Abstract
Background
We do not have a unified, scientifically tested theory of causation for obesity and its co-morbidities, nor do we have explanations for the mechanics of the metabolic/bariatric surgery procedures. Integral to proffered hypotheses are the actions of the hormones glucagon-like peptide-1 (GLP-1), peptide YY (PYY), and leptin. The objective of this study was to obtain blood levels of GLP-1, PYY, and leptin after stimulation of the terminal ileum and cecum by a static infusion of a food hydrolysate in morbidly obese patients undergoing a duodenal switch procedure.
Setting
University Hospital.
Methods
Plasma levels of GLP-1, PYY, and leptin were obtained at 0, 30, 60, 90, and 120 minutes after instillation of 240 mL of a food hydrolysate into the ileum or cecum.
Results
The mean ± SD GLP-1 values by cecal stimulation for 0, 30, 60, 90, and 120 minutes were: 41.3 ± 23.2; 39.6 ± 21.8; 38.9 ± 19.1; 47.4 ± 22.3; 51.7 ± 27.3 pM, and by ileal stimulation: 55.0 ± 32.8; 83.4 ± 16.1; 78.7 ± 23.8; 84.7 ± 23.5; 76.4 ± 25.6. The mean ± SD PYY values by cecal stimulation were: 62.1 ± 24.8; 91.1 ± 32.8; 102.1 ± 39.6; 119.6 ± 37.5; 130.3 ± 36.7, and by ileal stimulation: 73.8 ± 41.6; 138.1 ± 17.7; 149.5 ± 23.3; 165.7 ± 24.3; 155.5 ± 29.1. Percent change in PYY levels increased ~150%, GLP-1 increased ~50%, and leptin decreased ~20%.
Conclusion
Direct stimulation of the human terminal ileum and cecum by a food hydrolysate elicits significant plasma GLP-1 and PYY elevations and leptin decreases, peaking at 90–120 minutes. The ileal GLP-1 and PYY responses exceed those of the cecum, and the PYY effect is about 3-fold that of GLP-1. The results of this study question the satiety premise for ileal transposition.
Keywords: GLP-1, PYY, Leptin, Duodenal switch
The discipline of bariatric surgery evolved empirically from observation, patient experimentation, and subsequent outcomes dissemination by publication. Individuals with a markedly shortened intestine or a proximal alimentary tract obstruction lost weight. Applying this common knowledge by iatrogenic gastrointestinal restriction or massive gut exclusion met with weight loss success and the initiation of bariatric surgery [1]. After 30-plus years of clinical reports and founding of the 2 pioneer professional bariatric societies—American Society for Bariatric Surgery in 1983 and the International Federation for the Surgery of Obesity in 1995—the field began to appreciate that bariatric surgery was an aspect of metabolic surgery, similar to peptic ulcer surgery and hyperlipidemia surgery [2]. With this insight, and 2 milestone reports on the effect of metabolic/bariatric surgery on type 2 diabetes [3,4], a search for metabolic mechanisms of action rapidly gained momentum. The over simplified explanations of primarily restrictive, restrictive/malabsorptive, and primarily malabsorptive failed to provide a satisfactory understanding for the neurohormonal changes being described to accompany metabolic/bariatric surgery or to explain the effects of electronic gut stimulation on weight and obesity co-morbidities.
In addition to attempting to explain how the standard metabolic/bariatric operations elicit their weight and other metabolic effects, clinicians have been eager to use the newly elucidated hormonal knowledge to promote new operations. Primary among these experimental procedures are the duodenal-jejunal bypass [5,6] and ileal transposition into the jejunum or even the duodenum [7,8], each with a different primary objective. The former procedure bypasses the pylorus, duodenum, and proximal jejunum, mainly for excluding contact of food with the proximal bowel, whereas the latter specifically seeks rapid exposure of the ileum to food. Both innovations have in common earlier release from the ileum of the hormones glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), both stated to effect satiety and type 2 diabetes.
To obtain basic knowledge and to offer experimental data for mechanisms, speculations, and procedures justification, we have obtained the blood levels of GLP-1, PYY, and leptin after stimulation of the terminal ileum and cecum by a static infusion of a food hydrolysate in morbidly obese patients undergoing a duodenal switch procedure.
Methods
Protocol
In an Institutional Review Board approved study, with fully informed patient consent, Nutren Liquid Nutrition (20 g protein, 49 g carbohydrate, 26 g fat, and vitamins) was instilled either into the terminal ileum or into the cecum during the performance of a scheduled open duodenal switch. Blood samples were obtained at baseline and at 0, 30, 60, 90, and 120 minutes for analysis of circulating GLP-1, PYY, and leptin.
