Abstract
Understanding the sorption mechanisms for organophosphate flame retardants (OPFRs) on impervious surfaces is important to improve our knowledge of the fate and transport of OPFRs in indoor environments. The sorption processes of semivolatile organic compounds (SVOCs) on indoor surfaces are heterogeneous (multilayer sorption) or homogeneous (monolayer sorption). In this study, we adopted simplified Langmuir isotherm and Freundlich isotherm in a dynamic sink model to characterize the sorption dynamics of OPFRs on impervious surfaces such as stainless steel and made comparisons between the two models through a series of empty chamber studies. The tests involve two types of stainless steel chambers (53-L small chambers and 44-mL micro chambers) using tris(2-chloroethyl)phosphate (TCEP) and tris(1-chloro-2-propyl)phosphate TCPP) as target compounds. Our test results show that the dynamic sink model using Freundlich isotherm can better represent the sorption process in the empty small chamber. Micro chamber test results from this study show that the sink model using both simplified Langmuir isotherm and Freundlich isotherm can well fit the measured gas-phase concentrations of OPFRs. We further applied both models and the parameters obtained to predict the gas phase concentrations of OPFRs in a small chamber with an emission source. Comparisons between model predictions and measurements demonstrate the reliability and applicability of the sorption parameters.
Keywords: Organophosphate flame retardants, SVOCs, Surface sorption, Langmuir isotherm, Freundlich isotherm, Dynamic sink model
1 Introduction
Organophosphorus flame retardants (OPFRs) are produced and used widely as alternative additives in building materials and consumer products such as polyvinyl chloride (PVC) flooring, electrical and electronic products, furniture, textile coatings, and plastics (Van der Veen and De Boer, 2012; Wei et al., 2015; Wensing et al., 2005; Stapleton et al., 2009). Due to their low volatility, these chemicals are classified as semivolatile organic compounds (SVOCs) (Wensing et al., 2005). Because OPFRs are usually present as additives rather than chemically bonded to product materials, they can leach or escape from the original materials and are released to the environment (Wei et al., 2015; Stapleton et al., 2009; Hartmann et al., 2004). Acute adverse health effects associated with humans and animals have been found for OPFRs such as tris(2-chloroethyl) phosphate (TCEP), tris(1-chloro-2-propyl) phosphate (TCPP), and tris(1,3-dichloro-2-propyl) phosphate (TDCPP) (Bradman et al., 2014; Van den Eede et al., 2011; ATSDR, 2012). The occurrence of OPFRs is extensive, and they have been detected in various environmental media including indoor air (Hartmann et al., 2004; Bradman et al., 2014; Staaf and Östman, 2005), house dust (Bradman et al., 2014; Van den Eede et al., 2011; Bergh et al., 2011; Brommer et al., 2012; Dodson et al., 2012; Yang et al., 2012; Brandsma et al., 2014), water (Andersen et al., 2004; Barnes et al, 2008; Cristale et al., 2013; Wang et al., 2015), plants (Hyland et al., 2015), and biota (Sundkvist et al., 2010).
Human exposure to OPFRs is likely to occur through inhalation of indoor air and airborne particles, dermal absorption, and dietary and non-dietary ingestion (Wei et al., 2015; ATSDR, 2012; Weschler and Nazaroff, 2008 and 2012; Little et al., 2012; Stapleton et al., 2011). Emissions of OPFRs from source materials usually occur slowly, and the gas phase OPFRs are readily adsorbed to and/or absorbed by interior surfaces, airborne particles, and settled dust. Understanding the sorption mechanisms of OPFRs on indoor surfaces is a prerequisite to characterize their fate and transport in indoor environments and further develop strategies to limit exposures and protect human health.
Over the years, researchers have developed various models to describe the physical sorption of gas molecules on solid surfaces. Among all these models, the Langmuir isotherm (Langmuir, 1916) is one of the earliest and most commonly used models. The derivation of the Langmuir isotherm is based on the assumption that there is a monolayer of molecules on a homogeneous surface and all sorption sites are independent and identical. It can be presented as (Nazaroff and Alvarez-Cohen, 2001)
| (1) |
where qe (µg/m2) is the amount of chemical on the material surface at equilibrium, qmax (µg/m2) is the maximum sorption capacity on the surface, Ce (µg/m3) is the gas-phase concentration at equilibrium, and b (m3/µg) is the equilibrium Langmuir isotherm constant. By assuming that the chemical has very low partial pressure in air, which is often the case for air pollutants, the concentration of the chemical in the air is low enough so that the fraction of surface sites that are covered by the chemical is much less than 1. Then Equation 1 becomes
| (2) |
where KL (m) refers to the equilibrium partition coefficient in the simplified Langmuir-isotherm.
Unlike the Langmuir isotherm, the Freundlich isotherm (Freundlich, 1906) is an empirical model for sorption that is non-linear and reversible, and the Freundlich isotherm has gained popularity for describing multilayer or heterogeneous sorption, especially for organic compounds (Foo and Hameed, 2010). The Freundlich equation is usually expressed as
| (3) |
where KF (m3n-2×µg1−n) and n are the Freundlich isotherm constant and exponential. Based on the Langmuir and the Freundlich isotherms, researchers have proposed other empirical models for the purpose of achieving better data fitting and prediction. Such proposed models include the Sips model (Sips, 1948), the Koble-Corrigan model (Koble and Corrigan, 1952), and the Redlich-Peterson model (Redlich and Peterson, 1959), all of which integrate the Langmuir and the Freundlich isotherms in different forms. Overall, these models provide valuable information and methods for scientists to study the sorption behaviors of SVOCs.
In contrast to the development of isotherm models, very little has been improved in modeling the sorption dynamics of SVOCs on indoor surfaces. Xu and Little (2006) proposed a fundamental mass transfer model to predict SVOC emissions from PVC products. In this model, they adopted the Freundlich isotherm. In their later simplified SVOC emission model, Xu et al. (2012) used the simplified Langmuir isotherm to study the equilibrium relationship between the chamber surface and the gaseous SVOCs. The linear partition relationship between chamber surface/air SVOCs has been continuously adopted in more recent SVOC studies (Liang and Xu, 2014; Liu et al., 2015; Bi et al., 2015) as a basis to explain partitioning of phthalates on impervious surfaces. Despite its simplicity and convenience in use, the linear partitioning relationship should be applied cautiously to the sorption of SVOCs on indoor surfaces. A test house study conducted by Bi et al. (2015) showed that the surface concentration of phthalates on window glass is higher than the surface concentration of phthalates on plates and mirrors, though the physical properties of these surfaces are very similar. The surface concentration difference is explained by considering the organic film development on indoor surfaces, and has been found in previous studies (Liu et al., 2003; Butt et al., 2004; Diamond et al., 2000). A very recent study (Wallace et al., 2017) explicitly measured organic mass accumulation on Petri dishes or foils, further demonstrating the need to use multilayer sorption models. In such cases, the Langmuir isotherm with monolayer assumption may no longer be tenable. The Freundlich isotherm, thus may explain the growth of organic films on impervious urban surfaces (Wu et al., 2008). Therefore, there is an urgent need to compare the two isotherms and their applications in explaining partition effect of SVOCs under different indoor scenarios.
