Abstract
Malignant glioma is the most common primary brain carcinoma in the world and has a poor survival rate. Previous studies have demonstrated that p53 dysfunction contributes to the development and severity of malignant glioma. It has also been demonstrated that Newcastle disease virus (NDV) may be a viable candidate for the treatment of various types of cancer. In the present study, a p53 oncolytic agent delivered using recombinant NDV (rNDV-p53) was constructed and its anti-tumor effects in vitro and in vivo were assessed. Glioma cell lines and a xenograft mouse model were utilized to assess the ability of p53 and rNDV to promote apoptosis and induce immunotherapy, respectively. The mechanism of rNDV-p53 in glioma therapy was investigated using quantitative polymerase chain reaction and immunohistochemistry. Tumor-specific cytotoxic T-lymphocyte (CTL) responses and lymphocyte infiltration were also analyzed in glioma-bearing models. The results of the present study demonstrate that rNDV-p53 may be a potential therapeutic agent that improves the prognosis of mice with glioma. It was revealed that rNDV-p53 inhibits glioma cell growth and aggressiveness in vitro and in vivo compared with rNDV and p53 alone. The results also demonstrated that rNDV-p53 induced glioma cell apoptosis by upregulating apoptosis-related genes. In addition, the present study demonstrated that rNDV-p53 significantly stimulated CTL responses and lymphocyte infiltration whilst increasing the number of apoptotic bodies in vivo. Furthermore, rNDV-p53 therapy inhibited tumor regression and prolonged the survival of glioma-bearing mice. In conclusion, rNDV-p53 invoked an immune response against glioma cells, which may serve as a comprehensive immunotherapeutic schedule for glioma.
Keywords: glioma, recombinant Newcastle disease virus-p53, apoptosis, immunotherapy
Introduction
Glioma originates from glial cells and is the most aggressive and lethal primary brain tumor of the adult central nervous system (1,2). Patients with glioma are routinely treated with surgical resection combined with radiotherapy, chemotherapy and other comprehensive treatments (3,4). Severe symptoms of glioblastoma, including seizures and cerebral hemorrhage, make the effective treatment of this tumor a challenge (5). It has been demonstrated that that glioblastomas account for ~75% of all malignant brain tumors (6). The World Health Organization categorizes glioblastoma into four grades based on the pathological characteristics of malignancy and indicates that the five-year survival is lower than other types of cancer (7,8). Due to variations in infiltrative growth, the malignant grades of glioblastoma are diverse in appearance (9). Therefore, whilst glioblastoma has accrued clinical interest, developing an effective treatment for patients remains a challenge.
Oncolytic virotherapy may be a promising form of gene therapy for the treatment of various types of cancer. It utilizes a combination of viral oncolytic properties and functional genes to destroy malignant cells (10). Cassel and Murray (11) demonstrated that Newcastle disease virus (NDV) oncolysates may be a promising anticancer agent in patients with stage III malignant melanoma. The potential therapeutic effects of NDV have prompted research into the underlying mechanism of oncolytic viruses (12). Previous studies have demonstrated that NDV exhibits cancer cell selectively by inducing interferon (IFN) and inhibiting NDV replication via its sialic acid receptor. This is unusual, as the majority of tumor cells impair the IFN signaling pathway (13,14). NDV therefore replicates in tumor cells and induces a potent IFN immune response to inhibit tumor cell growth. However, previous studies have also demonstrated that NDV treatment alone is insufficient to fully inhibit tumor growth (15,16).
The tumor suppressor protein p53 is encoded by the tumor protein 53 gene and serves a primary role in a number of pathways, including cell cycle, cell growth, differentiation, apoptosis and cell death (17,18). It has been demonstrated that p53 regulates the variation and repair of cells when exposed to DNA-damaging agents, including ultraviolet radiation, toxins and certain drugs (19). Similar to various other cancers, the occurrence of glioma is associated with genetic mutations in p53 (20–24). The alteration or inactivation of p53 induced by mutations or its interaction with oncogene products of DNA tumor viruses may lead to cancer (25). Low p53 expression occurs in glioma, which leads to dysfunction of the p53-associated regulatory pathways (26). Therefore, dysregulation of p53 may contribute to the regression of glioma therapy.
In the present study, the therapeutic effects of recombinant (r)NDV-p53 in glioma cell lines and tumor models were assessed. The role of p53 as a molecular marker of glioma remains controversial as previous studies have demonstrated that no association exists between p53 and prognosis (27,28). However, the results of the present study demonstrate that rAd-p53 exhibits an anticancer effect in tumor-bearing mice. Intravenous injections of rNDV-p53 in pre-clinical examinations demonstrated that gene-targeted oncolytic virotherapy exhibited marked effects on glioma growth. The present study aimed to assess the efficacy and impact of p53 on the growth, aggressiveness, apoptosis, prognosis and immunoregulatory function of NDV in the treatment of glioma, as well as its effects on T lymphocyte infiltration, immunologic memory and specific toxicity in vivo. The results of the present study provide an insight into the pathophysiology of glioma and suggest that rNDV-p53 may serve as a potential anti-cancer oncolytic drug for the treatment of glioma.
Materials and methods
Ethics statement
The present study was performed in strict accordance with the Guide for the Care and Use of Laboratory Animals of Qianfoshan Hospital (Shandong, China). All experimental protocols involving animals were performed in accordance with the National Institutes of Health and approved by the Ethics Committee of Qianfoshan Hospital of Shandong Province (Shandong, China). All surgery and euthanasia were performed to minimize suffering. The use of human tissue samples was also approved by the Qianfoshan Hospital of Shandong Province.
Patient tissue samples
A total of 4 patients with glioma (2 females, 2 females, aged 46–62 years old) were recruited from the Qianfoshan Hospital of Shandong Province between January 2015 and May 2016. Glioma samples and adjacent non-tumor tissues were obtained from the same individuals. All patients signed a study-specific written informed consent prior to inclusion.
