Abstract
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are efficient in treating patients with non-small cell lung cancer (NSCLC) harboring EGFR activating mutations. Unfortunately, nearly all patients ultimately acquire resistance to EGFR-TKI treatment. Liver X receptors (LXRs) can regulate tumor growth in various cancer cell lines. The present study indicated that LXR agonist combined with gefitinib weakened Akt-nuclear factor (NF)-κB activation and inhibited the expression levels of apoptosis-related proteins in vitro. By contrast, LXR ligands alone exhibited no significant effect on gefitinib-resistant lung cells. In conclusion, the study provided evidence for the combination treatment of acquired TKI resistance in NSCLC.
Keywords: LXR ligands, gefitinib, drug resistance, lung cancer
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide (1), and non-small cell lung cancer (NSCLC) is the most common clinicopathological type. Although early diagnosis of lung cancer has developed in recent years, most patients are initially diagnosed with an advanced stage (2).
The epidermal growth factor receptor (EGFR) is a well characterized mutated oncogene in NSCLC with 10–20% cases in Western countries and is predominantly associated with adenocarcinoma histology. EGFR-mutated tumors are dependent on EGFR signaling for proliferation and survival (3,4). EGFR tyrosine kinase inhibitors (EGFR-TKIs) have shown dramatic therapeutic effects in patients with NSCLC harboring EGFR-activating mutations. Based on the positive results of several phase III clinical trials, National Comprehensive Cancer Network (NCCN) has recommended EGFR-TKI as the standard first-line therapy in NSCLC patients with sensitive EGFR mutations (5,6). However, nearly all patients eventually developed drug resistance after a median period of ~10 months (7). Thus, innovative treatment strategies are urgently needed for increasing sensibility to EGFR-TKI and improving the survival of patients with NSCLC.
Recently, a number of nuclear receptor superfamily, including liver X receptors (LXRs), have been shown to mediate tumor proliferation and enhance chemotherapeutic efficacy (8–11). LXRs (LXRα/NR1H3 and LXRβ/NR1H2) can be activated by natural ligands, including oxysterols and synthetic agonists GW3965 (12). High level of LXRα expression can be found in liver, kidney, intestine, fat tissue and macrophages, whereas LXRβ expression is ubiquitous (13). Some studies have demonstrated that LXR ligands exhibited anti-cancer activities in a variety of cancer cell lines. For example, LXR ligands can suppress the proliferation of breast, ovarian, prostate, colon and leukemia cancer cells in vitro (14). Recently, a study has found that LXR ligands combined with gefitinib could suppress cell cycle progression by inhibiting cyclinD1 and cyclinB expression in NSCLC cells (15).
Based on these previous reports, the present study aimed to investigate the effects of synthetic LXR ligands (GW3965) on increasing sensibility of gefitinib-resistant NSCLC cell to EGFR-TKI and explored their potential mechanisms.
Materials and methods
Cell lines and reagents
Human NSCLC H827 cell line harboring the EGFR exon 19 deletion (Del E746-A750) was obtained from Shanghai Institutes for Biological Sciences, Chinese Academy of Cell Resource Center, and maintained in RPMI-1640 supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C with 5% CO2 and at humidified atmosphere. Gefitinib (Iressa) was purchased from AstraZeneca, and GW3965 was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Cells were treated with gefitinib and GW3965 in RPMI-1640 supplemented with 5% FBS. Primary antibodies against MET, PTEN, AKT, p-Akt (Ser473), NF-κB, p-NF-κB, Bax, Bcl-2 and β-actin were obtained from cell signaling technology.
Generation of gefitinib-resistant H827 cells in vitro
To generate a resistant cell line, we exposed H827 cells to increasing concentrations of gefitinib according to previously described methods (16). The resistant H827 cells were passed 25 times in the absence of gefitinib and were found to maintain their resistance as confirmed by Cell Counting kit-8 (CCK-8; Dojindo, Kumamoto, Japan) assays. Six individual clones were isolated and all were confirmed independently to be resistant to gefitinib by CCK-8 assay. No significant change was observed in the sensitivity to gefitinib in parental cells during the period.
Cell proliferation assay
Cancer cells were seeded in 96-well plates and exposed to different doses of gefitinib alone, GW3965 alone, and both drugs for 96 h. Each combination of cell line and drug concentration was set up in 5 replicate wells and repeated at least thrice. Cell proliferation was measured by the CCK-8 assay. IC50 values were determined by interpolation from the dose-response curves. Among the six gefitinib-resistant clones, we selected the most sensitive to LXR ligands for further study.
