Abstract
Cells of tomato (Lycopersicon esculentum) growing in suspension gradually depleted their culture medium and caused a steady decrease in its osmolality. When confronted with a sudden change in medium osmolality (a hypo-osmotic or hyperosmotic shock), respectively, these cells responded with volume changes and stress symptoms such as rapid extracellular alkalinization, efflux of K+-ions, and induction of 1-aminocyclopropane-1-carboxylate synthase acid, the key enzyme of ethylene biosynthesis. This array of stress symptoms is well known from cultured plant cells treated with microbial elicitors. Compared with elicitor treatment, induction of responses by hyperosmotic shock was slow and occurred only after increases of approximately 200,000 Pa in osmotic pressure. In contrast, hypo-osmotic shock induced responses without measurable lag and faster than elicitor treatments. Measurable medium alkalinization was induced when medium osmolality was reduced by as little as approximately 10 mosmol, a change corresponding to only approximately 0.2 bar in osmotic pressure. Like treatment with elicitors, hypo-osmotic shock induced specific changes in protein phosphorylations as demonstrated by in vivo labeling with [33P]orthophosphate. Exposure of cells to consecutive up- and down-shifts in medium osmolality showed that sensing of osmotic changes occurred within seconds, whereas adaptation to new osmotic conditions proceeded over hours. In conclusion, suspension-cultured plant cells display rapid, easily measurable macroscopic responses to osmotic shock and provide an interesting model system to study osmoregulation, a key process in plant growth and development.
Plants are exposed to a variety of potentially adverse environmental conditions such as drought or salinity stress, flooding, anaerobiosis, unfavorable conditions with regard to light or temperature, mechanical stress (wind), wounding, and pathogen attack. To adapt to these conditions, plants have evolved mechanisms to sense these environmental parameters and stress factors. Cultured plant cells have been used as models to study osmotic stress (Yahraus et al., 1995; Cazalé et al., 1999), heavy metals (Hirt et al., 1989), ozone (Kangasjärvi et al., 1994), UV light (Hahlbrock and Scheel, 1989), or medium starvation for auxin (Leguay and Jouanneau, 1987). Most widely, cultured plant cells have been used as models to study chemoperception systems for microbial elicitors thought to signal the presence of potential pathogen to the plant cells (Ebel and Cosio, 1994; Boller, 1995).
Characteristic reactions of cultured plant cells to treatments with pathogens or elicitors include rapid alkalinization of the culture medium, efflux of K+ ions, influx of Ca2+, increased production of activated oxygen species, and production of the stress hormone ethylene (for reviews, see Dixon et al., 1994; Boller, 1995). The physiological role of these early responses is not well understood nor is their connection to defense mechanisms directed against pathogens such as phytoalexin production or induction of antimicrobial enzymes. Nevertheless, these responses can serve as early and easily measurable indicators of elicitor perception (Dixon et al., 1994; Boller, 1995).
Altered ion fluxes across the plasma membrane, activation of mitogen-activated protein (MAP) kinase pathways and induced production of reactive oxygen species have been observed also after treatment of plant cells in culture with mechanical stress or osmotic shock (Yahraus et al., 1995; Takahashi et al., 1997a; Cazalé et al., 1999, Mikolajczyk et al., 2000). In this report we compared responses of suspension-cultured tomato (Lycopersion esculentum) cells treated with elicitor preparations with the ones observed after hypo-osmotic or hyperosmotic shock. Sudden changes in the osmolality of the culture medium were found to have similar effects as treatment with elicitors and stimulated medium alkalinization, K+-efflux, and induction of 1-aminocyclopropane-1-carboxylate synthase acid (ACC), the enzyme catalyzing the first step in ethylene biosynthesis. Induction of responses by elicitor and hypo-osomotic shock were both dependent on protein phosphorylation and were paralleled by changed phosphorylation of specific proteins. Medium alkalinization, occurring as an early and easily measurable consequence of altered ion fluxes, was used as a bioassay to monitor and characterize the osmosensing system of the suspension cultured tomato cells. Cells proved particularly sensitive to hypo-osmotic conditions and responded to pressure changes as small as approximately 0.2 bar. A particularly tight link between stimulus and response was observed also with respect to the kinetics of induction, demonstrating a close and dynamic link of osmosensing and intracellular signaling in plant cells.
RESULTS
Changes in the Culture Medium during a Subculture Period
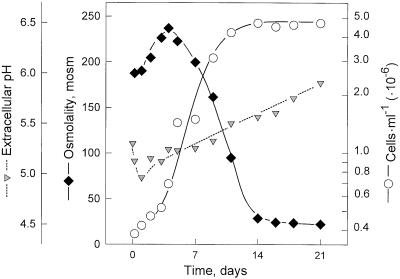
The tomato cells used throughout the present study were subcultured in 2-week intervals in a liquidMurashige and Skoog-type medium supplemented with 3% (w/v) Suc as described earlier (Felix et al., 1991a), using approximately 2 g of cells (fresh weight) for inoculation of the fresh medium. The medium of a freshly inoculated culture had an osmolality of 180 mosmol and a pH of approximately 5.3. During the first 3 d after subculture, the osmolality rose to 240 mosmol (Fig. 1), an increase attributable to the hydrolysis of Suc. Thereafter, it dropped continuously and reached a value of approximately 20 mosmol in the stationary phase (Fig. 1), indicating that the cells depleted the medium nearly completely. Similarly, the concentration of K+-ions in the culture medium dropped continuously from an initial value of 15 mm to approximately 2 mm (data not shown). The pH in the medium also underwent characteristic changes. It first decreased to a minimum of pH 4.9 after 1 d and then steadily increased to pH approximately 5.8 in the stationary phase (Fig. 1).
Figure 1.
Growth characteristics of suspension-cultured tomato cells. Cells (approximately 2 g of fresh weight) were inoculated into 50 mL of fresh medium at d 0. The cell number, extracellular pH, and medium osmolality were measured at different times after subculture.