Construction of rNDV
The expression system of NDV was used to construct the rNDV virion. The 585 base pair DNA sequence encoding human p53 (forward primer, 5′-TGGAGGAGCCGCAGTCAGAT-3′, reverse primer, 5′-ATATCGTCCGGGGACAGC-3′) and the enhanced green fluorescent protein (EGFP) were amplified from plasmid expression p53 (pMD-p53) using polymerase chain reaction [PCR; 25 µl volume: Primers, 1 µl, DNA polymerase (Takara Bio, Inc., Otsu, Japan), 1 µl dNTP, 2 µl Buffer, 2 µl water] and subcloned into rNDV plasmids using TA Cloning™ kit (cat. no. K200001; Thermo Fisher Scientific, Inc., Waltham, MA, USA) as described previously and named rNDV-P53 (29). The thermocycling conditions were as follows: 30 cycles of 95°C for 9 sec, 54.5°C for 5 sec and 72°C for 20 sec. The PCR products were analyzed by 1% agarose gel electrophoresis and photographed by Image Master VDS Gel Imaging system (Pharmacia Biotech, Uppsala, Sweden). rNDV-p53 and rNDV-EGFP plasmids were acquired. PCR and gene sequencing were used to select the correct clone and translated into E. coli. rNDV, rNDV-EGFP and rNDV-p53 (10 µg, constructed by the Department of Neurosurgery, Qianfoshan Hospital of Shandong Province) were generated through transfection into CEF1 cells (BeNa Biotechnology, Shanghai, China) using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Following 72 h transfection, rNDVs were propagated in specific pathogen free (SPF) embryonated chickens (Microbiology Laboratory, Shangdong University, Jinan, China). rNDV viruses were purified following a previously described protocol (30). NDV titers were determined using a TCID50 assay using the Reed-Muench method and recorded as plaque-forming units (pfu)/ml, following the protocol of a previous study (31). MOI was calculated by measuring TCID50.
MTT cytotoxicity
Glioblastoma cell lines G422 (cat. no. BNCC340295) and U251 (cat. no. BNCC337874) were purchased from Beijing BeiNa Institute of Biotechnology (Beijing, China) and cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere containing 5% CO2. G422 and U251 cells were then incubated with p53 (10 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), rNDV-EGFP, rNDV-p53 or PBS in a 96-well plate for 96 h at 37°C in triplicate for each condition for 48 h. A total of 20 µl MTT (5 mg/ml) in PBS solution was added to each well and cells were incubated for a further 4 h at 37°C. Medium was removed and 100 µl dimethylsulfoxide was added to dissolve the formazan crystals. Optical density was measured using an ELISA reader at a wavelength of 450 nm.
Animal analysis
A total of 45 SPF female BALB/c nude mice (6 weeks old; body weight, 26–32 g) were purchased from the Harbin Veterinary Research Institute (Harbin, China). All animals were housed in a temperature-controlled facility at 23±1°C and a relative humidity of 50±5%. Animals were subjected to a 12 h light/dark cycle and had ad libitum access to food and water. A total of 100 µl U251 cells at a density of 5×105 were injected into the right flank of mice. Treatment for tumor-bearing mice, rNDV-EGFP or rNDV-p53 was initiated when tumor diameters reached 6–8 mm at 7 days following inoculation. Mice with glioma were randomly divided into 3 groups (n=15) and injected intratumorally with 2×107 pfu rNDV-p53, rAd-EGFP or PBS. Treatment was performed once every other day for a total of 10 days. Tumor diameters were recorded once every 2 days and tumor volume was calculated by using the following formula: 0.52 × smallest diameter2 × largest diameter. Tumor volume was recorded over a 30 day period of observation following the 10 day treatment period. The survival rate of experimental mice was calculated in a long-term experiment conducted over 180 days using Kaplan-Meier method (32).
Cell culture and flow cytometric analysis (FACS)
Cell suspensions (5×106) from the tumors of treated mice were prepared for FACS on day 30. Tumor cell suspensions from experimental mice were filtered through a 100 µm nylon strainer. Tumor cells were then labeled with cluster of differentiation (CD)31 (1:500; cat. no. ab28364; Abcam, Cambridge, UK) and CD69 (1:500; cat. no. ab202909; Abcam) for 12 h at 4°C, followed by an incubation with goat anti-rabbit horseradish peroxidase (HRP)-conjugated immunoglobulin G (IgG; Alexa Fluor® 488, 1:1,000; cat. no. ab150077; Abcam) for 2 h at 37°C to assess the frequency of CD31 and CD69 cell subsets in the total number of infiltrated immune cells. Stained cells were analyzed using a FACScan flow cytometer. To assess cell apoptosis, G422 cells (1×106) were incubated with an Annexin V-fluorescein isothiocyanate/propidium iodide double staining kit (Beyotime Institute of Biotechnology, Haimen, China) for 15 min at room temperature according to the manufacturer's protocol. The ratios of apoptotic cells were measured using a Coulter EPICS XL Flow Cytometer and the results were analyzed using Expo32-ADC v. 1.2B software (Beckman Coulter, Inc., Brea, CA, USA).
Splenocyte collection and cytotoxic T cell (CTL) responses
Splenocytes were obtained from the spleens of experimental mice following treatment. The monoplast suspension was washed three times with PBS. U251 cells were inactivated with ethylalcohol (Sigma-Aldrich; Merck KGaA) for 30 min at 37°C. Inactivated U251 cells were used to incubate splenocytes. IFN-γ levels were assessed using a mouse IFN-γ Quantikine ELISA kit (MIF00; Bio-Rad Laboratories Inc., Hercules, CA, USA) in the supernatants obtained from cell culture fluid following a 72 h culture at 37°C and centrifugation at 3,000 × g for 10 min at room temperature. T cells (1×106) obtained from splenocytes were purified (33) and co-cultured with fresh U251 cells at 37°C for 4 h at effector:target ratios of 5:1, 15:1 and 45:1. CTL activity on target cells was determined using MTT cytotoxicity assays as previously described (34).
Tumor cell migration and invasion assays
G422 and U251 cells were cultured in DMEM and treated with rNDV-EGFP or rNDV-p53. Cells were then incubated in DMEM medium with 5% FBS for 48 h at 37°C using a Transwell insert (BD Biosciences, Franklin Lakes, NJ, USA) instead of a Matrigel invasion chamber to assess migration. In the invasion assay, rNDV or rNDV-p53-treated cells were suspended at a density of 5×104 in 200 µl serum-free DMEM. DMEM medium with 5% PBS were plated in the lower chamber of the BD BioCoat Matrigel invasion chamber (BD Biosciences). G422 and U251 cells and then plated in the upper chamber for 48 h at 37°C following the manufacturer's protocol. After 48 h, the cells that invaded through the membrane were fixed with 3% formaldehyde for 15 min at 37°C and stained with 0.5% crystal violet for 10 min at 37°C. The invasion and migration of tumor cells were assessed in a minimum of three randomly selected fields using an inverted microscope (Olympus BX51; Olympus Corporation, Tokyo, Japan) at ×40 magnification.