Sequencing of the EGFR gene
To determine the EGFR sequence of cells, DNA was extracted from each cell line using a QIA-amp DNA mini kit (Qiagen, Tokyo, Japan), and the exons encoding the intracellular domain (exons 18–21) were amplified by PCR. Primer sequences are shown in Table I (17). Sequencing was conducted using an ABI 3500 sequencer (ABI).
Table I.
Sequence of EGFR (at exons 18–21), β-actin, LXRα, LXRβ.
| Primer name | Primer sequence 5′ to 3′ |
|---|---|
| EGFR18-F | AGCATGGTGAGGGCTGAGGTGAC |
| EGFR18-R | ATATACAGCTTGCAAGGACTCTGG |
| EGFR19-F | CCAGATCACTGGGCAGCATGTGGCACC |
| EGFR19-R | AGCAGGGTCTAGAGCAGAGCAGCTGCC |
| EGFR20-F | GATCGCATTCATGCGTCTTCACC |
| EGFR20-R | TTGCTATCCCAGGAGCGCAGACC |
| EGFR21-F | TCAGAGCCTGGCATGAACATGACCCTG |
| EGFR21-R | GGTCCCTGGTGTCAGGAAAATGCTGG |
| β-actin-F | GATGAGATTGGCATGGCTTT |
| β-actin-R | CACCTTCACCGTTCCAGTTT |
| LXRα-F | TCTGGAGACATCTCGGAGGTA |
| LXRα-R | GGCCCTGGAGAACTCGAAG |
| LXRβ-F | AGAAGATTCGGAAACAACAGCA |
| LXRβ-R | GCTGGATCATTAGTTCTTGAGCC |
EGFR, epidermal growth factor receptor; F, forward; R, reverse; LXR, liver X receptor.
Colony formation assay
Clonogenic survival assays were prepared according to literature (18). Briefly, H827-7-2 and H827-7-4 cells were seeded at a density of 600 cells/well in flat-bottomed 6-well plates. According to pre-experimental results, cells were treated with 2 ml of gefitinib (1 µM) alone, LXR ligands (GW3965, 1 µM) alone, and both drugs diluted with the medium to appropriate concentrations after 24 h of incubation. Then, cells were cultured for an additional 15 days, and subsequently stained with Giemsa. Experiments were performed forthrice.
Cell apoptosis analysis
H827-7-2 and H827-7-4 cells were seeded in 6-well plates (2×104 cells/well) for 12 h and treated with gefitinib (5 µM) alone, LXR ligands (GW3965, 5 µM) alone, and both drugs for another 96 h, and then cells were harvested and washed twice with ice-cold PBS. Annexin V-FITC/PI staining was used to detect apoptotic cells. Three individual experiments were conducted.
Quantitative PCR (qPCR) analysis
Total RNA was isolated from H827-7-2 and H827-7-4 cells treated with GW3965 at different concentrations using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions and cDNA was synthesized with PrimeScript RT Master Mix and premix EX Taq™ Probe qPCR Mix (Takara Bio, Dalian, China) on a 7300 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Quantitative PCR was performed using SYBR Green PCR Mix (Roche, Mannheim, Germany). β-actin was used as an internal control to normalize the amount of total RNA in each sample. Primer sequences were summarized in Table I. The relative levels of gene expression were determined using the δδCt method relative to internal control gene β-actin. All reactions were repeated thrice for each sample.
Moreover, qPCR was performed using premix EX Taq™ Probe qPCR Mix on a 7300 real-time PCR system. The copy number ratio of MET to GAPDH, a housekeeping gene, was calculated using a genomic DNA sample. The sequences of the Taqman probe and primers for MET and GAPDH were previously described (19). Quantification was based on standard curves from a serial dilution of normal human genomic DNA. All specimens were analyzed intriplicates.
Western blot analysis
Cells were lysed in RIPA buffer (Beyotime Institute of Biotechnology, Haimen, China). Proteins were separated on 10% Bis-Tris Mini gels and transferred to a PVDF membrane. The primary antibodies used were AKT, p-Akt (Ser473), NF-κB, p-NF-κB, Bax, Bcl-2, MET, PTEN (1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA), and β-actin (1:1,000; Sigma-Aldrich). Proteins were detected with secondary antibodies (1:5,000; Immunology Consultanta Laboratory, Portland, OR, USA) and enhanced chemiluminescence solution (ECL; Beyotime Institute of Biotechnology).
Statistical analysis
Data were expressed as means ± SD. Statistical analysis was conducted using one-way ANOVA and Student's t-test with Graphpad 5.0 and SPSS 13.0. P<0.05 was considered statistically significant.
