Abstract
The current controversy about the “cohesion-tension” of water ascent in plants arises from the recent cryo-scanning electron microscopy (cryo-SEM) observations of xylem vessels content by Canny and coworkers (1995). On the basis of these observations it has been claimed that vessels were emptying and refilling during active transpiration in direct contradiction to the previous theory. In this study we compared the cryo-SEM data with the standard hydraulic approach on walnut (Juglans regia) petioles. The results of the two techniques were in clear conflict and could not both be right. Cryo-SEM observations of walnut petioles frozen intact on the tree in a bath of liquid nitrogen (LN2) suggested that vessel cavitation was occurring and reversing itself on a diurnal basis. Up to 30% of the vessels were embolized at midday. In contrast, the percentage of loss of hydraulic conductance (PLC) of excised petiole segments remained close to 0% throughout the day. To find out which technique was erroneous we first analyzed the possibility that PLC values were rapidly returned to zero when the xylem pressures were released. We used the centrifugal force to measure the xylem conductance of petiole segments exposed to very negative pressures and established the relevance of this technique. We then analyzed the possibility that vessels were becoming partially air-filled when exposed to LN2. Cryo-SEM observations of petiole segments frozen shortly after their xylem pressure was returned to atmospheric values agreed entirely with the PLC values. We confirmed, with water-filled capillary tubes exposed to a large centrifugal force, that it was not possible to freeze intact their content with LN2. We concluded that partially air-filled conduits were artifacts of the cryo-SEM technique in our study. We believe that the cryo-SEM observations published recently should probably be reconsidered in the light of our results before they may be used as arguments against the cohesion-tension theory.
The “cohesion-tension” (CT) theory of sap ascent in plants was proposed more than a century ago by Böhm (1893) and Dixon and Joly (1894). The theory postulates that (a) the xylem conduits form continuous water columns from the roots to the leaves, (b) the columns are held in place thanks to the capillary pressures that develop in the leaf mesophyll, and (c) leaf transpiration pulls water out of the xylem, which causes water absorption by the roots. A corollary of the theory is that high xylem tensions (negative pressures) must develop inside the xylem conduits. Over the past century a considerable amount of experimental data have been cumulated by plant physiologists, all consistent with the CT theory.
However, recent direct measurements of sap pressure with xylem pressure probes (Zimmermann et al., 1994) and direct cryo-scanning electron microscopy (cryo-SEM) observations of xylem vessels content during transpiration (Canny, 1997b, 1998b) have questioned the validity of the CT theory. New experiments with the xylem pressure probe (Wei et al., 1999a 1999b) have contradicted the previous observations and now support the CT theory. The cryo-SEM observations have never been refuted by any contradictory experiment and still represent a serious argument against the CT theory.
The cryo-SEM observations have revealed the presence of numerous air-filled vessels during the day in the roots (McCully et al., 1998; Berndt et al., 1999; Buchard et al., 1999; McCully, 1999; Pate and Canny, 1999; Shane and McCully, 1999) and shoots (Canny, 1997a, 1997b, 1998a, 1998b; Tyree et al., 1999) of many mono- and dicotyledonous species. According to these observations, embolism seems to form early in the morning, while the xylem pressure is still high (>−0.3 MPa) and seems to disappear in the afternoon while plant transpiration is high. Furthermore, distinct water droplets and air bubbles have been seen in the lumen of many vessels at the same time, which has been proposed as evidence for vessel refilling and embolism repair by an as yet unknown mechanism (Buchard et al., 1999; Canny, 1999; Holbrook and Zwieniecki, 1999; McCully, 1999; Tyree et al., 1999).
These observations clearly contradict the CT theory because (a) the presence of extensive embolism while transpiration is high negates the existence of continuous water columns and (b) the presence of free water droplets lying against the vessel wall negates the existence of large negative pressures. As a consequence, a new theory for the ascent of sap in plants has been proposed (Canny, 1995, 1998). If validated, the new “compensating pressure” theory will fundamentally change the way plant water relations are understood and a whole domain of the plant physiology will have to be reconsidered. Although the theory has been criticized theoretically (Tyree, 1997; Comstock, 1999) and experimentally (Stiller and Sperry, 1999), no alternative explanation has yet been proposed to account for the extensive cryo-SEM observations made by Canny and coworkers.
