Abstract
It has been suggested that endoplasmic reticulum stress (ERS) may induce apoptosis following spinal cord injury (SCI). Methotrexate (MTX) has been used as a long-term therapy regimen for rheumatoid arthritis. However, it is not clear whether MTX remediates SCI by inhibiting ERS. In the present study, to establish an in vitro ERS cell model, PC12 cells were pre-incubated with triglycerides (TG). MTT assays revealed that treatment with 1, 2.5, 5 and 10 µM TG decreased PC12 cell viability in a dose-dependent manner. Additionally, MTX treatment significantly reversed the TG-induced decrease in cell viability and increased apoptosis according to the flow cytometry assay (P<0.05). Notably, western blotting indicated that MTX significantly decreased levels of glucose-regulated protein (GRP)78, CCAAT-enhancer-binding protein homologous protein (CHOP) and caspase-12 expression (P<0.05), which were increased following treatment with TG. Furthermore, the in vivo role of MTX in a rat model of SCI was evaluated. The motor behavioral function of rats was improved following treatment with MTX according to Basso, Beattie and Bresnahan scoring (P<0.05). Terminal deoxynucleotidyl-transferase-mediated dUTP nick end staining indicated that there were no apoptotic cells present in sham rats. In the SCI model group, apoptotic cells were observed at day 7; however, the number of apoptotic cells was reduced following an additional 7 days of MTX administration. Furthermore, levels of ERS-associated proteins, including caspase-3, activating transcription factor 6, serine/threonine-protein kinase/endoribonuclease inositol-requiring enzyme 1 α, eukaryotic initiation factor 2 α and GRP78, were significantly increased following SCI; however, administration of MTX for 7 days significantly reversed this effect (P<0.05, P<0.01 and P<0.001). Therefore, MTX may improve SCI by suppressing ERS-induced apoptosis in vitro and in vivo.
Keywords: methotrexate, spinal cord injury, endoplasmic reticulum stress, apoptosis
Introduction
Spinal cord injury (SCI) poses a serious threat to human health and typically results in incomplete or complete loss of motor and sensory function (1). The prevalence of SCI is relatively high. Notably, the prevalence among the elderly (aged >60 years old) and the younger (aged 16–45 years old) have been estimated to be ~24 and 49%, respectively, which are primarily due to traffic collisions (2,3). Although various methods of treating SCI are currently used, these treatments are not effective since SCIs still result in substantial permanent morbidity and mortality (2). Therefore, it is important to develop a novel effective method of treating patients with SCI.
Apoptosis is a process of programmed cell death that is increased under pathological conditions (2). Apoptosis inhibits nerve function following SCI and this is the primary factor that causes secondary injury of the spinal cord (4). Previous studies have suggested that the mitochondrial and death receptor signaling pathways are the two primary signaling pathways that induce apoptosis (5–7). Furthermore, it has been demonstrated that endoplasmic reticulum stress (ERS) induces apoptosis (8). The endoplasmic reticulum (ER) is an important organelle in eukaryotic cells that regulates calcium ions and the processing and synthesis of proteins (9). When ER function is altered during pathological conditions, the ER will respond accordingly with an ERS reaction (10,11). Subsequently, the unfolded protein response (UPR), which is an adaptive response that predominantly functions to maintain normal ER function, is induced (12). There are three processes associated with UPR, which include the involvement of activating transcription factor (ATF) 6, serine/threonine-protein kinase/endoribonuclease inositol-requiring enzyme (IRE)1 and RNA-activated protein kinase-like ER kinase (PERK) (13). Under normal physiological conditions, these three proteins bind to an intra-ER chaperone, glucose-regulated protein (GRP)78, which is maintained in an inactive state in the ER membrane (13). However, in response to stress, GRP78 dissociates from the ER membrane to bind to misfolded proteins, thereby initiating the signal transduction processes to re-establish ER homeostasis (14). Although the UPR typically acts to maintain homeostasis in cells by removing misfolded proteins, elevated and sustained ER stress induces cell death (15). Therefore, therapeutic strategies targeting ER stress and its downstream apoptosis signaling pathways may be used to treat patients with SCI.
Methotrexate (MTX), which is characterized by anti-inflammatory and immunosuppressive functions, is a first-line prescription agent used in the treatment of rheumatoid arthritis (RA) (16,17). Due to its safety and tolerability, low doses of MTX has been used as a long-term therapy regimen for patients with RA (18). However, to the best of our knowledge, there have been no studies investigating whether MTX improves traumatic SCI by inhibiting ERS. Therefore, the present study aimed to explore the use of low-dose MTX in the treatment of a rat model of SCI as a means to uncover a potential inhibition mechanism of ERS.
Materials and methods
Establishment of a rat SCI model
A total of 72 specific-pathogen free male Sprague Dawley rats (weight, 220–250 g, 6–8 weeks old) were purchased from Shanghai Silaike Experimental Animal Limited Liability Company (Shanghai, China). Rats were maintained in the animal experimental center of Hongqi Hospital Affiliated to Mudanjiang Medical University and four animals were housed per each cage with a 12-h light/dark cycle. Room temperature was maintained at 23±1°C, humidity was maintained at ~60% and all rats had free access to food and water. Rats were randomly divided into three groups: A sham, an SCI and an MTX+SCI group. MTX (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) stock solutions were prepared in 0.1 mol/l NaOH at 0.5 mg/ml and diluted 1:10 in 0.1 mol/l phosphate-buffered saline (PBS) prior to use; the pH of the solution was adjusted to 7.4–7.6. For the sham group and SCI rats, no other treatments were performed. For the MTX+SCI group, a total of 24 h following SCI, rats were treated with 50 mg/kg MTX. A total of 8 rats were in the sham group, 32 rats were in the SCI group and 32 rats were in MTX+SCI group. Rats in the SCI and MTX+SCI groups were further divided into 1, 3, 7 and 14 day groups (n=8, each group) according to the different time points following injury.