Immunohistochemical staining
Tumor tissues were prepared and fixed in 4% paraformaldehyde for 2 h at 37°C. Tissues were deparaffinized in xylene and rehydrated in a graded alcohol series. Following washing with PBS for 15 min at room temperature, tissue sections (4 µm) were prepared and epitope retrieval was performed using Lab Vision™ Tris-HCl buffer for heat-induced epitope retrieval (cat. no. AP-9005-050; Thermo Fisher Scientific, Inc.). Paraffin-embedded sections were quenched with 3% hydrogen peroxide for 15 min and subsequently blocked with 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) for 15 min at 37°C. Sections were then incubated with antibodies against p53 (1:1,000; cat. no. ab1431), p21 (1:1,000; cat. no. ab1091919), caspase-3 (1:1,000; cat. no. ab13847), B cell lymphoma-2 (Bcl2; 1:1,000; cat. no. ab59348), Bcl-2 associated X (Bax; 1:1,000; cat. no. ab32503), Bcl-2 like 11 (Bim; 1:1,000; cat. no. ab32158), CD31 (1:1,000; cat. no. ab28364) and CD69 (1:1,000; cat. no. ab202909) at 4°C for 12 h following blocking. All primary antibodies were sourced from Abcam. All sections were washed three times with PBS and incubated with secondary antibodies HRP-conjugated IgG (1:5,000; PV-6001; OriGene Technologies Inc., Rockville, MD, USA) for 1 h at 37°C. Visualization was achieved using peroxidase-labeled streptavidin-biotin and diaminobenzidine (Advansta, Inc., Menlo Park, CA, USA) for at least 5 min at 37°C. The slides were examined with a Keyence Biozero BZ8100E fluorescence microscope (Keyence, Osaka, Japan) at a magnification of ×40.
Western blotting
G422 and U251 cells were treated with rNDV-EGFP or rNDV-p53 and homogenized in 10% RIPA buffer (Sigma-Aldrich; Merck KGaA) for 1 h at 37°C lysate buffer containing a protease-inhibitor. Cells were then centrifuged at 6,000 × g at 4°C for 10 min and supernatants were analyzed. Protein concentration was measured using a BCA protein assay kit (Thermo Fisher Scientific, Inc.). Protein samples (10 µg) were separated on 12.5% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA) SDS assays were performed as previously described (35). Membranes were then blocked with 5% skimmed milk for 1 h at 37°C and then incubated with the following primary antibodies: p53 (1:1,000; cat. no. ab1431), p21 (1:1,000; cat. no. ab1091919), caspase-3 (1:1,000; cat. no. ab13847), Bcl2 (1:1,000; cat. no. ab59348), Bax (1:1,000; cat. no. ab32503), Bim (1:1,000; cat. no. ab32158) and β-actin (1:1,000; cat. no. ab8226) for 12 h at 4°C. All primary antibodies were supplied by Abcam. The membranes were then incubated with goat anti-rabbit HRP-conjugated IgG secondary antibodies (1:5,000; cat. no. PV-6001; OriGene Technologies, Inc.) at 4°C for 24 h. Blots were imaged using WesternBright ECL Chemiluminescent HRP Substrate (Advansta).
TUNEL analysis
Tumor tissue sections were fixed with 4% paraformaldehyde solution for 2 h at 4°C. Sections were washed three times with PBS and then permeabilized by immersing cells slides in a 0.2% Triton X-100 solution with PBS for 14 min at 4°C. Subsequently, sections were incubated with an equilibration buffer for 14 min at 4°C and were then incubated with 50 µl reaction mixture at 37°C for 60 min and washed 3 times with PBS. The tissues or cells were incubated with 0.5 µg/ml DAPI (Sigma-Aldrich; Merck KGaA) in a humidified chamber in the dark at 37°C for 14 min. Following 3 washes with PBS, the terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling (TUNEL) Apo-Green Detection kit (Biotool, Stratech Scientific, Ltd., Suffolk, UK) was used according to the manufacturers protocol to detect TUNEL-positive cells. Finally, tissue section images were captured at 6 fields of view using a ZEISS LSM 510 confocal microscope (Zeiss AG, Oberkochen, Germany).
Statistical methods
All data are presented as the mean ± standard error of the mean. Unpaired data were analyzed using a Student's t-test. Comparisons between multiple groups were analyzed using one-way analysis of variance followed by Tukey's honest significance difference test. P<0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of rNDV and p53 expression in rAd-p53-infected cells in vitro
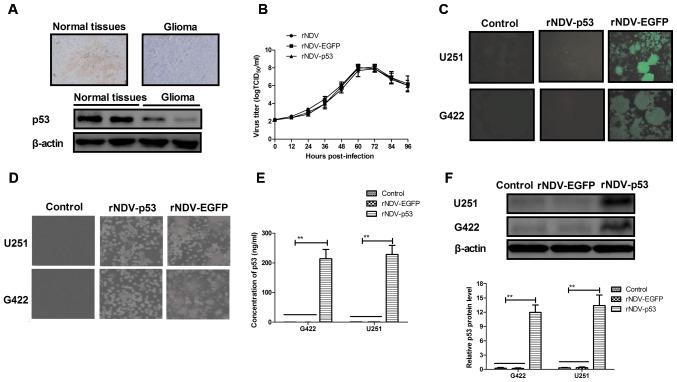
The expression of p53 was assessed in human glioma tissues using immunohistochemistry. It was demonstrated that p53 expression was decreased in human glioma tissues compared with normal adjacent tissue (non-tumor tissue situated around the site of glioma; Fig. 1A). In order to assess the efficiency of rNDV replication, a growth dynamics curve was constructed using CEF1 cells. The results indicated that growth was marginally affected by p53 and EGFP and viruses recovered parental titers following 60 h (Fig. 1B). It was also demonstrated that there was an increased expression of rNDV-EGFP in G422 and U251 cells (Fig. 1C). Increased formation of syncytium in rNDV-p53 treated cells compared with rNDV and controls (Fig. 1D) was also observed. In addition, the endogenous expression of p53 in G422 and U251 cells was assessed using ELISA and western blotting. The results demonstrated that p53 was highly expressed in G422 and U251 cells (Fig. 1E and F). Collectively, these results demonstrate that glioma cells exhibit decreased p53 expression and that rNDV-p53 selectively replicates and expresses p53 in glioma cell lines.
Figure 1.
Characteristics of rNDV and p53 expression in glioma tissues and rNDV-p53-infected cells. (A) p53 expression levels in human glioma tissues and adjacent normal tissue (magnification, ×20). (B) Virus titer of rNDV (control), rNDV-EGFP and rNDV-p53. (C) EGFP expression in rNDV-EGFP and rNDV-p53 infected G422 and U251 cells, as determined using a fluorescent microscope (magnification, ×40). (D) Numbers of rNDV-p53-treated cells in syncytium compared with rNDV-EGFP-treated cells and controls (magnification, ×40). (E) The concentration of p53 expressed in rNDV-p53 and rNDV-EGFP treated G422 and U251 cells using ELISA analysis. (F) Western blot analysis of p53 expressed in rNDV-p53 and rNDV-EGFP treated cells. **P<0.01 vs. control. rNDV, recombinant Newcastle disease virus; EGFP, enhanced green fluorescent protein.
