Abstract
In this review we explore the similarities between spinocerebellar ataxias and dystonias, and suggest potentially shared molecular pathways using a gene co-expression network approach. The spinocerebellar ataxias are a group of neurodegenerative disorders characterized by coordination problems caused mainly by atrophy of the cerebellum. The dystonias are another group of neurological movement disorders linked to basal ganglia dysfunction, although evidence is now pointing to cerebellar involvement as well. Our gene co-expression network approach identified 99 shared genes and showed the involvement of two major pathways: synaptic transmission and neurodevelopment. These pathways overlapped in the two disorders, with a large role for GABAergic signaling in both. The overlapping pathways may provide novel targets for disease therapies. We need to prioritize variants obtained by whole exome sequencing in the genes associated with these pathways in the search for new pathogenic variants, which can than be used to help in the genetic counseling of patients and their families.
Keywords: Spinocerebellar ataxia, Dystonia, Gene network, Synaptic transmission, Neurodevelopment, Neurodegeneration, Molecular pathways, Pathophysiology
1. Introduction
The cerebellar ataxias are a heterogeneous group of movement disorders characterized by degeneration of Purkinje cells (PCs) and atrophy of the cerebellum. Motor symptoms include loss of balance and coordination, unstable gait, dysarthria and abnormal eye movements. Cerebellar ataxias can be primary (genetic), congenital (brain malformations) or acquired (e.g. after stroke). The spinocerebellar ataxias (SCAs), the genetically dominant forms of cerebellar ataxia, have an estimated prevalence of 1–3 per 100,000 in Europe with onset usually occurring in adulthood (Durr, 2010). Dystonia is a neurological movement disorder characterized by involuntary muscle contractions that cause abnormal twisting movements and postures. It has many clinical manifestations, ranging from isolated and focal to generalized dystonia, or dystonia in combination with other neurological symptoms such as myoclonus or ataxia. The list of diseases that can cause or present with dystonia is extensive (Fung et al., 2013).
Many patients show a combination of cerebellar ataxia and dystonia. Dystonia is frequently seen in SCA2 (14%), SCA3 (24%) and SCA17 (53%), and regularly seen in SCA types 1, 6, 12, 14, 15/16 and 20, in ataxia telangiectasia, in Friedreich’s ataxia, and in ataxia with oculomotor apraxia (Neychev et al., 2011; Prudente et al., 2014; van Gaalen et al., 2011). Kuoppamaki and Van de Warrenburg reported eleven patients in total who showed early onset, primarily cervical, dystonia in combination with slowly progressive cerebellar ataxia. All had tested negative for the most common SCA types, although some patients had a positive family history, and all the patients showed cerebellar atrophy (Kuoppamaki et al., 2003; van de Warrenburg et al., 2007).
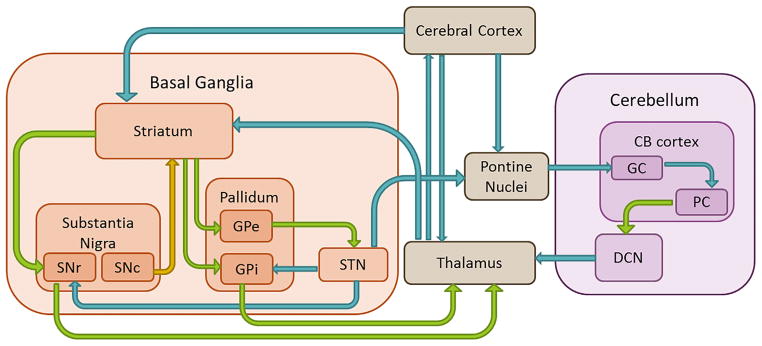
Taken into account the clinical and etiological heterogeneity, the exact pathophysiological mechanisms of SCA and dystonia are not exactly clear. For SCA, several etiological roles have been identified that lead to neurotransmission deficits and result in PC death, including transcriptional dysregulation, autophagy, mitochondrial defects and alterations in calcium homeostasis (Matilla-Dueñas et al., 2014). In dystonia, the basal ganglia have classically been attributed a key role. However, recent theories support a pathophysiological model in which dystonia is seen as a network disorder involving several brain regions, including the sensorimotor cortex, brainstem, thalamus and cerebellum (Neychev et al., 2011; Prudente et al., 2014). Nevertheless, it remains uncertain whether dysfunction of a single brain area, combined dysfunction of multiple areas, or abnormal communication between several brain areas leads to dystonia. Dystonia is regarded as a disorder of motor control (Hallett, 2011) involving the cerebellum (Shadmehr and Krakauer, 2008) and the basal ganglia, which are interconnected (Fig. 1). The cerebellum also plays a role in cerebellar ataxias. In this review, we therefore focus on the potentially shared pathophysiology of the cerebellum in SCA and dystonia.
Fig. 1.
Schematic representation of connections between basal ganglia and cerebellum. Blue arrows represent excitatory glutamatergic projections. Green arrows represent inhibitory GABAergic projections. Yellow arrow represents dopaminergic projections. CB = cerebellum; GC = granule cells; PC = Purkinje cells; DCN = deep cerebellar nuclei; STN = subthalamic nucleus; SNr = substantia nigra pars reticulata; SNc = substantia nigra pars compacta; GPe = globus pallidus externus; GPi = globus pallidus internus. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2. Evidence for overlap in pathology between ataxia and dystonia
2.1. Evidence from clinical studies
Evidence of cerebellar involvement in dystonia comes from several lines of research. By the beginning of the 20th century it had already been recognized that tumors in the posterior fossa could result in the abnormal postures of the head that we would now classify as dystonia (Batten, 1903; Extremera et al., 2008; Grey, 1916; Krauss et al., 1997). These clinical findings were replicated in a larger cohort of 25 cervical dystonia patients, in which almost half of the patients had a lesion in the brainstem or cerebellum, whereas lesions in the basal ganglia were seen in only a quarter of them (LeDoux and Brady, 2003). Batla et al. showed cerebellar abnormalities in 26 out of 188 (14%) cervical dystonia patients (Batla et al., 2015). For secondary blepharospasm, a focal form of dystonia, lesions were found mostly in the thalamus, with the remainder equally split between basal ganglia and cerebellum (Khooshnoodi et al., 2013). Other cases reported oromandibular dystonia and blepharospasm after cerebellar infarction (Akin et al., 2014; O’Rourke et al., 2006; Rumbach et al., 1995), hemidystonia caused by vertebral artery occlusion (Waln and LeDoux, 2010), and focal limb dystonia after isolated cerebellar tuberculoma (Alarcón et al., 2001).
Only small case series have been reported for neuropathological changes in dystonia, specifically in isolated cervical dystonia. A recent review showed that no pathological abnormalities were found in almost all reported cases that had a high probability of suffering from cervical dystonia (Prudente et al., 2013). These case studies have, however, several shortcomings. The major limitation is that most were focused on specific brain regions that did not include the cerebellum or the brainstem. The authors also found that the cerebellums of the cervical dystonia patients had significantly lower PC density compared to healthy controls (Prudente et al., 2013; Zoons and Tijssen, 2013). This implies a role for the cerebellum in dystonia, and it is further worth noting that loss of PCs is also associated with other neurodegenerative disorders, such as SCA.
2.2. Evidence from imaging studies
In addition to alterations in the sensorimotor cortices and the basal ganglia, structural abnormalities in the cerebellum or cerebellar projections have been found in several types of dystonia. Diffusion tensor imaging was used to assess microstructural white matter integrity in different non-hereditary isolated dystonias and demonstrated alterations varying from the white matter tracts underlying several cortical areas (Delmaire et al., 2009; Fabbrini et al., 2008) to connections to the cerebellar lobules and peduncles (Niethammer et al., 2011; Prell et al., 2013; Ramdhani et al., 2014; Sako et al., 2015; Simonyan et al., 2008; Yang et al., 2014). See reviews by Neychev et al. (2011) and Zoons et al. (2011) for a complete overview of the evidence for the role of the cerebellum based on imaging studies. More recent findings come from studies of several groups of hereditary dystonia. In DYT1, DYT6 and DYT11 patients, microstructural abnormalities were found close to the superior cerebellar peduncle (Carbon et al., 2008, 2004; van der Meer et al., 2012). A reduction in structural connectivity of the cerebellothalamic pathway in DYT1 and DYT6 patients was also seen by tractography (Argyelan et al., 2009).
Metabolic imaging using [18F]-fluorodeoxyglucose-PET also shows involvement of the cerebellum in dystonia. The most reported pattern of altered metabolic activity involves the basal ganglia, pre-motor and motor areas, and the cerebellum, and is present in both hereditary as well as non-hereditary isolated dystonia (Eidelberg et al., 1998). More recently, Niethammer et al. described a motor-related activation pattern characterized by cerebellothalamo-cortical motor circuits, that is increased in both hereditary and non-hereditary dystonia and even in non-manifesting carriers of DYT1 and DYT6 (Niethammer et al., 2011). Furthermore, the results of the eight voxel-based morphometry studies published to date have been inconsistent. An increase in gray matter volume of several parts of the cerebellum was seen in various types of isolated focal dystonia (Draganski et al., 2003; Obermann et al., 2007; Prell et al., 2013; Ramdhani et al., 2014; Simonyan and Ludlow, 2012), while a decrease in gray matter volume was shown in other studies (Delmaire et al., 2007; Piccinin et al., 2015; Ramdhani et al., 2014).
Lastly, cerebellar abnormalities have been frequently reported in a number of studies using fMRI. In addition to alterations in sensorimotor cortical areas and the basal ganglia, altered task-related activity (e.g. finger tapping, writing or speaking) was found in several cerebellar areas, including the cerebellar nuclei, posterior vermis, and paramedian cerebellar hemisphere. Again, however, the effects were inconsistent across and between different types of isolated dystonia (Beukers et al., 2010; Haslinger et al., 2005; Hu et al., 2006; Kadota et al., 2010; Obermann et al., 2010; Preibisch et al., 2001; Simonyan and Ludlow, 2010; Wu et al., 2010).
2.3. Evidence from electrophysiological studies
In the past few years, electrophysiological studies on dystonia have identified three common themes that explain its pathophysiology: loss of inhibition, maladaptive plasticity and defective sensorimotor integration (Hallett, 2011). Classically in isolated dystonia, there is a reduction in cortical inhibition (i.e. an increase in motor-evoked potentials and a reduction of the short intracortical inhibition using transcranial magnetic stimulation). However, in some types of dystonia (e.g. myoclonus dystonia), a contrasting pattern is seen that resembles the excitability profile of cerebellar pathology (Meunier et al., 2008; Talelli et al., 2011). Maladaptive plasticity, a second common theme, has been demonstrated in several types of dystonia (Quartarone and Pisani, 2011). It is known, for instance, that beyond the basal ganglia, the cerebellum also plays a role in plasticity and motor learning (Thompson and Steinmetz, 2009). Eye blink classical conditioning (EBCC) is a paradigm for associative motor learning that has been shown to be highly dependent on cerebellar functioning without basal ganglia involvement (Gerwig et al., 2007; Sommer et al., 1999). Teo and co-authors demonstrated significant abnormal EBCC in isolated dystonia patients (Teo et al., 2009), which could be normalized by continuous theta burst stimulation; this suggests a secondary role for the cerebellum (Hoffland et al., 2013). A study on sensorimotor adaptation using a motor learning paradigm that relies on both the cerebellum and the sensorimotor network (split-belt paradigm) demonstrated abnormalities in patients with blepharospasm and writer’s cramp (Hoffland et al., 2014). Defective sensorimotor adaptation was also seen in writer’s cramp patients performing a visuomotor adaptation task, suggesting that the cerebellum had lost its ability to modulate sensorimotor plasticity of the motor cortex (Hubsch et al., 2013). In contrast to these findings, normal sensorimotor adaptation was seen in cervical dystonia patients (Hoffland et al., 2014; Sadnicka et al., 2015). Experiments with a therapeutic focus also demonstrated cerebellar involvement in dystonia. In focal hand dystonia patients, cerebellar transcranial direct current stimulation improved handwriting and cyclic drawing kinematics, most likely by reducing cerebellar-brain inhibition (Bradnam et al., 2015). In cervical dystonia patients, two weeks of cerebellar stimulation resulted in a small but significant, clinical improvement of approximately 15% measured by the Toronto Western Spasmodic Torticollis Rating Scale (Koch et al., 2014).
While these effects of non-invasive stimulation have been modest or transient so far, they show that manipulating cerebellar physiology can influence the severity of dystonia. More direct manipulations of the cerebellum, analogous to pallidotomy or deep brain stimulation of the basal ganglia, may be required to produce robust and lasting effects. Cerebellar dentatectomy (Zervas, 1977) and direct electrical stimulation of the cerebellum (Davis, 2000) were once routinely applied as treatments for dystonia, but these procedures were abandoned because the benefits were unpredictable. However, the indication for these procedures was “hypertonia” or “cerebral palsy”, which could reflect any combination of dystonia, spasticity and/or rigidity, and this grouping together of many disorders with potentially different causes may have been responsible for the unpredictable outcomes. Now that the various types of hypertonia are better distinguished, more recent studies have begun to discriminate between the beneficial effects of dentatectomy (Teixeira et al., 2015) and cerebellar stimulation (Sokal et al., 2015), although further studies are still needed.
2.4. Evidence from animal models
In line with the evidence from patients who show both dystonia and cerebellar ataxia, several animal models confirm the role of the cerebellum in the etiology of dystonia. Ataxia and dystonia were identified in a mouse model (leaner) with a spontaneous missense mutation in the splice donor consensus sequence, at the 5′ end of the affected intron in Cacna1a, which encodes the voltage-gated calcium channel Cav2.1 (Doyle et al., 1997; Fletcher et al., 1996; Meier and MacPike, 1971). This phenotype is associated with abnormal physiological activity and slow degeneration of cerebellar Purkinje neurons (Heckroth and Abbott, 1994; Herrup and Wilczynski, 1982; Lau et al., 2004; Meier and MacPike, 1971; Ovsepian and Friel, 2012, 2008; Walter et al., 2006). Mutations in CACNA1A have been linked to a range of movement disorders including SCA6 and benign paroxysmal torticollis of infancy (Giffin et al., 2002; Zhuchenko et al., 1997). Notably, in the leaner mouse model, the dystonia abated while the ataxia worsened as PCs were lost over time (Raike et al., 2015). This finding is evidence for the hypothesis that PC-dysfunction leads to dystonia, whereas PC-loss causes ataxia. Additionally, Cacna1a null mice also exhibited dystonia and highly selective cerebellar degeneration, further confirming a key role for PC functioning in a shared pathology of dystonia and ataxia (Fletcher et al., 1996). Additionally, a mouse model for rapid-onset dystonia-Parkinsonism (RDP), which mimicked the effect of mutations in the α3 isoform of the Na(+)/K(+)-ATPase (sodium pump), exhibited ataxia quickly followed by a dystonic phenotype upon blockage of α3-sodium pumps with ouabain (Calderon et al., 2011). Fremont et al. showed that restricted cerebellar perfusion with ouabain is sufficient to induce dystonia, and in vivo recordings from these dystonic mice showed persistent high-frequency-burst firing of PCs (Fremont et al., 2014). Moreover, selective knock down of the α3-sodium pump in the substantia nigra resulted in a Parkinsonism phenotype, while knockdown in other basal ganglia regions had no apparent effect. Cerebellum-specific knockdown of the α3-sodium pump recapitulated the phenotype of the RDP mouse, which was again associated with altered intrinsic pacemaking of PCs, but it had no effect on the firing rate of neurons from the deep cerebellar nuclei (DCN) (see Fig. 1 for projections between brain regions) (Fremont et al., 2015). However, DCN neurons fired irregularly in dystonic animals compared to controls, a difference most likely caused by aberrant PC input to the DCN (Fremont et al., 2015). Furthermore, a disynaptic connection between the cerebellum and the basal ganglia via the thalamic intralaminar nuclei, was shown to underlie the dystonic phenotype of the RDP mouse, as lesions of the centrolateral nucleus alleviated the dystonia (Chen et al., 2014a).
In another model, Atcayji–hes mice, which show very low levels of the caytaxin protein, action-induced stiff dystonic legs can be turned into broad-based ataxic gait by partial cerebellectomy or lesions of the DCN (Luna-Cancalon et al., 2014). In contrast, homozygous missense mutations in ATCAY, the human orthologue of Atcay, cause autosomal recessive Cayman ataxia but not dystonia (Bomar et al., 2003). The dystonic phenotype in mice is most likely the consequence of increased repetitive firing of DCN neurons caused by absence of inhibitory repetitive firing of PCs. Thus, it is the combination of aberrant firing patterns of PCs and DCN neurons that likely exaggerates the hyperexcitability of the DCN neurons above a threshold sufficient to directly activate muscle groups resulting in dystonia and ataxia.
Finally, knockout of fgf14, the human disease gene underlying SCA27, resulted in ataxia and a paroxysmal hyperkinetic movement disorder mimicking a form of dystonia (Wang et al., 2002). With the notion that fgf14 was most abundantly expressed in cerebellar granule cells of the cerebellum, PC dysfunction rather than a direct PC deficit seems to cause the ataxia and dystonia-like phenotype.
Overall, these studies show a clear role for the cerebellum in the etiology of dystonia. We therefore hypothesize that shared genetic pathways might underlie the pathogenesis of dystonia and SCA, and that the cerebellum plays a major role in both disorders. To further elucidate this hypothesis, we first explore the current knowledge about the genetic background of both groups of disorders.
3. Genetics of dystonia and SCA
The introduction of high-throughput next generation sequencing (NGS), which rapidly detects all protein-coding variants, has led to the discovery of multiple disease genes for SCA (Table 1) and dystonia (Table 2). Exome sequencing has become common practice for gene identification of Mendelian forms of dystonia and SCA. To date, 16 genes have been identified using exome sequencing, including the recently discovered mutations in COL6A3 (Zech et al., 2015) and TRPC3 (Fogel et al., 2015). However, linkage analysis is still commonly used to pinpoint the region of interest and to filter exome sequencing results. Exome sequencing has its limitations, and an alternative method would be genome sequencing of the complete genome (coding and non-coding parts). However, genome sequencing remains relatively expensive and produces a very long list of putative candidates with undefined pathogenicity, many of which will be located in non-coding regions, making pathogenicity very difficult to establish. Furthermore, many diagnostic requests come from independently referred patients (singletons) who lack the additional affected family members necessary for co-segregation analysis. In reality, conventional genetic testing is usually only performed for the most common SCA and dystonia types, leaving a large group of patients genetically undiagnosed.
Table 1.
Known SCA genes.
| SCA type | OMIM Number | Locus | Gene Name | Mutation type | References |
|---|---|---|---|---|---|
| SCA1 | 164400 | 6p22.3 | ATXN1 | CAG repeat | (Orr et al., 1993) |
| SCA2 | 183090 | 12q24.13 | ATXN2 | CAG repeat | (Pulst et al., 1996; Sanpei et al., 1996) |
| SCA3 | 109150 | 14p32.12 | ATXN3 | CAG repeat | (Kawaguchi et al., 1994; Takiyama et al., 1994) |
| SCA5 | 600224 | 11q13.2 | SPTBN2 | Deletion, MM | (Bürk et al., 2004; Ikeda et al., 2006) |
| SCA6 | 183086 | 19p13.13 | CACNA1A | CAG repeat | (Zhuchenko et al., 1997) |
| SCA7 | 164500 | 3p14.1 | ATXN7 | CAG repeat | (David et al., 1997) |
| SCA8 | 608768 | 13q21 | ATXN8OS | CTG repeat (non-coding) | (Koob et al., 1999) |
| SCA10 | 603516 | 22q13.31 | ATXN10 | ATTCT repeat | (Grewal et al., 1998; Matsuura et al., 2000; Zu et al., 1999) |
| SCA11 | 604432 | 15q15.2 | TTBK2 | Deletion | (Houlden et al., 2007) |
| SCA12 | 604326 | 5q32 | PPP2R2B | CAG repeat (non-coding) | (Holmes et al., 1999) |
| SCA13 | 605259 | 19q13.33 | KCNC3 | MM | (Waters et al., 2006, 2005) |
| SCA14 | 605361 | 19q13.42 | PRKCG | MM | (Chen et al., 2003) |
| SCA15 | 606658 | 3p26.1 | ITPR1 | Deletion | (Storey et al., 2001; van de Leemput et al., 2007) |
| SCA16 | 606658 | 3p26.1 | ITPR1 | Deletion | (Storey et al., 2001; van de Leemput et al., 2007) |
| SCA17 | 607136 | 6q27 | TBP | CAG repeat | (Nakamura, 2001) |
| SCA19/22 | 607346 | 1p13.2 | KCND3 | MM | (Chung et al., 2003; Duarri et al., 2012; Lee et al., 2012; Verbeek et al., 2002) |
| SCA20 | 608687 | 11q12 | DAGLA | 12 genes duplication | (Knight et al., 2008, 2004) |
| SCA21 | 607454 | 1p36.33 | TMEM240 | MM | (Delplanque et al., 2014) |
| SCA23 | 610245 | 20p13 | PDYN | MM, frameshift | (Bakalkin et al., 2010; Verbeek et al., 2004) |
| SCA26 | 609306 | 19p13.3 | EEF2 | MM | (Hekman et al., 2012; Yu et al., 2005) |
| SCA27 | 609307 | 13q13.1 | FGF14 | MM | (van Swieten et al., 2003) |
| SCA28 | 610246 | 18p11.21 | AFG3L2 | MM | (Cagnoli et al., 2006; Di Bella et al., 2010) |
| SCA29 | 117360 | 3p26.1 | ITPR1 | MM | (Dudding et al., 2004; Huang et al., 2012) |
| SCA31 | 117210 | 16q22 | BEAN | TGGAA repeat (non-coding) | (Hirano et al., 2004; Sato et al., 2009) |
| SCA34 | 133190 | 6q14 | ELOVL4 | MM | (Cadieux-Dion et al., 2014) |
| SCA35 | 613908 | 20p13 | TGM6 | MM | (Wang et al., 2010) |
| SCA36 | 614153 | 20p13 | NOP56 | non-coding repeat | (Kobayashi et al., 2011) |
| SCA38 | 615957 | 6p12 | ELOVL5 | MM | (Di Gregorio et al., 2014) |
| SCA40 | 616053 | 14q32.12 | CCDC88C | MM | (Tsoi et al., 2014) |
| SCA41 | 616410 | 4q27 | TRPC3 | MM | (Fogel et al., 2015) |
| SCA42 | 616795 | 17q21.33 | CACNA1G | MM | (Coutelier et al., 2015a) |
MM = missense mutation.
