Abstract
Currently, ovarian cancer is identified as one of the leading causes of cancer-associated mortality in females. Despite numerous efforts that were made on developing novel treatments for ovarian cancer, the survival rate remains unsatisfactory. Considering the important regulatory role of miRNAs in different types of cancer, the present study aims to identify a novel therapeutic target for treatment of ovarian cancer. The expression of miR-149 was detected using reverse transcription-quantitative polymerase chain reaction in cancerous and normal cells. Furthermore, the effects of miR-149 on ovarian cancer cell activities were investigated using MTT assay, colony formation, flow cytometry and western blotting analysis. In the present study, it was revealed that microRNA (miR)-149 was significantly downregulated in ovarian cancer tissues and cell lines, and that the miR-149 expression was correlated with the patient prognosis. In addition, it was observed that forced expression of miR-149 increased the sensitivity of ovarian cancer cell to cisplatin. Based on bioinformatics analysis and luciferase assay, X-linked inhibitor of apoptosis (XIAP) was identified as a direct target gene of miR-149 in ovarian cancer cells. It was also demonstrated that XIAP expression was upregulated in the ovarian cancer tissues and cell lines, while it was negatively correlated with miR-149 in these tissues and cells. Furthermore, results revealed that ectopic expression of XIAP was able to abolish the miR-149-enhanced cell sensitivity to cisplatin. In conclusion, the present study revealed that miR-149 functioned as a tumor suppressor in the progression of ovarian cancer, increasing the sensitivity of ovarian cancer cells to cisplatin treatment.
Keywords: ovarian cancer, microRNA-149, X-linked inhibitor of apoptosis, chemoresistance
Introduction
Ovarian cancer is one of the leading causes of cancer-associated mortality in females. As epithelial ovarian cancer accounts for ~90% of all ovarian cancer cases worldwide, it is considered to be one of the most common types of gynecological tumor (1,2). Due to the lack of effective means for early diagnosis, the majority of patients are diagnosed at an advanced stage of the disease, and the 5-year survival rate of advanced ovarian cancer remains unsatisfactory, at only 20–25% (3). Cisplatin-mediated chemotherapy has been used to improve the prognosis and survival patients with advanced stage ovarian cancer (2). However, the clinical application of this therapeutic agent is limited by chemoresistance. Therefore, it is necessary to investigate the mechanism underlying the development of chemoresistance, which may assist in developing novel treatment strategies for ovarian cancer patients with chemoresistance.
MicroRNAs (miRNAs or miRs) are a class of small (18–25 nucleotides), endogenous, non-coding RNAs, which function by directly binding to the 3′-untranslated region (3′UTR) of their target mRNAs, causing translational inhibition of proteins and mRNA degradation (4). miRNAs have been documented to be involved in various biological processes, including tumorigenesis through the regulation of cell proliferation, apoptosis, differentiation, migration, invasion, epithelial-mesenchymal transition and chemoresistant phenotype formation (5–11). Several previous studies have reported that miR-149 serves crucial roles in the progression of various tumors. For instance, Chen et al (12) demonstrated that downregulated expression of miR-149 promoted apoptosis in side population cells that were sorted from the TSU prostate cancer cell lines. In addition, miR-149-3p-Wnt-1 signaling was observed to be involved in 18β-glycyrrhetinic acid-mediated gastric cancer suppression (13). However, the mechanism underlying the effect of miR-149 in ovarian cancer remains unclear.
In the present study, miR-149 expression was observed to be downregulated in ovarian cancer tissues and cell lines. Low miR-149 expression was associated with a poor patient prognosis, whereas forced expression of miR-149 increased the sensitivity of ovarian cancer cell to cisplatin treatment. Furthermore, it was demonstrated that X-linked inhibitor of apoptosis (XIAP) was a target of miR-149 and was involved in the function of miR-149 in ovarian cancer.
Patients and methods
Patients
A total of 58 human epithelial ovarian tumor tissues and adjacent normal ovarian tissues were obtained from the patients who received chemotherapy for ovarian cancer in the Department of Gynecology, Affiliated Hospital of Qingdao University (Qingdao, China) between September 2010 and October 2015. Patients were between 25 and 85 years of age, with a mean age of 44.5±3.4. According to the criteria of the International Federation of Gynecology and Obstetrics, International Gynecologic Cancer Society (14), these patients were divided into three stages (stage I, n=12; stage II, n=28; stage III, n=18). The exclusion criteria of samples are as follows: i) Patients who were pregnant when they were afflicted with ovarian cancer, ii) patients who were simultaneously afflicted with ovarian cancer and other malignant tumor types and iii) patients whose case or data were incomplete. According to the radiologic Response Evaluation Criteria in Solid Tumors (RECIST) guidelines, the 58 clinical tissues were divided into two groups immediately following administration of the chemotherapy regime (15). According to their response to chemotherapy, they were divided into the ‘sensitive’ (complete or partial response) and ‘insensitive’ (stable or progressive disease) groups. The research protocol was reviewed and approved by the Ethical Committee and Institutional Review Board of the Department of Gynecology, Affiliated Hospital of Qingdao University. Written informed consent was obtained from each patient included into the current study. Following detection of the expression level of miR-149 in all samples, the mean value of miR-149 was used as the cut-off value. Thus, the ovarian cancer samples were divided into two groups in accordance with the level of miR-149 expression (the miR-149 high expression group and miR-149 low expression group).
