Abstract
The identification significance of C-reactive protein (CRP) and procalcitonin (PCT) levels in the intensive care unit patients with combined infection and their prognostic effects of patients with sepsis was investigated. A total of 203 patients were divided into the sepsis (n=60) and the non-sepsis group (n=143). The predictive effects of CRP and PCT levels in patients in the intensive care unit on sepsis and their effects on the prognosis of patients with sepsis were analyzed. The results showed that CRP and PCT levels in patients in the sepsis were higher than those in the non-sepsis group (P<0.05); CRP and PCT levels in patients who died of sepsis at 1 week and 2 weeks after admission were not statistically different to those before admission (P>0.05); CRP and PCT levels in patients surviving sepsis at 1 week after admission were significantly decreased compared with those at admission (P<0.05). CRP and PCT levels in patients at 2 weeks after admission were significantly decreased compared with those at admission (P<0.05). CRP and PCT levels in patients who died of sepsis were higher than those surviving sepsis (P<0.05). Logistic regression analysis showed that the higher the CRP and PCT levels were, the worse the patients' conditions would be, and the higher the risk of death would be (r=0.732, P=0.012; r=0.826, P=0.007); besides, PCT had a higher value in predicting the poor prognosis of patients [PCT: Area under the curve (AUC)=0.734, CRP: AUC=0.699]; the univariate Cox regression analysis revealed that CRP, PCT and age may be the risk factors for poor prognosis in patients. CRP and PCT can be used to identify whether the patients in the intensive care unit are infected or not. The dynamic monitoring of CRP and PCT has important clinical significance in predicting the prognosis of patients with sepsis.
Keywords: intensive care unit, procalcitonin, C-reactive protein, sepsis, clinical significance
Introduction
Sepsis is a generalized systemic inflammatory disease with a high prevalence. There are approximately 18 million people on average suffering from sepsis per year around the world, and this number is still rising each year (1–3). Sepsis is one of the leading causes of death in the intensive care unit, and there are nearly 14,000 people worldwide die of secondary diseases on average every day (4,5). In recent years, although great strides have been made in the anti-infective treatment, the mortality rate of sepsis patients still ranges from 25 to 60% (6,7). Sepsis treatment is expensive, and the use of medical resources is also very serious (7,8). Therefore, strengthening the early diagnosis, treatment and prognosis of patients with sepsis plays a very significant role.
Recently, studies have shown that changes in C-reactive protein (CRP) and procalcitonin (PCT) levels can prompt the severity of sepsis, and CRP and PCT tests have advantages of short time and high sensitivity, which are of great significance for the diagnosis and differential diagnosis of early infection (9,10). CRP is a non-specific and inflammation-related protein that is produced in the liver and regulated by plasma interleukin-6 (IL-6). When infection or body damage occurs, the concentration of CRP will be greatly altered (11). PCT is a glycoprotein with no hormonal activity, whose sensitivity to viral and bacterial infections is high; for example, sepsis can lead to a large change in its level (12). Therefore, changes in CRP and PCT levels in patients in the intensive care unit were detected in this study, so as to explore their predictive and prognostic effects on sepsis.
Patients and methods
Study objects
A total of 203 patients aged 21–76 years admitted to the Intensive Care Unit of Qilu Hospital of Shandong University in Dongying (Dongying, China) from May 2014 to May 2016 were selected and divided into the sepsis (n=60) and the non-sepsis group (n=143). In the sepsis group, there were 21 mild-to-moderate cases, including 12 males and 9 females with an average age of 43.2±21.3 years, 19 severe cases, including 11 males and 8 females with an average age of 58.5±19.6 years, and 20 cases with septic shock, including 12 males and 8 females with an average age of 68.1±23.5 years. In the non-sepsis group, there were 143 cases with colorectal cancer, including 82 males and 61 females with an average age of 35.1±1.6 years. The diagnostic criteria were in line with the International Guidelines for Management of Severe Sepsis and Septic Shock (Version 2016). The study was approved by the Ethics Committee of Qilu Hospital of Shandong University in Dongying and informed consents were signed by the patients and/or guardians.