Table 2.
Known dystonia genes.
| Dystonia type | OMIM Number | Locus | Gene Name | Inheritance pattern | References |
|---|---|---|---|---|---|
| DYT1 | 128100 | 9q34.11 | TOR1A | AD | (Ozelius et al., 1997, 1992) |
| DYT2 | 224500 | 1p35.1 | HPCA | AR | (Charlesworth et al., 2015) |
| DYT3 | 314250 | Xq13.1 | TAF1 | XLR | (Haberhausen et al., 1995; Makino et al., 2007) |
| DYT4 | 128101 | 19p13.3 | TUBB4A | AD | (Hersheson et al., 2013) |
| DYT5 | 128230 | 14q22.2 | GCH1 | AD | (Ichinose et al., 1994; Nygaard et al., 1993) |
| DYT6 | 602629 | 8p11.21 | THAP1 | AD | (Almasy et al., 1997; Fuchs et al., 2009; Saunders-Pullman et al., 2007) |
| DYT8 | 118800 | 2q35 | PNKD | AD | (Fink et al., 1996; Rainier et al., 2004) |
| DYT9 | 601042 | 1p34.2 | SLC2A1 | AD | (Auburger et al., 1996; Weber et al., 2011) |
| DYT10 | 128200 | 16p11.2 | PRRT2 | AD | (Chen et al., 2011; Tomita et al., 1999; Wang et al., 2011) |
| DYT11 | 159900 | 7q21.3 | SGCE | AD | (Nygaard et al., 1999; Zimprich et al., 2001) |
| DYT12 | 128235 | 19q13.2 | ATP1A3 | AD | (De Carvalho Aguiar et al., 2004; Kramer et al., 1999) |
| DYT16 | 612067 | 2q31.2 | PRKRA | AR | (Camargos et al., 2008) |
| DYT18 | 61216 | 1p34.2 | SLC2A1 | AR | (Weber et al., 2008) |
| DYT23 | 614860 | 9q34.3 | CACNA1B | AD | (Groen et al., 2014a,b) |
| DYT24 | 615034 | 11p14.2 | ANO3 | AD | (Charlesworth et al., 2012) |
| DYT25 | 615073 | 18p11 | GNAL | AD | (Fuchs et al., 2013) |
| DYT26 | 616398 | 22q12.3 | KCTD17 | AD | (Mencacci et al., 2015a,b) |
| DYT27 | 616411 | 2q37.3 | COL6A3 | AR | (Zech et al., 2015) |
| 612716 | 2q13.2 | SPR | AR | (Bonafé et al., 2001) | |
| 605407 | 11p.15.5 | TH | AR | (Lüdecke et al., 1995) | |
| 9q34.11 | CIZ1 | AD | (Xiao et al., 2012) |
AD = Autosomal dominant; AR = Autosomal recessive; XLR = X-linked recessive.
Despite the many disease genes that have been identified, our knowledge of the underlying biological pathways and pathogenesis of dystonia and ataxia is still limited. The main biological pathway linked to dystonia before the introduction of NGS was dopamine synthesis, which was implicated via mutations in GCH1 and TH, which encode GTP cyclohydrolase 1 and tyrosine hydroxylase, respectively (Ichinose et al., 1994; Lüdecke et al., 1995). This finding was reinforced by the observation that these patients are levodopa (L-DOPA) responsive (Nygaard et al., 1988). However, the majority of dystonia patients do not respond to L-DOPA treatment, indicating that there are other biological pathways involved. At first, no main biological pathway was recognized for SCA, as the functions of ATXN1 and ATXN3 had not then been determined. Now, however, the main pathway involved has been identified as altered synaptic transmission due to mutations in KCNC3, KCND3, CACNA1A, ITPR1, TRPC3 and PDYN. These encode various ion channels, receptors and a neuropeptide precursor, affecting potassium (KCNC3 and KCND3), calcium (CACNA1A, ITPR1 and TRPC3) and opioid receptor signaling (PDYN). See reviews of the genetics of dystonia and SCA by (Balint and Bhatia, 2015; Matilla-Dueñas, 2012; Storey and Phil, 2014; Xiao et al., 2014) for further details.
4. Investigating the shared genetic background
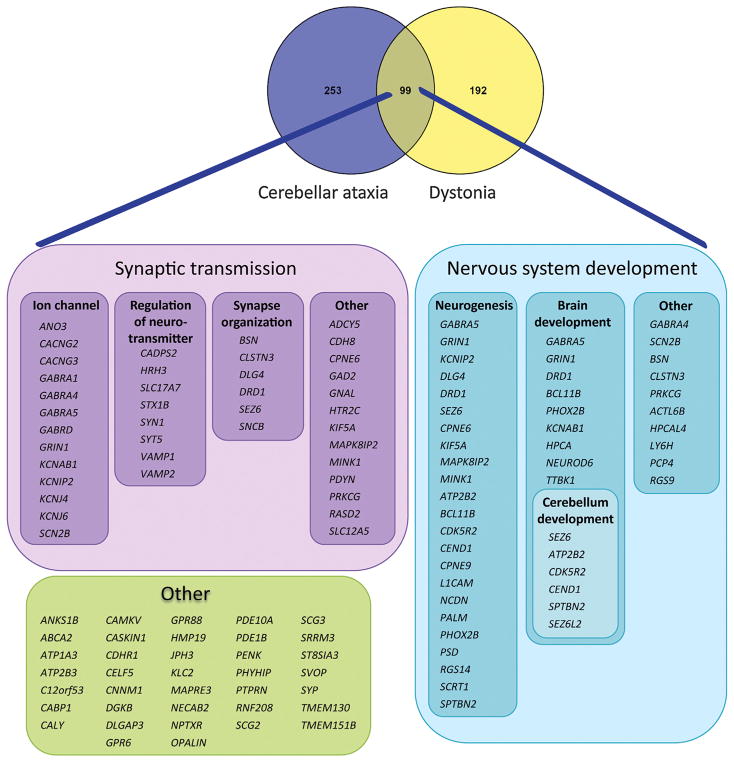
For this review we brought together dystonia and SCA genes to identify the shared genetic components and expose possible common underlying biological pathways. Our focus lies on dominantly inherited SCAs and dystonias and we have therefore used 16 autosomal dominant dystonia genes and 28 SCA genes, including polyQ-SCA genes, as input seeds for further analysis (Tables 1 and 2). To gain insight into the common biological pathways we used GeneNetwork (http://129.125.135.180:8080/GeneNetwork/cytoscape.html), a co-expression tool based on approximately 80,000 microarrays from Gene Expression Omnibus (Pers et al., 2015), and PANTHER software to analyze gene ontologies (Mi et al., 2013). Based on the assumption that co-expressed genes are more likely to be involved in similar biological pathways, this guilt-by-association approach enabled us to assess which genes generally tend to be activated simultaneously, and are thus under similar transcriptional regulation with known autosomal dominant dystonia and ataxia genes. By applying independent GeneNetwork analyses for both known autosomal dominant dystonia and ataxia genes (Supp. Figs. S1 and S2) and with manual assessment of the overlap in genes between these gene networks, we identified 99 genes shared by the two disorders (Fig. 2). No data was filtered out of the analysis. In contrast, when we compared either the dystonia gene network or the ataxia gene network with a “control” gene network based on 26 known autosomal dominant Charcot-Marie-Tooth (CMT) genes, we did not find any significant overlap (p = 0.2153 for CMT/SCA and p = 0.1222 for CMT/Dystonia), thereby validating our approach. Additionally, six of the 99 genes shared between dystonia and SCA were known dystonia or ataxia genes (ATP1A3, ANO3, GNAL, SPTBN2, PRKCG, and PDYN) and their presence further validates our hypothesis that shared co-expression networks likely point towards shared pathogenesis. Using PANTHER, we were able to categorize most of the 99 shared genes into two major neurological pathways: synaptic transmission and nervous system development (Fig. 2). We observed a 14-fold enrichment (p = 8.39E-31) for genes playing a role in synaptic transmission (39/99 genes; see Fig. 2) and a 3.8-fold enrichment (p = 8.96E-10) for genes involved in nervous system development (38/99 genes). However, not all genes fit into these two categories (see Fig. 2), indicating that there are other biological pathways/mechanisms playing a role in the molecular pathology of ataxia and dystonia. In the next section, we focus on these two major neurological pathways and highlight their role in disease pathogenesis.
Fig. 2.
Shared genes between SCA and dystonia. We found 99 genes are shared between the SCA and dystonia gene co-expression networks. Two main pathways were identified that involved synaptic transmission (39 genes) and nervous system development (38 genes).
4.1. Genes involved in synaptic transmission
4.1.1. Ion channels
We observed that 40 of the shared genes identified by our network approach were involved in synaptic transmission, a finding that was validated by recent studies showing that both dystonia and ataxia are characterized by altered excitability of neurons (Coutelier et al., 2015a; Duarri et al., 2015b; Groen et al., 2014a,b). One-third of the shared genes identified as involved in synaptic transmission encode ion channel subunits that regulate neuronal potassium, calcium or chloride signaling. Notably, 13.1% of the shared genes encode ion channels compared to 2% of all genes in the complete exome (p < 0.0001), further emphasizing the importance of ion channels in the etiology ataxia and dystonia. In contrast, no ion channels were present among the genes that were shared between CMT and SCA and between CMT and dystonia (data not shown).
The role of ion channels in the pathophysiology of both dystonia and SCA is underscored by the identification of human mutations leading to these diseases in CACNA1B (DYT23; voltage-gated calcium channel Cav2.2), KCNC3 (SCA13; voltage-gated potassium channel Kv3.3), KCND3 (SCA19; voltage-gated potassium channel Kv4.3), CACNA1A (SCA6; voltage-gated calcium channel Cav2.1), CACNA1G (SCA42; voltage-gated calcium channel Cav3.1), ITPR1 (SCA15/16; Type 1 Inositol 1,4,5-Trisphosphate Receptor) and TRPC3 (SCA41; Transient Receptor Potential Cation Channel, Subfamily C, Member 3) (Coutelier et al., 2015b; Duarri et al., 2012; Fogel et al., 2015; Groen et al., 2014a,b; Huang et al., 2012; Lee et al., 2012; Waters et al., 2006; Zhuchenko et al., 1997). Additionally, various ion channel mutations in mouse models lead to dystonia and/or ataxia including cacna1a knockout and cacna1a mutants, such as leaner mouse with generalized dystonia/ataxia, tottering mouse displaying ataxia plus paroxysmal generalized dystonia and rocker mouse exhibiting ataxia with paroxysmal focal dystonia. In contrast, lethargic cacna1b mutant mouse models appear to have ataxia with paroxysmal exertional dystonia (Meisler et al., 1997; Shirley et al., 2008). A putative role for calcium channels in human dystonia was proposed based on the fact that mice treated with an L-type calcium channel agonist developed a phenotype resembling generalized dystonia (Jinnah et al., 2000). Furthermore, in addition to ataxia, posture dystonia was observed in the opisthotonos mouse, which carries a spontaneous mutation in itpr1 (ITPR1 is the human SCA15/16 gene) that codes for the type 1 inositol 1,4,5-trisphosphate receptor, which is involved in calcium release from the endoplasmatic reticulum (Street et al., 1997). While many ion channels are associated with various forms of epilepsy, this topic lies outside the scope of this review and will not be described in further detail here. In the following paragraphs, we discuss the evidence for a role of the shared ion channel genes in the pathogenesis of dystonia and ataxia based on both human and mouse data and according to the pathway in which these genes function.
The shared genes shown to be involved in potassium signaling are KCHIP2 (Kv channel interacting protein 2), KCNAB1 (regulatory beta subunit Kvβ1), and KCNJ4 and KCNJ6 (inwardly rectifying potassium channels Kir2.3 and Kir3.2/GIRK2, respectively). KCHIP2 has been implicated in the pathogenesis of SCA19 (Duarri et al., 2015a) and mutations in KCNA1 encoding Kv1.1, the alpha subunit of Kvβ1, cause episodic ataxia (EA1) in humans (Browne et al., 1994). In mouse striatum, Kir2.3 is specifically located in matrix compartments, which may primarily influence motor circuits within basal ganglia, suggesting it has a role in dystonia (Prüss et al., 2003). A homozygous point mutation in the pore region of Girk2 (G156S) causes the weaver phenotype in mice, which is characterized by cerebellar granule cell death, dopaminergic neuronal cell death in substantia nigra, severe ataxia and spontaneous seizures. In contrast, heterozygous mutant mice showed a thinner granule cell layer and a disorganized PC layer, but no motor phenotype (Signorini et al., 1997). Furthermore, GIRK2 modulates the degree of opioid inhibition upon opioid receptor activation (Kotecki et al., 2015), a pathway known to be affected in SCA23 (Bakalkin et al., 2010). GIRK2 has also been shown to be a key determinant of the sensitivity of dopaminergic neurons of the ventral tegmental area to the motor-stimulatory effects of opioids (Kotecki et al., 2015). However, its effect in dopaminergic neurons of the substantia nigra is not yet known.
There is increasing evidence that GABAergic signaling is altered in various movement disorders, including dystonia, ataxia, epilepsy and tremor (Boecker, 2013). The shared GABA type A receptor subunits GABRA1, GABRA4, GABRA5 and GABRD identified in our network are ligand-gated chloride channels responsible for the inhibitory effects mediated by the neurotransmitter GABA (γ-aminobutyric acid) in the brain. Multiple cerebellar neurons use GABA as their output neurotransmitter, including GABAergic inhibitory projection neurons and small GABAergic interneurons in the cerebellar nuclei and Purkinje, Golgi, Lugaro, stellate, basket and candelabrum cells in the cerebellar cortex (Hori and Hoshino, 2012). Additionally, GABAergic projections are present in basal ganglia arising from striatum, globus pallidus and substantia nigra reticula (Fig. 1) (Segawa and Nomura, 2014). Loss of inhibition is thought to be a crucial causal component in the pathogenesis of dystonia (Quartarone and Hallett, 2013), and alterations in GABAA receptors may affect inhibition of action potential firing, thus contributing to disease (Hirose, 2014). Differential expression of GABAA receptors was observed in a SCA6 mouse model caused by mutations in Cacna1a (Kaja et al., 2015). In contrast, mice with PC-specific knockdown of Vgat, which encodes a vesicular GABA transporter, showed an ataxic phenotype that did not coincide with visible brain malformations at 40 weeks of age. This suggests that GABA release is important for PC functioning, and alterations in PC-specific GABA release cause ataxia (Kayakabe et al., 2014).
Shared genes CACNG2 and CACNG3, which encode voltage-dependent calcium channels γ-subunits 2 and 3, play a role in regulation of glutamate signaling by their function as transmembrane AMPA receptor (AMPAR) regulatory proteins (TARPs). The AMPAR-TARP complexes are involved in long-term potentiation and long-term depression, and therefore play a role in memory and motor learning, which has been implicated in various mouse ataxia models (Armbrust et al., 2014; Nomura et al., 2012; Wozniak et al., 2007). Stargazer mice show distinctive head-tossing and ataxic gait caused by mutant CACNG2 (TARP-γ2, stargazin) (Letts et al., 1998). In zebrafish, Cacng2a was shown to be required for trafficking of the AMPAR to the membrane that mediates normal AMPAR functioning (Roy et al., 2015). The role of glutamate in the pathogenesis of both dystonia and ataxia is further highlighted by a shared gene, GRIN1, which encodes the N-methyl-D-aspartate (NMDA)-type glutamate receptor subunit, GluN1. GLuN1 can interact with the dopamine receptor D1R (de Bartolomeis et al., 2014), and mutations in this gene are likely to affect both NMDA and dopamine signaling, causing non-syndromic intellectual disability and epileptic encephalopathy, encephalopathy with infantile-onset epilepsy, and hyperkinetic and stereotyped movement disorders (Hamdan et al., 2011; Ohba et al., 2015). Glutamate excitotoxicity has been postulated as a possible disease mechanism for various neurodegenerative disorders, including SCA (Miladinovic et al., 2015). The GRIN1 interaction with DR1 suggests that putative mutations in GRIN1 or alterations in GRIN1 expression may lead to aberrant dopamine signaling and subsequently to dystonia (Charlesworth et al., 2013).
Sodium signaling, amongst others mediated by the shared gene SCN2B that encodes the auxiliary β2-subunit of voltage-gated sodium channels, has only recently been implicated in dystonia. A spontaneous mouse mutant carrying a mutation in scna8 developed a chronic movement disorder with early onset tremor and adult onset dystonia (Jones et al., 2016). Given that Nav1.6 (encoded by scna8) is important for the initiation of action potentials, loss of Nav1.6 function might affect the repetitive firing of PCs. Thus, genes encoding components of sodium signaling could be considered as candidate genes for human movement disorders, including dystonia.
4.1.2. Regulation of neurotransmitters
Our search also identified a group of shared genes involved in the regulation of neurotransmitters, and a clear role for neurotransmitter regulation can be seen in the pathogenesis of dopamine-responsive dystonias. Most of the shared genes are associated with neurotransmitter-containing vesicles and thus play a role in the release of neurotransmitters and control synaptic transmission. Interestingly, the majority of these genes are involved in glutamatergic and GABAergic signaling. Shared genes SYT5 (synaptotagmin V) and CADPS2 (Ca2+-dependent activator protein for secretion) are both linked to dense-core vesicles. Syt5 is involved in Ca2+-dependent exocytosis in mouse brain (Saegusa et al., 2002), and cadps2 is involved in secretion of vesicles containing brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) that play a pivotal role in neuronal differentiation and survival (Sadakata et al., 2007). BDNF goes on to induce presynaptic glutamate release, increase NMDA-receptor density and increase the dendritic complexity of the GABAergic neurons (Caldeira et al., 2007; Vicario-Abejon et al., 1998). Furthermore, cadps2 knockout mice showed deficits in cerebellar development accompanied by aberrant motor coordination and eye movement (Sadakata et al., 2007). Solute carrier family 17, member 7, encoded by shared gene SLC17A7 (VGLUT1), is a vesicular glutamate transporter necessary for filling glutamate vesicles that thereby influences synaptic transmission efficiency (Bellocchio, 2000). VGLUT1-deficient mice appeared normal until postnatal week two, after which they started to lag in development and differ in movement and behavior, with death occurring between postnatal week P18 and P21 (Wojcik et al., 2004).
Among the shared genes identified in our search, there are also components of the SNARE-complex involved in exocytosis of neurotransmitter vesicles. We identified STX1B (syntaxin-1B) and vesicle-associated membrane proteins (VAMP1/synaptobrevin1 and VAMP2/synaptobrevin2) in our network. Decreased spontaneous GABAergic transmission frequencies were detected in STX1B knockout cerebellar cultures (Wu et al., 2015), whereas mutations in VAMP1 have been identified as causing dominant hereditary spastic ataxia (SPAX1) (Bourassa et al., 2012). Moreover, VAMP1-deficient mice (lethal-wasting (lew)) showed premature death at P15 and were found to have neurological deficits, including profound motor impairments that were most likely caused by reduced functioning of neuromuscular junctions (Liu et al., 2011; Nystuen et al., 2007). VAMP2-deficient mice demonstrated a critical role for VAMP2 in Ca2+-dependent vesicle exocytosis (Schoch, 2001) and in postsynaptic insertion of GluA1-containing AMPA receptors into the synaptic plasma membrane (Hussain and Davanger, 2015). However, an association with a human disorder has yet to be found. Another shared gene is SYN1 (synapsin I), which encodes a synaptic-vesicle-associated phosphoprotein involved in organization of the vesicles at the presynaptic terminal and in axonal development (Chin et al., 1995; Li et al., 1995).
The only gene in this shared group not specifically linked to vesicles is histamine receptor H3 (H3R), which is selectively expressed in brain (Lovenberg et al., 1999) and present on presynaptic terminals, where it acts as an autoreceptor inhibiting histamine production and release. H3R is also present on postsynaptic nerve terminals where it functions as a heteroreceptor that inhibits the release of other neurotransmitters such as norepinephrine, serotonin, GABA, acetylcholine, glutamate and dopamine (Schneider et al., 2014). While no mutations in H3R have been reported, hrh3-knockout mice showed reduced locomotor activity, indicating that H3R is a plausible candidate gene for movement disorders (Takahashi et al., 2002; Toyota et al., 2002).
