Abstract
We have identified many dark-inducible (din) genes that are expressed in Arabidopsis leaves kept in the dark. In the present study we addressed the question of how plant cells sense the depletion of sugars, and how sugar starvation triggers din gene expression in suspension-cultured cells of Arabidopsis. Depletion of sucrose in the medium triggered marked accumulation of din transcripts. Suppression of din gene expression by 2-deoxy-Glc, and a non-suppressive effect exerted by 3-O-methyl-Glc, suggested that sugar-repressible expression of din genes is mediated through the phosphorylation of hexose by hexokinase, as exemplified in the repression of photosynthetic genes by sugars. We have further shown that the signaling triggered by sugar starvation involves protein phosphorylation and dephosphorylation events, and have provided the first evidence that multiple pathways of protein dephosphorylation exist in sugar starvation-induced gene expression. An inhibitor of serine/threonine protein kinase, K-252a, inhibited din gene expression in sugar-depleted cells. Okadaic acid, which may preferentially inhibit type 2A protein phosphatases over type 1, enhanced the transcript levels of all din genes, except din6 and din10, under sugar starvation. Conversely, a more potent inhibitor of type 1 and 2A protein phosphatases, calyculin A, increased transcripts from din2 and din9, but decreased those from other din genes, in sugar-depleted cells. On the other hand, calyculin A, but not okadaic acid, completely inhibited the gene expression of chlorophyll a/b-binding protein under sugar starvation. These results indicate that multiple signaling pathways, mediated by different types of protein phosphatases, regulate gene expression during sugar starvation.
Sugars are major respiratory substrates in plant cells. However, plants easily fall into sugar starvation under conditions such as leaf senescence (Hensel et al., 1993), darkness (Brouquisse et al., 1998), and in post-harvest stages (Davies et al., 1996), all of which inevitably result in a significant decrease in photosynthesis.
Sugar starvation induces enzymatic activities related to the degradation of proteins (James et al., 1993; Moriyasu and Ohsumi, 1996), and the catabolism of fatty acids (Dieuaide et al., 1992) and amino acids (Brouquisse et al., 1992). These studies imply that plants survive sugar starvation by substituting protein and lipid catabolism for sugar catabolism (Journet et al., 1986; Yu, 1999). Besides these biochemical changes, sugar starvation has been shown to induce the expression of various genes (Yu et al., 1991; Graham et al., 1994; Chevalier et al., 1995; Koch, 1996; Prata et al., 1997). However, little is known about the mechanisms controlling gene expression associated with sugar starvation.
The mechanism of sugar-modulated gene expression has been studied extensively in yeast (Gancedo, 1998). Hexokinase and SNF1 protein kinase are known to play critical roles in sugar signaling in yeast (Gancedo, 1998). Several reports have suggested that plants have evolved a similar sugar sensing mechanism (Smeekens and Rook, 1997). One well-characterized example in plants involves a hexokinase-mediated sugar sensing system in the repression of photosynthetic genes by hexose (Jang and Sheen, 1994; Jang et al., 1997; Moore and Sheen, 1999). In a similar manner, the importance of phosphorylation of hexose by hexokinase was proposed for sugar suppression of non-photosynthetic genes (Graham et al., 1994; Prata et al., 1997; Umemura et al., 1998). Another key component in sugar signaling, a homolog of SNF1, has been isolated from a variety of plants (Halford and Hardie, 1998). Several plant homologs have been shown to complement snf1 mutations in yeast, suggesting that there might be an SNF1-dependent sugar-signaling pathway in plants (Halford and Hardie, 1998; Halford et al., 1999). However, no study has presented evidence for the involvement of plant SNF1 homologs in the regulation of gene expression under sugar starvation (Halford and Hardie, 1998).
Besides the sugar-sensing system mediated by hexokinase, the existence of a Suc-specific sensor and a hexose transporter-associated sensor has been suggested (Smeekens and Rook, 1997; Lalonde et al., 1999). Despite considerable progress in recent years, many crucial elements in these pathways are still unknown (Koch et al., 2000; Pego et al., 2000).
In our attempt to understand the response of plants to photosynthetically unfavorable light conditions, we have isolated and characterized dozens of dark-inducible (din) genes from Arabidopsis and radish, the transcripts of which accumulate in leaves kept in the dark (Azumi and Watanabe, 1991; Fujiki et al., 1997, 2000; Shimada et al., 1998; Nakabayashi et al., 1999; Nozawa et al., 1999). We found that application of 3% (w/v) Suc to detached leaves prevented dark-induced expression of din genes, suggesting that sugar deprivation plays a key role in din gene expression in leaves exposed to unfavorable light conditions (Fujiki et al., 2000).
In this study we took a pharmacological approach to identify signaling processes in sugar starvation-inducible gene expression in suspension-cultured cells of Arabidopsis, using a set of din genes (Table I) and the chlorophyll a/b-binding protein (Cab) gene as model genes. We found that the sugar sensing system for the suppression of din genes by sugars involved phosphorylation of hexose by hexokinase, as previously shown for the Cab gene. In addition, we have shown that protein phosphorylation and dephosphorylation events are involved in sugar starvation-induced gene expression. Furthermore, we have found that multiple pathways, coordinated by different protein phosphatases, control gene expression during sugar starvation. Application of okadaic acid enhanced transcript levels for all din genes, except din6 and din10, whereas calyculin A increased transcript levels for din2 and din9, but decreased those for other din genes during sugar starvation. In contrast, okadaic acid had no inhibitory effect on Cab gene expression, whereas calyculin A had a strong inhibitory effect, independent of sugar. These results reveal that multiple regulatory pathways lead to sugar starvation-induced gene expression, and that din genes constitute useful molecular markers for analysis of such regulation.
