitation, extended time on parenteral nutrition, and progressive loss of abdominal domain, further increasing the likelihood of post-operative hernia requiring another additional complex abdominal operation. Thus, there is a significant need to find pre-operative risk markers that can be used to predict poor outcomes, allowing for proper patient selection and operative timing.
In this retrospective cohort study, we show that pre-operative CT psoas density as our marker of muscle quality and physiologic reserve significantly correlates with other methods that measure malnutrition and frailty. Furthermore, patients in the lowest interquartile range of psoas muscle density, defining low muscle quality, had the highest risk of leak resulting in abscess or recurrent fistula, complications, and mortality across multiple outcomes including 1 year, and 3 year mortality as well as post-operative infirmity requiring discharge to a skilled nursing facility or long-term acute care hospital. When comparing CT psoas density to pre-operative serum albumin and MFI as a predictor of poor outcomes, density significantly outperforms these other measures. After multivariate adjustment, LMQ remained a significant predictor of poor outcomes. When psoas density was compared by quartile against our composite outcome of fistula-free survival, LMQ patients had the worst outcomes compared to the other quartiles.
Serum albumin is a well-known and utilized pre-operative risk assessment marker that has been shown to predict poor outcomes in gastrointestinal, cardiac, thoracic, and orthopedic surgery(22–24). In our study, serum albumin had a moderate and significant correlation with psoas HUAC, indicating that psoas density is capturing a significant part of the protein malnutrition and persistent inflammatory state represented by hypoalbuminemia. Our data did demonstrate an increased risk of complications with albumin < 3.0 g/dL. Although not reaching statistical significance, patients with hypoalbuminemia trended towards increased 1 year and 3year death, which is like findings in other studies.
ECF creates significant nutritional deficiencies, including protein loss. The small bowel secretes on average, approximately 75 g of protein, that, is usually re-absorbed as it travels distally in a closed-system GI tract, and that much of this is lost with the presence of an ECF. The significant net loss of protein/nitrogen from the alimentary tract, reduces availability of amino acids available for albumin and pre-albumin synthesis, likely explaining why a majority of the patients in our cohort (63%) had hypoalbuminemia < 3.0 g/dL. Current clinical guidelines recognize the significance of ongoing protein loss, recommending 1.5 – 2.0 g/kg/day of protein to account for GI losses, with more complex and proximal fistula requiring even further supplementation of up to 2.5 g/kg/day.
There was no association between serum pre-albumin and our outcomes in this study (data not shown). Furthermore, there was no correlation between pre-albumin and psoas HUAC. To specifically address why albumin, and not pre-albumin, was associated to outcomes, and in our study, we believe that this is due to the different half-lives of the proteins. It is well known that serum albumin has a half-life of 20–22 days. In contrast, pre-albumin has a half-life of only 2–4 days. Thus, measuring serum albumin is considered a better, overall marker of nutritional status, whereas pre-albumin is more relevant in the context of knowing whether a nutritional intervention is working, reflecting acute changes to nutritional status (25, 26). Thus, we conjecture that patients with normal pre-albumin values, but with low albumin values may be malnourished, but are only recently showing evidence of anabolism and improving nutritional status. Conversely, patients with relatively high albumin values, but low pre-albumin values may be showing evidence of beginning malnutrition, but may have enough relatively preserved reserve to tolerate ECF repair.
Both albumin and pre-albumin are acute phase reactants that can be significantly decreased by inflammation, even in the otherwise, nourished patient limiting their interpretation as specific markers of malnutrition. Therefore, they are not recommended to be interpreted as comprehensive markers of nutritional status that take the place of morphometric and history-based methods. However, several studies have validated albumin as a good predictor of morbidity making it still a clinically relevant marker, possibly because it captures information on both malnutrition and degree of inflammatory state. Thus, its routine measurement remains recommended in the latest ASPEN clinical guidelines for enterocutaneous fistula management (27).
The modified frailty index is an 11-point score created form the National Surgical Quality Improvement Program with the intent of helping hospitals risk adjust patients(8, 9). It considers a combination of chronic medical conditions, pre-operative functional status, and delirium to create a risk adjusted score. We did find a significant moderate correlation between MFI score and psoas muscle HUAC, indicating that psoas density is capturing some of the frailty and co-morbidities as the MFI. However, MFI score was not significantly associated with our poor outcomes of measure. Given that the MFI score tends to have more impact on mortality in lower risk operations, and the average score is substantially lower on average for patients undergoing safer gastrointestinal operations compared to our cohort, this likely reduced the discriminatory power of MFI in our ECF repair cohort(8).