4.1.3. Synapse organization
The third group of shared genes that emerged from our network analysis plays a role in the organization of synapses and encodes scaffolding proteins necessary for proper synapse formation and vesicular structures. On the postsynaptic site, we identified DLG4 (Disc large 4, or postsynaptic density protein 95; PSD95) and CLSTN3 (calsyntenin-3/alcadein-β) as shared genes between ataxia and dystonia. DLG4 is involved in clustering of NMDA-receptors, potassium channels and interacting proteins (Kim et al., 1996), some of which some, e.g. KCNC3, KCND3, and KCTD17, are already linked to ataxia or dystonia. Additionally, a DLG4 knockout mouse model showed enhanced long-term potentiation accompanied by impairments in spatial learning, but no motor phenotype (Migaud et al., 1998). CLSTN3 is a cadherin protein that forms a functional complex with α-neurexin and is mainly involved in regulating inhibitory synaptic functions including GABAergic signaling (Lu et al., 2014; Pettem et al., 2013; Um et al., 2014). We also identified dopamine receptor D1 (DRD1) in the network, which is a G-protein-coupled receptor for the neurotransmitter dopamine that also regulates NMDA receptor functions (Lee et al., 2002). Variants in D1R have been associated to tardive-like dystonia(Groen et al., 2014a,b) and D1R-knockout mice showed reduced dynorphin expression in the striatum and increased locomotor activity (Xu et al., 1994). However, loss of dopaminergic neurons does not necessarily lead to Parkinsonism in SCA2 and SCA3 patients (Schöls et al., 2015). Moreover, SEZ6 (seizure-related 6 homolog) and SNCB (β-synuclein) were identified in our search, and the exact functions of these genes are still unclear. However, sez-6 knockout mice do display altered dendritic branching, a smaller number of excitatory synapses and defects in excitatory synaptic transmission leading to reduced motor coordination (Gunnersen et al., 2007; Miyazaki et al., 2006). Bassoon, a presynaptic scaffold protein encoded by BSN, is primarily involved in ribbon synapses in retina and cochlear hair cells, and thus shows no direct link to movement disorders (Frank et al., 2010).
4.1.4. Other shared genes involved in synaptic transmission
We identified 13 other shared genes involved in synaptic transmission that did not match the GO-terms ion channel, neurotransmitter regulation or synapse organization. A number of these genes are involved in intracellular signal transduction. PRKCG, for example, encodes the neuron-specific protein kinase C gamma that mediates neuronal signal transduction. PRKCG is the causative gene for SCA14, in which dystonic phenotypes, including writer’s cramp, myoclonus and focal dystonia are regularly reported (Ganos et al., 2014; Miura et al., 2009; van de Warrenburg et al., 2003; Visser et al., 2007). We also identified MAPK8IP2 (mitogen-activated protein kinase 8-interacting protein 2/JNK-interacting protein 2 (JIP2)), a synaptic scaffolding protein involved in the JNK-signaling cascade (Yasuda et al., 1999). Jip2 knockout mice showed sensorimotor deficits and impaired social interaction due to changes in the morphology of dendritic arbors of PCs associating with aberrant NMDA-and AMPA-receptor-mediated glutamatergic signaling (Giza et al., 2010). Notably, Jip1/Jip2 double knockouts were severely ataxic (Kennedy et al., 2007), demonstrating a role for JIP2 in the underlying pathology of cerebellar ataxias.
Two other shared genes are directly implicated in dystonia: ADCY5 encoding adenylyl cyclase 5 (Ludwig and Seuwen, 2002) and GNAL encoding stimulatory G-protein α-subunit Gαorf (Hervé, 2011). Mutations in ADCY5 have been found to cause familial dyskinesia with facial myokymia (FDFM) (Chen et al., 2012, 2014b) and (benign) chorea with dystonia (Carapito et al., 2015; Mencacci et al., 2015a,b), while mutations in GNAL underlie adult-onset cervical and segmental dystonia (DYT25) (Fuchs et al., 2013). Additionally, RASD2 (RASD family, member 2/tumor endothelial marker 2 (TEM2)/Ras homolog enriched in striatum (RHES)) encodes a small GTPase that plays a role in dopaminergic signaling (Harrison and He, 2011; Quintero et al., 2008) and enhances L-dopa induced dyskinesia via activation of mTOR (Subramaniam et al., 2011), giving it a putative role in dystonia.
Three other shared genes, SLC12A5, HTR2C and KIF5A, are involved in regulation of receptor functioning, thereby mediating post-synaptic neurotransmission. SLC12A5 (solute carrier family 12, member 5/potassium-chloride cotransporter 2 (KCC2)) encodes a neuron-specific ion transporter that is the main extruder of the intracellular chloride used to create the chloride gradient necessary for GABA- and glycine-receptor functioning, and which ultimately promotes post-synaptic inhibition (Hübner et al., 2001; Rivera et al., 1999). KIF5A encodes a neuron-specific kinesin heavy chain protein involved in microtubule-based cargo transport of, among others, the GABAA-receptor (Campbell et al., 2014; Nakajima et al., 2012).
For the remainder of the genes in this group, brain functions are not yet completely established, but some, including GAD2, PDYN, MINK1 and CPNE6, seem to link to GABA neurotransmitter synthesis and synaptic plasticity (Almuedo-Castillo et al., 2011; Cerpa et al., 2009; Nakayama et al., 1998; Pan, 2012). Of this list, only PDYN, the precursor for the neuropeptides α-neoendorphin, dynorphin A (Dyn A) and dynorphin B (Dyn B), is directly linked to ataxia. Notably, dynorphins are involved in pain sensing, addiction and depression via opioid signaling (Hauser et al., 2005), while mutations in PDYN have been shown to cause SCA23 and suggestively affect glutamate signaling (Bakalkin et al., 2010). Lastly, CDH8 mediates Ca2+-dependent cell–cell adhesion involved in presynaptic organization and synaptic remodeling (Togashi et al., 2002). Cdh8 knockdown in rat primary neurons led to aberrant dendritic arborization and decreased self-avoidance of dendritic branches (Friedman et al., 2015).
4.2. Genes involved in neurodevelopment
Beyond the large group of genes involved in synaptic transmission, we identified many shared genes involved in development of the nervous system. Indeed, many disorders that combine dystonia with other neurological features are developmental (Fung et al., 2013). Neurodevelopmental abnormalities have already been linked to DYT1 dystonia and include alterations in the cerebello-thalamo-cortical pathways (Carbon and Eidelberg, 2009). Recently, cerebellar neurogenesis was found to be compromised in mouse models of DYT1 dystonia (Vanni et al., 2015), demonstrating that loss of Tor1A induces important developmental alterations in the cerebellum, and thereby contributing to the development of dystonia in DYT1 mutation carriers. To date, no clear evidence has been reported for alterations in brain development in the pathology of dominant hereditary ataxias. However, developmental delay has been reported for SCA27, SCA2, SCA5 and SCA13 (Di Fabio et al., 2012; Jacob et al., 2013; Planes et al., 2015; Waters et al., 2006). In SCA13, differences in axonal pathfinding were observed in zebrafish motor neurons expressing mutant F448L-KCNC3 (Issa et al., 2012), which is also indicative of aberrant neurogenesis. More evidence that abnormalities in cerebellum development and deficits in axon elongation may give rise to ataxia came from a mice lacking neuron navigator 2 (Nav2) (McNeill et al., 2011). These mice exhibited a small cerebellum at E17.5 and ataxia at age 5 months due to aberrant migration of post-mitotic granule cells. Given that all these examples showed a young age of onset or juvenile ataxia, in contrast to the late age of onset found in the majority of SCAs, it seems difficult to recocel them with playing a role in neurodevelopment in disorders with a late age of onset. However, some preliminary evidence has been reported for a role of neurodevelopment in Alzheimer’s disease and Parkinson’s disease (Doehner and Knuesel, 2010; Grilli et al., 2003; Wilkaniec et al., 2016). Therefore, we will now address the overlapping ataxia-dystonia network genes in the context of their putative role in the central nervous system (CNS) development, including neurogenesis and brain/cerebellum development.
4.2.1. Neurogenesis
The shared genes involved in this group are implicated in axon path finding, regulation of dendrite/axon extension, or dendrite morphogenesis. Many of these genes (GABRA5, GRIN1, SEZ6, ATP2B2, BCL11B, CDK5R2, CEND1 and SPTBN2) were also listed in the group for brain/cerebellum development and these will be discussed in the next section. Additionally, some genes that are involved in neurogenesis and/or neurodevelopment (GABRA5, GRIN1, SEZ6, KCNIP2, DLG4 (PSD-95), CPNE6, KIF5A, MAPK8IP2 and MINK1) are also involved in synaptic transmission and have already been discussed in the Synaptic transmission section.
Of the genes involved in neurogenesis, Copine1, which is encoded by CPNE9, regulates neurite outgrowth and differentiation of hippocampal progenitor cells (Park et al., 2012). In contrast, LCAM1 and NCDN, which encode cell adhesion molecule L1 and neurochondrin, respectively, modify neurite growth either in cerebellar neurons(Huang et al., 2013) or PC12 cells (Wang et al., 2013). The paired-like homeobox domain protein Phox2b is a key determinant of neuronal identity, as it is required for the initial phase in neuronal differentiation, giving rise to noradrenergic neurons that may be involved in the pathology of dystonia (Adams and Foote, 1988; Brunet and Pattyn, 2002). For PSD, RSG14 and SCRT1, not much is known about their role in neurogenesis. However, the relation of PSD to DLG4 (see section on Synaptic transmission), and the fact that RSG14 and SCRT1 are highly expressed during early postnatal development (Evans et al., 2014; Marín and Nieto, 2006), suggest a role for these genes in brain development.
4.2.2. Brain/cerebellum development
Data highlighting GABAergic signaling as a shared pathway in movement disorders including dystonia, ataxia, epilepsy and tremor, was mentioned in the Synaptic transmission section, but GABAergic signaling also plays a role in brain development. Cerebellar maturation was reported to depend on modulation of the pool of GABAergic interneurons via exogenous Sonic hedgehog (De Luca et al., 2015). For example, direct neuronal migrations of cortical interneurons expressing GABAB receptors are regulated by Gαi-Go-coupled receptor signaling in which the G-protein-regulator of neurite outgrowth 1, encoded by shared gene GRIN1, seemingly participates (López-Bendito et al., 2003; Masuho et al., 2008). In situ data showed that GRIN1 was expressed on a migrating subpopulation of neurons from the caudal rhombic lip to the pre-cerebellar nuclei of the brainstem, and moderate GRIN1 expression was seen in the cerebellum, suggesting it has a role in cerebellum development (Masuho et al., 2008; Wang et al., 2005). NEUROD6, a member of the basic helix-loop-helix transcription factors is involved in neuronal differentiation of GABAergic and glycinergic interneurons (Kay et al., 2011).
The role of the plasma membrane Ca2+-Pump 2 encoded by shared gene ATP2B2 in cerebellar development was established by studies of cerebellar organotypic slice cultures. Here, inhibition of the Ca2+-pump resulted in a reduction of the PC dendritic tree, confirming the importance of calcium signaling in controlling dendritic growth (Sherkhane and Kapfhammer, 2013). Mutations in ATP2B3, which is also expressed in the cerebellum, led to X-linked congenital cerebellar ataxia (Zanni et al., 2012), further emphasizing the importance of these pumps in the pathology of cerebellar ataxias. Additionally, cell-type-restricted and time-dependent expression of the neuron-specific calcium-binding protein hippocalcin, which is encoded by shared gene HPCA and underlies DYT2, was observed in the developing brain; this suggests that hippocalcin plays a role in neuronal differentiation in the early stages of development (Saitoh et al., 1994). Furthermore, mutations in overlapping gene SPTBN2 cause SCA5 (Ikeda et al., 2006), and mutant B-III-spectrin led to mislocalization and dysfunction of mGluR1α at dendritic spines (Armbrust et al., 2014). These findings further connect glutamatergic dysfunction and aberrant calcium homeostasis to brain/cerebellar development. Potassium signaling may also play a role in the proper development of the CNS, as differential regulation of distinct potassium channel beta subunits, including Kvbeta 1 (KCNAB1), was observed to mediate neuronal survival and maturation (Downen et al., 1999).
Neuronal-specific gene Cell Cycle Exit And Neuronal Differentiation 1 (CEND1) is required for normal cerebellar development. Mice lacking Cend1 showed impaired cerebellar development and motor dysfunction due to an increased proliferation of granule cell precursors, delayed granule cell migration, and alterations in PC differentiation (Sergaki et al., 2010). In contrast, CDK5R2, which encodes the neuronal-specific activator of CDK5 kinase, indirectly regulates dendrite development and axon guidance by regulating gene expression and phosphorylation of key targets such as Connexin 43 and PIPKI gamma 90 during brain development (Liang et al., 2015; Qi et al., 2015; Tojima et al., 2014). However, no direct role for Tau Tubulin Kinase 1, TTBK1, in brain development has yet been reported.
4.2.3. Other genes not tagged as involved in neurogenesis or brain cerebellum development
In this last group, ten genes were identified that did not fit the GO terms neurogenesis and/or brain-cerebellum development. We have already discussed GABRA4, SCN2B, BSN and PRKCG, which have been previously linked to synaptic transmission, but apparently they also play a role in CNS development. Of the remaining genes, not much is known about their respective functions or role in brain development. However, calcium signaling again plays a role, as CLSTN3, HPCAL4, PCP4 and Ly6h are all seemingly involved in mediating intracellular calcium homeostasis (Mouton-Liger et al., 2014; Pettem et al., 2013; Puddifoot et al., 2015).
Shared gene ACTL6B is part of a neuronal-specific chromatin-remodeling complex that mediates long-term memory formation via the coordinated regulation of gene expression (Vogel-Ciernia and Wood, 2014). This complex also contains the rBAF factor that regulates the expression of genes involved in the dendritic development required for synaptic plasticity and memory. Deficits in plasticity were also already reported in DYT1 dystonia mice and various ataxia mouse models (Huynh et al., 2009; Mark et al., 2015; Martella et al., 2014; Wozniak et al., 2007).
Shared gene RGS9, which encodes a regulator of G-protein signaling, was found to have a restricted expression in the basal ganglia and suggestively plays a role in local signaling pathways that include mu-opioid receptor signaling (Psifogeorgou et al., 2007; Thomas et al., 1998). Aberrant opioid signaling was reported in a hamster model of paroxysmal dystonia (Nobrega et al., 2004), and mutations in the precursor protein of the opioid dynorphin peptides, prodynorphin, cause SCA23 (Bakalkin et al., 2010). However, no clear evidence has been published with regard to its postulated role in CNS development. Further studies are required to establish a role for these shared genes in neurodevelopment.
5. Discussion
By combining gene co-expression networks for dystonia and SCA, we have identified a set of 99 shared genes that may play a role in the pathogenesis of both disorders. These results further validate the clinical observations of comorbidity of ataxia and dystonia and show a role for the cerebellum in dystonia. Additionally, a “control” network based on known CMT genes showed no significant overlap with dystonia or SCA gene network, confirming that dystonia and SCA are biologically closer related. The key shared molecular pathways between ataxia and dystonia were synaptic transmission and neurodevelopment, with the involvement of synaptic transmission in both disorders well recognized, as mutations in many ion channels have already been shown to cause these diseases (Charlesworth et al., 2012; Coutelier et al., 2015a; Duarri et al., 2012; Groen et al., 2014a,b; Lee et al., 2012; Waters et al., 2006; Zhuchenko et al., 1997). On the other hand, molecular pathways related to neurodevelopment had not been as clearly linked to ataxia and dystonia, although a recent review mentioned neurodevelopment as a common theme in dystonia (Domingo et al., 2016), and developmental defects have been described in DYT1 (Carbon and Eidelberg, 2009) and developmental delay is known in SCA27, SCA13, SCA5 and SCA2 (Di Fabio et al., 2012; Jacob et al., 2013; Planes et al., 2015; Waters et al., 2006). Moreover, genes involved in neurodevelopment may not exert their function solely during development of the CNS because they also appear to play a role in Alzheimer’s disease and Parkinson’s disease (Doehner and Knuesel, 2010; Grilli et al., 2003; Wilkaniec et al., 2016), thus providing a link between development and degeneration. Identifying common pathways may aid the development of therapies because restoring a pathway will help patients with different genetic backgrounds.
Within both major pathways, the importance of glutamatergic-, and especially GABAergic signaling was strikingly demonstrated by the identification of four GABA receptor genes in the overlapping gene set. While mutations in these genes are not currently known to cause SCA or dystonia, they have been linked to epilepsy (Cossette et al., 2012; Hirose, 2014; Kim, 2014), making them potential candidate genes for movement disorders. Furthermore, we also identified a number of genes that indirectly affect GABAergic signaling, including CADPS2, STX1B, H3R, SLC12A5, KIF5A, GAD2 and GRIN1. We therefore suggest prioritizing genes involved in GABAergic signaling in genetic studies of SCA and dystonia patients, as these genes are likely candidate genes in genetically unexplained cases. This is particularly important because GABAergic signaling can be targeted by drugs; this is a treatment approach used in multiple neuropsychiatric disorders (Goddard, 2016) that has also been proven to relieve rotenone-induced Parkinsonism-like symptoms (Sharma et al., 2016).
There were 37 genes that did not fit within the GO-terms synaptic transmission or nervous system development. This does not, however, mean that these genes are not involved in these pathways, but rather that their functions may be unknown or that they might have more than one function, in both cases this would contribute to their lack of representation in the GO annotations. Alternately, the existence of this group of genes may indicate the involvement of other molecular pathways, in addition to the two major ones we have described here. Other pathways have certainly been described as triggering SCA and/or dystonia pathology and include transcriptional dysregulation, mitochondrial defects, autophagy, dopaminergic signaling, ER stress and alterations in calcium homeostasis (Bragg et al., 2011; Matilla-Dueñas et al., 2014).
Further unraveling of the common pathways in SCA and dystonia will improve our understanding of disease pathologies and may open the way to novel therapies by identifying new drug targets. Furthermore, our sophisticated network approach strongly suggests that the molecular pathways of ataxia and dystonia are indeed closely related. Both should be tested for simultaneously in genetic diagnostics work on ataxia and dystonia patients. As up to 100 genes need to be screened, this will be easier to achieve with the implementation of disease-focused gene panels or dedicated exome strategies. Genes that have been identified in the gene set that overlaps between ataxia and dystonia should be prioritized when searching for disease gene mutations in patients wthout a genetic diagnosis, because variants in these genes are more likely to be involved in their disease pathology. Overall, this gene co-expression network provides useful insights into the possible shared molecular mechanisms of ataxia and dystonia and it will lead to a better understanding of their pathogenesis.
Supplementary Material
Acknowledgments
We thank Kate Mc Intyre and Jackie Senior for improving the manuscript. This work was funded by a Rosalind Franklin Fellowship from by the University of Groningen awarded to DSV and by grants to MAFT from the Fonds Nuts-Ohra, Prinses Beatrix Fonds, Gossweiler Foundation, Stichting wetenschapsfonds dystonie vereniging, Fonds Psychische Gezondheid, Phelps Stichting, and the Beatrix Kinderziekenhuis Fonds. Further funding came from unrestricted grants from Actelion, Merz, Ipsen, Allergan Farmaceutics and Medtronic and MAJT received an honorarium from the Merz expert meeting in Paris, January 2016. HAJ is supported in part by grants to the Dystonia Coalition from the Office of Rare Diseases Research in the USA National Center for Advancing Translational Studies (TR001456) and the NIH National Institute for Neurological Disorders and Stroke (NS067501). TJdK received grants from the Metabolic Power Fundation and Metakids foundation and Ride4Kids foundation (all non-profit) for studying movement disorders in metabolic diseases. He received research grants from Actelion pharmaceuticals (profit) for studying movement disorders in NP-C disease and received an honorarium for presenting at a sponsored meeting on NP-C. None of the funding obtained relates to the work presented here.
Abbreviations
- AMPAR
α-amino-3-hydroxyl-5methyl-4-isoxazole-proprionate Receptor
- BDNF
brain derived neurotrophic factor
- CB
cerebellum
- DCN
deep cerebellar nuclei
- EBCC
eye blink classical conditioning
- GABA
gamma aminobutyric acid
- NGS
next generation sequencing
- NMDA
N-methyl-D-aspartate
- PC
Purkinje Cell
- SCA
spinocerebellar ataxia
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neubiorev.2017.01.033.
