Abstract
Mitochondria were isolated from imbibed seeds of lentil (Lens culinaris) and Phaseolus vulgaris. We copurified two voltage-dependent anion channel from detergent solubilized mitochondria in a single purification step using hydroxyapatite. The two isoforms from P. vulgaris were separated by chromatofocusing chromatography in 4 m urea without any loss of channel activity. Channel activity of each isoform was characterized upon reconstitution into diphytanoyl phosphatidylcholine planar lipid bilayers. Both isoforms form large conductance channels that are slightly anion selective and display cation selective substates.
The exchange of solutes between the mitochondrial matrix and the cytoplasm proceeds through the two mitochondrial membranes. Small metabolites up to 6 kD are transported across the outer membrane through a pore-forming protein called VDAC (voltage-dependent anion-selective channel) or mitochondrial porin. VDAC channels have been purified, cloned, and characterized from a variety of organisms (Colombini, 1983; De Pinto et al., 1987; Kleene et al., 1987; Heins et al., 1994). Their channel characteristics are similar when reconstituted into planar lipid bilayers (Sorgato and Moran, 1993; Benz, 1994). At low membrane voltage (10 mV) the channel is mainly in the fully open state with a single channel conductance ranging from 3.6 to 4.5 nanoSiemens in 1 m KCl. In this state, the channel exhibits slight anion selectivity. Upon application of voltages above 20 mV, the channel switches to a low-conducting substate often called closed state with a concomitant change to cation selectivity (Benz et al., 1990). In this substate, the flow of negatively charged metabolites such as succinate, citrate (Hodge and Colombini, 1997), and adenine nucleotides (Benz et al., 1988; Rostovtseva and Colombini, 1997) through the channel is strongly reduced. Several substates have been identified (Colombini, 1986), but their specific properties have not been investigated in details. Treatments that induce closure of VDAC channels are known to inhibit mitochondrial respiration and enzyme activity (Liu and Colombini, 1992; Gellerich et al., 1993). These results suggest that VDAC channels not only provide the major pathway for metabolites across the mitochondrial outer membrane but that they also participate in the regulation of mitochondrial functions.
It is now well established that VDAC belongs to a small multigene family in human (Ha et al., 1993; Yu et al., 1995), mouse (Sampson et al., 1996), and plants (Heins et al., 1994; Elkeles et al., 1995; Roosens et al., 2000). There is increasing evidence indicating that more than one VDAC isoform is expressed in a homogeneous tissue (De Pinto et al., 1991). However, only one isoform has been purified from the outer mitochondrial membrane. In mammalian cells, each VDAC isoform might play a specific role in mitochondria and cell metabolism. For instance, cytosolic enzymes like glycerol kinase and hexokinase are known to bind VDAC (Adams et al., 1991). Experiments performed with two human VDAC isoforms expressed in a yeast vdac-minus mutant showed that only one of the isoforms binds hexokinase (Blachly-Dyson et al., 1993). This suggests that the mitochondrial VDAC could be part of a protein complex, but its physiological role remains to be elucidated.
Little is known about the regulation of plant VDAC isoforms and their physiological function. Functional expression of three wheat isoforms in yeast vdac-minus mutants revealed specific differences in conductance and channel-gating characteristics (Elkeles et al., 1997). The three vdac genes were differentially expressed in wheat floral tissues (Elkeles et al., 1995), suggesting that the various plant VDAC isoforms could be involved in specific processes.
Here we provide evidence that two VDAC isoforms are expressed in mitochondria isolated from cotyledons of Fabaceae seeds. Each isoform was purified from bean seeds, and its channel activity was characterized in planar lipid bilayers.
RESULTS
Purification of Two VDAC Isoforms
Seeds from two different genus of the Fabaceae family were screened for mitochondrial VDAC proteins. Two-hundred grams (dry weight) of seeds from Phaseolus vulgaris or lentil (Lens culinaris) were imbibed in tap water, and cotyledons were used as a source of mitochondria. The same protocols were used for the isolation of mitochondria and for the VDAC purification (see “Materials and Methods”) for the two different plant seeds. The final mitochondrial pellets contained 200 to 400 mg of proteins. This high yield is approximately 10 to 20 times larger than that reported for other plant tissues, e.g. coleoptiles or roots (Blumenthal et al., 1993).
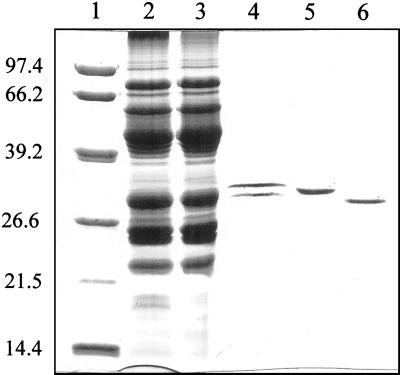
Membrane proteins were solubilized from whole mitochondria in the presence of 2% (v/v) Genapol X-080. Dry hydroxyapatite (HTP; 1 g of dry HTP/10 mg of protein) was added to the detergent-solubilized protein fraction. Unbounded proteins were collected and loaded on a 12% (w/v) SDS-polyacrylamide gel. As shown in Figure 1, two major protein bands with an apparent molecular mass of 31 and 32 kD were copurified with P. vulgaris (Fig. 1, lane 2). A similar pattern was obtained with seeds of mung bean (Vigna radiata) (data not shown). However, a single protein band with an apparent molecular mass of 31.5 kD was observed for lentil (Fig. 1, lane 3). Though the major proteins were obtained by this simple procedure, several contaminants could be easily observed when the gel was overloaded with proteins and silver stained indicating that further purification was required (see below).
Figure 1.