The aim of this study is to measure and characterize OPFR sorption dynamics on indoor impervious surfaces (e.g. stainless steel, glass and acrylic). The specific goals are as follows: 1) measure the sorption behavior of OPFRs on electro polished stainless steel chamber surfaces using a small chamber method and on Silicosteel® coated stainless steel using a micro chamber method; 2) compare simplified Langmuir isotherm and Freundlich isotherm in characterizing the mechanisms governing the sorption effect; 3) obtain sorption parameters for OPFRs from chamber tests; and 4) demonstrate the applicability of the obtained parameters in an OPFR emission small chamber test.
2 Materials and Methods
2.1 Chemicals
The chemicals, including liquid TCEP and TCPP, for chamber tests were obtained from ICL-IP America Inc. (Gallipolis Ferry, WV, USA). Certified TCEP and TCPP calibration standards were purchased from AccuStandard Inc. (New Haven, CT, USA). An isotopically-labeled compound, tributyl phosphate-d27 (99.5% purity, Cambridge Isotope Laboratories, Inc., Andover, MA) was used as the internal standard on the GC/MS system. Triphenyl phosphate-d15 (98% purity, Sigma Aldrich, St. Louis, MO, USA) was used as extraction recovery check standard (RCS). Chromatography-grade methylene chloride (Burdick and Jackson, Muskegon, MI, USA) and ethyl acetate (OmniSoly, Billerica, MA, USA) were used as solvents in extraction and cleaning without further purification. The solvents were regularly analyzed to monitor potential contamination with OPFRs.
2.2 Empty Small Chamber Sink Test
The test was conducted using two 53-L electro polished stainless steel chambers, one as the source chamber and the other as the empty sink test chamber (Fig. 1a and 1b). Both chambers were placed in a temperature-controlled incubator (Model SCN4-52, Environmental Equipment Co., Inc., Cincinnati, OH, USA) during the tests. The chambers were designed and constructed according to ASTM guide D5116-10 (ASTM, 2010). Each chamber includes a 5-cm diameter computer cooling fan (Model EC4020, Evercool Thermal Corp., Ltd., Taiwan) to provide air mixing in the chamber space. Measurements were conducted at 23 °C, 50% relative humidity (RH), and 1h−1 air change rate (ACH). More details of chamber setting and test procedure can be found in previous studies (Liu et al., 2014 and 2016).
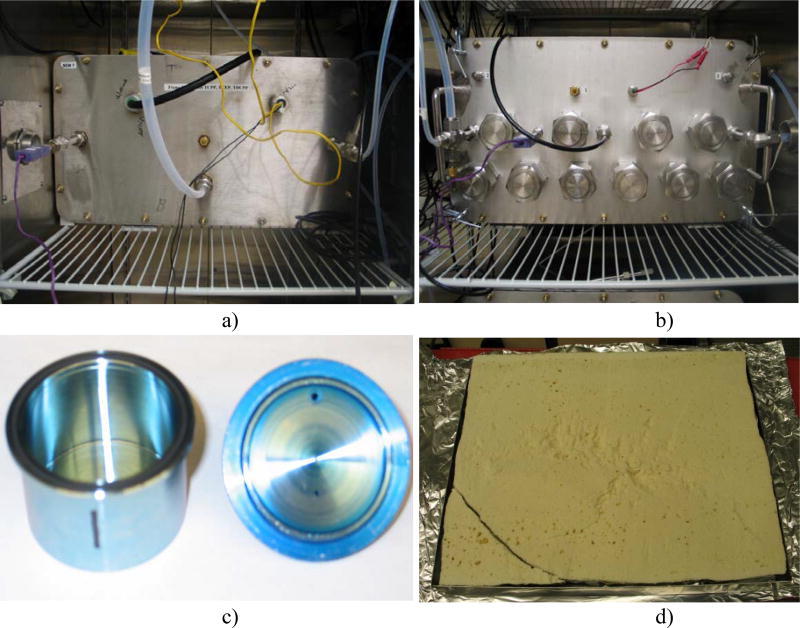
Fig. 1.
a) Source generation chamber; b) empty sink chamber; c) empty micro chamber with lid removed to show interior; and d) material for small chamber emission test.
The empty small chamber test was conducted by exposing the empty chamber to the OPFR gas-phase sources (detailed information on the OPFR source chamber setup can be found in Liu et al. (2016)). Prior to the tests, the empty chamber air background samples were collected and analyzed to check whether for the presence of contamination. Then, the empty chamber was dosed with OPFRs from the source chamber for approximately 809 hours, after which the chemical source air was disconnected, and the chamber was dosed with clean air for approximately 10261 hours until the gas-phase OPFRs became stable. Polyurethane foam (PUF) (small pre-cleaned certified, Supelco, St. Louis, MO, USA) samples were collected at the inlet and the faceplate of the empty chamber to monitor the gas-phase concentrations of OPFRs.
After completion of the empty chamber measurements, the chamber was removed from the incubator and placed, opened, in an exhaust hood at test elapse time 11206 hours. A series of wipe samples were then collected twice from the exposed surfaces in the chamber including all chamber wall surfaces and RH probe, thermocouple, inlet and exhaust manifolds that are used inside the chamber. The fan and its wires inside the chamber were not wiped because the toluene solvent used for wiping the stainless steel surface would likely break down the plastic material from which the fan was made leading to potential analytical interferences. The wipe sampling procedure followed the method outlined in ASTM D6661-10 (ASTM, 2006). In addition, the inlet and exhaust manifolds were rinsed with toluene once.