Detection methods
Venous blood (3 ml × 2) was drawn from all the subjects the morning after admission, placed into a vacuum tube containing anticoagulant and then sent to the Laboratory Medicine of Qilu Hospital of Shandong University in Dongying for the detection of CRP and PCT expression levels. Fasting venous blood samples of patients in the control group were extracted for detection during the physical examination. CRP level was measured by immunoturbidimetry, and kits were provided by Beijing Strong Biotechnologies, Inc. (Beijing, China). The detection was performed by using the Beckman Coulter AU5800 automatic biochemical analyzer (Beckman Coulter, Inc., Brea, CA, USA). The standard and accusative reagents were provided by the manufacturer, and experiments were conducted in strict accordance with the kit instructions. PCT level was tested with the QMT8000 Immunoquantitative Analyzer (Getein Biotech, Inc., Nanjing, Cina), and kits were also supplied by the company.
Observation indexes
Differences in CRP and PCT levels in patients at admission between the two groups were compared; differences in CRP and PCT levels in patients who died of sepsis and those surviving from the disease at 1 and 2 weeks after admission and those at admission were analyzed; predictive effects of CRP and PCT levels on sepsis in the intensive care unit patients and their influence on the prognosis of patients with sepsis were analyzed. The latest CRP and PCT levels detected before death were taken as the standard for the dead patients with the survival time less than 1 or 2 weeks, and the data of patients at 1 or 2 weeks after admission were included, respectively.
Statistical analysis
Statistical analysis was conducted using SPSS 19.0 software [AsiaAnalytics (formerly SPSS China), Shanghai, China]. Sex and treatment effects were compared by the χ2 test; measurement data were expressed as mean ± SD; the non-parametric Kolmogorov-Smirnov (K-S) test was selected to compare the data between the two groups, and comparisons among various groups were conducted by using the analysis of variance. The receiver operating characteristic (ROC) curves of CRP and PCT of subjects were drawn; logistic regression analysis was used to analyze the correlation of CRP and PCT with the poor prognosis of patients. Univariate Cox regression analysis was used to analyze the related factors affecting the prognosis of patients with sepsis. A P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical data
There were a total of 203 patients in the intensive care unit. In the sepsis group, there were 60 patients with colorectal cancer, including 35 males and 25 females with an average age of 56.6±21.5 years; in the non-sepsis group, there were 143 patients with colorectal cancer, including 82 males and 61 females with an average age of 35.1±11.6 years. There was a difference in age between the two groups (P<0.05), but no differences in other basic data such as sex were found (P>0.05) (Tables I–III).
Table I.
Comparisons of basic data between the two groups of patients.
| Basic data | Sepsis group | Non-sepsis group | P-value |
|---|---|---|---|
| No. of cases (n) | 60 | 143 | |
| Sex (male/female) | 35/25 | 82/61 | 0.579 |
| Age (years) | 56.6±21.5 | 40.1±11.6 | 0.036 |
| Smoking history, n (%) | 0.556 | ||
| Yes | 17 (28.33) | 33 (23.08) | |
| No | 43 (71.67) | 110 (76.92) | |
| Place of residence, n (%) | 0.372 | ||
| City | 41 (68.33) | 82 (57.34) | |
| Countryside | 19 (31.67) | 61 (42.66) |
Table III.
Prognosis of patients with sepsis.
| Item | Mild sepsis | Severe sepsis | Septic shock | P-value |
|---|---|---|---|---|
| No. of cases (n) | 21 | 19 | 20 | |
| Survival, n (%) | 19 (90.48) | 10 (52.63) | 5 (25.00) | 0.029 |
| Death, n (%) | 2 (9.52) | 9 (47.37) | 15 (75.00) |
Prognosis of patients with sepsis
As of May 2016, there were 26 death cases in the sepsis group, with a mortality rate of 43.33%. Septic shock occurred in most of them, with a mortality rate as high as 75%, which was significantly higher than those of patients with mild and severe sepsis (P<0.05). There was no case of death in the non-sepsis group, and systemic inflammatory responses in patients were controlled and did not develop into sepsis (Table III).
