Abstract
Tetrandrine (TET) exhibits biological activities, including anticancer activity. In Chinese medicine, TET has been used to treat hypertensive and arrhythmic conditions and has been demonstrated to induce cytotoxic effects on human cancer cell lines. However, to the best of the author's knowledge, no previous studies have revealed that TET affects cell metastasis in SW620 human colon cancer cells. The present study demonstrated that TET decreased the cell number and inhibited cell adhesion and mobility of SW620 cells. Furthermore, a wound healing assay was performed to demonstrate that TET suppressed cell movement, and Transwell chamber assays were used to reveal that TET suppressed the cell migration and invasion of SW620 cells. Western blotting demonstrated that TET significantly reduced protein expression levels of SOS Ras/Rac guanine nucleotide exchange factor 1, phosphatidylinositol 3-kinase, growth factor receptor bound protein 2, phosphorylated (p)-c Jun N-terminal kinase 1/2, p-p38, p38, 14-3-3, Rho A, β-catenin, nuclear factor-κB p65, signal transducer and activator of transcription-1 and cyclooxygenase-2, in comparison with untreated SW620 cells. Overall, the results of the present study suggested that TET may be used as a novel anti-metastasis agent for the treatment of human colon cancer in the future.
Keywords: tetrandrine, migration, invasion, matrix metalloproteinase-2, matrix metalloproteinase-9, nuclear factor-κB p65, SW620 human colon cancer cells
Introduction
In recent years, colorectal cancer remains a primary cause of morbidity and it is the fourth leading cause of cancer-associated mortality worldwide (1). In the USA (2) and Europe (3), colorectal cancer is the second leading cause of cancer-associated mortality. In China, it has been noted that the morbidity and mortality rates of colorectal cancer were increased compared with previous years (4). In Taiwan, colon cancer is the fourth most common type of cancer, accounting for 23.9 mortalities per 100,000 individuals, based on the 2014 report from the Department of Health, Executive Yuan, Taiwan (5). For patients with superficial cancer (Duke's staging of colorectal cancer), the 5-year survival rate was up to 90%; however, for patients with distant metastasis, the survival rate was ~9% (6,7). Therefore, it is well known that investigating the mechanisms underlying metastatic disease is critical for the treatment and development of metastatic prevention strategies of patients with cancer.
It is well known that tumor metastasis involves epithelial cancer cell adhesion, migration, invasion and angiogenesis for the development of cancer in other sites of the body (8,9). Furthermore, numerous factors are associated with tumor metastasis, including matrix metalloproteinases (MMPs) and urokinase plasminogen activator (uPA), which serve critical roles in degrading the extracellular matrix and basement membrane collagen for cancer cells to invade into new sites (10–12). Epithelial mesenchymal-transition (EMT) is an important process for epithelial cancer cell loss of polarity and cell to cell contact (13), and EMT is one of the initial and primary events in tumor progression (14). The fibroblast growth factor family has been revealed to be associated with tumor metastasis in EMT (15,16). Other factors, including secreted factors, cytokines, chemokines and growth factors have been revealed to be associated with the distinct modes of metastasis and subsequent mortality in tumors (17). A previous study demonstrated that activation of the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt) signaling pathway is involved in cancer cell metastasis (18). Therefore, numerous studies have aimed to investigate the use of novel compounds extracted from natural products as treatments for colon cancer cell metastasis (19–21).
Tetrandrine (TET), a bisbenzylisoquinoline alkaloid isolated from the root of Stephania tetrandra S. Moore, has been revealed to have biological activity, including cytotoxic effects, cell cycle arrest and induction of cell apoptosis in a number of human cancer cell lines (22–26). It was reported that TET suppresses proliferation, induces apoptosis and inhibits migration and invasion in human prostate cancer cells (27). It was also reported that TET regulates metastatic- and angiogenic-associated proteins, including vascular endothelial growth factor, hypoxia-inducible factor-1, integrin β5, endothelial cell specific molecule-1 and intercellular adhesion molecule-1 (28). Previously, it was demonstrated that TET targets epidermal growth factor receptor signaling and its downstream molecules contribute to the inhibition of epidermal growth factor (EGF)-induced HT29 cell metastasis in vitro (29). Furthermore, it was also reported that TET-loaded PVP-b-PCL nanoparticles more efficiently inhibit cell migration and invasion compared with free TET in A549 human lung cancer cells (30). Although it was reported that TET inhibits cell migration and invasion in human colon cancer HT29 cells via inhibition of EGF, whether nuclear factor (NF)-κB is involved in TET suppression of SW620 human colon cancer cell metastasis remains unclear. The present study revealed that TET inhibited cell migration and invasion of SW620 cells via the PI3K, NF-κB and mitogen-activated protein kinase signaling pathways.
Materials and methods
Chemicals and reagents
TET, dimethyl sulfoxide (DMSO) and propidium iodide were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Leibovitz's L-15 medium, fetal bovine serum (FBS), L-glutamine and antibiotics (penicillin-streptomycin) were purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Primary and secondary antibodies were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). Polyvinylidene difluoride (PVDF) membrane was obtained from EMD Millipore (Billerica, CA, USA).
Cell culture
The SW620 human colon cancer cell line was purchased from the Food Industry Research and Development Institute (Hsinchu, Taiwan). Cells were cultured in Leibovitz's L-15 medium supplemented with 10% FBS, 100 units/ml penicillin and 100 µg/ml streptomycin in a 75 cm2 tissue culture flask at 37°C in a humidified atmosphere containing 5% CO2 (31,32).
Cell viability assays
SW620 cells were seeded in a 96-well plate at a density of 1.5×104 cells/well and treated with TET at the final concentrations of 0, 0.2, 0.39, 0.78, 1.56, 3.12, 6.25, 12.5, 25 and 50 µM or 0.5% DMSO as the vehicle control. Following exposure to the drug for 24 or 48 h, 100 µl MTT (0.5 mg/ml; Sigma-Aldrich; Merck KGaA) was added to each well and the plates were incubated for an additional 4 h at 37°C. MTT solution in the medium was aspirated off. To achieve solubilization of the formazan crystals formed in viable cells, 200 µl DMSO was added to each well prior to evaluation of absorbance at a wavelength of 570 nm (33).