Interestingly, there appeared to be a dose-dependent effect on outcomes with decreasing psoas muscle attenuation. LMQ patients had significantly worse outcomes compared to the other quartiles, while IQR4 patients, representing those with the best quality muscle in our study, had overall, the best outcomes (Figure 3). This effect remained significant after risk adjustment (Table 4). IQR2 and 3 patients did not have a statistically significant difference in outcome compared to IQR4 after risk-adjustment, though this could be due to our study being underpowered to detect a difference with a smaller effect size. Lastly, patients with LMQ had worse composite fistula-free survival compared to the other quartiles (Figure 4) that, in contrast to the quartile ranges, continued to decline over time.
Unlike acute emergency surgery, fistula patients do not require surgical intervention within a specified period. Patients can be maintained with enteral/parenteral nutritional support and wound care/management for a prolonged period, allowing for improvements in nutritional status and control of chronic co-morbidities before pursuing elective repair(18, 19, 27). In fact, recent literature demonstrates that waiting for a longer interim before definitive repair significantly improves outcomes, with an average wait time of 12 months vs. 8 months having improved success and post-operative survival(18, 19).
We expect that surgeons could incorporate this tool into their clinical assessment to identify high-risk patients that would benefit from further time on non-operative management before repair, or may not be operative candidates. Additionally, dietitians and therapists could use this tool to identify patients who would benefit from further oral or parenteral nutritional interventions and physical therapy regimens. What remains unknown is the extent to which psoas muscle density can improve with nutritional and physical therapy. We posit that as nutritional deficiencies are corrected and inflammation resolves, physiologic reserve, and CT psoas density should improve. Thus, perhaps psoas muscle density can be obtained serially, allowing for an objective determination of when a patient is physiologically optimized for surgery, though the benefits of obtaining this information would need to be weighed carefully against the < 0.1% per 10 mSv increased risk of lifetime malignancy presented by CT scanning.
Our study has several limitations. Our data was gathered from a small cohort of 100 patients retrospectively, totaling the experience from a single institution over a 10-year span. In order to be generalizable, our results should be externally validated at other centers. Specifically, the set point that defines pathologically low muscle density has not been cross-validated, and it remains to be seen whether our cut off value of 32.6 HU is similar to other institutions. Interestingly, similar values have been proposed by other studies, ranging from 30 – 38.5 HU which may be a result of different muscle groups, CT modalities, and methods of calculation (6, 28). We chose to only include patients who had a preoperative CT scan of the abdomen pelvis with venous phase contrast. Our choice of CT scan in this study is due to this modality being the most commonly used to map pre-operative surgical anatomy at our institution. However, it is unknown how generalizable our results will be when comparing non-contrast or arterial-phase imaging. Time from fistula occurrence to ECF repair varied widely, likely representing differences in physician preference in the timing of operative repair. In addition, albumin and pre-albumin are acute phase reactant proteins that significantly decrease with inflammation. Thus, protein loss and poor oral intake and ECF related persistent inflammatory state alter levels. Consequently, this limits interpretation of these markers specifically addressing malnutrition. Lastly, psoas muscle measurements, which have been associated with poor outcomes across multiple studies, have yet to be robustly validated as an overall marker of body composition, thus, we cannot conclude that psoas density is representative of total muscle composition.
Future studies comparing comprehensive nutritional assessments and psoas density, albumin, pre-albumin, an MFI score would be valuable in determining which of these markers best captures information specifically on malnutrition. Furthermore, studies comparing psoas density to total body composition would validate this method as a representative marker of physiologic reserve. Lastly, ECF repair was performed by multiple surgeons across the different general surgery services at our institution, which likely increases variability in outcomes. To minimize this, all operative reports were reviewed to make sure similar procedures which involved lysis of adhesions, and bowel resection with primary anastomosis were performed.
In conclusion, this study demonstrates that CT-based psoas density measurements is associated with multiple poor outcomes, and may represent the underlying physiologic reserve of ECF patients. This easy to measure variable that can be obtained in a pre-operative CT scan identifies patients at risk of death, fistula recurrence, complications, and discharge to a dependent facility who may benefit from additional non-operative measures and time before definitive repair.
Table 5.