2.3 Empty Micro Chamber Sink Tests
Similar empty chamber measurements were also conducted in two micro chambers (Markes International, Llantrisant, UK) for OPFRs using the same source chamber in the small chamber tests as OPFR sources (Fig. 1a). As demonstrated in Fig. 1c, each micro chamber consists of an open-ended cylinder (cup) constructed of Silicosteel® coated stainless steel measuring 25 mm deep with a diameter of 45 mm and a volume of 44 mL.
Two empty micro chambers were connected in series to the source chamber with constant gas-phase OPFRs. The flow rate through each micro chamber was maintained at 195 mL/min, which resulted in an ACH of 266 h−1. Measurements were conducted under conditions of 23 °C, 50% RH. The empty micro chambers were dosed with OPFRs for 1300 hours to allow the gas-phase OPFRs to reach steady state. Thereafter, the source was disconnected and the chambers were flushed with clean air for 200 hours until the gas-phase OPFRs became stable. PUF samples were collected at the outlet of each chamber to monitor the gas-phase OPFR concentration change during the test period.
2.4 Small Chamber Emission Test
The test was conducted in a single 53-L electro polished stainless steel chamber in the temperature-controlled incubator at 23 °C, 50% RH and 1h−1 ACH. The chamber included a 5-cm diameter computer cooling fan to provide air mixing in the chamber space. Prior to the test, the empty chamber air background samples were collected and analyzed. Then, the chamber was opened and the tray with closed cell polyurethane foam (dimensions: 44.5 cm × 37.1 cm × 5.08 cm, density: 1.80 × 104 g/m3, Fig. 1d) as the TCPP source material was placed at the bottom of the chamber. The emission test lasted for approximately 1425 hours, at which we made sure it had reached the steady state. PUF samples were collected at the faceplate of the chamber to monitor the gas-phase concentrations of OPFRs.
2.5 Air Velocity Measurements
A Kanomax Climomaster handheld omni-directional heated sphere anemometer (Model A543, Kanomax USA, Inc) was used to measure the air velocities adjacent to chamber surfaces in the 53-L small chamber and the 44-mL micro chamber. To allow the use of the anemometer, we made clear plastic faceplate and lid for the small chamber and the micro chamber, respectively. The faceplate was manufactured with multiple holes so that the anemometer probe can be inserted into the chambers at different locations. There is only one hole on the lid for the micro chamber due to its small size. The air velocities at different chamber locations were measured with the chambers being set at the same test conditions as in the sorption tests. Setup for measuring air velocities near the chamber surfaces in both chambers is shown in Fig. S1 in SI.
2.6 Sample Extraction and Analysis
After sample collection, each PUF cartridge was capped in a glass holder, wrapped in aluminum foil, placed in a sealable plastic bag, and stored in the refrigerator at 4 °C until analysis. PUF samples were placed in individual 40-mL borosilicate glass amber I-Chem™ vials (Thermo Scientific, Waltham, MA, USA) with approximately 35 mL 1:1 methylene chloride/ethyl acetate MeCl2/EtOAC) and 50 µL of 5-µg/mL recovery check standard and extracted horizontally on the Multipurpose Lab Rotator (Barnstead International Model 2346, Thermo Scientific, Waltham, MA, USA) for 1 h. The extract was filtered through anhydrous sodium sulfate and further concentrated to approximately 1.5 mL using the RapidVap N2 Evaporation System (Model 791000, LabConco, Kansas City, MO, USA). The wipe samples from the empty small chamber sink test were extracted with 10 mL 1:1 methylene chloride/ethyl acetate (Fisher Scientific, Pittsburgh, PA, USA), 50 µL of 20 µg/mL extraction recovery check standard, d15-triphenyl phosphate (d15-TPP, Sigma-Aldrich, St. Louis, MO, USA). The extraction was performed using a sonicator (Ultrasonic Cleaner FS30, Fisher Scientific, Pittsburgh, PA, USA) for 30 min in a scintillation vial. The concentrated extract was then transferred to a 5-mL volumetric flask and brought up to volume with rinse solution from the concentration tube for GC/MS analysis followed the method described in Liu et al. (2016) and Table S1 in SI.
2.7 Quality Assurance and Control
Quality assurance project plans (QAPPs) were prepared and approved prior to measurements. The GC/MS calibration was verified by the internal audit program. Each batch of samples was analyzed along with its corresponding quality control samples. Extraction method blank and field blank samples were prepared and analyzed as well. All samples were extracted and analyzed with the criteria that the percentage recovery of the recovery check standards had to be within 100 ± 25%, and that the precision of the duplicate samples had to be within ± 25%. When the measured concentrations of OPFRs in the sample were above the highest calibration level, the extract was diluted and reanalyzed.
2.8 Dynamic Sink Model
The accumulation of gaseous OPFR in chamber obeys the law of mass conservation as described by
| (4) |
where y (µg/m3) is the gas-phase OPFR concentration in the chamber, V (m3) is the volume of the chamber, Q (m3/h) is the ventilation rate, yin (µg/m3) is the inlet gas-phase OPFR concentration, As (m2) is the area of surfaces inside the chamber, and Ss (µg/h) is the emission rate of OPFR source in the chamber. For the simulation of empty chamber tests, no emission sources are present in the chamber, in which case Ss in Equation 4 is equal to zero.
Assuming a boundary layer exists adjacent to the sorption surfaces (chamber surfaces), the amount of OPFRs accumulated on the surface is equal to the total mass transferred through the boundary layer from the gas phase, or
| (5) |
where hm (m/h) is the mass transfer coefficient through the boundary layer immediately adjacent to chamber surfaces and Ce (µg/m3) is the gas-phase OPFR concentration in the boundary layer. The values of hm in this study are calculated based on measured air velocities across sorption surfaces in each chamber using PARAMS (Guo, 2005). The relationship between the surface-phase concentration on the chamber surface, qe, and the gas-phase concentration adjacent to the chamber surface, Ce, follows either the previously mentioned simplified Langmuir isotherm (Equation 2) or Freundlich isotherm (Equation 3). Combining either Equation with Equations 4 and 5, we are able to solve the gas-phase OPFR concentration (y) in the empty chamber. With the measured data for y in the empty chamber, we can obtain KL or KF and n by fitting the data. In this study, we used MATLAB from MathWorks Inc. (Natick, MA, USA) to fit the data.
3 Results and Discussion
3.1 OPFRs in the Empty Small Chamber
The chamber test was conducted for approximately 11070 hours (416 days) to study the sorption mechanisms of OPFRs in a stainless steel small chamber. The OPFR concentrations in the chamber air are shown in Fig. 2a and 2b. At the end of the test (test elapse time 11206 hours), we opened the chamber and collected wipe samples on each surface to measure the surface-phase OPFR concentrations (Table S2).