The main objectives of our study was to repeat, for the first time by a different laboratory, the cryo-SEM observations using a similar technology and further test the validity of the technique. Until now, the cryo-SEM technique has never been directly compared with the traditional hydraulic way of measuring xylem embolism (Sperry et al., 1988). This method consists in measuring the loss of hydraulic conductance caused by the air embolism into the xylem. Therefore, we followed the diurnal time course of xylem embolism in the leaf rachis of a walnut tree (Juglans regia) concurrently with the two methods. We also dehydrated branches to different levels by means of three independent techniques (air dehydration, air pressurization, and centrifugation) and compared the results of the cryo-SEM and hydraulic methods.
If we were able to repeat the observations of Canny and coworkers in walnut, it would be certain that the results of the two methodologies would disagree. This is because measurements with the hydraulic technique have shown that embolisms develop in walnut petioles only when sap pressures drop below −1.2 MPa (Tyree et al., 1993), which is far lower than the minimum xylem pressure reached during a sunny day on a well-watered plant (Tyree et al., 1993 and see “Results”). One technique has to be misleading or artifactual. We conducted a series of tests to validate or discredit one of the methods.
With the hydraulic technique, the prevailing xylem pressures have to be released because samples are perfused with water under a positive pressure gradient. Canny (1998) has argued that “the compensating pressure of the living cell is able (…) to refill any cavitated vessels during the conductance measurement when the branch is supplied with water.” We tested this hypothesis by measuring the hydraulic conductivity of xylem segments while they were still subject to their prevailing very negative pressures.
A basic assumption of the cryo-SEM method is that the sap in the xylem conduits is frozen intact, which enables a direct in situ observation of the vessel lumen. However, the possibility exists that embolisms may form in the xylem conduits while the sap is freezing because of the presence of negative hydrostatic pressures. We tested this hypothesis by comparing samples frozen before and shortly after the pressures were reduced to atmospheric pressure. We also tested this hypothesis on a physical model of a xylem vessel. Altogether these experiments enabled us to address the validity of the cryo-SEM for assessing xylem embolisms and, therefore, the opportunity for introducing a new theory for the ascent of sap in plants.
RESULTS
Diurnal Time Courses
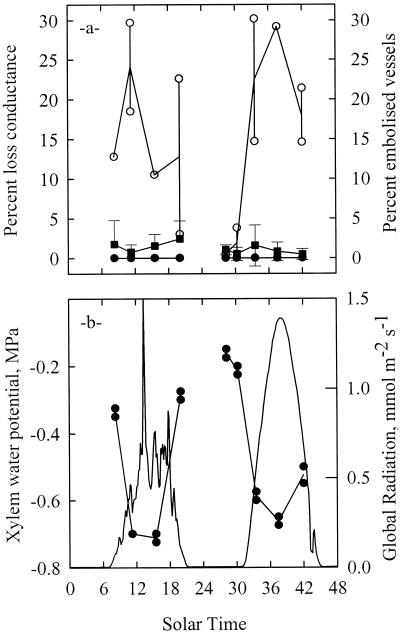
The diurnal time courses of xylem embolism and xylem water potential are shown in Figure 1 for two consecutive days. The xylem water potential (Ψx) was high before dawn and declined to a minimum of −0.7 MPa around midday. The variations in Ψx followed the variations in solar radiation (Fig. 1b). The amount of xylem embolism as assessed by the percentage of loss of hydraulic conductance remained extremely low throughout the 2 consecutive d (Fig. 1a). The values were within the detection limit of the technique and no significant diurnal trend could be identified.
Figure 1.
Change in xylem vessel functionality (a), water potential, and incident irradiance (b) in the petioles of a mature walnut tree during 2 consecutive d. Vessel functionality was estimated indirectly via the PLC (▪) or directly as the percentage of vessels seen air-filled on a cross-section in a cryo-microscope. Samples observed in the cryo-SEM were either frozen intact on the tree with LN2 (○) or frozen after the xylem pressure was released to zero (●). Each circle represents one sample and the lines are through the mean values. Error bars represent one sd (n = 10). Only when the xylem pressure was released prior to freezing was a good agreement found between the direct cryo-SEM and the indirect hydraulic methods.