References
- Adams LM, Foote SL. Possible involvement of brain noradrenergic neurons in dystonia. Adv Neurol. 1988;50:313–333. [PubMed] [Google Scholar]
- Akin A, Yilmaz R, Selcuk F, Akbostanci MC. Sudden onset of oromandibular dystonia after cerebellar stroke. Tremor Other Hyperkinet Mov (N Y) 2014;4:262. doi: 10.7916/D8C24TN3. http://dx.doi.org/10.7916/D8C24TN3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón F, Tolosa E, Muñoz E. Focal limb dystonia in a patient with a cerebellar mass. Arch Neurol. 2001;58:1125–1127. doi: 10.1001/archneur.58.7.1125. [DOI] [PubMed] [Google Scholar]
- Almasy L, Bressman SB, Raymond D, Kramer PL, Greene PE, Heiman GA, Ford B, Yount J, de Leon D, Chouinard S, Saunders-Pullman R, Brin MF, Kapoor RP, Jones AC, Shen H, Fahn S, Risch NJ, Nygaard TG. Idiopathic torsion dystonia linked to chromosome 8 in two Mennonite families. Ann Neurol. 1997;42:670–673. doi: 10.1002/ana.410420421. http://dx.doi.org/10.1002/ana.410420421. [DOI] [PubMed] [Google Scholar]
- Almuedo-Castillo M, Saló E, Adell T. Dishevelled is essential for neural connectivity and planar cell polarity in planarians. Proc Natl Acad Sci U S A. 2011;108:2813–2818. doi: 10.1073/pnas.1012090108. http://dx.doi.org/10.1073/pnas.1012090108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss HU, Bressman SB, Dhawan V, Eidelberg D. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci. 2009;29:9740–9747. doi: 10.1523/JNEUROSCI.2300-09.2009. http://dx.doi.org/10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbrust KR, Wang X, Hathorn TJ, Cramer SW, Chen G, Zu T, Kangas T, Zink AN, Oz G, Ebner TJ, Ranum LP. Mutant beta-III spectrin causes mGluR1alpha mislocalization and functional deficits in a mouse model of spinocerebellar ataxia type 5. J Neurosci. 2014;34:9891–9904. doi: 10.1523/JNEUROSCI.0876-14.2014. http://dx.doi.org/10.1523/JNEUROSCI.0876-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auburger G, Ratzlaff T, Lunkes A, Nelles HW, Leube B, Binkofski F, Kugel H, Heindel W, Seitz R, Benecke R, Witte OW, Voit T. A gene for autosomal dominant paroxysmal choreoathetosis/spasticity (CSE) maps to the vicinity of a potassium channel gene cluster on chromosome 1p, probably within 2 cM between D1S443 and D1S197. Genomics. 1996;31:90–94. doi: 10.1006/geno.1996.0013. http://dx.doi.org/10.1006/geno.1996.0013. [DOI] [PubMed] [Google Scholar]
- Bürk K, Zühlke C, König IR, Ziegler A, Schwinger E, Globas C, Dichgans J, Hellenbroich Y. Spinocerebellar ataxia type 5: clinical and molecular genetic features of a German kindred. Neurology. 2004;62:327–329. doi: 10.1212/01.wnl.0000103293.63340.c1. http://dx.doi.org/10.1212/01.WNL.0000103293.63340.C1. [DOI] [PubMed] [Google Scholar]
- Bakalkin G, Watanabe H, Jezierska J, Depoorter C, Verschuuren-Bemelmans C, Bazov I, Artemenko KA, Yakovleva T, Dooijes D, Van de Warrenburg BPC, Zubarev RA, Kremer B, Knapp PE, Hauser KF, Wijmenga C, Nyberg F, Sinke RJ, Verbeek DS. Prodynorphin mutations cause the neurodegenerative disorder spinocerebellar ataxia type 23. Am J Hum Genet. 2010;87:593–603. doi: 10.1016/j.ajhg.2010.10.001. http://dx.doi.org/10.1016/j.ajhg.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint B, Bhatia KP. Isolated and combined dystonia syndromes—an update on new genes and their phenotypes. Eur J Neurol. 2015;22:610–617. doi: 10.1111/ene.12650. http://dx.doi.org/10.1111/ene.12650. [DOI] [PubMed] [Google Scholar]
- Batla A, Sánchez MC, Erro R, Ganos C, Stamelou M, Balint B, Brugger F, Antelmi E, Bhatia KP. The role of cerebellum in patients with late onset cervical/segmental dystonia?-Evidence from the clinic. Parkinsonism Relat Disord. 2015 doi: 10.1016/j.parkreldis.2015.09.013. http://dx.doi.org/10.1016/j.parkreldis.2015.09.013. [DOI] [PubMed]
- Batten FE. On the diagnostic value of the position of the head in cases of cerebellar disease. Brain. 1903;26:71–80. http://dx.doi.org/10.1093/brain/26.1.71. [Google Scholar]
- Bellocchio EE. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;80(289):957–960. doi: 10.1126/science.289.5481.957. http://dx.doi.org/10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- Beukers RJ, Foncke EMJ, van der Meer JN, Nederveen AJ, de Ruiter MB, Bour LJ, Veltman DJ, Tijssen MAJ. Disorganized sensorimotor integration in mutation-positive myoclonus-dystonia: a functional magnetic resonance imaging study. Arch Neurol. 2010;67:469–474. doi: 10.1001/archneurol.2010.54. http://dx.doi.org/10.1001/archneurol.2010.54. [DOI] [PubMed] [Google Scholar]
- Boecker H. Imaging the role of GABA in movement disorders. Curr Neurol Neurosci Rep. 2013;13:385. doi: 10.1007/s11910-013-0385-9. http://dx.doi.org/10.1007/s11910-013-0385-9. [DOI] [PubMed] [Google Scholar]
- Bomar JM, Benke PJ, Slattery EL, Puttagunta R, Taylor LP, Seong E, Nystuen A, Chen W, Albin RL, Patel PD, Kittles R, Sheffield VC, Burmeister M. Mutations in a novel gene encoding a CRAL-TRIO domain cause human Cayman ataxia and ataxia/dystonia in the jittery mouse. Nat Genet. 2003;35:264–269. doi: 10.1038/ng1255. http://dx.doi.org/10.1038/ng1255. [DOI] [PubMed] [Google Scholar]
- Bonafé L, Thöny B, Penzien JM, Czarnecki B, Blau N. Mutations in the sepiapterin reductase gene cause a novel tetrahydrobiopterin-dependent monoamine-neurotransmitter deficiency without hyperphenylalaninemia. Am J Hum Genet. 2001;69:269–277. doi: 10.1086/321970. http://dx.doi.org/10.1086/321970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa CV, Meijer IA, Merner ND, Grewal KK, Stefanelli MG, Hodgkinson K, Ives EJ, Pryse-Phillips W, Jog M, Boycott K, Grimes DA, Goobie S, Leckey R, Dion PA, Rouleau GA. VAMP1 mutation causes dominant hereditary spastic ataxia in Newfoundland families. Am J Hum Genet. 2012;91:548–552. doi: 10.1016/j.ajhg.2012.07.018. http://dx.doi.org/10.1016/j.ajhg.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradnam LV, Graetz LJ, McDonnell MN, Ridding MC. Anodal transcranial direct current stimulation to the cerebellum improves handwriting and cyclic drawing kinematics in focal hand dystonia. Front Hum Neurosci. 2015;9:286. doi: 10.3389/fnhum.2015.00286. http://dx.doi.org/10.3389/fnhum.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg DC, Armata IA, Nery FC, Breakefield XO, Sharma N. Molecular pathways in dystonia. Neurobiol Dis. 2011;42:136–147. doi: 10.1016/j.nbd.2010.11.015. http://dx.doi.org/10.1016/j.nbd.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne DL, Gancher ST, Nutt JG, Brunt ERP, Smith EA, Kramer P, Litt M. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet. 1994;8:136–140. doi: 10.1038/ng1094-136. http://dx.doi.org/10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- Brunet JF, Pattyn A. Phox2 genes—from patterning to connectivity. Curr Opin Genet Dev. 2002;12:435–440. doi: 10.1016/s0959-437x(02)00322-2. [DOI] [PubMed] [Google Scholar]
- Cadieux-Dion M, Turcotte-Gauthier M, Noreau A, Martin C, Meloche C, Gravel M, Drouin CA, Rouleau G, Nguyen a, Cossette DK. Expanding the clinical phenotype associated with ELOVL4 mutation: study of a large French-Canadian family with autosomal dominant spinocerebellar ataxia and erythrokeratodermia. JAMA Neurol. 2014:4–9. doi: 10.1001/jamaneurol.2013.6337. http://dx.doi.org/10.1001/jamaneurol.2013.6337. [DOI] [PubMed]
- Cagnoli C, Mariotti C, Taroni F, Seri M, Brussino A, Michielotto C, Grisoli M, Di Bella D, Migone N, Gellera C, Di Donato S, Brusco A. SCA28, a novel form of autosomal dominant cerebellar ataxia on chromosome 18p11.22-q11.2. Brain. 2006;129:235–242. doi: 10.1093/brain/awh651. http://dx.doi.org/10.1093/brain/awh651. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Melo CV, Pereira DB, Carvalho RF, Carvalho AL, Duarte CB. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2007;35:208–219. doi: 10.1016/j.mcn.2007.02.019. http://dx.doi.org/10.1016/j.mcn.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Calderon DP, Fremont R, Kraenzlin F, Khodakhah K. The neural substrates of rapid-onset Dystonia-Parkinsonism. Nat Neurosci. 2011;14:357–365. doi: 10.1038/nn.2753. http://dx.doi.org/10.1038/nn.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargos S, Scholz S, Simón-Sánchez J, Paisán-Ruiz C, Lewis P, Hernandez D, Ding J, Gibbs JR, Cookson MR, Bras J, Guerreiro R, Oliveira CR, Lees A, Hardy J, Cardoso F, Singleton AB. DYT16, a novel young-onset dystonia-parkinsonism disorder: identification of a segregating mutation in the stress-response protein PRKRA. Lancet Neurol. 2008;7:207–215. doi: 10.1016/S1474-4422(08)70022-X. http://dx.doi.org/10.1016/S1474-4422(08)70022-X. [DOI] [PubMed] [Google Scholar]
- Campbell PD, Shen K, Sapio MR, Glenn TD, Talbot WS, Marlow FL. Unique function of Kinesin Kif5A in localization of mitochondria in axons. J Neurosci. 2014;34:14717–14732. doi: 10.1523/JNEUROSCI.2770-14.2014. http://dx.doi.org/10.1523/JNEUROSCI.2770-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carapito R, Paul N, Untrau M, Le Gentil M, Ott L, Alsaleh G, Jochem P, Radosavljevic M, Le Caignec C, David A, Damier P, Isidor B, Bahram S. A de novo ADCY5 mutation causes early-onset autosomal dominant chorea and dystonia. Mov Disord. 2015;30:423–427. doi: 10.1002/mds.26115. http://dx.doi.org/10.1002/mds.26115. [DOI] [PubMed] [Google Scholar]
- Carbon M, Eidelberg D. Abnormal structure-function relationships in hereditary dystonia. Neuroscience. 2009;164:220–229. doi: 10.1016/j.neuroscience.2008.12.041. http://dx.doi.org/10.1016/j.neuroscience.2008.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Kingsley PB, Su S, Smith GS, Spetsieris P, Bressman S, Eidelberg D. Microstructural white matter changes in carriers of the DYT1 gene mutation. Ann Neurol. 2004;56:283–286. doi: 10.1002/ana.20177. http://dx.doi.org/10.1002/ana.20177. [DOI] [PubMed] [Google Scholar]
- Carbon M, Kingsley PB, Tang C, Bressman S, Eidelberg D. Microstructural white matter changes in primary torsion dystonia. Mov Disord. 2008;23:234–239. doi: 10.1002/mds.21806. http://dx.doi.org/10.1002/mds.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerpa W, Toledo EM, Varela-Nallar L, Inestrosa NC. The role of Wnt signaling in neuroprotection. Drug News Perspect. 2009;22:579–591. doi: 10.1358/dnp.2009.10.1436817. http://dx.doi.org/10.1358/dnp.2009.10.1436817. [DOI] [PubMed] [Google Scholar]
- Charlesworth G, Plagnol V, Holmström KM, Bras J, Sheerin UM, Preza E, Rubio-Agusti I, Ryten M, Schneider SA, Stamelou M, Trabzuni D, Abramov AY, Bhatia KP, Wood NW. Mutations in ANO3 cause dominant craniocervical dystonia: ion channel implicated in pathogenesis. Am J Hum Genet. 2012;91:1041–1050. doi: 10.1016/j.ajhg.2012.10.024. http://dx.doi.org/10.1016/j.ajhg.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth G, Bhatia KP, Wood NW. The genetics of dystonia: new twists in an old tale. Brain. 2013;136:2017–2037. doi: 10.1093/brain/awt138. http://dx.doi.org/10.1093/brain/awt138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth G, Angelova PR, Bartolomé-Robledo F, Ryten M, Trabzuni D, Stamelou M, Abramov AY, Bhatia KP, Wood NW. Mutations in HPCA cause autosomal-recessive primary isolated dystonia. Am J Hum Genet. 2015;96:657–665. doi: 10.1016/j.ajhg.2015.02.007. http://dx.doi.org/10.1016/j.ajhg.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DH, Brkanac Z, Christophe Verlinde LMJ, Tan XJ, Bylenok L, Nochlin D, Matsushita M, Lipe H, Wolff J, Fernandez M, Cimino PJ, Thomas Bird D, Raskind WH. Missense mutations in the regulatory domain of PKCγ: a new mechanism for dominant nonepisodic cerebellar ataxia. Am J Hum Genet. 2003;72:839–849. doi: 10.1086/373883. http://dx.doi.org/10.1086/373883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ, Lin Y, Xiong ZQ, Wei W, Ni W, Tan GH, Guo SL, He J, Chen YF, Zhang QJ, Li HF, Lin Y, Murong SX, Xu J, Wang N, Wu ZY. Exome sequencing identifies truncating mutations in PRRT2 that cause paroxysmal kinesigenic dyskinesia. Nat Genet. 2011;43:1252–1255. doi: 10.1038/ng.1008. http://dx.doi.org/10.1038/ng.1008. [DOI] [PubMed] [Google Scholar]
- Chen YZ, Matsushita MM, Robertson P, Rieder M, Girirajan S, Antonacci F, Lipe H, Eichler EE, Nickerson DA, Bird TD, Raskind WH. Autosomal dominant familial dyskinesia and facial myokymia: single exome sequencing identifies a mutation in adenylyl cyclase 5. Arch Neurol. 2012;69:630–635. doi: 10.1001/archneurol.2012.54. http://dx.doi.org/10.1001/archneurol.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Fremont R, Arteaga-Bracho EE, Khodakhah K. Short latency cerebellar modulation of the basal ganglia. Nat Neurosci. 2014a;17:1767–1775. doi: 10.1038/nn.3868. http://dx.doi.org/10.1038/nn.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YZ, Friedman JR, Chen DH, Chan GCK, Bloss CS, Hisama FM, Topol SE, Carson AR, Pham PH, Bonkowski ES, Scott ER, Lee JK, Zhang G, Oliveira G, Xu J, Scott-Van Zeeland AA, Chen Q, Levy S, Topol EJ, Storm D, Swanson PD, Bird TD, Schork NJ, Raskind WH, Torkamani A. Gain-of-function ADCY5 mutations in familial dyskinesia with facial myokymia. Ann Neurol. 2014b;75:542–549. doi: 10.1002/ana.24119. http://dx.doi.org/10.1002/ana.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin LS, Li L, Ferreira A, Kosik KS, Greengard P. Impairment of axonal development and of synaptogenesis in hippocampal neurons of synapsin I-deficient mice. Proc Natl Acad Sci U S A. 1995;92:9230–9234. doi: 10.1073/pnas.92.20.9230. http://dx.doi.org/10.1073/pnas.92.20.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MY, Lu YC, Cheng NC, Soong BW. A novel autosomal dominant spinocerebellar ataxia (SCA22) linked to chromosome 1p21-q23. Brain. 2003;126:1293–1299. doi: 10.1093/brain/awg130. http://dx.doi.org/10.1093/brain/awg130. [DOI] [PubMed] [Google Scholar]
- Cossette P, Lachance-touchette P, Rouleau G. Jasper’s Basic Mechanisms of the Epilepsies. 4. Bethesda (MD): National Center for Biotechnology Information (US); 2012. Mutated GABA A receptor subunits in idiopathic generalized epilepsy; pp. 714–730. [PubMed] [Google Scholar]
- Coutelier M, Blesneac I, Monteil A, Monin M, Ando K, Mundwiller E, Brusco A, Le Ber I, Anheim M, Castrioto A, Duyckaerts C, Brice A, Durr A, Lory P, Stevanin G. A recurrent mutation in CACNA1G alters cav3.1 T-type calcium-channel conduction and causes autosomal-dominant cerebellar ataxia. Am J Hum Genet. 2015a;97:726–737. doi: 10.1016/j.ajhg.2015.09.007. http://dx.doi.org/10.1016/j.ajhg.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutelier M, Stevanin G, Brice A. Genetic landscape remodelling in spinocerebellar ataxias: the influence of next-generation sequencing. J Neurol. 2015b doi: 10.1007/s00415-015-7725-4. http://dx.doi.org/10.1007/s00415-015-7725-4. [DOI] [PubMed]
- David G, Abbas N, Stevanin G, Dürr A, Yvert G, Cancel G, Weber C, Imbert G, Saudou F, Antoniou E, Drabkin H, Gemmill R, Giunti P, Benomar A, Wood N, Ruberg M, Agid Y, Mandel JL, Brice A. Cloning of the SCA7 gene reveals a highly unstable CAG repeat expansion. Nat Genet. 1997;17:65–70. doi: 10.1038/ng0997-65. http://dx.doi.org/10.1038/ng0997-65. [DOI] [PubMed] [Google Scholar]
- Davis R. Cerebellar stimulation for cerebral palsy spasticity, function, and seizures. Arch Med Res. 2000;31:290–299. doi: 10.1016/s0188-4409(00)00065-5. [DOI] [PubMed] [Google Scholar]
- de Bartolomeis A, Buonaguro EF, Iasevoli F, Tomasetti C. The emerging role of dopamine-glutamate interaction and of the postsynaptic density in bipolar disorder pathophysiology: implications for treatment. J Psychopharmacol. 2014;28:505–526. doi: 10.1177/0269881114523864. http://dx.doi.org/10.1177/0269881114523864. [DOI] [PubMed] [Google Scholar]
- De Carvalho Aguiar P, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, Linazasoro G, Borg M, Tijssen MJ, Bressman SB, Dobyns WB, Brashear A, Ozelius LJ. Mutations in the Na+/K+-ATPase α3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron. 2004;43:169–175. doi: 10.1016/j.neuron.2004.06.028. http://dx.doi.org/10.1016/j.neuron.2004.06.028. [DOI] [PubMed] [Google Scholar]
- De Luca A, Parmigiani E, Tosatto G, Martire S, Hoshino M, Buffo A, Leto K, Rossi F. Exogenous Sonic hedgehog modulates the pool of GABAergic interneurons during cerebellar development. Cerebellum. 2015;14:72–85. doi: 10.1007/s12311-014-0596-x. http://dx.doi.org/10.1007/s12311-014-0596-x. [DOI] [PubMed] [Google Scholar]
- Delmaire C, Vidailhet M, Elbaz A, Bourdain F, Bleton JP, Sangla S, Meunier S, Terrier A, Lehéricy S. Structural abnormalities in the cerebellum and sensorimotor circuit in writer’s cramp. Neurology. 2007;69:376–380. doi: 10.1212/01.wnl.0000266591.49624.1a. http://dx.doi.org/10.1212/01.wnl.0000266591.49624.1a. [DOI] [PubMed] [Google Scholar]
- Delmaire C, Vidailhet M, Wassermann D, Descoteaux M, Valabregue R, Bourdain F, Lenglet C, Sangla S, Terrier A, Deriche R, Lehéricy S. Diffusion abnormalities in the primary sensorimotor pathways in writer’s cramp. Arch Neurol. 2009;66:502–508. doi: 10.1001/archneurol.2009.8. http://dx.doi.org/10.1001/archneurol.2009.8. [DOI] [PubMed] [Google Scholar]
- Delplanque J, Devos D, Huin V, Genet A, Sand O, Moreau C, Goizet C, Charles P, Anheim M, Monin ML, Buee L, Destee A, Grolez G, Delmaire C, Dujardin K, Dellacherie D, Brice A, Stevanin G, Strubi-Vuillaume I, Durr A, Sablonniere B. TMEM240 mutations cause spinocerebellar ataxia 21 with mental retardation and severe cognitive impairment. Brain. 2014;137:2657–2663. doi: 10.1093/brain/awu202. http://dx.doi.org/10.1093/brain/awu202. [DOI] [PubMed] [Google Scholar]
- Di Bella D, Lazzaro F, Brusco A, Plumari M, Battaglia G, Pastore A, Finardi A, Cagnoli C, Tempia F, Frontali M, Veneziano L, Sacco T, Boda E, Brussino A, Bonn F, Castellotti B, Baratta S, Mariotti C, Gellera C, Fracasso V, Magri S, Langer T, Plevani P, Di Donato S, Muzi-Falconi M, Taroni F. Mutations in the mitochondrial protease gene AFG3L2 cause dominant hereditary ataxia SCA28. Nat Genet. 2010;42:313–321. doi: 10.1038/ng.544. http://dx.doi.org/10.1038/ng.544. [DOI] [PubMed] [Google Scholar]
- Di Fabio R, Santorelli F, Bertini E, Balestri M, Cursi L, Tessa A, Pierelli F, Casali C. Infantile childhood onset of spinocerebellar ataxia type 2. Cerebellum. 2012;11:526–530. doi: 10.1007/s12311-011-0315-9. http://dx.doi.org/10.1007/s12311-011-0315-9. [DOI] [PubMed] [Google Scholar]
- Di Gregorio E, Borroni B, Giorgio E, Lacerenza D, Ferrero M, Lo Buono N, Ragusa N, Mancini C, Gaussen M, Calcia A, Mitro N, Hoxha E, Mura I, Coviello DA, Moon YA, Tesson C, Vaula G, Couarch P, Orsi L, Duregon E, Papotti MG, Deleuze JF, Imbert J, Costanzi C, Padovani A, Giunti P, Maillet-Vioud M, Durr A, Brice A, Tempia F, Funaro A, Boccone L, Caruso D, Stevanin G, Brusco A. ELOVL5 mutations cause spinocerebellar ataxia 38. Am J Hum Genet. 2014;95:209–217. doi: 10.1016/j.ajhg.2014.07.001. http://dx.doi.org/10.1016/j.ajhg.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehner J, Knuesel I. Reelin-mediated signaling during normal and pathological forms of aging. Aging Dis. 2010;1:12–29. [PMC free article] [PubMed] [Google Scholar]