Cell lines
In total, four ovarian cancer cell lines (HG-SOC, HO8910, SKOV-3 and ES2) and the immortalized normal fallopian tube epithelial FTE187 cell line were purchased from the Cell Bank of the Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). The cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS, Gibco; Thermo Fisher Scientific, Inc.), 100 µl ampicillin and 100 µl streptomycin at 37°C in a humidified atmosphere with 95% air and 5% CO2.
Cell transfection
HO8910 and SKOV-3 cells were preserved in RPMI-1640 (Invitrogen; Thermo Fisher Scientific, Inc.), supplemented with 100 µl penicillin/streptomycin and 10% fetal bovine serum until transfection. Cells were incubated until 80% confluence was reached, and then transfected for 15 min at 37°C. For overexpression and knockdown of XIAP, cells were separately transfected with pcDNA3.1-XIAP or small interfering RNA (siRNA)-XIAP vectors (both from GenePharma Co., Ltd., Shanghai, China). At the same time, the negative controls (the pcDNA3.1 empty vector and si-NC) were transfected into cells for comparison. The negative controls were obtained from GenePharma Co., Ltd. For overexpression and knockdown of miR-149, cells were separately transfected with miR-149 mimics and miR-149 inhibitors and miR-NC (Shanghai GenePharma Co., Ltd., Shanghai, China). All transfections were performed using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from the cells or tissues was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The concentration of RNA was quantified using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific, Inc., Wilmington, DE, USA). RNA concentration was determined using the following formula; optical density (OD)260 nm × dilution × ratio 0.04 ug/ul. cDNA synthesized from total RNA was reverse transcribed with the Transcriptor High Fidelity cDNA synthesis kit (Roche Applied Science, Mannheim, Germany) using the miRNA-specific primer or oligo (dT) and the random hexamer-primers. The relative expression level of mRNA was detected by SYBR-Green qRT-PCR assay (Bio-Rad Laboratories Inc, Hercules, CA, USA). qPCR was then performed to measure the miR-149 and XIAP expression levels using SYBR-Green PCR Master Mix on an ABI 7500 PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) following the manufacturer's instructions. The primers sequences used for this experiment were as follows: miR-149 forward, 5′-GGCTCTGGCTCCGTGTCTT-3′, and reverse, CAGTGCAGGGTCCGAGGTATT; U6 forward, 5′-CAAATTCGTGAAGCGTTCCATA-3′ and reverse, 5′-AGTGCAGGGTCCGAGGTATTC-3′; GAPDH forward, CCTGTACGCCAACACAGTGC and reverse, ATACTCCTGCTTGCTGATCC. qPCR conditions were as follows; pre-denaturation at 95°C for 10 min followed by 45 cycles at 95°C for 15 sec and 40 cycles at 60°C for 1 min. The miR-149 or XIAP levels were calculated with the 2−ΔΔCq method (16), and were normalized to U6 RNA or GAPDH mRNA levels, respectively. Control levels were defined as 1.0, and the miRNA or mRNA expression levels were presented relative to the fold change of the corresponding control. All assays were performed in triplicate.
Bioinformatics analysis
By employing the online miRNA target binding site analysis miRanda software (www.microrna.org/microrna/home.do), ~8,955 prediction targets of miR-149 were identified. Among these 8,955 prediction targets, the current study focused on investigating the role of XIAP, which is the study object of our research group.
Dual-luciferase activity assay
A XIAP 3′-UTR luciferase reporter gene plasmid was constructed by inserting it into the pGL3 vector (Promega Corporation, Madison, WI, USA), and the fragment containing putative binding sites for miR-149 was amplified. The plasmids pXIAP-wild-type (WT) and pXIAP-mutated (MUT) and miR-149 overexpressing vector were generated via subcloning the downstream luciferase vector using Fugene (Promega Corporation). Dual-luciferase reporter experiments were then performed. Briefly, human ovarian cancer cells (SKOV-3, 5×104) seeded into 96-well plates and then co-transfected with 100 ng pXIAP-WT or pXIAP-MUT and with miR-149 mimics or scramble oligonucleotide in the presence of 50 nM Lipofectamine 2000 (Life Technologies, Carlsbad, USA). After 48 h, cells were assayed using the Dual-Glo Luciferase Assay kit (Promega Corp., Madison, WI, USA), according to the manufacturer's instructions. The SpectraMax M5 microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA) was used to analyze the results.