Multivariate-Adjusted Likelihood Ratio of Poor Outcomes after ECF Repair by Interquartile Range
| IQR1 (95% CI) | p-value | IQR2 (95% CI) | p-value | IQR3 (95% CI) | p-value | IQR4 (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|---|
| 1-year Leak | 2.41 (0.62–9.42) | 0.21 | 0.77 (0.18–3.31) | 0.73 | 1.29 (0.32–5.25) | 0.72 | 1.00 (N/A) | (N/A) |
| 1-year Mortality | 25.78 (1.81–367.6) | 0.02* | 3.78 (0.23–61.50) | 0.35 | 6.04 (0.42–87.19) | 0.19 | 1.00 (N/A) | (N/A) |
| 3-year Mortality | 10.19 (1.55–67.12) | 0.02* | 1.24 (0.17–9.19) | 0.83 | 3.58 (0.38–26.87) | 0.22 | 1.00 (N/A) | (N/A) |
| 30-Day Complication | 15.52 (1.64–146.58) | 0.02* | 1.38 (0.36–5.21) | 0.64 | 1.84 (0.48–6.97) | 0.37 | 1.00 (N/A) | (N/A) |
| Dependent Discharge | 6.03 (1.30–28.03) | 0.02* | 1.60 (0.30–8.49) | 0.55 | 2.83 (0.56–14.15) | 0.21 | 1.00 (N/A) | (N/A) |
Likelihood ratio based on comparisons to IQR4, controlling for BMI, Time from CT Scan to Operative Repair, Multiple Tracts, Small Bowel Fistula Location, Albumin < 3.0, MFI ≥ 2 ECF; Enterocutaneous fistula, IQR; interquartile range, CI; confidence interval.
marks significance below α = 0.05.
Clinical Relevancy Statement.
Enterocutaneous fistula (ECF) represents a challenging surgical problem. Persistent loss of fluid and protein, electrolyte derangements, significant malnutrition, and persistent inflammatory state leave patients in a state of recursive physiologic decline, accounting for historically high rates of complications, mortality, and recurrence. Here we find that CT psoas density can predict poor outcomes in elective ECF repair. This easy to measure variable can be obtained in pre-operative CT scans and can identify patients at risk of poor outcomes who may benefit from additional nutritional/physical interventions and time before definitive repair.
ABBREVIATIONS
- BMI
Body mass index
- CPT
Current procedural terminology
- CT
Computerized tomography
- EAF
Entero-atmospheric fistula
- ECF
Enterocutaneous fistula
- HU
Hounsfield unit
- HUAC
Hounsfield unit average calculation
- IBD
Inflammatory bowel disease
- IQR
Interquartile range
- LMQ
Low muscle quality
- LPA
Left psoas area
- LPHU
Left psoas Hounsfield unit
- MFI
Modified frailty index
- OR
Odds ratio
- PACS
Picture archiving system
- RPA
Right psoas area
- RPHU
Right psoas Hounsfield unit
- RR
Relative risk
- PN
Parenteral nutrition
Footnotes
Financial Disclosures: None declared
Conflicts of Interest: None declared
References
- 1.Rosenberg IH. Sarcopenia: Origins and Clinical Relevance. Journal of Nutrition. 1997;127:990–1. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 2.Janssen I, Heymsfield S, Ross R. Low Relative Skeletal Muscle Mass (Sarcopenia) in Older Persons Is Associated with Functional Impairment and Physical Disability. Journal of the American Geriatric Society. 2002;50(5):889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 3.Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211(2):271–8. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones KI, Doleman B, Scott S, Lund JN, Williams JP. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis. 2015;17(1):O20–6. doi: 10.1111/codi.12805. [DOI] [PubMed] [Google Scholar]
- 5.Joglekar S, Asghar A, Mott SL, Johnson BE, Button AM, Clark E, et al. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol. 2015;111(6):771–5. doi: 10.1002/jso.23862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buettner S, Wagner D, Kim Y, Margonis GA, Makary MA, Wilson A, et al. Inclusion of Sarcopenia Outperforms the Modified Frailty Index in Predicting 1-Year Mortality among 1,326 Patients Undergoing Gastrointestinal Surgery for a Malignant Indication. J Am Coll Surg. 2016;222(4):397–407. e2. doi: 10.1016/j.jamcollsurg.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velanovich V, Antoine H, Swartz A, Peters D, Rubinfeld I. Accumulating deficits model of frailty and postoperative mortality and morbidity: its application to a national database. J Surg Res. 2013;183(1):104–10. doi: 10.1016/j.jss.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Farhat JS, Velanovich V, Falvo AJ, Horst HM, Swartz A, Patton JH, Jr, et al. Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg. 2012;72(6):1526–30. doi: 10.1097/TA.0b013e3182542fab. discussion 30–1. [DOI] [PubMed] [Google Scholar]
