Abstract
The demand for hospice services as well as for ‘well-dying’ of terminal patients is increasing as patient financial burden is decreasing due to National Health Insurance coverage for hospice care. Hospice institutions utilize interdisciplinary teams comprising doctors, nurses, dietitians, and other health staffs to provide comprehensive patient management. This report examined the nutritional status of a hospice patient from admission to death as well as the nutrition management of this patient in the hospice ward through nutrition interventions performed by a dietitian in the interdisciplinary team. The patient in the present case was a 74-year-old man diagnosed with pancreatic head cancer who died after 26 days of hospice care following transfer from the general ward. During hospice care, the dietitian monitored the patient's nutritional status and performed 8 nutrition interventions, but his oral intake decreased as the patient's symptoms worsened. The average energy intake rates were 30% and 17% of required rates for oral and artificial nutrition, respectively. In line with a report suggesting that the main focus of nutrition in palliative care should be on improving the quality of life and reducing worry in patients, rather than aggressive nutritional management, there is a need for nutrition interventions that are personalized to individual patients by monitoring progress and offering continuous counseling from the time of admission. In addition, further studies such as comparative analysis of nutritional management in Korean hospice ward will be needed for better nutrition management for terminally ill patients.
Keywords: Hospice care, Nutrition intervention, Artificial nutrition, Interdisciplinary team
INTRODUCTION
Hospice care aims to comprehensively assess and treat terminally ill patients or patients in the process of dying as well as to assist their families by providing physical, psychosocial, and spiritual support including the alleviation of pain and symptoms, as outlined in the Act on Decisions on Life-Sustaining Treatment for Patients in Hospice and Palliative Care or at the End of Life (Article 2, Subparagraph 6). Since 2015, the demand for hospice wards has increased as the medical cost burden for palliative care has decreased due to the resolution by the National Health Insurance on health insurance fees for hospice care inpatient treatment in palliative care institutions for terminal cancer patients [1]. Matching this increased demand on hospice wards, interdisciplinary hospice care teams comprising doctors, nurses, dietitians, and social workers provide comprehensive services [2]. The dietitians on these teams monitor nutrition support, provide meal management for terminal patients and are responsible for overall nutrition interventions. Nutrition support through nutrition interventions is essential for terminal patients to assure the dignity and value of a human being until the end of life; as stated in the Act on Decisions on Life-Sustaining Treatment for Patients in Hospice and Palliative Care or at the End of Life (Article 19, Paragraph 2), “when deciding and acting on withholding and withdrawing life-sustaining treatment, medical practice for pain relief, nutrient supply, water supply, and simple supply of oxygen should not be neglected or halted.” Meals served to hospice patients will be tried in various forms as long as oral intake is possible. However, due to anorexia, dysphagia, weakness, and confusion, which are the main symptoms experienced by patients at the end of life, oral intake decreases and is accompanied by weight loss. Consequently, artificial nutrition (AN) is required in a subset of patients [3]. According to the literature, approximately 50% of hospice patients are affected by malnutrition and weight loss, and infection increases due to malnutrition [4], which may diminish patient quality of life [3]. Therefore, nutrition management should be continuously performed in order to delay deterioration and improve the quality of life of hospice patients.
The aim of this report was to examine the state of overall patient monitoring and nutrition support in an interdisciplinary medical treatment environment from admission to death in the case of a terminally ill cancer patient in hospice care.
CASE
The subject was a 74-year-old male patient diagnosed with pancreatic head cancer who received computer-controlled radiation therapy for approximately 2 months after diagnosis. He was subsequently admitted to the hemato-oncology department due to poor oral intake and hematochezia on May 17, 2017. During the treatment, he was placed on the waiting list for the hospice ward on June 17, 2017 and was transferred to a hospice care unit on June 26, 2017. The patient died on July 22, 2017, the 26th day of hospice care (after a total of 66 days of hospitalization).
Patient's body mass index of 24 kg/m2 was calculated based on height (166 cm) and weight (66 kg) on the first day of admission. The patient was able to ingest a general or liquid diet after at least 20 days of nothing per os (NPO) in the general ward due to hematochezia.
The interdisciplinary hospice care team in the study hospital comprises doctors, nurses, dietitians, and social workers, as described by the International Association for Hospice & Palliative Care [2], and periodically performed monitoring. In addition, a dietitian additionally performed monitoring and 8 nutrition interventions after the patient was transferred to the hospice ward (Table 1).