Table I.
Dark-inducible genes in Arabidopsis examined in this study
| Gene Name | Gene Product | Reference |
|---|---|---|
| din1 | Sulfide dehydrogenase (SEN1) | Shimada et al. (1998) |
| din2 | β-Glucosidase | Fujiki et al. (1997) |
| din3 | BCKDH E2-subunita | Fujiki et al. (2000) |
| din4 | BCKDH E1β-subunit | Fujiki et al. (2000) |
| din6 | Asparagine synthetase (ASN1) | Nozawa et al. (1999) |
| din9 | Mannose-6-phosphate isomerase | Fujiki et al. (1997) |
| din10 | Seed imbibition protein | Fujiki et al. (1997) |
BCKDH, Branched-chain α-keto acid dehydrogenase.
RESULTS
Expression of din Genes in Suc-Starved Cells
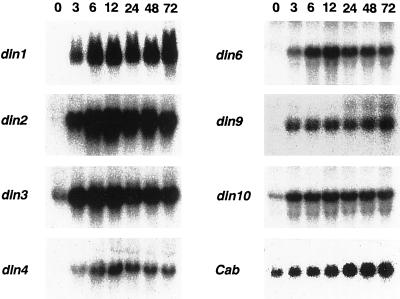
Northern-blot analysis was performed to examine the accumulation of transcripts from din genes in Arabidopsis suspension-cultured cells subjected to Suc starvation. Cells were transferred to a Suc-free medium, and then collected from the medium after varying periods of time up to 72 h. Transcripts from all din genes examined began to accumulate immediately after the depletion of Suc, and transcript levels reached a maximum at 12 h of Suc starvation. (Fig. 1).
Figure 1.
Time course analysis of the expression of din genes during Suc starvation. Total RNA was isolated from cells incubated in the absence of Suc for varying lengths of time up to 72 h. An equal amount of RNA (20 μg) was loaded in each lane and analyzed by northern-blot hybridization. In this study we examined the expression of the Cab gene as a control for sugar-repressible genes.
In contrast, when cells were incubated in a Suc-free medium for 12 h and then returned to a Suc-containing medium, the transcripts accumulated in sugar-starved cells disappeared within 4 h of Suc feeding (data not shown). These results suggest that the expression of din genes is repressed by Suc. Because it is well known that the expression of photosynthetic genes is repressed by sugars (Jang and Sheen, 1994), we examined the expression of the Cab gene as a typical example of a sugar-repressible gene. The transcript level of the Cab gene in Suc-fed cells was maintained at a basal level (Fig. 1, time 0). Once Suc was removed from the medium, the repression by sugar was eliminated, and the transcript levels of the Cab gene began to increase with kinetics similar to those observed for din genes. This implied that din genes and the Cab gene share, in part, a common mechanism for sugar-repressible gene expression.
Effect of Glc Analogs on the Expression of din Genes
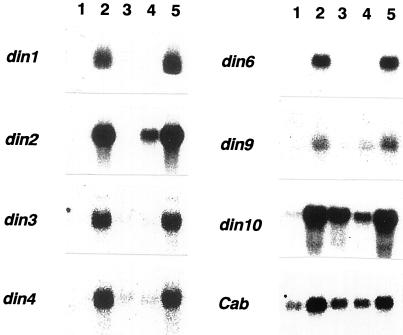
Several studies using Glc analogs and inhibitors of hexokinase have described a putative role for hexokinase and/or the phosphorylation of hexose in sugar repression of gene expression (Jang and Sheen, 1994; Prata et al., 1997; Umemura et al., 1998). We examined whether the phosphorylation of hexose by hexokinase is involved in the regulation of din gene expression by using Glc analogs. Seven-day-old cells were washed with a Suc-free medium and incubated for 12 h with a fresh medium containing 10 mm Glc, 10 mm 3-O-methyl-Glc (3-OMG), or 0.5 mm 2-deoxy-Glc (2-d-Glc). 3-OMG cannot be phosphorylated by hexokinase, and thus generates no sugar repression signal through hexokinase (Jang and Sheen, 1994, and refs. therein). In contrast, 2-d-Glc can be phosphorylated by hexokinase in plant cells, and in turn can initiate hexokinase-mediated sugar signaling, but cannot be easily metabolized further (Jang and Sheen, 1994; and refs. therein). The application of 2-d-Glc abolished the accumulation of transcripts from the din genes, whereas 3-OMG did not suppress din gene expression (Fig. 2). These expression patterns apparently resembled that of the Cab gene, which is repressed by sugars in a hexokinase-dependent manner (Jang and Sheen, 1994; see also Fig. 2), suggesting that din genes and the Cab gene are subject to a common sugar-sensing mechanism through the phosphorylation of hexose. However, it should be noted that the expression of the Cab gene was not completely suppressed even in the presence of 2% (w/v) Suc, 10 mm Glc, or 0.5 mm 2-d-Glc in our experimental system (Fig. 2). The Cab gene is known to be positively regulated by light (Terzaghi and Cashmore, 1995; Argüello-Astorga and Herrera-Estrella, 1998), and so the expression of this gene might have been, in part, induced by light since the cells were incubated under illumination in our system. In contrast, din genes are not positively regulated by light (Fujiki et al., 2000).