Operative infusion technique
Subsequent to anesthesia induction and open exposure of the abdominal cavity, the Nutren hydrolysate infusion was carried out at the start of the duodenal switch procedure. The common channel (~100 cm from the ileocecal valve) and the biliopancreatic and enteric (Roux) limbs (~half each of the small bowel length proximal to the common channel) were identified by placement of transmesenteric marking sutures. When the terminal ileum was randomly selected for the hydrolysate infusion, encircling vascular loops were placed at the ileocecal junction and just below the marking suture for the proximal end of the common channel. The distal vascular loop was tightened to block flow into the cecum. An enterotomy was made into the ileum where the jejunoileostomy for the duodenal switch would be situated, a soft catheter threaded into the terminal ileum, and the 240 mL of infusate instilled through the catheter with the aid of an assistant outside the operative field. The proximal vascular loop below the enterotomy was then tightened to occlude the terminal ileum containing the hydrolysate. For the cecal instillation, the vascular loop at the ileocecal valve was tightened for proximal bowel occlusion (the cecum was not occluded distally), and the hydrolysate injected via a catheter through the base of the appendix before completion of the appendectomy. The 240 mL of infusate was infused over 2–3 minutes by hand pressure on the plunger of a 60-mL syringe. At the end of the operation, all vascular loops were removed. There were no untoward events in any of the subject patients.
GLP-1, PYY, leptin analyses
GLP-1, PYY, and leptin plasma concentration analyses by the enzyme-linked immunosorbent assay were conducted by the EMD Millipore Corporation (St. Charles, MO).
Statistics
Statistical analyses were performed by repeated measure analysis of variance (ANOVA) across time and between groups; multiple statistical derivations (e.g., confidence intervals, standard errors) are provided in the tables and figures, and P values are given in the text. P values < .05 were considered significant.
Results
The number of patient samples, mean values, standard deviations, 95% confidence intervals, and minimal and maximum values for cecal and ileal infusions are shown in Table 1 for GLP-1, in Table 2 for PYY, and in Table 3 for leptin.
Table 1.
Measured changes in GLP-1 over time in both cecum and ileum
| Time | Location | N | Mean | Std. deviation | 95% Confidence… | 95% Confidence… | Minimum | Maximum | P value |
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Lower boundary | Upper boundary | ||||||||
| 0 min | Cecum | 7 | 41.3 | 23.2 | 19.9 | 62.7 | 7.2 | 66.2 | .42 |
| Ileum | 5 | 55.0 | 32.8 | 14.2 | 95.7 | 10.8 | 96.0 | ||
| 30 min | Cecum | 7 | 39.6 | 21.8 | 19.4 | 59.8 | 13.1 | 78.1 | <.01 |
| Ileum | 4 | 83.4 | 16.1 | 57.7 | 109.0 | 68.8 | 105.0 | ||
| 60 min | Cecum | 7 | 38.9 | 19.1 | 21.2 | 56.6 | 13.9 | 68.2 | <.01 |
| Ileum | 5 | 78.7 | 23.8 | 49.2 | 108.2 | 49.3 | 101.0 | ||
| 90 min | Cecum | 7 | 47.4 | 22.3 | 26.7 | 68.0 | 16.3 | 74.3 | .02 |
| Ileum | 5 | 84.7 | 23.5 | 55.5 | 113.9 | 56.0 | 111.0 | ||
| 120 min | Cecum | 7 | 51.7 | 27.3 | 26.5 | 77.0 | 17.9 | 92.4 | .14 |
| Ileum | 5 | 76.4 | 25.6 | 44.6 | 108.2 | 35.7 | 95.6 | ||
Comparisons performed by repeated measures ANOVA. There was a significant difference between cecum and ileum at 30-, 60- and 90-minutes. P values <.05 were considered significant.
Table 2.