Alkalinization of the Culture Medium after Osmotic Shock
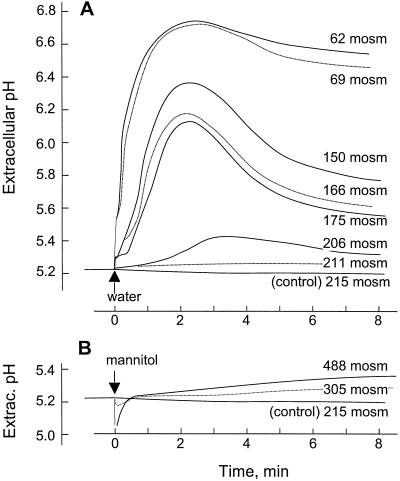
During a subculture cycle, cells experience considerable but steady changes in the osmolality of their incubation medium. We examined the reaction of cells in their exponential growth phase to sudden changes in the osmolality of the growth medium, adding either water (hypo-osmotic shock) or medium supplemented with 1 m mannitol (hyperosmotic shock) to the suspension. In both cases, the cells showed a rapid response, as reflected by changes of the pH in the growth medium (Fig. 2).
Figure 2.
Induction of the alkalinization response by hypo-osmotic and hyperosmotic shock. Medium osmolality in cells (5 d after subculture, 215 mosmol) was changed in a sudden manner to the values indicated by replacing the medium surrounding the cells with medium diluted with water (A, hypo-osmotic shock) or medium supplemented with mannitol (B, hyperosmotic shock). For controls, the medium was removed and added again without further addition.
Hypo-osmotic shock caused a particularly rapid and strong alkalinization response. For example, lowering the osmolality in a 5-d-old culture from 215 to 62 mosmol, the extracellular pH increased without apparent lag and reached a maximum approximately 1.5 pH units above the initial value of 5.2 within less than 3 min (Fig. 2A). The alkalinization response depended on the strength of the stimulus and lowering the osmolality of the medium to a smaller degree led to smaller and more transient alkalinization of the medium (Fig. 2A). The smallest change in osmolality that still induced a significant pH increase was a reduction from 215 to 206 mosmol, corresponding to a reduction of the osmotic pressure of approximately 0.2 bar (20 kPa), caused by diluting the suspension with 0.05 volumes of water.
Hyperosmotic shock also provoked extracellular alkalinization, but at a much weaker and slower rate. For example, a sudden increase in the osmolality of the medium to 305 or 488 mosmol caused a slow, continuous rise of the pH by 0.1 or 0.2 unit over a period of 8 min (Fig. 2B).
The experimental treatment of removal and re-addition of the culture medium itself did not induce alkalinization (Fig. 2, control cells). Also, no alkalinization response was observed when the culture medium was diluted or replaced with iso-osmotic solutions of mannitol, KCl, or l-Pro in water (data not shown), indicating that alkalinization response is due to changes in the osmolality of the medium and not due to removal or dilution of components present in the incubation medium of the cells.
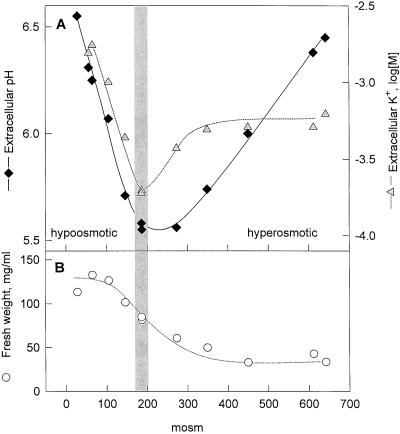
Cell Volume Changes and K+ Efflux in Cells Treated under Hypo-Osmotic and Hyperosmotic Conditions
Extracellular alkalinization in response to osmotic shock was paralleled by a concomitant efflux of K+ ions from the cells (Fig. 3A). To measure changes in K+ levels more accurately cells were first pre-incubated in a medium with a reduced K+ concentration (0.1 mm KCl). Treatment of these cells for 15 min under hypo-osmotic or hyperosmotic conditions resulted in strongly increased levels of K+ ions in the extracellular medium. Similar to the changes of the extracellular pH, these changes depended on the strength of the osmotic challenge applied (Fig. 3A). In cells incubated at a density of approximately 0.25 g fresh weight mL−1, K+ concentration remained at approximately 0.1 mm under iso-osmotic conditions (Fig. 3, gray bar) but increased to approximately 2 mm and approximately 0.6 mm under hypo-osmotic and hyperosmotic conditions, respectively (Fig. 3A), indicating bulk net efflux of K+ ions under both conditions.
Figure 3.
Alkalinization response, K+-efflux, and change in cell volume after hypo-osmotic and hyperosmotic shock. Cells (6 d after subculture, 190 mosmol) were washed and pre-incubated for approximately 1 h in an iso-osmotic solution containing 5 mm NaCl, 1 mm CaCl2, 0.1 mm KCl, and an appropriate amount of mannitol. A, Extracellular pH and extracellular K+ concentration measured 15 min after replacing the assay medium with media of different osmolality. Gray bar indicates treatment of the suspension under iso-osmotic conditions (190 mosmol). B, Fresh weight of the cells after 15 min in media of different osmolality.
As expected for turgescent plant cells with elastic cell walls, the osmolality of the culture medium also affected the cell volume (Fig. 3B). Compared with cells treated under iso-osmotic conditions, the volume of the cells, determined as the fresh weight of cells after removal of the incubation medium, increased by approximately 50% after 15 min in media with <100 mosmol and decreased by approximately 50% after incubation in media with >300 mosmol, respectively.