Adhesion assay
SW620 cells (1×106 cells/well) were cultured with 0, 1, 5 and 10 µM TET for 48 h at 37°C in 12-well plates, which were pre-coated with type I collagen (10 µg/ml) (Merck KGaA, Darmastadt, Germany) for 60 min at room temperature. Unattached cells were removed and attached cells were mixed in 1% glutaraldehyde (Sigma-Aldrich; Merck KGaA) supplemented with PBS for 20 min, and stained with 0.02% crystal violet solution for 5 min at room temperature. Ethanol (70%) was used to dissolve crystal violet in the stained cells. Optical density (O.D.) was evaluated at 570 nm using a microplate reader with a reference of 405 nm. The adhesion ability (percentage of adhesive cells, %) was determined by measuring the treated cells compared with the control cells (34).
Wound healing assay
SW620 cells (5×105 cells/well) were cultured in 6-well plate until cell growth reached 100% confluence. A sterile yellow micropipette tip was used to scrape the cell monolayers in the well and cells were washed with PBS three times. Cells were then cultured in medium containing 0, 1, 5 and 10 µM TET for 24 and 48 h at 37°C. Cells were examined and imaged using an inverted microscope (×100 magnification) (32,34).
Invasion and migration assays
Evaluation of SW620 cell invasion was performed using Matrigel-coated Transwell cell culture chambers (8 µm pore size). Cells (8×104 cells/well) were seed in the upper chamber and incubated with Leibovitz's L-15 medium supplemented with 0% FBS, and 0 or 10 µM TET for 48 h at 37°C. Leibovitz's L-15 medium supplemented with 10% FBS was placed in the lower chamber. The non-invaded cells were removed using a cotton swab on the upper surface of the membrane and the invaded cells on the lower surface of the membrane were fixed with 4% cold formaldehyde, stained with 0.1% crystal violet for 15 min at room temperature and then imaged using an inverted light microscope (×200 magnification). The invaded cells in the chamber were counted. For the determination of cell migration, the same invasion assay was performed with the membrane coated without Matrigel, as previously described (34). Cell migration was quantified by ImageJ (version 1.49o software, National Institutes of Health, Bethesda, MD, USA) based on the change in the area of the cell-free gap before and after TET stimulation:
Western blot analysis
SW620 cells (6×106) were plated in 10-cm dishes and incubated with 0, 1, 5, 10, 20 and 30 µM TET for 48 h at 37°C, subsequently the cells were collected and lysed in a lysis buffer [40 mM Tris-HCl (pH 7.4), 10 mM EDTA, 120 mM NaCl, 1 mM dithiothreitol, 0.1% Nonide P-40]. The total protein concentration from each treatment was evaluated as previously described (34). A total of 30 µg protein was separated by SDS-PAGE (5% stacking gel and 10–12% separation gel) for western blot analysis. The gel was transferred to a PVDF membrane and the membrane was blocked in 5% fat-free dry milk solution in PBS containing 0.1% Tween-20 for 1 h at room temperature, and then incubated with primary antibodies overnight at 4°C. The phospho-Jun N-terminal kinase (p-JNK) 1/2 (sc-6254), p-38 (sc-136210), phospho-p-38 (sc-166182), ras homolog family member A (Rho A; sc-418), growth factor receptor bound protein 2 (GRB2; sc-503) and 14-3-3 protein σ (sc-100638) antibodies were supplied by Santa-Cruz Biotechnology, Inc. (Dallas, TX, USA, dilution 1:1,000). The anti-matrix metalloproteinase (MMP)-1 (MAB13439) and tissue inhibitor of metalloproteinase (TIMP)-1 (AB6007) antibodies were supplies by Merck Millipore Corp. (Billerica, MA, USA; dilution, 1:1,000). The Son of sevenless homolog (SOS)-1 (610095, dilution, 1:250), phosphoinositide 3-kinase (PI3K) (610046, dilution, 1:2,500), signal transducer and activator of transcription 1 (STAT1) (610115, dilution, 1:1,000), cyclooxygenase-2 (Cox-2) (610204, dilution, 1:500) and -nuclear factor kappa B (NF-κB p65) (610868, dilution, 1:500) antibodies were obtained from BD Biosciences (Bedford, MA, USA). The anti-MMP-2 (ab7032, dilution, 1:1,000) antibody was obtained from Abcam (Cambridge, MA, USA), and the MMP-9 (GTX32122, dilution, 1:1,000) antibody was supplied by GeneTex, Inc. (Irvine, CA, USA) for the β-Catenin (C2206, dilution, 1:4,000) and β-actin (A5316, dilution, 1:10,000) antibodies were supplied by Sigma-Aldrich (St. Louis, MO, USA). Subsequently, the membranes were incubated with secondary antibodies [horseradish peroxidase (HRP)-conjugated mouse immunoglobulin G (IgG; GTX213112) and rabbit HRP-conjugated IgG secondary antibodies (GTX213110), dilution, 1:5,000; GeneTex, Irvine, CA, USA] for 1 h at room temperature. Proteins were visualized using enhanced chemiluminescencereagents (GE Healthcare, Chicago, IL, USA) to stain, as previously described (34).
Statistical analysis
All data are expressed as the mean ± standard deviation. Differences between groups were analyzed by one-way analysis of variance. Statistical comparisons were made using Tukey's test (SigmaPlot for Windows v12.0; Systat Software, Inc., San Jose, CA), and P<0.05 was considered to indicate a statistically significant difference. Differences between two groups were determined using the unpaired Student's t-test (SigmaPlot for Windows version 10.0; Systat Software, Inc., San Jose, CA), and P<0.01 was considered to indicate a statistically significant difference.
Results
TET decreases the cell viability of SW620 cells
SW620 cells were treated with TET (0, 0.2, 0.39, 0.78, 1.56, 3.12, 6.25, 12.5, 25 and 50 µM) for 24 and 48 h prior to collection of the cells to determine the percentage of total viable cell number (Fig. 1). The data indicated a significant dose-dependent reduction of living SW620 cells treated with TET at 0.2–50 µM concentrations for 24 and 48 h (P<0.001). Thus the present study selected 0, 1, 5 and 10 µM for cell migration and invasion experiments.