Characterization of p53 expressed by rNDV-p53 treated glioma cells
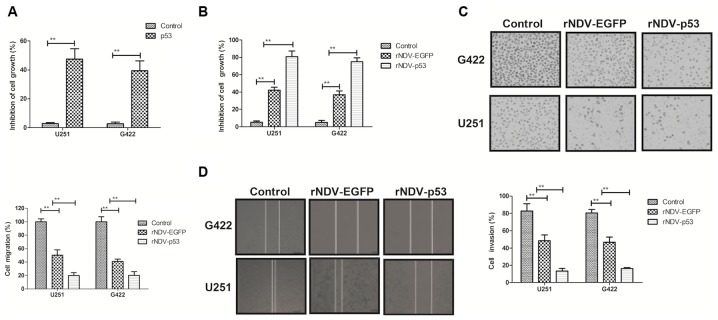
The biological activity of p53 in rNDV-p53-transfected glioma cells was assessed. It was demonstrated that p53 (10 mg/ml) significantly inhibited the growth of G422 and U251 cells (P<0.01; Fig. 2A). The inhibitory effects of rNDV-p53 on glioma cells were observed in vitro. The rate of inhibition was significantly increased in cells treated with rNDV-p53 compared with those that received PBS and rNDV-EGFP (P<0.01; Fig. 2B). The expression of p53, delivered by rNDV-p53, significantly suppressed the migration and invasion of G422 and U251 cells compared with those treated with rNDV-EGFP (P<0.01; Fig. 2C and D). These results indicate that rNDV-p53 inhibits the growth and aggressiveness of glioma cells.
Figure 2.
Inhibitory effects of rNDV-p53 on glioma tumor cells. The inhibitory effects of (A) p53 and (B) rNDV-p53/rNDV-EGFP treatment on G422 and U251 cell growth. (C) Migration and (D) invasion of G422 and U251 cells treated with rNDV-p53, rNDV-EGFP or PBS. **P<0.01 vs. control. rNDV, recombinant Newcastle disease virus; EGFP, enhanced green fluorescent protein.
rNDV-p53 downregulates anti-apoptosis and activated pro-apoptosis proteins
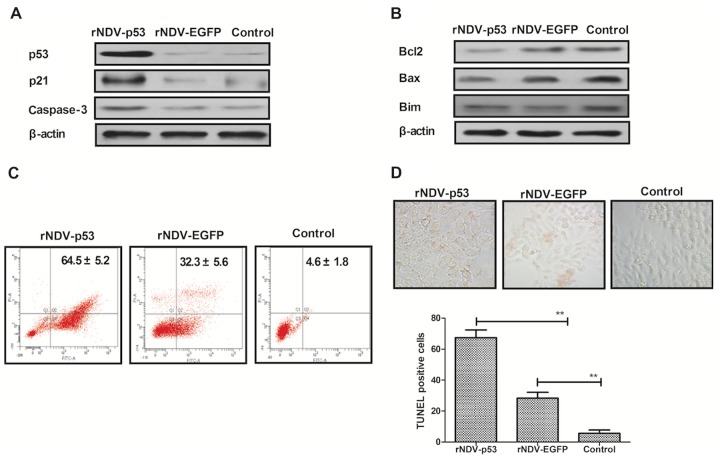
It has been demonstrated that U251 is more aggressive than other glioma cell lines (36). U251 cells were therefore utilized to assess the mechanism of rNDV-p53-mediated apoptosis. To investigate whether rNDV-p53 upregulates p53 downstream proteins and enhances glioma cell apoptosis, the expression of p53, p21 and caspase-3 was examined following treatment for 48 h (Fig. 3A). The rNDV-p53 virus markedly enhanced the transcriptional activity of p53, p21 and caspase-3. rNDV-p53 treatment also markedly inhibited the expression of anti-apoptotic proteins, including Bcl-2, Bim and Bax in U251 cells (Fig. 3B). In addition, it was demonstrated that rNDV-p53 promotes U251 cell apoptosis at a multiplicity of infection (MOI) of 5 for 24 h when compared with rNDV-EGFP infected and control cells (Fig. 3C). In addition, TUNEL staining analysis demonstrated a significantly increased number of TUNEL-positive cells in rNDV-p53 treated cells (MOI=5) following 48 h incubation (P<0.01; Fig. 3D). These results indicate that rNDV-p53 promotes glioma cell apoptosis.
Figure 3.
rNDV-p53 promotes and activates anti-apoptotic proteins. (A) p53, p21 and caspase-3 levels in U251 cells following treatment with rNDV-p53, rNDV-EGFP or controls. (B) Bcl-2, Bim and Bax expression in U251 cells following treatment with rNDV-p53, rNDV-EGFP or controls. (C) rNDV-p53 treatment for 24 h promoted U251 cell apoptosis at an MOI of 5, compared with rNDV-EGFP and control treated cells. (D) TUNEL-positive U251 cells incubated for 48 h with rNDV-p53, rNDV-EGFP or control (MOI=5; magnification, ×40). **P<0.01 vs. control. rNDV, recombinant Newcastle disease virus; EGFP, enhanced green fluorescent protein; Bcl-2, B cell lymphoma-2; Bim, Bcl-2 like 11; Bax, Bcl-2 associated X; MOI, multiplicity of infection.
In vivo anti-tumor efficacy of rNDV-p53
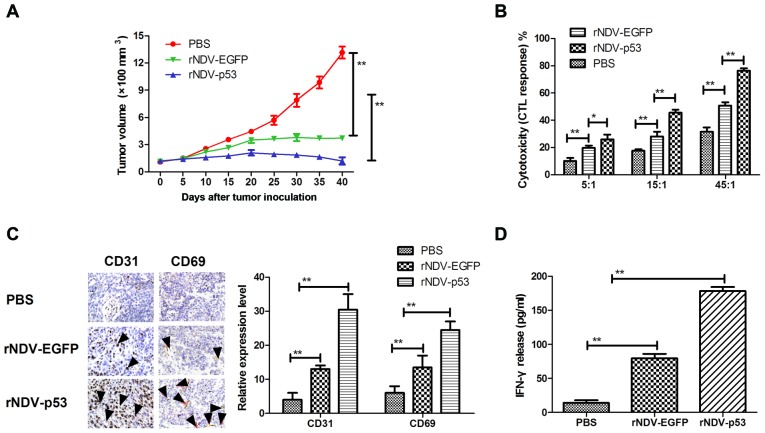
In order to assess the therapeutic effects of rNDV-p53, mice were xenografted with glioma tissue and treated with rNDV-p53 via intravenous injection with PBS and rAd-EGFP as a control. The results demonstrated that rNDV-p53 significantly inhibited glioma growth compared with rNDV-EGFP and PBS treated mice over the 30 day observation period (P<0.01; Fig. 4A). In addition, CTL responses against U251 cells were assessed. Mice treated with rNDV-p53 developed a stronger CTL response against U251 cells compared with rNDV-EGFP and PBS groups (Fig. 4B). Furthermore, CD65 and CD31 cell infiltration was upregulated in tumors following treatment with rNDV-EGFP (Fig. 4C). Mice treated with rNDV-p53 also exhibited a significantly higher IFN-γ release compared with those that received rNDV-EGFP and PBS treatment (P<0.01; Fig. 4D).