- 10.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–8. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Justiniano CF, Evans DC, Cook CH, Eiferman DS, Gerlach AT, Beery PR, 2nd, et al. Comorbidity-polypharmacy score: a novel adjunct in post-emergency department trauma triage. J Surg Res. 2013;181(1):16–9. doi: 10.1016/j.jss.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodpaster B, Carlson C, Visser M, Kelley D, Scherzinger A, Harris T, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. Journal of Applied Physiology. 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 13.Reeves ND, Maganaris CN, Narici MV. Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol. 2004;91(1):116–8. doi: 10.1007/s00421-003-0961-9. [DOI] [PubMed] [Google Scholar]
- 14.Proctor D, PCOB, EJA, KSN Comparison of techniques to estimate total body skeletal mass in people of different age groups. Am J Physiol. 1999;(489–495):277. doi: 10.1152/ajpendo.1999.277.3.E489. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Heo M, Lee R, Kotler D, Withers R, Heymsfield S. Muscularity in adult humans: proportion of adipose tissue-free body mass as skeletal muscle. Am J Hum Biol. 2001;13(5):612–9. doi: 10.1002/ajhb.1099. [DOI] [PubMed] [Google Scholar]
- 16.Edmunds L, Williams G, Welch C. External Fistulas Arising from the Gastrointestinal Tract. Annals of Surgery. 1960;152(93):445–69. doi: 10.1097/00000658-196009000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altomare D, Serio G, Pannarale O, Lupo L, Palasciano N, Memeo V, et al. Prediction of mortality by logistic regression analysis in patients with postoperative enterocutaneous fistulae. British Journal of Surgery. 1990;77(4):450–3. doi: 10.1002/bjs.1800770428. [DOI] [PubMed] [Google Scholar]
- 18.Hollington P, Mawdsley J, Lim W, Gabe SM, Forbes A, Windsor AJ. An 11-year experience of enterocutaneous fistula. Br J Surg. 2004;91(12):1646–51. doi: 10.1002/bjs.4788. [DOI] [PubMed] [Google Scholar]
- 19.Rahbour G, Gabe SM, Ullah MR, Thomas GP, Al-Hassi HO, Yassin NA, et al. Seven-year experience of enterocutaneous fistula with univariate and multivariate analysis of factors associated with healing: development of a validated scoring system. Colorectal Dis. 2013;15(9):1162–70. doi: 10.1111/codi.12363. [DOI] [PubMed] [Google Scholar]
- 20.Ravindran P, Ansari N, Young C, Solomon M. Definitive surgical closure of enterocutaneous fistula: outcome and factors predictive of increased postoperative morbidity. Colorectal Dis. 2014;16(3):209–18. doi: 10.1111/codi.12473. [DOI] [PubMed] [Google Scholar]
- 21.Martinez J, Luque-de-Leon E, Ballinas-Oseguera G, Mendez J, Juarez-Oropeza M, Ramon-Ramos R. Factors predictive of recurrence and mortality after surgical repair of enterocutaneous fistula. J Gastrointest Surg. 2012;16(1):156–63. doi: 10.1007/s11605-011-1703-7. [DOI] [PubMed] [Google Scholar]
- 22.Bendersky V, Sun Z, Adam MA, Rushing C, Kim J, Youngwirth L, et al. Determining the Optimal Quantitative Threshold for Preoperative Albumin Level Before Elective Colorectal Surgery. J Gastrointest Surg. 2017 doi: 10.1007/s11605-017-3370-9. [DOI] [PubMed] [Google Scholar]
- 23.Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri S. Preoperative Serum Albumin Level as a Predictor of Operative Mortality and Morbidity. Archives of Surgery. 1999;134:35–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 24.Hickman D, Miller R, Rombeau J, Twomey P, Frey C. Serum albumin and body weight as predictors of postoperative course in colorectal cancer. Journal of Parenteral and Enteral Nutrition. 1980;4(3):314–6. doi: 10.1177/014860718000400315. [DOI] [PubMed] [Google Scholar]
- 25.Shenkin A. Serum Prealbumin: Is It a Marker of Nutritional Status or of Risk of Malnutrition. Clinical Chemistry. 2006;52(12):2177–9. doi: 10.1373/clinchem.2006.077412. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein L, Leukhardt-Fairfield C, Pleban W, Rudolph R. Usefulness of data on albumin and prealbumin concentrations in determining effectiveness of nutritional support. Clin Chem. 1989;35(2):271–4. [PubMed] [Google Scholar]
- 27.Kumpf VJ, de Aguilar-Nascimento JE, Diaz-Pizarro Graf JI, Hall AM, McKeever L, Steiger E, et al. ASPEN-FELANPE Clinical Guidelines. JPEN J Parenter Enteral Nutr. 2017;41(1):104–12. doi: 10.1177/0148607116680792. [DOI] [PubMed] [Google Scholar]
- 28.Goodpaster B, Thaete F, Kelley D. Composition of skeletal muscle evaluated with computer tomography. Ann N Y Acad Sci. 2000;904:18–24. doi: 10.1111/j.1749-6632.2000.tb06416.x. [DOI] [PubMed] [Google Scholar]