VDAC purification from plant seed cotyledons. Mitochondria were isolated from cotyledons of imbibed seeds from P. vulgaris and lentil. Whole mitochondria were treated with 2% (v/v) Genapol X-080 and solubilized proteins were subjected to a HTP-batch purification. The SDS gel shows the unbounded protein fractions recovered from the HTP batch obtained from P. vulgaris (lane 2) and lentil (lane 3). The VDACs migrate to approximately 31 kD. Lane 1, Mr markers, their corresponding Mr are given on the left. The gel was silver stained.
N-Terminal Amino Acid Sequencing
To identify the purified proteins from P. vulgaris and from lentil they were separated by 12% (w/v) SDS-PAGE, blotted on a polyvinylidene difluoride membrane, and subjected to Edman degradation. What appeared as a single protein band on SDS-gels for lentil (Fig. 1, lane 3) gave rise to two highly homologous sequences with 80% identical amino acids, thus representing two different isoforms. The two proteins from P. vulgaris had 76% identical N-terminal sequences and displayed also high homologies (80%–95% identity) to the two lens sequences. Alignment of the N-terminal sequences obtained from the lens and kidney bean proteins with that of other plant VDACs (Fig. 2) indicated a high homology. The sequence homologies to the two potato isoforms (pom 36 and pom 34, two VDAC isoforms from potato; see Fig. 2) ranged between 73% and 95% identical amino acids. The homology to the three wheat VDAC isoforms (Ta I, Ta II, and Ta III in Fig. 2) was in the range of 55% to 68% sequence identity.
Figure 2.
Alignment of plant VDAC N-terminal amino acid sequences. The two sequences from lentil, Lc I and Lc II, and the two sequences from kidney bean, VDAC 31 and VDAC 32, were aligned with the two VDAC isoforms from potato (pom 34 and pom 36; Heins et al., 1994) and the three VDAC isoforms from wheat (Ta I, Ta II, and Ta III; Elkeles et al., 1995). The bold letters indicate amino acid residues identical in all sequences.
Two-Dimensional Gel Electrophoresis
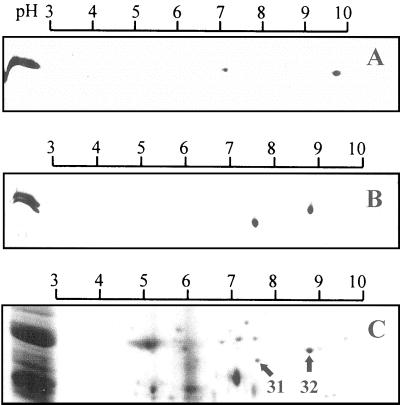
In the first dimension, proteins were separated on gel strips with a linear pH gradient (pH 3–10) according to their respective pI. The second dimension separated the proteins according to their Mr by 12% (w/v) SDS-PAGE. Figure 3 (A and B) shows the two-dimensional (2-D) maps obtained from the purified VDACs of the different plant seeds. The single 31.5-kD band of lentil VDAC (on the left side of the 2-D gel) separated into two polypeptides with pI of 7.1 and 9.8, respectively (Fig. 3A). This confirms that the two VDAC proteins are distinct isoform. The pI of the 31-kD isoform and of the 32-kD isoform from P. vulgaris is 7.6 and 8.8, respectively (Fig. 3B).
Figure 3.
2-D-gel electrophoresis. The 2-D maps compare the VDAC isoforms from the different plant seed mitochondria. A, Purified VDAC isoforms from lentil; B, purified VDAC isoforms from P. vulgaris; C, whole mitochondria. Proteins were separated in the first dimension on immobilineDry strips with a linear pH-gradient from pH 3 to 10. The second dimension was standard SDS-12% (w/v) PAGE. The gels A and B were silver stained. The gel C is a section of the 2-D map of whole mitochondria (250 μg of protein) from P. vulgaris stained with Coomassie Blue.
2-D-gel electrophoresis was carried out with whole mitochondria isolated from P. vulgaris. Approximately 60 polypeptides were detected on a 2-D map of whole mitochondria. In Figure 3C, only a section of the 2-D map is presented. Analysis of the scanned 2-D maps with the Melanie II software revealed that the 32-kD isoform is 3 times more abundant than the 31-kD isoform.
Separation of Two VDAC Isoforms
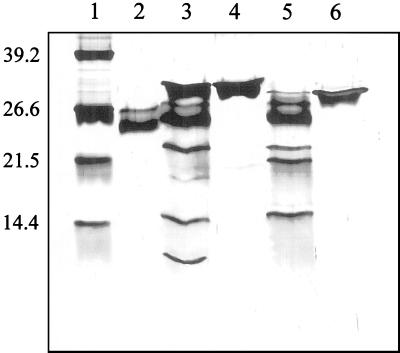
Both anion-exchange columns and cation exchange columns were assayed. However, no separation of the two isoforms was achieved. DEAE anion-exchange column eliminated most of the minor contaminating proteins still present after the HTP purification, but the two VDAC isoforms co-eluted (data not shown). Taking into account the specific pI of each VDAC protein, a chromatofocusing column was used to separate the proteins. Chromatofocusing chromatography was performed using a pH gradient from pH 9.4 to 7.0 but preliminary experiments had shown that no separation occurred unless in the presence of 4 m urea. After the HTP purification step, the unbounded protein fraction was concentrated five to six times (Centripep-30, Amicon, Beverly, MA) and dialyzed overnight against a buffer solution containing 4 m urea (see “Materials and Methods”). This fraction was loaded onto the chromatofocusing column, and the eluted proteins were analyzed by SDS-PAGE. The fractions that eluted at approximately pH 8.9 contained the upper protein band VDAC 32 (Fig. 4, lane 5). A second peak eluted at around pH 8.2 and contained the lower protein band VDAC 31 (Fig. 4, lane 6). The SDS gel in Figure 4 shows the electrophoretic pattern of proteins collected at different steps of the purification protocol. The final yield was approximately 0.25% to 0.5% of the total mitochondrial protein for VDAC 32. A similar yield has been obtained with corn (0.12%), wheat (0.6%) (Aljamal et al., 1993; Blumenthal et al., 1993), and animals (De Pinto et al., 1987). The yield of VDAC 31 was approximately one-half of VDAC 32.