Detection results of CRP
At admission, the average level of CRP in the sepsis was obviously higher than that in the non-sepsis group (P<0.05). In the sepsis group, the average CRP level in patients with septic shock was higher than that in patients with severe sepsis and mild sepsis (P<0.05); the average CRP level in patients with severe sepsis was higher than that in patients with mild sepsis (P<0.05). Comparisons of CRP levels in patients who died of sepsis at 1 and 2 weeks after admission and those at admission showed that there were no changes (P>0.05); CRP level in patients surviving sepsis at 1 week after admission was clearly decreased compared with that at admission (P<0.05); CRP level in those at 2 weeks after admission was significantly reduced compared with that at admission (P<0.05). The average level of CRP of patients who died of sepsis was higher than that of those who survived (P<0.05) (Figs. 1 and 2 and Table IV).
Figure 1.
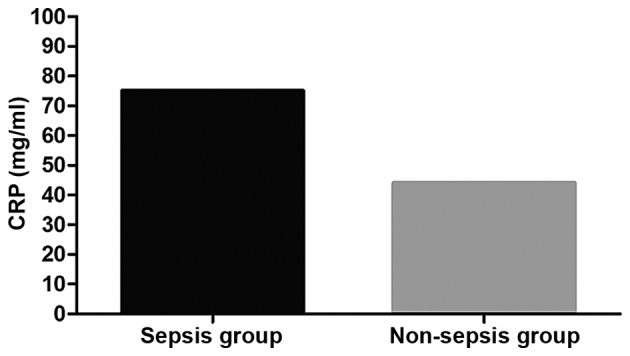
Detection results of CRP in the sepsis and the non-sepsis group. The average level of CRP in the sepsis group at admission is significantly higher than that in the non-sepsis group (P<0.05). CRP, C-reactive protein.
Figure 2.
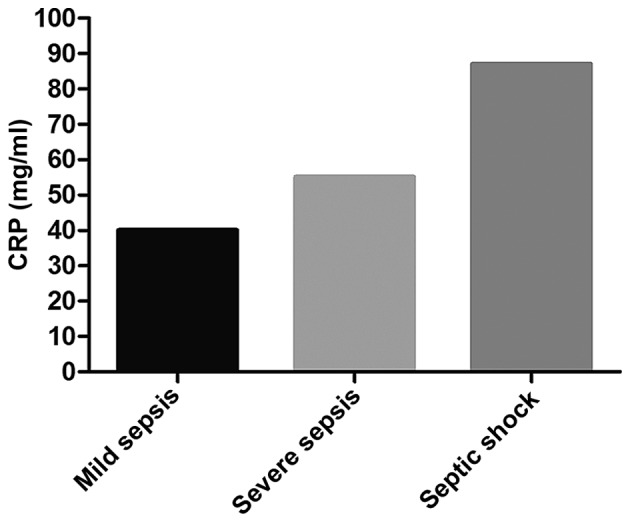
Detection results of CRP in each subgroup of patients in the sepsis group. In the sepsis group, the average CRP level in patients with septic shock is higher than those in patients with severe sepsis and mild sepsis (P<0.05), and the average CRP level in patients with severe sepsis is higher than that in patients with mild sepsis (P<0.05). CRP, C-reactive protein.
Table IV.
Comparisons of the detection results of CRP between patients surviving sepsis and those who died (mg/ml).
| Time | Patient surviving sepsis | Patient died of sepsis | t-value | P-value |
|---|---|---|---|---|
| No. of cases (n) | 34 | 26 | ||
| At admission | 52.2±11.3 | 64.5±10.9 | 2.365 | 0.047 |
| 1 week after admission | 42.1±8.4a | 68.2±10.4c | 2.933 | 0.036 |
| 2 weeks after admission | 35.2±7.7b | 69.5±9.4d | 3.114 | 0.027 |
Compared with that at admission, level is decreased (P<0.05)
compared with that at admission, there is no change (P>0.05). CRP, C-reactive protein.