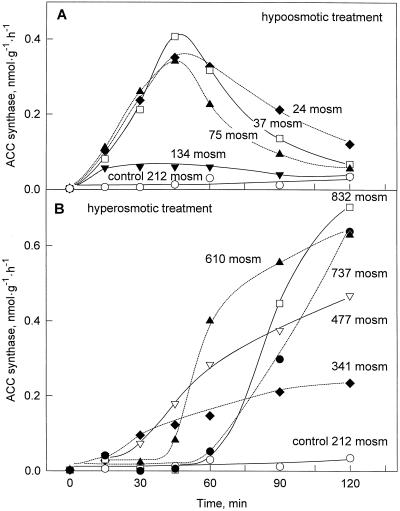
Induction of ACC Synthase
Both hypo- and hyperosmotic shock caused a strong increase in the activity of ACC synthase, the enzyme catalyzing the first step in ethylene biosynthesis (Fig. 4). Stimulation depended on the strength of the osmotic change applied and occurred particularly rapidly after hypo-osmotic shock with clearly elevated enzyme activities after 15 min of treatment. Induction under hypo-osmotic conditions was transient with maximal activity observed approximately 45 min after the reduction of osmolality (Fig. 4A). Hyperosmotic shock, in contrast, caused a slower but more persistent induction of the enzyme (Fig. 4B). The activities reached after 120 min of treatment depended on the strength of the hyperosmotic shock in a steady manner. However, the time courses of induction exhibited a more complex pattern with more rapid onset of induction for weaker and slower onset for stronger increases in osmolality of the medium. Cells transferred to high osmoticum also displayed a delayed onset of ACC synthase induction when treated with the fungal elicitor xylanase (from Trichoderma viride) (data not shown), indicating that induction of ACC synthase was possible only after adjustment of the cells to the hyperosmotic conditions. Induction of ACC synthase by hyperosmotic conditions was similarly observed when sorbitol, glycerol, or KCl were used as osmotica (data not shown).
Figure 4.
Induction of ACC synthase activity by osmotic shock. Cells (5 d after subculture, 212 mosmol) were transferred to media adjusted to lower osmolalities by water (A) or to higher osmolalities by mannitol (B).
Reversibility of Hypo-Osmotic Stress and Inhibition of Alkalinization Response
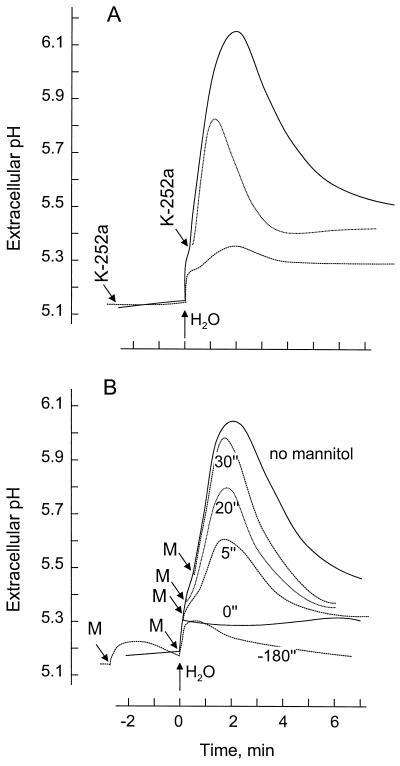
The alkalinization response of cells subjected to hypo-osmotic conditions was strongly, although not completely, inhibited by a pretreatment with 1 μm K-252a (Fig. 5A). K-252a added after the onset of the response led to an arrest of pH increase within <1 min. Reversal of the hypo-osmotic condition by the addition of an appropriate amount of mannitol solution affected the alkalinization response with comparable kinetics (Fig. 5B). When osmolality was restored shortly after the onset of alkalinization, the pH increase continued for 1 to 2 min after the addition of osmoticum. Addition of an equivalent dose of mannitol either before or concomitantly with water prevented the alkalinization response. In contrast, mannitol added to cells had no effect on the alkalinization response induced by fungal stimuli such as chitin fragments or xylanase (data not shown). Although the reversion of osmolality rapidly stopped induction, a 5-s treatment under hypo-osmotic conditions was sufficient to induce a pH increase (ΔpHmax) that was nearly one-half as big as continued treatment at low osmolality (Fig. 5B).
Figure 5.
Inhibition of the alkalinization response by the protein kinase inhibitor K-252a and by reversion of hypo-osmotic stress. A, Cells (6 d after subculture, 191 mosmol) were treated with a hypo-osmotic shock (133 mosmol) by the addition of water (t = 0 min) and with 1 μm K-252a as indicated. B, Cells were treated with water (t = 0 min) and an amount of mannitol (M) sufficient to revert the osmolality back to approximately 191 mosmol as indicated.
Adaptation to Hyperosmotic Conditions
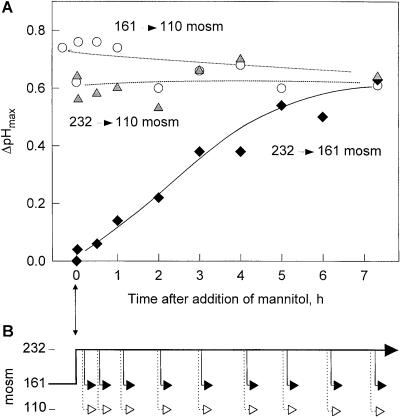
To study the adaptation process to hyperosmotic conditions, cells were subjected to a weak hyperosmotic treatment by adjusting the osmolality from 161 to 232 mosmol with addition of mannitol (Fig. 6). When these cells were shifted back to 161 mosmol after 3 min, there was no significant alkalinization response (Fig. 6). Cells incubated in the hyperosmotic medium for a longer time started to display an alkalinization response when shifted back to the original osmolality of 161 mosmol. The extent of this alkalinization response increased gradually over 5 h and reached a plateau of ΔpHmax of approximately 0.6. In contrast, mannitol-treated cells when shifted down to 110 mosmol showed a constant alkalinization response throughout the experiment (Fig. 6). This response resembled one of the control cells not treated with mannitol after shifts from 161 to 110 mosmol, indicating that mannitol treatment did not generally inhibit response to hypo-osmotic treatment. In summary, these results indicate that cells adapt to higher osmolality in a process that proceeds over several hours.
Figure 6.
Adaptation of cells to hyperosmotic conditions. Cells (7 d after subculture, 161 mosmol) were subjected to a mild hyperosmotic shock (232 mosmol) by the addition of mannitol at time zero. A, At different times, aliquots of these cells were tested for their alkalinization response when transferred to approximately 161 mosmol or approximately 110 mosmol. Control cells (○) were treated in parallel without increasing the osmolality of their medium, and then subjected to a hypo-osmotic shock (approximately 110 mosmol). B, Schematic representation of osmolality changes in A for cells treated with mannitol at time t = 0 h (thick line) and transfer to approximately 161 mosmol (arrows with black heads) and approximately 110 mosmol (arrows with white heads). Treatment of control cells are not represented in this scheme.