Figure 4.
Purification and separation of two VDAC isoforms from P. vulgaris. Lane 1, Mr marker, the corresponding Mr are shown on the left; lane 2, whole mitochondria (30-μg of protein); lane 3, Genapol X-080 solubilized proteins (30 μg of protein); lane 4, supernatant after the HTP purification step (5 μg of protein). Note that the higher detergent concentration (2%, v/v) slightly retarded the migration of the proteins; lane 5 and lane 6, VDAC 32 and VDAC 31, respectively, after chromatofocusing chromatography. The 12% (w/v) polyacrylamide gel is Coomassie Blue stained.
Peptide Map
Peptide mapping was performed to get more insight into structural differences of the two VDAC proteins from P. vulgaris. Peptide maps of the two purified isoforms were obtained by digestion with protease V8 from Staphylococcus aureus. This protease specifically hydrolyzes the peptide bonds at the C-termini of Glu residues. Digestion was carried out in an electrophoresis sample buffer at room temperature for 1.5 h. Peptides were separated by 15% (w/v) SDS-PAGE and silver stained. As shown in Figure 5, each isoform led to a specific proteolytic pattern indicating differences in their primary structure. VDAC 32 (Fig. 5, lane 4) was cleaved in three peptides of 22, 15, and 9 kD (Fig. 5, lane 3). VDAC 31 (Fig. 5, lane 6) was cleaved in three peptides of 22, 20, and 15 kD (Fig. 5, lane 5).
Figure 5.
Peptide map. Comparison of the peptide pattern from two VDAC isoforms from P. vulgaris. Digestion was done with S. aureus protease V8. Protease V8 (0.25 μg) was added to the purified proteins dissolved in the gel electrophoresis sample buffer (2% SDS [w/v], 62.5 mm Tris [tris(hydroxymethyl)aminomethane]-HCl, pH 6.8, 5% [v/v] mercaptoethanol, 10% [v/v] glycerol) and incubated at room temperature for 1.5 h. The reaction was stopped by boiling for 5 min and the samples were subjected to 15% (w/v) SDS-PAGE. Lane 1, Mr standard; lane 2, 0.25 μg of protease V8; lane 3, VDAC 32 + protease V8; lane 4, VDAC 32; lane 5, VDAC 31 + protease V8; lane 6, VDAC 31. The gel was silver stained.
Functional Characterization
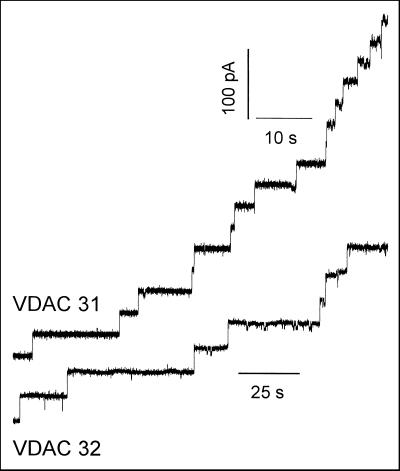
Mitochondrial VDAC proteins are known to form large conductance channels when reconstituted into planar lipid bilayers. To demonstrate that the purification procedure used here did not alter the function of the proteins, they were added to the cis side of a planar lipid bilayer at a concentration of 50 to 100 ng/mL and an electrical potential difference of +10 mV was applied to the membrane. At this voltage, VDAC is mainly in its fully open state, and each insertion of a channel into the membrane yielded to a discrete current step as illustrated in Figure 6. The initial current increments occurred with rather uniform amplitudes (Fig. 6). The histogram shows a uniform amplitude distribution of the different current steps with a mean of 3.7 ± 0.1 nS (n = 79) and 4.0 ± 0.1 nS (n = 83) for VDAC 31 and VDAC 32, respectively. These conductances correspond to the fully open state of a single channel and are consistent with those reported for VDAC from other sources (Colombini, 1989; Benz, 1994).
Figure 6.
Reconstitution of kidney bean VDAC isoforms into planar lipid bilayer. Stepwise increase of the membrane current after addition of the individual VDAC isoforms. Upper current trace, VDAC 31; lower current trace, VDAC 32. The lipid bilayer was formed from 1% (w/v) diphytanoyl phosphatidylcholine in n-decane. The bath solution was symmetrical 1 m KCl, 10 mm HEPES (4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid), pH 7.2. Fifty to 100 ng of the purified and separated VDAC isoforms were added to the cis side. The membrane voltage was +10 mV.
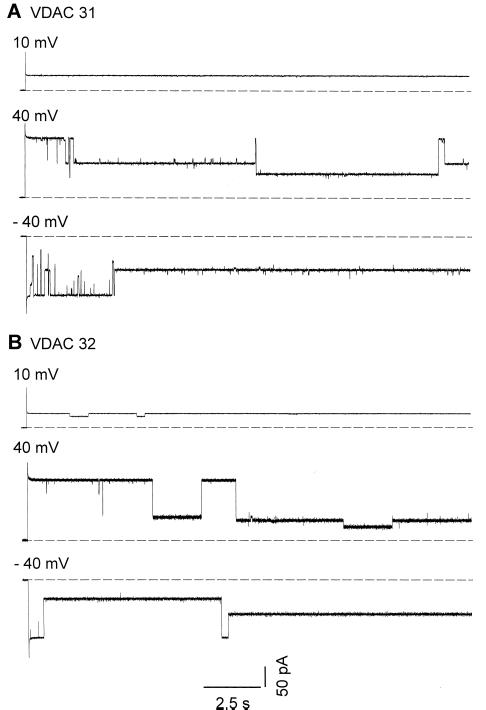
Data available for VDAC from plant mitochondria have been collected from multichannel records. These results suggested that plant VDAC have two conducting states (Aljamal et al., 1993). Here we report on single-channel experiments. Figure 7 shows the time course of the current fluctuations recorded in symmetrical conditions (1 m KCl) at different voltages. The single-channel records indicate that the VDAC has several subconducting states (Fig. 7). At least four different subconducting states ranging from 0.5 to 3 nS were recorded for each isoform. Transitions between the fully open state and the subconducting states were reversible.