Results
Effect of combined treatment of GW3965 and gefitinib
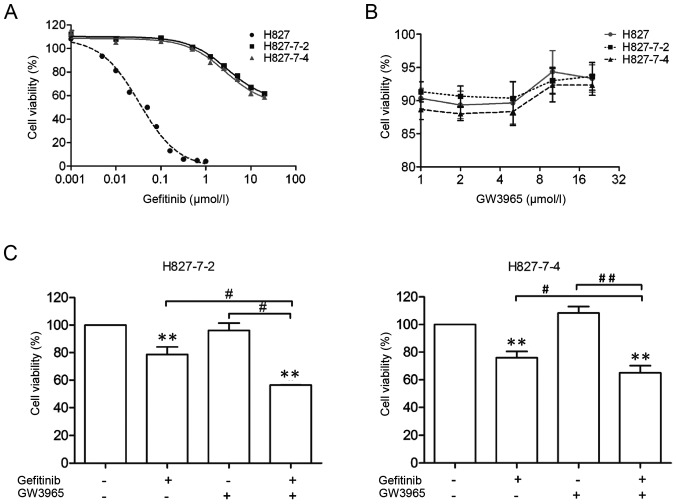
After establishing six monoclonal gefitinib-resistant H827 cell lines, we characterized their drug resistance indices through cell proliferation assays. As illustrated in Table II, ~300-fold increase was observed in IC50 for each monoclonal cell line compared with those of parental H827 cells (Fig. 1A).
Table II.
Lung cancer inhibitory concentration 50 values (IC50) for treatment with gefitinib.
| Human lung cancer cells | Gefitinib (IC5048 h) |
|---|---|
| H827 | 0.0401±0.042 µM |
| H827-7–2 | >20 µM |
| H827-7–4 | >20 µM |
IC50, half maximal inhibitory concentration.
Figure 1.
H827-7-2 and H827-7-4 cells resistant to gefitinib. (A and B) Cell lines were treated with the indicated doses of GW3965 or gefitinib for 96 h. The viability of cells was determined using the CCK-8 assay. (C) H827-7-2 and H827-7-4 cells were treated with gefitinib alone or combined with LXR ligands for 96 h. **P<0.001 compared with control; #P<0.01, ##P<0.001 between one treatment alone. CCK-8, cell counting kit-8; LXR, liver X receptor.
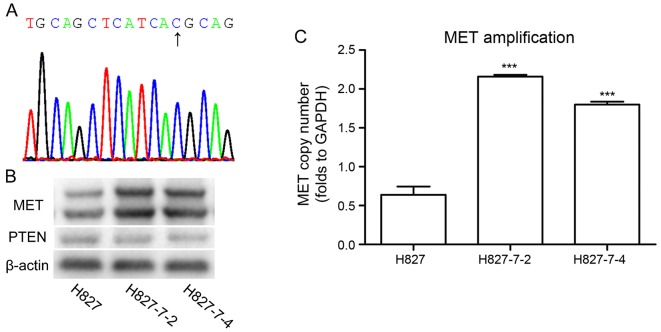
We found that 5 µM GW3965 had no inhibitory effects on the growth of TKI-resistant lung cancer cell lines and its monoclonal cell lines (Fig. 1B). Therefore, we continued using this concentration for further analysis. The amount of gefitinib (5 µM), which was less than 50% of the inhibitory concentration (IC50) had no significant effect on all monoclonal cells (Fig. 1A). Then, we explored the combinational therapeutic potential of GW3965 and gefitinib, in monoclonal cell lines. Combined treatment showed significant growth inhibitory proliferation compared with each drug alone in H827-7-2 and H827-7-4 cells (Fig. 1C). These data suggested that GW3965 may increase the sensitivity of NSCLC to EGFR-TKI. To exclude the mechanisms of acquired resistance, we conducted the DNA sequencing of EGFR at exons 18–21 and examined genetic alterations, including the levels of MET (20) and PTEN (21) and the well-known T790M mutation in these two monoclonal cells (Table I). T790M mutation at exon 20 was not observed in the H827-7-2 and H827-7-4 cells (Fig. 2A). The expression of PTEN was similar in both monoclonal cells, whereas MET was higher in H827-7-2 and H827-7-4 cells than in parental H827 cells (Fig. 2B and C).
Figure 2.
(A) T790M mutation was not observed in H827-7-2 and H827-7-4 cells by direct sequencing. (B) Western blot analysis was performed on H827: H827-7-2 and H827-7-4 cells to detect the expression levels of MET and PTEN. (C) The copy number ratio of MET to GAPDH, was calculated using a genomic DNA sample. ***P<0.0001 vs. H827.