Response to Two Consecutive Treatments with Hypo-Osmotic Stress
Alkalinization after mild hypo-osmotic shock (Δmosmol of <60 mosmol) was transient, and extracellular pH returned close to its original value within 10 min (Fig. 2A). This could indicate rapid adaptation to lower osmoticum. Because restoring the osmotic condition (a mild hyperosmotic treatment) did not induce any measurable response, this process of adaptation could not be tested directly as described above for adaptation to hyperosmotic conditions. To test adaptive processes, cells were subjected to two consecutive treatments under hypo-osmotic conditions. In the experiment summarized in Figure 7, a batch of cells (178 mosmol) was first treated at 130 mosmol for 10 min, and then medium osmolality was restored to the original value of 178 mosmol by the addition of mannitol. At different times after this pretreatment, aliquots of the cells were then tested for alkalinization response to a second hypo-osmotic treatment at 130 mosmol (Fig. 7). Already after 3 min of restoration of the original osmolality, the cells showed a low but significant alkalinization response (Fig. 7). Thereafter, responsiveness of the cells to hypo-osmotic shock rapidly recovered, and, within 60 min the ΔpHmax of the alkalinization response reached the same values observed in control cells not subjected to the hypo-osmotic pretreatment (Fig. 7). Thus the transient character of the extracellular alkalinization after hypo-osmotic shock seemed not to reflect complete adaptation to lower osmoticum but rather represented a new dynamic equilibrium of alkalinization and processes readjusting the pH to its original value.
Figure 7.
Alkalinization response to consecutive hypo-osmotic shock treatments. Cells (6 d after subculture, 178 mosmolm) were pretreated for 10 min under hypo-osmotic conditions (130 mosmol) before osmolality of medium was restored to 178 mosmol by mannitol (time zero). A, Alkalinization in aliquots of the cells subjected to a second hypo-osmotic shock (approximately 130 mosmol) at the times indicated. Control cells (○) exposed to hypo-osmotic shock (approximately 130 mosmol) without pretreatment. B, Schematic representation of osmolality changes in A for cells pretreated under hypo-osmotic conditions (thick line and arrows with black heads) and control cells (dotted line and arrows with white heads).
Induction of Myelin Basic Protein (MBP) Kinase Activity
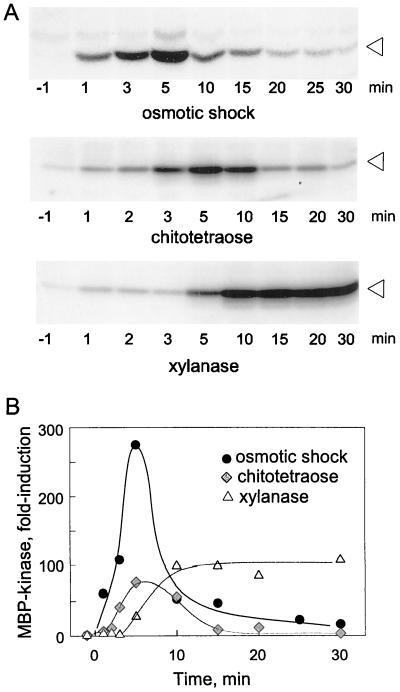
Rapid activation of MAP kinase-like enzyme activities have been reported in various plant cell cultures treated with elicitors, wounding, and mechanical stress (Seo et al., 1995; Suzuki and Shinshi, 1995) but also after osmotic shock (Seo et al., 1995; Takahashi et al., 1997a; Cazalé et al., 1999; Mikolajczyk et al., 2000). We tested whether hypo-osmotic stress would similarly stimulate activity of MBP-kinase(s) in tomato cells. An MBP-kinase, migrating with an apparent Mr of approximately 51,000 on SDS-PAGE, was rapidly and strongly induced after treatment with hypo-osmotic stress (Fig. 8). Activation was visible after 1 min of treatment and reached a maximum after 5 min. Treatment with the elicitors chitotetraose and xylanase also induced MBP kinase activity although with somewhat slower kinetics (Fig. 8).
Figure 8.
MBP kinase activity after hypo-osmotic shock and elicitor treatment. A, Cells were treated by hypo-osmotic shock (lowering medium osmolality from 212 to 40 mosmol), xylanase (10 μg mL−1), or chitotetraose (10 nm) as indicated. At different time points, aliquots of cells were tested for MBP kinase activity by in-gel kinase assays and analyzed by using a PhosphorImager. Arrows mark position of kinase activity migrating with an apparent Mr of approximately 51. B, Quantitative analysis of MBP kinase activity observed in A. Values are expressed as relative increase over values before treatment.
Changes in Protein Phosphorylation
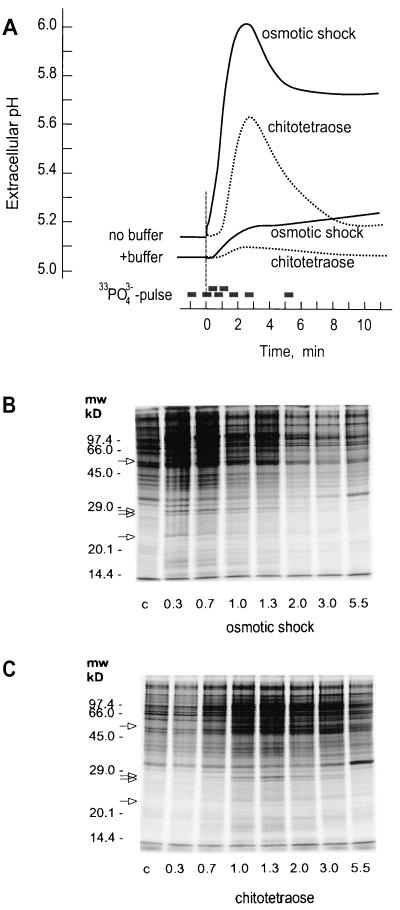
Induction of MBP kinase activities is usually taken as indicator for the activation of kinase cascades leading to increased phosphorylations of an array of target proteins. In previous experiments we have observed that the onset of the alkalinization response induced by fungal elicitors, chitin fragments, and calyculin A is paralleled by specific changes in the pattern of protein phosphorylation (Felix et al., 1991b, 1993, 1994). For these studies we developed a labeling technique that involves short, 30-s labeling of cells with [33P]orthophosphate and subsequent analysis of the labeled phosphoproteins by SDS-PAGE and autoradiography. For this, cell cultures were supplemented with 10 mm MES (4-morpholine-ethanesulfonic acid)/KOH (pH 5.1) to dampen the extracellular alkalinization and allow uniform uptake of orthophosphate throughout the experiments. When cells were labeled at different times after application of osmotic shock or, for comparison, with chitotetraose (bars in Fig. 9A), the first changes in the pattern of protein phosphorylation became visible 1 min after addition of chitotetraose (Fig. 9C) and already 20 s after lowering the osmolality of the culture medium (Fig. 9B). After both treatments, the pattern of more than 10 newly labeled bands appeared reproducibly at the onset of the alkalinization process. Within the resolution of this one-dimensional analysis, these changes were identical for both treatments, and they were also indistinguishable from the changes after treatments of cells with fungal xylanase and calyculin A described earlier (Felix et al., 1994).