Figure 1.

TET decreases the percentage of viable SW620 cells. Cells (1.5×104 cells/well) were treated with 0, 0.2, 0.39, 0.78, 1.56, 3.12, 6.25, 12.5, 25 and 50 µM TET or 0.5% dimthylsulfoxide as a vehicle control for 24 and 48 h. Cell growth inhibition was assessed by MTT assay. The values with different letters were significantly different from each other, P<0.05 (Tukey's test). TET, tetrandrine.
TET decreases the cell adhesion of SW620 cells
SW620 cells were cultured with 0, 1, 5 and 10 µM TET for 48 h and the total percentage of adhesion was determined and presented in Fig. 2, [1 µM (87.36±0.71%, P<0.05); 5 µM (79.22±0.18%, P<0.05); 10 µM (78.72±0.18%, P<0.05) compared to untreated control cells (100.00±0.18%)]. Based on these results, it was indicated that TET at 1–10 µM for 48 h treatment significantly reduced cell adhesion in SW620 cells in vitro.
Figure 2.

TET decreases the cell adhesion of SW620 cells. SW620 cells were cultured with 0, 1, 5 and 10 µM TET for 48 h at 37°C in 12-well plates, which were pre-coated with type I collagen (10 µg/ml) for 60 min, and the attached cells were mixed with 1% glutaraldehyde in PBS for 20 min and stained with 0.02% crystal violet solution for 5 min. Ethanol (70%) was used to dissolve crystal violet in the stained cells. Optical density was evaluated at 570 nm using a microplate reader with a reference of 405 nm. The total percentage of adhesion was determined based on the cells that had adhered compared with the control. The results are presented as the mean ± standard deviation (n=3). The values with different letters were significantly different from each other, P<0.05 (Tukey's test). TET, tetrandrine.
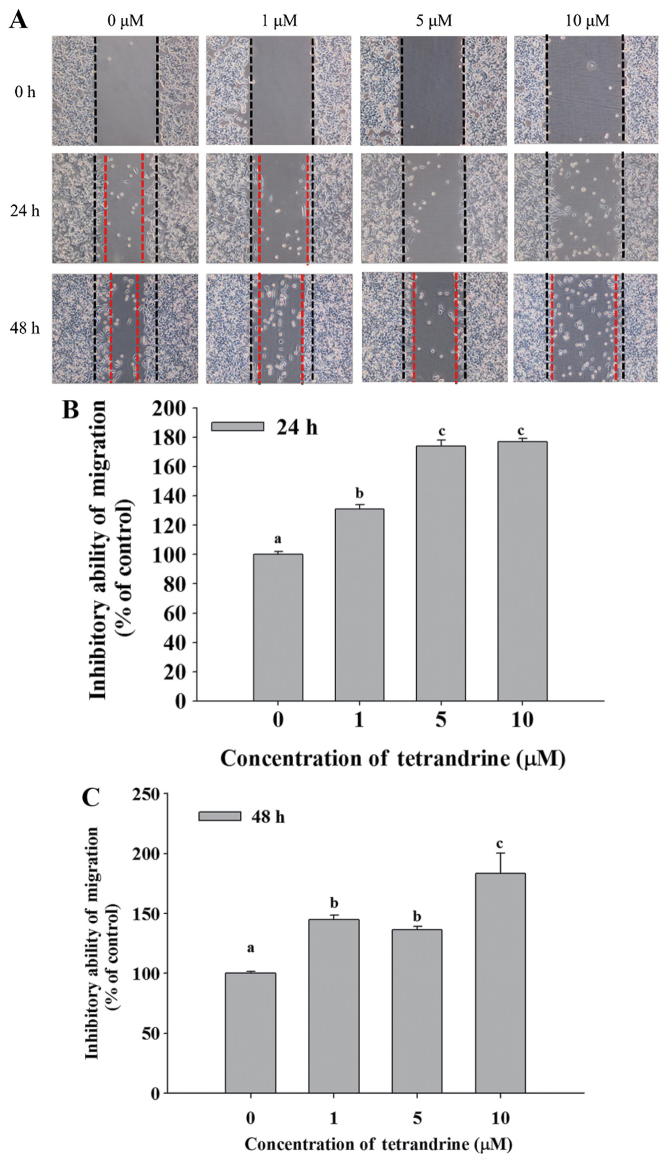
TET decreases cell mobility of SW620 cells
Cell mobility was evaluated using a wound healing assay. SW620 cells were cultured in 6-well plates and the cell monolayers were scraped and then cultured in medium containing 0, 1, 5 and 10 µM TET for 24 and 48 h (Fig. 3). Fig. 3A demonstrated that closure of the scraped area at the highest dose of TET was decreased compared with the control. TET significantly reduced cell mobility, and increased the ability to inhibit migration at 24 and 48 h up to 176.74 and 183.45% in the 10 µM TET treated cells, respectively, compared with control cells (Fig. 3B and C).
Figure 3.
TET decreases cell mobility of SW620 cells. Cell mobility was evaluated using a wound healing assay. SW620 cells were cultured in a 6-well plate, the cell monolayers were scraped and then cultured in medium containing 0, 1, 5 and 10 µM TET for 24 and 48 h. (A) The cell mobility rates were examined and imaged using contrast phase microscopy (×200 magnification). The (B) 24 h and (C) 48 h percentage of inhibition of cell motilities were determined. The values with different letters were significantly different from each other, P<0.05 (Tukey's test). TET, tetrandrine.
TET inhibits the migration and invasion of SW620 cells
Transwell migration and invasion assays were performed to investigate the inhibitory role of TET on SW620 cell migration and invasion, the results are presented in Fig. 4. The results indicated that TET significantly (P<0.05) inhibited cell invasion by 35% for 10 µM TET treated cells for 48 h (P<0.01; Fig. 4A), and inhibited cell migration by 35% for 10 µM TET treated cells for 48 h compared with the control cells (P<0.01; Fig. 4B).
Figure 4.