LXR ligands increase gefitinib-induced apoptosis in H827-7-2 and H827-7-4 cells
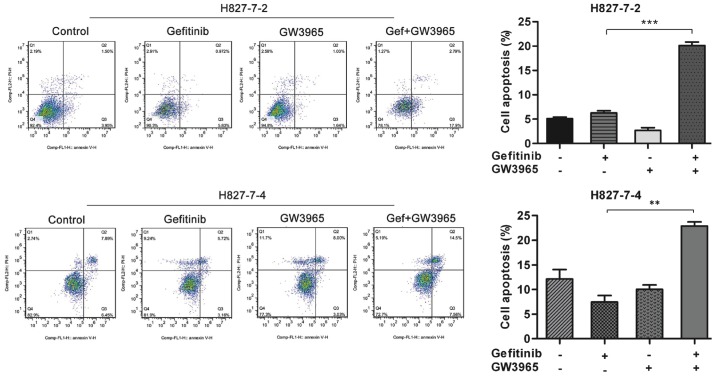
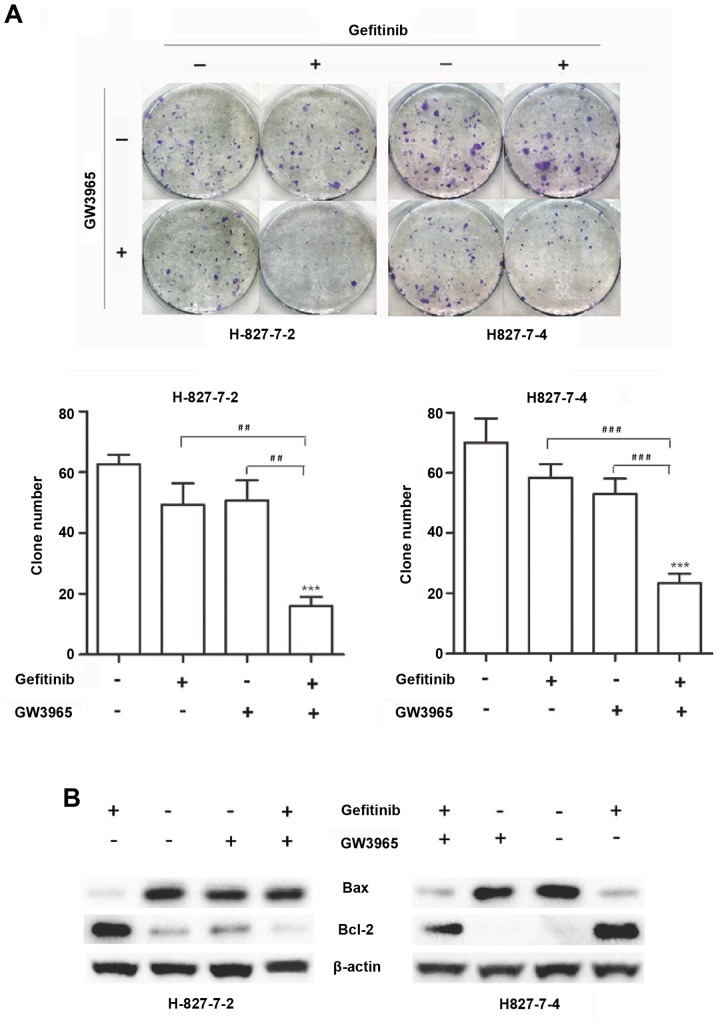
We then analyzed the induction of apoptosis in the two monoclonal cells treated with GW3965 alone, gefitinib alone, or in combination. As shown in Fig. 3, flow cytometric analysis revealed that the percentage of apoptosis induced by gefitinib in H827-7-2 cells was 6.802%. When GW3965 was added together with gefitinib, the percentage of apoptosis dramatically increased to 20.69%. Similarly, the percentage of apoptosis induced by gefitinib increased from 8.88 to 22.06% after treatment with GW3965 in H827-7-4 cells (Fig. 3). Colony-forming assays revealed that GW3965 alone did not enhance the apoptosis of H827-7-2 and H827-7-4 cells, but the combination therapy significantly augmented apoptosis (Fig. 4A).
Figure 3.
GW3965 (5 µM) in combination with gefitinib (5 µM) enhanced apoptosis of H827-7-2 and H827-7-4 cells. **P<0.001 and ***P<0.0001 vs. gefitinib treatment alone.
Figure 4.
(A) Colony formation assays in H827-7-2 and H827-7-4 cells. ***P<0.0001 vs. untreated group; ##P<0.001 and ###P<0.0001 between one treatment alone. (B) Western blot analysis was performed to detect the expression levels of Bcl-2 and Bax with different treatments.