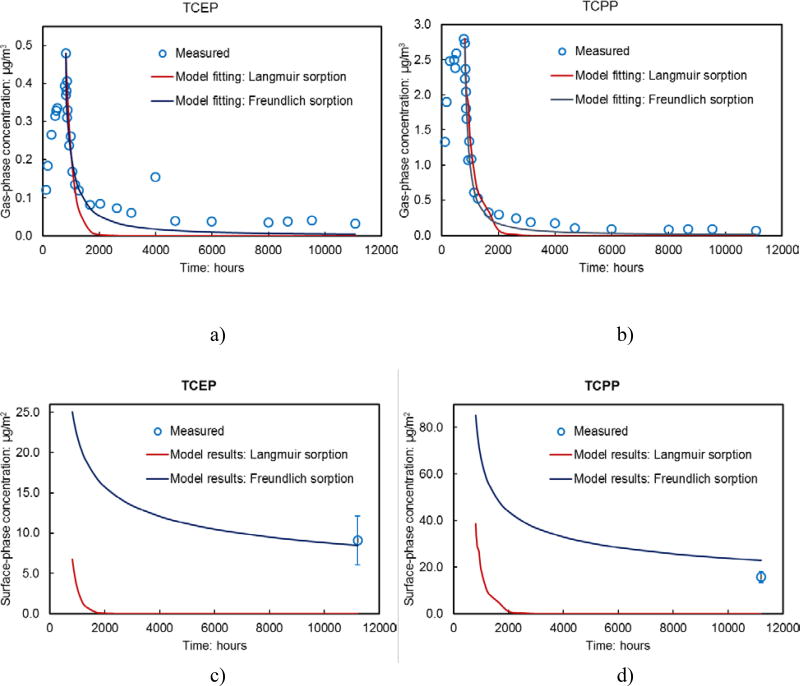
Fig. 2.
OPFR concentrations measured in the empty stainless steel small chamber with model fittings. a) Gas-phase TCEP concentrations, b) Gas-phase TCPP concentrations, c) Model predicted chamber wall TCEP concentrations over time and the wipe test results at 11206 hours, and d) Model predicted chamber wall TCPP concentrations over time and the wipe test results at 11206 hours.
The dynamic sink model (Equation 4) using Langmuir and Freundlich isotherms was used to fit the experimental data (Parameters are in Table 1). We fit the gas-phase OPFR concentrations measured after the source was disconnected from the chamber (Fig. 2a and 2b). The reasons are that there were limited data points during the dosing time (0 – 809 h) and that long desorption period (809–11070 h) will help to better understand differences of the two isotherms. Although the Langmuir and Freundlich isotherms behave similarly in fitting the gas-phase OPFR concentrations in the chamber, the advantage of the Freundlich isotherm was very pronounced when we compared the calculated surface-phase TCEP and TCPP concentrations with measured results (Fig. 2c and 2d). In examining the decay of gas-phase TCEP and TCPP concentrations, we found that the concentration declined drastically (~ 90 % for both compounds) within 1200 h (809 – 2000 h) and remained at a low level with small change for the rest of the test. The study of the development of organic films on impervious surfaces by others (Diamond et al., 2000; Liu et al., 2003; Bi et al., 2015), suggested that organic films formed on the surfaces very quickly at first with a much slower process thereafter. With the assumption that the sorption process is reversible, the decomposition of organic films may occur very quickly at first (a slow development rate indicates a fast decomposition rate) followed by a slower decay process. The OPFRs decay in the small chamber shown in Figs 2a and 2b agreed with the observations from others. One possible explanation of the organic film formation/decomposition is that an impervious surface has a stronger affinity for gaseous SVOCs than the organic films developed upon it. Another explanation is that the difference between the surface phase and gas phase concentrations serves as one of the key factors for the growth/decay of the organic film in the chamber. In addition, it is unknown if the sorption process on the chamber surfaces is the same as the development of organic films in different indoor environments. Therefore, further research is needed to investigate the relationship between the sorption process and the development of organic films.
Table 1.
TCEP and TCPP model parameters in the empty small chamber test.
| Parameters at 23 °C | TCEP | TCPP | |
|---|---|---|---|
| Chamber volume (V)a | m3 | 0.053 | |
| Air change rate(ACH)a | h−1 | 1.01 | |
| Interior chamber surface area (As)a | m2 | 0.88 | |
| Average air velocity in chambera | m/s | 0.17 | |
|
| |||
| Inlet gas phase concentrationa | µg/m3 | 2.13 | 7.88 |
| Mass transfer coefficient (hm)b | m/h | 1.98 | 1.82 |
| Langmuir constant (KL)c | m | 14.1 | 13.8 |
| R2 (Langmuir isotherm)d | 0.97 | 0.97 | |
| Freundlich constant (KF)c | m3n−2×µg1−n | 31.6 | 67.1 |
| Freundlich exponential (n)c | 0.24 | 0.26 | |
| R2 (Freundlich isotherm)d | 0.97 | 0.99 | |
Measured in test.
Determined using PARAMS (Guo, 2005). The average air velocity across the interior chamber surfaces was measured as 0.17 m/s. Parameters used for the calculation are listed in Table S4 in SI.
Obtained from model fitting.
R2 is the correlation coefficient, which represents the percentage of variability in the dependent variable.
The mass balance analysis was performed to investigate the significance of the sorption of OPFRs on the chamber surfaces. (Table 2). As shown in Table 2, the adsorbed OPFR mass on the chamber surfaces is dominant comparing to the remaining gas-phase OPFR mass in the chamber at the end of the test. The Langmuir equation indicates that a low gas-phase concentration corresponded a low surface-phase concentration. In contrast, the fitted values of n in the Freundlich equation for TCEP and TCPP were 0.24 and 0.26, respectively, which suggested that the surface-phase concentration could potentially stay relatively high, although the gas-phase concentration was low. For future tests on measuring the sorption kinetics of SVOCs, in addition to measuring the gas-phase concentrations of target compounds, it is necessary to also measure the chamber surface-phase concentrations to determine the appropriate surface/gas partition relationship. The mass balance results explicitly show that the OPFRs adsorbed on all surfaces (including chamber walls, PH probe, thermocouple and inlet and outlet manifolds that made of stainless steel with holes) is considerably larger than that on the chamber wall, which demonstrates the significance of other surface sorption in this test. Notably, the percentages of total mass recovered to the dosed mass are 65.36% and 63.64% for TCEP and TCPP, respectively (Table 2). The difference may due to the loss on the fan and its wires, during wipe and rinse sampling, different time between gas phase sampling and wipe/rinse sampling and analysis process. Data in Table 2 demonstrates that using the small chamber for SVOC emission measurement will underestimate their emission rates and cause significant uncertainty if the sink effect is not taken into account. Measures that may help reduce the sink effect during the emissions testing include using smaller chambers and constructing the chamber with materials that are weak or no sinks for SVOCs.