Figure 7.
Single-channel recordings. Current traces of single VDAC channels in planar lipid bilayer at different applied voltages. A, VDAC 31; B, VDAC 32. Single channels of the individual VDAC isoforms from P. vulgaris were reconstituted into planar lipid bilayer made from diphytanoyl phosphatidylcholine in n-decane. The experimental solution was 1 m KCl, 10 mm HEPES, pH 7.2. The dashed line indicates the zero current level.
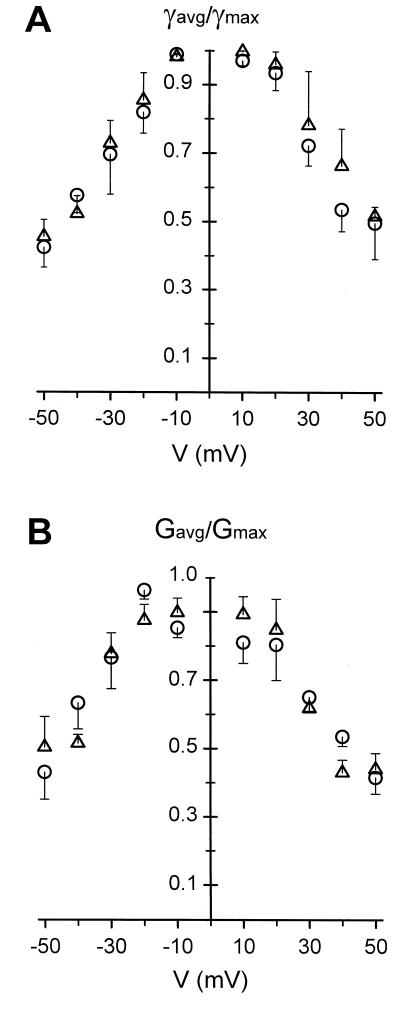
The voltage-dependence of multichannel-containing membrane is usually obtained from the relationship between the probability of being in the fully open state and the voltage applied to the membrane (Schein et al., 1976; Colombini et al., 1996). Practically, the normalized conductance G/G0 is plotted versus the voltage, where G is the steady-state membrane conductance at a given voltage and G0 is the membrane conductance when all channels are open. To compare the voltage-dependence of single-channel records to that of multichannel experiments, single-channel data were collected during 20 s at various voltages. The magnitude of the current flowing through the channel in its fully open state was measured, and we have calculated the average current flowing through the channel during 20 s. These two currents were converted into a conductance (γ and γavg, respectively) using the Ω law. The ratio γavg/γ was plotted against the voltage. As shown in Figure 8A, both VDAC 31 and VDAC 32 display a similar bell-shaped voltage-dependence. Multichannel data collected from planar lipid bilayer containing up to 10 channels show no differences with single-channel data (Fig. 8B).
Figure 8.
Voltage dependence of the VDAC isoforms. A, Single channel; B, multichannel. The two conductance-voltage plots were derived as described in the text from 20-s records of current flowing through planar bilayer containing a single VDAC channel (A) or up to 10 channels (B) at various voltages. (▵), VDAC 31; (○), VDAC 32. Vertical bars represent the se of the mean, n = 5 in A and n = 4 in B, respectively. The experimental solution was 1 m KCl, 10 mm HEPES, pH 7.2.
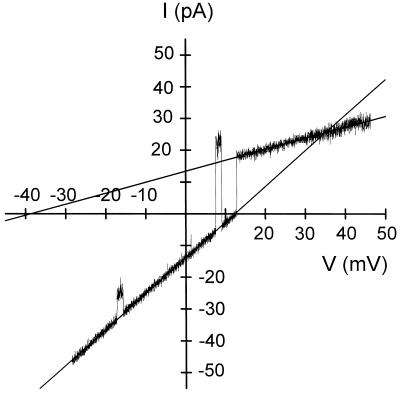
To get information about the ion selectivity of the channel, a symmetrical 10 mHz triangular wave was applied to the lipid bilayer membrane containing a single channel. In a first step, experiments were performed in asymmetric 500/100 mm KCl (cis/trans) conditions. The concentration of the trans compartment was then raised to 500 mm KCl. Both voltage and current were digitized and used to draw a current-voltage curve as illustrated for VDAC 32 in asymmetric KCl solution (Fig. 9). The large- and the low-conducting states had a conductance of 1.2 and 0.35 nS, respectively. Similar results were obtained for both VDAC 31 and VDAC 32. The reversal potential of the large conducting state was 12.5 mV ± 0.4 (n = 7), which according to the Goldman-Hodgkin-Katz equation corresponds to a permeability ratio PK+/PCl− equal to 0.47. Thus the large-conducting state was slightly anion selective, whereas the low conducting state, which had a permeability ratio PK+/PCl− = 44, was strongly cation selective. This change in selectivity upon a transition between the fully open state and a subconducting state is a typical feature of VDAC.
Figure 9.
Ion selectivity of VDAC. Current-voltage relationship of a single VDAC 32 channel to which a voltage ramp from −50 to 50 mV was applied. The salt concentration in the cis and trans compartments were 0.5 m and 0.1 m KCl, respectively (10 mm HEPES, pH 7.2). The purified proteins were added to the cis compartment. Data were filtered at 60 Hz and sampled at 250 Hz. The conductance (slope) and the reversal potentials (at I = 0) were calculated from the extrapolated regression lines of the different conducting states of the channel.