Figure 2.
Effects of Glc analogs on the expression of din genes. Seven-day-old cells were rinsed and incubated for 12 h with fresh medium containing 10 mm Glc (lane 3), 0.5 mm 2-d-Glc (lane 4), or 10 mm 3-OMG (lane 5). RNA isolated from non-treated (2% [w/v] Suc, lane 1) and Suc-starved (lane 2) cells was used as a control. Each lane was loaded with 10 μg of RNA.
The application of 0.5 mm 2-d-Glc incompletely suppressed the expression of din2 and din10 (Fig. 2, lane 4). Because expression of din2 and din10 was strongly suppressed in the presence of 2% (w/v) Suc (Fig. 2, lane 1), the suppression of din2 and din10 may require a higher concentration of sugar than did other din genes.
Requirement of Protein Synthesis for the Expression of din Genes during Sugar Starvation
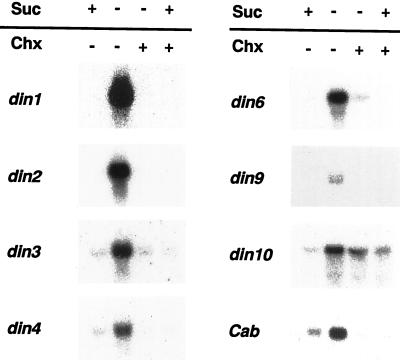
We examined whether protein synthesis is required in the process of din gene expression during sugar starvation. Addition to the culture medium of 20 μm cycloheximide, an inhibitor of cytosolic translation, completely blocked the sugar starvation-induced accumulation of transcripts from all din genes except din10 and partly abolished din10 expression (Fig. 3, lane 3). This result suggested that the sugar starvation-induced expression of din genes requires the synthesis of new proteins.
Figure 3.
Effect of cycloheximide on the expression of din genes during Suc starvation. For the treatment of cycloheximide, cells were pre-incubated for 1 h with 20 μm of cycloheximide (chx). Cells were incubated for 12 h either in a Suc-free (−) or a Suc-containing medium (+), with or without cycloheximide. Each lane was loaded with 10 μg of RNA.
With respect to din10 expression, a higher concentration of cycloheximide was required for its complete inhibition in sugar-starved cells (data not shown). In addition, it should be noted that the suppression of din10 expression by Suc seemed to be partially relieved by 20 μm cycloheximide (Fig. 3, lane 4). These results suggest that regulation of the expression of din10 differs, to a certain degree, from that of other din genes. Sheu et al. (1996) reported that cycloheximide, at concentrations ranging from 20 to 300 μm, blocked the suppression of a rice α-amylase gene (αAmy3) by Suc, resulting in marked accumulation of its transcripts. In contrast, we found that cycloheximide at 200 μm, but not at 20 μm, inhibited the accumulation of din10 transcripts (data not shown). These results imply that the regulation of din10 gene expression by sugars is distinct not only from that of other din genes, but also from that of an αAmy3.
Effects of Inhibitors of Protein Kinases and Phosphatases on the Expression of din Genes
Protein phosphorylation and dephosphorylation events are known to regulate numerous biological processes (Luan, 1998). By using various inhibitors of protein kinases and phosphatases, we examined whether protein phosphorylation and dephosphorylation events are involved in the expression of din genes during Suc starvation. These inhibitors were dissolved in dimethyl sulfoxide, and used at concentrations known to be effective in Arabidopsis cell culture (Christie and Jenkins, 1996). In our preliminary experiments, dimethyl sulfoxide alone did not alter the expression pattern of din genes in cells with or without Suc (data not shown).
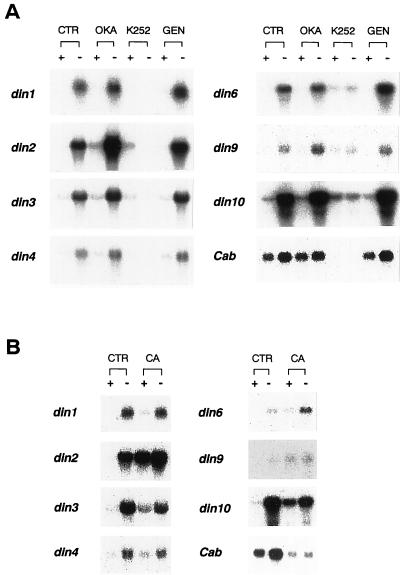
Incubation of cells with 4 μm K-252a, a general Ser/Thr protein kinase inhibitor, prevented the accumulation of din transcripts in sugar-starved cells. In contrast, 75 μm genistein, a Thy/His kinase inhibitor, had no effect on gene expression (Fig. 4A). These results suggest that Ser/Thr protein kinases, but not Thy/His kinases, are involved in the processes of din gene expression during sugar starvation. Accumulation of the transcripts from the Cab gene was completely inhibited by K-252a, but not by genistein (Fig. 4A).
Figure 4.