The diurnal time course of the percentage of embolized vessels as assessed by the cryogenic technique is shown on Figure 1a. The xylem vessels of the samples frozen after the xylem pressure was released were always entirely water-filled. In contrast, samples frozen on the tree were entirely water-filled only early in the morning when Ψx was higher than approximately −0.25 MPa. When Ψx decreased because of the transpiration pull, the percent of embolized vessels drastically increased up to 30% around midday and tended to decrease thereafter. The vessels still air-filled at the end of the 1st d were apparently all water-filled the following morning. If we accept that the cryo-SEM observations accurately reflected the native state of xylem prior to freezing, then we would conclude that, in the morning, the embolized vessels contained only a little air, often in the form of an air bubble in the middle of the lumen and that in the middle of the day, the embolized vessels were more emptied, water being seen only on one side of the vessel (Fig. 2), or in the form of droplets lying against the wall.
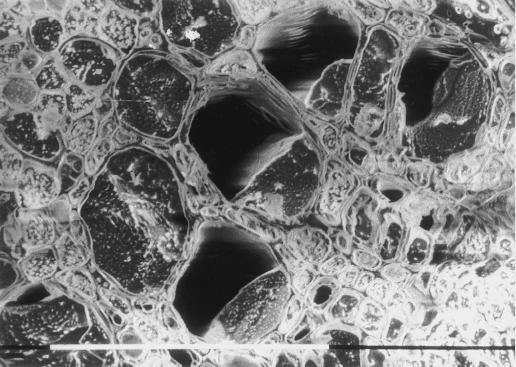
Figure 2.
Representative cryo-SEM observation of a walnut petiole collected at midday on a field grown tree. The petioles was frozen intact on the tree during active leaf transpiration and while the xylem water potential was around −0.7 MPa. The cross-section was observed uncoated at −150°C and 5 kV. Vessels were either entirely water filled (vessels on the left side of the picture) or one-half-filled with sap (right side). When xylem pressures were relaxed shortly before freezing all the vessels entirely filled with sap.
Vulnerablity Curves (VCs)
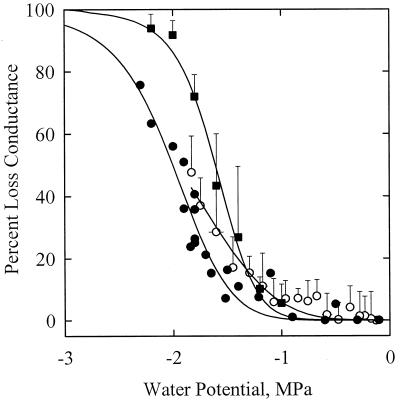
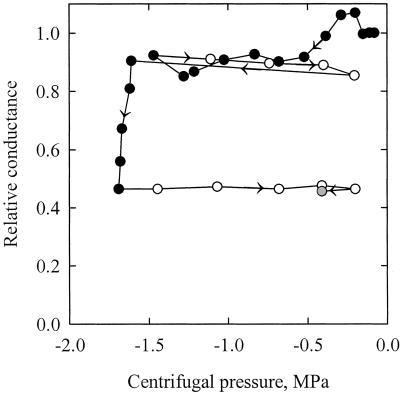
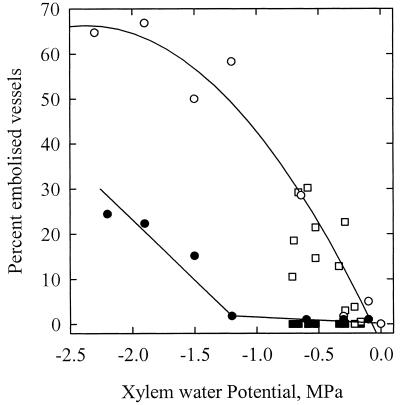
The VC obtained in 1999 with the air pressurization technique was not statistically different from the curves obtained by Tyree et al. (1992) with the air-dehydration and air-pressurization techniques (Fig. 3; Table I). The percentage of loss of hydraulic conductance (PLC) values significantly increased for Ψx values lower than −1.2 MPa, and a 50 PLC was obtained for Ψx equal to −1.5 MPa. Samples centrifuged and measured after centrifugation with the hydraulic technique exhibited significantly lower PLC values (about a 0.4 MPa shift). This shift might be due to the fact that the x axis in Figure 3 represents the minimum negative pressure at the middle of the sample, which is lower than average negative pressure in the sample (Alder et al., 1997). When the hydraulic conductance was measured on samples still exposed to their prevailing negative centrifugal pressures, a vulnerability curve similar to the first three curves was obtained, but with a significantly lower slope. Figure 4 shows the results of one experiment in details. The relative change in conductance is expressed as a function of the centrifugal pressure. The negative pressure was first decreased to −1.5 MPa, returned to −0.2 MPa, decreased to −1.7 MPa, and finally returned to −0.2 MPa. The conductance was measured anew at −0.4 MPa after the sample was left for 23 min at atmospheric pressure. The changes in conductance were very small between −0.05 and −1.5 MPa. At more negative pressures, a significant and irreversible drop in conductance was noticed. The loss of conductance was still not reversed 23 min after embolism induction.