Western blot analysis
Total proteins were extracted from the cells or tissues using radioimmunoprecipitation assay lysis buffer (Thermo Fisher Scientific, Inc.). Protein concentration was determined using the following formula: [(1.45 × (OD280 nm −0.74 × OD260 nm) × dilution factor]. Next, the total protein samples were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrophoretically transferred to polyvinylidene difluoride membranes (Roche Diagnostics, Basel, Switzerland). The membranes were then blocked using 3% non-fat milk for ~30 min at 4°C, followed by incubation with primary antibodies against XIAP (1:5,000; cat. no. ab21278; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and GAPDH (1:5,000; cat. no. ab9485; Abcam, Cambridge, MA USA) at 4°C overnight. Subsequently, the membranes were probed with horseradish peroxidase-labeled secondary antibody (1:5,000; cat. no. ab205718; Abcam), and the signals were visualized using an enhanced chemiluminescence detection system (cat. no. NEL100001EA; PerkinElmer, Inc., Waltham, MA, USA). All antibodies were incubated at room temperature for 1–2 h. Quantity One 1-D analysis software (version 4.6.5; Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to quantify results. GAPDH was used as the control.
In vitro chemosensitivity assay
An MTT assay (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was conducted to examine the chemosensitivity of cells to cisplatin. Briefly, cells (500 cells/well) were cultured in 96-well plates and then treated with cisplatin at different concentrations (0, 2.5, 5, 10, 20, 40, 80 µM). Cells were then incubated for 1 h at room temperature. After 48 h of incubation, MTT solution (5 mg/ml; 20 µl) was added to each well for 4 h, followed by removal of the media and addition of 100 µl dimethyl sulfoxide to each well. The relative number of surviving cells was subsequently assessed by measuring the optical density of the cell lysates at a wavelength of 560 nm. All assays were conducted in triplicate.
Colony formation assay
In order to examine the colony formation ability of cells, 500 cells per well were seeded into 6-well plates and incubated in RPMI 1640 supplemented with 10% FBS at 37°C. After 2 weeks, the cells were fixed in 4% paraformaldehyde for 15 min and stained with 0.1% crystal violet. Subsequently, the number of visible colonies in the plates was counted manually.
Flow cytometric analysis of apoptosis
In order to investigate cell apoptosis, flow cytometry was conducted using an Annexin V-FITC Apoptosis Detection kit (BD Biosciences, Franklin Lakes, NJ, USA), according to the manufacturer's protocol. All samples were assayed in triplicate.
Statistical analysis
All data are expressed as the mean ± standard deviation. Student's t-test or one-way analysis of variance was used to determine any statistically significant differences among the groups using SPSS version 17.0 software (SPSS, Inc., Chicago, IL, USA). Log-rank test and Kaplan-Meier was used for survival analysis. All tests performed were two-sided. The correlation between miR-149 and XIAP expression levels in the 58 cases of ovarian tumor tissues was analyzed by Spearman's correlation analysis. P<0.05 was considered to indicate a difference that was statistically significant. All the experiments were repeated at least three times with each sample examined in triplicate.
Results
miR-149 expression is downregulated in chemosensitive and chemo-insensitive ovarian cancer tissues, and ovarian cancer cell lines
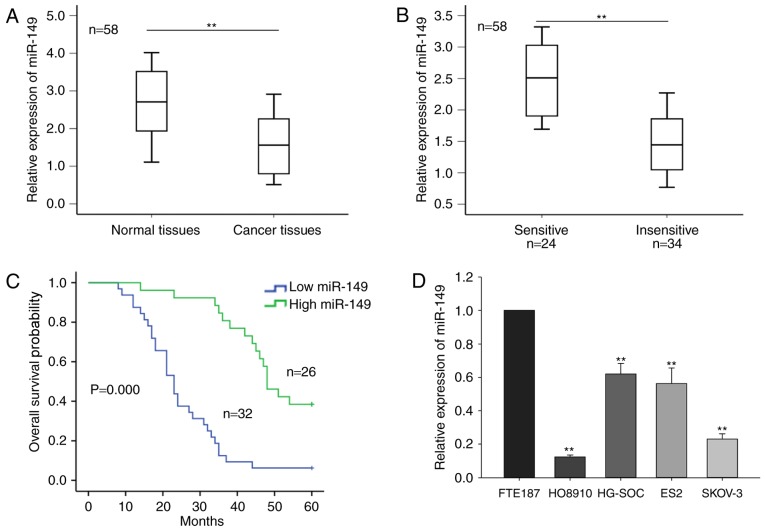
In the current study, the level of miR-149 in 58 ovarian tumor tissues and the corresponding normal tissues was initially measured. As shown in Fig. 1A, miR-149 expression was significantly downregulated in cancer tissues compared with that in the normal tissues (P<0.01). Furthermore, based on the radiologic RECIST guidelines, the 58 cases of ovarian tumor were classified as sensitive (complete or partial response) and insensitive (stable or progressive disease) to cisplatin-based chemotherapy. As demonstrated in Fig. 1B, a significantly higher level of miR-149 expression was observed in the chemosensitive group (n=24) compared with the chemo-insensitive group (n=34), as detected by RT-qPCR. The mean value of the miR-149 expression level of the samples was regarded as the cut-off value, and the ovarian cancer samples were divided into two groups according to the level of miR-149 expression (miR-149 high expression group and miR-149 low expression group). Furthermore, survival analysis indicated that a low level of miR-149 was associated with poor prognosis of the patients (Fig. 1C).