Measured changes in PYY over time in both cecum and ileum
| Time | Location | N | Mean | Std. Deviation | 95% Confidence… | 95% Confidence… | Minimum | Maximum | P value |
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Lower boundary | Upper boundary | ||||||||
| 0 min | Cecum | 7 | 62.1 | 24.8 | 39.1 | 85.1 | 41.2 | 111.7 | .55 |
| Ileum | 5 | 73.8 | 41.6 | 22.1 | 125.5 | 41.2 | 133.8 | ||
| 30 min | Cecum | 7 | 91.1 | 32.8 | 60.8 | 121.5 | 41.2 | 127.8 | .02 |
| Ileum | 5 | 138.1 | 17.7 | 116.1 | 160.2 | 115.0 | 154.3 | ||
| 60 min | Cecum | 6 | 102.1 | 39.6 | 60.5 | 143.7 | 52.6 | 162.0 | .04 |
| Ileum | 5 | 149.5 | 23.3 | 120.6 | 178.4 | 126.2 | 177.1 | ||
| 90 min | Cecum | 7 | 119.6 | 37.5 | 84.9 | 154.2 | 52.6 | 163.5 | .04 |
| Ileum | 5 | 165.7 | 24.3 | 135.6 | 195.9 | 140.3 | 196.9 | ||
| 120 min | Cecum | 7 | 130.3 | 36.7 | 96.4 | 164.2 | 81.6 | 184.5 | .23 |
| Ileum | 5 | 155.5 | 29.1 | 119.3 | 191.7 | 113.8 | 187.0 | ||
Comparisons performed by repeated measures ANOVA. There was a significant difference between cecum and ileum at 30-, 60- and 90-minutes. P values <.05 were considered significant.
Table 3.
Measured changes in Leptin over time in both cecum and ileum
| Time | Location | N | Mean | Std. Deviation | 95% Confidence… | 95% Confidence… | Minimum | Maximum | P value |
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Lower boundary | Upper boundary | ||||||||
| 0 min | Cecum | 7 | 34500 | 10419 | 24865 | 44136 | 16720 | 51196 | .90 |
| Ileum | 5 | 33433 | 19591 | 9108 | 57758 | 18004 | 67026 | ||
| 30 min | Cecum | 7 | 32733 | 10722 | 22817 | 42649 | 18237 | 54207 | .55 |
| Ileum | 5 | 27640 | 18118 | 5143 | 50137 | 12403 | 58182 | ||
| 60 min | Cecum | 7 | 31937 | 11480 | 20735 | 41969 | 13776 | 52404 | .71 |
| Ileum | 5 | 27843 | 20471 | 2425 | 53261 | 14381 | 63513 | ||
| 90 min | Cecum | 7 | 30210 | 10014 | 20948 | 39471 | 16512 | 50172 | .61 |
| Ileum | 5 | 25695 | 19348 | 1671 | 49718 | 11540 | 59354 | ||
| 120 min | Cecum | 7 | 28483 | 9672 | 19538 | 37429 | 16570 | 48276 | .84 |
| Ileum | 5 | 26412 | 24967 | −4589 | 57413 | 6426 | 69962 | ||
Comparisons performed by repeated measures ANOVA. There were no significant differences between cecum and ileum. P values <.05 were considered significant.
The mean ± SD GLP-1 values by cecal stimulation for 0, 30, 60, 90, and 120 minutes, were (rounded): 41.3 ± 23.2; 39.6 ± 21.8; 38.9 ± 19.1; 47.4 ± 22.3; and 51.7 ± 27.3 pM. The mean ± SD GLP-1 values by ileal stimulation for the same time intervals were 55.0 ± 32.8; 83.4 ± 16.1; 78.7 ± 23.8; 84.7 ± 23.5; and 76.4 ± 25.6 pM. Total GLP-1 concentration levels compared by repeated measures ANOVA for ileal to cecal stimulation for the same time intervals were P = .416, .007, .009, .019, and .145.
The mean ± SD PYY values by cecal stimulation for 0, 30, 60, 90, and 120 minutes were (rounded): 62.1 ± 24.8; 91.1 ± 32.8; 102.1 ± 39.6; 119.6 ± 37.5; and 130.3 ± 36.7. The mean ± SD PYY values by ileal stimulation for the same time intervals were 73.8 ± 41.6; 138.1 ± 17.7; 149.5 ± 23.3; 165.7 ± 24.3; and 155.5 ± 29.1. Total PYY concentration levels compared by repeated measures ANOVA for ileal to cecal stimulation for the same time intervals were P = .554, .016, .043, .037, and .232.
The mean ± SD leptin values by cecal stimulation for 0, 30, 60, 90, and 120 minutes were (rounded) −345 ± 104; −327 ± 107; −319 ± 115; −302 ± 100; and −285 ± 97. The mean ± SD leptin values for ileal stimulation for the same time intervals were: −344 ± 196; −276 ± 181; −278 ± 205; 257 ± 193; and −264 ± 250. Total leptin concentration levels compared by repeated measures ANOVA for ileal to cecal stimulation for the same time intervals were P = 0.90, .55, .71, .61, and .84.