Figure 3.
PLC in the xylem of walnut petioles exposed to a water stress. The water stress was provoked by exposing excised petioles to different pneumatic pressures (▪) or different centrifugal forces (○). Samples exposed to a centrifugal force were either measured while the xylem was still under centrifugal force with negative xylem pressure (○) or shortly after the xylem pressure was returned to 0 MPa (●). The error bars represent ± 1 sd. Each closed circle represents a different sample. The different techniques yielded close results. Whatever the technique, the threshold water potential for embolism induction was always less than −1.0 MPa.
Table I.
Parameters of the logistic functions fitted to the experimental vulnerability curves constructed with different techniques
| Parameter | Dehydration | Pressurization
|
Centrifugation
|
||
|---|---|---|---|---|---|
| P = 0 | P < 0 | ||||
| 1992 | 1992 | 1999 | 1999 | 1999 | |
| Ψ50 | −1.47 ± 0.09a | −1.53 ± 0.07a | −1.61 ± 0.04a | −2.00 ± 0.03b | −1.96 ± 0.05b |
| S | −9.94 ± 4.08ab | −8.10 ± 2.23ab | −8.40 ± 2.23a | −7.33 ± 0.98a | −4.12 ± 0.52b |
The logistic function given in equation 1 has two parameters: Ψ50, the xylem pressure (MPa) provoking 50% loss of conductance, and s, a slope parameter. The vulnerability curves were obtained on walnut petioles air dehydrated, air pressurized, or centrifuged. Petioles centrifuged were either measured before (P < 0) or after (P = 0) the negative xylem pressure was returned to zero. Experiments conducted in 1992 were obtained by Tyree et al. (1993) on similar plant material to that used in this study (1999). Data that have a letter in common are not significantly different at P = 0.05.
Figure 4.
Relative change in the hydraulic conductance of a petiole segment exposed to different centrifugal pressures (x axis). The conductance was measured while the segment was still under negative pressure. The arrows indicate the time course of the experiment. The segment was exposed to decreasing (black symbols) or increasing (white symbols) pressures. The hydraulic conductance decreased significantly only when the negative pressure became less than −1.5 MPa. The change in conductance was still not reversed 23 min after the petiole was exposed to zero pressure (gray symbol).
The VCs obtained with the cryogenic technique yielded contrasting results depending on whether the samples were frozen before or after the xylem pressure was released (Fig. 5). When pressure was released before freezing, the percentage of embolized vessels remained close to zero for prevailing pressures less negative than −1.2 MPa. The number of emptied vessels increased only at more negative pressures. These observations were consistent with the curves obtained with the hydraulic technique. When samples were frozen while still under negative pressure from the rotational pull, the percentage of embolized vessels was considerably higher and was found to increase for pressures as high as −0.3 MPa. At −1.2 MPa, 60% of the vessels were embolized.
Figure 5.
Percentage of embolized vessels present in the xylem of walnut petioles exposed to a water stress. The water stress was induced in the xylem by the transpiration pull for leaves collected in the field (squares) or by exposing excised petioles to different centrifugal forces (circles). Samples were either frozen with LN2 while the xylem was still under negative pressure (white symbols) or shortly after the xylem pressure was returned to 0 MPa (black symbols). The percentage of vessels containing air in their lumens was counted on a frozen cross-section in a cryo-SEM. Each point represents one sample. When the samples were frozen while the xylem was under negative pressures, the percentage of air-filled vessels was much higher (compare white and black symbols).