Figure 1.
miR-149 expression is downregulated in ovarian cancer tissues and cell lines. The miR-149 expression levels in (A) ovarian cancer tissues and corresponding normal tissues, as well as in (B) sensitive and insensitive ovarian cancer tissues to chemotherapy, were measured by RT-qPCR. (C) Correlation between the expression level of miR-149 and patient prognosis was analyzed by Kaplan-Meier survival analysis. (D) miR-149 expression levels in four ovarian cancer cell lines (HG-SOC, HO8910, SKOV-3 and ES2) and one immortalized normal fallopian tube epithelial cell line (FTE187) were measured by RT-qPCR. Error bars represent the mean ± standard deviation of at least three independent experiments. **P<0.01 vs. control group. XIAP, X-linked inhibitor of apoptosis; miR, microRNA; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
Furthermore, the current study determined the level of miR-149 in four ovarian cancer cell lines, namely HG-SOC, HO8910, SKOV-3 and ES2, and the immortalized normal fallopian tube epithelial FTE187 cell line. As presented in Fig. 1D, miR-149 expression was significantly reduced in the four ovarian cancer cell lines, as compared with that in the normal cells. In addition, the level of miR-149 in HO8910 and SKOV-3 cells was relatively lower in comparison with that in the other two cancer cell lines; thus, the HO8910 and SKOV-3 cell lines were selected for use in subsequent assays. These results indicated that miR-149 may be involved in the progression of ovarian cancer.
Ectopic expression of miR-149 increases the sensitivity of ovarian cancer cells to cisplatin, inhibits cell proliferation and induces cell apoptosis
To determine the function of miR-149 in ovarian cells, the two cell lines HO8910 and SKOV-3 were selected as model cells, since they exhibited the lowest expression levels in RT-qPCR assay. These cells were transfected with miR-149 mimics to induce overexpression of this miRNA, or with miR-negative control (NC), which served as the negative control group (Fig. 2A). The results of the MTT assay revealed that the overexpression of miR-149 significantly increased the sensitivity of HO8910 and SKOV-3 cells to cisplatin with the increasing concentration of cisplatin, compared with that of the control transfected cells (Fig. 2B). Colony formation assays also revealed that ectopic expression of miR-149 significantly inhibited the HO8910 and SKOV-3 cell proliferation as compared with that of miR-NC-transfected cells (Fig. 2C). Additionally, flow cytometry analysis indicated that overexpression of miR-149 markedly increased the apoptosis rate in the two cell lines (Fig. 2D). These findings revealed that miR-149 may function as a tumor suppressor in ovarian cancer.
Figure 2.
Ectopic expression of miR-149 increased the sensitivity of ovarian cells to cisplatin, inhibited cell proliferation and induced cell apoptosis. (A) HO8910 and SKOV-3 were transfected with miR-149 mimics, and the transfection efficiency was confirmed by reverse transcription-quantitative polymerase chain reaction. (B) Sensitivity of HO8910 and SKOV-3 cells transfected with miR-149 mimics to cisplatin treatment was determined by MTT assay. (C) Colony formation assays were performed to measure the effect of miR-149 on HO8910 and SKOV-3 cell proliferation. (D) Flow cytometry analysis was used to analyze the effect of miR-149 on the HO8910 and SKOV-3 cell apoptosis rate. Error bars represent the mean ± standard deviation of at least three independent experiments. **P<0.01 vs. NC group. XIAP, X-linked inhibitor of apoptosis; miR, microRNA; NC, negative control.