Thus, total GLP-1 and PYY concentration levels only peaked after 90–120 minutes. Measured values were both significantly higher after ileal compared to cecal stimulation at 30, 60, and 90 minutes. The leptin concentrations decreased reciprocally to the GLP-1 and PYY values.
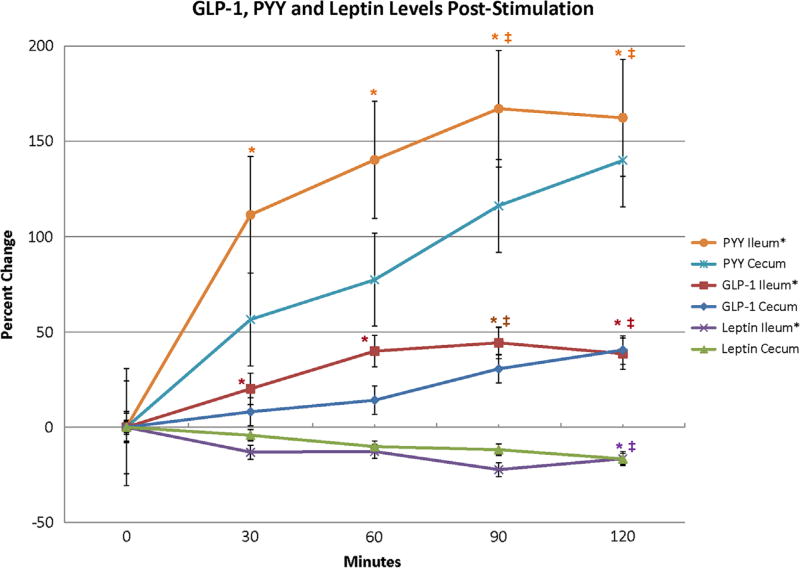
The percent change in GLP-1, PYY, and leptin levels post-stimulation are shown in Fig. 1. PYY levels after ileal stimulation rose above 150% at 90 minutes and then plateaued; PYY after cecal stimulation continued to rise and nearly reached 150% at 120 minutes. The GLP-1 levels rose concurrently but never exceeded 50%. The leptin levels consistently dropped below baseline reaching approximately −20% at 90 minutes. The steepest ascent slope for the PYY concentrations after both ileal and cecal stimulation was in the first 30 minutes with a less steep rise to their measured maximums. The steepest ascent slope for GLP-1 after ileal stimulation continued up to 60 minutes, where the GLP-1 concentration started to plateau. The steepest ascent for GLP-1 after cecal stimulation occurred after 60 minutes, between 60–90 minutes, and this concentration curve was still rising at 120 minutes.
Fig. 1.
Comparison of GLP-1, PYY, and leptin changes from baseline between cecum and ileum. All values compared using repeated measures ANOVA. Data represented as mean ± SE. *P < .05 for ileum compared to baseline; ‡P < .05 for cecum compared to baseline.
Discussion
Currently, we have a plethora of information on gut and other hormones, incretins, vagal and complementary neural pathways, the gastric pacemaker, and hypothalamic functions, attempting to explain why metabolic/bariatric surgery works and how the different operative procedures influence these mechanisms. Based on these data, several hypotheses have emerged to explain mechanisms of action. Some of them are quite plausible but at times contradictory. We have yet to derive a unified, scientifically tested, hypothesis of causation that is also explanatory of the effects of intercession.