Capillary Tubes
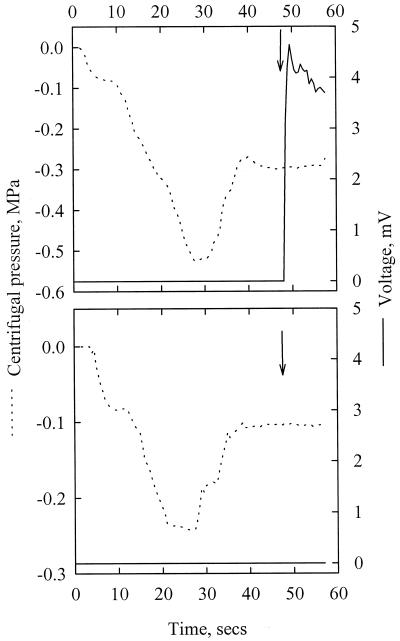
When water-filled capillaries were centrifuged and suddenly cooled with liquid nitrogen (LN2), two situations were observed. First, when the centrifugal pressure was lower than approximately −0.1 MPa, the water column consistently broke when LN2 was poured and the tube was entirely emptied. Figure 6A shows the results of one typical experiment with a 0.25-mm capillary exposed to a centrifugal pressure of −0.3 MPa when frozen. The column rupture was detected about 0.6 s after LN2 was poured. Second, when the centrifugal pressure was higher than −0.1 MPa (Fig. 6B) water was not expulsed out of the capillary and ice could be observed in the lumen. On many occasions the capillary tube was found broken, probably because of volume expansion with water freezing. The threshold centrifugal pressure that provoked the water column rupture upon freezing (−0.1 MPa) was not different between the two types of capillaries.
Figure 6.
Typical results of the experiments with capillary tubes. Water-filled glass capillary tubes (a 0.25-mm internal diameter) were exposed to different centrifugal pressures (dotted line, left y axis) and suddenly frozen with LN2 (arrows). When the negative pressure was lower than approximately −0.12 MPa (top), water was expulsed out of the capillary upon freezing because of a breakdown of the water column. This caused a short cut in an electrical circuit and a voltage increased (plain line, right y axis). When the centrifugal pressure was higher than −0.12 MPa (bottom), the water column did not break.
Cooling Rates
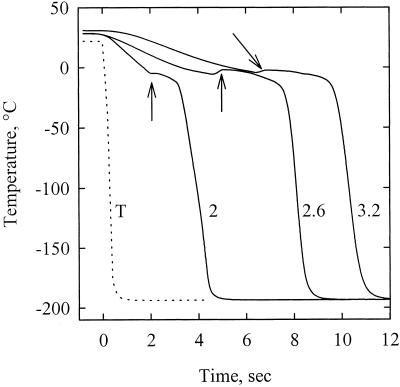
Figure 7 shows the changes in temperature of thermocouples and petiole segments exposed to LN2 at time zero (only representative experiments are shown for clarity). The cooling rates of thermocouples immersed in LN2 was 512 °K s−1. Three distinct phases were observed during the cooling of petiole segments. The temperature was first found to decrease linearly at a mean rate of 10.2 (sd of 3.4) °K s−1 down to a slightly negative value. The temperature stabilized or even increased for a period from 0.4 to 4.4 s (sd = 1.4 s and mean = 2.4 s). This phase probably corresponded to the water freezing exotherm. The temperature then rapidly declined to LN2 temperature (sd = 32.5 K s−1 and mean = 126.5 K s−1).
Figure 7.
Time course of temperature of three petiole segments immersed in a bath of liquid nitrogen at time = 0 s (plain curves). The arrows indicate the onset of the freezing exotherms. The numbers on the graph correspond to the sample diameter (millimeters). The dotted line was obtained by immersing a thermocouple directly into the LN2 bath.
DISCUSSION
In this study we compared the standard hydraulic method for assessing the degree of xylem embolism with the recently introduced cryo-SEM method. These two measurement approaches were in clear conflict and would lead to dramatically different conclusions about cavitation dynamics in plants. The cryo-SEM observations performed in this study on walnut petioles frozen while still attached to the tree clearly confirmed the observations of Canny and coworkers on many different plant materials (see refs. listed in the introduction). If we accept that the cryo-SEM data accurately reflect the xylem content prior to measurement, then we would conclude that vessels were distinctly water-filled at predawn, became partially air-filled in the morning, and refilled late in the afternoon and at night. Up to 30% of the vessels were embolized in the middle of the day. We have also confirmed these observations on tall fescue leaves (H. Cochard, P. Martre, and J.L. Durand, unpublished data).