Figure 4.
Anti-tumor efficacy of rNDV-p53 in U251-bearing mice. (A) rNDV-p53 treatment inhibited glioma growth over a 40 day observation. (B) CTL responses against rNDV-p53, rNDV-EGFP or PBS treated U251 cells. (C) CD31 and CD69 expression and (D) tumor-specific IFN-γ release from stimulated splenocytes in the murine glioma model analyzed using ELISA. The black arrows indicate CD31 and CD69-positive cells (magnification, ×20). *P<0.05 vs control; **P<0.01 vs. control. rNDV, recombinant Newcastle disease virus; EGFP, enhanced green fluorescent protein; CTL, cytotoxic T lymphocytes; CD, cluster of differentiation; IFN-γ, interferon-γ.
rNDV-p53 induces glioma tumor apoptosis and prolongs survival in vivo
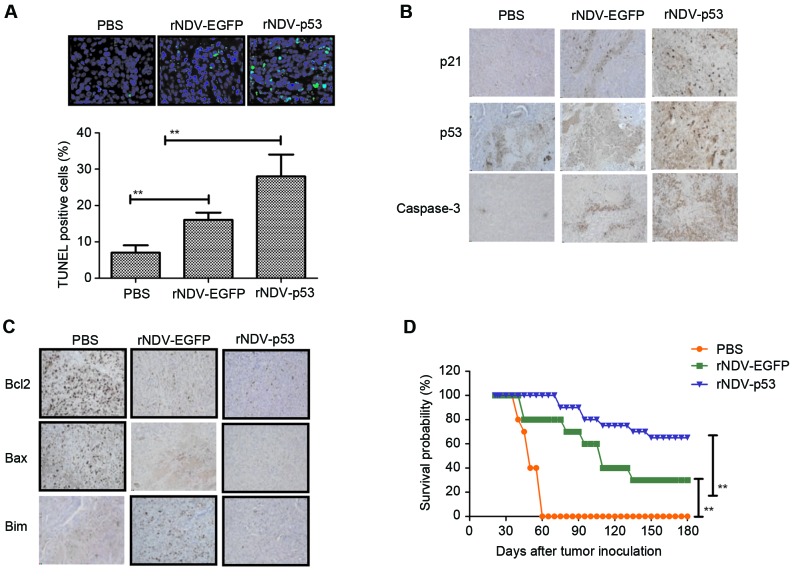
The obtained results indicated that rNDV-p53 inhibits the progression of U251-bearing mice. Apoptosis rates in tumors following treatment with rNDV-p53, rNDV-EGFP or PBS was verified using TUNEL staining (Fig. 5A) and it was demonstrated that tumor growth was inhibited. Additionally, the downstream molecules of p53, including p21 and caspase 3, were assessed by performing immunohistochemical staining. Results demonstrated that rNDV-p53 treatment upregulated these molecules in murine tumors (Fig. 5B). Further analysis revealed that rNDV-p53 treatment induced the downregulation of anti-apoptotic proteins, including Bcl-2, Bim and Bax compared with mice that received rNDV-EGFP and PBS (Fig. 5C). The long-term survival rate of mice following drug treatment was calculated to be 180 days. The results of the current study demonstrated that rNDV-p53 significantly prolonged the survival of mice compared with control groups (P<0.01; Fig. 5D). These data suggest that rNDV-p53 efficiently inhibits glioma and eliminates tumor cells by initiating apoptosis, which contributes to long-term tumor-free survival.
Figure 5.
Cell apoptosis and mice survival rate in rNDV-p53 treated glioma. (A) TUNEL-positive glioma cells following treatment with rNDV-p53, rNDV-EGFP or PBS (magnification, ×20). (B) p53, p21 and caspase3 and (C) Bcl-2, Bax and Bim expression in tumors from experimental mice following treatment with rNDV-p53, rNDV-EGFP or PBS (magnification, ×20). (D) Survival rates of rNDV-p53, rNDV-EGFP or PBS treated U251-bearing mice over a 180-day observation. **P<0.01 vs. control. rNDV, recombinant Newcastle disease virus; EGFP, enhanced green fluorescent protein; Bcl-2, B cell lymphoma-2; Bax, Bcl-2 associated X; Bim, Bcl-2 like 11.
Discussion
The results of the present current study indicate that rNDV-p53 enhances the anti-cancer potential of rNDV and p53 and exhibits strong inhibitory effects on glioma cells in vitro and in vivo. It was also demonstrated that rNDV-p53 induces an immune response against U251 cells. NDV-mediated p53 gene expression exhibited increased anti-tumor effects through NDV mediated cell death and p53-mediated apoptosis induction. Anti-apoptotic protein expression levels were significantly increased in glioma cells following treatment with rNDV-p53, indicating that the apoptosis-resistance observed in various types of cancer had been attenuated. In addition, it was demonstrated that rNDV-p53 treatment initiates an anti-tumor immune response, protecting mouse tissues. These results indicate that disruption to rNDV-p53-glioma interactions may be an effective future anti-cancer target.
NDV was utilized as an anti-tumor agent based on a previous study that examined a patient with cervical carcinoma (37). The oncolytic mechanism of NDV has been examined in various carcinomas using reverse genetic technology (38), which involves the insertion of certain functional genes or proteins into the genome of NDV to enhance the therapeutic effects of oncolytic virotherapy (39). Keshelava et al (40) assessed the efficacy of NDV and neoadjuvant therapy in 84 patients with breast cancer. The results of the study indicated that the LaSotha strain of NDV was an efficient and safe from of immunotherapy and neoadjuvant treatment. In addition, Schulze et al (41) examined NDV immunization combined with modified colorectal cancer cells in patients with colorectal cancer. It was demonstrated that patient survival rate improved following treatment, indicating that this therapy may have anti-cancer effects. Additionally, Bai et al (42) revealed that genetically engineered NDV expressing interleukin (IL)-2 may be a potential candidate for cancer immunotherapy in patients with hepatic carcinoma and melanoma. Chai et al (43) assessed the use of rNDV in patients with lung cancer, generated using reverse genetics based on the oncolytic D90 strain and carrying a gene encoding EGFP. Furthermore, NDV may trigger U251 glioma cell autophagy to enhance viral replication and promote the apoptosis of tumor cells (44). It has also been demonstrated that NDV inhibits the decrease in Rac1 gene expression exhibited in glioma tumors (45). In the present study, an rNDV encoding p53 was constructed to assess efficacy in glioma cells and animals. The results indicate that rNDV-p53 inhibits glioma cell growth and aggressiveness in vitro as well as suppressing tumor growth through the accumulation and infiltration of T lymphocytes and apoptosis in vivo.