The rat SCI model was established as previously described (19). Rats in the sham surgery group underwent all aspects of the surgery except for contusion thoracic injury. Following 8 h fasting, rats were anesthetized via intraperitoneal injection with 10% chloral hydrate (400 mg/kg), according to a previous study (20). Subsequently, rats were placed in a prone position and an incision (~2.5 cm in length) was made in the middle of the back. Skin was cut layer by layer and the T8 to T10 vertebral plates were exposed. Total laminectomy was performed for the T9 vertebral plate to expose the spinal dura mater. T8 and T10 spinous processes were fixated using forceps. A Kirschner wire (10 g) was inserted into the catheter that was inserted into the aorta with a weight, which fell freely from a 25-mm height. Following this, a semicircular slice (4×2 mm) made from thin plastic was hit, and the wire was immediately removed, resulting in incomplete injury of the rat spinal cord. The incision was sutured layer by layer. Following the strike, rats exhibiting a tail-wagging reflex, retraction flutter in the lower limbs and body, and flaccid paralysis in the lower limbs in an awake state represented successful model construction. For MTX treatment, MTX was delivered intrathecally once per day (50 mg/kg) after SCI for 3 days and a dose of 50 mg/kg MTX was selected based on the results of a previous study, which indicated that the median lethal dose of MTX in rats was 2,288.5 mg/kg (21). No changes in motion or mental state were observed in rats from the SCI, SCI+MTX-treated or the sham group in the present study. Therefore, the dose of MTX selected was considered to be safe. The present study was approved by the Animal Ethics Committee of the Hongqi Hospital Affiliated to Mudanjiang Medical University (Mudanjiang, China).
Basso, Beattie and Bresnahan (BBB) scoring
Behavioral scoring was performed at different time points (n=8, each time point) following SCI surgery. The recovery of hind limb motor function was observed and scored using the BBB scoring system (22). All behavioral observations were performed at the same time (8:00 p.m.) to avoid variations in the movement of animals between day and night. Furthermore, an inclined plane test was performed. Behavior was evaluated days 1, 3, 5, 7 and 14 days postoperatively using the modified Rivlin's method (23), which tested the ability of animals to balance on elevated wooden beams. A simple device was constructed containing a moveable plate with an adjustable angle of 0–90°. The rat's head was placed faced forward, and the angle of inclination between the inclined plane and the horizontal plane was increased gradually, until the rats were unable to maintain a constant position (45°) for 5 sec. The angle was considered to be the critical value and then recorded.
Cell culture
PC12 cells were purchased from the Chinese Academy of Medical Sciences (Beijing, China). Cells were cultured in high-glucose Dulbecco's modified Eagle's medium/F12 (GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 100 U/ml penicillin and 100 U/ml streptomycin in 25-cm2 culture flasks at 37°C in a humidified atmosphere containing 5% CO2.
MTT colorimetric assay
Triglycerides (TG, Sigma-Aldrich; Merck KGaA) were used to establish the in vitro ERS cell model. To investigate the influence of TG on PC12 cell viability, PC12 cells were seeded in 96-well tissue culture plates at a density of 5×104 cells per well in DMEM medium. When the confluence reached 70%, 1, 2.5, 5 and 10 µM TG was added to each well and the cells were incubated at 37°C for 48 h. Cell viability was examined with MTT assay kits (Sigma-Aldrich; Merck KGaA). The blue formazan products in the cells were dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich; Merck KGaA) and spectrophotometrically measured at a wavelength of 550 nm. All experiments were performed in triplicate.
Drug treatment
In brief, 106 PC12 cells were seeded into the 6-well plates for 24 h at 37°C. To establish the in vitro ERS cell model, 5 µM TG were dissolved in DMSO and applied to PC12 cells for 24 h. Cells in the normal control (NC) group was treated with DMSO for 24 h. Subsequently, PC12 cells were treated with 100 ng/ml MTX dissolved in PBS for 48 h and subsequently administered to the cells.
Western blotting
Rats were anesthetized with intraperitoneal injection of 400 mg/kg 10% chloral hydrate (20) and subjected to perfusion with fixatives. Spinal cords were stripped bluntly through the incision on the back of the rat, resulting in a single 1-cm spinal cord segment with the damaged part at the center. The spinal cords were then processed into homogenized tissue using a homogenizer and then the homogenates were centrifuged at 10,000 × g for 15 min at 4°C. Following this, spinal cord tissues or PC12 cells were treated with radioimmunoprecipitation assay buffer (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) containing 1% (v/v) phenylmethylsulfonyl fluoride (Beijing Solarbio Science & Technology Co., Ltd.), 0.3% (v/v) protease inhibitor (Sigma-Aldrich; Merck KGaA) and 0.1% (v/v) phosphorylated proteinase inhibitor (Sigma-Aldrich; Merck KGaA). A BCA protein assay kit (Pierce; Thermo Fisher Scientific, Inc.) was used to determine the protein concentration. Subsequently, supernatants were extracted from the lysates following centrifugation at 11,000 × g at 4°C for 15 min. Equal amounts of protein (15 µg/lane) were separated using 10% SDS-PAGE at 300 mA for 2 h and transferred onto a polyvinylidene fluoride membrane, as previously reported (10). Nonspecific binding was blocked using 8% (w/v) milk in Tris-buffered saline with Tween-20 (TBS-T) for 2 h at room temperature. The following primary antibodies were used: β-actin (cat. no. 4970), cleaved-caspase-3 (cat. no. 9664), GRP78 (cat. no. 3177), CHOP (cat. no. 5554), caspase-12 (cat. no. 2202), ATF6 (cat. no. 65880), IRE1α (cat. no. 3294) and eukaryotic initiation factor 2 (eIF2)α (cat. no. 5324; all 1:1,000 dilution; Cell Signaling Technology, Inc., Danvers, MA, USA). Following several washes with Tris-buffered saline with Tween-20, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5,000; ZB-2306; Zhongshan Gold Bridge Biological Technology Co., Beijing, China) for 2 h at room temperature and then washed with TBS-T. Proteins were detected using enhanced chemiluminescence RapidStep™ ECL, according to the manufacturer's protocol (cat. no. 345818; Merck KGaA). ImageJ 1.8.0 (National Institutes of Health, Bethesda, MD, USA) was applied to quantify the relative protein levels. GAPDH was used as an internal control.