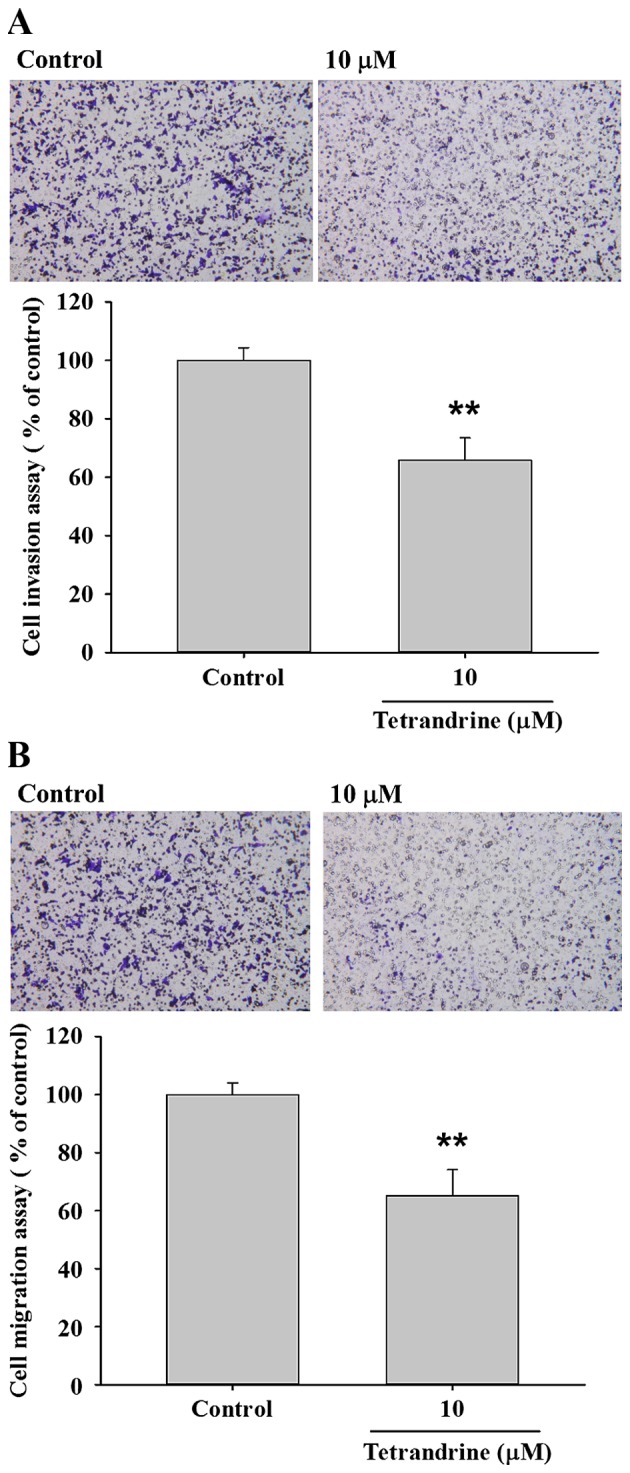
TET inhibits migration and invasion of SW620 cells. Transwell cell migration and invasion assays were used to investigate the inhibition of TET on SW620 cell migration and invasion. (A) Cell invasion was imaged using contrast phase microscopy (×100 magnification) and percentage of inhibition of cell invasion. (B) Cell migration was imaged using contrast phase microscopy (X100) and percentage of inhibition of cell migration. **P<0.01 (Student's t-test) vs. control. TET, tetrandrine.
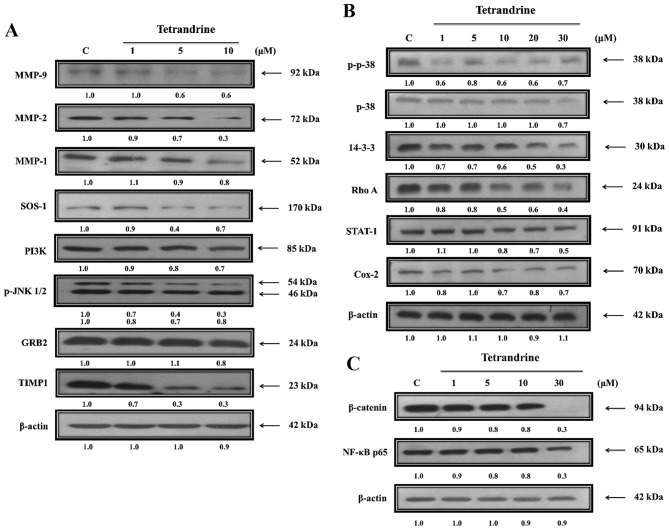
TET alters expression levels of proteins associated with migration and invasion of SW620 cells
The present study further investigated the role of upstream regulated proteins associated with SW620 cell migration and invasion following exposure to TET (Fig. 5). TET significantly reduced protein expression levels of MMP-9, MMP-2, MMP-1, SOS Ras/Rac guanine nucleotide exchange factor 1 (SOS-1), PI3K, phosphorylated (p)-c Jun N-terminal kinase (JNK)1/2, growth factor receptor bound protein 2 (GRB2) and TIMP metallopeptidase inhibitor 1 (TIMP1; Fig. 5A), p-p38, p38, 14-3-3, Rho A, signal transducer and activator of transcription-1 (STAT-1) and cyclooxygenase-2 (Cox-2; Fig. 5B), β-catenin and NF-κB (Fig. 5C). The protein expression levels were decreased in TET-treated cells compared with untreated-cells. TET inhibited the p38, JNK and Rho A signaling pathways by reducing PI3K, Cox-2 and NF-κB p65 expression levels, which induced MMP-2/-9 downregulation (Fig. 6).
Figure 5.
TET alters the expression levels of proteins associated with migration and invasion of SW620 cells. Cells were treated with various concentrations of TET for 48 h and then total proteins were quantified and apoptosis associated proteins were examined by western blotting. (A) MMP-9, MMP-2 and MMP-1, SOS-1, PI3K, p-JNK1/2, GRB2, TIMP1. (B) p-p38, p38, 14-3-3, Rho A, STAT-1 and Cox-2. (C) β-catenin and NF-κB p65. TET, tetrandrine. MMP, matrix metalloproteinase; SOS-1, SOS Ras/Rac guanine nucleotide exchange factor 1; PI3K, phosphatidylinositol 3 kinase; p, phosphorylated; JNK1/2, c Jun N-terminal kinase; GRB2, growth factor receptor bound protein 2; TIMP1, TIMP metallopeptidase inhibitor 1; STAT-1, signal transducer and activator of transcription-1; Cox-2, cyclooxygenase-2; NF-κB, nuclear factor-κB.
