Abstract
Myrtus communis (myrtle) is well known for its therapeutic effects pertaining to the major secondary metabolites including essential oils (EOs). EOs are composed of volatile compounds and simply evaporate or decompose leading to their instability. Preparation of EOs niosomal formulation may be a promising approach to deal with these obstacles. Niosomal formulations of myrtle essential oil (nMEO) were provided using non-ionic surfactants and cholesterol (Chol). In the next steps, vesicle size, zeta potential, percentage of entrapment efficiency (EE%) and physical stability of nMEO were investigated. Finally, the effect of myrtle essential oil (MEO) and nMEO on microbial growth inhibition were assessed. Values for nMEO size and zeta potential ranged from 6.17 ± 0.32 to 7.24 ± 0.61 (μm) and -20.41 ± 0.17 to -31.75 ± 0.45 (mV), respectively. Higher degrees of EE% were obtained by F6 formulation (Span/Tween 60:Chol (50:50 molar ratio)). Moreover, niosomes have been reported to be stable at 4 °C during a three-month time period. It was revealed that nMEO F6 formulation inhibited growth of Staphylococcus aureus, Staphylococcus epidermidis, Serratia marcescens, and Bacillus subtilis at concentrations lower than that of MEO. Overall, it was found that stable multilamellar vesicles were formed in the presence of 0.5% MEO and F6 formulation. This formulation also exhibited better antibacterial activity than MEO.
Keywords: Antibacterial activity; Essential oil; Myrtle, Myrtus communis; Niosome
INTRODUCTION
For many years, aromatic plants, such as myrtle, have been widely used for their medicinal properties (1). Different parts of myrtle such as leaves, berries, branches, and fruits have been widely applied in the traditional medicine (2). Moreover, not only have myrtle leaves been utilized for treatment of diarrhea, stomachache, hyperglycemia, cough, and pulmonary disorders but they have also been externally used as an antiseptic, disinfectant, and for mouth ulcer, burn, and wound healing (1,3). Such therapeutic potentials may be related to myrtle's major secondary metabolites including polyphenols and essential oils (EOs) (4).
EOs are natural multi-component systems composed mainly of both terpenes and some other non-terpene components (5). Low chemical and physical stability of the EOs as well as their lipophilic nature have been considered as the important obstacle to the preparation and storage of the EOs formulations.
In terms of physical properties, EOs are volatile compounds which can easily evaporate and/or decompose even at room temperature due to very low boiling point and direct exposure to light or oxygen, respectively (6). Furthermore, conventional formulations of EOs can oftentimes cause skin irritation and sensitization. Several approaches including the application of vesicular systems such as niosomes (7) have been developed to resolve these problems. Niosomes are non-ionic surfactants and have been used in drug (8) or gene delivery (9). In drug delivery, niosomes are capable of increasing skin penetration and acting as a local depot to provide sustained release of topically applied drugs in the dermis. Due to properties such as biodegradability, non-toxicity, non-immunogenicity, low cost, and superior chemical and storage stabilities, niosomes offer several advantages over other vesicular systems (10,11). EOs’ entrapment in the hydrophobic region of niosomes can improve their permeability and stability features, consequently reducing skin irritation properties of EOs (12). Niosomal formulations can improve the stratum corneum functions by attenuating water loss from trans-epidermal skin and increasing skin smoothness by replacing its lost lipids. Furthermore, the residence time of drug in the stratum corneum and epidermis increases and its systemic absorption decreases following application of niosomal systems (13,14). It was previously shown by Manosroi, et al. that niosomal encapsulation of Mimusops elengi flower extract reduced extract cytotoxicity on human skin fibroblast and also enhanced its physical stability (15).
In the current study, at first myrtle essential oil (MEO) was extracted and analyzed and then the niosomal myrtle essential oil (nMEO) were prepared and characterized in for their size, zeta potential, percentage of entrapment efficiency (EE%), and stability. Finally, functionality of nMEO for microbial growth inhibition was measured against Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Micrococcus luteus, Serratia marcescens, and Bacillus subtilis. These bacterial species are responsible for wound contamination, wound colonization, and clinical infections. Also they may cause opportunistic skin infections in immune-suppressed patients. Gram-positive coccid such as S. aureus, S. epidermidis, and M. luteus may origin from wound infections, impetigo, furuncles, abscesses, and carbuncles. The Gram-negative bacilli including E. coli and S. marcescens are part of normal intestinal flora. However, in some situation they may cause infection in complicating burn injuries and also surgical site (16,17). In this study, we hypothesized that nMEO is more efficient than MEO in drug releasing behavior.
MATERIALS AND METHODS
Materials
Sorbitane laurate (Span 20), sorbitane monopalmitate (Span 40), sorbitane stearate (Span 60), Sorbitane monooleate (span 80), and polysorbate 20, 40, 60, and 80 were purchased from Sigma Chemical (St. Louis, USA). 2-(p-iodophenyl)-3-(p-nitrophenyl)-5phenyl tetrazolium chloride (INT), cholesterol (Chol), Muller-Hinton agar medium, Muller-Hinton broth medium, chloroform, and ethanol were all provided from Merck Company (Darmstadt, Germany). Filter membrane polytetrafluoroethylene (PTFE) pore size 0.22 μm was procured from Sigma-Aldrich (St. Louis, USA). All other reagents were of analytical grade.