Apoptosis assay
To determine the effects of TG or MTX on the apoptosis of PC12 cells, PC cells (50–60% confluence) were treated with 5 µM TG for 24 h at 37°C. Once they reached 50–60% confluence, PC12 cells were incubated with or without 100 ng/ml MTX at 37°C for 48 h. Subsequently, cells were washed with 1X PBS three times and an Annexin V-fluorescein (FITC)-propidium iodide (PI) Apoptosis kit (Beyotime Institution of Biotechnology, Shanghai, China) according to the manufacturer's protocol. Cells were analyzed by FC500 flow cytometry instrument equipped with CXP software (Beckman Coulter, Bethesda, MA, USA).
Terminal deoxynucleotidyl-transferase-mediated dUTP nick end (TUNEL)
In the SCI model group, apoptotic cells in the spinal cord were examined after 7 days of SCI, while the number of apoptotic cells in the MTX group were evaluated after 7 days post-administration of MTX. Four-µm-thick spinal cord tissues were acquired following fixation, dehydration, paraffin embedding and serial sectioning. Nuclear fragmentation was detected using TUNEL staining with an In Situ Cell Death Detection kit (Roche Diagnostics, Indianapolis, IN, USA) according to the supplier's instructions. The transfected cells were fixed using 4% paraformaldehyde for 30 min, followed by incubation with TUNEL buffer for 1 h at 37°C. After rinsing with PBS, the number of TUNEL-positive apoptotic cells and the total number of cells in five different random high-power fields were counted using a microscope (Olympus Corporation, Tokyo, Japan) at a magnification of 400. The percentage of apoptotic cells was calculated as the ratio of the number of TUNEL-positive cells to the total number of cells.
Statistical analysis
Data were expressed as the mean ± standard error of the mean, as indicated. Each experiment was performed in triplicate. Multiple comparisons were performed using one-way analysis of variance followed by Tukey's multiple comparison test. P<0.05 was considered to indicate a statistically significant difference. The data were analyzed using SPSS software, version 13.0 (SPSS, Inc., Chicago, IL, USA).
Results
MTX inhibits TG-induced PC12 cell apoptosis
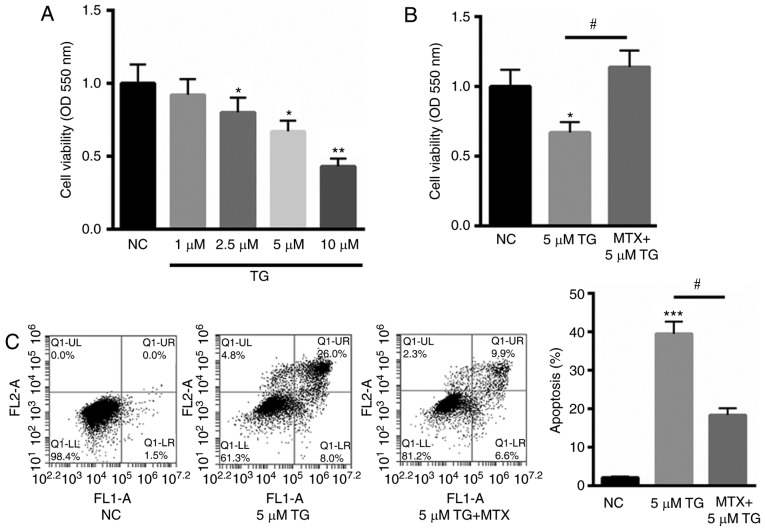
PC12 cells are cancer cells that have been used as a cell model for the study of SCI (24–26) and were therefore selected for use in the present study. PC12 cells were treated with TG to establish an in vitro ERS cell model. As indicated in Fig. 1A, treatment with 1, 2.5, 5 and 10 µM TG decreased cell viability to 92, 80, 67 and 43%, in a dose-dependent manner. As 10 µM TG reduced >50% cell viability, for subsequent experiments, 5 µM TG was selected to stimulate PC12 cells. Subsequently, PC12 cells were stimulated with TG and assessed in the presence or absence of 100 ng/ml MTX. The results indicated that MTX significantly reversed the TG-induced reduction in cell viability (P<0.05; Fig. 1B). Furthermore, the protective role of MTX in TG-induced PC12 cell apoptosis was assessed using Annexin V-FITC staining. Compared with the normal control (NC), 5 µM TG significantly increased cell apoptosis (P<0.001). However, treatment with MTX significantly reduced this effect (P<0.05; Fig. 1C). These results indicate that MTX may inhibit TG-induced apoptosis and may be involved in ERS.
Figure 1.