Figure 9.
Kinetics of alkalinization and kinetics of changes in protein phosphorylation in response to hypo-osmotic shock and chitotetraose. A, Effect of hypo-osmotic shock (lowering medium osmolality from 194 to 142 mosmol) or chitotetraose (10 nm) on the extracellular pH in the presence or absence of 10 mm MES (K+) buffer, pH 5.1. Bars above the time scale indicate the labeling periods used in B and C for the [33P]phosphate pulses. B and C, SDS-PAGE and autoradiography of cell extracts from tomato cells treated with hypo-osmotic shock (B) or chitotetraose (C) and labeled with 30-s pulses of [33P]phosphate in the presence of 10 mm MES (K+) buffer, pH 5.1. First lane (c), Extract of cells pulse-labeled 1 min before treatment by hypo-osmotic shock or chitotetraose. Numbers below the lanes indicate the time (min) at which the pulse-labeling period ended.
DISCUSSION
The tomato cells used in this study have previously been characterized with respect to their responses to microbial elicitors like fungal-derived glycopeptides, chitin fragments, ergosterol, xylanase, and bacterial flagellin (Basse et al., 1993; Felix et al., 1993, 1999). In the cases further studied, specific high-affinity binding site residing on the surface of the cells could be identified (Basse et al., 1993; Baureithel et al., 1994), suggesting perception of these different elicitors via an array of different receptors. Although with characteristically different kinetics, these different receptors seem to activate identical intracellular signaling elements and early responses. These elicitor responses, including rapid changes in ion fluxes across the plasma membrane, release of reactive oxygen species, increased biosynthesis of ethylene, and the induction of the phenylpropanoid pathway are symptoms common to plants under attack by pathogens or exposed to wounding (Dixon et al., 1994; Ebel and Cosio, 1994; Baron and Zambryski, 1995). Elements of these stress symptoms have also been observed in plant cells treated with heavy metals (Hirt et al., 1989), ozone (Kangasjärvi et al., 1994), UV irradiation (Hahlbrock and Scheel, 1989), medium starvation for auxin (Leguay and Jouanneau, 1987), and osmotic shock (Yahraus et al., 1995; Takahashi et al., 1997b). The multitude of different stress conditions leading to apparently common symptoms suggests induction processes via converging signaling pathways. However, an example for separate pathways has been described for the induction of Phe ammonia lyase activity by elicitor preparations and UV light where induction of two distinct Phe ammonia lyase genes have been implicated (Hahlbrock and Scheel, 1989). Similarly, different sources of active oxygen species appeared to be involved in the oxidative burst induced by different types of stress (Allan and Fluhr, 1997).
In this study, responses of suspension-cultured tomato cells to sudden changes in osmotic conditions were compared with previously reported responses to treatment with elicitors. Both, hypo-osmotic and hyperosmotic stress conditions activated responses and stimulated medium alkalinization, efflux of K+-ions, and induction of ACC synthase. The induction of these responses differed considerably for the two forms of stress with regard to kinetics and sensitivity. Responses to hyperosmotic shock, observed after an increase in medium osmolality of >100 mosmol or a change in osmotic potential of >2 bar, were induced with a delay and proceeded for a prolonged time. Responses to hypo-osmotic shock, in contrast, were induced very rapidly and very sensitively. Lowering medium osmolality by as little as 10 mosmol, corresponding to a changes in osmotic potential of approximately 0.2 bar, caused significant induction of medium alkalinization. Thus, perception of hypo-osmotic conditions appeared to be directly and tightly linked to cellular signaling mechanisms.
It might be argued that osmotic shock could confer a general permeability or leakiness to cell membranes or even bursting of the cells that could explain K+ efflux and medium alkalinization as a passive process. However, induction of ACC synthase depends on active metabolism of intact cells. Also, as previously described for Ca2+ influx and oxidative burst induced by hypo-osmotic shock (Takahashi et al., 1997b; Cazalé et al., 1999), induction of responses was strongly inhibited by protein kinase inhibitors like K-252a, indicating dependence on a functional intracellular signaling mechanism. Signaling by hypo-osmotic shock was correlated with a rapid increase in MBP-kinase activity (Cazalé et al., 1999; Fig. 8) and specific changes in pattern of de novo phosphorylated proteins (Fig. 9). Thereby, within the limits of the one-dimensional gel electrophoretic analysis performed in this study, these changes were indistinguishable from the changes induced by elicitors.
Osmotic shock leads to a rapid change of cell turgor and results in changes in cell volume (Fig. 3). Efflux of K+-ions after hypo-osmotic shock could counteract the osmotic pressure applied and lead to a decrease of turgor pressure. In contrast, efflux of K+ after hyperosmotic shock would decrease intracellular osmolality and cause further decrease of turgor. The K+ efflux amounts to approximately 3 mmol kg−1 cells within 15 to 20 min, an amount that has little effect on intracellular osmolality. Also, experimentally altered medium osmolality remained stable for the duration of the experiments, indicating that efflux and uptake of solutes including K+ did not significantly counteract the osmotic stress applied. However, in intact plant tissues, where the volume of the extracellular fluid is only a fraction of the intracellular volume, an equivalent K+ efflux might alter the osmolality of the extracellular fluid in an analogous situation more drastically.