Table 2.
Mass balance of TCEP and TCPP in the empty small chamber test.
| TCEP | TCPP | ||
|---|---|---|---|
| Mass doseda | µg | 92.24 | 341.25 |
| Mass exhaustedb | µg | 43.08 | 188.93 |
| Mass remained in chamberc | µg | 0.0022 | 0.0045 |
| Mass adsorbed on all surfacesd | µg | 14.02 | 22.14 |
| Mass adsorbed on chamber surfacese | µg | 7.99 | 13.97 |
| Total mass recoveredf | µg | 60.29 | 217.16 |
| Recovery ratiog | µg | 65.36% | 63.64% |
Dosed OPFR to the empty chamber from the source chamber during the dosing period of the test. Mass dosed = Inlet concentration × Dosing time (809 h).
Exhausted OPFR through ventilation during the whole test. Mass exhausted is the integration of measured gas-phase OPFR concentration over time during the whole test (0 – 11070 h)
Gas-phase OPFR remained in the chamber at the end of the test (t = 11070 h).
Adsorbed OPFR on all surfaces (including chamber wall, RH probe, thermocouple and etc.) when the chamber was opened (t = 11206 h). Note that the wipe test can only be conducted when the chamber is open and therefore the surface-phase OPFR concentration was measured later than the last gas-phase concentration was measured.
Adsorbed OPFR on chamber surfaces only.
Mass recovered = Mass exhausted + Mass remained in chamber + Mass adsorbed on all surfaces.
Recovery ratio = Mass recovered/Mass dosed.
It is noted that the small chamber does not have advantages for SVOC emission tests because of the significant sink effect. We conducted this research for two reasons: (1) to better understand the chamber sink effect and find ways to minimize it for the dual chamber system used for sorption tests of consumer products and building materials (Liu et al., 2014 and 2016); (2) to help researchers who use chamber test data and mass transfer parameters for indoor SVOC source emission and fate and transport modeling, given that stainless steel is the most widely used and convenient type of materials for configuring test chambers.
3.2 OPFRs in Empty Micro Chambers
The empty micro chamber tests were conducted in duplicate with identical conditions (i.e., temperature, ACH, inlet gas-phase TCEP and TCPP concentrations, etc.). The gas-phase TCEP and TCPP concentrations measured in the micro chambers are shown in Fig. 3. The gas-phase TCEP and TCPP concentrations in the micro chambers reached steady state in approximately 300 h and 100 h, respectively. Models were used to fit the data in two chambers and it was found that both the Langmuir and Freundlich isotherms provided good fitting results (R2 > 0.9) with the experimental data (Fig. 3 and Table 3). The comparison of the micro chamber and the small chamber tests, however, does not necessarily contradict the conclusion drawn from the empty small chamber test that Freundlich isotherm was better in describing sorption kinetics of OPFRs on stainless steel chamber surfaces. One possible reason is that the measured desorption period in the micro chamber is much shorter than that in the small chamber. As a result, we were unable to determine whether or not the Freundlich isotherm was superior in describing surface/gas partition for OPFRs in the micro chambers.
Fig. 3.
Gas-phase OPFR concentrations measured in micro chambers with model fitting. a) TCEP in chamber 1, b) TCPP in chamber 1, c) TCEP in chamber 2, and d) TCPP in chamber 2.
Table 3.
TCEP and TCPP parameters in empty micro chamber testsa
| Parameters at 23 °C | TCEP | TCPP | |
|---|---|---|---|
| Chamber volume (V)b | mL | 44.0 | |
| Ventilation rate (Q)b | mL/min | 195.0 | |
| Air change rate (ACH)b | h−1 | 265.9 | |
| Interior chamber surface area (As)b | m2 | 0.020 | |
| Average air velocity in chamberb | m/s | 0.09 | |
| Inlet gas phase concentrationb | µg/m3 | 1.02 | 5.49 |
| Mass transfer coefficient (hm)c | m/h | 4.92 | 4.55 |
|
| |||
| Langmuir isotherm constant (KL, µC 1)d | m | 25.7 | 6.02 |
| R2 (Langmuir isotherm, Test 1)d | 0.94 | 0.95 | |
|
| |||
| Langmuir isotherm constant (KL, µC 2)d | m | 33.1 | 6.38 |
| R2 (Langmuir isotherm, Test 2)d | 0.91 | 0.90 | |
|
| |||
| Freundlich isotherm constant (KF, µC 1)d | m3n−2×µg1−n | 36.5 | 43.5 |
| Freundlich isotherm exponential (n, µC 1)d | 0.30 | 0.28 | |
| R2 (Freundlich isotherm, Test 1)d | 0.93 | 0.94 | |
|
| |||
| Freundlich isotherm constant (KF, µC 2)d | m3n−2×µg1−n | 39.5 | 36.5 |
| Freundlich isotherm exponential (n, µC 2)d | 0.29 | 0.28 | |
| R2 (Freundlich isotherm, µC 2)d | 0.93 | 0.91 | |
Parameters were obtained by fitting experimental results of Chamber 1 and Chamber 2.
Measured in test.
Determined using program PARAMS (Guo, 2005). The average air velocity across the interior chamber surfaces was measured as 0.09 m/s. Parameters used for the calculation are listed in Table S4 in SI.
Obtained from model results.
The simplified Langmuir isotherm failed to predict well-agreed surface-phase OPFR concentrations with test results from the small chamber. Interestingly, when the Freundlich exponentials in the small chamber and those in the micro chambers were compared, they were very close for both TCEP and TCPP: n = 0.24 (small chamber) vs. n = 0.30 and 0.29 (micro chamber test 1 and 2), and n = 0.26 (small chamber) vs. n = 0.28 and 0.28 (micro chamber test 1 and 2), respectively. This implied the sorption of OPFRs on both chamber surfaces was close to each other. It is worth noting that the interior surfaces of the micro chambers are Silicosteel® coated stainless steel, which are different from the electro-polished surfaces of the small chamber. A very recent study (Wu et al., 2017) shows that the surface roughness plays an important factor in the sorption process. However, though the interior surfaces of the micro chambers differ from that of the small chamber, they are both polished and have a roughness factor (true surface area/area footprint) close to 1 (Wu et al., 2017). For monolayer sorption, the contact was only between the gaseous molecules and the impervious surface, whereas for multilayer sorption, the contact was between the gas molecules themselves after the organic film was formed on the impervious surface. The physical properties of the impervious surface may influence only the initial sorption of a chemical. However, further research is still needed to verify whether the conclusion can be generalized to other categories of SVOCs.