Effects of inhibitors of protein phosphatases and protein kinases on the expression of din genes. A, Cells were pre-incubated for 1 h with 1 μm okadaic acid (OKA), 4 μm K-252a (K252), or 75 μm genistein (GEN). Cells were rinsed and incubated with each inhibitor for 12 h in a Suc-free (−) or a Suc containing (+) medium. Cells were also incubated for 12 h in the absence of inhibitors (CTR). B, Cells were incubated with 1 μm of calyculin A (CA) for 12 h, either in a Suc-free (−) or a Suc-containing (+) medium. Each lane was loaded with 10 μg of RNA.
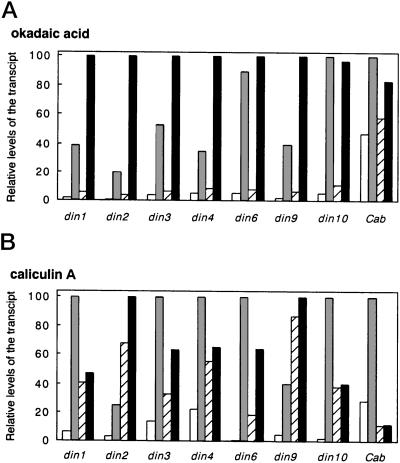
Figure 5 summarizes the relative mRNA levels of din genes and the Cab gene in cells treated with protein phosphatase inhibitors. The application of 1 μm okadaic acid, known to preferentially inhibit protein phosphatase type 2A (PP2A) over type 1 (PP1; Cohen et al., 1990), enhanced transcript levels of all din genes, except din6 and din10, in sugar-depleted cells (Figs. 4A and 5A). On the other hand, okadaic acid had little inhibitory effect on din6 and din10 expression under sugar starvation. The Cab gene expression similarly was not influenced by okadaic acid (Figs. 4A and 5A).
Figure 5.
Effects of protein phosphatase inhibitors on the relative mRNA levels of din genes. Relative levels of the transcripts were estimated by quantitation of signals on the blotted membranes (Fig. 4), with a Bio Imaging Analyzer BAS2000 (Fuji Photo Film, Tokyo). The results were expressed as a percentage of the respective maximum level for each gene. Cells were incubated in a Suc-containing (white bars) or a Suc-free (shaded bars) medium for 12 h in the absence of inhibitors. For the treatment of inhibitors, cells were incubated with okadaic acid (A) or calyculin A (B) for 12 h in a Suc-containing (hatched bars) or a Suc-free (black bars) medium.
We also examined the effect of 1 μm calyculin A, a more potent inhibitor of PP1 and PP2A (Cohen et al., 1990; Figs. 4B and 5B). Calyculin A enhanced transcript levels of din2 and din9, but reduced those of other din genes in Suc-starved cells. In contrast, transcript levels of all din genes were enhanced by the addition of calyculin A in the Suc-fed cells. In particular, sugar-mediated suppression of din2 and din9 seemed to be profoundly influenced by calyculin A (Fig. 5B). Furthermore, the expression patterns of din genes were completely different from that of the Cab gene, since the basal transcript level of Cab in the sugar-fed cells was significantly decreased by calyculin A, and the induction of Cab expression by sugar starvation was completely inhibited by calyculin A (Figs. 4B and 5B). These results suggest that there are multiple pathways for the regulatory processes in sugar-modulated gene expression, i.e. with respect to protein dephosphorylation events.
DISCUSSION
We have previously shown that the expression of a variety of genes is induced in leaves kept in the dark, and in senescing leaves (Azumi and Watanabe, 1991; Fujiki et al., 1997, 2000; Nakabayashi et al., 1999; Nozawa et al., 1999). Under these conditions plants suffer sugar starvation as a direct consequence of the cessation of photosynthesis. The gene products encoded by din genes (Table I) include proteins related to the catabolism of β-glucoside (din2), of amino acids (din3, din4, and din6), of Man (din9), and of the raffinose family oligosaccharides (din10). All of these enzymes would significantly contribute to plant survival under conditions of sugar starvation by providing alternate energy sources in place of photosynthate (Fujiki et al., 1997, 2000). Thus din genes will be useful molecular markers for research into sugar starvation. It is well known that sugar starvation triggers dramatic biochemical changes in the metabolism of lipids and amino acids in many plant species (Journet et al., 1986; Yu, 1999). Few experiments, however, have been undertaken to investigate the regulation of gene expression induced by sugar starvation. To investigate the molecular events occurring in sugar-starved cells, we adopted a suspension-cultured cell system, and succeeded in illustrating a part of the signaling process of gene expression including din genes and the Cab gene during sugar starvation. Transcripts from all din genes accumulated rapidly in Arabidopsis suspension-cultured cells transferred to a Suc-free medium. Conversely, transcripts disappeared immediately after sugar-starved cells were transferred back to a Suc-containing medium. These results confirmed our previous results that the dark-induced expression of din genes in leaves is repressed by Suc, but not by mannitol (Fujiki et al., 1997, 2000).