- Domingo A, Erro R, Lohmann K. Novel dystonia genes: clues on disease mechanisms and the complexities of high-throughput sequencing. Mov Disord. 2016;31:471–477. doi: 10.1002/mds.26600. http://dx.doi.org/10.1002/mds.26600. [DOI] [PubMed] [Google Scholar]
- Downen M, Belkowski S, Knowles H, Cardillo M, Prystowsky MB. Developmental expression of voltage-gated potassium channel β subunits. Dev Brain Res. 1999;117:71–80. doi: 10.1016/s0165-3806(99)00100-5. http://dx.doi.org/10.1016/S0165-3806(99)00100-5. [DOI] [PubMed] [Google Scholar]
- Doyle J, Ren X, Lennon G, Stubbs L. Mutations in the Cacnl1a4 calcium channel gene are associated with seizures, cerebellar degeneration, and ataxia in tottering and leaner mutant mice. Mamm Genome. 1997;8:113–120. doi: 10.1007/s003359900369. http://dx.doi.org/10.1007/s003359900369. [DOI] [PubMed] [Google Scholar]
- Draganski B, Thun-Hohenstein C, Bogdahn U, Winkler J, May A. Motor circuit gray matter changes in idiopathic cervical dystonia. Neurology. 2003;61:1228–1231. doi: 10.1212/01.wnl.0000094240.93745.83. [DOI] [PubMed] [Google Scholar]
- Duarri A, Jezierska J, Fokkens M, Meijer M, Schelhaas HJ, den Dunnen WFA, van Dijk F, Verschuuren-Bemelmans C, Hageman G, van de Vlies P, Küsters B, van de Warrenburg BP, Kremer B, Wijmenga C, Sinke RJ, Swertz MA, Kampinga HH, Boddeke E, Verbeek DS. Mutations in potassium channel kcnd3 cause spinocerebellar ataxia type 19. Ann Neurol. 2012;72:870–880. doi: 10.1002/ana.23700. http://dx.doi.org/10.1002/ana.23700. [DOI] [PubMed] [Google Scholar]
- Duarri A, Lin MA, Fokkens MR, Meijer M, Smeets CJLM, Nibbeling EAR, Boddeke E, Sinke RJ, Kampinga HH, Papazian DM, Verbeek DS. Spinocerebellar ataxia type 19/22 mutations alter heterocomplex Kv4.3 channel function and gating in a dominant manner. Cell Mol Life Sci. 2015a;72:3387–3399. doi: 10.1007/s00018-015-1894-2. http://dx.doi.org/10.1007/s00018-015-1894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarri A, Nibbeling EAR, Fokkens MR, Meijer M, Boerrigter M, Verschuuren-Bemelmans CC, Kremer BPH, van de Warrenburg BP, Dooijes D, Boddeke E, Sinke RJ, Verbeek DS. Functional analysis helps to define KCNC3 mutational spectrum in dutch ataxia cases. PLoS One. 2015b;10:e0116599. doi: 10.1371/journal.pone.0116599. http://dx.doi.org/10.1371/journal.pone.0116599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudding TE, Friend K, Schofield PW, Lee S, Wilkinson IA, Richards RI. Autosomal dominant congenital non-progressive ataxia overlaps with the SCA15 locus. Neurology. 2004;63:2288–2292. doi: 10.1212/01.wnl.0000147299.80872.d1. http://dx.doi.org/10.1212/01.WNL.0000147299.80872.D1. [DOI] [PubMed] [Google Scholar]
- Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol. 2010;9:885–894. doi: 10.1016/S1474-4422(10)70183-6. http://dx.doi.org/10.1016/S1474-4422(10)70183-6. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Antonini A, Kazumata K, Nakamura T, Dhawan V, Spetsieris P, deLeon D, Bressman SB, Fahn S. Functional brain networks in DYT1 dystonia. Ann Neurol. 1998;44:303–312. doi: 10.1002/ana.410440304. http://dx.doi.org/10.1002/ana.410440304. [DOI] [PubMed] [Google Scholar]
- Evans PR, Lee SE, Smith Y, Hepler JR. Postnatal developmental expression of regulator of G protein signaling 14 (RGS14) in the mouse brain. J Comp Neurol. 2014;522:186–203. doi: 10.1002/cne.23395. http://dx.doi.org/10.1002/cne.23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Extremera VC, Alvarez-Coca J, Rodríguez GA, Pérez JM, de Villanueva JLR, Díaz CP. Torticollis is a usual symptom in posterior fossa tumors. Eur J Pediatr. 2008;167:249–250. doi: 10.1007/s00431-007-0453-8. http://dx.doi.org/10.1007/s00431-007-0453-8. [DOI] [PubMed] [Google Scholar]
- Fabbrini G, Pantano P, Totaro P, Calistri V, Colosimo C, Carmellini M, Defazio G, Berardelli A. Diffusion tensor imaging in patients with primary cervical dystonia and in patients with blepharospasm. Eur J Neurol. 2008;15:185–189. doi: 10.1111/j.1468-1331.2007.02034.x. http://dx.doi.org/10.1111/j.1468-1331.2007.02034.x. [DOI] [PubMed] [Google Scholar]
- Fink JK, Rainer S, Wilkowski J, Jones SM, Kume A, Hedera P, Albin R, Mathay J, Girbach L, Varvil T, Otterud B, Leppert M. Paroxysmal dystonic choreoathetosis: tight linkage to chromosome 2q. Am J Hum Genet. 1996;59:140–145. [PMC free article] [PubMed] [Google Scholar]
- Fletcher CF, Lutz CM, O’sullivan TN, Shaughnessy JD, Hawkes R, Frankel WN, Copeland NG, Jenkins NA. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell. 1996;87:607–617. doi: 10.1016/s0092-8674(00)81381-1. http://dx.doi.org/10.1016/S0092-8674(00)81381-1. [DOI] [PubMed] [Google Scholar]
- Fogel BL, Hanson SM, Becker EBE. Do mutations in the murine ataxia gene TRPC3 cause cerebellar ataxia in humans? Mov Disord. 2015;30:284–286. doi: 10.1002/mds.26096. http://dx.doi.org/10.1002/mds.26096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank T, Rutherford MA, Strenzke N, Neef A, Pangršič T, Khimich D, Fejtova A, Fetjova A, Gundelfinger ED, Liberman MC, Harke B, Bryan KE, Lee A, Egner A, Riedel D, Moser T. Bassoon and the synaptic ribbon organize Ca2+ channels and vesicles to add release sites and promote refilling. Neuron. 2010;68:724–738. doi: 10.1016/j.neuron.2010.10.027. http://dx.doi.org/10.1016/j.neuron.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremont R, Calderon DP, Maleki S, Khodakhah K. Abnormal high-frequency burst firing of cerebellar neurons in rapid-onset dystonia-parkinsonism. J Neurosci. 2014;34:11723–11732. doi: 10.1523/JNEUROSCI.1409-14.2014. http://dx.doi.org/10.1523/JNEUROSCI.1409-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremont R, Tewari A, Khodakhah K. Aberrant Purkinje cell activity is the cause of dystonia in a shRNA-based mouse model of Rapid Onset Dystonia–Parkinsonism. Neurobiol Dis. 2015;82:200–212. doi: 10.1016/j.nbd.2015.06.004. http://dx.doi.org/10.1016/j.nbd.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman LG, Riemslagh FW, Sullivan JM, Mesias R, Williams FM, Huntley GW, Benson DL. Cadherin-8 expression, synaptic localization, and molecular control of neuronal form in prefrontal corticostriatal circuits. J Comp Neurol. 2015;523:75–92. doi: 10.1002/cne.23666. http://dx.doi.org/10.1002/cne.23666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T, Gavarini S, Saunders-Pullman R, Raymond D, Ehrlich ME, Bressman SB, Ozelius LJ. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat Genet. 2009;41:286–288. doi: 10.1038/ng.304. http://dx.doi.org/10.1038/ng.304. [DOI] [PubMed] [Google Scholar]
- Fuchs T, Saunders-Pullman R, Masuho I, Luciano MS, Raymond D, Factor S, Lang AE, Liang TW, Trosch RM, White S, Ainehsazan E, Hervé D, Sharma N, Ehrlich ME, Martemyanov K, Bressman SB, Ozelius LJ. Mutations in GNAL cause primary torsion dystonia. Nat Genet. 2013;45:88–92. doi: 10.1038/ng.2496. http://dx.doi.org/10.1038/ng.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung VSC, Jinnah HA, Bhatia K, Vidailhet M. Assessment of patients with isolated or combined dystonia: an update on dystonia syndromes. Mov Disord. 2013;28:889–898. doi: 10.1002/mds.25549. http://dx.doi.org/10.1002/mds.25549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganos C, Zittel S, Minnerop M, Schunke O, Heinbokel C, Gerloff C, Zühlke C, Bauer P, Klockgether T, Münchau A, Bäumer T. Clinical and neurophysiological profile of four German families with spinocerebellar ataxia type 14. Cerebellum. 2014;13:89–96. doi: 10.1007/s12311-013-0522-7. http://dx.doi.org/10.1007/s12311-013-0522-7. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Kolb FP, Timmann D. The involvement of the human cerebellum in eyeblink conditioning. Cerebellum. 2007;6:38–57. doi: 10.1080/14734220701225904. http://dx.doi.org/10.1080/14734220701225904. [DOI] [PubMed] [Google Scholar]
- Giffin NJ, Benton S, Goadsby PJ. Benign paroxysmal torticollis of infancy: four new cases and linkage to CACNA1A mutation. Dev Med Child Neurol. 2002;44:490–493. doi: 10.1017/s0012162201002407. http://dx.doi.org/10.1017/S0012162201002407. [DOI] [PubMed] [Google Scholar]
- Giza J, Urbanski MJ, Prestori F, Bandyopadhyay B, Yam A, Friedrich V, Kelley K, D’Angelo E, Goldfarb M. Behavioral and cerebellar transmission deficits in mice lacking the autism-linked gene islet brain-2. J Neurosci. 2010;30:14805–14816. doi: 10.1523/JNEUROSCI.1161-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.1161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard AW. Cortical and subcortical gamma amino acid butyric acid deficits in anxiety and stress disorders: clinical implications. World J Psychiatry. 2016;6:43–53. doi: 10.5498/wjp.v6.i1.43. http://dx.doi.org/10.5498/wjp.v6.i1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal RP, Tayag E, Figueroa KP, Zu L, Durazo A, Nunez C, Pulst SM. Clinical and genetic analysis of a distinct autosomal dominant spinocerebellar ataxia. Neurology. 1998;51:1423–1426. doi: 10.1212/wnl.51.5.1423. http://dx.doi.org/10.1212/WNL.51.5.1423. [DOI] [PubMed] [Google Scholar]
- Grey EG. Studies on the localization of cerebellar tumors: the position of the head and suboccipital discomforts. Ann Surg. 1916;63:129–139. doi: 10.1097/00000658-191602000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M, Toninelli GF, Uberti D, Spano PF, Memo M. Alzheimer’s disease linking neurodegeneration with neurodevelopment. Funct Neurol. 2003;18:145–148. [PubMed] [Google Scholar]
- Groen JL, Andrade A, Ritz K, Jalalzadeh H, Haagmans M, Bradley TEJ, Jongejan A, Verbeek DS, Nürnberg P, Denome S, Hennekam RCM, Lipscombe D, Baas F, Tijssen MAJ. CACNA1B mutation is linked to unique myoclonus-dystonia syndrome. Hum Mol Genet. 2014a;24:987–993. doi: 10.1093/hmg/ddu513. http://dx.doi.org/10.1093/hmg/ddu513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen JL, Ritz K, Warner TT, Baas F, Tijssen MAJ. DRD1 rare variants associated with tardive-like dystonia: a pilot pathway sequencing study in dystonia. Park Relat Disord. 2014b;20:782–785. doi: 10.1016/j.parkreldis.2014.04.002. http://dx.doi.org/10.1016/j.parkreldis.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Gunnersen JM, Kim MH, Fuller SJ, De Silva M, Britto JM, Hammond VE, Davies PJ, Petrou S, Faber ESL, Sah P, Tan SS. Sez-6 proteins affect dendritic arborization patterns and excitability of cortical pyramidal neurons. Neuron. 2007;56:621–639. doi: 10.1016/j.neuron.2007.09.018. http://dx.doi.org/10.1016/j.neuron.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Hübner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 2001;30:515–524. doi: 10.1016/s0896-6273(01)00297-5. http://dx.doi.org/10.1016/S0896-6273(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Haberhausen G, Schmitt I, Köhler A, Peters U, Rider S, Chelly J, Terwilliger JD, Monaco AP, Müller U. Assignment of the dystonia-parkinsonism syndrome locus DYT3, to a small region within a 1.8-Mb YAC contig of Xq13.1. Am J Hum Genet. 1995;57:644–650. [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Neurophysiology of dystonia: the role of inhibition. Neurobiol Dis. 2011;42:177–184. doi: 10.1016/j.nbd.2010.08.025. http://dx.doi.org/10.1016/j.nbd.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan FF, Gauthier J, Araki Y, Lin DT, Yoshizawa Y, Higashi K, Park AR, Spiegelman D, Dobrzeniecka S, Piton A, Tomitori H, Daoud H, Massicotte C, Henrion E, Diallo O, Shekarabi M, Marineau C, Shevell M, Maranda B, Mitchell G, Nadeau A, D’Anjou G, Vanasse M, Srour M, Lafrenière RG, Drapeau P, Lacaille JC, Kim E, Lee JR, Igarashi K, Huganir RL, Rouleau GA, Michaud JL. Excess of de novo deleterious mutations in genes associated with glutamatergic systems in nonsyndromic intellectual disability. Am J Hum Genet. 2011;88:306–316. doi: 10.1016/j.ajhg.2011.02.001. http://dx.doi.org/10.1016/j.ajhg.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison LM, He Y. Rhes and AGS1/Dexras1 affect signaling by dopamine D1 receptors through adenylyl cyclase. J Neurosci Res. 2011;89:874–882. doi: 10.1002/jnr.22604. http://dx.doi.org/10.1002/jnr.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Dresel C, Castrop F, Roettinger M, Ceballos-Baumann AO. Silent event-related fMRI reveals reduced sensorimotor activation in laryngeal dystonia. Neurology. 2005;65:1562–1569. doi: 10.1212/01.wnl.0000184478.59063.db. http://dx.doi.org/10.1212/01.wnl.0000184478.59063.db. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Aldrich JV, Anderson KJ, Bakalkin G, Christie MJ, Hall ED, Knapp PE, Scheff SW, Singh IN, Vissel B, Woods AS, Yakovleva T, Shippenberg TS. Pathobiology of dynorphins in trauma and disease. Front Biosci. 2005;10:216–235. doi: 10.2741/1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckroth JA, Abbott LC. Purkinje cell loss from alternating sagittal zones in the cerebellum of leaner mutant mice. Brain Res. 1994;658:93–104. doi: 10.1016/s0006-8993(09)90014-2. http://dx.doi.org/10.1016/S0006-8993(09)90014-2. [DOI] [PubMed] [Google Scholar]
- Hekman KE, Yu GY, Brown CD, Zhu H, Du X, Gervin K, Undlien DE, Peterson A, Stevanin G, Clark HB, Pulst SM, Bird TD, White KP, Gomez CM. A conserved eEF2 coding variant in SCA26 leads to loss of translational fidelity and increased susceptibility to proteostatic insult. Hum Mol Genet. 2012;21:5472–5483. doi: 10.1093/hmg/dds392. http://dx.doi.org/10.1093/hmg/dds392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K, Wilczynski SLL. Cerebellar cell degeneration in the leaner mutant mouse. Neuroscience. 1982;7:2185–2196. doi: 10.1016/0306-4522(82)90129-4. http://dx.doi.org/10.1016/0306-4522(82)90129-4. [DOI] [PubMed] [Google Scholar]
- Hersheson J, Mencacci NE, Davis M, MacDonald N, Trabzuni D, Ryten M, Pittman A, Paudel R, Kara E, Fawcett K, Plagnol V, Bhatia KP, Medlar AJ, Stanescu HC, Hardy J, Kleta R, Wood NW, Houlden H. Mutations in the autoregulatory domain of β-tubulin 4a cause hereditary dystonia. Ann Neurol. 2013;73:546–553. doi: 10.1002/ana.23832. http://dx.doi.org/10.1002/ana.23832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé D. Identification of a specific assembly of the G protein golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum. Front Neuroanat. 2011;5:1–9. doi: 10.3389/fnana.2011.00048. http://dx.doi.org/10.3389/fnana.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano R, Takashima H, Okubo R, Tajima K, Okamoto Y, Ishida S, Tsuruta K, Arisato T, Arata H, Nakagawa M, Osame M, Arimura K. Fine mapping of 16q-linked autosomal dominant cerebellar ataxia type III in Japanese families. Neurogenetics. 2004;5:215–221. doi: 10.1007/s10048-004-0194-z. http://dx.doi.org/10.1007/s10048-004-0194-z. [DOI] [PubMed] [Google Scholar]
- Hirose S. Mutant GABA(A) receptor subunits in genetic (idiopathic) epilepsy. Prog Brain Res. 2014;213:55–85. doi: 10.1016/B978-0-444-63326-2.00003-X. http://dx.doi.org/10.1016/B978-0-444-63326-2.00003-X. [DOI] [PubMed] [Google Scholar]
- Hoffland BS, Kassavetis P, Bologna M, Teo JTH, Bhatia KP, Rothwell JC, Edwards MJ, van de Warrenburg BP. Cerebellum-dependent associative learning deficits in primary dystonia are normalized by rTMS and practice. Eur J Neurosci. 2013;38:2166–2171. doi: 10.1111/ejn.12186. http://dx.doi.org/10.1111/ejn.12186. [DOI] [PubMed] [Google Scholar]
- Hoffland BS, Veugen LC, Janssen MMHP, Pasman JW, Weerdesteyn V, van de Warrenburg BP. A gait paradigm reveals different patterns of abnormal cerebellar motor learning in primary focal dystonias. Cerebellum. 2014;13:760–766. doi: 10.1007/s12311-014-0594-z. http://dx.doi.org/10.1007/s12311-014-0594-z. [DOI] [PubMed] [Google Scholar]
- Holmes SE, O’Hearn EE, McInnis MG, Gorelick-Feldman DA, Kleiderlein JJ, Callahan C, Kwak NG, Ingersoll-Ashworth RG, Sherr M, Sumner AJ, Sharp AH, Ananth U, Seltzer WK, Boss MA, Vieria-Saecker AM, Epplen JT, Riess O, Ross CA. Expansion of a novel CAG trinucleotide repeat in the 5′ region of PPP2R2 B is associated with SCA12. Nat Genet. 1999;23:391–392. doi: 10.1038/70493. http://dx.doi.org/10.1038/70493. [DOI] [PubMed] [Google Scholar]
- Hori K, Hoshino M. GABAergic neuron specification in the spinal cord, the cerebellum, and the cochlear nucleus. Neural Plast. 2012;2012:1–11. doi: 10.1155/2012/921732. http://dx.doi.org/10.1155/2012/921732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlden H, Johnson J, Gardner-Thorpe C, Lashley T, Hernandez D, Worth P, Singleton AB, Hilton D, Holton J, Revesz T, Davis MB, Giunti P, Wood NW. Mutations in TTBK2, encoding a kinase implicated in tau phosphorylation, segregate with spinocerebellar ataxia type 11. Nat Genet. 2007;39:1434–1436. doi: 10.1038/ng.2007.43. http://dx.doi.org/10.1038/ng.2007.43. [DOI] [PubMed] [Google Scholar]
- Hu X, Wang L, Liu H, Zhang S. Functional magnetic resonance imaging study of writer’s cramp. Chin Med J (Engl) 2006;119:1263–1271. [PubMed] [Google Scholar]
- Huang L, Warman J, Carter MT, Friend KL, Dudding TE, Schwartzentruber J, Zou R, Schofield PW, Douglas S, Bulman DE, Boycott KM. Missense mutations in ITPR1 cause autosomal dominant congenital nonprogressive spinocerebellar ataxia. Orphanet J Rare Dis. 2012;7:67. doi: 10.1186/1750-1172-7-67. http://dx.doi.org/10.1186/1750-1172-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Hu J, Li Y, Zhuyun Yang Z, Zhu H, Zhou L, Ma K, Schachner M, Xiao Z, Li Y. The cell adhesion molecule L1 regulates the expression of FGF21 and enhances neurite outgrowth. Brain Res. 2013;1530:13–21. doi: 10.1016/j.brainres.2013.07.043. http://dx.doi.org/10.1016/j.brainres.2013.07.043. [DOI] [PubMed] [Google Scholar]
- Hubsch C, Roze E, Popa T, Russo M, Balachandran A, Pradeep S, Mueller F, Brochard V, Quartarone A, Degos B, Vidailhet M, Kishore A, Meunier S. Defective cerebellar control of cortical plasticity in writer’s cramp. Brain. 2013;136:2050–2062. doi: 10.1093/brain/awt147. http://dx.doi.org/10.1093/brain/awt147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Davanger S. Postsynaptic VAMP/Synaptobrevin facilitates differential vesicle trafficking of GluA1 and GluA2 AMPA receptor subunits. PLoS One. 2015;10:e0140868. doi: 10.1371/journal.pone.0140868. http://dx.doi.org/10.1371/journal.pone.0140868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh DP, Maalouf M, Silva AJ, Schweizer FE, Pulst SM. Dissociated fear and spatial learning in mice with deficiency of ataxin-2. PLoS One. 2009;4:e6235. doi: 10.1371/journal.pone.0006235. http://dx.doi.org/10.1371/journal.pone.0006235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose H, Ohye T, Takahashi E, Seki N, Hori T, Segawa M, Nomura Y, Endo K, Tanaka H, Tsuji S, Fujita K, Nagatsu T. Hereditary progressive dystonia with marked diurnal fluctuation caused by mutations in the GTP cyclohydrolase I gene. Nat Genet. 1994;8:236–242. doi: 10.1038/ng1194-236. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Dick KA, Weatherspoon MR, Gincel D, Armbrust KR, Dalton JC, Stevanin G, Dürr A, Zühlke C, Bürk K, Clark HB, Brice A, Rothstein JD, Schut LJ, Day JW, Ranum LPW. Spectrin mutations cause spinocerebellar ataxia type 5. Nat Genet. 2006;38:184–190. doi: 10.1038/ng1728. http://dx.doi.org/10.1038/ng1728. [DOI] [PubMed] [Google Scholar]