Nevertheless, we consider it more likely that the changes in H+ and K+-ions in the extracellular medium are symptoms reflecting intracellular processes involved in signaling stress responses as hypothesized for the corresponding fluxes of H+, K+, Cl−, and Ca2+ ions observed in cells after elicitor treatments (Mathieu et al., 1991; Atkinson et al., 1993; Nürnberger et al., 1997). Candidates for intracellular changes that could serve as second messengers activating downstream responses include depolarization of the plasma membrane (Mathieu et al., 1991; Kuchitsu et al., 1993), cytoplasmic acidification (Horn et al., 1992; Roos et al., 1998), and increased levels of Ca2+ in the cytoplasma (Chandra et al., 1997; Takahashi et al., 1997a, 1997b).
It is interesting that the induction of the alkalinization response by strong hypo-osmotic shock started without apparent lag and occurred faster than after treatments with microbial stimuli. In general, lag times were characteristic for a particular stimulus and the lag phases increased in the same relative order with hypo-osmotic shock < chitin fragments < flagellin < xylanase < ergosterol in the tomato cells investigated here but also in cells of the wild tomato Lycopersicon peruvianum and tobacco cells (data not shown). The spectrum of microbial stimuli recognized and the duration of the responses induced depended on the particular cell culture analyzed. Whether these differences reflect differences intrinsic to the plant species or whether they originated by variation and selection processes during the years of in vitro growth remains to be tested. However, all plant cell cultures tested so far, including soybean, Arabidopsis, tobacco, potato, tomato, L. peruvianum, and rice, reacted with an alkalinization response when challenged with hypo-osmotic conditions (data not shown), indicating that the capacity for osmosensing is a general characteristic highly conserved among different plant species.
A tight link between stimulus and response was evident in experiments with treatment of the cells under hypo-osmotic conditions and subsequent restoration of the original medium osmolality. On the one hand, exposure to low osmolality for only few seconds triggered a measurable alkalinization response and, on the other hand, restoration led to rapid arrest of the induction process. In contrast, adaptation to changes in medium osmolality proceeded slowly over time spans in the order of hours. For example (Fig. 6), cells transferred to elevated osmoticum only slowly developed an alkalinization response to restoration of the original osmotic conditions. Thus, restoration of medium conditions was not sensed as a hypo-osmotic stress corresponding to an equivalent change in osmolality in fully adapted cells. Since these cells respond “normally” to lowering osmolality below the original values, this hints at a “memory” of the plant cells for the original conditions. It also indicates that the osmosensing system of the cells responds to absolute values of osmotic pressure rather than to relative changes.
Osmoregulation is an important aspect of cellular metabolism in all organisms confronted with changes in the extracellular water potential. Elements important for osmoregulation have been identified in bacteria, yeast, and mammalian cells. In bacteria, several osmoregulated operons have been described for which genes of two-component pathways act as upstream regulatory elements (Csonka and Hanson, 1991). The response of yeast to high osmolality similarly depends on a two-component system (Maeda et al., 1994). It is most interesting that a hybrid-type His kinase was identified recently in Arabidopsis that could functionally complement yeast mutants defective in osmosensing (Urao et al., 1999). Osmoregulation involves activation of MAP kinase cascades in yeast (Brewster et al., 1993), in mammalian cells (Han et al., 1994), and in plant cells (Yahraus et al., 1995; Takahashi et al., 1997a; Cazalé et al., 1999; Mikolajczyk et al., 2000). Thus, osmoregulation from bacteria to man appears to involve similar elements for signal perception and transduction. Common to these different systems is also the lack of knowledge about the physicochemical parameters sensed by these osmosensing systems. In bacteria several osmoreceptors have been identified genetically and some of the corresponding genes code for proteins with structures of chemoreceptors (Csonka and Hanson, 1991). However, the chemical identity of the putative ligand for these chemosensors remains unknown. Alternatively, osmotic changes could be sensed via hydrostatic pressure, corresponding to the turgor of the cell, by a mechanism functioning as a baroreceptor or by stretch- or mechanosensitive ion channels. Ion channels of this type have been characterized in yeast (Gustin et al., 1988) and in plants (Ding and Pickard, 1993).
Osmosensing in plants can be expected to be of functional importance for adaptation to rapidly changing levels of water supply. Cultured plant cells show rapid responses even to minor changes in the osmolality of their culture medium and should provide suitable experimental systems to study the mechanism of osmosensing in plants.
MATERIALS AND METHODS
Chemicals and Elicitor Preparations
Xylanase (from Trichoderma viride) and K-252a were obtained from Fluka (Buchs, Switzerland), N,N′,N",N′"-tetraacetyl-chitotetraose was from Seikagaku (Tokyo), and calyculin A was from LC-Services (Woburn, MA). Xylanase was used as crude preparation or obtained in a partially purified form after ion-exchange chromatography on a CM-Trisacryl column (Sepracor-IBF Biotechnics, Villeneuve-la-Garenne, France).
Cell Culture
The tomato cell line Msk8 (Koornneef et al., 1987) was maintained in suspension culture by biweekly subculture. In brief, approximately 2 g of cells (fresh weight), harvested by filtration after passage through a steel sieve (100-μm mesh size) to remove bigger aggregates, were inoculated in 50 mL of liquid Murashige and Skoog-type medium, and were supplemented with 3% (w/v) Suc and vitamins (Adams and Townsend, 1983) as described by Felix et al. (1991a).
Measurement of Osmolality
Cells were removed by filtration from aliquots of the cell suspension, and the osmolality of the medium was assayed with an osmometer (“μOsmette;” Precision Systems, Natick, MA).