Furthermore, we examined the effect of GW3965 on apoptotic proteins. Cells were treated with gefitinib (5 µM) alone, LXR ligand (GW3965, 5 µM) alone, or both drugs for 96 h. Western blot analysis showed that gefitinib enhanced Bcl-2 expression and decreased Bax expression compared with that of the control. GW3965 treatment with gefitinib weakened the effect of cell apoptotic proteins compared with gefitinib alone (Fig. 4B). Overall, these in vitro data suggest that gefitinib resistant cells have a stress response to gefitinib, but GW3965 can reduce this stress response to gefitinib in the H827-7-2 and H827-7-4 cells. The combination of GW3965 with gefitinib resensitized the resistant cells to TKIs. Reduced stress response may be one of the important mechanisms underlying the synergistic effects of LXR ligands on gefitinib.
Effect of GW3965 on the transcriptional level of nuclear receptor LXR
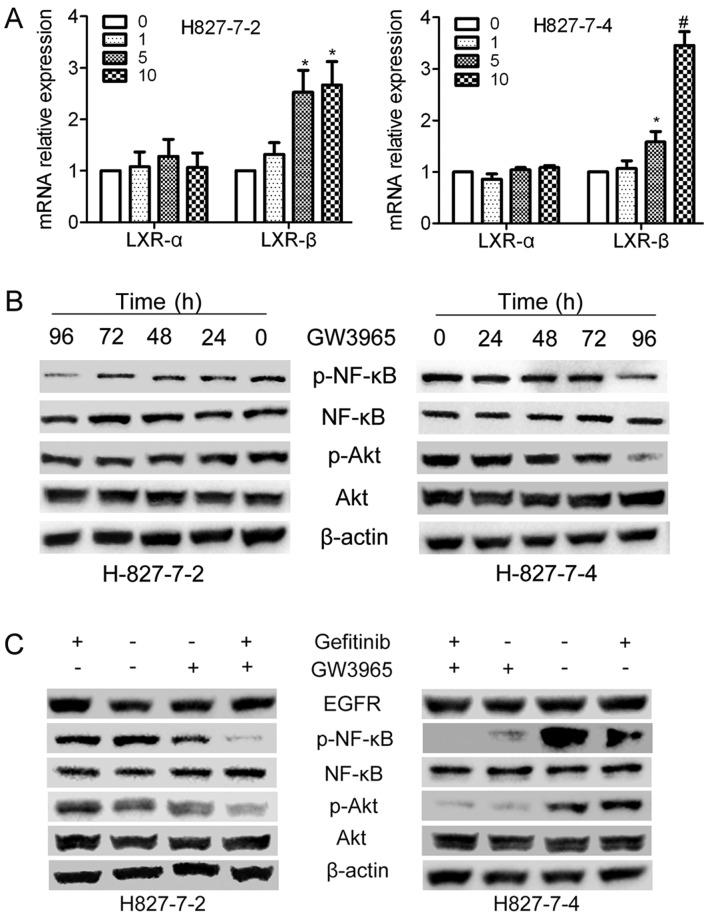
As GW3965 activates LXR receptors, we then applied Quantitative PCR assay to confirm whether GW3965 affects the level of LXRs receptors (LXRα and LXRβ) in the cell nucleus. As shown in Fig. 5A, with the treatment of GW3965 in H827-7-2 and H827-7-4 cells, the expression level of LXRα did not exhibit any differences, However, GW3965 increased the expression level of LXRβ distinctly at a higher dose (5 and 10 µM), whereas it had no effect at low dose (1 µM).
Figure 5.
GW3965 sensitizes gefitinib by inhibiting Akt-NF-κB activation: (A) H827-7-2 and H827-7-4 cells were treated with increasing concentrations of LXR ligands. mRNA expression levels of LXRα and LXRβ were measured using the qPCR assay. (*P<0.01 and #P<0.001 compared with control). (B) H827-7-2 and H827-7-4 cells were treated with GW3965 for different hour. The expression of AKT/p-AKT and NF-κB/p-NF-κB were detected by western blot analysis. (C) H827-7-2 and H827-7-4 cells were treated with gefitinib alone or combined with LXR ligands for 96 h. Western blot analysis was performed to detect the expressions of AKT, p-AKT, NF-κB and p-NF-κB. LXR, liver X receptor.
GW3965 treatment with gefitinib influences the activation of AKT-NF-κB
To further investigate the underlying mechanism of apoptosis introduced by GW3965 combined with gefitinib in H827-7-2 and H827-7-4 cells, these two cells were first treated with GW3965 (5 µM) separately at various periods (0–96 h). The phosphorylation of AKT and NF-κB was decreased after treatment with T0901317 for 96 h (Fig. 5B). Therefore, we analyzed the expression of EGFR and its downstream genes in two cells treated with gefitinib (5 µM) alone, LXR ligands (GW3965, 5 µM) alone or both drugs for 96 h. As shown in Fig. 5C, the phosphorylation levels of AKT and NF-κB when combined with GW3965 were downregulated compared with that of gefitinib alone.