Figure 6.
The possible signaling pathways for TET inhibited cell mobility, adhesion, migration and invasion in SW620 cells in vitro. TET, tetrandrine; ECM, extracellular matrix; GRB2, growth factor receptor bound protein 2; SOS-1, SOS Ras/Rac guanine nucleotide exchange factor 1; PI3K, phosphatidylinositol 3 kinase; p, phosphorylated; JNK1/2, c Jun N-terminal kinase; MAP, mitogen-activated protein; NF-κB, nuclear factor-κB; STAT-1, signal transducer and activator of transcription-1; MMP, matrix metalloproteinase.
Discussion
Previous studies have demonstrated that cancer cells exhibit extensive invasive and migratory abilities, which are factors that may block the effectiveness of clinical treatments against cancer, including chemotherapy (35,36). Cancer cell metastasis involves a complex multistep process, which includes cell movement and cell adhesion accompanied with migration, invasion and angiogenesis to develop new tumors in other sites of body (37,38). Therefore, investigators focus on the inhibition of cancer cell migration and invasion, as an anticancer strategy. It has previously been reported that TET induces cancer cell death via cell cycle arrest and induction of apoptosis in numerous human cancer cell lines; however, there is no available information to demonstrate TET inhibiting migration and invasion in human colon cancer SW620 cells. The present study investigated the effects of TET on adhesion, migration and invasion of SW620 cells in vitro.
Firstly, the present study examined the cytotoxic effects of TET on SW620 cells in vitro and the results indicated that TET induced cell death in a dose-dependent manner. Therefore, 1, 5 and 10 µM TET treatments were selected for further experiments. The present study also investigated cell adhesion of SW620 cells following exposure to 0, 1, 5 and 10 µM TET for 48 h and the results indicated that TET inhibited cell adhesion in a concentration-dependent manner. It is well documented that wound healing is one of the methods for examining cancer cell mobility (39,40); thus, the results from the wound healing assay indicated that TET inhibited cell mobility in SW620 cells in a dose-dependent manner. The Transwell assay has been recognized to be effective in the analysis of cell migration and invasion (41,42). The present study performed Transwell assays to investigate cell migration and invasion of SW620 cells following exposure to TET in vitro. The findings indicated that TET significantly inhibited cell migration and invasion when compared with the control groups. Based on these observations, the present study suggested that TET suppressed cell migration and invasion via the inhibition of cell attachment (adhesion) to the basement membrane.
MMPs, a family of zinc-dependent proteases, serve essential roles in defining how cells interact with their surrounding microenvironment (43). It was reported that increased expression levels of MMPs are associated with increased levels of cancer cell angiogenesis, migration and invasion (44); thus, MMPs have previously been used as drug targets (45). Therefore, the present study first examined the protein expression levels of MMP-2 and MMP-9 in SW620 cells following exposure to various concentrations of TET, and the results indicated that TET decreased the protein expression levels of MMP-2, MMP-9, MMP-1 and TIMP1 in a concentration-dependent manner, which was revealed by western blotting. MMP-2 and MMP-9 serve important roles in cancer invasion and metastasis (46,47). Furthermore, results indicated that TET suppressed the protein expression levels of SOS-1, PI3K, GRB2 and p-JNK1/2 in SW620 cells. SOS-1 and GRB2 have been observed in HT 29 colon cancer cells (48). To the best of the author's knowledge, the present study is the first demonstrate that TET inhibited the protein expression levels of SOS-1 and GRB2. GRB2-associated binding protein 2 serves a critical role in the proliferation and migration of various types of cancer (49). Therefore, further investigations are required to understand the role of SOS-1 and GRB2 in cancer cell metastasis. The results of the present study also revealed that TET inhibited the protein expression levels of PI3K in SW620 cells. PI3K/Akt and extracellular signal regulated kinase pathways are involved in growth factor-mediated colon cancer proliferation (50). It was reported that 17β-estradiol treatment inhibited prostaglandin E2-induced uPA, MMP-9 and cellular motility by suppressing activation of JNK1/2 in LoVo human colon cancer cells (51).
The results of the present study demonstrated that TET inhibited the protein expression levels of p-p38, p38, 14-3-3 and Rho A in SW620 cells. p-p38 and p38 were significantly reduced in TET-treated SW620 cells compared with untreated cells. It was previously reported that in SW620 human colon cancer-derived metastatic cells, nicotine stimulates the invasion and metastasis of colon cancer cells in vitro via activation of the p38 MAPK downstream signaling pathway (52). The present study revealed that TET significantly reduced the protein expression levels of 14-3-3 in SW620 cells in a dose-dependent manner. It was previously demonstrated that 14-3-3 protein overexpression promotes lung cancer progression when combined with HSP27 overexpression (53). A previous study revealed that in patient colorectal cancer samples, Rho A is associated with the invasion of lymph nodes and blood vessels, thus, Rho A may be a promising target for cancer treatment (54).
The results of the present study additionally indicated that TET significantly suppressed the protein expression levels of β-catenin and NF-κB p65 in SW620 cells. β-catenin is a 92-kDa cellular protein and a member of the Wnt signaling pathway that has been revealed to serve an important role in colorectal cancer tumorigenesis (55,56), and is associated with E-cadherin in maintaining cellular adhesion (57). The aberrant activation of β-catenin increases its translocation to the nucleus in colorectal cancer (58). Therefore, targeting the Wnt/β-catenin signaling pathway to develop novel chemotherapeutic agents against colon cancer may be a promising strategy. NF-κB is a transcription factor closely associated with cell survival, proliferation and metastasis (59). It is well documented that agents blocking the NF-κB signaling pathway may act as therapeutic agents to treat inflammation and cancer (60). The results of the present study indicated that TET inhibited cell migration and invasion of SW620 cells via inhibition of NF-κB. It was also revealed that TET suppressed the protein expression levels of STAT1 and Cox-2 in SW620 cells. Constitutive overexpression of STAT1 in tumor cells is correlated with protection of tumor cells to genotoxic stress following doxorubicin (61) or cisplatin (62) treatment. Cox-2 has tumor promoting properties and is expressed in approximately 40–50% of colonic adenomas and in 80–90% of colorectal carcinomas (63,64). Cox-2 is also associated with cancer cell invasion (65), serves an important role in carcinogenesis and therefore has the potential to be used as a novel anticancer therapeutic target (66,67).