DISCUSSION
VDAC allows the transport through the outer mitochondrial membrane of molecules up to 6 kD. Several genes encoding VDAC proteins have been cloned from plant species. This suggests that several isoforms of VDAC can exist in plants. However, these plant isoforms have not yet been purified and separated. In the present work, we describe a protocol, which permits to purify and separate two VDAC isoforms from plant seed mitochondria without loss of activity. Our data provide evidence that mitochondria from seeds of Fabaceae contain two VDAC isoforms, which can be distinguished on the basis of their respective pI. In Phaseolus two isoforms with a specific apparent Mr were separated on a denaturating gel. However, both isoforms have the same apparent Mr in lentil. These results are in perfect agreement with those showing that the yeast Saccharomyces cerevisea contains two genes encoding for two VDAC isoforms that are simultaneously expressed in mitochondria (Blachly-Dyson et al., 1997).
Previous purifications of a plant VDAC protein from maize and wheat (Aljamal et al., 1993; Blumenthal et al., 1993) revealed that VDAC formed a single band on one-dimensional SDS-PAGE. Our results suggest that this does not mean that mitochondria of these plants contain a single VDAC protein. 2-D electrophoresis would be required to assess that different VDAC isoforms exist in mitochondria of these plants.
One of the main biochemical differences between VDAC isoforms is their pI. From the primary sequence of all plant VDACs cloned up to now it can be calculated that VDAC proteins should have a theoretical pI at alkaline pH. In Lens, one of the isoforms has a remarkably high pI of 9.8 and the two isoforms have by far the greatest difference in their respective pIs, 2.7-pH units. Such a high pI is not specific for plants, and a VDAC protein with a pI of approximately 10 was found in Dictyostelium (Troll et al., 1992). The theoretical pIs calculated from the primary sequences of the two potato VDACs, (pom 36, pI 7.78 and pom 34, pI 8.68; Heins et al., 1994) are very similar to those found in kidney bean. Analysis of 2-D maps obtained from whole mitochondria indicated a 3-fold amount of VDAC 32 over VDAC 31. This suggests a differential expression of two VDAC isoforms in mitochondria from plant seeds. Analysis of mRNA demonstrated differential expression of vdac genes in wheat (Elkeles et al., 1995). The expression pattern of the three wheat VDAC isoforms varied within the examined tissues and with the developmental state of the embryo. The different transcript levels suggested the possibility of different functions for the isoforms (Elkeles et al., 1995). Differential expression of the kidney bean isoforms could be an indication that they play different physiological roles in plant seeds.
VDAC genes have been isolated from plants, animals, and fungi. The sequence homology between VDACs of these organisms is poor. In yeast and animals the amino terminal sequence of the VDAC is not conserved between species. This does not seem to be the case in plants. The N-terminal sequences of VDAC isoforms from the dicotyledon plants like kidney beans, lens, and potato have a higher homology to each other (73%–95%) than to the monocotyledon wheat (55%–65%). This could reflect the evolutionary diversity of vdac genes in monocotyledons and dicotyledons (Elkeles et al., 1995). The N-terminal sequence of VDAC proteins has the characteristics of an amphipathic α-helix and may be involved in targeting the cytoplasmatically synthesized proteins to the mitochondrial outer membrane (Kleene et al., 1987; Pfaller et al., 1990). Digestion of the two VDACs purified from bean seeds with the protease V8 led to specific peptide mapping, which indicates that sequence differences between the two isoforms are not restricted to the amino terminal domain but occur throughout the protein.
Despite the poor homology between the cDNA sequences of VDACs cloned from plants, yeasts, and animals their channel properties are highly conserved among these different organisms. The basic channel features of each kidney bean VDAC isoform are consistent with other known VDACs (Colombini, 1989; Benz, 1994). Both proteins form large conductance channels in the fully open state. The two isoforms exhibited slight differences in their single channel conductances similar to that reported for the wheat VDAC isoforms (Elkeles et al., 1997). Both isoforms closed in a voltage-dependent manner upon application of electrical potential differences above 20 mV to the planar lipid bilayer. Closures occurred at both positive and negative voltages. The interpretation of the gating properties is made difficult since there are at least four subconducting states, but they are not systematically observed at each voltage. The properties of the four substates have not been investigated in detail here, and further work will be required to fully understand the gating mechanism of the VDAC of mitochondria.
The similar channel characteristics of the two different VDAC isoforms from P. vulgaris raise the question of their specific function. There is now increasing evidence indicating that VDAC from mammals and yeast could participate in several cellular processes like metabolite flux (Rostovtseva and Colombini, 1996, 1997; Hodge and Colombini, 1997), apoptosis (Zoratti and Szabo, 1995; Shimizu et al., 1999), binding to receptor (McEnery et al., 1992), and cytoskeleton (Linden and Karlsson, 1996). It is reasonable to assume that each isoform could have a different role. This assertion is supported by experimental evidence indicating a differential regulation of VDACs. For instance, it has been demonstrated that the two human VDAC isoforms expressed in yeast had different binding properties to hexokinase, HVDAC1 having a strong affinity for the kinase (Blachly-Dyson et al., 1993). Upon binding to the VDAC the kinase is activated. Therefore, although two VDACs have identical channel properties they can be involved in different regulating processes. The function of plant VDACs has not been investigated in details, and the role of the different isoforms found in plants will deserve further work.
MATERIALS AND METHODS
Plant Material and Chemicals
Seeds from Phaseolus vulgaris var Streamline and lentil (Lens culinaris) were purchased from a local store; Genapol X-080 was from Fluka (Buchs, Switzerland); and hydroxyapatite (BioGel HTP gel) came from Bio-Rad Laboratories (Hercules, CA). Percoll and chromatofocusing media (Polybuffer exchanger PBE 94 and Polybuffer PB 96) were obtained from Pharmacia Biotech (Uppsala, Sweden). Urea was from Merck (Darmstadt, Germany); protease V8 from Staphylococcus aureus was obtained from Roche Diagnostics (Indianapolis, IN).