Table 1. Summary of each nutrition intervention.
| Visits | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th |
|---|---|---|---|---|---|---|---|---|
| Diet order | High-protein liquid diet | High-protein liquid diet | High-protein liquid diet | High-protein soft diet | General diet | General diet | NPO | NPO |
| Oral intake*, kcal/% | 600/45 | 500/40 | 600/45 | 680/50 | 230/20 | 150/10 | 30/2 | 0/0 |
| AN type | Lipid-free 2 in 1 preparation | Lipid-free 2 in 1 preparation | - | 5% Dextrose saline | 5% Dextrose saline, Amino acid preparation | 5% Dextrose saline | 5% Dextrose saline, Lipid preparation | 5% Dextrose saline |
| AN intake*, kcal/% | 251/20 | 251/20 | 0/0 | 85/7 | 140/10 | 140/10 | 585/45 | 85/7 |
| Total intake*, kcal/% | 851/65 | 751/60 | 600/45 | 765/60 | 370/30 | 290/20 | 615/45 | 85/7 |
| Albumin†, g/dL | 2.32 | - | - | - | 2.17 | - | 2.02 | - |
| CRP‡, mg/dL | 77.01 | - | - | - | 79.51 | - | 112.04 | - |
| Creatinine§, mg/dL | 1.03 | - | - | - | 1.38 | - | 1.63 | - |
| eGFR, mL/min/1.73 m2 | 75.15 | - | - | - | 53.62 | - | 44.25 | - |
| Symptoms | General weakness, powerlessness, abdominal pain, fatigue | General weakness, melena | General weakness, input/output imbalance | General weakness, pain, dizziness, anxiety | General weakness, fatigue, abdominal pain, constipation, disabled mobility | General weakness, powerlessness, drowsy mental state, delirium, insomnia | General weakness, dyspnea, nausea | General weakness, pain, insomnia, nausea/vomiting |
| Nutrition interventions | Provide watery kimchi soup at every meal, apple juice instead of milk, and normal thin rice gruel instead of black sesame thin rice gruel for snacks meals | Encourage to intake orally within acceptable limits | Encourage to oral intake within acceptable limits | Encourage to eat small amounts of soft protein food and to eat the side dishes provided in the hospital meals | Encouraged to frequently consume small amounts of food | Encourage to oral intake within acceptable limits | - | - |
AN, artificial nutrition; CRP, C-reactive protein; eGFR, epidermal growth factor receptor; NPO, nothing per os.
*Percentage (%) indicated ratio of total energy intake to required energy intake; †Normal range of albumin: 3.5–5.2 (g/dL); ‡Normal range of CRP: 0.0–5.0 (mg/dL); §Normal range of creatinine: 0.67–1.17 (mg/dL).
1st intervention (2017-06-27, day #2 of hospice)
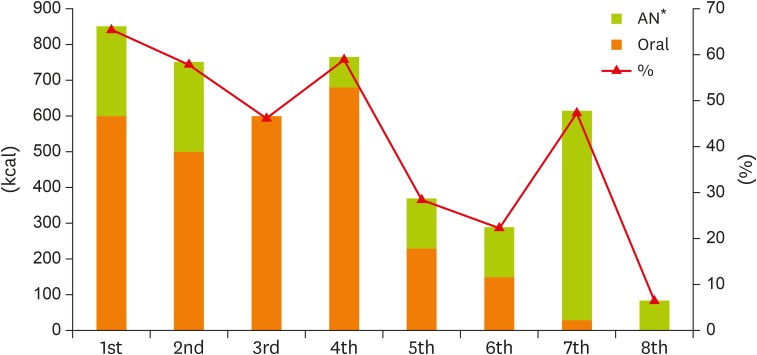
The patient complained about symptoms including weakness, powerlessness, abdominal pain, and fatigue. A high-protein liquid diet was prescribed for his hospital meals (Table 2) and approximately 45% of his required caloric intake was covered through the intake of thin rice gruel (150 mL), watery kimchi soup, oral nutrition supplement (ONS, 50 mL), fruit juice (720–900 mL per day), and a snack of thin rice gruel 150 mL (Figure 1). For AN, a lipid-free 2 in 1 preparation was provided and approximately 65% of the required caloric intake was covered through oral intake and AN. For nutrition intervention, the meals were adjusted to provide watery kimchi soup at every meal, apple juice instead of milk, and normal thin rice gruel instead of black sesame thin rice gruel for snack meals based on the personal preferences of the patient.
Table 2. The composition of high-protein liquid diet.
| Breakfast | Snack meal | Lunch | Snack meal | Dinner |
|---|---|---|---|---|
| Rice gruel | Gruel* | Rice gruel | Gruel* | Rice gruel |
| Watery kimchi soup | Instant soup | Watery kimchi soup | ||
| Soy milk+skim milk powder | High-protein ONS† | Steamed egg | ||
| Rice beverage | Milk+skim milk powder | Mango juice |
ONS, oral nutrition supplement.
*Various kinds of gruel are served by 10-day cycle.
Figure 1.
Changes in energy intake through oral and AN.
AN, artificial nutrition.