The p53 tumor suppressor gene is mutated in a variety of cancers and is therefore an important area of cancer research (46). Elucidating the scope of p53 mutations allows for the better understanding of cancer etiology and the molecular pathogenesis of neoplasia (47,48). The detection of p53 abnormalities may have diagnostic, prognostic and therapeutic implications in patients with cancer (49,50). A previous study demonstrated that p53 mutations are important for tumor classification and gliomagenesis (19). The most common p53 mutations identified in glioma occur in the DNA-binding domain, specifically within six hotspot mutation sites (51). p53 protects against neoplastic transformation and exhibits effects on certain processes, including cell cycle modulation, DNA repair, apoptosis, senescence, angiogenesis and metabolism, resulting in the formation of a complex signaling network (50,52). However, the distinct effect of p53 in glioma therapy is yet to be elucidated.
Immunotherapy involving anti-tumor surface antigens that is used alongside other therapeutic methods, including chemoradiotherapy and surgery, has been observed to have therapeutic activities in animal models of different types of cancer (53–55). The use of anti-neoplastic agents and immunotherapy is an effective therapy for tumor cells with specific recognition molecules, antigen domains or receptors (56–59). The results of the present study indicate that rNDV-p53 stimulates the immune system induce glioma cell death in U251-bearing mice. Additionally, p53 gene therapy delivered by NDV is an effective gene delivering system.
The present study demonstrates that rNDV-p53 treatment inhibits murine glioma growth by stimulating T cell proliferation, memory T cell responses, CTL responses and IFN-γ release targeted against tumor cells. The mechanism of rNDV-p53-mediated anti-glioma therapy was also assessed and the results demonstrated a significant increase in tumor cell apoptotic rate. In conclusion, the present study indicates that rNDV-p53 possesses potential beneficial effects for the oncolytic efficacy of NDV by expressing p53 in the treatment of glioma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science Foundation of Shandong Province (grant no. 2013ZRB14142) and the Development Foundation of Shandong Medical and Health Science and Technology (grant no. 2016WS0480).
Availability of data and materials
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Authors' contributions
XF, HL, YC, XH analyzed and interpreted the data. XF, XH, CH, GL performed the experiments, and GL was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was performed in strict accordance with the Guide for the Care and Use of Laboratory Animals of Qianfoshan Hospital (Shandong, China). All experimental protocols involving animals were performed in accordance with the National Institutes of Health and approved by the Ethical Committee of Qianfoshan Hospital of Shandong Province (Shandong, China). All surgery and euthanasia were performed to minimize suffering. The use of human tissue samples was also approved by the Qianfoshan Hospital of Shandong Province and all patients signed a study-specific written informed consent prior to inclusion.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
References
- 1.Lu M, Zhang X, Zhang M, Chen H, Dou W, Li S, Dai J. Non-model segmentation of brain glioma tissues with the combination of DWI and fMRI signals. Biomed Mater Eng. 2015;26:S1315–S1324. doi: 10.3233/BME-151429. [DOI] [PubMed] [Google Scholar]
- 2.Chow KK, Naik S, Kakarla S, Brawley VS, Shaffer DR, Yi Z, Rainusso N, Wu MF, Liu H, Kew Y, et al. T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther. 2013;21:629–637. doi: 10.1038/mt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Englot DJ, Berger MS, Chang EF, Garcia PA. Characteristics and treatment of seizures in patients with high-grade glioma: A review. Neurosurg Clin N Am. 2012;23:227–235. doi: 10.1016/j.nec.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Siu A, Wind JJ, Iorgulescu JB, Chan TA, Yamada Y, Sherman JH. Radiation necrosis following treatment of high grade glioma-a review of the literature and current understanding. Acta neurochir(Wien) 2012;154:191–201. doi: 10.1007/s00701-011-1228-6. [DOI] [PubMed] [Google Scholar]
- 5.Taphoorn MJ, Bottomley A. Health-related quality of life and symptom research in glioblastoma multiforme patients. Expert Rev Pharmacoecon Outcomes Res. 2005;5:763–774. doi: 10.1586/14737167.5.6.763. [DOI] [PubMed] [Google Scholar]
- 6.Delgado-López PD, Corrales-Garcia EM. Survival in glioblastoma: A review on the impact of treatment modalities. Clin Transl Oncol. 2016;18:1062–1071. doi: 10.1007/s12094-016-1497-x. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin R, Capparella J, Brown A. Classification of glioblastoma multiforme in adults by molecular genetics. Cancer J. 2003;9:82–90. doi: 10.1097/00130404-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Ortega A, Sarmiento JM, Ly D, Nuño M, Mukherjee D, Black KL, Patil CG. Multiple resections and survival of recurrent glioblastoma patients in the temozolomide era. J Clin Neurosci. 2016;24:105–111. doi: 10.1016/j.jocn.2015.05.047. [DOI] [PubMed] [Google Scholar]
- 9.Koekkoek JA, Postma TJ, Heimans JJ, Reijneveld JC, Taphoorn MJ. Antiepileptic drug treatment in the end-of-life phase of glioma patients: A feasibility study. Support Care Cancer. 2015;24:1633–1638. doi: 10.1007/s00520-015-2930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaRocca CJ, Davydova J. Oncolytic virotherapy increases the detection of microscopic metastatic disease at time of staging laparoscopy for pancreatic adenocarcinoma. EBioMedicine. 2016;7:15–16. doi: 10.1016/j.ebiom.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassel WA, Murray DR. A ten-year follow-up on stage II malignant melanoma patients treated postsurgically with Newcastle disease virus oncolysate. Med Oncol Tumor Pharmacother. 1992;9:169–171. doi: 10.1007/BF02987752. [DOI] [PubMed] [Google Scholar]
- 12.Yaacov B, Eliahoo E, Lazar I, Ben-Shlomo M, Greenbaum I, Panet A, Zakay-Rones Z. Selective oncolytic effect of an attenuated Newcastle disease virus (NDV-HUJ) in lung tumors. Cancer Gene Ther. 2008;15:795–807. doi: 10.1038/cgt.2008.31. [DOI] [PubMed] [Google Scholar]