3.3 OPFR in Emission the Small Chamber
To validate the parameters obtained from the previous empty small chamber test, a small chamber study with an aged spray polyurethane foam as a source was conducted. Using the Langmuir and Freundlich parameters obtained from the empty chamber studies for the sink effect (Table 4), we were able to simulate the emission process of TCPP in the chamber. During the small chamber emission test, the chamber was ventilated with clean air, and the gas-phase TCPP was emitted from the aged spray polyurethane foam. The governing equation for the small chamber emission test (yin = 0) based on Equation 4 is
| (6) |
Table 4.
Model input parameters for TCPP in small chamber foam emission test.
| Parameters at 23 °C | ||
|---|---|---|
| Chamber volume (V)a | m3 | 0.053 |
| Air change rate(ACH)a | h−1 | 1.01 |
| Interior chamber surface area (As)a | m2 | 0.88 |
| Average air velocity in chambera | m/s | 0.17 |
| Mass transfer coefficient on chamber surfaces (hm)b | m/h | 1.824 |
| Emission surface area (A0)a | m2 | 0.165 |
| Air velocity above emission surfacea | m/s | 0.25 |
| Mass transfer coefficient on emission surface (hm0)b | m/h | 2.210 |
| TCPP concentration in the foam (C0)a | µg/m3 | 2.73×109 |
| Material (foam)/air partition coefficient (K)c | dimensionless | 5.27×107 |
| Gas-phase TCPP concentration near emission surface (y0)c | µg/m3 | 51.9 |
| Langmuir constant (KL)d | m | 13.8 |
| Freundlich constant (KF)d | m3n− 2×µg1−n | 67.1 |
| Freundlich exponential (n)d | 0.26 |
Measured in test.
Determined using program PARAMS (Guo, 2005). The average air velocity across the interior chamber surfaces and emission surface are 0.17 m/s and 0.25 m/s, respectively.
Calculated using Method P24 in Guo (2002). The vapor pressure of TCPP is 1.05×10−5 mm Hg (Liu et al., 2016), y0 = C0/K.
Obtained from model fitting results in empty chamber measurements in Table 2.
For the source emission, Ss, it is assumed that the gas-phase TCPP concentration in the layer immediately adjacent to the foam material is linearly related to its material phase concentration and remained constant throughout the test and if we let y0 (µg/m3), hm0 (m/h), and A0 (m2) be the TCPP concentration above the source surface, the mass transfer coefficient, and the emission area, respectively, Ss in Equation 6 can be further described by
| (7) |
In Equation 7, y0 was calculated based on the linear partition relationship between the gas-phase and material-phase concentrations:
| (8) |
where C0 (µg/m3) is the material-phase TCPP concentration and K is the material/gas partition coefficient, which can be determined by the following equation (Guo, 2002):
| (9) |
where P (mm Hg) is the vapor pressure of TCPP.
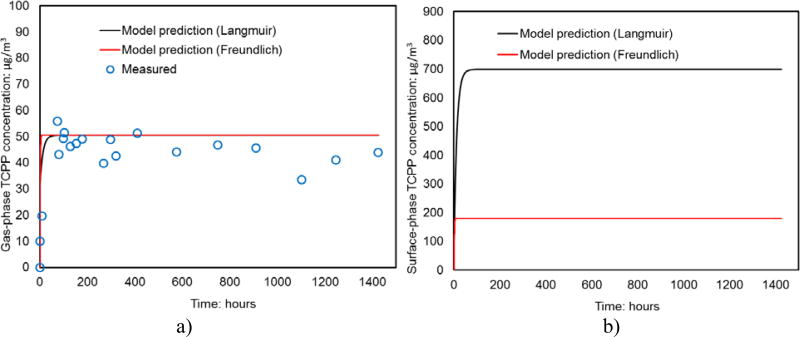
As shown in Fig. 4, both Langmuir and Freundlich isotherms provided very close predictions of the gas-phase TCPP concentrations in the chamber. However, the surface-phase TCPP concentrations predicted by Langmuir isotherm are over three times higher than that by Freundlich isotherm in the emission test (Fig. 4 b). Unfortunately, there is a lack of experimental data on surface-phase TCPP concentration in this test to further verify if Freundlich isotherm can better represent the sorption dynamics. It is important to know from the model results that cautions should be taken when predicting surface-phase SVOC concentrations on indoor impervious models using Langmuir, Freundlich or other models in future studies. Direct surface-phase concentration measurements can be necessary to further verify the sorption model.
Fig. 4.
a) Gas-phase TCPP concentrations measured in the foam emission test in the small chamber with Langmuir and Freundlich sorption model predictions; b) Langmuir and Freundlich sorption model predictions of surface-phase TCPP concentrations in the small chamber.
3.4 Limitations of the Present Study
Although the tests in this study have provided some important parameters for characterizing OPFR sorption dynamics for the first time in the literature, some limitations of the present study should be considered. First, the Langmuir and Freundlich parameters in the empty small chamber test were obtained by fitting the gas-phase OPFR concentrations only in the desorption period. Theoretically, fitting Langmuir and/or Freudlich parameters from sorption or desorption or both should yield same results given that both isotherms are sorption/desorption reversible; therefore, a change of dosing time will not affect the fitting results. However, neither model could fit perfectly the measured data in the empty small chamber method. Some attempts were made to propose new models that involve variable sorption parameters at different stages of the sorption process, which calls for future research. Second, the chamber tests have been applied to characterize sorption kinetics of TCEP and TCPP on stainless steel surfaces; more research is needed to study the sorption for other types of SVOCs on various other indoor surfaces. Third, the models used for the small chamber tests did not differentiate surfaces inside the chamber (i.e. chamber walls, thermocouple, RH probe, fan, etc.), which needs to be improved in future research. Fourth, Equation 9 used in the emission test is a rough estimation of material/air partition coefficients for all materials and thus there is uncertainty associated with the vapor pressure of TCPP reported in the literature (Liu et al., 2016), which may result in uncertainty in obtaining the K value. In addition, more accurate experimental methods are needed for measuring material/air partition coefficients. Fifth, the Langmuir isotherm used in the dynamic sink model is simplified. Additional isotherms including the comprehensive Langmuir and the Redlich-Peterson isotherms should be investigated in the future research to further understand the sorption phenomena.