To move forward in this quest, we need to study in-depth portions of the total physiologic/biochemical mosaic of metabolic surgery and its subdivisions of bariatric surgery, diabetes surgery, etc. The actions of hormones, in particular the gastrointestinal hormones, have occupied extensive attention. Among them, the intestinal hormones GLP-1 and PYY have been well characterized, as has the adipocyte-derived cytokine leptin. GLP-1 and PYY, both elaborated by the L-cells of the intestinal mucosa, seem to work in concert, either eliciting the same metabolic responses or augmenting the actions of the other. Their infusion-elicited gastrointestinal properties include the reduction of hunger and increase in the sensation of satiety by stimulation of the hypothalamic arcuate nucleus [9–11]; and the “ileal brake” effect of delayed gastric emptying, delayed mouth to cecum transit time, and decreased jejunal wave pressure [12–14]. Curiously, PYY exhibits 2 active circulating forms: PYY (1–36), which essentially increases appetite and promotes weight gain, and PYY (3–36), formed from the cleavage of the tyrosine-proline residues from the N-terminal of the polypeptide, which does the opposite, centrally decreasing appetite and promoting weight loss [15–19]. PYY also inhibits pentagastrine-stimulated gastric acid secretion [20] and the cephalic phase of pancreatic exocrine secretion [21,22]. With respect to pancreatic endocrine function, GLP-1 and PYY contribute significantly to the incretin effect, defined as the concentration of insulin released by oral glucose stimulation that exceeds the insulin concentration elicited by the same amount of intravenous glucose [23]. PYY effects insulin secretion indirectly by inhibiting the action of gastrin-stimulating peptide and gastrin-releasing peptide [24,25]. The influence of GLP-1 on pancreatic endocrine function is more direct and appears to be more powerful. GLP-1 stimulates glucose-dependent insulin secretion, preinsulin gene expression, β-cell proliferation and antiapoptotic pathways, and inhibits glucagon release [26,27]. It has been shown that GLP-1 secretion is reduced in patients with type 2 diabetes [28,29], which may be responsible for the hyperglycemia of this disease [30].
The natural stimulation mechanisms for the release of GLP-1 and PYY into the bloodstream have been well studied. Both hormones are secreted in increasing amounts as a function of the caloric content of ingested food [31]. Influencing factors include the intestinal site of stimulation [32–34], bile acids [35], central neural and vagal mechanisms [36–38], and other hormones (vasoactive intestinal peptide, [39] gastrin [40]). GLP-1 and PYY are interdependent in a biofeedback autoregulation loop [41].
The relative intestinal secretion capacity for PYY has been well defined by Adrian et al [31]: 6 pmol/g in duodenum, 5 pmol/g in jejunum, 84 pmol/g in terminal ileum, and higher concentrations throughout the colon (ascending 82 pmol/g, sigmoid 196 pmol/g, rectum 480 pmol/g). It would appear that there is minimal proximal concentration of PYY with increasing concentrations in descending segments of bowel with essentially equal concentrations in the terminal ileum and cecum. These studies have been corroborated by immunoreactivity techniques [42–45].
Leptin forms a triad with GLP-1 and PYY in weight regulating mechanisms, possibly providing an autoregulatory function. Leptin stimulates GLP-1 secretion and GLP-1 suppresses leptin levels [46].
This study has shown that (1) direct stimulation of the human terminal ileum and cecum by a food hydrolysate elicits significant plasma GLP-1 and PYY elevations and leptin decreases over 120 minutes, (2) these responses peak at 90–120 minutes, (3) the ileal GLP-1 and PYY elevations exceed those of the cecum, and (4) the hydrolysate effect on the PYY plasma concentration is about 3-fold that of GLP-1.
The fact that both GLP-1 and PYY levels peaked only after 90–120 minutes by direct ileal and cecal stimulation make the effect of these hormones on immediate postprandial satiety questionable. It is unlikely that the translocation of a segment of ileum by ileal transposition would enhance the immediate postprandial release of GLP-1 and PYY over that obtained by direct stimulation in situ. The importance of these findings for metabolic surgery for type 2 diabetes is currently unclear. There are recent studies demonstrating limited deterioration of glucose tolerance on blockade of GLP-1 action [47,48]. These reports seem to diminish the importance of GLP-1 secretion in the amelioration of type 2 diabetes.
Both a strength and weakness of this study is the experimental condition under which it was performed. To the best of our knowledge, this is the first report of measurement of plasma GLP-1, PYY, and leptin levels after direct ileal and cecal stimulation by a food hydrolysate in humans and, in particular, obese humans. At the same time, the study was conducted under general anesthesia and in individuals who had been NPO for 12 hours. These factors could influence the hormonal release pattern tested by diminishing or augmenting the results. There are no known studies of the effects of anesthesia on the secretion of gut hormones. Furthermore, the study protocol does not truly replicate homeostatic human physiology. After all, ingested nutrients do not remain captive in a small segment of the gastrointestinal tract under normal circumstances. The bowel preparation (magnesium citrate) and the patients being NPO for 12 hours may also have an effect on gut hormone secretion. Finally, the obese human hormonal response may well be different from the response in nonobese patients, especially in those with type 2 diabetes.