Treatments to Impose Sudden Changes of Medium Osmolality
Osmolality of the culture medium was changed by partially or completely removing the medium using narrow tipped pipettes or filtration and by replacing the removed portion of the medium with an equal volume of assay medium, i.e. water, for the low osmolality treatment or medium supplemented with 1 m mannitol, sorbitol, or KCl, as indicated, for the high osmolality treatment. As a control treatment, medium was removed and then reapplied in an analogous way. In the tomato cells used in this study, this procedure did not induce any of the responses measured.
Alkalinization Response and Extracellular K+ Concentration
Aliquots (2.5 mL) of the cell suspension, 4 to 10 d after subculture, were incubated in open vials on a rotary shaker at 120 cycles min−1. The pH of the culture medium was continuously measured with small combined glass electrodes (Metrohm, Herisau, Switzerland), and the values were registered using pen recorders. Results are shown as tracings of these pH profiles or as the maximal pH increase (ΔpHmax) read from these curves. In different batches of cells ΔpHmax to a given stimulus was dependent on the age of the culture, the density of cells, and the initial extracellular pH. Within one batch of cells, as used in bioassays for the experiments shown, alkalinization to replicate treatments varied little (mean sd of <15%).
Extracellular K+ concentration was measured with a K+-sensitive polymer membrane electrode (Metrohm) in combination with an Ag/AgCl reference electrode. To reduce the initial concentration of K+, cells were washed and pre-incubated for 1 to 3 h in a medium with 5 mm NaCl, 1 mm CaCl2, 0.1 mm KCl, and the amount of mannitol necessary to reach the required osmolality.
Measurement of ACC Synthase
Activity of ACC synthase was measured in permeabilized cells as described before (Spanu et al., 1990).
In Vivo Pulse-Labeling with [33P]Phosphate
In vivo labeling with ortho-phosphate was carried out as described before (Felix et al., 1994). In brief, 0.2 mL aliquots of cell suspension containing approximately 50 mg of cells were added to 10 μCi carrier-free [33P]phosphate (NEN, Boston). Incubations were stopped 30 s later by addition of 0.3 mL of trichloroacetic acid (10%, w/v) containing 10 mm ATP and freezing in liquid nitrogen. Samples were thawed in a sonicator bath and centrifuged for 2 min at 12,000g. Pellets were washed twice with 1.0 mL 80% (v/v) acetone/20% (v/v) 20 mm Tris-HCl, pH 7.4, and extracted with 100 μL of SDS-sample buffer at 95°C for 5 min. After centrifugation, 30 μL of the supernatants were subjected to SDS-PAGE (Laemmli, 1970) and autoradiography.
In Gel Protein Kinase Assay
Samples (200 μL) of cell cultures were collected at the time points indicated, mixed with an equal volume of 10% (w/v) trichloroacetic acid, and frozen in liquid nitrogen. After thawing and ultrasonication, pellets were collected by centrifugation (10 min, 12,000g) and washed twice with 80% (v/v) acetone/20% (v/v) Tris-MES (2-[N-morpholino]ethanesulfonic acid) buffer (20 mm, pH 8.0). Proteins were solubilized from pellets with SDS-sample buffer and separated by SDS-PAGE on gels containing 12% (w/v) acrylamide and 0.2% (w/v) MBP (Sigma, St. Louis). Proteins in gels were denatured, renatured, and assayed for kinase activity as described (Suzuki and Shinshi, 1995). Radioactivity in dried gels was analyzed and quantified using a Phosphorimager (Molecular Dynamics, Sunnyvale, CA).
Reproducibility
The results shown in the figures represent single experiments that are representative for several independent repetitions.
ACKNOWLEDGMENT
We thank T. Meindl (Friedrich Miescher-Institute) for critical reading of the manuscript.
LITERATURE CITED
- Adams TL, Townsend JA. A new procedure for increasing the efficiency of protoplast plating and clone selection. Plant Cell Rep. 1983;2:165–168. doi: 10.1007/BF00270093. [DOI] [PubMed] [Google Scholar]
- Allan AC, Fluhr R. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell. 1997;9:1559–1572. doi: 10.1105/tpc.9.9.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M, Bina J, Sequeira L. Phosphoinositide breakdown during the K+/H+ exchange response of tobacco to Pseudomonas syringae pv. syringae. Mol Plant-Microbe Interact. 1993;6:253–260. [Google Scholar]
- Baron C, Zambryski PC. The plant response in pathogenesis, symbiosis, and wounding: variations on a common theme. Annu Rev Genet. 1995;29:107–129. doi: 10.1146/annurev.ge.29.120195.000543. [DOI] [PubMed] [Google Scholar]
- Basse CW, Fath A, Boller T. High affinity binding of glycopeptide elicitor to tomato cells and microsomal membranes and displacement by specific glycan suppressors. J Biol Chem. 1993;268:14724–14731. [PubMed] [Google Scholar]
- Baureithel K, Felix G, Boller T. Specific, high affinity binding of chitin fragments to tomato cellls and membranes: competitive inhibition of binding by derivatives of chitooligosaccharides and a Nod factor of Rhizobium. J Biol Chem. 1994;269:17931–17938. [PubMed] [Google Scholar]
- Boller T. Chemoperception of microbial signals in plant cells. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:189–214. [Google Scholar]
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Cazalé A-C, Droillard M-J, Wilson C, Heberle-Bors E, Barbier-Brygoo H, Laurière C. MAP kinase activation by hypoosmotic stress of tobacco cell suspensions: towards the oxidative burst response? Plant J. 1999;19:297–307. doi: 10.1046/j.1365-313x.1999.00528.x. [DOI] [PubMed] [Google Scholar]
- Chandra S, Stennis M, Low PS. Measurement of Ca2+ fluxes during elicitation of the oxidative burst in aequorin-transformed tobacco cells. J Biol Chem. 1997;272:28274–28280. doi: 10.1074/jbc.272.45.28274. [DOI] [PubMed] [Google Scholar]
- Csonka LN, Hanson AD. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- Ding JP, Pickard BG. Mechanosensory calcium-selective cation channels in epidermal cells. Plant J. 1993;3:83–110. doi: 10.1111/j.1365-313x.1993.tb00013.x. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Harrison MJ, Lamb CJ. Early events in the activation of plant defense responses. Annu Rev Phytopathol. 1994;32:479–501. [Google Scholar]