*Percentage (%) indicated ratio of total energy intake to required energy intake.
2nd intervention (2017-06-30, day #4)
The patient could take only 2 meals per day as his condition gradually deteriorated. From the high-protein liquid diet, 150 mL thin rice gruel, watery kimchi, scorched rice soup, and 720–900 mL of fruit juice per day were taken and his oral intake was reduced by approximately 100 kcal. AN was provided as before. ONS was not administered due to the low compliance by the patient.
3rd intervention (2017-07-04, day #8)
To control the patient's input/output, a diuretic was prescribed and the AN was discontinued. Additionally, a vitamin K preparation (vitamin K1, 30 mg) was provided by doctor's order to control hemorrhage. Oral intake was maintained similar to that in the previous intervention.
4th intervention (2017-07-06, day #10)
The patient's pain became severe and he also suffered from dizziness and anxiety. At the patient's request, the high-protein soft diet was changed to high-protein liquid diet, a half-bowl of glutinous rice and seasoned seaweed were consumed as private foods brought from outside the hospice, and watery kimchi soup and scorched rice soup were taken as hospital meals. Due to his decreased ability to digest, the patient was unable to eat dinner and instead drank 800 mL of fruit juice per day. As dextrose saline was additionally provided, his total intake covered approximately 60% of the required calories, but his protein intake was as low as approximately 15% of required levels. To supplement his protein intake, the patient was instructed to eat small amounts of soft protein foods and was encouraged to eat the side dishes provided with the hospital meals.
5th intervention (2017-07-11, day #15)
The patient experienced impaired mobility, complained of fatigue and general weakness, and his defecation was irregular. At his request, his food prescription was changed to a general diet and the amount of intake was reduced by approximately 450 kcal through the intake of 2 dollops of rice or scorched rice at every meal, a small amount of meat gravy as an outside food source, and 400 mL of juice per day. For AN, the dextrose saline and the amino acid preparation were provided by doctor's order and his total intake covered approximately 30% of the required caloric intake. The patient was encouraged to frequently consume small amounts of food as his condition worsened.
6th intervention (2017-07-14, day #18)
The patient's weakness became severe and he suffered from intermittent symptoms of delirium and insomnia due to mental deterioration. With the intake of 2 dollops of rice, seasoned seaweed, and 200 mL of juice per day, his food intake was further reduced by approximately 35%. AN was provided as in the fifth intervention.
7th intervention (2017-07-18, day #22)
The patient complained of dyspnea and nausea and NPO was prescribed. For AN, Dextrose saline and the lipid preparation were provided. C-reactive protein and creatinine levels were further elevated. Only 1 or 2 sips of juice were taken orally; just enough to moisten the mouth.
8th intervention (2017-07-20, day #24)
The complaints of symptoms including pain, insomnia, and nausea/vomiting were more frequent and management of the pre-death phase began. Minimal infusion solution was supplied, including dextrose saline; in addition, there was no oral intake of food or water. The patient died on July 22, 2017, the 26th day of hospice stay.
DISCUSSION
Generally, weight loss occurs as cancer progresses due to accelerated metabolism [3], whereas the digestive functions and the amount of required energy decrease in the terminal stages [5]. Nutrition interventions for hospice patients have limitations because there are no guidelines for the estimation of the recommended nutritional intake based on changes in the condition of patients near the end of life. In this case, considering that the patient was in a near bed-ridden state, the required intake was estimated using the Harris-Benedict equation, which is generally used for the estimation of resting energy expenditure [6].
In the present case, the average oral and AN intake during the hospice period were approximately 30% and 17% of the required caloric intake, respectively. The patient's intake declined due to a loss of appetite caused by various symptoms. In response, his meals were adjusted through nutrition interventions by a dietitian based on the patient's personal preferences. The patient was encouraged to take soft protein food to supplement his protein intake, and oral intake was continuously encouraged by guiding the patient to try easily digestible foods by increasing the intake of small amounts of food. However, there was almost no improvement in oral intake despite of these nutrition interventions.
Although ONS is often recommended as a nutrition intervention to supplement nutrition when oral intake decreases [7], the consistent intake of ONS by patients in the hospice ward can be difficult due to repulsion to its taste, diarrhea, etc. As the patient in this case also had low ONS compliance, ONS was ineffective.
Generally, active nutrition interventions are considered to improve the nutrition condition of patients, but there are reports that excessive nutrition interventions may not only result in frustration on the part of the administering staff but also result in patient discomfort [8]. Therefore, a careful approach based on the patient's condition is required, especially when performing nutrition interventions in patients near the end of life. In this case, the prescription of a liquid diet was recommended and maintained as the patient's condition worsened, but in the later stage, oral intake was maintained with a general diet as requested by the patient.