Detection results of PCT
The average PCT level in the sepsis group was significantly higher than that in the non-sepsis group (P<0.05). In the sepsis group, the average level of PCT in patients with septic shock was higher than that in patients with severe sepsis and mild sepsis (P<0.05); the average PCT level in patients with severe sepsis was higher than that in patients with mild sepsis (P<0.05). Comparison of the average PCT level in patients who died of sepsis at 1 week and 2 weeks after admission and that at admission showed there were no changes (P>0.05). PCT level in patients surviving from sepsis at 1 week after admission was overtly lowered compared with that at admission (P<0.05); PCT level in those at 2 weeks after admission significantly declined compared with that at admission (P<0.05). The average level of PCT in patients who died of sepsis was higher than that in patients surviving from it (P<0.05) (Figs. 3 and 4 and Table V).
Figure 3.
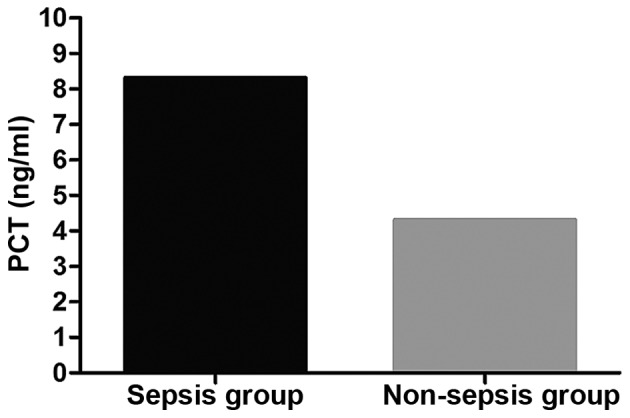
Detection results of PCT in the sepsis and the non-sepsis group. The average PCT level in the sepsis is significantly higher than that in the non-sepsis group (P<0.05). PCT, procalcitonin.
Figure 4.
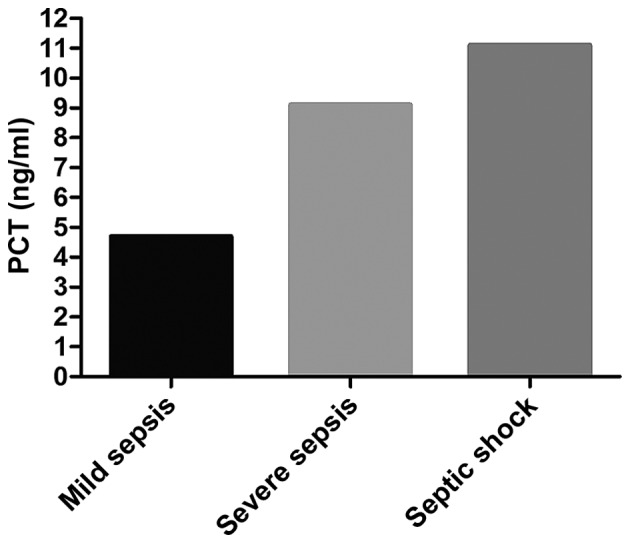
Detection results of PCT in each subgroup of patients in the sepsis group. In the sepsis group, the average level of PCT in patients with septic shock is higher than those in patients with severe sepsis and mild sepsis (P<0.05), and the average PCT level in patients with severe sepsis is higher than that in patients with mild sepsis (P<0.05). PCT, procalcitonin.
Table V.
Comparisons of the detection results of PCT between patients surviving sepsis and those who died (ng/ml).
| Time | Patient surviving sepsis | Patient died of sepsis | t-value | P-value |
|---|---|---|---|---|
| No. of cases (n) | 34 | 26 | ||
| At admission | 5.8±1.2 | 7.8±1.6 | 2.269 | 0.038 |
| 1 week after admission | 3.4±0.9a | 8.9±2.1c | 3.012 | 0.024 |
| 2 weeks after admission | 2.1±0.4b | 8.1±1.4d | 3.985 | 0.015 |
Compared with that at admission, level is decreased (P<0.05)
compared with that at admission, there is no change (P>0.05). PCT, procalcitonin.