It has been suggested that hexokinase is involved in an early sensory event in the suppression of photosynthetic genes by hexose (Jang and Sheen, 1994; Jang et al., 1997; Moore and Sheen, 1999). In an alternate manner, hexokinase, which is primarily responsible for catalyzing the first step of glycolysis, may play a role in sugar-repressible gene expression through changing AMP and/or ATP levels (Halford et al., 1999). We found that 2-d-Glc, but not 3-OMG, could mimic the repression effect of Glc on the expression of din genes, as already exemplified for photosynthetic genes such as the Cab gene. These results are consistent with the notion that the phosphorylation of hexose generates the signal for the sugar-repressible expression of not only photosynthetic genes, but also of the numerous dark-inducible genes. Thus far, many studies have investigated the responses of genes to increasing levels of sugars, i.e. induction or repression of gene expression by sugars. Hexokinase is involved in one such mechanism of sensing increasing levels of sugars. In the present study, we addressed the question of how plant cells sense the depletion of sugars, and how sugar starvation leads to gene expression. We have demonstrated that the induction of din gene expression in Suc-depleted cells requires cytoplasmic protein synthesis. Furthermore, we found that signaling in sugar starvation includes protein phosphorylation and dephosphorylation events. These results indicate that the processes in sugar starvation-induced gene expression are complex.
The induction of din gene expression by Suc starvation is inhibited by K-252a. This indicates that Ser/Thr protein kinases play a role in the induction of din gene expression during sugar starvation. SNF1 Ser/Thr protein kinase is thought to be a metabolic sensor in yeast (Gancedo, 1998) and possibly in plants because SNF1 homologs from several plant species have been shown to complement the snf1 mutation in yeast (Halford and Hardie, 1998). Ikeda et al. (1999) showed that transcripts from the wpk4 gene, a SNF1 homolog in wheat, accumulate in wheat seedlings after the removal of Suc from the culture medium. Hence it will be of interest to examine whether plant SNF1 homologs regulate gene expression triggered by sugar starvation.
To date, the effects of okadaic acid and calyculin A on sugar-regulated genes have been studied only in a limited number of cases, such as the sugar-repressible expression of an αAmy3 (Lue and Lee, 1994), and the sugar-inducible expression of the β-amylase and sporamin genes (Takeda et al., 1994). Although these studies only considered a small number of genes, the conclusions drawn may indicate that protein dephosphorylation mediates the regulation of gene expression in the presence of sugars. However, whether protein dephosphorylation events are involved in the regulation of gene expression under sugar starvation was not examined. Furthermore, no previous study has revealed the complex nature of sugar-modulated gene expression involving multiple signaling pathways regulated by different types of protein phosphatases. In the present study we examined the expression of a variety of din genes and the Cab gene, using okadaic acid and calyculin A, and found that sugar starvation-induced gene expression was mediated by protein dephosphorylation events. Furthermore, we found that the effects of okadaic acid and calyculin A on sugar-modulated gene expression vary among the din genes and the Cab gene, indicating that the mechanism of sugar-regulated gene expression is more complicated than previously envisaged.
In sugar-depleted cells, okadaic acid enhanced the accumulation of transcripts from all din genes, except din6 and din10. Okadaic acid, however, exerted little or no enhancement on the accumulation of transcripts from din6, din10, or Cab in sugar-depleted cells, indicating that these genes are regulated in a slightly different manner from other din genes under sugar starvation. Therefore, we propose the hypothesis that an okadaic acid-sensitive protein phosphatase negatively regulates the expression of all din genes, except din6 and din10, in response to a sugar starvation signal, e.g. by inhibiting transcription and/or destabilizing transcripts. In addition, okadaic acid produced a weak enhancement of transcript levels of all din genes, even in Suc-fed cells where gene expression was suppressed by sugar (Fig. 5A). Thus another okadaic acid-sensitive protein dephosphorylation event is proposed to negatively regulate the basal machinery, rather than the sugar-specific machinery, for the accumulation of transcripts. Because this enhancement effect of okadaic acid was not strong enough to cancel the suppression effect of sugar, okadaic acid-sensitive protein dephosphorylation did not seem to play a major role in the sugar-mediated suppression of din genes or the Cab gene.
In contrast to okadaic acid, calyculin A decreased transcript levels of all din genes, except din2 and din9, in Suc-depleted cells. This suggested that, with the exception of din2 and din9, the induction of din genes by sugar starvation requires a calyculin A-sensitive protein phosphatase that positively regulates gene expression in response to a sugar starvation signal, e.g. by stimulating transcription and/or stabilizing transcripts. In contrast, this calyculin A-sensitive protein phosphorylation system did not seem to apply to din2 and din9 expression, since the expression of these genes in sugar-depleted cells was enhanced by calyculin A. However, another calyculin A-sensitive protein phosphatase seemed to negatively regulate the expression of din2 and din9 in the presence of sugar because transcript levels of these genes in sugar-fed cells were strongly enhanced by calyculin A (Fig. 5B). In accord with the above results, din genes can be divided into three groups. The first group includes din1, din3, and din4, which are negatively controlled by an okadaic acid-sensitive phosphatase, and positively controlled by a calyculin A-sensitive phosphatase in sugar-starved cells. The second group includes din6 and din10 genes that are little affected by an okadaic acid-sensitive phosphatase, but are positively regulated by a calyculin A-sensitive phosphatase during sugar starvation. The third group includes din2 and din9, which appear to be negatively controlled in sugar-depleted cells by protein phosphatases sensitive to okadaic acid and calyculin A. In addition, the expression of din2 and din9 appear to be negatively controlled by another calyculin A-sensitive protein phosphatase in sugar-fed cells. The expression pattern of the Cab gene was, in part, similar to that of din6 and din10, since okadaic acid had little effect on Cab gene expression. However, calyculin A had a strong inhibitory effect on Cab gene expression independently of the presence of Suc. Hence it is likely that a calyculin A-sensitive protein phosphatase may be responsible for the basal machinery of Cab gene expression under illumination.