In conclusion, the present study revealed that TET suppressed cell mobility, adhesion, invasion and migration in SW620 cells via the inhibition of metastasis-associated proteins such as MMP-2/-9.
Therefore, the results of the present study suggested that TET may be a potential candidate for developing preventive agents against human colon cancer metastasis.
Acknowledgements
The present study was supported by the China Medical University Beigang Hospital, Yunlin, Taiwan (grant no. CMUBH R103-011).
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Labianca R, Beretta GD, Kildani B, Milesi L, Merlin F, Mosconi S, Pessi MA, Prochilo T, Quadri A, Gatta G, et al. Colon cancer. Crit Rev Oncol Hematol. 2010;74:106–133. doi: 10.1016/j.critrevonc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Li M, Gu J. Changing patterns of colorectal cancer in China over a period of 20 years. World J Gastroenterol. 2005;11:4685–4688. doi: 10.3748/wjg.v11.i30.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ministry of Health and Welfare: The cancer mortality report of the Department of Health. Taiwan: 2014. [Google Scholar]
- 6.Camp ER, Ellis LM. CCR 20th Anniversary Commentary: RAS as a Biomarker for EGFR-targeted therapy for colorectal cancer-from concept to practice. Clin Cancer Res. 2015;21:3578–3580. doi: 10.1158/1078-0432.CCR-14-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 8.Hazan RB, Qiao R, Keren R, Badano I, Suyama K. Cadherin switch in tumor progression. Ann N Y Acad Sci. 2004;1014:155–163. doi: 10.1196/annals.1294.016. [DOI] [PubMed] [Google Scholar]
- 9.Makrilia N, Kollias A, Manolopoulos L, Syrigos K. Cell adhesion molecules: Role and clinical significance in cancer. Cancer Invest. 2009;27:1023–1037. doi: 10.3109/07357900902769749. [DOI] [PubMed] [Google Scholar]
- 10.Babykutty S, Suboj P, Srinivas P, Nair AS, Chandramohan K, Gopala S. Insidious role of nitric oxide in migration/invasion of colon cancer cells by upregulating MMP-2/9 via activation of cGMP-PKG-ERK signaling pathways. Clin Exp Metastasis. 2012;29:471–492. doi: 10.1007/s10585-012-9464-6. [DOI] [PubMed] [Google Scholar]
- 11.Dung TD, Feng CC, Kuo WW, Pai P, Chung LC, Chang SH, Hsu HH, Tsai FJ, Lin YM, Huang CY. Suppression of plasminogen activators and the MMP-2/-9 pathway by a Zanthoxylum avicennae extract to inhibit the HA22T human hepatocellular carcinoma cell migration and invasion effects in vitro and in vivo via phosphatase 2A activation. Biosci Biotechnol Biochem. 2013;77:1814–1821. doi: 10.1271/bbb.2013E2. [DOI] [PubMed] [Google Scholar]
- 12.Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- 13.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalluri R. EMT: When epithelial cells decide to become mesenchymal-like cells. J Clin. Invest. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Rieger-Christ KM, Lee P, Zagha R, Kosakowski M, Moinzadeh A, Stoffel J, Ben-Ze'ev A, Libertino JA, Summerhayes IC. Novel expression of N-cadherin elicits in vitro bladder cell invasion via the Akt signaling pathway. Oncogene. 2004;23:4745–4753. doi: 10.1038/sj.onc.1207629. [DOI] [PubMed] [Google Scholar]
- 19.Tong W, Wang Q, Sun D, Suo J. Curcumin suppresses colon cancer cell invasion via AMPK-induced inhibition of NF-κB, uPA activator and MMP9. Oncol Lett. 2016;12:4139–4146. doi: 10.3892/ol.2016.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han M, Song Y, Zhang X. Quercetin suppresses the migration and invasion in human colon cancer Caco-2 cells through regulating toll-like receptor 4/nuclear factor-kappa B Pathway. Pharmacogn Mag. 2016;12(Suppl 2):S237–S244. doi: 10.4103/0973-1296.182154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yun JH, Kim KA, Yoo G, Kim SY, Shin JM, Kim JH, Jung SH, Kim J, Nho CW. Phenethyl isothiocyanate suppresses cancer stem cell properties in vitro and in a xenograft model. Phytomedicine. 2017;30:42–49. doi: 10.1016/j.phymed.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Kuo PL, Lin CC. Tetrandrine-induced cell cycle arrest and apoptosis in Hep G2 cells. Life Sci. 2003;73:243–252. doi: 10.1016/S0024-3205(03)00266-2. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Kang GH, Kim KC, Kim KM, Park DI, Choi BT, Kang HS, Lee YT, Choi YH. Tetrandrine-induced cell cycle arrest and apoptosis in A549 human lung carcinoma cells. Int J Oncol. 2002;21:1239–1244. [PubMed] [Google Scholar]
- 24.Meng LH, Zhang H, Hayward L, Takemura H, Shao RG, Pommier Y. Tetrandrine induces early G1 arrest in human colon carcinoma cells by down-regulating the activity and inducing the degradation of G1-S-specific cyclin-dependent kinases and by inducing p53 and p21Cip1. Cancer Res. 2004;64:9086–9092. doi: 10.1158/0008-5472.CAN-04-0313. [DOI] [PubMed] [Google Scholar]
- 25.Chen XL, Ren KH, He HW, Shao RG. Involvement of PI3K/AKT/GSK3beta pathway in tetrandrine-induced G1 arrest and apoptosis. Cancer Biol Ther. 2008;7:1073–1078. doi: 10.4161/cbt.7.7.6142. [DOI] [PubMed] [Google Scholar]
- 26.McCubrey JA, Basecke J, Cervello M, Martelli AM, Franklin RA. GSK-3beta is a critical mediator of tetrandrine induced cell cycle arrest and cytotoxicity. Cancer Biol Ther. 2008;7:1079. doi: 10.4161/cbt.7.7.6519. [DOI] [PubMed] [Google Scholar]