4 Conclusions
In this study, we conducted the first tests to characterize OPFR sorption on stainless steel surfaces in both small chambers and micro chambers. Test results showed that sorption of OPFR by the 44-mL micro chamber was insignificant compared to the 53-L small chamber. In analyzing the experimental results, we found that the Freundlich isotherm can better characterize the sorption kinetics of TCEP and TCPP in the chambers. This paper reported the first hand data of the Freundlich isotherm parameters for OPFRs on stainless steel surfaces. The surface-phase TCEP and TCPP concentrations predicted by the Freundlich isotherm and the comparison with measured data in the empty chamber further supported the organic film theory that the organic films formed on surfaces quickly at first followed by a much slower process. More experimental data are needed for further investigation of the organic film development on indoor surfaces. Therefore, caution should be taken when these parameters are applied to predict OPFR concentrations in real-world situations.
Supplementary Material
Acknowledgments
We thank Edgar Folk IV from Jacobs Technology Inc. for helping with the GC/MS instrument operation and the small chamber tests, Mark Barnes from U.S. EPA for preparing the foam materials, and former employee of U.S. EPA, Dr. Zhishi Guo, for his insightful comments.
Footnotes
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. EPA. Mention of trade names or commercial products does not constitute endorsement or recommendation for use by the U.S. EPA.
References
- Andersen JA, Grundmann A, Bester K. Organophosphorus flame retardants and plasticisers in surface water. Sci. Total. Environ. 2007;332:155–166. doi: 10.1016/j.scitotenv.2004.04.021. [DOI] [PubMed] [Google Scholar]
- ASTM. D6661-01 Field collection of organic compounds from surfaces using wipe sampling. ASTM International; West Conshohocken, PA: 2006. [Google Scholar]
- ASTM. D5116-10 Standard guide for small-scale environmental chamber determinations of organic emissions from indoor materials/products. ASTM International; West Conshohocken, PA: 2010. [Google Scholar]
- Bradman A, Castorina R, Gaspar F, Nishioka M, Colón M, Weathers W, Egeghy PP, Maddalena R, Williams J, Jenkinse PL, McKonea TE. Flame retardant exposures in California early childhood education environments. Chemosphere. 2014;116:61–66. doi: 10.1016/j.chemosphere.2014.02.072. [DOI] [PubMed] [Google Scholar]
- Bergh C, Torgrip R, Emenius G, Östman C. Organophosphate and phthalate esters in air and settled dust-a multi-location indoor study. Indoor Air. 2011;21:67–76. doi: 10.1111/j.1600-0668.2010.00684.x. [DOI] [PubMed] [Google Scholar]
- Brommer S, Harrad S, den Eede NV, Covaci A. Concentrations of organophosphate esters and brominated flame retardants in German indoor dust samples. J. Environ. Monit. 2012;14:2482–2487. doi: 10.1039/c2em30303e. [DOI] [PubMed] [Google Scholar]
- Brandsma SH, de Boer J, van Velzen MJM, Leonards PEG. Organophosphorus flame retardants (PFRs) and plasticizers in house and car dust and the influence of electronic equipment. Chemosphere. 2014;116:3–9. doi: 10.1016/j.chemosphere.2014.02.036. [DOI] [PubMed] [Google Scholar]
- Barnes KK, Kolpin DW, Furlong ET, Zhang SD, Meyer MT, Barber LB. A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States – 1) groundwater. Sci. Total Environ. 2008;402:192–200. doi: 10.1016/j.scitotenv.2008.04.028. [DOI] [PubMed] [Google Scholar]
- Brunauer S, Emmett PH, Teller E. Adsorption of gases in multimolecular layers. J Amer. Chem. Soc. 1938;60:309–319. [Google Scholar]
- Bi C, Liang Y, Xu Y. Fate and transport of phthalates in indoor environments and the influence of temperature: A case study in a test house. Environ. Sci. Technol. 2015;49:9674–9681. doi: 10.1021/acs.est.5b02787. [DOI] [PubMed] [Google Scholar]
- Butt CM, Diamond ML, Truong J, Ikonomou MG, ter Schure AFH. Spatial distribution of polybrominated diphenyl ethers in southern Ontario as measured in indoor and outdoor window organic films. Environ. Sci. Technol. 2004;38:724–731. doi: 10.1021/es034670r. [DOI] [PubMed] [Google Scholar]
- Cristale J, Katsoyiannis A, Sweetman AJ, Jones KC, Lacorte S. Occurrence and risk assessment of organophosphorus and brominated flame retardants in the River Aire (UK) Environ. Pollut. 2013;179:194–200. doi: 10.1016/j.envpol.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Dodson RE, Perovich LJ, Covaci A, den Eede NV, Ionas AC, Dirtu AC, Brody JG, Rudel RA. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ. Sci. Technol. 2012;46:13056–13066. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ML, Gingrich SE, Fertuck K, McCarry BE, Stern GA, Billeck B, Grift B, Brooker D, Yager TD. Evidence for organic film on an impervious urban surface: Characterization and potential teratogenic effects. Environ. Sci. Technol. 2000;34:2900–2908. [Google Scholar]
- Foo KY, Hameed BH. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010;156:2–10. [Google Scholar]
- Freundlich HMF. Over the adsorption in solution. J. Phys. Chem. 1906;57:385–471. [Google Scholar]
- Guo Z. Program PARAMS users guide. U.S. Environmental Protection Agency; Washington, DC: 2005. EPA/600/R-05/066 (NTIS PB2006-102000) [Google Scholar]
- Guo Z. Review of indoor emission source models. Part 2. Parameter estimation. Environ. Pollut. 2002;120:551–564. doi: 10.1016/s0269-7491(02)00188-4. [DOI] [PubMed] [Google Scholar]
- Hartmann PC, Bürgi D, Gier W. Organophosphate flame retardants and plasticizers in indoor air. Chemosphere. 2004;57:781–787. doi: 10.1016/j.chemosphere.2004.08.051. [DOI] [PubMed] [Google Scholar]
- Hyland KC, Blaine AC, Dickenson ERV, Higgins CP. Accumulation of contaminants of emerging concern in food crops, part one: Edible strawberries and lettuce grown in reclaimed water. Environ. Toxicol. Chem. 2015;34:2213–2221. doi: 10.1002/etc.3066. [DOI] [PubMed] [Google Scholar]