Okadaic acid was shown, in cell-free extracts, to inhibit PP2A at a low concentration (1 nm), and PP1 at a higher concentration (1 μm; Cohen et al., 1990). Calyculin A inhibits PP2A with a potency equal to that of okadaic acid, and inhibits PP1 with 10- to 100-fold greater potency than okadaic acid. Although it is difficult to manipulate the effective concentrations of these inhibitors in vivo, the differential effects of okadaic acid and calyculin A observed in this study may be explained by the presence of hypothetical protein phosphatases (PP1 and PP2A) in plant cells that exhibit differential sensitivity to these inhibitors. Thus we suggest that PP1 and PP2A play different roles in sugar-modulated gene expression. PP2A, which may be inhibited by okadaic acid and calyculin A, appears to negatively regulate the sugar starvation-inducible expression of din1, din2, din3, din4, and din9, but not that of din6, din10, and Cab genes. Another PP2A may be involved in the basal machinery of transcription and/or the stabilization of transcripts in a negative manner, which may be responsible for the accumulation of transcripts from all din genes in sugar-fed cells in the presence of okadaic acid (and possibly calyculin A as well). PP1, which is inhibited by calyculin A, but not by okadaic acid, may positively regulate sugar starvation-inducible expression of all din genes, except din2 and din9. With respect to din2 and din9, another PP1 may play a major role in the negative control of gene expression in the presence of Suc, because calyculin A, but not okadaic acid, strongly enhances transcript levels of din2 and din9 in sugar-fed cells (Fig. 5). However, it was not possible to clarify whether PP1, as well as PP2A, negatively controls din2 and din9 expression in the absence of Suc, because calyculin A potentially inhibits PP2A as okadaic acid does.
We observed that calyculin A, but not okadaic acid, has a strong inhibitory effect on Cab expression under illumination, which suggests that PP1 may be involved in the activation of Cab gene expression. In a similar manner, PP1 inhibited by calyculin A, but not by okadaic acid, appears to be essential for light-dependent activation of the expression of other photosynthetic genes (Sheen, 1993). However, the regulatory role of PP1 in light-induced expression of photosynthetic genes, including the Cab gene, may be different from that in the sugar-regulated expression of din genes since calyculin A completely inhibited the induction of the Cab gene, but not that of the din genes by sugar starvation. Kurotani et al. (1999) recently showed that protein phosphatases are responsible for the light-induced accumulation of Cab transcripts through stabilization of the transcripts, rather than by changing the transcription rate. It will be interesting to determine whether the inhibitors examined in this study are responsible for changing the transcription rate and/or the stability of transcripts from din genes.
In conclusion, the sugar-sensing mechanism mediated by the phosphorylation of hexose was found to be common to all din genes and to the Cab gene. However, we have shown for the first time that the signaling pathways leading to sugar starvation-induced gene expression differ among various genes, including the din genes and the Cab gene. We have demonstrated that protein phosphorylation and dephosphorylation play critical roles in the sugar-regulated expression of din genes. We propose the hypothesis that a calyculin A-sensitive protein phosphatase, probably PP1, is partly responsible for the expression of din genes, except din2 and din9 during Suc starvation. By contrast, another PP1 may be responsible for the suppression of din2 and din9 gene expression by sugars. On the other hand, protein phosphatases that were inhibited by okadaic acid and calyculin A, probably PP2A, may be involved in the destabilization of the transcripts, and/or in the suppression of transcription of din genes, except din6 and din10 in sugar-depleted cells. Identification and characterization of the protein kinases and phosphatases responsible for such regulation will be important in revealing the signaling pathways leading to gene expression during sugar starvation. We believe that the Arabidopsis suspension-cultured cell system and the din genes are useful tools for further investigation of the molecular mechanisms of sugar starvation-induced gene expression.
MATERIALS AND METHODS
Plant Materials
The Arabidopsis suspension-cultured cell line T87 (Axelos et al., 1992) was obtained from the RIKEN Plant Cell Bank (Tsukuba, Japan). Cells were grown in 80 mL of Gamborg B5 medium (Wako Pure Chemical Industries, Osaka), containing 2% (w/v) Suc and 2.5 μm 2,4-dichlorophenoxyacetic acid at 23°C under continuous illumination at a photon flux density of 40 μmol m−2 s−1. The cell suspension was maintained by transplanting 2 mL of 12-d-old cells to fresh medium.
For Suc-starvation treatment, 7-d-old T87 cells were rinsed and incubated with fresh medium devoid of Suc. As a control, cells were washed and incubated with fresh medium containing 2% (w/v) Suc. Inhibitors were added to the culture medium at concentrations that were reported to be effective in an Arabidopsis suspension-cultured cell system (Christie and Jenkins, 1996). Cells were collected on filter paper by vacuum filtration and were immediately frozen in liquid nitrogen.
Northern-Blot Hybridization
Isolation of total RNA from T87 cells and northern-blot analysis were performed as described previously (Fujiki et al., 2000). The distribution of radioactivity on the blotted membranes was analyzed with a Bio Imaging Analyzer BAS2000 (Fuji Photo Film). Full-length cDNA inserts for din1 (sen1, Oh et al., 1996) din2, din3, and din4 genes, and partial cDNA fragments for din6, din9, and din10 genes were used as hybridization probes (Table I). A cDNA clone for the Cab gene in Arabidopsis (GenBank accession no. P27521) was obtained from the Arabidopsis Biological Resource Center at Ohio State University. Experiments with inhibitors were repeated two to four times, and similar results were observed in each case.