- 27.Liu W, Kou B, Ma ZK, Tang XS, Lv C, Ye M, Chen JQ, Li L, Wang XY, He DL. Tetrandrine suppresses proliferation, induces apoptosis, and inhibits migration and invasion in human prostate cancer cells. Asian J Androl. 2015;17:850–853. doi: 10.4103/1008-682X.142134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao JL, Ji X, He TC, Zhang Q, He K, Zhao Y, Chen SH, Lv GY. Tetrandrine suppresses cancer angiogenesis and metastasis in 4T1 tumor bearing mice. Evid Based Complement. Alternat Med. 2013;2013:265061. doi: 10.1155/2013/265061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horng CT, Yang JS, Chiang JH, Lu CC, Lee CF, Chiang NN, Chen FA. Inhibitory effects of tetrandrine on epidermal growth factor-induced invasion and migration in HT29 human colorectal adenocarcinoma cells. Mol Med Rep. 2016;13:1003–1009. doi: 10.3892/mmr.2015.4635. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Hou Z, Zhang H, Kong H, Li X, Wang H, Xie W. An efficient Trojan delivery of tetrandrine by poly(N-vinylpyrrolidone)-block-poly(ε-caprolactone) (PVP-b-PCL) nanoparticles shows enhanced apoptotic induction of lung cancer cells and inhibition of its migration and invasion. Int J Nanomedicine. 2014;9:231–242. doi: 10.2147/IJN.S55541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang JS, Li DM, Ma Y, He N, Gu Q, Wang FS, Jiang SQ, Chen BQ, Liu JR. γ-Tocotrienol induces paraptosis-like cell death in human colon carcinoma SW620 cells. PLoS One. 2013;8:e57779. doi: 10.1371/journal.pone.0057779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang YM, Velmurugan BK, Kuo WW, Chen YS, HO TJ, Tsai CT, Ye CX, Tsai CH, Tsai FJ, Huang CY. Inhibitory effect of alpinate Oxyphyllae fructus extracts on Ang II-induced cardiac pathological remodeling-related pathways in H9c2 cardiomyoblast cells. Biomedicine. 2013;3:148–152. doi: 10.1016/j.biomed.2013.05.001. [DOI] [Google Scholar]
- 33.Park WH, Seol JG, Kim ES, Hyun JM, Jung CW, Lee CC, Kim BK, Lee YY. Arsenic trioxide-mediated growth inhibition in MC/CAR myeloma cells via cell cycle arrest in association with induction of cyclin-dependent kinase inhibitor, p21, and apoptosis. Cancer Res. 2000;60:3065–3071. [PubMed] [Google Scholar]
- 34.Lai KC, Hsu SC, Yang JS, Yu CC, Lein JC, Chung JG. Diallyl trisulfide inhibits migration, invasion and angiogenesis of human colon cancer HT-29 cells and umbilical vein endothelial cells, and suppresses murine xenograft tumour growth. J Cell Mol Med. 2015;19:474–484. doi: 10.1111/jcmm.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verhoeff JJ, van Tellingen O, Claes A, Stalpers LJ, van Linde ME, Richel DJ, Leenders WP, van Furth WR. Concerns about anti-angiogenic treatment in patients with glioblastoma multiforme. BMC Cancer. 2009;9:444. doi: 10.1186/1471-2407-9-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng SH, Jian JJ, Tsai SY, Chan KY, Yen LK, Chu NM, Tan TD, Tsou MH, Huang AT. Prognostic features and treatment outcome in locoregionally advanced nasopharyngeal carcinoma following concurrent chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 1998;41:755–762. doi: 10.1016/S0360-3016(98)00092-3. [DOI] [PubMed] [Google Scholar]
- 37.Gupta GP, Massague J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Huang YL, Chu YL, Ho CT, Chung JG, Lai CI, Su YC, Kuo YH, Sheen LY. Antcin K, an active triterpenoid from the fruiting bodies of basswood-cultivated antrodia cinnamomea, inhibits metastasis via suppression of integrin-mediated adhesion, migration, and invasion in human hepatoma cells. J Agric Food Chem. 2015;63:4561–4569. doi: 10.1021/jf5059304. [DOI] [PubMed] [Google Scholar]
- 39.Park SJ, Kong HK, Kim YS, Lee YS, Park JH. Inhibition of S-adenosylhomocysteine hydrolase decreases cell mobility and cell proliferation through cell cycle arrest. Am J Cancer Res. 2015;5:2127–2138. [PMC free article] [PubMed] [Google Scholar]
- 40.Wu ZY, Lien JC, Huang YP, Liao CL, Lin JJ, Fan MJ, Ko YC, Hsiao YP, Lu HF, Chung JG. Casticin inhibits A375.S2 human melanoma cell migration/invasion through downregulating NF-κB and matrix metalloproteinase-2 and −1. Molecules. 2016;21:384. doi: 10.3390/molecules21030384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji BC, Hsiao YP, Tsai CH, Chang SJ, Hsu SC, Liu HC, Huang YP, Lien JC, Chung JG. Cantharidin impairs cell migration and invasion of A375.S2 human melanoma cells by suppressing MMP-2 and −9 through PI3K/NF-κB signaling pathways. Anticancer Res. 2015;35:729–738. [PubMed] [Google Scholar]