Discussion
LXR is a ligand-dependent nuclear receptor. Previous study demonstrated that LXR had antiproliferative effects on cancer cells (22). Our results demonstrated that GW3965 exhibited no cytotoxicity in H827-7-2 and H827-7-4 cells. However, the proliferation rates of the two cells being treated with GW3965 combined with gefitinib were obviously inhibited when compared with that of gefitinib alone.
We found that GW3965 sensitized gefitinib in the two cells by inducing apoptosis and inhibiting colony formation. To further investigate whether GW3965 could affect cells apoptotic proteins, we detected the protein expression of Bcl-2 and Bax. Western blot analysis showed that gefitinib increased the expression level of Bcl-2 and decreased the expression level of Bax compared with that in the control. These results further illustrated that lung cancer cells had been resistant to gefitinib (23). As expected, GW3965 alone did not change the expression level of cell apoptotic proteins compared with that in the control. Meanwhile, when GW3965 was combined with gefitinib, the expression levels of Bcl-2 decreased while Bax increased compared with that in gefitinib alone.
In addition, synthetic agonists including TO-901317 and GW3965, were biphasic activators of LXRα and LXRβ (24). Compared with endogenous ligands, synthetic agonists could activate LXR more effectively and generate stronger effect on the transcription of downstream target genes (25,26). Colon cancer cell apoptosis could be induced by the LXRβ-dependent pathway (27). However, LXR could be synergistic in human carcinomas owning to signaling interactions mediated through LXRα (28). In this study, the expression level of LXRβ increased with higher dose of GW3965, prompting that LXRβ may be the primary subtype expressed in our experimental models.
Human NSCLCs with activating EGFR mutations showed an excellent response to treatment with EGFR-TKIs, including gefitinib and erlotinib. However, most patients with prolonged exposure to the drug developed relapse of cancer with drug resistance (29). Two principal mechanisms accounting for ~50% of acquired resistance were secondary mutations of threonine-to-methionine substitution at amino acid position 790 (T790M) of EGFR and amplification of the N-methyl-N0-nitro-N-nitroso-guanidine (MNNG) HOS-transforming gene (MET) oncogene (30–32). In this study, T790M mutation at exon 20 was not observed in the cells, but we found that the expression level of MET was higher in H827-7-2 and H827-7-4 cells than that in parental H827 cells, whereas PTEN had similar expression between two cell lines.
Various molecular mechanisms of acquired resistance to EGFR-TKIs in lung cancer were reactivation of EGFR downstream signaling pathways, especially Akt-NF-κB signal pathway (33). MET amplification could lead to gefitinib resistance in lung cancer by activating ERBB3-phosphoinositide 3-kinase (PI3K)/Akt (34). NF-κB was known to have a role in survival signaling and could promote the transcription of anti-apoptotic genes. Previous study found that inhibition of NF-κB enhanced apoptosis in EGFR-mutant lung cancer models (35). Furthermore, the inhibition of NF-κB could enhance EGFR TKI-induced apoptosis (36). Previous studies demonstrated that the LXR agonist could inhibit activation of the EGFR-AKT-NF-κB pathway and also had a potential synergistic effect with EGFR-TKI treatment (37,38). The phosphorylation of Akt and NF-κB reduced time dependently with GW3965 treatment in the two cells in our study. Thus, we hypothesized that the Akt-NF-κB signaling pathway could be suppressed by the LXR agonist, so that it could sensitize gefitinib-resistant non-small cell lung cancer cells to gefitinib treatment. Our results proved this hypothesis and confirmed the inhibitory effect of the LXR agonist on the Akt-NF-κB signaling pathway. The combination of gefitinib with GW3965 suppressed Akt-NF-κB signaling pathway, which promoted cell apoptotsis and inhibited cell proliferation.