ACKNOWLEDGMENTS
We thank Mr. Atsuhiko Aoyama for his excellent technical assistance. We also thank Professor Hong Gil Nam of Pohang University of Science and Technology (Kyungbuk, Korea) for providing a cDNA clone for the Arabidopsis sen1 gene.
Footnotes
This work was supported by the “Research for the Future” Program of the Japan Society for the Promotion of Science (no. JSPS–RFTF96L00601 to A.W.) and by the Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists (no. 4206 to Y.F.).
LITERATURE CITED
- Argüello-Astorga G, Herrera-Estrella L. Evolution of light-regulated plant promoters. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:525–555. doi: 10.1146/annurev.arplant.49.1.525. [DOI] [PubMed] [Google Scholar]
- Axelos M, Curie C, Mazzolini L, Bardet C, Lescure B. A protocol for transient gene expression in Arabidopsis thaliana protoplasts isolated from cell suspension cultures. Plant Physiol Biochem. 1992;30:123–128. [Google Scholar]
- Azumi Y, Watanabe A. Evidence for a senescence-associated gene induced by darkness. Plant Physiol. 1991;95:577–583. doi: 10.1104/pp.95.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouquisse R, Gaudillère JP, Raymond P. Induction of a carbon-starvation-related proteolysis in whole maize plants submitted to light/dark cycles and to extended darkness. Plant Physiol. 1998;117:1281–1291. doi: 10.1104/pp.117.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouquisse R, James F, Pradet A, Raymond P. Asparagine metabolism and nitrogen distribution during protein degradation in sugar-starved maize root tips. Planta. 1992;188:384–395. doi: 10.1007/BF00192806. [DOI] [PubMed] [Google Scholar]
- Chevalier C, Bourgeois E, Pradet A, Raymond P. Molecular cloning and characterization of six cDNAs expressed during glucose starvation in excised maize (Zea mays L.) root tips. Plant Mol Biol. 1995;28:473–485. doi: 10.1007/BF00020395. [DOI] [PubMed] [Google Scholar]
- Christie JM, Jenkins GI. Distinct UV-B and UV-A/blue light signal transduction pathways induce chalcone synthase gene expression in Arabidopsis cells. Plant Cell. 1996;8:1555–1567. doi: 10.1105/tpc.8.9.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Holmes CFB, Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990;15:98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Davies KM, Seelye JF, Irving DE, Borst WM, Hurst PL, King GA. Sugar regulation of harvest-related genes in asparagus. Physiol Plant. 1996;111:877–883. doi: 10.1104/pp.111.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieuaide M, Brouquisse R, Pradet A, Raymond P. Increased fatty acid β-oxidation after glucose starvation in maize root tips. Plant Physiol. 1992;99:595–600. doi: 10.1104/pp.99.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y, Sato T, Ito M, Watanabe A. Isolation and characterization of cDNA clones for the E1β and E2 subunits of branched-chain α-keto acid dehydrogenase complex in Arabidopsis. J Biol Chem. 2000;275:6007–6013. doi: 10.1074/jbc.275.8.6007. [DOI] [PubMed] [Google Scholar]
- Fujiki Y, Sato T, Yoshikawa Y, Ito M, Watanabe A. Dark-induced expression of the genes for branched-chain alpha-keto acid dehydrogenase complex in Arabidopsis leaves (abstract no. 400) Plant Physiol. 1997;114:S-95. [Google Scholar]
- Gancedo JM. Yeast carbon catabolite repression. Microbiol Mol Biol Rev. 1998;62:334–361. doi: 10.1128/mmbr.62.2.334-361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham IA, Denby KJ, Leaver CJ. Carbon catabolite repression regulates glyoxylate cycle gene expression in cucumber. Plant Cell. 1994;6:761–772. doi: 10.1105/tpc.6.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford NG, Hardie DG. SNF1-related protein kinases: global regulators of carbon metabolism in plants? Plant Mol Biol. 1998;37:735–748. doi: 10.1023/a:1006024231305. [DOI] [PubMed] [Google Scholar]
- Halford NG, Purcell PC, Hardie DG. Is hexokinase really a sugar sensor in plants? Trends Plant Sci. 1999;4:117–120. doi: 10.1016/s1360-1385(99)01377-1. [DOI] [PubMed] [Google Scholar]
- Hensel LL, Grbić V, Baumgarten DA, Bleecker AB. Developmental and age-related processes that influence the longevity and senescence of photosynthetic tissues in Arabidopsis. Plant Cell. 1993;5:553–564. doi: 10.1105/tpc.5.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Koizumi N, Kusano T, Sano H. Sucrose and cytokinin modulation of WPK4, a gene encoding a SNF1-related protein kinase from wheat. Plant Physiol. 1999;121:813–820. doi: 10.1104/pp.121.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James F, Brouquisse R, Pradet A, Raymond P. Changes in proteolytic activities in glucose-starved maize root tips: regulation by sugars. Plant Physiol Biochem. 1993;31:845–856. [Google Scholar]