- Issa FA, Mock AF, Sagasti A, Papazian DM. Spinocerebellar ataxia type 13 mutation that is associated with disease onset in infancy disrupts axonal pathfinding during neuronal development. Dis Model Mech. 2012;5:921–929. doi: 10.1242/dmm.010157. http://dx.doi.org/10.1242/dmm.010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob FD, Ho ES, Martinez-Ojeda M, Darras BT, Khwaja OS. Case of infantile onset spinocerebellar ataxia type 5. J Child Neurol. 2013;28:1292–1295. doi: 10.1177/0883073812454331. http://dx.doi.org/10.1177/0883073812454331. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Sepkuty JP, Ho T, Yitta S, Drew T, Rothstein JD, Hess EJ. Calcium channel agonists and dystonia in the mouse. Mov Disord. 2000;15:542–551. doi: 10.1002/1531-8257(200005)15:3<542::AID-MDS1019>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Jones JM, Dionne L, Dell’Orco J, Parent R, Krueger JN, Cheng X, Dib-Hajj SD, Bunton-Stasyshyn RK, Sharkey LM, Dowling JJ, Murphy GG, Shakkottai VG, Shrager P, Meisler MH. Single amino acid deletion in transmembrane segment D4S6 of sodium channel Scn8a (Nav1.6) in a mouse mutant with a chronic movement disorder. Neurobiol Dis. 2016;89:36–45. doi: 10.1016/j.nbd.2016.01.018. http://dx.doi.org/10.1016/j.nbd.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota H, Nakajima Y, Miyazaki M, Sekiguchi H, Kohno Y, Amako M, Arino H, Nemoto K, Sakai N. An fMRI study of musicians with focal dystonia during tapping tasks. J Neurol. 2010;257:1092–1098. doi: 10.1007/s00415-010-5468-9. http://dx.doi.org/10.1007/s00415-010-5468-9. [DOI] [PubMed] [Google Scholar]
- Kaja S, Payne AJ, Nielsen EØ, Thompson CL, van den Maagdenberg AMJM, Koulen P, Snutch TP. Differential cerebellar GABAA receptor expression in mice with mutations in CaV2.1 (P/Q-type) calcium channels. Neuroscience. 2015;304:198–208. doi: 10.1016/j.neuroscience.2015.07.044. http://dx.doi.org/10.1016/j.neuroscience.2015.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Okamoto T, Taniwaki M, Aizawa M, Inoue M, Katayama S, Kawakami H, Nakamura S, Nishimura M, Akiguchi I, Kimura J, Narumiya S, Kakizuka A. CAG expansions in a novel gene for Machado-Joseph Disease at chromosome 14q32.1. Nat Genet. 1994;8:221–228. doi: 10.1038/ng1194-221. http://dx.doi.org/10.1038/ng1194-221. [DOI] [PubMed] [Google Scholar]
- Kay JN, Voinescu PE, Chu MW, Sanes JR. Neurod6 expression defines new retinal amacrine cell subtypes and regulates their fate. Nat Neurosci. 2011;14:965–972. doi: 10.1038/nn.2859. http://dx.doi.org/10.1038/nn.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayakabe M, Kakizaki T, Kaneko R, Sasaki A, Nakazato Y, Shibasaki K, Ishizaki Y, Saito H, Suzuki N, Furuya N, Yanagawa Y. Motor dysfunction in cerebellar Purkinje cell-specific vesicular GABA transporter knockout mice. Front Cell Neurosci. 2014;7:286. doi: 10.3389/fncel.2013.00286. http://dx.doi.org/10.3389/fncel.2013.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy NJ, Martin G, Ehrhardt AG, Cavanagh-Kyros J, Kuan CY, Rakic P, Flavell RA, Treistman SN, Davis RJ. Requirement of JIP scaffold proteins for NMDA-mediated signal transduction. Genes Dev. 2007;21:2336–2346. doi: 10.1101/gad.1563107. http://dx.doi.org/10.1101/gad.1563107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khooshnoodi MA, Factor SA, Jinnah HA. Secondary blepharospasm associated with structural lesions of the brain. J Neurol Sci. 2013;331:98–101. doi: 10.1016/j.jns.2013.05.022. http://dx.doi.org/10.1016/j.jns.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Cho KO, Rothschild A, Sheng M. Heteromultimerization and NMDA receptor-clustering activity of chapsyn-110, a member of the PSD-95 family of proteins. Neuron. 1996;17:103–113. doi: 10.1016/s0896-6273(00)80284-6. http://dx.doi.org/10.1016/S0896-6273(00)80284-6. [DOI] [PubMed] [Google Scholar]
- Kim JB. Channelopathies. Korean J Pediatr. 2014;57:1–18. doi: 10.3345/kjp.2014.57.1.1. http://dx.doi.org/10.3345/kjp.2014.57.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight MA, Gardner RJM, Bahlo M, Matsuura T, Dixon JA, Forrest SM, Storey E. Dominantly inherited ataxia and dysphonia with dentate calcification: spinocerebellar ataxia type 20. Brain. 2004;127:1172–1181. doi: 10.1093/brain/awh139. http://dx.doi.org/10.1093/brain/awh139. [DOI] [PubMed] [Google Scholar]
- Knight MA, Hernandez D, Diede SJ, Dauwerse HG, Rafferty I, van de Leemput J, Forrest SM, Gardner RJM, Storey E, van Ommen GJB, Tapscott SJ, Fischbeck KH, Singleton AB. A duplication at chromosome 11q12.2–11q12.3 is associated with spinocerebellar ataxia type 20. Hum Mol Genet. 2008;17:3847–3853. doi: 10.1093/hmg/ddn283. http://dx.doi.org/10.1093/hmg/ddn283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Abe K, Matsuura T, Ikeda Y, Hitomi T, Akechi Y, Habu T, Liu W, Okuda H, Koizumi A. Expansion of intronic GGCCTG hexanucleotide repeat in NOP56 causes SCA36, a type of spinocerebellar ataxia accompanied by motor neuron involvement. Am J Hum Genet. 2011;89:121–130. doi: 10.1016/j.ajhg.2011.05.015. http://dx.doi.org/10.1016/j.ajhg.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Porcacchia P, Ponzo V, Carrillo F, Cáceres-Redondo MT, Brusa L, Desiato MT, Arciprete F, Di Lorenzo F, Pisani A, Caltagirone C, Palomar FJ, Mir P. Effects of two weeks of cerebellar theta burst stimulation in cervical dystonia patients. Brain Stimul. 2014;7:564–572. doi: 10.1016/j.brs.2014.05.002. http://dx.doi.org/10.1016/j.brs.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Koob MD, Moseley ML, Schut LJ, Benzow K, Bird TD, Day JW, Ranum LP. An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8) Nat Genet. 1999;21:379–384. doi: 10.1038/7710. http://dx.doi.org/10.1038/7710. [DOI] [PubMed] [Google Scholar]
- Kotecki L, Hearing M, McCall NM, Marron Fernandez de Velasco E, Pravetoni M, Arora D, Victoria NC, Munoz MB, Xia Z, Slesinger P, Weaver CD, Wickman K. GIRK channels modulate opioid-induced motor activity in a cell type- and subunit-dependent manner. J Neurosci. 2015;35:7131–7142. doi: 10.1523/JNEUROSCI.5051-14.2015. http://dx.doi.org/10.1523/JNEUROSCI.5051-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PL, Mineta M, Klein C, Schilling K, de Leon D, Farlow MR, Breakefield XO, Bressman SB, Dobyns WB, Ozelius LJ, Brashear A. Rapid-onset dystonia-parkinsonism: linkage to chromosome 19q13. Ann Neurol. 1999;46:176–182. doi: 10.1002/1531-8249(199908)46:2<176::aid-ana6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Krauss J, Seeger W, Jankovic J. Cervical dystonia associated with tumors of the posterior fossa. Mov Disord. 1997;12:443–447. doi: 10.1002/mds.870120329. http://dx.doi.org/10.1002/mds.870120329. [DOI] [PubMed] [Google Scholar]
- Kuoppamaki M, Giunti P, Quinn N, Wood N, Bhatia K. Slowly progressive cerebellar ataxia and cervical dystonia: clinical presentation of a new form of spinocerebellar ataxia? Mov Disord. 2003;18:200–206. doi: 10.1002/mds.10308. [DOI] [PubMed] [Google Scholar]
- López-Bendito G, Luján R, Shigemoto R, Ganter P, Paulsen O, Molnár Z. Blockade of GABA(B) receptors alters the tangential migration of cortical neurons. Cereb Cortex. 2003;13:932–942. doi: 10.1093/cercor/13.9.932. [DOI] [PubMed] [Google Scholar]
- Lüdecke B, Dworniczak B, Bartholomé K. A point mutation in the tyrosine hydroxylase gene associated with Segawa’s syndrome. Hum Genet. 1995;95:123–125. doi: 10.1007/BF00225091. http://dx.doi.org/10.1007/BF00225091. [DOI] [PubMed] [Google Scholar]
- Lau FC, Frank TC, Nahm SS, Stoica G, Abbott LC. Postnatal apoptosis in cerebellar granule cells of homozygous leaner (tg1a/tg1a) mice. Neurotox Res. 2004;6:267–280. doi: 10.1007/BF03033437. [DOI] [PubMed] [Google Scholar]
- LeDoux MS, Brady KA. Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord. 2003;18:60–69. doi: 10.1002/mds.10301. http://dx.doi.org/10.1002/mds.10301. [DOI] [PubMed] [Google Scholar]
- Lee FJS, Xue S, Pei L, Vukusic B, Chéry N, Wang Y, Wang YT, Niznik HB, Yu X, Liu F. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–230. doi: 10.1016/s0092-8674(02)00962-5. http://dx.doi.org/10.1016/S0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- Lee YC, Durr A, Majczenko K, Huang YH, Liu YC, Lien CC, Tsai PC, Ichikawa Y, Goto J, Monin ML, Li JZ, Chung MY, Mundwiller E, Shakkottai V, Liu TT, Tesson C, Lu YC, Brice A, Tsuji S, Burmeister M, Stevanin G, Soong BW. Mutations in KCND3 cause spinocerebellar ataxia type 22. Ann Neurol. 2012;72:859–869. doi: 10.1002/ana.23701. http://dx.doi.org/10.1002/ana.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett FS, Mori Y, Campbell KP, Frankel WN. The mouse stargazer gene encodes a neuronal Ca2+-channel gamma subunit. Nat Genet. 1998;19:340–347. doi: 10.1038/1228. http://dx.doi.org/10.1038/1228. [DOI] [PubMed] [Google Scholar]
- Li L, Chin LS, Shupliakov O, Brodin L, Sihra TS, Hvalby O, Jensen V, Zheng D, McNamara JO, Greengard P. Impairment of synaptic vesicle clustering and of synaptic transmission, and increased seizure propensity, in synapsin I-deficient mice. Proc Natl Acad Sci U S A. 1995;92:9235–9239. doi: 10.1073/pnas.92.20.9235. http://dx.doi.org/10.1073/pnas.92.20.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Ye T, Zhou X, Lai KO, Fu AKY, Ip NY. Cdk5 regulates activity-dependent gene expression and dendrite development. J Neurosci. 2015;35:15127–15134. doi: 10.1523/JNEUROSCI.1443-15.2015. http://dx.doi.org/10.1523/JNEUROSCI.1443-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sugiura Y, Lin W. The role of synaptobrevin1/VAMP1 in Ca2+-triggered neurotransmitter release at the mouse neuromuscular junction. J Physiol. 2011;589:1603–1618. doi: 10.1113/jphysiol.2010.201939. http://dx.doi.org/10.1113/jphysiol.2010.201939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Roland BL, Wilson SJ, Jiang X, Pyati J, Huvar A, Jackson MR, Erlander MG. Cloning and functional expression of the human histamine H3 receptor. Mol Pharmacol. 1999;55:1101–1107. http://dx.doi.org/10.1124/mol.55.6.1101. [PubMed] [Google Scholar]
- Lu Z, Wang Y, Chen F, Tong H, Reddy MVVVS, Luo L, Seshadrinathan S, Zhang L, Holthauzen LMF, Craig AM, Ren G, Rudenko G. Calsyntenin-3 molecular architecture and interaction with neurexin 1α. J Biol Chem. 2014;289:34530–34542. doi: 10.1074/jbc.M114.606806. http://dx.doi.org/10.1074/jbc.M114.606806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig MG, Seuwen K. Characterization of the human adenylyl cyclase gene family: CDNA, gene, structure, and tissue distribution of the nine isoforms. J Recept Signal Transduct. 2002;22:79–110. doi: 10.1081/rrs-120014589. http://dx.doi.org/10.1081/RRS-120014589. [DOI] [PubMed] [Google Scholar]
- Luna-Cancalon K, Sikora KM, Pappas SS, Singh V, Wulff H, Paulson HL, Burmeister M, Shakkottai VG. Alterations in cerebellar physiology are associated with a stiff-legged gait in Atcayjihes mice. Neurobiol Dis. 2014;67:140–148. doi: 10.1016/j.nbd.2014.03.020. http://dx.doi.org/10.1016/j.nbd.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Kaji R, Ando S, Tomizawa M, Yasuno K, Goto S, Matsumoto S, Tabuena MD, Maranon E, Dantes M, Lee LV, Ogasawara K, Tooyama L, Akatsu H, Nishimura M, Tamiya G. Reduced neuron-specific expression of the TAF1 gene is associated with X-linked dystonia-parkinsonism. Am J Hum Genet. 2007;80:393–406. doi: 10.1086/512129. http://dx.doi.org/10.1086/512129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín F, Nieto MA. The expression of Scratch genes in the developing and adult brain. Dev Dyn. 2006;235:2586–2591. doi: 10.1002/dvdy.20869. http://dx.doi.org/10.1002/dvdy.20869. [DOI] [PubMed] [Google Scholar]
- Mark MD, Krause M, Boele HJ, Kruse W, Pollok S, Kuner T, Dalkara D, Koekkoek S, De Zeeuw CI, Herlitze S. Spinocerebellar ataxia type 6 protein aggregates cause deficits in motor learning and cerebellar plasticity. J Neurosci. 2015;35:8882–8895. doi: 10.1523/JNEUROSCI.0891-15.2015. http://dx.doi.org/10.1523/JNEUROSCI.0891-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella G, Maltese M, Nisticò R, Schirinzi T, Madeo G, Sciamanna G, Ponterio G, Tassone A, Mandolesi G, Vanni V, Pignatelli M, Bonsi P, Pisani A. Regional specificity of synaptic plasticity deficits in a knock-in mouse model of DYT1 dystonia. Neurobiol Dis. 2014;65:124–132. doi: 10.1016/j.nbd.2014.01.016. http://dx.doi.org/10.1016/j.nbd.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Masuho I, Mototani Y, Sahara Y, Asami J, Nakamura S, Kozasa T, Inoue T. Dynamic expression patterns of G protein-regulated inducer of neurite outgrowth 1 (GRIN1) and its colocalization with Galphao implicate significant roles of Galphao-GRIN1 signaling in nervous system. Dev Dyn. 2008;237:2415–2429. doi: 10.1002/dvdy.21686. http://dx.doi.org/10.1002/dvdy.21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matilla-Dueñas A, Ashizawa T, Brice A, Magri S, McFarland KN, Pandolfo M, Pulst SM, Riess O, Rubinsztein DC, Schmidt J, Schmidt T, Scoles DR, Stevanin G, Taroni F, Underwood BR, Sánchez I. Consensus paper: pathological mechanisms underlying neurodegeneration in spinocerebellar ataxias. The Cerebellum. 2014;13:269–302. doi: 10.1007/s12311-013-0539-y. http://dx.doi.org/10.1007/s12311-013-0539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matilla-Dueñas A. The ever expanding spinocerebellar ataxias. editorial. Cerebellum. 2012;11:821–827. doi: 10.1007/s12311-012-0376-4. http://dx.doi.org/10.1007/s12311-012-0376-4. [DOI] [PubMed] [Google Scholar]
- Matsuura T, Yamagata T, Burgess DL, Rasmussen A, Grewal RP, Watase K, Khajavi M, McCall AE, Davis CF, Zu L, Achari M, Pulst SM, Alonso E, Noebels JL, Nelson DL, Zoghbi HY, Ashizawa T. Large expansion of the ATTCT pentanucleotide repeat in spinocerebellar ataxia type 10. Nat Genet. 2000;26:191–194. doi: 10.1038/79911. http://dx.doi.org/10.1038/79911. [DOI] [PubMed] [Google Scholar]
- McNeill EM, Klöckner-Bormann M, Roesler EC, Talton LE, Moechars D, Clagett-Dame M. Nav2 hypomorphic mutant mice are ataxic and exhibit abnormalities in cerebellar development. Dev Biol. 2011;353:331–343. doi: 10.1016/j.ydbio.2011.03.008. http://dx.doi.org/10.1016/j.ydbio.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier H, MacPike AD. Three syndromes produced by two mutant genes. J Hered. 1971;62:297–302. doi: 10.1093/oxfordjournals.jhered.a108176. [DOI] [PubMed] [Google Scholar]
- Meisler MH, Sprunger LK, Plummer NW, Escayg A, Jones JM. Ion channel mutations in mouse models of inherited neurological disease. Ann Med. 1997;29:569–574. doi: 10.3109/07853899709007484. [DOI] [PubMed] [Google Scholar]
- Mencacci NE, Erro R, Wiethoff S, Hersheson J, Ryten M, Balint B, Ganos C, Stamelou M, Quinn N, Houlden H, Wood NW, Bhatia KP. ADCY5 mutations are another cause of benign hereditary chorea. Neurology. 2015a doi: 10.1212/WNL.0000000000001720. http://dx.doi.org/10.1212/wnl.0000000000001720. [DOI] [PMC free article] [PubMed]
- Mencacci NE, Rubio-Agusti I, Zdebik A, Asmus F, Ludtmann MHR, Ryten M, Plagnol V, Hauser AK, Bandres-Ciga S, Bettencourt C, Forabosco P, Hughes D, Soutar MMP, Peall K, Morris HR, Trabzuni D, Tekman M, Stanescu HC, Kleta R, Carecchio M, Zorzi G, Nardocci N, Garavaglia B, Lohmann E, Weissbach A, Klein C, Hardy J, Pittman AM, Foltynie T, Abramov AY, Gasser T, Bhatia KP, Wood NW. A missense mutation in KCTD17 causes autosomal dominant myoclonus-dystonia. Am J Hum Genet. 2015b;96:938–947. doi: 10.1016/j.ajhg.2015.04.008. http://dx.doi.org/10.1016/j.ajhg.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Lourenco G, Roze E, Apartis E, Trocello JM, Vidailhet M. Cortical excitability in DYT-11 positive myoclonus dystonia. Mov Disord. 2008;23:761–764. doi: 10.1002/mds.21954. http://dx.doi.org/10.1002/mds.21954. [DOI] [PubMed] [Google Scholar]
- Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8:1551–1566. doi: 10.1038/nprot.2013.092. http://dx.doi.org/10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RGM, Morrison JH, O’Dell TJ. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. http://dx.doi.org/10.1038/24790. [DOI] [PubMed] [Google Scholar]
- Miladinovic T, Nashed M, Singh G. Overview of glutamatergic dysregulation in central pathologies. Biomolecules. 2015;5:3112–3141. doi: 10.3390/biom5043112. http://dx.doi.org/10.3390/biom5043112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, Nakagawara H, Kaida H, Sugita M, Noda K, Motomura K, Ohyagi Y, Ayabe M, Aizawa H, Ishibashi M, Taniwaki T. Expansion of the phenotypic spectrum of SCA14 caused by the Gly128Asp mutation in PRKCG. Clin Neurol Neurosurg. 2009;111:211–215. doi: 10.1016/j.clineuro.2008.09.013. http://dx.doi.org/10.1016/j.clineuro.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Hashimoto K, Uda A, Sakagami H, Nakamura Y, Saito S, Nishi M, Kume H, Tohgo A, Kaneko I, Kondo H, Fukunaga K, Kano M, Watanabe M, Takeshima H. Disturbance of cerebellar synaptic maturation in mutant mice lacking BSRPs, a novel brain-specific receptor-like protein family. FEBS Lett. 2006;580:4057–4064. doi: 10.1016/j.febslet.2006.06.043. http://dx.doi.org/10.1016/j.febslet.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Mouton-Liger F, Sahún I, Collin T, Lopes Pereira P, Masini D, Thomas S, Paly E, Luilier S, Même S, Jouhault Q, Bennaï S, Beloeil JC, Bizot JC, Hérault Y, Dierssen M, Créau N. Developmental molecular and functional cerebellar alterations induced by PCP4/PEP19 overexpression: implications for Down syndrome. Neurobiol Dis. 2014;63:92–106. doi: 10.1016/j.nbd.2013.11.016. http://dx.doi.org/10.1016/j.nbd.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Yin X, Takei Y, Seog DH, Homma N, Hirokawa N. Molecular motor KIF5A is essential for GABA(A) receptor transport, and KIF5A deletion causes epilepsy. Neuron. 2012;76:945–961. doi: 10.1016/j.neuron.2012.10.012. http://dx.doi.org/10.1016/j.neuron.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Nakamura K. SCA17, a novel autosomal dominant cerebellar ataxia caused by an expanded polyglutamine in TATA-binding protein. Hum Mol Genet. 2001;10:1441–1448. doi: 10.1093/hmg/10.14.1441. http://dx.doi.org/10.1093/hmg/10.14.1441. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Yaoi T, Yasui M, Kuwajima G. N-copine: a novel two C2-domain-containing protein with neuronal activity-regulated expression. FEBS Lett. 1998;428:80–84. doi: 10.1016/s0014-5793(98)00497-9. http://dx.doi.org/10.1016/S0014-5793(98)00497-9. [DOI] [PubMed] [Google Scholar]