XIAP is a direct target of miR-149 in the ovarian cancer cells
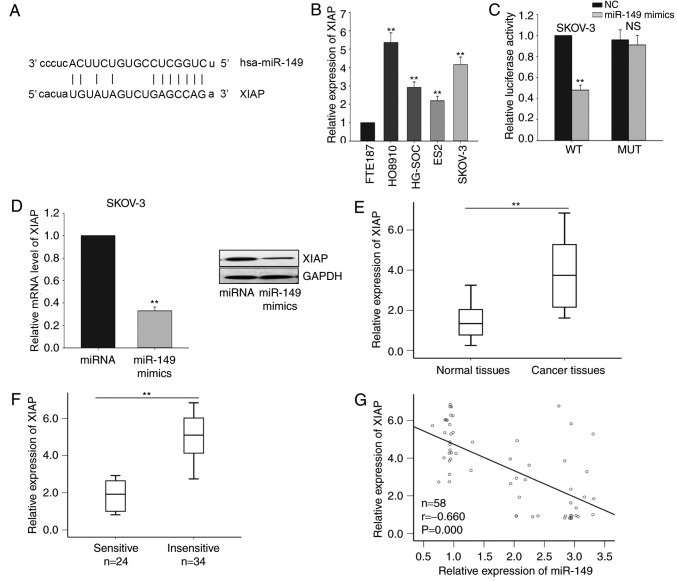
Based on the results of bioinformatics analysis using the miRanda software, 8,955 predicted targets of miR-149 were identified. Among these predicted targets, XIAP was observed to be a potential target gene of miR-149 (Fig. 3A), and this gene was selected for further investigation. Next, the level of XIAP in the four ovarian cancer cell lines (HG-SOC, HO8910, SKOV-3 and ES2) and the immortalized normal fallopian tube epithelial cell line (FTE187) was detected. As illustrated in Fig. 3B, the mRNA levels of XIAP was significantly increased in the four ovarian cancer cells, particularly in HO8910 and SKOV-3 cells. Subsequently, the interaction between miR-149 and XIAP was measured by a dual-luciferase reporter assay in SKOV-3 cells. As demonstrated in Fig. 3C, miR-149 overexpression by mimic transfection significantly inhibited the luciferase activity of the XIAP-WT reporter, but not of the XIAP-MUT 3′UTR reporter. Furthermore, forced expression of miR-149 significantly inhibited XIAP expression at the mRNA and protein levels in SKOV-3 cells (Fig. 3D). Furthermore, the level of XIAP in the ovarian cancer tissues was measured. It was revealed that XIAP was significantly upregulated in ovarian cancer tissues compared with that in the corresponding normal tissues (Fig. 3E). A lower level of XIAP was observed in the chemosensitive group when compared with that in the chemo-insensitive group (Fig. 3F). Additionally, XIAP expression was inversely correlated with miR-149 expression in ovarian cancer tissues in accordance with the Pearson correlation results. Collectively, all results indicate that miR-149 negatively regulated XIAP in ovarian cancer cells (Fig. 3G).
Figure 3.
XIAP is a direct target of miR-149 in ovarian cancer cells. (A) Bioinformatics analysis was used to predict the binding site for miR-149 in the 3′-untranslated region of XIAP. (B) RT-qPCR was performed to measure the level of XIAP in four ovarian cancer cells (HG-SOC, HO8910, SKOV-3 and ES2) and one immortalized normal fallopian tube epithelial (FTE187) cell lines. (C) Dual-luciferase reporter assay was performed to confirm the interaction between miR-149 and XIAP. (D) mRNA and protein levels of XIAP in response to forced expression of miR-149 in SKOV-3 cells. The mRNA levels of XIAP in (E) ovarian cancer and corresponding normal tissues, as were as in (F) sensitive and insensitive ovarian cancer tissues, were measured by RT-qPCR. (G) The correlation between miR-149 and XIAP was analyzed by Spearman's correlation analysis. Error bars represent the mean ± standard deviation of at least three independent experiments. **P<0.01 vs. corresponding control group. XIAP, X-linked inhibitor of apoptosis; miR, microRNA; NC, negative control; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; WT, wild-type; MUT, mutated; NS, non-significant.