Logistic regression analysis and ROC analysis
Logistics regression analysis showed that PCT and CRP levels in patients in the intensive care unit were closely related to the severity of sepsis and the prognosis of patients. The higher the PCT and CRP levels were, the more severe the sepsis and the worse the prognosis would be (r=0.826, P=0.007; r=0.732, P=0.012). With death as the end of the prognosis of patients, the values of PCT and CRP in predicting the death of patients were relatively great, and their areas under the curve (AUC) were 0.734 and 0.699, respectively, and 95% confidence intervals (95% CIs) were 0.665–0.874 and 0.601–0.792, respectively (Fig. 5).
Figure 5.
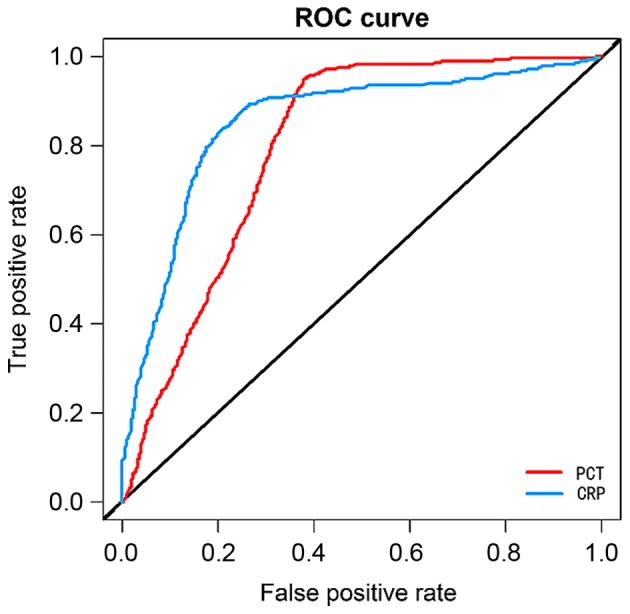
ROC regression analyses of PCT and CRP. With death as the end of the prognosis of patients, the values of PCT and CRP in predicting the death of patients are relatively great, and their AUC are 0.734 and 0.699, respectively, and 95% CIs are 0.665–0.874 and 0.601–0.792, respectively. ROC, receiver operating characteristic; PCT, procalcitonin; CRP, C-reactive protein; AUC, area under the curve.
Univariate Cox regression analysis
PCT level at 6.9 ng/ml represented that the specificity and sensitivity of the poor prognosis of patients with sepsis were 73.6 and 77.5%, respectively, so PCT=6.9 ng/ml was taken as the critical point between high concentration and low concentration. CRP concentration at 55.7 mg/l indicated that the specificity and sensitivity of the poor prognosis of patients were 64.6 and 77.4%, respectively, so CRP=55.7 mg/l was taken as the critical point between high concentration and low concentration. Univariate Cox regression analysis revealed that CRP, PCT and age might be risk factors for the poor prognosis of patients with sepsis (Table VI).
Table VI.
Univariate Cox regression analysis.
| Univariate analysis | ||
|---|---|---|
| Factors | HR (95% CI) | P-value |
| Sex (male vs. female) | 0.734 (0.247–2.356) | 0.792 |
| Age (<50 vs. ≥50 years) | 2.145 (1.549–4.566) | 0.012 |
| CRP (low vs. high) | 2.141 (1.269–2.724 | 0.013 |
| PCT (low vs. high) | 3.044 (1.258–7.336) | 0.011 |
CRP, C-reactive protein; PCT, procalcitonin; HR, hazard ratio; CI, confidence interval.
Discussion
Sepsis is one of the major causes of patient's death in the intensive care unit. It leads to the body's use of a large amount of sugar, lipids and proteins, thus changing the energy metabolism mode and the rate of energy utilization of patients, which will cause an additional burden and may also cause concurrence with hypoproteinemia in patients (13,14). Moreover, the body's resistance to sepsis-induced tissue damage and inflammatory responses can further undermine the body's metabolic balance, and even cause organ failure (15,16). Therefore, it is very important to predict the occurrence of sepsis in patients in the intensive care unit, control and treat sepsis in patients in the intensive care unit, and improve patients' quality of life and survival time. In this study, the predictive values of PCT and CRP for sepsis in patients in the intensive care unit and the prognostic values for patients with sepsis were explored by examining changes in PCT and CRP levels in patients in the intensive care unit.