- Neychev VK, Gross RE, Lehéricy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42:185–201. doi: 10.1016/j.nbd.2011.01.026. http://dx.doi.org/10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M, Carbon M, Argyelan M, Eidelberg D. Hereditary dystonia as a neurodevelopmental circuit disorder: evidence from neuroimaging. Neurobiol Dis. 2011;42:202–209. doi: 10.1016/j.nbd.2010.10.010. http://dx.doi.org/10.1016/j.nbd.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega J, Parkes J, Wong P, Raymond R, Richter A. Altered expression of preproenkephalin and prodynorphin mRNA in a genetic model of paroxysmal dystonia. Brain Res. 2004;1015:87–95. doi: 10.1016/j.brainres.2004.04.046. http://dx.doi.org/10.1016/j.brainres.2004.04.046. [DOI] [PubMed] [Google Scholar]
- Nomura T, Kakegawa W, Matsuda S, Kohda K, Nishiyama J, Takahashi T, Yuzaki M. Cerebellar long-term depression requires dephosphorylation of TARP in Purkinje cells. Eur J Neurosci. 2012;35:402–410. doi: 10.1111/j.1460-9568.2011.07963.x. http://dx.doi.org/10.1111/j.1460-9568.2011.07963.x. [DOI] [PubMed] [Google Scholar]
- Nygaard TG, Marsden CD, Duvoisin RC. Dopa-responsive dystonia. Adv Neurol. 1988;50:377–384. [PubMed] [Google Scholar]
- Nygaard TG, Wilhelmsen KC, Risch NJ, Brown DL, Trugman JM, Gilliam TC, Fahn S, Weeks DE. Linkage mapping of dopa-responsive dystonia (DRD) to chromosome 14q. Nat Genet. 1993;5:386–391. doi: 10.1038/ng1293-386. http://dx.doi.org/10.1038/ng1293-386. [DOI] [PubMed] [Google Scholar]
- Nygaard TG, Raymond D, Chen C, Nishino I, Greene PE, Jennings D, Heiman GA, Klein C, Saunders-Pullman RJ, Kramer P, Ozelius LJ, Bressman SB. Localization of a gene for myoclonus-dystonia to chromosome 7q21–q31. Ann Neurol. 1999;46:794–798. doi: 10.1002/1531-8249(199911)46:5<794::aid-ana19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Nystuen AM, Schwendinger JK, Sachs AJ, Yang AW, Haider NB. A null mutation in VAMP1/synaptobrevin is associated with neurological defects and prewean mortality in the lethal-wasting mouse mutant. Neurogenetics. 2007;8:1–10. doi: 10.1007/s10048-006-0068-7. http://dx.doi.org/10.1007/s10048-006-0068-7. [DOI] [PubMed] [Google Scholar]
- O’Rourke K, O’Riordan S, Gallagher J, Hutchinson M. Paroxysmal torticollis and blepharospasm following bilateral cerebellar infarction. J Neurol. 2006;253:1644–1645. doi: 10.1007/s00415-006-0202-3. http://dx.doi.org/10.1007/s00415-006-0202-3. [DOI] [PubMed] [Google Scholar]
- Obermann M, Yaldizli O, De Greiff A, Lachenmayer ML, Buhl AR, Tumczak F, Gizewski ER, Diener HC, Maschke M. Morphometric changes of sensorimotor structures in focal dystonia. Mov Disord. 2007;22:1117–1123. doi: 10.1002/mds.21495. http://dx.doi.org/10.1002/mds.21495. [DOI] [PubMed] [Google Scholar]
- Obermann M, Vollrath C, de Greiff A, Gizewski ER, Diener HC, Hallett M, Maschke M. Sensory disinhibition on passive movement in cervical dystonia. Mov Disord. 2010;25:2627–2633. doi: 10.1002/mds.23321. http://dx.doi.org/10.1002/mds.23321. [DOI] [PubMed] [Google Scholar]
- Ohba C, Shiina M, Tohyama J, Haginoya K, Lerman-Sagie T, Okamoto N, Blumkin L, Lev D, Mukaida S, Nozaki F, Uematsu M, Onuma A, Kodera H, Nakashima M, Tsurusaki Y, Miyake N, Tanaka F, Kato M, Ogata K, Saitsu H, Matsumoto N. GRIN1 mutations cause encephalopathy with infantile-onset epilepsy, and hyperkinetic and stereotyped movement disorders. Epilepsia. 2015;56:841–848. doi: 10.1111/epi.12987. http://dx.doi.org/10.1111/epi.12987. [DOI] [PubMed] [Google Scholar]
- Orr HT, Chung M, Banfi S, Kwiatkowski TJ, Servadio A, Beaudet AL, McCall AE, Duvick LA, Ranum LPW, Zoghbi HY. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993;4:221–226. doi: 10.1038/ng0793-221. http://dx.doi.org/10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- Ovsepian SV, Friel DD. The leaner P/Q-type calcium channel mutation renders cerebellar Purkinje neurons hyper-excitable and eliminates Ca2+-Na+ spike bursts. Eur J Neurosci. 2008;27:93–103. doi: 10.1111/j.1460-9568.2007.05998.x. http://dx.doi.org/10.1111/j.1460-9568.2007.05998.x. [DOI] [PubMed] [Google Scholar]
- Ovsepian SV, Friel DD. Enhanced synaptic inhibition disrupts the efferent code of cerebellar Purkinje neurons in leaner Cav2.1 Ca2+ channel mutant mice. Cerebellum. 2012;11:666–680. doi: 10.1007/s12311-010-0210-9. http://dx.doi.org/10.1007/s12311-010-0210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozelius LJ, Kramer PL, de Leon D, Risch N, Bressman SB, Schuback DE, Brin MF, Kwiatkowski DJ, Burke RE, Gusella JF. Strong allelic association between the torsion dystonia gene (DYT1) andloci on chromosome 9q34 in Ashkenazi Jews. Am J Hum Genet. 1992;50:619–628. [PMC free article] [PubMed] [Google Scholar]
- Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, de Leon D, Brin MF, Raymond D, Corey DP, Fahn S, Risch NJ, Buckler AJ, Gusella JF, Breakefield XO. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. http://dx.doi.org/10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- Pan ZZ. Transcriptional control of Gad2. Transcription. 2012;3:68–72. doi: 10.4161/trns.19511. http://dx.doi.org/10.4161/trns.19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park N, Yoo JC, Ryu J, Hong SG, Hwang EM, Park JY. Copine1 enhances neuronal differentiation of the hippocampal progenitor HiB5 cells. Mol Cells. 2012;34:549–554. doi: 10.1007/s10059-012-0235-7. http://dx.doi.org/10.1007/s10059-012-0235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pers TH, Karjalainen JM, Chan Y, Westra HJ, Wood AR, Yang J, Lui JC, Vedantam S, Gustafsson S, Esko T, Frayling T, Speliotes EK, Boehnke M, Raychaudhuri S, Fehrmann RSN, Hirschhorn JN, Franke L. Biological interpretation of genome-wide association studies using predicted gene functions. Nat Commun. 2015;6:5890. doi: 10.1038/ncomms6890. http://dx.doi.org/10.1038/ncomms6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettem KL, Yokomaku D, Luo L, Linhoff MW, Prasad T, Connor SA, Siddiqui TJ, Kawabe H, Chen F, Zhang L, Rudenko G, Wang YT, Brose N, Craig AM. The specific α-neurexin interactor calsyntenin-3 promotes excitatory and inhibitory synapse development. Neuron. 2013;80:113–128. doi: 10.1016/j.neuron.2013.07.016. http://dx.doi.org/10.1016/j.neuron.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinin CC, Piovesana LG, Santos MCA, Guimarães RP, De Campos BM, Rezende TJR, Campos LS, Torres FR, Amato-Filho AC, França MC, Lopes-Cendes I, Cendes F, D’Abreu A. Diffuse decreased gray matter in patients with idiopathic craniocervical dystonia: a voxel-based morphometry study. Front Neurol. 2015;5:1–11. doi: 10.3389/fneur.2014.00283. http://dx.doi.org/10.3389/fneur.2014.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planes M, Rooryck C, Vuillaume ML, Besnard L, Bouron J, Lacombe D, Arveiler B, Goizet C. SCA27 is a cause of early-onset ataxia and developmental delay. Eur J Paediatr Neurol. 2015;19:271–273. doi: 10.1016/j.ejpn.2014.11.013. http://dx.doi.org/10.1016/j.ejpn.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Prüss H, Wenzel M, Eulitz D, Thomzig A, Karschin A, Veh RW. Kir2 potassium channels in rat striatum are strategically localized to control basal ganglia function. Mol Brain Res. 2003;110:203–219. doi: 10.1016/s0169-328x(02)00649-6. http://dx.doi.org/10.1016/S0169-328X(02)00649-6. [DOI] [PubMed] [Google Scholar]
- Preibisch C, Berg D, Hofmann E, Solymosi L, Naumann M. Cerebral activation patterns in patients with writer’s cramp: a functional magnetic resonance imaging study. J Neurol. 2001;248:10–17. doi: 10.1007/s004150170263. [DOI] [PubMed] [Google Scholar]
- Prell T, Peschel T, Köhler B, Bokemeyer MH, Dengler R, Günther A, Grosskreutz J. Structural brain abnormalities in cervical dystonia. BMC Neurosci. 2013;14:123. doi: 10.1186/1471-2202-14-123. http://dx.doi.org/10.1186/1471-2202-14-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudente CN, Pardo CA, Xiao J, Hanfelt J, Hess EJ, LeDoux MS, Jinnah HA. Neuropathology of cervical dystonia. Exp Neurol. 2013;241:95–104. doi: 10.1016/j.expneurol.2012.11.019. http://dx.doi.org/10.1016/j.expneurol.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudente CN, Hess EJ, Jinnah HA. Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience. 2014;260:23–35. doi: 10.1016/j.neuroscience.2013.11.062. http://dx.doi.org/10.1016/j.neuroscience.2013.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psifogeorgou K, Papakosta P, Russo SJ, Neve RL, Kardassis D, Gold SJ, Zachariou V. RGS9-2 is a negative modulator of mu-opioid receptor function. J Neurochem. 2007;103:617–625. doi: 10.1111/j.1471-4159.2007.04812.x. http://dx.doi.org/10.1111/j.1471-4159.2007.04812.x. [DOI] [PubMed] [Google Scholar]
- Puddifoot CA, Wu M, Sung RJ, Joiner WJ. Ly6h regulates trafficking of alpha7 nicotinic acetylcholine receptors and nicotine-induced potentiation of glutamatergic signaling. J Neurosci. 2015;35:3420–3430. doi: 10.1523/JNEUROSCI.3630-14.2015. http://dx.doi.org/10.1523/JNEUROSCI.3630-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulst SM, Nechiporuk A, Nechiporuk T, Gispert S, Chen XN, Lopes-Cendes I, Pearlman S, Starkman S, Orozco-Diaz G, Lunkes A, DeJong P, Rouleau GA, Auburger G, Korenberg JR, Figueroa C, Sahba S. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet. 1996;14:269–276. doi: 10.1038/ng1196-269. http://dx.doi.org/10.1038/ng1196-269. [DOI] [PubMed] [Google Scholar]
- Qi G-J, Chen Q, Chen L-J, Shu Y, Bu L-L, Shao X-Y, Zhang P, Jiao F-J, Shi J, Tian B. Phosphorylation of connexin 43 by Cdk5 modulates neuronal migration during embryonic brain development. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9190-6. http://dx.doi.org/10.1007/s12035-015-9190-6. [DOI] [PubMed]
- Quartarone A, Hallett M. Emerging concepts in the physiological basis of dystonia. Mov Disord. 2013;28:958–967. doi: 10.1002/mds.25532. http://dx.doi.org/10.1002/mds.25532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartarone A, Pisani A. Abnormal plasticity in dystonia: disruption of synaptic homeostasis. Neurobiol Dis. 2011;42:162–170. doi: 10.1016/j.nbd.2010.12.011. http://dx.doi.org/10.1016/j.nbd.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Quintero GC, Spano D, Lahoste GJ, Harrison LM. TheRas homologRhes affects dopamineD1 and D2 receptor-mediated behavior in mice. Neuropharmacol Neurotoxicol. 2008;19:1563–1566. doi: 10.1097/WNR.0b013e3283118434. http://dx.doi.org/10.1097/WNR.0b013e32831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raike RS, Hess EJ, Jinnah HA. Dystonia and cerebellar degeneration in the leaner mouse mutant. Brain Res. 2015;1611:56–64. doi: 10.1016/j.brainres.2015.03.011. http://dx.doi.org/10.1016/j.brainres.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainier S, Thomas D, Tokarz D, Ming L, Bui M, Plein E, Zhao X, Lemons R, Albin R, Delaney C, Alvarado D, Fink JK. Myofibrillogenesis regulator 1 gene mutations cause paroxysmal dystonic choreoathetosis. Arch Neurol. 2004;61:1025–1029. doi: 10.1001/archneur.61.7.1025. http://dx.doi.org/10.1001/archneur.61.7.1025. [DOI] [PubMed] [Google Scholar]
- Ramdhani RA, Kumar V, Velickovic M, Frucht SJ, Tagliati M, Simonyan K. What’s special about task in dystonia? A voxel-based morphometry and diffusion weighted imaging study. Mov Disord. 2014;29:1141–1150. doi: 10.1002/mds.25934. http://dx.doi.org/10.1002/mds.25934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. http://dx.doi.org/10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Roy B, Ahmed KT, Cunningham ME, Ferdous J, Mukherjee R, Zheng W, Chen X-Z, Ali DW. Zebrafish TARP Cacng2 is required for the expression and normal development of AMPA receptors at excitatory synapses. Dev Neurobiol. 2015:2. doi: 10.1002/dneu.22327. http://dx.doi.org/10.1002/dneu.22327. [DOI] [PubMed]
- Rumbach L, Barth P, Costaz A, Mas J. Hemidystonia consequent upon ipsilateral vertebral artery occlusion and cerebellar infarction. Mov Disord. 1995;10:522–525. doi: 10.1002/mds.870100424. http://dx.doi.org/10.1002/mds.870100423. [DOI] [PubMed] [Google Scholar]
- Sadakata T, Kakegawa W, Mizoguchi A, Washida M, Katoh-Semba R, Shutoh F, Okamoto T, Nakashima H, Kimura K, Tanaka M, Sekine Y, Itohara S, Yuzaki M, Nagao S, Furuichi T. Impaired cerebellar development and function in mice lacking CAPS2, a protein involved in neurotrophin release. J Neurosci. 2007;27:2472–2482. doi: 10.1523/JNEUROSCI.2279-06.2007. http://dx.doi.org/10.1523/JNEUROSCI.2279-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadnicka A, Teo JT, Kojovic M, Pareés I, Saifee TA, Kassavetis P, Schwingenschuh P, Katschnig-Winter P, Stamelou M, Mencacci NE, Rothwell JC, Edwards MJ, Bhatia KP. All in the blink of an eye: new insight into cerebellar and brainstem function in DYT1 and DYT6 dystonia. Eur J Neurol. 2015;22:762–767. doi: 10.1111/ene.12521. http://dx.doi.org/10.1111/ene.12521. [DOI] [PubMed] [Google Scholar]
- Saegusa C, Fukuda M, Mikoshiba K. Synaptotagmin V is targeted to dense-core vesicles that undergo calcium-dependent exocytosis in PC12 cells. J Biol Chem. 2002;277:24499–24505. doi: 10.1074/jbc.M202767200. http://dx.doi.org/10.1074/jbc.M202767200. [DOI] [PubMed] [Google Scholar]
- Saitoh S, Takamatsu K, Kobayashi M, Noguchi T. Expression of hippocalcin in the developing rat brain. Brain Res Dev Brain Res. 1994;80:199–208. doi: 10.1016/0165-3806(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Sako W, Fujita K, Vo A, Rucker JC, Rizzo JR, Niethammer M, Carbon M, Bressman SB, Uluğ AM, Eidelberg D. The visual perception of natural motion: abnormal task-related neural activity in DYT1 dystonia. Brain. 2015;138:3598–3609. doi: 10.1093/brain/awv282. http://dx.doi.org/10.1093/brain/awv282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanpei K, Takano H, Igarashi S, Sato T, Oyake M, Sasaki H, Wakisaka A, Tashiro K, Ishida Y, Ikeuchi T, Koide R, Saito M, Sato A, Tanaka T, Hanyu S, Takiyama Y, Nishizawa M, Shimizu N, Nomura Y, Segawa M, Iwabuchi K, Eguchi I, Tanaka H, Takahashi H, Tsuji S. Identification of the spinocerebellar ataxia type 2 gene using a direct identification of repeat expansion and cloning technique. Direct Nat Genet. 1996;14:277–284. doi: 10.1038/ng1196-277. http://dx.doi.org/10.1038/ng1196-277. [DOI] [PubMed] [Google Scholar]
- Sato N, Amino T, Kobayashi K, Asakawa S, Ishiguro T, Tsunemi T, Takahashi M, Matsuura T, Flanigan KM, Iwasaki S, Ishino F, Saito Y, Murayama S, Yoshida M, Hashizume Y, Takahashi Y, Tsuji S, Shimizu N, Toda T, Ishikawa K, Mizusawa H. Spinocerebellar ataxia type 31 is associated with inserted penta-nucleotide repeats containing (TGGAA)n. Am J Hum Genet. 2009;85:544–557. doi: 10.1016/j.ajhg.2009.09.019. http://dx.doi.org/10.1016/j.ajhg.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders-Pullman R, Raymond D, Senthil G, Kramer P, Ohmann E, Deligtisch A, Shanker V, Greene P, Tabamo R, Huang N, Tagliati M, Kavanagh P, Soto-Valencia J, Aguiar P, de C, Risch N, Ozelius L, Bressman S. Narrowing the DYT6 dystonia region and evidence for locus heterogeneity in the Amish-Mennonites. Am J Med Genet A. 2007;143A:2098–2105. doi: 10.1002/ajmg.a.31887. http://dx.doi.org/10.1002/ajmg.a.31887. [DOI] [PubMed] [Google Scholar]
- Schöls L, Reimold M, Seidel K, Globas C, Brockmann K, Karsten Hauser T, Auburger G, Bürk K, Den Dunnen W, Reischl G, Korf HF, Brunt ER, Rüb U. No parkinsonism in SCA2 and SCA3 despite severe neurodegeneration of the dopaminergic substantia nigra. Brain. 2015;138:3316–3326. doi: 10.1093/brain/awv255. http://dx.doi.org/10.1093/brain/awv255. [DOI] [PubMed] [Google Scholar]
- Schneider EH, Neumann D, Seifert R. Modulation of behavior by the histaminergic system: lessons from HDC-, H3R- and H4R-deficient mice. Neurosci Biobehav Rev. 2014;47:101–121. doi: 10.1016/j.neubiorev.2014.07.020. http://dx.doi.org/10.1016/j.neubiorev.2014.07.020. [DOI] [PubMed] [Google Scholar]
- Schoch S. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;80(294):1117–1122. doi: 10.1126/science.1064335. http://dx.doi.org/10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- Segawa M, Nomura Y. Genetics and pathophysiology of primary dystonia with special emphasis on DYT1 and DYT5. Semin Neurol. 2014;34:306–311. doi: 10.1055/s-0034-1386768. [DOI] [PubMed] [Google Scholar]
- Sergaki MC, Guillemot F, Matsas R. Impaired cerebellar development and deficits in motor coordination in mice lacking the neuronal protein BM88/Cend1. Mol Cell Neurosci. 2010;44:15–29. doi: 10.1016/j.mcn.2010.01.011. http://dx.doi.org/10.1016/j.mcn.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. http://dx.doi.org/10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Jamwal S, Kumar P. Beneficial effect of antidepressants against rotenone induced Parkinsonism like symptoms in rats. Pathophysiology. 2016;23:123–134. doi: 10.1016/j.pathophys.2016.03.002. http://dx.doi.org/10.1016/j.pathophys.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Sherkhane P, Kapfhammer JP. The plasma membrane Ca2+-ATPase2 (PMCA2) is involved in the regulation of Purkinje cell dendritic growth in cerebellar organotypic slice cultures. Neural Plast. 2013;2013:321685. doi: 10.1155/2013/321685. http://dx.doi.org/10.1155/2013/321685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley TL, Rao LM, Hess EJ, Jinnah HA. Paroxysmal dyskinesias in mice. Mov Disord. 2008;23:259–264. doi: 10.1002/mds.21829. http://dx.doi.org/10.1002/mds.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorini S, Liao YJ, Duncan SA, Jan LY, Stoffel M. Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc Natl Acad Sci U S A. 1997;94:923–927. doi: 10.1073/pnas.94.3.923. http://dx.doi.org/10.1073/pnas.94.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Ludlow CL. Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: an fMRI study. Cereb Cortex. 2010;20:2749–2759. doi: 10.1093/cercor/bhq023. http://dx.doi.org/10.1093/cercor/bhq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Ludlow CL. Abnormal structure-function relationship in spasmodic dysphonia. Cereb Cortex. 2012;22:417–425. doi: 10.1093/cercor/bhr120. http://dx.doi.org/10.1093/cercor/bhr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Tovar-Moll F, Ostuni J, Hallett M, Kalasinsky VF, Lewin-Smith MR, Rushing EJ, Vortmeyer AO, Ludlow CL. Focal white matter changes in spasmodic dysphonia: a combined diffusion tensor imaging and neuropathological study. Brain. 2008;131:447–459. doi: 10.1093/brain/awm303. http://dx.doi.org/10.1093/brain/awm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal P, Rudaś M, Harat M, Szylberg Ł, Zieliński P. Deep anterior cerebellar stimulation reduces symptoms of secondary dystonia in patients with cerebral palsy treated due to spasticity. Clin Neurol Neurosurg. 2015;135:62–68. doi: 10.1016/j.clineuro.2015.05.017. http://dx.doi.org/10.1016/j.clineuro.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Sommer M, Grafman J, Clark K, Hallett M. Learning in Parkinson’s disease: eyeblink conditioning, declarative learning, and procedural learning. J Neurol Neurosurg Psychiatry. 1999;67:27–34. doi: 10.1136/jnnp.67.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey E, Phil D. Genetic cerebellar ataxias. Semin Neurol. 2014:280–292. doi: 10.1055/s-0034-1386766. [DOI] [PubMed] [Google Scholar]