MTX inhibited TG-induced PC12 cell apoptosis. (A) MTT assay of PC12 cells treated with 1, 2.5, 5, and 10 µM TG. (B) MTX treatment significantly reversed the TG-induced decrease in cell viability by 46.7%. (C) Flow cytometry analysis of PC12 cells treated with 5 µM TG in the presence or absence of MTX. FL1-A on the X-axis represents the relative fluorescence intensity value; FL2-A on the Y-axis represents the relative fluorescence intensity value. Data are presented as the mean ± standard error of the mean. *P<0.05, **P<0.01 and ***P<0.001 vs. NC; #P<0.05. NC, normal control; MTX, methotrexate; TG, triglycerides; OD, optical density.
MTX inhibits TG-induced ERS in PC12 cells
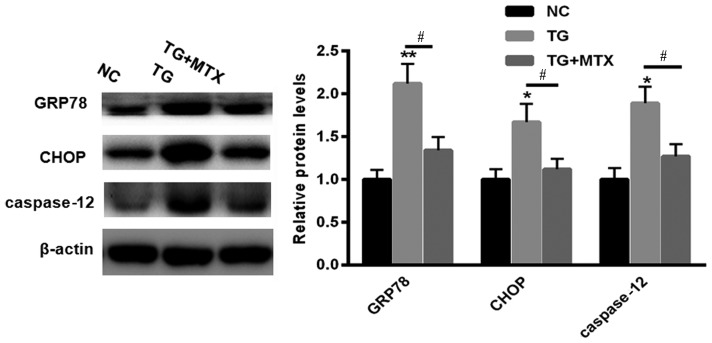
To explore the role of MTX in TG-induced ERS, levels of GRP78, CHOP and caspase-12 expression in PC12 cells incubated with 5 µM TG in the presence or absence of MTX were assessed using western blotting. Levels of GRP78, CHOP and caspase-12 expression were all significantly increased compared with the NC group following TG treatment (P<0.01 or P<0.05; Fig. 2). However, following administration of MTX, levels of GRP78, CHOP and caspase-12 expression were all significantly decreased compared with the PC12 cells that received TG treatment alone (all P<0.05; Fig. 2). These results suggest that MTX may inhibit the ERS induced by TG in PC12 cells.
Figure 2.
MTX reversed the effects of TG on the levels of GRP78, CHOP and caspase-12 expression in PC12 cells. Western blotting was performed to assess the levels of GRP78, CHOP and caspase-12 expression in PC12 cells treated with 5 µM TG in the presence or absence of MTX. Data are presented as the mean ± standard error of the mean. *P<0.05 and **P<0.01 vs. NC; #P<0.05. GRP78, glucose-regulated protein 78; CHOP, CCAAT-enhancer-binding protein homologous protein; NC, normal control (dimethyl sulfoxide); MTX, methotrexate; TG, triglycerides; NC, normal control.
MTX improves motor behavioral function in rats with SCI
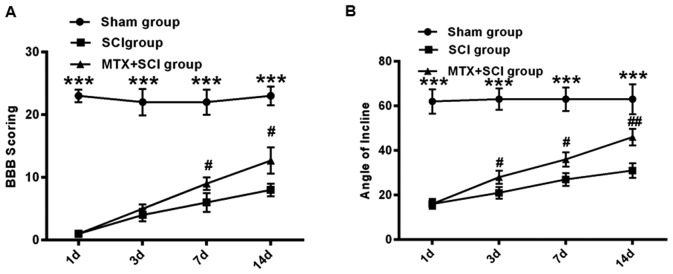
The effect of MTX on ERS was further assessed in the SCI rat model. Following SCI, the hind legs of rats were paralyzed and the rats exhibited limited movement. To assess the hind limb function recovery following MTX administration, the rats in the sham, SCI and MTX+SCI groups were observed and their limb function was scored. Using the BBB scoring system, hind limb function recovery was assessed in rats 1, 3, 7 and 14 days following SCI. Compared with the sham group, severe paralysis was identified in all rats that had undergone SCI (P<0.001; Fig. 3A). However, on days 7 and 14, movement capacity was significantly improved in the MTX+SCI group compared with the SCI group (P<0.05; Fig. 3A). After 14 days, the BBB score was increased by 12.7±2.1 in the MTX+SCI group compared with that in the SCI group (P<0.05; Fig. 3A). Additionally, as determined by inclined plane test, the hind limb strength of rats in the sham group was strong. Rats in the sham group were able to stand on a 65° sloped plate for ≥5 sec. The hind limb strength of rats in the SCI group was significantly weaker, and they were unable to stand on the sloped plate for ≥5 sec (P<0.001). However, rats from the MTX-SCI group exhibited significantly strengthened hind limb force compared with the SCI group 3 and 7 days after SCI initiation (P<0.05), and this improvement was continued 14 days after SCI induction (P<0.01; Fig. 3B).
Figure 3.
MTX improved motor behavioral function in rats with SCI. (A) BBB scoring and the (B) inclined plate test were performed on rats in the sham, SCI and MTX+SCI groups (n=8 rats per group). Data are presented as the mean ± standard error of the mean. ***P<0.001 vs. Sham group; #P<0.05 and ##P<0.01 vs. SCI group. BBB, Basso, Beattie and Bresnahan; SCI, spinal cord injury; MTX, methotrexate.