In this study, changes in PCT and CRP levels in 203 intensive care unit patients were measured, and the results revealed that patients with sepsis had higher levels of PCT and CRP than non-sepsis patients, suggesting that PCT and CRP may be related to the occurrence of sepsis. However, no patients without sepsis was found to develop into patients with sepsis in this study. Therefore, whether changes in PCT and CRP levels have values in predicting sepsis needs to be further investigated. The study of Su et al (17) found that PCT cannot be completely used to predict the risk of sepsis after transplantation. The most sensitive indicator for neonatal sepsis diagnosis is CRP (18). However, there are few studies on whether CRP can be used as a predictor for sepsis, so more studies are needed to analyze whether these two markers can be predictors for sepsis.
In our study, patients with sepsis were further subdivided in detail according to different components, which showed that with the aggravation of sepsis in patients, PCT and CRP levels were also increased. Therefore, logistics regression analysis was used to analyze the relationships of PCT and CRP levels with the severity of sepsis, which revealed that the higher the PCT and CRP levels were, the more severe the sepsis in patients would be. Univariate Cox regression analysis also manifested that PCT and CRP might be risk factors for the poor prognosis of patients with sepsis. Studies of Savva et al (19), and Ashour et al (20) proved that soluble triggering receptor expressed on myeloid cells 1 (sTREM-1) and PCT have very good effects in assessing the severity of sepsis. Currently, there is little research on the value of CRP in assessing the severity of sepsis. However, Huo et al (21) found that autophagy-related 16-like 1 (ATG16L1) gene polymorphism is closely related to the severity of sepsis. Whether ATG16L1 affects PCT and CRP levels is worth further investigation.
During this study, there were 26 cases of death (43.33%). The prognosis of patients with sepsis was also analyzed. Logistic regression analysis showed that the higher the PCT and CRP levels were, the higher the risk of poor prognosis would be. Further ROC curve analysis revealed that PCT and CRP have good values in the prognosis of patients with sepsis. PCT is a good indicator for the diagnosis and prognosis of sepsis, and PCT and CRP levels are closely related to the severity of infection and organ dysfunction (22). A study of Franekova et al (23) also revealed that serum PCT and CRP can predict the prognosis of children with sepsis, which is consistent with our results. A study of Sonawane et al (24) indicated that CRP can also be used as an early predictor of sepsis in patients with thermal burns. Therefore, PCT and CRP are good indicators for the diagnosis and prognosis of sepsis, but their joint diagnostic values remain to be further explored.
In conclusion, the detection of changes in CRP and PCT levels has great clinical value in assessing the prognosis of patients with sepsis. High-level CRP and PCT indicate a poor prognosis in patients with sepsis.
Table II.
Subgroups of the sepsis group (n=60).
| Subgroups | Mild sepsis | Severe sepsis | Septic shock | P-value |
|---|---|---|---|---|
| No. of cases (n) | 21 | 19 | 20 | |
| Sex (male/female) | 12/9 | 11/8 | 12/8 | 0.732 |
| Average age (years) | 43.2±21.3 | 58.5±19.6 | 68.1±23.5a | 0.664 |
Compared with those in patients with mild sepsis, there is a difference in the average age (t=3.125, P=0.032).