Extraction of the essential oil
The aerial parts of myrtle were collected from Haji Abad, Iran during July/August 2015 and then identified by Dr. Mirtajadini at Department of Botany, Shahid Bahonar University of Kerman, Kerman, Iran. Voucher specimen was prepared (KF 1356) and deposited at the herbarium of the Faculty of Pharmacy, Kerman University of medical sciences, Kerman, Iran. Moreover, MEO was obtained from dried harvested leaves by hydro distillation assay in a Clevenger apparatus (18). The oil was dried over anhydrous sodium sulfate and stored in -20 °C for future analysis.
Analysis of the essential oil
The composition of the volatile constituents of MEO was determined using gas chromatography/mass spectrometry (GC/MS) analyses, which was performed via a GC/MS-QP5050 equipped with a DB-5MS capillary column (40 m × 1.8 mm (internal diameter)), film thickness: 0.18 μm) and a QP5050 mass selective detector. In order to detect GC/MS, an electron ionizations system with ionization energy of 70 eV was adopted. Helium was the carrier gas at a flow rate of 1 mL/min. Injector and detector line temperature were set at 260 °C and 230 °C, respectively. Moreover, column temperature was initially kept at 60 °C for 5 min, after which it was gradually increased to 260 °C at a 5 °C/min rate. One μL diluted samples were injected manually and the split ratio was equaled 1/40.
The components of the oil were identified by comparing their retention index (RI) with Kovats’ index (KI). RI is relative to C8-C24 n-alkenes series injected to the GC/MS in the present study. KI was obtained from mass spectra published by Adams (19). Further identifications were made by matching the mass spectra with those stored in the Wiley 275.L mass spectral library of the GC/MS data system using computer.
Niosome preparation
The formulations were prepared using film hydration method as previously described (20). A precise amount of 1200 μmol of the nonionic surfactants (Span and Tween) and Chol in different molar ratios, 50:50, 60:40, and 70:30 were dissolved in chloroform in a round-bottom flask. Afterwards, 2.5 mL of MEO solution 2% in chloroform was added to the lipid phase. After removing the organic solvents at 50 °C under vacuum in a rotary evaporator (EYELA SB-1200, Japan), the film was hydrated with 10 mL deionized water with a gentle rotation at 50 °C for 60 min to produce an aqueous niosomal suspension containing 0.5% MEO.
Vesicle size determination
Malvern particle size analyzer (Malvern Instruments, MasterSizer X-100, UK) was utilized to measure size, whose distribution derived by this technique was volume-based and measured by dynamic light scattering method (21).
Zeta potential determination
The zeta potential values for nMEO were obtained by high resolution laser doppler electrophoretic technique using WALLIS zeta potential analyzer (Corduan, France).
Microscopic observation
Optical microscopy (Leitz, HM-LUX3, Germany) and scanning electron microscopy (KYKY-EM3200, China) were taken to indicate the number and surface morphologic differences between formulated nMEO prepared with different surfactants and different molar ratios.
Determination of encapsulation efficiency
The EE% of nMEO was determined by separating the non-entrapped drug using centrifuge (Vision/VS-35SMTi, Korea) at 59000 g for 30 min at 6 °C. The amounts of active constituent in the supernatant and also in the pellets were determined by GC/MS analyses. Afterwards, the EE% was calculated using following equation:
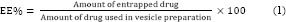
Vesicle stability study
The physical stability of the selected formulations was assessed in terms of vesicles size as described earlier. According to the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guidelines, Iran is categorized in zone II. As far as accelerated and intermediate testing condition is concerned, formulations were stored under three conditions such as 4 °C, 25 °C with relative humidity (RH) of 30%, and 40 °C with RH of 70% for 3 months (22). Then, vesicle sizes were examined during 24 h, 2 weeks, 1 and 3 months after preparation.
Evaluation of myrtle essential oil release
The in vitro release study was performed for F6 formulation, using static vertical diffusion Franz cells with an effective diffusion area of 1.5 cm2 and a receptor phase volume of 15 mL (Ashke-shisheh Co., Iran). The PTFE filter membrane pore size 0.22 μm was soaked in ethanol 50% for 24 h before the experiments. The membrane was fixed between donor and receptor compartments. The receptor compartment was filled with 50% ethanol 98° and 50% distilled water, then it was continuously stirred and thermostated at 37 ± 1 °C throughout the experiment. The donor compartment was filled with 1 mL nMEO. Total MEO solution and empty niosomal formulation were used as the control group. One mL sample was withdrawn at fixed time intervals from receptor compartment and replaced with an equal volume of fresh acceptor phase to ensure sink conditions. The permeated drug concentrations were measured by GC/MS analyses.
Bacteria
S. aureus (PTCC 1112), S. epidermidis (PTCC 1114), E. coli (PTCC 1330), M. luteus (PTCC 1110), S. marcescens (PTCC 1621), and B. subtilis (PTCC 1023) were obtained from the Department of Microbiology, School of Medicine, Kerman University of Medical Sciences, Kerman, Iran. They were maintained in Muller-Hinton agar slants at 4 °C throughout the study.
Antibacterial activity
The minimum inhibitory concentration (MIC) was determined by broth microdilution method which deals with preparing two-fold dilutions of the antimicrobial agent (23). A series of twofold dilutions were dispensed in 96-well microtitration plate (100 μL in each well). Then 10 μL of inoculum containing 105 CFU/mL bacteria were added to each well and incubated at 37 °C for 24 h. After incubation, 20 μL of INT solution 0.5% (w/v) was added to the wells and the microplates were further incubated for 30 min at 37 °C. A well-defined pink color in the wells signified positive microbial growth. Finally, the lowest concentration of the formulation that prevented bacterial growth was recorded as the MIC value.