- 42.Liao CL, Lai KC, Huang AC, Yang JS, Lin JJ, Wu SH, Wood Gibson W, Lin JG, Chung JG. Gallic acid inhibits migration and invasion in human osteosarcoma U-2 OS cells through suppressing the matrix metalloproteinase-2/-9, protein kinase B (PKB) and PKC signaling pathways. Food Chem Toxicol. 2012;50:1734–1740. doi: 10.1016/j.fct.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 43.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shia CS, Suresh G, Hou YC, Lin YC, Chao PD, Juang SH. Suppression on metastasis by rhubarb through modulation on MMP-2 and uPA in human A549 lung adenocarcinoma: An ex vivo approach. J Ethnopharmacol. 2011;133:426–433. doi: 10.1016/j.jep.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 45.Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: Outside-in signaling and relationship to tumor progression. Biochim Biophys Acta. 2012;1825:29–36. doi: 10.1016/j.bbcan.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: A review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 47.Dutta A, Li J, Lu H, Akech J, Pratap J, Wang T, Zerlanko BJ, FitzGerald TJ, Jiang Z, Birbe R, et al. Integrin αvβ6 promotes an osteolytic program in cancer cells by upregulating MMP2. Cancer Res. 2014;74:1598–1608. doi: 10.1158/0008-5472.CAN-13-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lai KC, Hsu SC, Kuo CL, Ip SW, Yang JS, Hsu YM, Huang HY, Wu SH, Chung JG. Phenethyl isothiocyanate inhibited tumor migration and invasion via suppressing multiple signal transduction pathways in human colon cancer HT29 cells. J Agric Food Chem. 2010;58:11148–11155. doi: 10.1021/jf102384n. [DOI] [PubMed] [Google Scholar]
- 49.Matsumura T, Sugimachi K, Takahashi Y, Uchi R, Sawada G, Ueda M, Hirata H, Sakimura S, Ueo H, Takano Y, et al. Clinical significance of GAB2, a scaffolding/docking protein acting downstream of EGFR in human colorectal cancer. Ann Surg Oncol. 2014;21(Suppl 4):S743–S749. doi: 10.1245/s10434-014-3889-x. [DOI] [PubMed] [Google Scholar]
- 50.Waseem T, Duxbury M, Ashley SW, Robinson MK. Ghrelin promotes intestinal epithelial cell proliferation through PI3K/Akt pathway and EGFR trans-activation both converging to ERK 1/2 phosphorylation. Peptides. 2014;52:113–121. doi: 10.1016/j.peptides.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 51.Hsu HH, Hu WS, Lin YM, Kuo WW, Chen LM, Chen WK, Hwang JM, Tsai FJ, Liu CJ, Huang CY. JNK suppression is essential for 17β-estradiol inhibits prostaglandin E2-induced uPA and MMP-9 expressions and cell migration in human LoVo colon cancer cells. J Biomed Sci. 2011;18:61. doi: 10.1186/1423-0127-18-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiang T, Fei R, Wang Z, Shen Z, Qian J, Chen W. Nicotine enhances invasion and metastasis of human colorectal cancer cells through the nicotinic acetylcholine receptor downstream p38 MAPK signaling pathway. Oncol Rep. 2016;35:205–210. doi: 10.3892/or.2015.4363. [DOI] [PubMed] [Google Scholar]
- 53.Zhao GY, Ding JY, Lu CL, Lin ZW, Guo J. The overexpression of 14-3-3ζ and Hsp27 promotes non-small cell lung cancer progression. Cancer. 2014;120:652–663. doi: 10.1002/cncr.28452. [DOI] [PubMed] [Google Scholar]
- 54.Jeong D, Park S, Kim H, Kim CJ, Ahn TS, Bae SB, Kim HJ, Kim TH, Im J, Lee MS. RhoA is associated with invasion and poor prognosis in colorectal cancer. Int J Oncol. 2016;48:714–722. doi: 10.3892/ijo.2015.3281. [DOI] [PubMed] [Google Scholar]
- 55.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 56.White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142:219–232. doi: 10.1053/j.gastro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Norwood MG, Bailey N, Nanji M, Gillies RS, Nicholson A, Ubhi S, Darnton JJ, Steyn RS, Womack C, Hughes A, et al. Cytoplasmic beta-catenin accumulation is a good prognostic marker in upper and lower gastrointestinal adenocarcinomas. Histopathology. 2010;57:101–111. doi: 10.1111/j.1365-2559.2010.03587.x. [DOI] [PubMed] [Google Scholar]
- 58.Kobayashi M, Honma T, Matsuda Y, Suzuki Y, Narisawa R, Ajioka Y, Asakura H. Nuclear translocation of beta-catenin in colorectal cancer. Br J Cancer. 2000;82:1689–1693. doi: 10.1054/bjoc.1999.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/MCB.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fryknas M, Dhar S, Oberg F, Rickardson L, Rydåker M, Göransson H, Gustafsson M, Pettersson U, Nygren P, Larsson R, Isaksson A. STAT1 signaling is associated with acquired crossresistance to doxorubicin and radiation in myeloma cell lines. Int J Cancer. 2007;120:189–195. doi: 10.1002/ijc.22291. [DOI] [PubMed] [Google Scholar]
- 62.Roberts D, Schick J, Conway S, Biade S, Laub PB, Stevenson JP, Hamilton TC, O'Dwyer PJ, Johnson SW. Identification of genes associated with platinum drug sensitivity and resistance in human ovarian cancer cells. Br J Cancer. 2005;92:1149–1158. doi: 10.1038/sj.bjc.6602447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hasegawa K, Ichikawa W, Fujita T, Ohno R, Okusa T, Yoshinaga K, Sugihara K. Expression of cyclooxygenase-2 (COX-2) mRNA in human colorectal adenomas. Eur J Cancer. 2001;37:1469–1474. doi: 10.1016/S0959-8049(01)00137-X. [DOI] [PubMed] [Google Scholar]
- 64.Peek RM., Jr Prevention of colorectal cancer through the use of COX-2 selective inhibitors. Cancer Chemother Pharmacol. 2004;54(Suppl 1):S50–S56. doi: 10.1007/s00280-004-0887-x. [DOI] [PubMed] [Google Scholar]
- 65.Dempke W, Rie C, Grothey A, Schmoll HJ. Cyclooxygenase-2: A novel target for cancer chemotherapy? J Cancer Res Clin Oncol. 2001;127:411–417. doi: 10.1007/s004320000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giercksky KE. COX-2 inhibition and prevention of cancer. Best Pract Res Clin Gastroenterol. 2001;15:821–833. doi: 10.1053/bega.2001.0237. [DOI] [PubMed] [Google Scholar]
- 67.Zhang H, Sun XF. Overexpression of cyclooxygenase-2 correlates with advanced stages of colorectal cancer. Am J Gastroenterol. 2002;97:1037–1041. doi: 10.1111/j.1572-0241.2002.05625.x. [DOI] [PubMed] [Google Scholar]