- 13.Liang W, Wang H, Sun TM, Yao WQ, Chen LL, Jin Y, Li CL, Meng FJ. Application of autologous tumor cell vaccine and NDV vaccine in treatment of tumors of digestive tract. World J Gastroenterol. 2003;9:495–498. doi: 10.3748/wjg.v9.i3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song KY, Wong J, Gonzalez L, Sheng G, Zamarin D, Fong Y. Antitumor efficacy of viral therapy using genetically engineered Newcastle disease virus [NDV(F3aa)-GFP] for peritoneally disseminated gastric cancer. J Mol Med (Berl) 2010;88:589–596. doi: 10.1007/s00109-010-0605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puhlmann J, Puehler F, Mumberg D, Boukamp P, Beier R. Rac1 is required for oncolytic NDV replication in human cancer cells and establishes a link between tumorigenesis and sensitivity to oncolytic virus. Oncogene. 2010;29:2205–2216. doi: 10.1038/onc.2009.507. [DOI] [PubMed] [Google Scholar]
- 16.Janke M, Peeters B, de Leeuw O, Moorman R, Arnold A, Fournier P, Schirrmacher V. Recombinant Newcastle disease virus (NDV) with inserted gene coding for GM-CSF as a new vector for cancer immunogene therapy. Gene Ther. 2007;14:1639–1649. doi: 10.1038/sj.gt.3303026. [DOI] [PubMed] [Google Scholar]
- 17.Dastjerdi MN, Valiani A, Mardani M, Ra MZ. Adenosine A1 receptor modifies P53 expression and apoptosis in breast cancer cell line Mcf-7. Bratisl Lek Listy. 2016;117:242–246. doi: 10.4149/bll_2016_046. [DOI] [PubMed] [Google Scholar]
- 18.Ohara M, Matsuura K, Akimoto E, Noma M, Doi M, Nishizaka T, Kagawa N, Itamoto T. Prognostic value of Ki67 and p53 in patients with estrogen receptor-positive and human epidermal growth factor receptor 2-negative breast cancer: Validation of the cut-off value of the Ki67 labeling index as a predictive factor. Mol Clin Oncol. 2016;4:648–654. doi: 10.3892/mco.2016.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karsy M, Neil JA, Guan J, Mahan MA, Colman H, Jensen RL. A practical review of prognostic correlations of molecular biomarkers in glioblastoma. Neurosurg Focus. 2015;38:E4. doi: 10.3171/2015.1.FOCUS14755. [DOI] [PubMed] [Google Scholar]
- 20.Yamanishi Y, Boyle DL, Green DR, Keystone EC, Connor A, Zollman S, Firestein GS. p53 tumor suppressor gene mutations in fibroblast-like synoviocytes from erosion synovium and non-erosion synovium in rheumatoid arthritis. Arthritis Res Ther. 2005;7:R12–R18. doi: 10.1186/ar1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohgaki H, Eibl RH, Schwab M, Reichel MB, Mariani L, Gehring M, Petersen I, Höll T, Wiestler OD, Kleihues P. Mutations of the p53 tumor suppressor gene in neoplasms of the human nervous system. Mol Carcinog. 1993;8:74–80. doi: 10.1002/mc.2940080203. [DOI] [PubMed] [Google Scholar]
- 22.Sakai E, Rikimaru K, Ueda M, Matsumoto Y, Ishii N, Enomoto S, Yamamoto H, Tsuchida N. The p53 tumor-suppressor gene and ras oncogene mutations in oral squamous-cell carcinoma. Int J Cancer. 1992;52:867–872. doi: 10.1002/ijc.2910520606. [DOI] [PubMed] [Google Scholar]
- 23.Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: Clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855–4878. [PubMed] [Google Scholar]
- 24.Rivlin N, Brosh R, Oren M, Rotter V. Mutations in the p53 Tumor suppressor gene: Important milestones at the various steps of tumorigenesis. Genes Cancer. 2011;2:466–474. doi: 10.1177/1947601911408889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yue Q, Yulong G, Liting Q, Shuai Y, Delong L, Yubao L, Lili J, Sidang L, Xiaomei W. Mutations in and expression of the tumor suppressor gene p53 in egg-type chickens infected with subgroup j avian leukosis virus. Vet Pathol. 2014;52:1052–1056. doi: 10.1177/0300985814560232. [DOI] [PubMed] [Google Scholar]
- 26.Sitarek P, Skala E, Toma M, Wielanek M, Szemraj J, Nieborowska-Skorska M, Kolasa M, Skorski T, Wysokińska H, Śliwiński T. A preliminary study of apoptosis induction in glioma cells via alteration of the Bax/Bcl-2-p53 axis by transformed and non-transformed root extracts of Leonurus sibiricus L. Tumour Biol. 2016;37:8753–8764. doi: 10.1007/s13277-015-4714-2. [DOI] [PubMed] [Google Scholar]
- 27.Chen GX, Zheng LH, Liu SY, He XH. rAd-p53 enhances the sensitivity of human gastric cancer cells to chemotherapy. World J Gastroenterol. 2011;17:4289–4297. doi: 10.3748/wjg.v17.i38.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie Q, Liang BL, Wu YH, Zhang J, Chen MW, Liu HY, Gu XF, Xu J. Synergistic anticancer effect of rAd/P53 combined with 5-fluorouracil or iodized oil in the early therapeutic response of human colon cancer in vivo. Gene. 2012;499:303–308. doi: 10.1016/j.gene.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Walker A, Taylor J, Rowe D, Summers D. A method for generating sticky-end PCR products which facilitates unidirectional cloning and the one-step assembly of complex DNA constructs. Plasmid. 2008;59:155–162. doi: 10.1016/j.plasmid.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Yan F, Zheng Y, Huang L. Adenovirus-mediated combined anti-angiogenic and pro-apoptotic gene therapy enhances antitumor efficacy in hepatocellular carcinoma. Oncol Lett. 2013;5:348–354. doi: 10.3892/ol.2012.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai FL, Yu YH, Tian H, Ren GP, Wang H, Zhou B, Han XH, Yu QZ, Li DS. Genetically engineered Newcastle disease virus expressing interleukin-2 and TNF-related apoptosis-inducing ligand for cancer therapy. Cancer Biol Ther. 2014;15:1226–1238. doi: 10.4161/cbt.29686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abd El Hafeez S, Torino C, D'Arrigo G, Bolignano D, Provenzano F, Mattace-Raso F, Zoccali C, Tripepi G. An overview on standard statistical methods for assessing exposure-outcome link in survival analysis (Part II): The kaplan-meier analysis and the cox regression method. Aging Clin Exp Res. 2012;24:203–206. doi: 10.1007/BF03325249. [DOI] [PubMed] [Google Scholar]
- 33.Greaves MF, Brown G. Purification of human T and B lymphocytes. J Immunol. 1974;112:420–423. [PubMed] [Google Scholar]