To monitor the antimicrobial activities of MEO and nMEO, the disk diffusion method was employed (24). Inoculate containing 105 CFU/mL bacteria was used to uniformly lawn Muller-Hinton agar plates. The two-fold MEO dilutions ranging from 4000 to 250 μg/100 μL were prepared by dissolving them in 10% dimethylsulfoxide (DMSO) with 0.5% (v/v) Tween 80. Muller-Hinton-broth with 0.5% (v/v) Tween 80 was used for preparing nMEO two-fold dilutions ranging from 2000 to 250 μg/100 μL. Empty sterilized discs (6.4 mm) were impregnated with 100 μL of different concentrations and placed on the agar surface. Paper disc moistened with 10% DMSO or empty niosome were used as a vehicle control and blank, respectively. Also antibiotic discs including ciprofloxacin and gentamicin were used as positive controls. After 24 h of incubation at 37 °C, the zone of inhibition was measured.
Statistical analysis
Results are expressed as mean ± standard deviations. The statistical analyses were performed by one-way ANOVA, and Tukey's post hoc test using the Graph Pad Prism V. 5 software. Significances were considered at P-values of less than 0.05.
RESULTS
Chemical composition of myrtle essential oil
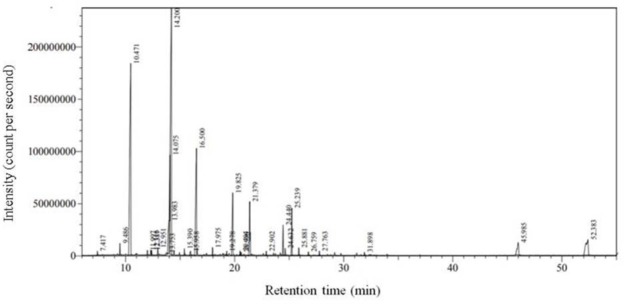
By using hydrodistillation method, 0.94 mL MEO was extracted from 100 g of dried leaves. The main components of MEO consists of 1,8-cineole (31.19%), α-pinene (22. 95%), linalool (12.14%), α-terpineol (5.06%), nonadecane (4.91%), linalyl acetate (4.41%), and β-phellandrene (4.26%) (Table 1, Fig. 1).
Table 1.
Chemical compositions of myrtle essential oil
Figure 1.
The GC/MS chromatogram of myrtle essential oil extracted by hydrodistillation.
Vesicle forming ability of surfactants
In the presence of Span/Tween (S/T) 20 and S/T 80 surfactants, the yielded vesicles were low along with formation of many separated crystals. By contrast, multilamellar vesicles (MLVs) were obtained from the S/T 40 and S/T 60 surfactants in the presence of Chol and 0.5% MEO.
Vesicle size
The mean volume diameters (dv) of the formulated vesicles with different compositions are shown in Table 2. The results of the study have demonstrated that in the S/T 60 formulations, vesicle size increased as a result of rises in the Chol content from 30 to 50 molar ratios.
Table 2.
Effect of surfactant type and cholesterol on size, zeta potential and EE% of niosomal myrtle essential oil (nMEO)
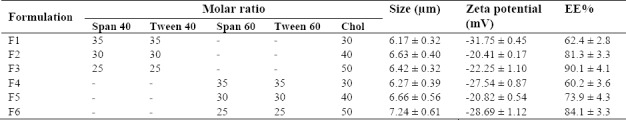
In line with our findings, the vesicle size in retinol liposomal formulations increased as the Chol content did so (25). According to findings of the present study, S/T 60 have larger vesicles size than S/T 40 related to their longer alkyl chain.
Zeta potential values
The zeta potential values of nMEO formulations are listed in Table 2. We have found that empty niosome of S/T 40 and S/T 60 in the presence of Chol has zeta potential values of 2.96 ± 0.05 to 4.13 ± 0.03 mV. In the present study, relatively high and negative zeta potential values were achieved, in spite of not incorporating any amphiphile molecules in the nisomes and is likely to be related to the multi-component nature of MEO.
Shape of niosomes
As depicted in Fig. 2, the optical and scanning electron micrographs of nMEO illustrated that the vesicles were nearly spherical in shape, uniform in size, and more often were multi-lamellar. MLVs formation may be related to our nMEO preparation method. Coordinately, previous report has shown that film hydration method usually leads to MLVs (26).
Figure 2.
Photomicrographs of niosomsl myrtle essential oil formulation F6 (A), scanning electron microscope (SEM); (B), optical microscope. Vesicles are spherical in shape and exist in dispersed and aggregate collections. Have been seen under (A), 20000×; and (B), 400× magnifications.
Entrapment efficiency
Of all the formulations, F3 formulation showed highest EE% (90.1 ± 4.1%). As shown in Table 2, S/T 40 formulations, compared to S/T 60, significantly have higher EE% (P < 0.05). In addition, increasing the amount of Chol from 30 to 50, molar ratios significantly increased (P < 0.05) the EE% in all nMEO formulations.