MTX reduces apoptosis in rats with SCIs
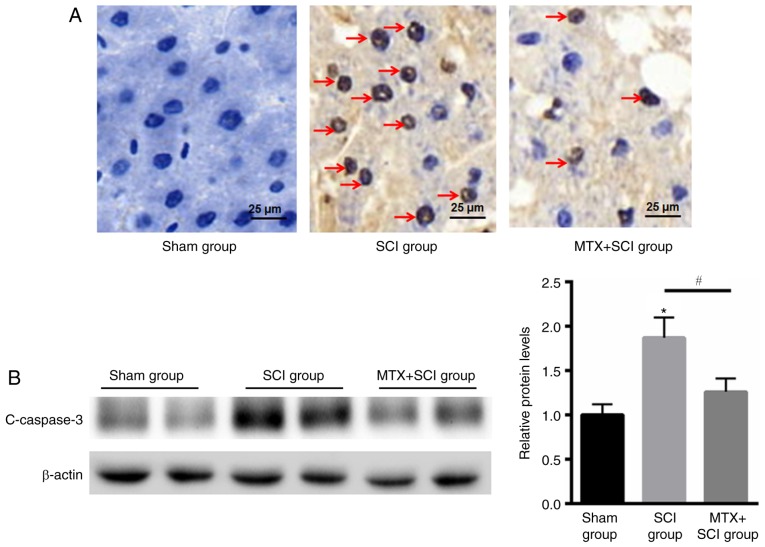
TUNEL staining indicated that there were no apoptotic cells present in rats from the sham group. In the SCI model group apoptotic cells were identified after 7 days, but the number of apoptotic cells was decreased in the MTX group after an additional 7 days (Fig. 4A). The caspase family serves an important role in mediating apoptosis. Caspase-3 is a key molecule that functions in various signaling pathways associated with apoptotic signaling (27). To determine the anti-apoptotic effect of MTX in rat spinal cord nerve cells, levels of cleaved caspase-3 expression were assessed using western blotting. The results indicated that levels of cleaved caspase-3 expression were significantly increased in rats that had undergone SCI compared with the sham group; however, MTX treatment significantly attenuated this effect at day 7 (P<0.05; Fig. 4B). These data suggested that MTX may protect rats from SCI by suppressing apoptosis.
Figure 4.
MTX reduced apoptosis in rats with SCI. (A) Terminal deoxynucleotidyl-transferase-mediated dUTP nick end staining (magnification, ×40) indicated that the sham group demonstrated no obvious cell apoptosis with blue staining, whereas an increased number of apoptotic cells in SCI group on day 7 and improvement of cell apoptosis after MTX treatment on day 7 were identified (TUNEL-positive nuclei were stained brown and TUNEL-negative nuclei were stained blue; red arrows indicated apoptotic cells). (B) Western blotting of c-caspase-3 protein expression in the sham, SCI and MTX+SCI group (n=8 rats per group). Data are presented as the mean ± standard error of the mean. *P<0.05 vs. Sham; #P<0.05. SCI, spinal cord injury; MTX, methotrexate; c-caspase-3 (cleaved-caspase-3).
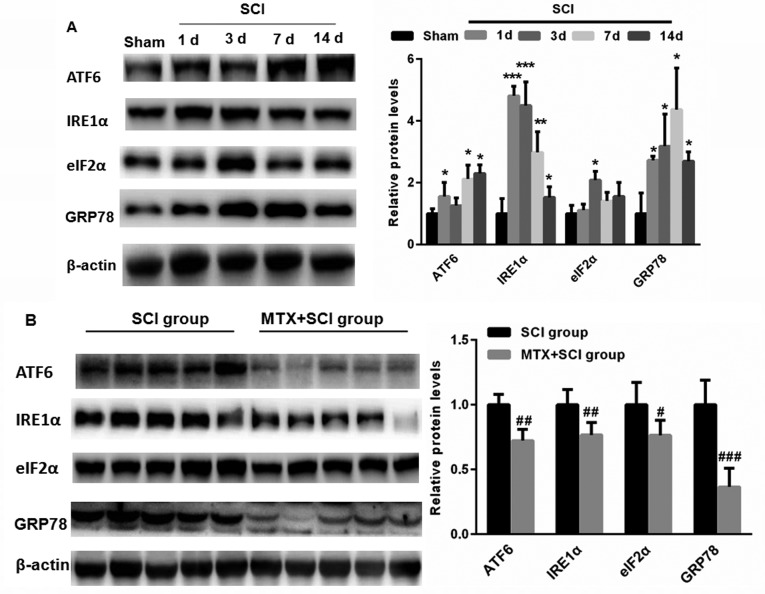
MTX inhibits the expression of GRP78 in cells in the region of SCI
To detect whether SCI induces ERS, levels of ATF6, IRE1α, eIF2α and GRP78 expression were assed using western blotting. Compared with sham group, levels of ATF6, IRE1α, eIF2α and GRP78 expression were significantly increased following SCI (P<0.05, P<0.01 and P<0.001; Fig. 5A). This suggests that SCI may induce ERS. Notably, on day 7, levels of ATF6, IRE1α, eIF2α and GRP78 expression were significantly decreased in the MTX+SCI group compared with the SCI group on day 7 days (each band represented one rat; P<0.05, P<0.01 and P<0.001; Fig. 5B). These data indicate that MTX may protect against ERS.
Figure 5.
MTX inhibited the expression of endoplasmic reticulum stress-associated proteins in the region of SCI. (A) Compared with the sham group, levels of ATF6, IRE1α, eIF2α and GRP78 expression were significantly increased in rats following SCI. (B) Following treatment with MTX, levels of ATF6, IRE1α, eIF2α and GRP78 expression were significantly decreased compared with the SCI group (n=8 rats per group). Data are presented as the mean ± standard error of the mean. *P<0.05, **P<0.01, ***P<0.001 vs. Sham; #P<0.05, ##P<0.01, ###P<0.001 vs. SCI. SCI, spinal cord injury; MTX, methotrexate; ATF6, activating transcription factor 6; IRE1α, serine/threonine-protein kinase/endoribonuclease inositol-requiring enzyme 1 α; eIF2α, eukaryotic initiation factor 2 α; GRP78, glucose-regulated protein 78; c-caspase-3, cleaved caspase-3.