Currently, ileal transposition is being practiced in obese patients or overweight patients with type 2 diabetes in the belief that the ileal hormonal effect will not only be beneficial but enhanced by moving an ileal segment higher in the gastrointestinal tract. It may be so, however, this assumption is not supported by the findings of this study. Furthermore, the proponents of ileal transposition seem to give little credence to the studies showing that duodenal stimulation, either by direct GLP-1 and PYY release from the L-cells located in the duodenum or by stimulation of the distal gastrointestinal tract via a neuro syncytium or a hormonal messenger [49,50], elicits an equivalent GLP-1 and PYY response to that of the ileum before the nutrients reach the ileum [36,49,51,52]. Finally, in a study in Goto-Kakizaki rats, we have found a statistically significant rise in the postprandial plasma GLP-1 levels after ileal excision (unpublished data). Thus, these animals totally deprived of their ileum exhibit the response hoped for by ileal transposition.
Research based on metabolic surgery is building a database of hormonal findings that will, in time, help to explain the precise mechanisms for metabolic/bariatric operations and possibly the etiologies of obesity and type 2 diabetes.
Acknowledgments
This study was funded by a Research Service Pilot grant from University of Minnesota Clinical and Translational Science Institute (CTSI).
Footnotes
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
References
- 1.Buchwald H, Buchwald JN. Evolution of operative procedures for the management of morbid obesity 1950–2000. Obes Surg. 2002;12:705–17. doi: 10.1381/096089202321019747. [DOI] [PubMed] [Google Scholar]
- 2.Buchwald H, Varco RL, editors. Metabolic surgery. New York: Grune and Stratton; 1978. [Google Scholar]
- 3.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–52. doi: 10.1097/00000658-199509000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scopinaro N, Adami GF, Marinari GMFS, et al. Biliopancreatic diversion. World J Surg. 1998;22:936–46. doi: 10.1007/s002689900497. [DOI] [PubMed] [Google Scholar]
- 5.Klein S, Fabbrini E, Patterson BW, et al. Moderate effect of duodenal-jejunal bypass surgery on glucose homeostasis in patients with type 2 diabetes. Obesity. 2012;20:1266–72. doi: 10.1038/oby.2011.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen R, Caravatto PP, Correa JL, et al. Glycemic control after stomach-sparing duodenal-jejunal bypass surgery in diabetic patients with low body mass index. Surg Obes Relat Dis. 2012;8:375–80. doi: 10.1016/j.soard.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Gagner M. Surgical treatment of nonseverely obese patients with type 2 diabetes mellitus: sleeve gastrectomy with ileal transposition (SGIT) is the same as the neuroendocrine brake (NEB) procedure or ileal interposition associated with sleeve gastrectomy (II-SG), but ileal interposition with diverted sleeve gastrectomy (II-DSG) is the same as duodenal switch. Surg Endosc. 2001;25:655–6. doi: 10.1007/s00464-010-1221-9. [DOI] [PubMed] [Google Scholar]
- 8.DePaula AL, Stival AR, DePaula CC, Halpern A, Vencio S. Surgical treatment of type 2 diabetes in patients with BMI below 35: mid-term outcomes of the laparoscopic ileal interposition associated with a sleeve gastrectomy in 202 consecutive cases. J Gastrointest Surg. 2012;16:967–76. doi: 10.1007/s11605-011-1807-0. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez EJ, Whitcomb DC, Vigna SR, Taylor IL. Saturable binding of circulating peptide YY in the dorsal vagal complex of rats. Am J Physiol Gastrointest Liver Physiol. 1994;226:G511–6. doi: 10.1152/ajpgi.1994.266.3.G511. [DOI] [PubMed] [Google Scholar]
- 10.Druce MR, Small CJ, Bloom SR. Minireview: gut peptides regulating satiety. Endocrinology. 2004;145:2660–5. doi: 10.1210/en.2004-0089. [DOI] [PubMed] [Google Scholar]
- 11.Korner J, Leibel RL. To eat or not to eat – how the gut talks to the brain. N Engl J Med. 2003;349:926–8. doi: 10.1056/NEJMp038114. [DOI] [PubMed] [Google Scholar]