Stability studies
The stability results are summarized in Table 3. Niosome size was not changed significantly during 3 months at 4 °C, a finding that confirmed physical stability of niosomal formulation in the above-mentioned condition as shown in Fig. 3. Nonetheless, size of vesicles significantly was increased during storage time at 40 °C with RH of 70% (P < 0.05). Moreover, the results also showed that increasing the Chol content from 30 to 50 molar ratios enhances stability at 4 °C during 3 months. Furthermore, it has been revealed that smaller vesicles are thermodynamically unstable (27).
Table 3.
Assessment of niosomal myrtle essential oil physical stability
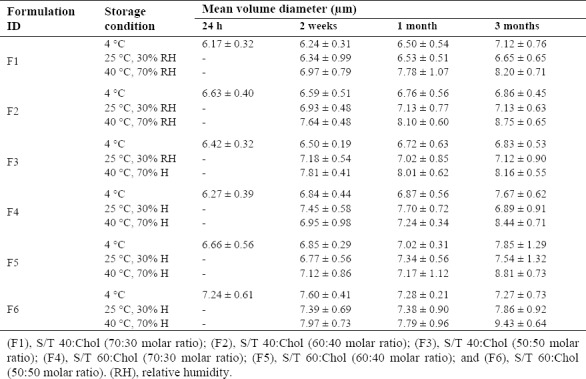
Figure 3.
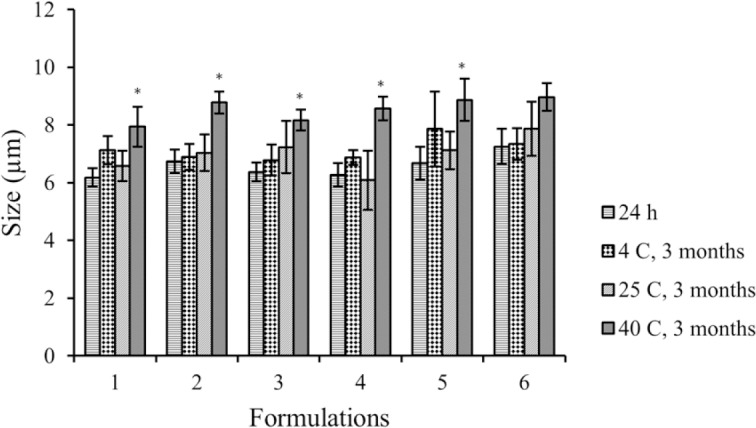
Size distribution changes of niosomal myrtle essential oil during 3 months storage at different temperatures. (F1), S/T 40:Chol (70:30 molar ratio); (F2), S/T 40:Chol (60:40 molar ratio); (F3), S/T 40:Chol (50:50 molar ratio); (F4), S/T 60:Chol (70:30 molar ratio); (F5), S/T 60:Chol (60:40 molar ratio); (F6), S/T 60:Chol (50:50 molar ratio).
In the present study, therefore, relative stability of nMEO during 3 months storage at 4 °C is likely to be associated with large vesicle size of all prepared nMEO.
The maximum and minimum mean size changes, during 3 months storage was for F2 formulation stored at 40 °C with RH of 70%, and F6 formulation stored at 4 °C (Fig. 4), respectively. Thus, F6 formulation was considered as an optimum formulation because of good stability during storage and high EE%.
Figure 4.
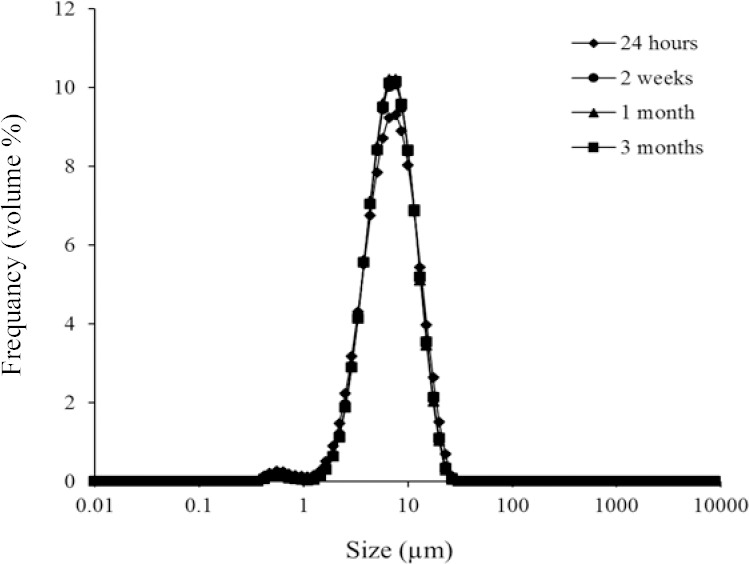
Size distribution changes of F6 formulation during storage at 4 °C as an indicator of physical stability of vesicles. (F6), S/T 60:Chol (50:50 molar ratio).
Myrtle essential oil release
As shown in Fig. 5, the release profiles were biphasic. MEO solutions yielded a release percentage of about 38% after 1.5 h through PTFE filter membrane pore size 0.2 μm whereas the niosomal formulation F6 demonstrated only 21% drug release after 1.5 h. The active constituents released from the nMEO did not reach the plateau after 4 h.
Figure 5.