miR-149 increases the sensitivity of SKOV-3 cells to cisplatin and inhibits ovarian cancer cell proliferation in a XIAP-dependent manner
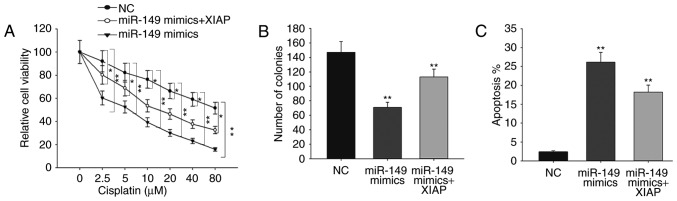
To further confirm the role of XIAP in ovarian cancer, rescue assays were performed. As shown in Fig. 4A, forced expression of XIAP was able to abolish the responses of miR-149-overexpressing ovarian cancer cells to cisplatin. The results of the colony formation assay revealed that miR-149 mimics decreased cell proliferation in comparison with NC, while the decreased proliferation was increased by XIAP (Fig. 4B). Furthermore, flow cytometry analysis demonstrated that the increased apoptosis rate caused by miR-149 mimics was reversed by XIAP overexpression (Fig. 4C). All these findings indicated that the function of miR-149 in ovarian cancer was exerted in an XIAP-dependent manner.
Figure 4.
miR-149 increased the sensitivity of SKOV-3 cells to cisplatin and inhibited ovarian cancer cell proliferation in an XIAP-dependent manner. (A) The sensitivity of SKOV-3 cells co-transfected with miR-149 mimics and XIAP expression vector to cisplatin treatment was measured by an MTT assay. (B) Colony formation and (C) flow cytometry assays were performed to detect the proliferation ability and apoptosis rate, respectively, of SKOV-3 cells co-transfected with miR-149 mimics and XIAP expression vector. Error bars represent the mean ± standard deviation of at least three independent experiments. *P<0.05 and **P<0.01, vs. corresponding control group. XIAP, X-linked inhibitor of apoptosis; miR, microRNA; NC, negative control.
Discussion
Drug resistance is identified as a major obstacle in tumor chemotherapy, and miRNAs have been reported to serve critical roles in this process (17–23). To date, several studies have identified that miR-149 exerts a critical function in numerous types of tumors. For instance, Jin et al (24) demonstrated that the tumor suppressor miR-149-5p was associated with cellular migration, proliferation and apoptosis in renal cell carcinoma. Fan et al (25) also reported that miR-149 promoted cell proliferation and suppressed apoptosis by mediating JunB in T-cell acute lymphoblastic leukemia. In addition, Luo et al (26) indicated that miR-149 repressed the metastasis of hepatocellular carcinoma by targeting protein phosphatase 1F, an actin-regulatory protein. Despite the existence of numerous studies investigating miR-149 in several tumors, the biological function and molecular mechanism of miR-149 in ovarian cancer remain unclear to date.
In the present study, miR-149 expression was demonstrated to be significantly downregulated in chemosensitive and chemo-insensitive ovarian cancer tissues, as well as in ovarian cancer cell lines. Furthermore, miR-149 overexpression suppressed the ovarian cancer cell proliferation and increased the sensitivity of these cells to cisplatin treatment. XIAP, as a member of the inhibitors of apoptosis family, has been reported in numerous types of human cancer, and is associated with chemical or radiation resistance (27–30). Recent studies indicated that expression of XIAP may be regulated by miRNAs (31–34). In the current study, it was observed that XIAP is a direct target gene of miR-149 in ovarian cancer. Ectopic expression of miR-149 inhibited the luciferase activity of the XIAP-WT reporter, but not of the XIAP-MUT reporter, suggesting that miR-149 was able to directly regulate the XIAP expression. Furthermore, miR-149 overexpression suppressed XIAP expression at the mRNA and protein levels. It was also observed that XIAP was overexpressed in ovarian cancer tissues and cells, and that its expression was negatively correlated with that of miR-149 expression in ovarian cancer tissues. Additionally, rescue assays revealed that overexpression of XIAP was able to abolish the increased cisplatin sensitivity and weaken the proliferative ability of ovarian cancer cells transfected with miR-149 mimics. These findings indicated that miR-149 functioned as a tumor suppressor in ovarian cancer and increased the sensitivity of ovarian cancer to cisplatin, at least partially, through targeting XIAP. However, certain limitations remain in the current study. For example, the present study only elucidated the effects of miR-149 on proliferation and apoptosis of ovarian cancer cells, the metastatic conditions still need to be explored. We performed experiments in only four ovarian cancer cell lines due to some limitations. We will make experiments in more cell lines in future. Future investigations should examine the role of other target genes of miR-149 and the upstream regulatory pathway of miR-149.