- 12.Savage AP, Adrian TE, Carolan G, Chatterjee VK, Bloom SR. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut. 1987;28:166–70. doi: 10.1136/gut.28.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiller RC, Trotman IF, Adrian TE, et al. Further characterization of the ‘ileal brake’ reflex in man—effect of ileal infusion of partial digests of fat, protein, and starch on jejunal mortality and release of neurotensin, enteroglucagon, and peptide YY. Gut. 1988;29:1042–51. doi: 10.1136/gut.29.8.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spiller RC, Trotman IF, Higgins BE, et al. The ileal brake–inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25:365–74. doi: 10.1136/gut.25.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballantyne GH. Peptide YY(1–36) and peptide (YY(3–36): Part I. Distribution, release and actions. Obes Surg. 2006;16:651–8. doi: 10.1381/096089206776944959. [DOI] [PubMed] [Google Scholar]
- 16.Sileno AP, Brandt GC, Spann BM, Quay SC. Lower mean weight after 14 days intravenous administration peptide YY(3–36) (PYY(3–36) in rabbits. Int J Obes. 2006;30:68–72. doi: 10.1038/sj.ijo.0803067. [DOI] [PubMed] [Google Scholar]
- 17.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 18.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349:941–8. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 19.Neary NM, Small CJ, Druce MR, et al. Peptide YY3–36 and glucagon-like peptide-17–36 inhibit food intake additively. Endocrinology. 2005;146:5120–7. doi: 10.1210/en.2005-0237. [DOI] [PubMed] [Google Scholar]
- 20.Adrian TE, Savage AP, Sagor GR, et al. Effect of peptide YY on gastric, pancreatic, and biliary function in humans. Gastroenterology. 1985;89:484–9. doi: 10.1016/0016-5085(85)90442-1. [DOI] [PubMed] [Google Scholar]
- 21.Lluis F, Gomez G, Fujimura M, Greeley GH, Jr, Thompson JC. Peptide YY inhibits nutrient-, hormonal-, and vagally-stimulated pancreatic exocrine secretion. Pancreas. 1987;2:454–62. doi: 10.1097/00006676-198707000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Hosotani R, Inoue K, Kogire M, et al. Effect of natural peptide YY on pancreatic secretion and cholecystokinin release in conscious dogs. Dig Dis Sci. 1989;34:468–73. doi: 10.1007/BF01536273. [DOI] [PubMed] [Google Scholar]
- 23.Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol. 2009;587:27–32. doi: 10.1113/jphysiol.2008.164012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lius F, Salva JA, Thompson JC. Peptide YY inhibits the insulinotropic action of gastric inhibitory polypeptide. Gastroenterology. 1989;96:690–4. [PubMed] [Google Scholar]
- 25.Guo YS, Singh P, DeBouno JF, Thompson JC. Effect of peptide YY on insulin release stimulated by 2-deoxyglucose and neuropeptides in dogs. Pancreas. 1988;3:128–34. doi: 10.1097/00006676-198804000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Lim GE, Brubaker PL. Glucagon-like peptide 1 secretion by the L-cell. The view from within. Diabetes. 2006;55:S70–7. [Google Scholar]
- 27.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretin of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–23. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 29.Mannucci E, Ognibene A, Cremasco F, et al. Glucagon-like peptide (GLP)-1 and leptin concentrations in obese patients with type 2 diabetes mellitus. Diabet Med. 2000;17:713–9. doi: 10.1046/j.1464-5491.2000.00367.x. [DOI] [PubMed] [Google Scholar]
- 30.Nauck M, Stockmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- 31.Adrian TE, Ferre G-L, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–7. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 32.Greeley GH, Jr, Hashimoto T, Izukura M, et al. A comparison of intraduodenally and intracolonically administered nutrients on the release of peptide-YY in the dog. Endocrinology. 1989;125:1761–5. doi: 10.1210/endo-125-4-1761. [DOI] [PubMed] [Google Scholar]
- 33.Zhang T, Brubaker PL, Thompson JC, Greeley GH., Jr Characterization of peptide-YY release in response to intracolonic infusion of amino acids. Endocrinology. 1993;132:553–7. doi: 10.1210/endo.132.2.8093875. [DOI] [PubMed] [Google Scholar]
- 34.Aponte GW, Fink AS, Meyer JH, Tatemoto K, Taylor IL. Regional distribution and release of peptide YY with fatty acids of different chain length. Am J Physiol. 1985;249:G745–50. doi: 10.1152/ajpgi.1985.249.6.G745. [DOI] [PubMed] [Google Scholar]