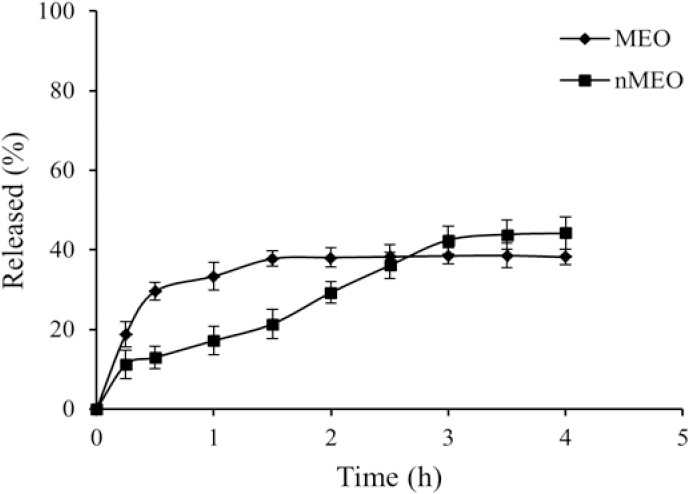
Release profile of myrtle essential oil (MEO) and F6 formulation (nMEO) from PTFE membrane, 0.2 μm, in ethanol 50% at 37 ºC (mean ± SD, n = 3). (F6), S/T 60:Chol (50:50 molar ratio).
The released data were analyzed mathematically according to zero order, first-order, Higuchi's, and Peppa's equations. The data were best fitted to Peppa's equation (R2 = 0.818) for MEO and first order (R2 = 0.977) for nMEO.
Antibacterial activity
The main constituents of MEO as shown by current results were 1,8-cineole, α-pinene, linalool, α-terpineol, nonadecane, linalyl acetate, and β-phellandrene. The potent antibacterial activity of EOs has mostly been related to oxygenated terpenes, such as 1,8-cineole, linalool and α-terpineol. Randrianarivelo, et al. demonstrated that potent antibacterial activity of MEO can be related to 1,8-cineole and linalool components (28). Moreover, linalool has previously been reported to be an antimicrobial agent (29).
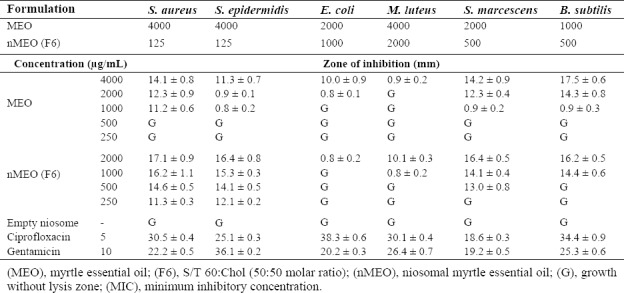
As presented in Table 4, MEO and F6 formulation have antibacterial activity against the selected microorganisms. No lysis zone was observed in control group consisting of empty niosomes treated-bacteria. In the zone of inhibition determination, nMEO inhibited growth of S. aureus, S.epidermidis, S. marcescens, and B. subtilis at lower concentrations than MEO did. Furthermore, the results revealed that F6 formulation were slightly more active against Gram-positive than Gram-negative bacteria. Furthermore, F6 formulation is more effective against S. aureus and S. epidermidis compared to MEO. This may be depends on the permeability of niosome to the membrane of Gram-positive coccid.
Table 4.
Diameter of zone of inhibition and MIC (ìg/mL) against bacteria by myrtle essential oil (MEO) and F6 formulation
DISCUSSION
Up to now, a plenty of studies have confirmed diverse therapeutic potentials of EOs including antifungal, antibacterial, antioxidant, cytotoxic, anti-diabetic, and anti-nociceptive features (5). Additionally, MEO has shown potent antioxidant, anti-mutagenic, antimicrobial, antiviral, and antifungal effects. According to ample evidence, EOs’ therapeutic effects are likely to be associated with their unique components. For instance, the potent antibacterial activity of EOs has mostly been related to the oxygenated terpenoids such as 1,8-cineole, linalool, and α-terpineol. Likewise, α-caryophyllene, a well-known component of MEO and another EOs, has been reported to function effectively as an antiviral (1). However, several evidences have indicated that environmental factors as well as other ones such as harvesting season, vegetative period of plant, and length of distillation affect the content of EOs chemical compositions (1,30). Mahboubi, et al. considered 1,8-cineole (36.1%), α-pinene (22.5%), and linalool (8.4%) to be the main constituents of MEO, collected from Iran, whose composition content order is similar to that of the results obtained from present study (31). Contrarily, other studies determined the constituents of MEO have yielded varying results. For example, the main constituents of Turkian MEO include linalool (36.5%) and linalyl acetate (16.3%) whereas Italian samples are comprised of α-pinene and 1,8-cineole (32). Despite having potent therapeutic potentials, EOs are unstable due to evaporation or decomposition even at room temperature (6). To overcome these problems, several methods are developed to stabilize EOs. Previous studies have shown that solid lipid nanoparticles were capable of reducing rapid evaporation of essential oil (33). Liolios, et al. study indicated that encapsulation of carvacrol and thymol in liposome dramatically increased antimicrobial activity (34). Unique features of niosomes including superior chemical and storage stabilities makes them a promising carrier for MEO in topical formulations compared to liposomes. However, different niosomal formulations present various physical properties that influence the EE% of active compounds. Hence, we have analyzed the effects of various molar ratios of different surfactants on features of niosomal formulations in the presence of Chol. We found that vesicle yield was low in the presence of Span 20 and Span 80 which could be due to short alkyl chain (C12) of Span 20 and unsaturated alkyl chain of Span 80 (35). Based on findings of this study, S/T 60 formulations have larger vesicles sizes while S/T 40 formulations have higher EE% which increases with increment of the amount of Chol from 30 to 50 molar ratios (P < 0.05). In line with our results, a previous study has revealed that alkyl chain length and hydrophilic-lipophilic balance (HLB) of used surfactants have a significant effect on size and EE% of formulations (36). Moreover, the length of alkyl chain in turn influences HLB value of the surfactant. Therefore, the higher EE% will be achieved with the higher HLB values of the surfactants (37). Ruckmani, et al. showed that a decrease in EE% values was accompanied by increased length of the alkyl chain of surfactants (37) while Guinedi, et al. reported that lower HLB surfactants led to higher EE% (38).