Isolation of Mitochondria
Mitochondria were isolated from plant seeds of P. vulgaris, lentil, or mung bean (Vigna radiata) following the protocol of Douce et al. (1987) with some minor modifications. Usually 200 g (dry weight) of seeds were soaked for 12 h (lentil) or 24 h (P. vulgaris) in aerated tap water at room temperature. The seed coat and the embryo were removed. The cotyledons were homogenized in 500 mL of extraction media at low speed for 2 × 3 s in a 1-L blender (Waring, New Hartford, CT). The homogenate was squeezed through a nylon net (60-μm pores), and the homogenization step was repeated once. Mitochondria were obtained after the differential centrifugation steps and were further purified on a 28% (v/v) Percoll gradient. The final mitochondrial pellet was resuspended in 0.3 m mannitol, 10 mm HEPES, and 1 mm EDTA, pH 7.2.
Mitochondrial fractions were assayed by measuring the cytochrome c oxidase activity (Maeshima et al., 1987). Contaminations with other organelles were assessed by the catalase activity (peroxisomes) and the Glc-6-phosphate dehydrogenase activity (plastids) according to Douce et al. (1987). After differential centrifugation, cytochrome c oxidase activity increased approximately 10-fold, the catalase activity decreased 10 times, and no Glc-6-phosphate dehydrogenase activity was detected. Protein concentration was determined with the bichinchonic acid-kit from Pierce Chemical (Rockford, IL) using bovine serum albumin (Sigma, St. Louis) as the standard.
VDAC Purification
VDAC were purified from whole mitochondria (De Pinto et al., 1989; Heins et al., 1994). Mitochondria (300–400 mg of protein) were solubilized in a solution containing 2% (v/v) Genapol X-080, 1 mm EDTA, and 10 mm HEPES, pH 7.2 (final protein concentration: 5 mg/mL). The suspension was frozen overnight (−20°C), thawed at room temperature, incubated for 30 min on ice, and non-solubilized proteins were pelleted at 35,000 rpm for 60 min (Beckman L7, rotor Ti60; Beckman Instruments, Fullerton, CA). Hydroxyapatite (1g/10 mg proteins) was added in a batch procedure to the detergent solubilized proteins. The unbounded proteins (approximately 2.5 mg/100 mg total mitochondrial protein) in the resulting supernatant were highly enriched in VDAC.
Separation of the Two VDAC-Isoforms from Kidney Bean Mitochondria
The HTP-purified VDAC isoforms fraction was concentrated five to six times (Centriprep-30, Amicon) and dialyzed overnight against the start buffer. The two VDAC isoforms from kidney bean mitochondria were separated by low-pressure chromatofocusing chromatography. Chromatography with polybuffer exchanger PBE 94 was performed according to the instruction manual from Pharmacia with a pH-gradient from pH 9.4 to 7.0. The start buffer was 25 mm ethanolamine, 0.1% (v/v) Genapol X-080, and 4 m urea, pH 9.4. Two-milliliter aliquots of the sample were loaded onto the chromatofocusing column (2 cm2 × 5 cm), and proteins were eluted with the Polybuffer 96 10-fold diluted in 0.1% (v/v) Genapol X-080 and 4 m urea (pH 7.0). The collected fractions were checked by SDS-PAGE for proteins, and those containing the same proteins were pooled and concentrated (Centriprep-30, Amicon). To remove the ampholytes and the urea present in the elution buffer, the proteins were applied to a gel-filtration column (Sephadex G-75) equilibrated with 0.1% (v/v) Genapol and 10 mm HEPES (pH 7.2). The column was run at a low flow-rate of 7.5 cm/h to avoid precipitation of proteins during the elimination of urea.
2-D-PAGE
The first dimension (isoelectric focusing) was carried out on ImmobilineDryStrip (11 cm) with a linear pH gradient from pH 3 to 10 (Pharmacia) in a Multiphor II apparatus (Pharmacia) according to the manufacturer for 24 h at 20°C. The second dimension was carried out in vertical 12% (w/v) SDS-PAGE on 140 × 160 × 1.5-mm slab gels (Laemmli, 1970). The gels were stained either by Coomassie Blue or by silver, scanned (AGFA) and analyzed with the Melanie II software from Bio-Rad Laboratories.
Amino Acid Sequence Analysis
The HTP-purified VDAC proteins were separated by 12% (w/v) SDS-PAGE and electroblotted onto polyvinylidene difluoride membranes. The blot was stained with Coomassie Blue (0.1% [w/v] in 50% [v/v] methanol). Amino acid microsequence analysis was performed by automated Edman degradation on an LF3400 protein-peptide microsequencer equipped with an on-line Gold 126 microgradient high-pressure liquid chromatography system and a model 168 Diode Array detector (Beckman Instruments, Fullerton, CA). All samples were sequenced using standard Beckman sequencer procedure 4. Alkylation of Cys with acrylamide was performed in situ as described by Brune (1992). The phenylthiohydantonin amino acid derivatives were quantitatively identified by reverse phase HPLC on an ODS Sperogel microphenylthiohydantoin column (3-μm-diameter particles, 2 × 150 mm; Beckman Instruments).
Peptide Mapping
The VDAC isoforms (2–3 μg) were mixed with SDS gel electrophoresis sample buffer (Laemmli, 1970) and 0.25 μg of protease V8 from S. aureus in a final volume of 40 μL (Cleveland et al., 1977). Incubation was done at room temperature for 1 to 4 h, and the reaction was stopped by boiling the sample for 5 min. The peptides were separated by 15% (w/v) SDS-PAGE (Laemmli, 1970) and silver stained.