- 34.Zamarin D, Vigil A, Kelly K, Garcia-Sastre A, Fong Y. Genetically engineered Newcastle disease virus for malignant melanoma therapy. Gene Ther. 2009;16:796–804. doi: 10.1038/gt.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wai-Hoe L, Wing-Seng L, Ismail Z, Lay-Harn G. SDS-PAGE-Based quantitative assay for screening of kidney stone disease. Biol Proced Online. 2009;11:145–160. doi: 10.1007/s12575-009-9007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang R, Wang R, Chen Q, Chang H. Inhibition of autophagy using 3-methyladenine increases cisplatin-induced apoptosis by increasing endoplasmic reticulum stress in U251 human glioma cells. Mol Med Rep. 2015;12:1727–1732. doi: 10.3892/mmr.2015.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoeck M, Marland-Noske C, Manasterski M, Zawatzky R, Horn S, Möbus V, Schlag P, Schirrmacher V. In vitro expansion and analysis of T lymphocyte microcultures obtained from the vaccination sites of cancer patients undergoing active specific immunization with autologous Newcastle-disease-virus-modified tumour cells. Cancer Immunol Immunother. 1993;37:240–244. doi: 10.1007/BF01518517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vigil A, Martinez O, Chua MA, Garcia-Sastre A. Recombinant Newcastle disease virus as a vaccine vector for cancer therapy. Mol Ther. 2008;16:1883–1890. doi: 10.1038/mt.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schirrmacher V, Fournier P. Newcastle disease virus: A promising vector for viral therapy, immune therapy and gene therapy of cancer. Methods Mol Biol. 2009;542:565–605. doi: 10.1007/978-1-59745-561-9_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keshelava VV, Dobrovol'skaia Nlu, Chazova NL, Bershchanskaia AM, Podol'skaia MV, Garmarnik TV, Mel'nikova NV. Neoadjuvant therapy of breast cancer using Newcastle disease virus. Vopr Onkol. 2009;55:433–435. [PubMed] [Google Scholar]
- 41.Schulze T, Kemmner W, Weitz J, Wernecke KD, Schirrmacher V, Schlag PM. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: Results of a prospective randomized trial. Cancer Immunol Immunother. 2009;58:61–69. doi: 10.1007/s00262-008-0526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bai F, Niu Z, Tian H, Li S, Lv Z, Zhang T, Ren G, Li D. Genetically engineered Newcastle disease virus expressing interleukin 2 is a potential drug candidate for cancer immunotherapy. Immunol Lett. 2014;159:36–46. doi: 10.1016/j.imlet.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Chai Z, Zhang P, Fu F, Zhang X, Liu Y, Hu L, Li X. Oncolytic therapy of a recombinant Newcastle disease virus D90 strain for lung cancer. Virol J. 2014;11:84. doi: 10.1186/1743-422X-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng C, Zhou Z, Jiang K, Yu S, Jia L, Wu Y, Liu Y, Meng S, Ding C. Newcastle disease virus triggers autophagy in U251 glioma cells to enhance virus replication. Arch Virol. 2012;157:1011–1018. doi: 10.1007/s00705-012-1270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mustafa Z, Shamsuddin HS, Ideris A, Ibrahim R, Jaafar H, Ali AM, Abdullah JM. Viability reduction and Rac1 gene downregulation of heterogeneous ex-vivo glioma acute slice infected by the oncolytic Newcastle disease virus strain V4UPM. Biomed Res Int. 2013;2013:248507. doi: 10.1155/2013/248507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bressy C, Hastie E, Grdzelishvili VZ. Combining oncolytic virotherapy with p53 tumor suppressor gene therapy. Mol Ther Oncolytics. 2017;5:20–40. doi: 10.1016/j.omto.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 48.Hamzehloie T, Mojarrad M, Hasanzadeh Nazarabadi M, Shekouhi S. The role of tumor protein 53 mutations in common human cancers and targeting the murine double minute 2-p53 interaction for cancer therapy. Iran J Med Sci. 2012;37:3–8. [PMC free article] [PubMed] [Google Scholar]
- 49.Fagin JA. Tumor suppressor genes in human thyroid neoplasms: p53 mutations are associated undifferentiated thyroid cancers. J Endocrinol Invest. 1995;18:140–142. doi: 10.1007/BF03349723. [DOI] [PubMed] [Google Scholar]
- 50.Harris CC, Hollstein M. Clinical implications of the p53 tumor-suppressor gene. N Engl J Med. 1993;329:1318–1327. doi: 10.1056/NEJM199310283291807. [DOI] [PubMed] [Google Scholar]
- 51.Casson AG, Evans SC, Gillis A, Porter GA, Veugelers P, Darnton SJ, Guernsey DL, Hainaut P. Clinical implications of p53 tumor suppressor gene mutation and protein expression in esophageal adenocarcinomas: Results of a ten-year prospective study. J Thorac Cardiovasc Surg. 2003;125:1121–1131. doi: 10.1067/mtc.2003.176. [DOI] [PubMed] [Google Scholar]
- 52.Kuczyk MA, Serth J, Hervatin C, Arndt H, Derendorf L, Thon WF, Jonas U. Detection of P53 tumor-suppressor-gene protein in bladder tumors and prostate cancer: Possible clinical implications. World J Urol. 1994;12:345–351. doi: 10.1007/BF00184117. [DOI] [PubMed] [Google Scholar]
- 53.Thomas AA, Ernstoff MS, Fadul CE. Immunotherapy for the treatment of glioblastoma. Cancer J. 2012;18:59–68. doi: 10.1097/PPO.0b013e3182431a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larsen CJ. Cellular immunotherapy and glioblastoma: A hopeful treatment? Bull Cancer. 2011;98:457. [PubMed] [Google Scholar]
- 55.Varghese S, Rabkin SD, Nielsen GP, MacGarvey U, Liu R, Martuza RL. Systemic therapy of spontaneous prostate cancer in transgenic mice with oncolytic herpes simplex viruses. Cancer Res. 2007;67:9371–9379. doi: 10.1158/0008-5472.CAN-07-0674. [DOI] [PubMed] [Google Scholar]
- 56.Husain SR, Behari N, Kreitman RJ, Pastan I, Puri RK. Complete regression of established human glioblastoma tumor xenograft by interleukin-4 toxin therapy. Cancer Res. 1998;58:3649–3653. [PubMed] [Google Scholar]
- 57.Debinski W, Gibo DM, Obiri NI, Kealiher A, Puri RK. Novel anti-brain tumor cytotoxins specific for cancer cells. Nat Biotechnol. 1998;16:449–453. doi: 10.1038/nbt0598-449. [DOI] [PubMed] [Google Scholar]
- 58.Bera TK, Viner J, Brinkmann E, Pastan I. Pharmacokinetics and antitumor activity of a bivalent disulfide-stabilized Fv immunotoxin with improved antigen binding to erbB2. Cancer Res. 1999;59:4018–4022. [PubMed] [Google Scholar]
- 59.Ghetie MA, Richardson J, Tucker T, Jones D, Uhr JW, Vitetta ES. Antitumor activity of Fab' and IgG-anti-CD22 immunotoxins in disseminated human B lymphoma grown in mice with severe combined immunodeficiency disease: Effect on tumor cells in extranodal sites. Cancer Res. 1991;51:5876–5880. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.