Furthermore, there are cases where AN is attempted to improve the quality of life when oral intake is no longer possible. However, not only the pros and cons of such an attempt but also the opinions of the patients' families on cost and other issues should be considered [9]. Hence, individualized clinical prescriptions are required [10].
According to the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines [11], AN is recommended when life expectancy is at least 2–3 months; however, nutrition support does not extend survival when life expectancy is low. Hui et al. [3] also did not recommend AN for patients with life expectancy of less than 1 month. After the patient was transferred to the hospice, the AN supply was reduced to 17% in contrast to AN supplying 40%–80% of the required intake through the central line in the general ward.
Nevertheless, there are insufficient evidence-based guidelines for reducing or ceasing AN supply toward the end of life [12]. According to the systematic literature review by Raijmakers et al. [13], AN contribution to calorie intake ranges from 3% to 53% during the week before death.
The present case study had several limitations. First, given the nature of the hospice ward, blood testing, weight measurement, and other tests were not regularly performed; in addition, there were insufficient objective data for assessing the nutritional status because it was difficult to have a thorough communication with the patient due to his deteriorating condition. Second, active nutrition interventions were difficult because there was no evidence for the recommended nutritional intake for hospice patients.
However, it was found that a nutrition management system is needed that takes into account the particularities of hospice patients by monitoring the change of condition from admission to death and the change of nutritional intake accordingly. Moreover, it is meaningful to have served as a team member of hospice care which provides a meal meeting the patient's needs through periodic interventions, so that the patient's condition can be maintained to meet the comfortable dying.
In conclusion, patient compliance can be increased by providing meals that meet their requests by paying attention not only to their condition, but also to their practical needs. In addition, it is important to have a systematic team approach to control the supply of AN when oral intakes are observed to maintain a certain level of nutrition supply. In order to have more systematic team approach, it is necessary to establish systematic guidelines for Korean nutrition management based on various systematic studies and to accumulate nutrition management data tailored to the patients' particular circumstances.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.Kim CG. Hospice & palliative care policy in Korea. Korean J Hosp Palliat Care. 2017;20:8–17. [Google Scholar]
- 2.Doyle D, Woodruff R. The IAHPC manual of palliative care. 2nd ed. Houston (TX): IAHPC Press; 2008. [Google Scholar]
- 3.Hui D, Dev R, Bruera E. The last days of life: symptom burden and impact on nutrition and hydration in cancer patients. Curr Opin Support Palliat Care. 2015;9:346–354. doi: 10.1097/SPC.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marley K, Cunningham KA. Food for thought: improving nutritional assessment and management in the hospice setting. BMJ Support Palliat Care. 2015;5(Suppl 3):A28. [abstract] [Google Scholar]
- 5.Compassion and Support at the End of Life (US) Signs of dying [Internet] 2009. [cited 2017 November 15]. Available from https://www.compassionandsupport.org/index.php/for_patients_families/death_dying/signs_of_dying.
- 6.Bosaeus I, Daneryd P, Svanberg E, Lundholm K. Dietary intake and resting energy expenditure in relation to weight loss in unselected cancer patients. Int J Cancer. 2001;93:380–383. doi: 10.1002/ijc.1332. [DOI] [PubMed] [Google Scholar]
- 7.Adelaide Hills Community Health Service (AU) Diet and nutrition in palliative care: a guide for clients and carers. Mount Barker: Adelaide Hills Community Health Service; 2012. [Google Scholar]
- 8.Cimino JE. The role of nutrition in hospice and palliative care of the cancer patient. Topics Clin Nutr. 2003;18:154–161. [Google Scholar]
- 9.Dalal S, Bruera E. Dehydration in cancer patients: to treat or not to treat. J Support Oncol. 2004;2:467–479. [PubMed] [Google Scholar]
- 10.Shils ME, Shike M. Modern nutrition in health and disease. 10th ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 11.Bozzetti F, Arends J, Lundholm K, Micklewright A, Zurcher G, Muscaritoli M. ESPEN. ESPEN guidelines on parenteral nutrition: non-surgical oncology. Clin Nutr. 2009;28:445–454. doi: 10.1016/j.clnu.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Kim DY, Lee SM, Lee KE, Lee HR, Kim JH, Lee KW, Lee JS, Lee SN. An evaluation of nutrition support for terminal cancer patients at teaching hospitals in Korea. Cancer Res Treat. 2006;38:214–217. doi: 10.4143/crt.2006.38.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raijmakers NJ, van Zuylen L, Costantini M, Caraceni A, Clark J, Lundquist G, Voltz R, Ellershaw JE, van der Heide A. OPCARE9. Artificial nutrition and hydration in the last week of life in cancer patients. A systematic literature review of practices and effects. Ann Oncol. 2011;22:1478–1486. doi: 10.1093/annonc/mdq620. [DOI] [PubMed] [Google Scholar]