- 35.Adrian TE, Ballantyne GH, Longo WE, et al. Deoxycholate is an important releaser of peptide YY and enteroglucagon from the human colon. Gut. 1993;34:1219–24. doi: 10.1136/gut.34.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onaga T, Zabielski R, Kato S. Multiple regulation of peptide YY secretion in the digestive tract. Peptides. 2002;23:279–90. doi: 10.1016/s0196-9781(01)00609-x. [DOI] [PubMed] [Google Scholar]
- 37.Zhang T, Uchida T, Gomez G, Lluis F, Thompson JC, Greeley GH., Jr Neural regulation of peptide YY secretion. Regulatory Peptides. 1993;48:321–8. doi: 10.1016/0167-0115(93)90160-a. [DOI] [PubMed] [Google Scholar]
- 38.Rudnicki M, Rigel DF, McFadden DW. Vagal cooling blocks circulating neuropeptide Y (NPY), peptide YY (PYY), and pancreatic polypeptide (PP) release. J Surg Res. 1991;51:40–5. doi: 10.1016/0022-4804(91)90067-v. [DOI] [PubMed] [Google Scholar]
- 39.Ballantyne GH, Goldenring JR, Savoca PE, et al. Cyclic AMP-mediated release of peptide YY (PYY) from the isolated perfused rabbit distal colon. Regulatory Peptides. 1993;47:117–26. doi: 10.1016/0167-0115(93)90415-5. [DOI] [PubMed] [Google Scholar]
- 40.Greeley GH, Jr, Jeng YJ, Gomez G, et al. Evidence for regulation of peptide-YY release by the proximal gut. Endocrinology. 1989;124:1438–43. doi: 10.1210/endo-124-3-1438. [DOI] [PubMed] [Google Scholar]
- 41.Naslund E, Bogefors J, Skogar S, et al. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol Regul Integr Comp Physiol. 1999;277:R910–6. doi: 10.1152/ajpregu.1999.277.3.R910. [DOI] [PubMed] [Google Scholar]
- 42.Ali-Rachedi A, Varndell IM, Adrian TE, et al. Peptide YY (PYY) immunoreactivity is co-stored with glucagon related immunoreactants in endocrine cells of the gut and pancreas. Histochem. 1984;80:487–91. doi: 10.1007/BF00495439. [DOI] [PubMed] [Google Scholar]
- 43.Bottcher G, Sjolund K, Ekblad E, Hakanson R, Schwartz TW, Sundler F. Coexistence of peptide YY and glicentin immunoreactivity in endocrine cells of the gut. Regulatory Peptides. 1984;8:261–6. doi: 10.1016/0167-0115(84)90034-x. [DOI] [PubMed] [Google Scholar]
- 44.Bottcher G, Alumets J, Hakanson R, Sundler F. Coexistence of glicentin and peptide YY in colorectal L-cells in cat and man. An electron microscopic study. Regulatory Peptides. 1986;13:283–91. doi: 10.1016/0167-0115(86)90046-7. [DOI] [PubMed] [Google Scholar]
- 45.Nilsson O, Bilchik AJ, Goldenring JR, Ballantyne GH, Adrian TE, Modlin IM. Distribution and immunocytochemical colocalization of peptide YY and enteroglucagon in endocrine cells of the rabbit colon. Endocrinology. 1991;129:139–48. doi: 10.1210/endo-129-1-139. [DOI] [PubMed] [Google Scholar]
- 46.Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52:252–9. doi: 10.2337/diabetes.52.2.252. [DOI] [PubMed] [Google Scholar]
- 47.Jimenez A, Casamitjana R, Viaplana-Masclans J, Lacy A, Vidal J. GLP-1 action and glucose tolerance in subjects with remission of type 2 diabetes after gastric bypass surgery. Diabetes Care. 2013;36:2062–9. doi: 10.2337/dc12-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah M, Law JH, Micheletto F, et al. The contribution of endogenous glucagon-like peptide-1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes. doi: 10.2337/db13-0954. published ahead of print October 2, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberge JN, Brubaker PL. Regulation of intestinal proglucagon-derived peptide secretion by glucose-dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology. 1993;133:233–40. doi: 10.1210/endo.133.1.8319572. [DOI] [PubMed] [Google Scholar]
- 50.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999;140:1687–94. doi: 10.1210/endo.140.4.6643. [DOI] [PubMed] [Google Scholar]
- 51.Rask E, Olsson T, Soderberg S, et al. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care. 2001;24:1640–5. doi: 10.2337/diacare.24.9.1640. [DOI] [PubMed] [Google Scholar]
- 52.Pilichiewicz AN, Little TJ, Brennan IM, et al. Effects of load and duration, of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am J Physiol. 2006;290:R668–77. doi: 10.1152/ajpregu.00606.2005. [DOI] [PubMed] [Google Scholar]