Discussion
MTX is the most commonly used disease-modifying anti-rheumatic agent and is also effective at treating patients with breast cancer (28,29). Previous studies have demonstrated that MTX induces greater cytotoxicity and growth inhibition in leukemia and other malignant cell types compared with other treatments (30,31). It has been demonstrated that sustained ERS is a major contributor to MTX-induced cell death, as indicated by the induction of several unfolded protein response markers, which ultimately induce apoptosis (32). Furthermore, MTX prevents epidural fibrosis via the ERS signaling pathway (33). The present study aimed to evaluate the possible protective effects and the potential mechanisms of low-dose MTX in rats with SCI and in PC12 cells.
In the present study, an in vitro cell model of ERS was established. PC12 cells were treated with 1, 2.5, 5 and 10 µM TG, and MTT assays demonstrated that TG decreased PC12 cell viability in a dose-dependent manner. Additionally, flow cytometric analysis indicated that TG significantly increased the apoptosis of PC12 cells. It has been suggested that, during the early stages of ERS, upregulation of GRP78 may aid cell survival by removing misfolded proteins; however, prolonged elevation of ERS levels may induce cell death (34). In addition to the activation of GRP78 during sustained ERS, the activation of CHOP, p53 upregulated modulator of apoptosis and caspase-12 is induced, thereby further promoting cell death (12,35). In accordance with previous studies, the results of the present study indicated that levels of the ERS-associated proteins GRP78, CHOP and caspase-12 were significantly increased in PC12 cells following treatment with TG. An increase in cell apoptosis and the expression of ERS-associated proteins was identified in a rat model of SCI. The results of the present study imply that ERS may be involved in this process.
Apoptosis is a type of programmed cell death that differs from necrosis (36). This process serves an important role in SCI, particularly during secondary injury. By blocking apoptosis, cells may survive and retain more nerve function (37). Neuronal apoptosis following SCI is an important pathological process and often leads to secondary injury in SCI (38). It has been reported that excessive apoptosis may result in decreased functioning of the spinal cord anterior horn and the spinal nerve fibers (38).
The ERS response regulates the homeostasis of organisms by modulating protein degradation and protein folding (39). Under normal physiological conditions, the ER chaperone GRP78 combines with PERK, ATF6 and IRE1, and is maintained in its inactive state (40). When the UPR is activated, unfolded proteins accumulate in the ER, which then trigger the ERS-induced cell apoptosis (41). GRP78, CHOP/GADDl53 and caspase-12 are ERS-specific transcription factors that are not activated in either the death receptor or the mitochondrial signaling pathways (42). In the present study, the motor behavioral function of rats with SCI was significantly improved following MTX treatment on day 7. Furthermore, the present study revealed that decreased neuronal cell apoptosis was accompanied by significantly reduced levels of GRP78, CHOP and caspase-12 expression, indicating that the neuroprotective effects of MTX may be associated with the inhibition of ERS-induced apoptosis.
In conclusion, the present study revealed that MTX treatment increased neuronal survival in the region of SCI and improved the functional recovery of rats with acute SCI. According to the results from the present in vivo and in vitro experiments, it was suggested that the protective effect of MTX in neuronal cells may be mediated by the inhibition of ERS-induced apoptosis. These data suggest that MTX is suitable for the treatment of SCI as it is able to reduce ERS-induced apoptosis. However, further studies are required to determine the most effective application of MTX in the treatment of SCI.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from Doctoral Fund of Mudanjiang Medical University (grant no. MDJU-20170234).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
FR performed the experiments and analyzed the data. WT performed the experiments. XG and KL performed part of the animal experiments. JW designed the whole experiments, analyzed the data and gave final approval of the version to be published.
Ethics approval and consent to participate
The present study was approved by the Animal Ethics Committee of the Hongqi Hospital Affiliated to Mudanjiang Medical University (Mudanjiang, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Motiei-Langroudi R, Sadeghian H. Traumatic spinal cord injury: Long-term motor, sensory, and urinary outcomes. Asian Spine J. 2017;11:412–418. doi: 10.4184/asj.2017.11.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shank CD, Walters BC, Hadley MN. Management of acute traumatic spinal cord injuries. Handb Clin Neurol. 2017;140:275–298. doi: 10.1016/B978-0-444-63600-3.00015-5. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Tang Y, Allen V, DeVivo MJ. Aging and spinal cord injury: External causes of injury and implications for prevention. Top Spinal Cord Inj Rehabil. 2015;21:218–226. doi: 10.1310/sci2103-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JY, Maeng S, Kang SR, Choi HY, Oh TH, Ju BG, Yune TY. Valproic acid protects motor neuron death by inhibiting oxidative stress and endoplasmic reticulum stress-mediated cytochrome C release after spinal cord injury. J Neurotrauma. 2014;31:582–594. doi: 10.1089/neu.2013.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Yang J, Li Z, Liang F, Wang Y, Su Q, Li C. Hyperbaric oxygen treatment protects against spinal cord injury by inhibiting endoplasmic reticulum stress in rats. Spine (Phila Pa 1976) 2015;40:E1276–E1283. doi: 10.1097/BRS.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 6.Matsuyama D, Watanabe M, Suyama K, Kuroiwa M, Mochida J. Endoplasmic reticulum stress response in the rat contusive spinal cord injury model-susceptibility in specific cell types. Spinal Cord. 2014;52:9–16. doi: 10.1038/sc.2013.118. [DOI] [PubMed] [Google Scholar]