- Ho YS, Porter JF, McKay G. Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: Copper, nickel and lead single component systems. Water Air Soil Pollut. 2002;141:1–33. [Google Scholar]
- Koble RA, Corrigan TE. Adsorption isotherms for pure hydrocabrons. J. Ind. Eng. Chem. 1952;44:383–387. [Google Scholar]
- Langmuir I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Amer. Chem. Soc. 1916;38:2221–2295. [Google Scholar]
- Liang Y, Xu Y. Improved method for measuring and characterizing phthalate emissions from building materials and its application to exposure assessment. Environ. Sci. Technol. 2014;48:4475–4484. doi: 10.1021/es405809r. [DOI] [PubMed] [Google Scholar]
- Little JC, Weschler CJ, Nazaroff WW, Liu Z, Cohen Hubal EA. Rapid methods to estimate potential exposure to semivolatile organic compounds in the indoor environment. Environ. Sci. Technol. 2012;46:11171–8. doi: 10.1021/es301088a. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhang Y, Benning JL, Little JC. The effect of ventilation on indoor exposure to semivolatile organic compounds. Indoor Air. 2015;25:285–296. doi: 10.1111/ina.12139. [DOI] [PubMed] [Google Scholar]
- Liu QT, Chen R, McCarry BE, Diamond ML, Bahavar B. Characterization of polar organic compounds in the organic film on indoor and outdoor glass windows. Environ. Sci. Technol. 2003;37:2340–2349. doi: 10.1021/es020848i. [DOI] [PubMed] [Google Scholar]
- Liu X, Allen MR, Roache NF. Characterization of organophosphorus flame retardants’ sorption on building materials and consumer products. Atmos. Environ. 2016;140:333–341. [Google Scholar]
- Liu X, Roache NF, Allen MR. Development of a small chamber method for SVOC sink effect study. The 13th International Conference on Indoor Air Quality and Climate; Hong Kong; China. July 07 – 12, 2014. [Google Scholar]
- Nazaroff WW, Alvarez-Cohen L. Environmental Engineering Science. John Wiley and Sons, Inc; 2001. [Google Scholar]
- Redlich O, Peterson DL. A useful adsorption isotherm. J. Phys. Chem. 1959;63:1024–1024. [Google Scholar]
- Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ. Sci. Technol. 2009;43:7490–5. doi: 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staaf T, Östman C. Organophosphate triesters in indoor environments. J. Environ. Monit. 2005;7:883–887. doi: 10.1039/b506631j. [DOI] [PubMed] [Google Scholar]
- Sundkvist AM, Olofsson U, Haglind P. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J. Environ. Monit. 2010;12:943–951. doi: 10.1039/b921910b. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A. Identification of flame retardants in polyurethane foam collected from baby products. Environ. Sci. Technol. 2011;45:5323–5331. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sips R. On the structure of a catalyst surface. J. Chem. Phys. 1948;16:490–495. [Google Scholar]
- U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry. [accessed 10.24.17];Toxicology profile for phosphate ester flame retardants. 2012 Sep; http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=1119&tid=239. [PubMed]
- Van der Veen I, De Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88:1119–1153. doi: 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- Van den Eede N, Dirtu AC, Neels H, Covaci A. Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ. Int. 2011;37:454–61. doi: 10.1016/j.envint.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Wallace LA, Ott WR, Weschler CJ, Lai AC. Desorption of SVOCs from heated surfaces in the form of ultrafine particles. Environ. Sci. Technol. 2017;51:1140–1146. doi: 10.1021/acs.est.6b03248. [DOI] [PubMed] [Google Scholar]
- Wei G, Li D, Zhuo M, Liao Y, Xie Z, Guo T, Li J, Zhang S, Liang Z. Organophosphorus flame retardants and plasticizers: sources, occurrence, toxicity and human exposure. Environ. Pollut. 2015;196:29–46. doi: 10.1016/j.envpol.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Wensing M, Uhde E, Salthammer T. Plastics additives in the indoor environment-flame retardants and plasticizers. Sci. Total Environ. 2005;339:19–40. doi: 10.1016/j.scitotenv.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Wang R, Tang J, Xie Z, Mi W, Chen Y, Wolschke H, Tian C, Pan X, Luo Y, Ebinghaus R. Occurrence and spatial distribution of organophosphate ester flame retardants and plasticizers in 40 rivers draining into the Bohai Sea, North China. Environ. Pollut. 2015;198:172–178. doi: 10.1016/j.envpol.2014.12.037. [DOI] [PubMed] [Google Scholar]
- Weschler CJ, Nazaroff WW. Semivolatile organic compounds in indoor environments. Atmos Environ. 2008;42:9018–9040. [Google Scholar]
- Weschler CJ, Nazaroff WW. SVOC exposure indoors: fresh look at dermal pathways. Indoor Air. 2012;22:356–77. doi: 10.1111/j.1600-0668.2012.00772.x. [DOI] [PubMed] [Google Scholar]
- Wu RW, Harner T, Diamond ML. Evolution rates and PCB content of surface films that develop on impervious urban surfaces. Atmos. Environ. 2008;42:6131–6143. [Google Scholar]
- Wu Y, Eichler CM, Leng W, Cox SS, Marr LC, Little JC. Adsorption of phthalates on impervious indoor surfaces. Environ. Sci. Technol. 2017;51:2907–2913. doi: 10.1021/acs.est.6b05853. [DOI] [PubMed] [Google Scholar]
- Xu Y, Little JC. Predicting emissions of SVOCs from polymeric materials and their interaction with airborne particles. Environ. Sci. Technol. 2006;40:456–461. doi: 10.1021/es051517j. [DOI] [PubMed] [Google Scholar]
- Xu Y, Liu Z, Park J, Clausen PA, Benning JL, Little JC. Measuring and predicting the emission rate of phthalate plasticizer from vinyl flooring in a specially-designed chamber. Environ. Sci. Technol. 2012;46:12534–12541. doi: 10.1021/es302319m. [DOI] [PubMed] [Google Scholar]
- Yang F, Ding J, Huang W, Xie W, Liu W. Particle size-specific distributions and preliminary exposure assessments of organophosphate flame retardants in office air particulate matter. Environ. Sci. Technol. 2012;48:63–70. doi: 10.1021/es403186z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.