Planar Lipid Bilayer Measurements
Planar lipid bilayers were formed from a n-decane solution (10 mg/mL) of diphytanoyl phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL) on a 200-μm diameter hole drilled in a polystyrene cup according to Mueller et al. (1962). Experiments were performed at room temperature. Triple distilled water was millipore (0.25 μm) filtered. The method used for channel recording was similar to that previously described (Fuks and Homblé, 1995). In brief, each compartment on both sides of the lipid bilayer was filled with 1 m KCl and connected to an Ag/AgCl electrode through a salt bridge (1 m KCl, 2% [w/v] agar). The current was measured with a BLM-120 amplifier (Bio-Logic, Claix, France) and stored on a DTR-1204 digital audio tape recorder (Bio-Logic). The data were low-pass filtered (300 Hz) and sampled at 1 kHz using the Strathclyde Electrophysiology Software (Dempster, 1993) kindly provided by John Dempster. Electrical potentials were defined as cis with respect to trans, which was held at ground.
ACKNOWLEDGMENTS
We thank G. Vandenbussche and A. Vlérick for scientific discussion and advice.
Footnotes
This work was supported by a European Commission Research Training grant (no. ERBFMBICT971977 to H.A.). F.H. is a Research Director from the National Fund for Scientific Research (Belgium).
LITERATURE CITED
- Adams V, Griffin L, Towbin J, Gelb B, Worley K, McCabe ER. Porin interaction with hexokinase and glycerol kinase: metabolic microcompartmentation at the outer mitochondrial membrane. Biochem Med Metab Biol. 1991;45:271–291. doi: 10.1016/0885-4505(91)90032-g. [DOI] [PubMed] [Google Scholar]
- Aljamal JA, Genchi G, De Pinto V, Stefanizz L, De Santis A, Benz R, Palmieri F. Purification and characterization of porin from corn (Zea mays L.) mitochondria. Plant Physiol. 1993;102:615–621. doi: 10.1104/pp.102.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R. Permeation of hydrophilic solutes through mitochondrial outer membranes: review on mitochondrial porins. Biochim Biophys Acta. 1994;1197:167–196. doi: 10.1016/0304-4157(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Benz R, Kottke M, Brdiczka D. The cationically selective state of the mitochondrial outer membrane pore: a study with intact mitochondria and reconstituted mitochondrial porin. Biochim Biophys Acta. 1990;1022:311–318. doi: 10.1016/0005-2736(90)90279-w. [DOI] [PubMed] [Google Scholar]
- Benz R, Wojtczak L, Bosch W, Brdiczka D. Inhibition of adenine nucleotide transport through the mitochondrial porin by a synthetic polyanion. FEBS Lett. 1988;231:75–80. doi: 10.1016/0014-5793(88)80706-3. [DOI] [PubMed] [Google Scholar]
- Blachly-Dyson E, Song J, Wolfgang WJ, Colombini M, Forte M. Multicopy suppressors of phenotypes resulting from the absence of yeast VDAC encode a VDAC-like protein. Mol Cell Biol. 1997;17:5727–5738. doi: 10.1128/mcb.17.10.5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachly-Dyson E, Zambronicz EB, Yu WH, Adams V, McCabe ER, Adelman J, Colombini M, Forte M. Cloning and functional expression in yeast of two human isoforms of the outer mitochondrial membrane channel, the voltage-dependent anion channel. J Biol Chem. 1993;268:1835–1841. [PubMed] [Google Scholar]
- Blumenthal A, Kahn K, Beja O, Galun E, Colombini M, Breiman A. Purification and characterization of the voltage-dependent anion-selective channel protein from wheat mitochondrial membranes. Plant Physiol. 1993;101:579–587. doi: 10.1104/pp.101.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune DC. Alkylation of cysteine with acrylamide for protein sequence analysis. Anal Biochem. 1992;207:285–290. doi: 10.1016/0003-2697(92)90013-w. [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Fischer SG, Kirschner MW, Laemmli UK. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977;252:1102–1106. [PubMed] [Google Scholar]
- Colombini M. Purification of VDAC (voltage-dependent anion-selective channel) from rat liver mitochondria. J Membr Biol. 1983;74:115–121. doi: 10.1007/BF01870500. [DOI] [PubMed] [Google Scholar]
- Colombini M. Voltage gating in VDAC: toward a molecular mechanism. In: Miller C, editor. Ion Channel Reconstitution. New York: Plenum Publishing; 1986. pp. 533–552. [Google Scholar]
- Colombini M. Voltage gating in the mitochondrial channel, VDAC. J Membr Biol. 1989;111:103–111. doi: 10.1007/BF01871775. [DOI] [PubMed] [Google Scholar]
- Colombini M, Blachly-Dyson E, Forte M. VDAC, a channel in the outer mitochondrial membrane. In: Narahashi T, editor. Ion Channels. Vol. 4. New York: Plenum Publishing; 1996. pp. 169–202. [DOI] [PubMed] [Google Scholar]
- De Pinto V, Benz R, Palmieri F. Interaction of non-classical detergents with the mitochondrial porin: a new purification procedure and characterization of the pore-forming unit. Eur J Biochem. 1989;183:179–187. doi: 10.1111/j.1432-1033.1989.tb14911.x. [DOI] [PubMed] [Google Scholar]
- De Pinto V, Ludwig O, Krause J, Benz R, Palmieri F. Porin pores of mitochondrial outer membranes from high and low eukaryotic cells: biochemical and biophysical characterization. Biochim Biophys Acta. 1987;894:109–119. doi: 10.1016/0005-2728(87)90180-0. [DOI] [PubMed] [Google Scholar]
- De Pinto V, Zara V, Benz R, Gnoni GV, Palmieri F. Characterization of pore-forming activity in liver mitochondria from Anguilla anguilla: two porins in mitochondria? Biochim Biophys Acta. 1991;1061:279–286. doi: 10.1016/0005-2736(91)90293-h. [DOI] [PubMed] [Google Scholar]