In conclusion, our results demonstrated that the LXR agonist GW3965 could effectively enhanced gefitinib-resistant cell sensitivity to gefitinib. In addition, the results suggested that the resensitization of H827-7-2 and H827-7-4 was achieved by the inhibited activation of Akt-NF-κB pathway, which mediated by the LXRβ expression. Overall, these findings provided evidence for the combination treatment of acquired TKI resistance NSCLC.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (no. 81372396) and the Natural Science Foundation of Jiangsu Province (nos. BK20141016 and BK20141017).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Jeong Y, Xie Y, Lee W, Bookout AL, Girard L, Raso G, Behrens C, Wistuba II, Gadzar AF, Minna JD, Mangelsdorf DJ. Research resource: Diagnostic and therapeutic potential of nuclear receptor expression in lung cancer. Mol Endocrinol. 2012;26:1443–1454. doi: 10.1210/me.2011-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukohara T, Engelman JA, Hanna NH, Yeap BY, Kobayashi S, Lindeman N, Halmos B, Pearlberg J, Tsuchihashi Z, Cantley LC, et al. Differential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutations. J Natl Cancer Ins. 2005;97:1185–1194. doi: 10.1093/jnci/dji238. [DOI] [PubMed] [Google Scholar]
- 4.Amann J, Kalyankrishna S, Massion PP, Ohm JE, Girard L, Shigematsu H, Peyton M, Juroske D, Huang Y, Salmon Stuart J, et al. Aberrant epidermal growth factor receptor signaling and enhanced sensitivity to EGFR inhibitors in lung cancer. Cancer Res. 2005;65:226–235. [PubMed] [Google Scholar]
- 5.Soria JC, Mok TS, Cappuzzo F, Jänne PA. EGFR-mutated oncogene-addicted non-small cell lung cancer: Current trends and future prospects. Cancer Treat Rev. 2012;38:416–430. doi: 10.1016/j.ctrv.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen KS, Neal JW. First-line treatment of EGFR-mutant non-small-cell lung cancer: The role of erlotinib and other tyrosine kinase inhibitors. Biologics. 2012;6:337–345. doi: 10.2147/BTT.S26558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong CR, Jänne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nature Med. 2013;19:1389–1400. doi: 10.1038/nm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong H, Guo P, Zhai Y, Zhou J, Uppal H, Jarzynka MJ, Song WC, Cheng SY, Xie W. Estrogen deprivation and inhibition of breast cancer growth in vivo through activation of the orphan nuclear receptor liver X receptor. Mol Endocrinol. 2007;21:1781–1790. doi: 10.1210/me.2007-0187. [DOI] [PubMed] [Google Scholar]
- 9.Vedin LL, Lewandowski SA, Parini P, Gustafsson JA, Steffensen KR. The oxysterol receptor LXR inhibits proliferation of human breast cancer cells. Carcinogenesis. 2009;30:575–579. doi: 10.1093/carcin/bgp029. [DOI] [PubMed] [Google Scholar]
- 10.Villablanca EJ, Raccosta L, Zhou D, Fontana R, Maggioni D, Negro A, Sanvito F, Ponzoni M, Valentinis B, Bregni M, et al. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat Med. 2010;16:98–105. doi: 10.1038/nm.2074. [DOI] [PubMed] [Google Scholar]
- 11.Scoles DR, Xu X, Wang H, Tran H, Taylor-Harding B, Li A, Karlan BY. Liver X receptor agonist inhibits proliferation of ovarian carcinoma cells stimulated by oxidized low density lipoprotein. Gynecol Oncol. 2010;116:109–116. doi: 10.1016/j.ygyno.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Lo Sasso G, Bovenga F, Murzilli S, Salvatore L, Di Tullio G, Martelli N, D'Orazio A, Rainaldi S, Vacca M, Mangia A, et al. Liver X receptors inhibit proliferation of human colorectal cancer cells and growth of intestinal tumors in mice. Gastroenterology. 2013;144(1497–1507):e1–e13. doi: 10.1053/j.gastro.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Bischoff ED, Daige CL, Petrowski M, Dedman H, Pattison J, Juliano J, Li AC, Schulman IG. Non-redundant roles for LXRalpha and LXRbeta in atherosclerosis susceptibility in low density lipoprotein receptor knockout mice. J Lipid Res. 2010;51:900–906. doi: 10.1194/jlr.M900096-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuu CP, Lin HP. Antiproliferative effect of LXR agonists T0901317 and 22(R)-hydroxycholesterol on multiple human cancer cell lines. Anticancer Res. 2010;30:3643–3648. [PubMed] [Google Scholar]
- 15.Wairagu PM, Park KH, Kim J, Choi JW, Kim HW, Yeh BI, Jung SH, Yong SJ, Jeong Y. Combined therapeutic potential of nuclear receptors with receptor tyrosine kinase inhibitors in lung cancer. Biochem Biophys Res Commun. 2014;447:490–495. doi: 10.1016/j.bbrc.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Morgillo F, Cascone T, D'Aiuto E, Martinelli E, Troiani T, Saintigny P, De Palma R, Heymach JV, Berrino L, Tuccillo C, Ciardiello F. Antitumour efficacy of MEK inhibitors in human lung cancer cells and their derivatives with acquired resistance to different tyrosine kinase inhibitors. Br J Cancer. 2011;105:382–392. doi: 10.1038/bjc.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al. EGF receptor gene mutations are common in lung cancers from ‘never smokers’ and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu X, Xing L, Jiao Y, Xu J, Wang X, Han A, Yu J. BTG2 overexpression increases the radiosensitivity of breast cancer cells in vitro and in vivo. Oncology Res. 2013;20:457–465. doi: 10.3727/096504013X13685487925211. [DOI] [PubMed] [Google Scholar]