These observations are clearly in contradiction with (a) the measurements of the xylem negative pressure with the pressure chamber and (b) the amount of xylem embolism measured with the hydraulic technique. The first contradiction results from the size of the water droplets and the air bubbles seen in the lumen of the partially embolized vessels. The water in these vessels was certainly in connection with the surrounding water-filled vessels through the pits in the wall and must then have been at a same water potential. These droplets and bubbles were as large as 50 μm in diameter, which would correspond to a capillary pressure of −6 kPa (Jurin's law), two orders of magnitude higher than the value given by the pressure balance technique (−0.7 MPa).
Second, the hydraulic conductivity measurements were inconsistent with the percentage of vessels partially filled with air. If we assume that these conduits were non-conductive then we would expect at least 30 PLC compared with 1 PLC measured at midday. This is a rough estimate because we do not account for the diameter distribution of the vessels and of the redundancy of the xylem (Tyree et al., 1994). These profound discrepancies between the different techniques imply that one of them has to be misleading or artifactual. Let us first analyze a possible artifact with the hydraulic technique.
Possible Artifacts with the Hydraulic Technique
We quoted in the introduction one argument of Canny (1998) suggesting that the embolized vessels are refilled by the time their hydraulic conductance is measured. Following Sperry et al. (1988), the PLC of excised xylem segments is determined by measuring the flow rate through the segments placed under a positive water pressure head (usually a few kPa). There is evidence from the literature (Tyree and Yang, 1992) that under these circumstances embolized conduits do refill, but only after several hours. However, the possibility remains that partially embolized conduits may refill much faster and that the hydraulic technique underestimates the actual PLC values. The experiment we conducted with the centrifugal force is a test of Canny's argument. Here we measured the hydraulic conductance of excised segments while still exposed to negative pressures. The hydraulic conductance was therefore determined with the in vivo water status. Starting with fully hydrated samples and high xylem pressures (low rotational speed) one would have expected a gradual reduction in conductance when the conduits were becoming partially air-filled with decreasing negative pressures. However, the hydraulic measurements failed to detect any extensive change in conductance (less than 10% PLC) when the xylem pressure varied from 0 to −1 MPa. The conductance decreased only when pressures dropped below −1.2 MPa, a value that precisely corresponded to the threshold water potential value for cavitation as determined with the other hydraulic techniques on samples returned to zero water potential. Once the conductance was reduced, our data confirmed that it was not rapidly reversed when the pressures were released. From these experiences we can reject Canny's argument and conclude that the measure of the PLC value on a sample soon after its xylem pressure has been released to atmospheric pressure yields the correct value. So why was the conductance constant in the range of 0 to −1 MPa when the cryo-SEM observations revealed the presence of many air-filled vessels? Let us now analyze the possibility of artifacts with the cryo-SEM technique.
Possible Artifacts with the Cryo-SEM Technique
Samples frozen intact on the tree at predawn when the xylem water potential is high show virtually no air-filled vessels. Samples frozen intact on the tree at midday when Ψx is low show many air-filled vessels. Samples frozen at the same time shortly after the Ψx is returned to zero show no air-filled vessels. Samples frozen while exposed to high centrifugal forces (e.g. −1.2 MPa) show many air-filled vessels. Comparable samples frozen shortly after the angular speed was returned to zero show only water-filled conduits. From this series of facts we hypothesized that the presence of partially air-filled vessels must correlate with the presence of a low water potential in the sap upon freezing.
The possibility that the technique was vitiated by artifacts caused by embolisms produced by the freezing of the xylem columns while it was under negative pressure from the pull of transpiration was discarded by McCully (1999) and Shane and McCully (1999). The test used by these authors consisted in freezing the same root segment at two locations, the second one being distal to the first one and frozen a few seconds later. The second segment was thus frozen in the absence of a transpiration pull. The two samples exhibited similar vessel contents. In these experiments however, xylem pressures in the second segment were not released to atmospheric pressure and probably remained at a level close to the prevailing pressures in the intact roots. A water potential gradient between the xylem conduits and the soil will continue to exist until the hydraulic root capacitance is recharged. It is the same phenomenon that explains why root absorption lags behind leaf transpiration in the evening. Therefore, these tests failed to demonstrate that vessel emptying was not produced by the pressures existing in the sap during freezing. The tests we conducted by comparing samples frozen with prevailing sap pressures to samples frozen shortly after the pressures were reduced to atmospheric pressure do demonstrate indeed that vessel emptying was caused by the hydrostatic pressures in the sap and occurred during freezing.