In conclusion, the findings of the current study revealed that miR-149 was downregulated in ovarian cancer cells and tissues. Forced expression of miR-149 suppressed the ovarian cancer cell proliferation and promoted the response of ovarian cancer cells to cisplatin via targeting XIAP. These data suggested that the miR-149/XIAP signaling pathway may be a potential therapeutic target for patients with ovarian cancer.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Tian S, Zhang M, Chen X, Liu Y, Lou G. MicroRNA-595 sensitizes ovarian cancer cells to cisplatin by targeting ABCB1. Oncotarget. 2016;7:87091–87099. doi: 10.18632/oncotarget.13526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flavin R, Smyth P, Barrett C, Russell S, Wen H, Wei J, Laios A, O'Toole S, Ring M, Denning K, et al. miR-29b expression is associated with disease-free survival in patients with ovarian serous carcinoma. Int J Gynecol Cancer. 2009;19:641–647. doi: 10.1111/IGC.0b013e3181a48cf9. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Miao Y, Zhang LF, Guo R, Liang S, Zhang M, Shi S, Shang-Guan CF, Liu MF, Li B. (18)F-FDG PET/CT for monitoring the response of breast cancer to miR-143-based therapeutics by targeting tumor glycolysis. Mol Ther Nucleic Acids. 2016;5:e357. doi: 10.1038/mtna.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai M, Wang Z, Zhang J, Zhou H, Jin L, Bai R, Weng Y. Adam17, a Target of Mir-326, Promotes Emt-Induced Cells Invasion in Lung Adenocarcinoma. Cell Physiol Biochem. 2015;36:1175–1185. doi: 10.1159/000430288. [DOI] [PubMed] [Google Scholar]
- 7.Perry MM, Baker JE, Gibeon DS, Adcock IM, Chung KF. Airway smooth muscle hyperproliferation is regulated by microRNA-221 in severe asthma. Am J Respir Cell Mol Biol. 2014;50:7–17. doi: 10.1165/rcmb.2013-0067OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Leary L, Sevinc K, Papazoglou IM, Tildy B, Detillieux K, Halayko AJ, Chung KF, Perry MM. Airway smooth muscle inflammation is regulated by microRNA-145 in COPD. FEBS Lett. 2016;590:1324–1334. doi: 10.1002/1873-3468.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldwin S, Deighan C, Bandeira E, Kwak KJ, Rahman M, Nana-Sinkam P, Lee LJ, Paulaitis ME. Analyzing the miRNA content of extracellular vesicles by fluorescence nanoparticle tracking. Nanomedicine. 2017;13:765–770. doi: 10.1016/j.nano.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Goeppert B, Ernst C, Baer C, Roessler S, Renner M, Mehrabi A, Hafezi M, Pathil A, Warth A, Stenzinger A, et al. Cadherin-6 is a putative tumor suppressor and target of epigenetically dysregulated miR-429 in cholangiocarcinoma. Epigenetics. 2016;11:780–790. doi: 10.1080/15592294.2016.1227899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xi S, Inchauste S, Guo H, Shan J, Xiao Z, Xu H, Miettenen M, Zhang MR, Hong JA, Raiji MT, et al. Cigarette smoke mediates epigenetic repression of miR-217 during esophageal adenocarcinogenesis. Oncogene. 2015;34:5548–5559. doi: 10.1038/onc.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Zhao J, Luo Y, Wang Y, Jiang Y. Downregulated expression of miRNA-149 promotes apoptosis in side population cells sorted from the TSU prostate cancer cell line. Oncol Rep. 2016;36:2587–2600. doi: 10.3892/or.2016.5047. [DOI] [PubMed] [Google Scholar]
- 13.Cao D, Jia Z, You L, Wu Y, Hou Z, Suo Y, Zhang H, Wen S, Tsukamoto T, Oshima M, et al. 18β-glycyrrhetinic acid suppresses gastric cancer by activation of miR-149-3p-Wnt-1 signaling. Oncotarget. 2016;7:71960–71973. doi: 10.18632/oncotarget.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assem H, Rambau PF, Lee S, Ogilvie T, Sienko A, Kelemen LE, Köbel M. High-grade endometrioid carcinoma of the ovary: A clinicopathologic study of 30 cases. Am J Surg Pathol. 2018 Jan 5; doi: 10.1097/PAS.0000000000001016. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 15.Guenther LM, Rowe RG, Acharya PT, Swenson DW, Meyer SC, Clinton CM, Guo D, Sridharan M, London WB, Grier HE, et al. Response evaluation criteria in solid tumors (RECIST) following neoadjuvant chemotherapy in osteosarcoma. Pediatr Blood Cancer. 2017 Dec 18; doi: 10.1002/pbc.26896. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Sun M, Guo J, Ma L, Jiang H, Gu L, Wen H, Liao S, Chen J, Zeng B, et al. 3-O-(Z)-coumaroyloleanolic acid overcomes Cks1b-induced chemoresistance in lung cancer by inhibiting Hsp90 and MEK pathways. Biochem Pharmacol. 