The results of the present study also revealed that all nMEO were stable at 4 °C during 3 months. Additionally, the results showed that increasing Chol content from 30 to 50 molar ratios enhances stability at 4 °C during 3 months. Chol increases the membrane rigidity and stability by altering fluidity of chains in bilayers and increasing the degree of orientational order (20,39). A previous study determined the stability of Gymnema sylvestre extract-loaded niosome through assessing the changes occurring in color, pH, and EE% before and after 90 days of being stored at 4 ± 1 °C, 25 ± 2 °C and 37 ± 2 °C. Their results showed the formulations were found to be stable at 4 ± 1 °C (22). Much consistent with findings of the present study, another study reported similar results about the size of vesicles under the same conditions (35) and the increase in the size may be related to aggregation of vesicles during the storage time (40). Of all the formulations, F6 had higher stability and lower size changes during storage time and high EE%. Based on the above-mentioned reasons, F6 formulation was selected as the preferred formulation for the next steps including the evaluation of release profile and antibacterial activity. The rate of active constituent release should be determined in order to achieve an optimal delivery system with desired release characteristics for formulation. However, the rate of release and drug retention from niosomal formulation depends on type of drug, so that it can change significantly. In addition, the drug efflux from the vesicles is affected by the chemical structure of the cholesterol and non-ionic surfactants (26). In many cases, the drug release profile of niosomal formulations the same as our result is biphasic such as acid ascorbic and α-tocopherol (26).
The initial rapid phase may have wide implications for desorption of drug from the surface of niosomes. Also, the second phase is likely to be associated with the entrapped drug being released from the formulation which is a time-consuming process. Being a stabilizing agent, Chol slowed down release of active constituent. Furthermore, the amount of drug released is related to the EE%, whose high amounts led to less drug release (26). It may be concluded that gradual release of MEO may decrease its decomposition and as well increase its efficacy during time. nMEO inhibited the growth of S. aureus, S. epidermidis, S. marcescens, and B. subtilis at lower concentrations than those of MEO. Similarly, in a study conducted by Salvagnini, et al., MEO has been reported to show an antibacterial activity against S. aureus, S. epidermidis, E. coli, B. subtilis, and S. marcescens (41). Moreover, Yadegarinia, et al., demonstrated that MEO functions against E. coli, S. aureus, and Candida albicans (42).
Alteration of bacterial cell membrane permeability, adsorption of vesicles, or the potential fusion to bacterial cell were considered as mechanisms capable of increasing antibacterial activity or decreasing bacterial resistance in nisin/EDTA nanoniosomes. Niosome encapsulation of nisin/EDTA could be gradually released in the medium, hence resulting in longer and higher antibacterial activity during the time (43). Previous investigations have indicated that several parameters, such as size, lamellarity, and Chol content could influence the antibacterial activity of niosomal formulations. Akbari, et al. showed that MICs of niosomal ciprofloxacin were two to eight-fold higher than the free drug against E. coli and S. aureus and, moreover, that the best antibacterial effect was achieved with the S/T 40: Chol (50:50 molar ratio) (44).
CONCLUSION
Encapsulation of MEO in niosomal formulation consisting of nonionic surfactants and Chol could be a promising strategy to improve the efficacy and stability of MEO. MLVs were obtained from S/T 40 and S/T 60 surfactants in the presence of Chol and 0.5% MEO. Moreover, higher EE% and stability was achieved by F6 formulation. The release profile followed first order kinetics. The results of the present study proved that, in comparison to MEO, encapsulation of MEO in F6 formulation enhanced antibacterial activity against some bacterial spices.
ACKNOWLEDGEMENTS
The content of this paper is extracted from the Ph.D. thesis (Grant No. 94/644) submitted by Mahboobeh Raeiszadeh which was financially supported by the Deputy of Research, Kerman University of Medical Sciences, Kerman, I.R. Iran.