- 7.Ohri SS, Maddie MA, Zhang Y, Shields CB, Hetman M, Whittemore SR. Deletion of the pro-apoptotic endoplasmic reticulum stress response effector CHOP does not result in improved locomotor function after severe contusive spinal cord injury. J Neurotrauma. 2012;29:579–588. doi: 10.1089/neu.2011.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang ZL, Zhou ZG, Chen Y, Li XT, Sun YS. Support vector machines model of computed tomography for assessing lymph node metastasis in esophageal cancer with neoadjuvant chemotherapy. J Comput Assist Tomogr. 2017;41:455–460. doi: 10.1097/RCT.0000000000000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohri SS, Maddie MA, Zhao Y, Qiu MS, Hetman M, Whittemore SR. Attenuating the endoplasmic reticulum stress response improves functional recovery after spinal cord injury. Glia. 2011;59:1489–1502. doi: 10.1002/glia.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohri SS, Mullins A, Hetman M, Whittemore SR. Inhibition of GADD34, the stress-inducible regulatory subunit of the endoplasmic reticulum stress response, does not enhance functional recovery after spinal cord injury. PLoS One. 2014;9:e109703. doi: 10.1371/journal.pone.0109703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penas C, Guzmán MS, Verdú E, Forés J, Navarro X, Casas C. Spinal cord injury induces endoplasmic reticulum stress with different cell-type dependent response. J Neurochem. 2007;102:1242–1255. doi: 10.1111/j.1471-4159.2007.04671.x. [DOI] [PubMed] [Google Scholar]
- 12.Tka Hadj Ayed K, Boussaid Mahfoudh A, Zaouali MA, Kammoun R, Bejaoui M, Ghoul Mazgar S, Catafau Rosello J, Ben Abdennebi H. Melatonin modulates endoplasmic reticulum stress and Akt/GSK3-beta signaling pathway in a rat model of renal warm ischemia reperfusion. Anal Cell Pathol (Amst) 2015;2015:635172. doi: 10.1155/2015/635172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halliday M, Mallucci GR. Review: Modulating the unfolded protein response to prevent neurodegeneration and enhance memory. Neuropathol Appl Neurobiol. 2015;41:414–427. doi: 10.1111/nan.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben Mosbah I, Alfany-Fernández I, Martel C, Zaouali MA, Bintanel-Morcillo M, Rimola A, Rodés J, Brenner C, Roselló-Catafau J, Peralta C. Endoplasmic reticulum stress inhibition protects steatotic and non-steatotic livers in partial hepatectomy under ischemia-reperfusion. Cell Death Dis. 2010;1:e52. doi: 10.1038/cddis.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Shao H, Liu W, Gu W, Shu X, Mo Y, Chen X, Zhang Q, Jiang M. Endoplasmic reticulum stress and oxidative stress are involved in ZnO nanoparticle-induced hepatotoxicity. Toxicol Lett. 2015;234:40–49. doi: 10.1016/j.toxlet.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtis JR, Wright GC, Strand V, Davis CS, Hitraya E, Sasso EH. Reanalysis of the multi-biomarker disease activity score for assessing disease activity in the abatacept versus adalimumab comparison in biologic-naive rheumatoid arthritis subjects with background methotrexate study: Comment on the Article by Fleischmann et al. Arthritis Rheumatol. 2017;69:863–865. doi: 10.1002/art.39981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleischmann R, Mease PJ, Schwartzman S, Hwang LJ, Soma K, Connell CA, Takiya L, Bananis E. Efficacy of tofacitinib in patients with rheumatoid arthritis stratified by background methotrexate dose group. Clin Rheumatol. 2017;36:15–24. doi: 10.1007/s10067-016-3436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito Y, Hozumi K, Okada Y, Kurimoto S. Adalimumab with methotrexate in treatment-naive Japanese patients with rheumatoid arthritis at risk of progressive structural joint damage: A postmarketing observational study. Rheumatol Ther. 2017;4:151–166. doi: 10.1007/s40744-017-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Li J, Liang S, Lin B, Peng Q, Zhao P, Cui J, Rao Y. Effect of hypoxia-inducible factor-1/vascular endothelial growth factor signaling pathway on spinal cord injury in rats. Exp Ther Med. 2017;13:861–866. doi: 10.3892/etm.2017.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vachon P, Faubert S, Blais D, Comtois A, Bienvenu JG. A pathophysiological study of abdominal organs following intraperitoneal injections of chloral hydrate in rats: comparison between two anaesthesia protocols. Lab Anim. 2000;34:84–90. doi: 10.1258/002367700780578082. [DOI] [PubMed] [Google Scholar]
- 21.Nakase Y, Hagiwara A, Kin S, Fukuda K, Ito T, Takagi T, Fujiyama J, Sakakura C, Otsuji E, Yamagishi H. Intratumoral administration of methotrexate bound to activated carbon particles: Antitumor effectiveness against human colon carcinoma xenografts and acute toxicity in mice. J Pharmacol Exp Ther. 2004;311:382–387. doi: 10.1124/jpet.104.069450. [DOI] [PubMed] [Google Scholar]
- 22.Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 23.Rivlin AS, Tator CH. Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg. 1977;47:577–581. doi: 10.3171/jns.1977.47.4.0577. [DOI] [PubMed] [Google Scholar]