- Jang JC, León P, Zhou L, Sheen J. Hexokinase as a sugar sensor in higher plants. Plant Cell. 1997;9:5–19. doi: 10.1105/tpc.9.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JC, Sheen J. Sugar sensing in higher plants. Plant Cell. 1994;6:1665–1679. doi: 10.1105/tpc.6.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet EP, Bligny R, Douce R. Biochemical changes during sucrose deprivation in higher plant cells. J Biol Chem. 1986;261:3193–3199. [PubMed] [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Koch KE, Ying Z, Wu Y, Avigne WT. Multiple paths of sugar-sensing and a sugar/oxygen overlap for genes of sucrose and ethanol metabolism. J Exp Bot. 2000;51:417–427. doi: 10.1093/jexbot/51.suppl_1.417. [DOI] [PubMed] [Google Scholar]
- Kurotani K, Hata S, Izui K. Light-regulated expression of the gene for C4-form phosphoenolpyruvate carboxylase in maize: destabilization of mRNA by okadaic acid. Plant Cell Physiol. 1999;40:423–430. [Google Scholar]
- Lalonde S, Boles E, Hellmann H, Barker L, Patrick JW, Frommer WB, Ward JM. The dual function of sugar carriers: transport and sugar sensing. Plant Cell. 1999;11:707–726. doi: 10.1105/tpc.11.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S. Protein phosphatases and signaling cascades in higher plants. Trends Plant Sci. 1998;3:271–275. [Google Scholar]
- Lue MY, Lee HT. Protein phosphatase inhibitors enhance the expression of an α-amylase gene, αAmy3, in cultured rice cells. Biochem Biophys Res Commun. 1994;205:807–816. doi: 10.1006/bbrc.1994.2737. [DOI] [PubMed] [Google Scholar]
- Moore B, Sheen J. Plant sugar sensing and signaling: a complex reality. Trends Plant Sci. 1999;4:250. doi: 10.1016/s1360-1385(99)01433-8. [DOI] [PubMed] [Google Scholar]
- Moriyasu Y, Ohsumi Y. Autophagy in tobacco suspension-cultured cells in response to sucrose starvation. Plant Physiol. 1996;111:1233–1241. doi: 10.1104/pp.111.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabayashi K, Ito M, Kiyosue T, Shinozaki K, Watanabe A. Identification of clp genes expressed in senescing Arabidopsis leaves. Plant Cell Physiol. 1999;40:504–514. doi: 10.1093/oxfordjournals.pcp.a029571. [DOI] [PubMed] [Google Scholar]
- Nozawa A, Ito M, Hayashi H, Watanabe A. Dark-induced expression of genes for asparagine synthetase and cytosolic glutamine synthetase in radish cotyledons is dependent on the growth stage. Plant Cell Physiol. 1999;40:942–948. [Google Scholar]
- Oh SA, Lee SY, Chung IK, Lee CH, Nam HG. A senescence-associated gene of Arabidopsis thaliana is distinctively regulated during natural and artificially induced leaf senescence. Plant Mol Biol. 1996;30:739–754. doi: 10.1007/BF00019008. [DOI] [PubMed] [Google Scholar]
- Pego JV, Kortstee AJ, Huijser C, Smeekens SCM. Photosynthesis, sugars and the regulation of gene expression. J Exp Bot. 2000;51:407–416. doi: 10.1093/jexbot/51.suppl_1.407. [DOI] [PubMed] [Google Scholar]
- Prata RTN, Williamson JD, Conkling MA, Pharr DM. Sugar repression of mannitol dehydrogenase activity in celery cells. Plant Physiol. 1997;114:307–314. doi: 10.1104/pp.114.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Protein phosphatase activity is required for light-inducible gene expression in maize. EMBO J. 1993;12:3497–3505. doi: 10.1002/j.1460-2075.1993.tb06024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu JJ, Yu TS, Tong WF, Yu SM. Carbohydrate starvation stimulates differential expression of rice α-amylase genes that is modulated through complicated transcriptional and post-transcriptional processes. J Biol Chem. 1996;271:26998–27004. doi: 10.1074/jbc.271.43.26998. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Wu GJ, Watanabe A. A protein encoded by din1, a dark-inducible and senescence-associated gene of radish, can be imported by isolated chloroplasts and has sequence similarity to sulfide dehydrogenase and other small stress proteins. Plant Cell Physiol. 1998;39:139–143. doi: 10.1093/oxfordjournals.pcp.a029350. [DOI] [PubMed] [Google Scholar]
- Smeekens S, Rook F. Sugar sensing and sugar-mediated signal transduction in plants. Plant Physiol. 1997;115:7–13. doi: 10.1104/pp.115.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Mano S, Ohto M, Nakamura K. Inhibitors of protein phosphatases 1 and 2A block the sugar-inducible gene expression in plants. Plant Physiol. 1994;106:567–574. doi: 10.1104/pp.106.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi WB, Cashmore AR. Light-regulated transcription. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:445–474. [Google Scholar]
- Umemura T, Perata P, Futsuhara Y, Yamaguchi J. Sugar sensing and α-amylase gene repression in rice embryos. Planta. 1998;204:420–428. doi: 10.1007/s004250050275. [DOI] [PubMed] [Google Scholar]
- Yu SM. Cellular and genetic responses of plants to sugar starvation. Plant Physiol. 1999;121:687–693. doi: 10.1104/pp.121.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SM, Kuo YH, Sheu G, Sheu YJ, Liu LF. Metabolic derepression of α-amylase gene expression in suspension-cultured cells of rice. J Biol Chem. 1991;266:21131–21137. [PubMed] [Google Scholar]