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
QL contributed to design of the study and was responsible for detection of CRP and PCT levels. He also drafted and revised the manuscript. XG analyzed and interpreted statistical analysis. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Qilu Hospital of Shandong University in Dongying (Dongying, China) and informed consents were signed by the patients and/or guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agyeman PKA, Schlapbach LJ, Giannoni E, Stocker M, Posfay-Barbe KM, Heininger U, Schindler M, Korten I, Konetzny G, Niederer-Loher A, et al. Epidemiology of blood culture-proven bacterial sepsis in children in Switzerland: a population-based cohort study. Lancet Child Adolesc Health. 2017;1:123–133. doi: 10.1016/S2352-4642(17)30010-X. [DOI] [PubMed] [Google Scholar]
- 3.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 5.Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, Artigas A, Schorr C, Levy MM. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: Results from a guideline-based performance improvement program. Crit Care Med. 2014;42:1749–1755. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 6.Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: Time for change. Lancet. 2013;381:774–775. doi: 10.1016/S0140-6736(12)61815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, Iwashyna TJ. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 8.Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, Fanizza C, Caspani L, Faenza S, Grasselli G, et al. ALBIOS Study Investigators: Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370:1412–1421. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 9.Yang AP, Liu J, Yue LH, Wang HQ, Yang WJ, Yang GH. Neutrophil CD64 combined with PCT, CRP and WBC improves the sensitivity for the early diagnosis of neonatal sepsis. Clin Chem Lab Med. 2016;54:345–351. doi: 10.1515/cclm-2015-0277. [DOI] [PubMed] [Google Scholar]
- 10.Henriquez-Camacho C, Losa J. Biomarkers for sepsis. BioMed Res Int. 2014;2014:547818. doi: 10.1155/2014/547818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao L, Liu X, Zhang D, Xu F, Chen Q, Hong Y, Feng G, Shi Q, Yang B, Xu L. Early diagnosis of bacterial infection in patients with septicopyemia by laboratory analysis of PCT, CRP and IL-6. Exp Ther Med. 2017;13:3479–3483. doi: 10.3892/etm.2017.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Zhou L. Diagnostic value of C-reactive protein and procalcitonin for bacterial infection in acute exacerbations of chronic obstructive pulmonary disease. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014;39:939–943. doi: 10.11817/j.issn.1672-7347.2014.09.013. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 13.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM, Shankar-Hari M, Singer M, et al. Assessment of clinical criteria for sepsis: For the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372:1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 15.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5:4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su L, Feng L, Song Q, Kang H, Zhang X, Liang Z, Jia Y, Feng D, Liu C, Xie L. Diagnostic value of dynamics serum sCD163, sTREM-1, PCT, and CRP in differentiating sepsis, severity assessment, and prognostic prediction. Mediators Inflamm. 2013;2013:969875. doi: 10.1155/2013/969875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Wang H, Liu J, Chen B, Li G. Serum soluble triggering receptor expressed on myeloid cells-1 and procalcitonin can reflect sepsis severity and predict prognosis: A prospective cohort study. Mediators Inflamm. 2014;2014:641039. doi: 10.1155/2014/641039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savva A, Plantinga TS, Kotanidou A, Farcas M, Baziaka F, Raftogiannis M, Orfanos SE, Dimopoulos G, Netea MG, Giamarellos-Bourboulis EJ. Association of autophagy-related 16-like 1 (ATG16L1) gene polymorphism with sepsis severity in patients with sepsis and ventilator-associated pneumonia. Eur J Clin Microbiol Infect Dis. 2014;33:1609–1614. doi: 10.1007/s10096-014-2118-7. [DOI] [PubMed] [Google Scholar]
- 20.Ashour FH, Maghraby HM, Hassan AS. Procalcitonin as a diagnostic and prognostic marker of sepsis in critically Ill patients in intensive care unit. Egypt J Hosp Med. 2017;68 [Google Scholar]
- 21.Huo JM, Huo R, Hu L, Lu SW, Zu J. Value of procalcitonin, high sensitivity C-reactive protein and pancreatic stone protein in predicting prognosis of children with sepsis. Sichuan Da Xue Xue Bao Yi Xue Ban. 2017;48:422–426. (In Chinese) [PubMed] [Google Scholar]
- 22.John J, Chisthi MM, Kuttanchettiyar KG. C-reactive protein: An early predictor of sepsis in patients with thermal burns. Int Surg J. 2017;4:628–632. doi: 10.18203/2349-2902.isj20170204. [DOI] [Google Scholar]
- 23.Franekova J, Kieslichova E, Brezina A, Brodska H, Secnik P, Jabor A. Presepsin can replace procalcitonin in the prediction of sepsis in transplant patients after antithymocyte globulin administration. Clin Chem Lab Med. 2015;53:S533. [Google Scholar]
- 24.Sonawane VB, Gaikwad SU, Kadam NN, Gavhane J. Comparative study of diagnostic markers in neonatal sepsis. J Nepal Paediatr Soc. 2014;34:111–114. doi: 10.3126/jnps.v34i2.9788. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.