- 24.Qiao Y, Peng C, Li J, Wu D, Wang X. Spinal cord ischemia-reperfusion causes damage of neurocyte by inhibiting RAP2C. Neurol Res. 2017;39:877–884. doi: 10.1080/01616412.2017.1352120. [DOI] [PubMed] [Google Scholar]
- 25.Tian R, Shi R. Dimercaprol is an acrolein scavenger that mitigates acrolein-mediated PC-12 cells toxicity and reduces acrolein in rat following spinal cord injury. J Neurochem. 2017;141:708–720. doi: 10.1111/jnc.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee DH, Seubert S, Huhn K, Brecht L, Rötger C, Waschbisch A, Schlachetzki J, Klausmeyer A, Melms A, Wiese S, et al. Fingolimod effects in neuroinflammation: Regulation of astroglial glutamate transporters? PLoS One. 2017;12:e0171552. doi: 10.1371/journal.pone.0171552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia Y, Xia H, Chen D, Liao Z, Yan Y. Mechanisms of autophagy and apoptosis mediated by JAK2 signaling pathway after spinal cord injury of rats. Exp Ther Med. 2017;14:1589–1593. doi: 10.3892/etm.2017.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JH, Im SA, Byun JM, Kim KH, Kim JS, Choi IS, Kim HJ, Lee KH, Kim TY, Han SW, et al. Cyclophosphamide, methotrexate and 5-fluorouracil as palliative treatment for heavily pretreated patients with metastatic breast cancer: A multicenter retrospective analysis. J Breast Cancer. 2017;20:347–355. doi: 10.4048/jbc.2017.20.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fekry B, Esmaeilniakooshkghazi A, Krupenko SA, Krupenko NI. Ceramide synthase 6 is a novel target of methotrexate mediating its antiproliferative effect in a p53-dependent manner. PLoS One. 2016;11:e0146618. doi: 10.1371/journal.pone.0146618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giordano L, Akinyede O, Bhatt N, Dighe D, Iqbal A. Methotrexate-induced neurotoxicity in hispanic adolescents with high-risk acute leukemia-a case series. J Adolesc Young Adult Oncol. 2017;6:494–498. doi: 10.1089/jayao.2016.0094. [DOI] [PubMed] [Google Scholar]
- 31.Bohme D, Krieghoff J, Beck-Sickinger AG. Double methotrexate-modified neuropeptide Y analogues express increased toxicity and overcome drug resistance in breast cancer cells. J Med Chem. 2016;59:3409–3417. doi: 10.1021/acs.jmedchem.6b00043. [DOI] [PubMed] [Google Scholar]
- 32.Kuznetsov JN, Leclerc GJ, Leclerc GM, Barredo JC. AMPK and Akt determine apoptotic cell death following perturbations of one-carbon metabolism by regulating ER stress in acute lymphoblastic leukemia. Mol Cancer Ther. 2011;10:437–447. doi: 10.1158/1535-7163.MCT-10-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen H, Yan L, Wang J, Sun Y, Li X, Zhao S, Wang D, Zhu G, Liang Y. Methotrexate prevents epidural fibrosis through endoplasmic reticulum stress signalling pathway. Eur J Pharmacol. 2017;796:131–138. doi: 10.1016/j.ejphar.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 34.Sharma S, Sarkar J, Haldar C, Sinha S. Melatonin reverses fas, E2F-1 and endoplasmic reticulum stress mediated apoptosis and dysregulation of autophagy induced by the herbicide atrazine in murine splenocytes. PLoS One. 2014;9:e108602. doi: 10.1371/journal.pone.0108602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuñón MJ, San-Miguel B, Crespo I, Laliena A, Vallejo D, Álvarez M, Prieto J, González-Gallego J. Melatonin treatment reduces endoplasmic reticulum stress and modulates the unfolded protein response in rabbits with lethal fulminant hepatitis of viral origin. J Pineal Res. 2013;55:221–228. doi: 10.1111/jpi.12063. [DOI] [PubMed] [Google Scholar]
- 36.Sui T, Ge DW, Yang L, Tang J, Cao XJ, Ge YB. Mitomycin C induces apoptosis in human epidural scar fibroblasts after surgical decompression for spinal cord injury. Neural Regen Res. 2017;12:644–653. doi: 10.4103/1673-5374.205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He Z, Zhou Y, Huang Y, Wang Q, Zheng B, Zhang H, Li J, Liu Y, Wu F, Zhang X, et al. Dl-3-n-butylphthalide improves functional recovery in rats with spinal cord injury by inhibiting endoplasmic reticulum stress-induced apoptosis. Am J Transl Res. 2017;9:1075–1087. [PMC free article] [PubMed] [Google Scholar]
- 38.Pei JP, Fan LH, Nan K, Li J, Dang XQ, Wang KZ. HSYA alleviates secondary neuronal death through attenuating oxidative stress, inflammatory response, and neural apoptosis in SD rat spinal cord compression injury. J Neuroinflammation. 2017;14:97. doi: 10.1186/s12974-017-0870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C, Shi D, Song X, Chen Y, Wang L, Zhang X. Calpain inhibitor attenuates ER stress-induced apoptosis in injured spinal cord after bone mesenchymal stem cells transplantation. Neurochem Int. 2016;97:15–25. doi: 10.1016/j.neuint.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 40.Xue Q, Li C, Chen J, Guo H, Li D, Wu X. The protective effect of the endoplasmic reticulum stress-related factors BiP/GRP78 and CHOP/Gadd153 on noise-induced hearing loss in guinea pigs. Noise Health. 2016;18:247–255. doi: 10.4103/1463-1741.192481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu SP, Wang ZG, Zhao YZ, Wu J, Shi HX, Ye LB, Wu FZ, Cheng Y, Zhang HY, He S, et al. Gelatin nanostructured lipid carriers incorporating nerve growth factor inhibit endoplasmic reticulum stress-induced apoptosis and improve recovery in spinal cord injury. Mol Neurobiol. 2016;53:4375–4386. doi: 10.1007/s12035-015-9372-2. [DOI] [PubMed] [Google Scholar]
- 42.Feng J, Chen X, Sun X, Wang F, Sun X. Expression of endoplasmic reticulum stress markers GRP78 and CHOP induced by oxidative stress in blue light-mediated damage of A2E-containing retinal pigment epithelium cells. Ophthalmic Res. 2014;52:224–233. doi: 10.1159/000363387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.