- 19.Harada D, Takigawa N, Ochi N, Ninomiya T, Yasugi M, Kubo T, Takeda H, Ichihara E, Ohashi K, Takata S, et al. JAK2-related pathway induces acquired erlotinib resistance in lung cancer cells harboring an epidermal growth factor receptor-activating mutation. Cancer Sci. 2012;103:1795–1802. doi: 10.1111/j.1349-7006.2012.02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer. 2009;10:281–289. doi: 10.3816/CLC.2009.n.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suda K, Tomizawa K, Osada H, Maehara Y, Yatabe Y, Sekido Y, Mitsudomi T. Conversion from the ‘oncogene addiction’ to ‘drug addiction’ by intensive inhibition of the EGFR and MET in lung cancer with activating EGFR mutation. Lung Cancer. 2012;76:292–299. doi: 10.1016/j.lungcan.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Derangère V, Chevriaux A, Courtaut F, Bruchard M, Berger H, Chalmin F, Causse SZ, Limagne E, Végran F, Ladoire S, et al. Liver X receptor β activation induces pyroptosis of human and murine colon cancer cells. Cell Death Differ. 2014;21:1914–1924. doi: 10.1038/cdd.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98:2603–2614. doi: 10.1182/blood.V98.9.2603. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Yu DD, Yan DL, Hu Y, Chen D, Liu Y, Zhang HD, Yu SR, Cao HX, Feng JF. Liver X receptor as a drug target for the treatment of breast cancer. Anticancer Drugs. 2016;27:373–382. doi: 10.1097/CAD.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 25.Mitro N, Vargas L, Romeo R, Koder A, Saez E. T0901317 is a potent PXR ligand: Implications for the biology ascribed to LXR. FEBS Lett. 2007;581:1721–1726. doi: 10.1016/j.febslet.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 26.Collins JL, Fivush AM, Watson MA, Galardi CM, Lewis MC, Moore LB, Parks DJ, Wilson JG, Tippin TK, Binz JG, et al. Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J Med Chem. 2002;45:1963–1966. doi: 10.1021/jm0255116. [DOI] [PubMed] [Google Scholar]
- 27.Courtaut F, Derangère V, Chevriaux A, Ladoire S, Cotte AK, Arnould L, Boidot R, Rialland M, Ghiringhelli F, Rébé C. Liver X receptor ligand cytotoxicity in colon cancer cells and not in normal colon epithelial cells depends on LXRβ subcellular localization. Oncotarget. 2015;6:26651–26662. doi: 10.18632/oncotarget.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabitova L, Restifo D, Gorin A, Manocha K, Handorf E, Yang DH, Cai KQ, Klein-Szanto AJ, Cunningham D, Kratz LE, et al. Endogenous sterol metabolites regulate growth of EGFR/KRAS-dependent tumors via LXR. Cell Rep. 2015;12:1927–1938. doi: 10.1016/j.celrep.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cadranel J, Ruppert AM, Beau-Faller M, Wislez M. Therapeutic strategy for advanced EGFR mutant non-small-cell lung carcinoma. Crit Rev Oncol Hematol. 2013;88:477–493. doi: 10.1016/j.critrevonc.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–2899. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- 32.Turke AB, Zejnullahu K, Wu YL, Song Y, Dias-Santagata D, Lifshits E, Toschi L, Rogers A, Mok T, Sequist L, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 34.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 35.Bivona TG, Hieronymus H, Parker J, Chang K, Taron M, Rosell R, Moonsamy P, Dahlman K, Miller VA, Costa C, et al. FAS and NF-κB signalling modulate dependence of lung cancers on mutant EGFR. Nature. 2011;471:523–526. doi: 10.1038/nature09870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakuma Y, Yamazaki Y, Nakamura Y, Yoshihara M, Matsukuma S, Koizume S, Miyagi Y. NF-κB signaling is activated and confers resistance to apoptosis in three-dimensionally cultured EGFR-mutant lung adenocarcinoma cells. Biochem Biophys Res Commun. 2012;423:667–671. doi: 10.1016/j.bbrc.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Guo D, Reinitz F, Youssef M, Hong C, Nathanson D, Akhavan D, Kuga D, Amzajerdi AN, Soto H, Zhu S, et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov. 2011;1:442–456. doi: 10.1158/2159-8290.CD-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng O, Ostrowski RP, Liu W, Zhang JH. Activation of liver X receptor reduces global ischemic brain injury by reduction of nuclear factor-kappaB. Neuroscience. 2010;166:1101–1109. doi: 10.1016/j.neuroscience.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]