2017;135:35–49. doi: 10.1016/j.bcp.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Pan CW, Jin X, Zhao Y, Pan Y, Yang J, Karnes RJ, Zhang J, Wang L, Huang H. AKT-phosphorylated FOXO1 suppresses ERK activation and chemoresistance by disrupting IQGAP1-MAPK interaction. EMBO J. 2017;36:995–1010. doi: 10.15252/embj.201695534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma H, Yokoyama S, Saiki I, Hayakawa Y. Chemosensitizing effect of saikosaponin B on B16F10 melanoma cells. Nutr Cancer. 2017;69:505–511. doi: 10.1080/01635581.2017.1285407. [DOI] [PubMed] [Google Scholar]
- 20.D'Angelo D, Mussnich P, Arra C, Battista S, Fusco A. Critical role of HMGA proteins in cancer cell chemoresistance. J Mol Med (Berl) 2017;95:353–360. doi: 10.1007/s00109-017-1520-x. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Huang J, Liu Z, Liang Q, Zhang N, Jin Y. FAM83A is amplified and promotes cancer stem cell-like traits and chemoresistance in pancreatic cancer. Oncogenesis. 2017;6:e300. doi: 10.1038/oncsis.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pu Y, Zhao F, Wang H, Cai S. MiR-34a-5p promotes multi-chemoresistance of osteosarcoma through down-regulation of the DLL1 gene. Sci Rep. 2017;7:44218. doi: 10.1038/srep44218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Zhang B, Shi T, Qin H. miR-182 promotes tumor growth and increases chemoresistance of human anaplastic thyroid cancer by targeting tripartite motif 8. Onco Targets Ther. 2017;10:1115–1122. doi: 10.2147/OTT.S110468. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Jin L, Li Y, Liu J, Yang S, Gui Y, Mao X, Nie G, Lai Y. Tumor suppressor miR-149-5p is associated with cellular migration, proliferation and apoptosis in renal cell carcinoma. Mol Med Rep. 2016;13:5386–5392. doi: 10.3892/mmr.2016.5205. [DOI] [PubMed] [Google Scholar]
- 25.Fan SJ, Li HB, Cui G, Kong XL, Sun LL, Zhao YQ, Li YH, Zhou J. miRNA-149* promotes cell proliferation and suppresses apoptosis by mediating JunB in T-cell acute lymphoblastic leukemia. Leuk Res. 2016;41:62–70. doi: 10.1016/j.leukres.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Luo G, Chao YL, Tang B, Li BS, Xiao YF, Xie R, Wang SM, Wu YY, Dong H, Liu XD, Yang SM. miR-149 represses metastasis of hepatocellular carcinoma by targeting actin-regulatory proteins PPM1F. Oncotarget. 2015;6:37808–37823. doi: 10.18632/oncotarget.5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riley A, Jordan LE, Holcik M. Distinct 5′ UTRs regulate XIAP expression under normal growth conditions and during cellular stress. Nucleic Acids Res. 2010;38:4665–4674. doi: 10.1093/nar/gkq241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 29.Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH, Monks A, et al. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000;6:1796–1803. [PubMed] [Google Scholar]
- 30.Pardo OE, Lesay A, Arcaro A, Lopes R, Ng BL, Warne PH, McNeish IA, Tetley TD, Lemoine NR, Mehmet H, et al. Fibroblast growth factor 2-mediated translational control of IAPs blocks mitochondrial release of Smac/DIABLO and apoptosis in small cell lung cancer cells. Mol Cell Biol. 2003;23:7600–7610. doi: 10.1128/MCB.23.21.7600-7610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding WB, Wang YX, Dong CW. microRNA15b induced SMCC7721 apoptosis via down-regulation of XIAP. Eur Rev Med Pharmacol Sci. 2017;21:542–548. [PubMed] [Google Scholar]
- 32.Li X, Chen W, Zeng W, Wan C, Duan S, Jiang S. microRNA-137 promotes apoptosis in ovarian cancer cells via the regulation of XIAP. Br J Cancer. 2017;116:66–76. doi: 10.1038/bjc.2016.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han J, Liu Z, Wang N, Pan W. MicroRNA-874 inhibits growth, induces apoptosis and reverses chemoresistance in colorectal cancer by targeting X-linked inhibitor of apoptosis protein. Oncol Rep. 2016;36:542–550. doi: 10.3892/or.2016.4810. [DOI] [PubMed] [Google Scholar]
- 34.Wu Q, Yan H, Tao SQ, Wang XN, Mou L, Chen P, Cheng XW, Wu WY, Wu ZS. XIAP 3′-untranslated region as a ceRNA promotes FSCN1 function in inducing the progression of breast cancer by binding endogenous miR-29a-5p. Oncotarget. 2017;8:16784–16800. doi: 10.18632/oncotarget.15159. [DOI] [PMC free article] [PubMed] [Google Scholar]