- Storey E, Gardner RJ, Knight MA, Kennerson ML, Tuck RR, Forrest SM, Nicholson GA. A new autosomal dominant pure cerebellar ataxia. Neurology. 2001;57:1913–1915. doi: 10.1212/wnl.57.10.1913. [DOI] [PubMed] [Google Scholar]
- Street VA, Bosma MM, Demas VP, Regan MR, Lin DD, Robinson LC, Agnew WS, Tempel BL. The type 1 inositol 1,4,5-trisphosphate receptor gene is altered in the opisthotonos mouse. J Neurosci. 1997;17:635–645. doi: 10.1523/JNEUROSCI.17-02-00635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Napolitano F, Mealer RG, Kim S, Errico F, Barrow R, Shahani N, Tyagi R, Snyder SH, Usiello A. Rhes, a striatal-enriched small G protein, mediates mTOR signaling and L-DOPA-induced dyskinesia. Nat Neurosci. 2011;15:191–193. doi: 10.1038/nn.2994. http://dx.doi.org/10.1038/nn.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Suwa H, Ishikawa T, Kotani H. Targeted disruption of H3 receptors results in changes in brain histamine tone leading to an obese phenotype. J Clin Invest. 2002;110:1791–1799. doi: 10.1172/JCI200215784. http://dx.doi.org/10.1172/JCI15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiyama Y, Oyanagi S, Kawashima S, Sakamoto H, Saito K, Yoshida M, Tsuji S, Mizuno Y, Nishizawa M. A clinical and pathologic study of a large Japanese family with Machado-Joseph disease tightly linked to the DNA markers on chromosome 14q. Neurology. 1994;44:1302–1308. doi: 10.1212/wnl.44.7.1302. [DOI] [PubMed] [Google Scholar]
- Talelli P, Hoffland BS, Schneider SA, Edwards MJ, Bhatia KP, van de Warrenburg BPC, Rothwell JC. A distinctive pattern of cortical excitability in patients with the syndrome of dystonia and cerebellar ataxia. Clin Neurophysiol. 2011;122:1816–1819. doi: 10.1016/j.clinph.2011.02.029. http://dx.doi.org/10.1016/j.clinph.2011.02.029. [DOI] [PubMed] [Google Scholar]
- Teixeira MJ, Schroeder HK, Lepski G. Evaluating cerebellar dentatotomy for the treatment of spasticity with or without dystonia. Br J Neurosurg. 2015:1–6. doi: 10.3109/02688697.2015.1025697. http://dx.doi.org/10.3109/02688697.2015.1025697. [DOI] [PubMed]
- Teo JTH, van de Warrenburg BPC, Schneider S, Rothwell JC, Bhatia KP. Neurophysiological evidence for cerebellar dysfunction in primary focal dystonia. J Neurol Neurosurg Psychiatry. 2009;80:80–83. doi: 10.1136/jnnp.2008.144626. http://dx.doi.org/10.1136/jnnp.2008.144626. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Danielson PE, Sutcliffe JG. RGS9: a regulator of G-protein signalling with specific expression in rat and mouse striatum. J Neurosci Res. 1998;52:118–124. doi: 10.1002/(SICI)1097-4547(19980401)52:1<118::AID-JNR11>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Steinmetz JE. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience. 2009;162:732–755. doi: 10.1016/j.neuroscience.2009.01.041. http://dx.doi.org/10.1016/j.neuroscience.2009.01.041. [DOI] [PubMed] [Google Scholar]
- Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. http://dx.doi.org/10.1016/S0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Tojima T, Itofusa R, Kamiguchi H. Steering neuronal growth cones by shifting the imbalance between exocytosis and endocytosis. J Neurosci. 2014;34:7165–7178. doi: 10.1523/JNEUROSCI.5261-13.2014. http://dx.doi.org/10.1523/JNEUROSCI.5261-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita H, Nagamitsu S, Wakui K, Fukushima Y, Yamada K, Sadamatsu M, Masui A, Konishi T, Matsuishi T, Aihara M, Shimizu K, Hashimoto K, Mineta M, Matsushima M, Tsujita T, Saito M, Tanaka H, Tsuji S, Takagi T, Nakamura Y, Nanko S, Kato N, Nakane Y, Niikawa N. Paroxysmal kinesigenic choreoathetosis locus maps to chromosome 16p11.2-q12.1. Am J Hum Genet. 1999;65:1688–1697. doi: 10.1086/302682. http://dx.doi.org/10.1086/302682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyota H, Dugovic C, Koehl M, Laposky AD, Weber C, Ngo K, Wu Y, Lee DH, Yanai K, Sakurai E, Watanabe T, Liu C, Chen J, Barbier AJ, Turek FW, Fung-Leung WP, Lovenberg TW. Behavioral characterization of mice lacking histamine H(3) receptors. Mol Pharmacol. 2002;62:389–397. doi: 10.1124/mol.62.2.389. [DOI] [PubMed] [Google Scholar]
- Tsoi H, Yu ACS, Chen ZS, Ng NKN, Chan AYY, Yuen LYP, Abrigo JM, Tsang SY, Tsui SKW, Tong TMF, Lo IFM, Lam STS, Mok VCT, Wong LKS, Ngo JCK, Lau K-F, Chan T-F, Chan HYE. A novel missense mutation in CCDC88C activates the JNK pathway and causes a dominant form of spinocerebellar ataxia. J Med Genet. 2014:1–6. doi: 10.1136/jmedgenet-2014-102333. http://dx.doi.org/10.1136/jmedgenet-2014-102333. [DOI] [PMC free article] [PubMed]
- Um JW, Pramanik G, Ko JS, Song MY, Lee D, Kim H, Park KS, Südhof TC, Tabuchi K, Ko J. Calsyntenins function as synaptogenic adhesion molecules in concert with neurexins. Cell Rep. 2014;6:1096–1109. doi: 10.1016/j.celrep.2014.02.010. http://dx.doi.org/10.1016/j.celrep.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaalen J, Giunti P, van de Warrenburg BP. Movement disorders in spinocerebellar ataxias. Mov Disord. 2011;26:792–800. doi: 10.1002/mds.23584. http://dx.doi.org/10.1002/mds.23584. [DOI] [PubMed] [Google Scholar]
- van Swieten JC, Brusse E, de Graaf BM, Krieger E, van de Graaf R, de Koning I, Maat-Kievit A, Leegwater P, Dooijes D, Oostra BA, Heutink P. A mutation in the fibroblast growth factor 14 gene is associated with autosomal dominant cerebellar ataxia. Am J Hum Genet. 2003;72:191–199. doi: 10.1086/345488. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Leemput J, Chandran J, Knight MA, Holtzclaw LA, Scholz S, Cookson MR, Houlden H, Gwinn-Hardy K, Fung HC, Lin X, Hernandez D, Simon-Sanchez J, Wood NW, Giunti P, Rafferty I, Hardy J, Storey E, Gardner RJM, Forrest SM, Fisher EMC, Russell JT, Cai H, Singleton AB. Deletion at ITPR1 underlies ataxia in mice and spinocerebellar ataxia 15 in humans. PLoS Genet. 2007;3:e108. doi: 10.1371/journal.pgen.0030108. http://dx.doi.org/10.1371/journal.pgen.0030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Warrenburg BPC, Verbeek DS, Piersma SJ, Hennekam FAM, Pearson PL, Knoers NVAM, Kremer HPH, Sinke RJ. Identification of a novel SCA14 mutation in a Dutch autosomal dominant cerebellar ataxia family. Neurology. 2003;61:1760–1765. doi: 10.1212/01.wnl.0000098883.79421.73. http://dx.doi.org/10.1212/01.WNL.0000098883.79421.73. [DOI] [PubMed] [Google Scholar]
- van de Warrenburg BPC, Giunti P, Schneider SA, Quinn NP, Wood NW, Bhatia KP. The syndrome of (predominantly cervical) dystonia and cerebellar ataxia: new cases indicate a distinct but heterogeneous entity. J Neurol Neurosurg Psychiatry. 2007;78:774–775. doi: 10.1136/jnnp.2006.105841. http://dx.doi.org/10.1136/jnnp.2006.105841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer JN, Beukers RJ, van der Salm SMA, Caan MWA, Tijssen MAJ, Nederveen AJ. White matter abnormalities in gene-positive myoclonus-dystonia. Mov Disord. 2012;27:1666–1672. doi: 10.1002/mds.25128. http://dx.doi.org/10.1002/mds.25128. [DOI] [PubMed] [Google Scholar]
- Vanni V, Puglisi F, Bonsi P, Ponterio G, Maltese M, Pisani A, Mandolesi G. Cerebellar synaptogenesis is compromised in mouse models of DYT1 dystonia. Exp Neurol. 2015;271:457–467. doi: 10.1016/j.expneurol.2015.07.005. http://dx.doi.org/10.1016/j.expneurol.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Verbeek DS, Schelhaas JH, Ippel EF, Beemer F, Pearson PL, Sinke RJ. Identification of a novel SCA locus (SCA19) in a Dutch autosomal dominant cerebellar ataxia family on chromosome region 1p21-q21. Hum Genet. 2002;111:388–393. doi: 10.1007/s00439-002-0782-7. http://dx.doi.org/10.1007/s00439-002-0782-7. [DOI] [PubMed] [Google Scholar]
- Verbeek DS, van de Warrenburg BP, Wesseling P, Pearson PL, Kremer HP, Sinke RJ. Mapping of the SCA23 locus involved in autosomal dominant cerebellar ataxia to chromosome region 20p 13-12.3. Brain. 2004;127:2551–2557. doi: 10.1093/brain/awh276. http://dx.doi.org/10.1093/brain/awh276. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejon C, Collin C, McKay RDG, Segal M. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J Neurosci. 1998;18:7256–7271. doi: 10.1523/JNEUROSCI.18-18-07256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser JE, Bloem BR, van de Warrenburg BPC. PRKCG mutation (SCA-14) causing a ramsay hunt phenotype. Mov Disord. 2007;22:1024–1026. doi: 10.1002/mds.21414. http://dx.doi.org/10.1002/mds.21414. [DOI] [PubMed] [Google Scholar]
- Vogel-Ciernia A, Wood MA. Neuron-specific chromatin remodeling: a missing link in epigenetic mechanisms underlying synaptic plasticity, memory, and intellectual disability disorders. Neuropharmacology. 2014;80:18–27. doi: 10.1016/j.neuropharm.2013.10.002. http://dx.doi.org/10.1016/j.neuropharm.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waln O, LeDoux MS. Delayed-onset oromandibular dystonia after a cerebellar hemorrhagic stroke. Parkinsonism Relat Disord. 2010;16:623–625. doi: 10.1016/j.parkreldis.2010.07.010. http://dx.doi.org/10.1016/j.parkreldis.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Walter JT, Alviña K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci. 2006;9:389–397. doi: 10.1038/nn1648. http://dx.doi.org/10.1038/nn1648. [DOI] [PubMed] [Google Scholar]
- Wang Q, Bardgett ME, Wong M, Wozniak DF, Lou J, McNeil BD, Chen C, Nardi A, Reid DC, Yamada K, Ornitz DM. Ataxia and paroxysmal dyskinesia in mice lacking axonally transported FGF14. Neuron. 2002;35:25–38. doi: 10.1016/s0896-6273(02)00744-4. [DOI] [PubMed] [Google Scholar]
- Wang VY, Rose MF, Zoghbi HY. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron. 2005;48:31–43. doi: 10.1016/j.neuron.2005.08.024. http://dx.doi.org/10.1016/j.neuron.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Wang JLJ, Yang X, Xia K, Hu ZM, Weng L, Jin X, Jiang H, Zhang P, Shen L, Guo JF, Li N, Li YR, Lei LF, Zhou J, Du J, Zhou YF, Pan Q, Wang JLJ, Wang JLJ, Li RQ, Tang BS. TGM6 identified as a novel causative gene of spinocerebellar ataxias using exome sequencing. Brain. 2010;133:3510–3518. doi: 10.1093/brain/awq323. http://dx.doi.org/10.1093/brain/awq323. [DOI] [PubMed] [Google Scholar]
- Wang JL, Cao L, Li XH, Hu ZM, Li JD, Zhang J, Liang Y, San A, Li N, Chen SQ, Guo JF, Jiang H, Shen L, Zheng L, Mao X, Yan WQ, Zhou Y, Shi YT, Ai SX, Dai MZ, Zhang P, Xia K, Chen SD, Tang BS. Identification of PRRT2 as the causative gene of paroxysmal kinesigenic dyskinesias. Brain. 2011;134:3493–3501. doi: 10.1093/brain/awr289. http://dx.doi.org/10.1093/brain/awr289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Duan X, Ren Y, Liu Y, Huang M, Liu P, Wang R, Gao G, Zhou L, Feng Z, Zheng W. FoxO3a negatively regulates nerve growth factor-induced neuronal differentiation through inhibiting the expression of neurochondrin in PC12 cells. Mol Neurobiol. 2013;47:24–36. doi: 10.1007/s12035-012-8357-7. http://dx.doi.org/10.1007/s12035-012-8357-7. [DOI] [PubMed] [Google Scholar]
- Waters MF, Fee D, Figueroa KP, Nolte D, Müller U, Advincula J, Coon H, Evidente VG, Pulst SM. An autosomal dominant ataxia maps to 19q13: Allelic heterogeneity of SCA13 or novel locus? Neurology. 2005;65:1111–1113. doi: 10.1212/01.wnl.0000177490.05162.41. http://dx.doi.org/10.1212/01.wnl.0000177490.05162.41. [DOI] [PubMed] [Google Scholar]
- Waters MF, Minassian NA, Stevanin G, Figueroa KP, Bannister JPA, Nolte D, Mock AF, Evidente VGH, Fee DB, Müller U, Dürr A, Brice A, Papazian DM, Pulst SM. Mutations in voltage-gated potassium channel KCNC3 cause degenerative and developmental central nervous system phenotypes. Nat Genet. 2006;38:447–451. doi: 10.1038/ng1758. http://dx.doi.org/10.1038/ng1758. [DOI] [PubMed] [Google Scholar]
- Weber YG, Storch A, Wuttke TV, Brockmann K, Kempfle J, Maljevic S, Margari L, Kamm C, Schneider SA, Huber SM, Pekrun A, Roebling R, Seebohm G, Koka S, Lang C, Kraft E, Blazevic D, Salvo-Vargas A, Fauler M, Mottaghy FM, Münchau A, Edwards MJ, Presicci A, Margari F, Gasser T, Lang F, Bhatia KP, Lehmann-Horn F, Lerche H. GLUT1 mutations are a cause of paroxysmal exertion-induced dyskinesias and induce hemolytic anemia by a cation leak. J Clin Invest. 2008;118:2157–2168. doi: 10.1172/JCI34438. http://dx.doi.org/10.1172/JCI34438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber YG, Kamm C, Suls A, Kempfle J, Kotschet K, Schüle R, Wuttke TV, Maljevic S, Liebrich J, Gasser T, Ludolph AC, Van Paesschen W, Schöls L, De Jonghe P, Auburger G, Lerche H. Paroxysmal choreoathetosis/spasticity (DYT9) is caused by a GLUT1 defect. Neurology. 2011:959–964. doi: 10.1212/WNL.0b013e31822e0479. [DOI] [PubMed] [Google Scholar]
- Wilkaniec A, Czapski GA, Adamczyk A. Cdk5 at crossroads of protein oligomerization in neurodegenerative diseases: facts and hypotheses. J Neurochem. 2016;136:222–233. doi: 10.1111/jnc.13365. http://dx.doi.org/10.1111/jnc.13365. [DOI] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A. 2004;101:7158–7163. doi: 10.1073/pnas.0401764101. http://dx.doi.org/10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak DF, Xiao M, Xu L, Yamada KA, Ornitz DM. Impaired spatial learning and defective theta burst induced LTP in mice lacking fibroblast growth factor 14. Neurobiol Dis. 2007;26:14–26. doi: 10.1016/j.nbd.2006.11.014. http://dx.doi.org/10.1016/j.nbd.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Fairhall SL, McNair NA, Hamm JP, Kirk IJ, Cunnington R, Anderson T, Lim VK. Impaired sensorimotor integration in focal hand dystonia patients in the absence of symptoms. J Neurol Neurosurg Psychiatry. 2010;81:659–665. doi: 10.1136/jnnp.2009.185637. http://dx.doi.org/10.1136/jnnp.2009.185637. [DOI] [PubMed] [Google Scholar]
- Wu YJ, Tejero R, Arancillo M, Vardar G, Korotkova T, Kintscher M, Schmitz D, Ponomarenko A, Tabares L, Rosenmund C. Syntaxin 1 B is important for mouse postnatal survival and proper synaptic function at the mouse neuromuscular junctions. J Neurophysiol. 2015;114:2404–2417. doi: 10.1152/jn.00577.2015. http://dx.doi.org/10.1152/jn.00577.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Uitti RJ, Zhao Y, Vemula SR, Perlmutter JS, Wszolek ZK, Maraganore DM, Auburger G, Leube B, Lehnhoff K, Ledoux MS. Mutations in CIZ1 cause adult onset primary cervical dystonia. Ann Neurol. 2012;71:458–469. doi: 10.1002/ana.23547. http://dx.doi.org/10.1002/ana.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Vemula SR, LeDoux MS. Recent advances in the genetics of dystonia. Curr Neurol Neurosci Rep. 2014;14:462. doi: 10.1007/s11910-014-0462-8. http://dx.doi.org/10.1007/s11910-014-0462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM, Tonegawa S. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994;79:729–742. doi: 10.1016/0092-8674(94)90557-6. http://dx.doi.org/10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]
- Yang J, Luo C, Song W, Guo X, Zhao B, Chen X, Huang X, Gong Q, Shang HF. Diffusion tensor imaging in blepharospasm and blepharospasm-oromandibular dystonia. J Neurol. 2014;261:1413–1424. doi: 10.1007/s00415-014-7359-y. http://dx.doi.org/10.1007/s00415-014-7359-y. [DOI] [PubMed] [Google Scholar]
- Yasuda J, Whitmarsh AJ, Cavanagh J, Sharma M, Davis RJ. The JIP group of mitogen-activated protein kinase scaffold proteins. Mol Cell Biol. 1999;19:7245–7254. doi: 10.1128/mcb.19.10.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu GY, Howell MJ, Roller MJ, Xie TD, Gomez CM. Spinocerebellar ataxia type 26 maps to chromosome 19p13.3 adjacent to SCA6. Ann Neurol. 2005;57:349–354. doi: 10.1002/ana.20371. http://dx.doi.org/10.1002/ana.20371. [DOI] [PubMed] [Google Scholar]
- Zanni G, Calì T, Kalscheuer VM, Ottolini D, Barresi S, Lebrun N, Montecchi-Palazzi L, Hu H, Chelly J, Bertini E, Brini M, Carafoli E. Mutation of plasma membrane Ca2+ ATPase isoform 3 in a family with X-linked congenital cerebellar ataxia impairs Ca2+ homeostasis. Proc Natl Acad Sci U S A. 2012;109:14514–14519. doi: 10.1073/pnas.1207488109. http://dx.doi.org/10.1073/pnas.1207488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zech M, Lam DD, Francescatto L, Schormair B, Salminen AV, Jochim A, Wieland T, Lichtner P, Peters A, Gieger C, Lochmüller H, Strom TM, Haslinger B, Katsanis N, Winkelmann J. Recessive mutations in the α3 (VI) collagen gene COL6A3 cause early-onset isolated dystonia. Am J Hum Genet. 2015;96:883–893. doi: 10.1016/j.ajhg.2015.04.010. http://dx.doi.org/10.1016/j.ajhg.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervas NT. Long-term review of dentatectomy in dystonia musculorum deformans and cerebral palsy. Acta Neurochir. 1977;24:49–51. doi: 10.1007/978-3-7091-8482-0_7. [DOI] [PubMed] [Google Scholar]
- Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, Dobyns WB, Subramony SH, Zoghbi HY, Lee CC. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansion in the α1A-voltage-dependent calcium channel. Nat Genet. 1997;15:62–69. doi: 10.1038/ng0197-62. http://dx.doi.org/10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Grabowski M, Asmus F, Naumann M, Berg D, Bertram M, Scheidtmann K, Kern P, Winkelmann J, Müller-Myhsok B, Riedel L, Bauer M, Müller T, Castro M, Meitinger T, Strom TM, Gasser T. Mutations in the gene encoding epsilon-sarcoglycan cause myoclonus-dystonia syndrome. Nat Genet. 2001;29:66–69. doi: 10.1038/ng709. http://dx.doi.org/10.1038/ng709. [DOI] [PubMed] [Google Scholar]
- Zoons E, Tijssen MJ. Pathologic changes in the brain in cervical dystonia pre- and post-mortem—a commentary with a special focus on the cerebellum. Exp Neurol. 2013;247:130–133. doi: 10.1016/j.expneurol.2013.04.005. http://dx.doi.org/10.1016/j.expneurol.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Zoons E, Booij J, Nederveen AJ, Dijk JM, Tijssen MAJ. Structural, functional and molecular imaging of the brain in primary focal dystonia—a review. Neuroimage. 2011;56:1011–1020. doi: 10.1016/j.neuroimage.2011.02.045. http://dx.doi.org/10.1016/j.neuroimage.2011.02.045. [DOI] [PubMed] [Google Scholar]
- Zu L, Figueroa KP, Grewal R, Pulst SM. Mapping of a new autosomal dominant spinocerebellar ataxia to chromosome 22. Am J Hum Genet. 1999;64:594–599. doi: 10.1086/302247. http://dx.doi.org/10.1086/302247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.