REFERENCES
- 1.Aleksic V, Knezevic P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol Res. 2014;169(4):240–254. doi: 10.1016/j.micres.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Alipour G, Dashti S, Hosseinzadeh H. Review of pharmacological effects of Myrtus communis L. and its active constituents. Phytother Res. 2014;28(8):1125, 1136. doi: 10.1002/ptr.5122. [DOI] [PubMed] [Google Scholar]
- 3.Canon of Medicine. Tehran, Iran: Soroosh Publisher; 1986. Avicenna; pp. 56–58. [Google Scholar]
- 4.Gardeli A, Papageorgiou V, Mallouchos A, Kibouris T, Komaitis M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: evaluation of antioxidant capacity of methanolic extracts. Food Chem. 2008;107(3):1120, 1130. [Google Scholar]
- 5.Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res. 2007;21(4):308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 6.Hosseini SF, Zandi M, Rezaei M, Farahmandghavi F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: preparation, characterization and in vitro release study. Carbohydr Polym. 2013;95(1):50–56. doi: 10.1016/j.carbpol.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 7.Manosroi A, Kietthanakorn BO, Chankhampan C, Khositsuntiwong N, Manosroi W, Abe M, et al. Physical characteristics and biological activities of Thai flower extracts loaded in niosomes. Month. 2013;40(4):603–617. [Google Scholar]
- 8.Zarkesh Kh, Khazaeli P, Pardakhty A, Rezaifar M. Preparation and physicochemical characterization of topical niosomal formulation of minoxidil and tretinoin. Glob J Pharmaceu Sci. 2017;3(2):001–006. [Google Scholar]
- 9.Nematollahi MH, Torkzadeh-Mahanai M, Pardakhty A, Ebrahimi Meimand HA, Asadikaram G. Ternary complex of plasmid DNA with NLS-Mu-Mu protein and cationic niosome for biocompatible and efficient gene delivery: a comparative study with protamine and lipofectamine. Artif Cells Nanomed Biotechnol. 2017:1–11. doi: 10.1080/21691401.2017.1392316. [DOI] [PubMed] [Google Scholar]
- 10.Puras G, Mashal M, Zárate J, Agirre M, Ojeda E, Grijalvo S, et al. A novel cationic niosome formulation for gene delivery to the retina. J Control Release. 2014;174:27–36. doi: 10.1016/j.jconrel.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Basiri L, Rajabzadeh G, Bostan A. α-Tocopherol-loaded niosome prepared by heating method and its release behavior. Food Chem. 2017;221:620–628. doi: 10.1016/j.foodchem.2016.11.129. [DOI] [PubMed] [Google Scholar]
- 12.Budhiraja A, Dhingra G. Development and characterization of a novel antiacne niosomal gel of rosmarinic acid. Drug Deliv. 2015;22(6):723–730. doi: 10.3109/10717544.2014.903010. [DOI] [PubMed] [Google Scholar]
- 13.Patel J, Ketkar S, Patil S, Fearnley J, Mahadik KR, Paradkar AR. Potentiating antimicrobial efficacy of propolis through niosomal-based system for administration. Integr Med Res. 2015;4(2):94–101. doi: 10.1016/j.imr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Junyaprasert VB, Singhsa P, Suksiriworapong J, Chantasart D. Physicochemical properties and skin permeation of Span 60/Tween 60 niosomes of ellagic acid. Int J Pharm. 2012;423(2):303–311. doi: 10.1016/j.ijpharm.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 15.Manosroi A, Chutoprapat R, Abe M, Manosroi W, Manosroi J. Anti-aging efficacy of topical formulations containing niosomes entrapped with rice bran bioactive compounds. Pharm Biol. 2012;50(2):208–224. doi: 10.3109/13880209.2011.596206. [DOI] [PubMed] [Google Scholar]
- 16.Weckesser S, Engel K, Simon-Haarhaus B, Wittmer A, Pelz K, Schempp CM. Screening of plant extracts for antimicrobial activity against bacteria and yeasts with dermatological relevance. Phytomedicine. 2007;14(7-8):508–516. doi: 10.1016/j.phymed.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Basualdo C, Sgroy V, Finola MS, Marioli JM. Comparison of the antibacterial activity of honey from different provenance against bacteria usually isolated from skin wounds. Vet Microbiol. 2007;124(3-4):375–381. doi: 10.1016/j.vetmic.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 18.Ghasemi dehkordi N, Sajadi SE, Ghanadi A, Amanzadeh Y, Azadbakht M, Asghari Gh, et al. Iranian Herbal Pharmacopeia. 1st ed. Tehran: Iranian Ministry of Health and Medical Education Publications; 2002. pp. 23–24. [Google Scholar]
- 19.Adams RP. Identification of Essential Oil Components by Gas Chromatography/ Quadrupole Mass Spectroscopy. 3rd ed. Carol Stream, Illinois: Allured Publishing Corporation; 2004. pp. 261–263. [Google Scholar]
- 20.Nematollahi MH, Pardakhty A, Torkzadeh-Mahanai M, Mehrabani M, Asadikaram Gh. Changes in physical and chemical properties of niosome membrane induced by cholesterol: a promising approach for niosome bilayer intervention. RSC Adv. 2017;7(78):49463–49472. [Google Scholar]
- 21.Khazaeli P, Pardakhty A, Shoorabi H. Caffeine-loaded niosomes: characterization and in vitro release studies. Drug Deliv. 2007;14(7):447–452. doi: 10.1080/10717540701603597. [DOI] [PubMed] [Google Scholar]