- Ebel J, Cosio EG. Elicitors of plant defense responses. Int Rev Cytol. 1994;148:1–36. [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18:265–276. doi: 10.1046/j.1365-313x.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- Felix G, Grosskopf DG, Regenass M, Basse CW, Boller T. Elicitor-induced ethylene biosynthesis in tomato cells: characterization and use as a bioassay for elicitor action. Plant Physiol. 1991a;97:19–25. doi: 10.1104/pp.97.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Grosskopf DG, Regenass M, Boller T. Rapid changes of protein phosphorylation are involved in transduction of the elicitor signal in plant cells. Proc Natl Acad Sci USA. 1991b;88:8831–8834. doi: 10.1073/pnas.88.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Regenass M, Boller T. Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J. 1993;4:307–316. [Google Scholar]
- Felix G, Regenass M, Spanu P, Boller T. The protein phosphatase inhibitor calyculin A mimics elicitor action in plant cells and induces rapid hyperphosphorylation of specific proteins as revealed by pulse-labeling with [33P]phosphate. Proc Natl Acad Sci USA. 1994;91:952–956. doi: 10.1073/pnas.91.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin MC, Zhou X-L, Martinac B, Kung C. A mechanosensitive ion channel in the yeast plasma membrane. Science. 1988;242:762–765. doi: 10.1126/science.2460920. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K, Scheel D. Physiology and molecular biology of phenylpropenoid metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:347–369. [Google Scholar]
- Han J, Lee J-D, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Hirt H, Casari G, Barta A. Cadmium-enhanced gene expression in suspension-culture cells of tobacco. Planta. 1989;179:414–414. doi: 10.1007/BF00391089. [DOI] [PubMed] [Google Scholar]
- Horn MA, Meadows RP, Apostol I, Jones CR, Gorenstein DG, Heinstein PF, Low PS. Effect of elicitation and changes in extracellular pH on the cytoplasmic and vacuolar pH of suspension-cultured soybean cells. Plant Physiol. 1992;98:680–686. doi: 10.1104/pp.98.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangasjärvi J, Talvinen J, Utriainen M, Karjalainen R. Plant defense systems induced by ozone. Plant Cell Environ. 1994;17:783–794. [Google Scholar]
- Koornneef M, Hanhart CJ, Martinelli L. A genetic analysis of cell culture traits in tomato. Theor Appl Genet. 1987;74:633–641. doi: 10.1007/BF00288863. [DOI] [PubMed] [Google Scholar]
- Kuchitsu K, Kikuyama M, Shibuya N. N-Acetylchitooligosaccharides, biotic elicitors for phytoalexin production, induce transient membrane depolarization in suspension-cultured rice cells. Protoplasma. 1993;174:79–81. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leguay JJ, Jouanneau JP. Auxin (2,4-dichlorophenoxyacetic acid) starvation and treatment with glucan elicitor isolated from Phytophthora megasperma induces similar responses in soybean-cultured cell suspensions. Dev Genet. 1987;8:351–364. [Google Scholar]
- Maeda T, Wurgler-Murphy SM, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- Mathieu Y, Kurkdjian A, Xia H, Guern J, Koller A, Spiro MD, O'Neill M, Albersheim P, Darvill A. Membrane responses induced by oligogalacturonides in suspension-cultured tobacco cells. Plant J. 1991;1:333–343. doi: 10.1046/j.1365-313X.1991.t01-10-00999.x. [DOI] [PubMed] [Google Scholar]
- Mikolajczyk M, Awotunde OS, Muszynska G, Klessig DF, Dobrowolska G. Osmotic stress induces rapid activation of a salicylic acid-induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell. 2000;12:165–178. [PMC free article] [PubMed] [Google Scholar]
- Nürnberger T, Wirtz W, Nennstiel D, Hahlbrock K, Jabs T, Zimmermann S, Scheel D. Signal perception and intracellular signal transduction in plant pathogen defense. J Recept Signal Transduct Res. 1997;17:127–136. doi: 10.3109/10799899709036598. [DOI] [PubMed] [Google Scholar]
- Roos W, Evers S, Hieke M, Tschope M, Schumann B. Shifts of intracellular pH distribution as a part of the signal mechanism leading to the elicitation of benzophenanthridine alkaloids: phytoalexin biosynthesis in cultured cells of Eschscholzia californica. Plant Physiol. 1998;118:349–364. doi: 10.1104/pp.118.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y. Tobacco MAP kinase: a possible mediator in wound signal transduction pathways. Science. 1995;270:1988–1992. doi: 10.1126/science.270.5244.1988. [DOI] [PubMed] [Google Scholar]
- Spanu P, Felix G, Boller T. Inactivation of stress induced 1-aminocyclopropane carboxylate synthase in vivo differs from substrate-dependent inactivation in vitro. Plant Physiol. 1990;93:1482–1485. doi: 10.1104/pp.93.4.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Shinshi H. Transient activation and tyrosine phosphorylation of a protein kinase in tobacco cells treated with a fungal elicitor. Plant Cell. 1995;7:639–647. doi: 10.1105/tpc.7.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Isobe M, Knight MR, Trewavas AJ, Muto S. Hypo-osmotic shock induces increases in cytosolic Ca2+ in tobacco suspension-culture cells. Plant Physiol. 1997b;113:587–594. doi: 10.1104/pp.113.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Isobe M, Muto S. An increase in cytosolic calcium ion concentration precedes hypoosmotic shock-induced activation of protein kinases in tobacco suspension culture cells. FEBS Lett. 1997a;401:202–206. doi: 10.1016/s0014-5793(96)01472-x. [DOI] [PubMed] [Google Scholar]
- Urao T, Yakubov B, Satoh R, Yamaguchi-Shinozaki K, Seki M, Hirayama T, Shinozaki K. A transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell. 1999;11:1743–1754. doi: 10.1105/tpc.11.9.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahraus T, Chandra S, Legendre L, Low PS. Evidence for a mechanically induced oxidative burst. Plant Physiol. 1995;109:1259–1266. doi: 10.1104/pp.109.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]