- Dempster J. Computer Analysis of Electrophysiological Signals. London: Academic Press; 1993. [Google Scholar]
- Douce R, Bourguignon J, Brouquisse R, Neuburger M. Isolation of plant mitochondria: general principles and criteria of integrity. Methods Enzymol. 1987;148:403–415. [Google Scholar]
- Elkeles A, Breiman A, Zizi M. Functional differences among wheat voltage-dependent anion channel (VDAC) isoforms expressed in yeast: indication for the presence of a novel VDAC-modulating protein? J Biol Chem. 1997;272:6252–6260. doi: 10.1074/jbc.272.10.6252. [DOI] [PubMed] [Google Scholar]
- Elkeles A, Devos KM, Graur D, Zizi M, Breiman A. Multiple cDNAs of wheat voltage-dependent anion channels (VDAC): isolation, differential expression, mapping and evolution. Plant Mol Biol. 1995;29:109–124. doi: 10.1007/BF00019123. [DOI] [PubMed] [Google Scholar]
- Fuks B, Homblé F. A voltage-dependent porin-like channel in the inner envelope membrane of plant chloroplasts. J Biol Chem. 1995;270:9947–9952. doi: 10.1074/jbc.270.17.9947. [DOI] [PubMed] [Google Scholar]
- Gellerich FN, Wagner M, Kapischke M, Wicker U, Brdiczka D. Effect of macromolecules on the regulation of the mitochondrial outer membrane pore and the activity of adenylate kinase in the inter-membrane space. Biochim Biophys Acta. 1993;1142:217–227. doi: 10.1016/0005-2728(93)90150-e. [DOI] [PubMed] [Google Scholar]
- Ha H, Hajek P, Bedwell DM, Burrows PD. A mitochondrial porin cDNA predicts the existence of multiple human porins. J Biol Chem. 1993;268:12143–12149. [PubMed] [Google Scholar]
- Heins L, Mentzel H, Schmid A, Benz R, Schmitz UK. Biochemical, molecular, and functional characterization of porin isoforms from potato mitochondria. J Biol Chem. 1994;269:26402–26410. [PubMed] [Google Scholar]
- Hodge T, Colombini M. Regulation of metabolite flux through voltage-gating of VDAC channels. J Membr Biol. 1997;157:271–279. doi: 10.1007/s002329900235. [DOI] [PubMed] [Google Scholar]
- Kleene R, Pfanner N, Pfaller R, Link TA, Sebald W, Neupert W, Tropschug M. Mitochondrial porin of Neurospora crassa: cDNA cloning, in vitro expression and import into mitochondria. EMBO J. 1987;6:2627–2633. doi: 10.1002/j.1460-2075.1987.tb02553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linden M, Karlsson G. Identification of porin as a binding site for MAP2. Biochem Biophys Res Commun. 1996;218:833–836. doi: 10.1006/bbrc.1996.0148. [DOI] [PubMed] [Google Scholar]
- Liu MY, Colombini M. Regulation of mitochondrial respiration by controlling the permeability of the outer membrane through the mitochondrial channel, VDAC. Biochim Biophys Acta. 1992;1098:255–260. doi: 10.1016/s0005-2728(05)80344-5. [DOI] [PubMed] [Google Scholar]
- Maeshima M, Hattori T, Asahi T. Purification of complexes II and IV from plant mitochondria. Methods Enzymol. 1987;148:491–501. [Google Scholar]
- McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci USA. 1992;89:3170–3174. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P, Rudin DO, Tien H, Wescott WC (1962) Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature 979–980 [DOI] [PubMed]
- Pfaller R, Kleene R, Neupert W. Biogenesis of mitochondrial porin: the import pathway. Experientia. 1990;46:153–161. doi: 10.1007/BF02027311. [DOI] [PubMed] [Google Scholar]
- Roosens N, Al Bitar, Jacobs M, Homblé F. Characterization of a cDNA encoding a rice mitochondria VDAC and its gene expression studied upon plant development and osmotic stress. Biochim Biophys Acta. 2000;1463:470–476. doi: 10.1016/s0005-2736(99)00246-1. [DOI] [PubMed] [Google Scholar]
- Rostovtseva T, Colombini M. ATP flux is controlled by a voltage-gated channel from the mitochondrial outer membrane. J Biol Chem. 1996;271:28006–28008. doi: 10.1074/jbc.271.45.28006. [DOI] [PubMed] [Google Scholar]
- Rostovtseva T, Colombini M. VDAC channels mediate and gate the flow of ATP: implications for the regulation of mitochondrial function. Biophys J. 1997;72:1954–1962. doi: 10.1016/S0006-3495(97)78841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson MJ, Lovell RS, Craigen WJ. Isolation, characterization, and mapping of two mouse mitochondrial voltage-dependent anion channel isoforms. Genomics. 1996;33:283–288. doi: 10.1006/geno.1996.0193. [DOI] [PubMed] [Google Scholar]
- Schein SJ, Colombini M, Finkelstein A. Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J Membr Biol. 1976;30:99–120. doi: 10.1007/BF01869662. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Sorgato MC, Moran O. Channels in mitochondrial membranes: knowns, unknowns, and prospects for the future. Crit Rev Biochem Mol Biol. 1993;28:127–171. doi: 10.3109/10409239309086793. [DOI] [PubMed] [Google Scholar]
- Troll H, Malchow D, Muller TA, Humbel B, Lottspeich F, Ecke M, Gerisch G, Schmid A, Benz R. Purification, functional characterization, and cDNA sequencing of mitochondrial porin from Dictyostelium discoideum. J Biol Chem. 1992;267:21072–21079. [PubMed] [Google Scholar]
- Yu WH, Wolfgang W, Forte M. Subcellular localization of human voltage-dependent anion channel isoforms. J Biol Chem. 1995;270:13998–14006. doi: 10.1074/jbc.270.23.13998. [DOI] [PubMed] [Google Scholar]
- Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]