- 22.Kamble B, Talreja S, Gupta A, Patil D, Pathak D, Moothedath I, et al. Development and biological evaluation of Gymnema sylvestre extract-loaded nonionic surfactant-based niosomes. Nanomedicine (Lond) 2013;8(8):1295–1305. doi: 10.2217/nnm.12.162. [DOI] [PubMed] [Google Scholar]
- 23.Valgas C, de Souza SM, Smânia EFA, Smânia A., Jr Screening methods to determine antibacterial activity of natural products. Braz J Microbiol. 2007;38(2):369–380. [Google Scholar]
- 24.Prabuseenivasan S, Jayakumar M, Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC complement Altern Med. 2006;6(1):39. doi: 10.1186/1472-6882-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SC, Lee KE, Kim JJ, Lim SH. The effect of cholesterol in the liposome bilayer on the stabilization of incorporated retinol. J Liposome Res. 2005;15(3-4):157–166. doi: 10.1080/08982100500364131. [DOI] [PubMed] [Google Scholar]
- 26.Varshosaz J, Taymouri S, Pardakhty A, Asadi-Shekaari M, Babaee A. Niosomes of ascorbic acid and α-tocopherol in the cerebral ischemia-reperfusion model in male rats. BioMed Res Int 2014. 2014:816103. doi: 10.1155/2014/816103. doi:10.1155/2014/816103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moazeni E, Gilani K, Sotoudegan F, Pardakhty A, Najafabadi AR, Ghalandari R, et al. Formulation and in vitro evaluation of ciprofloxacin containing niosomes for pulmonary delivery. J Microencapsul. 2010;27(7):618–627. doi: 10.3109/02652048.2010.506579. [DOI] [PubMed] [Google Scholar]
- 28.Randrianarivelo R, Sarter S, Odoux E, Brat P, Lebrun M, Romestand B, et al. Composition and antimicrobial activity of essential oils of Cinnamosma fragrans. Food Chem. 2009;114(2):680–684. [Google Scholar]
- 29.Carson CF, Riley TV. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J Appl Bacteriol. 1995;78(3):264–269. doi: 10.1111/j.1365-2672.1995.tb05025.x. [DOI] [PubMed] [Google Scholar]
- 30.Mahmoudvand H, Ezzatkhah F, Sharififar F, Sharifi I, Dezaki ES. Antileishmanial and cytotoxic effects of essential oil and methanolic extract of Myrtus communis L. Korean J Parasitol. 2015;53(1):21–27. doi: 10.3347/kjp.2015.53.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahboubi M, Bidgoli FG. In vitro synergistic efficacy of combination of amphotericin B with Myrtus communis essential oil against clinical isolates of Candida albicans. Phytomedicine. 2010;17(10):771–774. doi: 10.1016/j.phymed.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Senatore F, Formisano C, Napolitano F, Rigano D, Özcan M. Chemical composition and antibacterial activity of essential oil of Myrtus communis L. growing wild in Italy and Turkey. J Essent Oil Bear Plants. 2006;9(2):162–169. [Google Scholar]
- 33.Lai F, Wissing SA, Müller RH, Fadda AM. Artemisia arborescens L essential oil-loaded solid lipid nanoparticles for potential agricultural application: preparation and characterization. AAPS PharmSciTech. 2006;7(1):E10. doi: 10.1208/pt070102. [DOI] [PubMed] [Google Scholar]
- 34.Liolios CC, Gortzi O, Lalas S, Tsaknis J, Chinou I. Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chem. 2009;112(1):77–83. [Google Scholar]
- 35.Varshosaz J, Pardakhty A, Hajhashemi VI, Najafabadi AR. Development and physical characterization of sorbitan monoester niosomes for insulin oral delivery. Drug Deliv. 2003;10(4):251–262. doi: 10.1080/drd_10_4_251. [DOI] [PubMed] [Google Scholar]
- 36.Hao YM, Li K. Entrapment and release difference resulting from hydrogen bonding interactions in niosome. Int J Pharm. 2011;403(1-2):245–253. doi: 10.1016/j.ijpharm.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 37.Ruckmani K, Sankar V. Formulation and optimization of zidovudine niosomes. AAPS PharmSciTech. 2010;11(3):1119–1127. doi: 10.1208/s12249-010-9480-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guinedi AS, Mortada ND, Mansour S, Hathout RM. Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of acetazolamide. Int J Pharm. 2005;306(1-2):71–82. doi: 10.1016/j.ijpharm.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 39.Bayindir ZS, Yuksel N. Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J Pharm Sci. 2010;99(4):2049–2060. doi: 10.1002/jps.21944. [DOI] [PubMed] [Google Scholar]
- 40.Lawrence J, Chauhan S, Lawrence SM, Barlow D. The formation, characterization and stability of non-ionic surfactant vesicles. STP Pharm Sci. 1996;6(1):49–60. [Google Scholar]
- 41.Salvagnini LE, Oliveira JRS, Santos LEd, Moreira RRD, Pietro RCLR. Evaluation of the antibacterial activity of Myrtus communis L.(Myrtaceae) leaves. Rev Bras Farmacogn. 2008;18(2):241–244. [Google Scholar]
- 42.Yadegarinia D, Gachkar L, Rezaei MB, Taghizadeh M, Astaneh SA, Rasooli I. Biochemical activities of Iranian Mentha piperita L. and Myrtus communis L. essential oils. 2006;67(12):1249–1255. doi: 10.1016/j.phytochem.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 43.Kopermsub P, Mayen V, Warin C. Nanoencapsulation of nisin and ethylene diamine tetra acetic acid in niosomes and their antibacterial activity. J Sci Res. 2012;4(2):457–465. [Google Scholar]
- 44.Akbari V, Abedi D, Pardakhty A, Sadeghi-Aliabadi H. Ciprofloxacin nano-niosomes for targeting intracellular infections: an in vitro evaluation. J Nanopart Res. 2013;15(4):1556–1570. [Google Scholar]