The breakdown of the water column in the xylem conduits upon freezing was confirmed by our experiments with the capillary tubes. The water columns inside the capillaries were able to support very low pressures without cavitation. However, when centrifuged and suddenly exposed to LN2 they consistently broke. Our results confirmed the finding of Lybeck (1959) who found that a water column exposed to a centrifugal pressure of −0.18 MPa immediately broke when a piece of dry ice was brought into contact with its central part. In our study it was possible to freeze them intact only when they were exposed to a pressure above −0.1 MPa. This threshold value may correspond to the partial pressure of vapor. However, this exact threshold might not hold in xylem conduits.
The cooling rates we measured on our samples immersed in LN2 were relatively low. The complete freezing of a petiole segment took several seconds. Cavitation events are known to last for a few milliseconds only (Tyree and Sperry, 1989). With such low cooling rates it is probably very unlikely that we could freeze the vessel content intact. Although our experiments were not specifically designed to address the physicochemical reasons for the artifacts produced by the cryo-SEM technique, we may tentatively find some explanations. It is possible that water may cavitate when tiny air bubbles are expulsed while the ice is forming (air is soluble in water, not in ice). In addition, ice crystals may act as catalysts during the formation of gas seeds. The critical size of seeds (i.e. the size of the air bubbles, which ultimately results in cavitation) is decreasing with increasing tension. This is a first reason that may explain why more artifacts were seen during the day. Sap velocity may also exacerbate the problem. Because water is incompressible and vessel walls are rigid, sap pressure in the vicinity of the bubbles will rapidly diminish unless water can exit the vessel. There are at least two reasons for water to exit the vessels. First, water may move and freeze in the intercellular air spaces. Second, water may migrate to rehydrated living cells. These cells may be located very close to the xylem vessels (parenchyma or ray cells) whose content usually freeze at a lower temperature. Water may also rehydrate cells in the leaves or sustain the transpiration stream. It is thus likely that the xylem vessels may be even more susceptible to freezing artifact under transpirational conditions. This could explain why vessels were more air-filled in the middle of the day than in the morning. We believe that the water droplets lying against the cell wall were probably frozen while they were aspirated by the surrounding conduits. More experiments are definitively needed to understand why and how artifacts form with the cryo-SEM technique.
The freezing procedure we used in this study (immersion in LN2) was not rapid enough to freeze intact the vessel content of transpiring walnut petioles. Canny (1997a, 1997b) used the same technique to assess the vessel content in the petioles of sunflower plants. Because sunflowers have petioles at least twice as wide as walnut, the cooling rate was probably even slower in his study. In the most recent papers (McCully et al., 1998; McCully, 1999; Shane and McCully, 1999) a more efficient technique has been used for freezing samples. The technique consists in pressing tissue to a polished copper surface at LN2 temperature. Cooling rates as high as 25,000 K s−1 are claimed to be achieved. However, no difference has been found between the two freezing procedures (McCully et al., 1998; Berndt et al., 1999) so this technique may still not be fast enough. We do not known if the fastest freezing procedures (such as propane at LN2 temperature) may be fast enough to freeze intact the vessel contents.
Hydraulic and Cryo-SEM Techniques Reconciled
Cryo-SEM observations of samples frozen shortly after the xylem pressure was released were very consistent with the hydraulic measurements. The PLC values remained close to zero throughout the day in the petioles of a walnut tree under field conditions and all the vessels were filled with water and the percentage of air-fill vessels in samples exposed to centrifugal forces followed closely the changes in PLC values. This suggests that the xylem in walnut petioles does not exhibit any diurnal variation in hydraulic conductivity, contrary to what has been found in other woody species (Salleo et al., 1996; Zwieniecki and Holbrook, 1998; Tyree et al., 1999).
CONCLUSIONS
First, by measuring the hydraulic conductance of petiole segments while they were exposed to low pressures we have demonstrated that the hydraulic technique introduced by Sperry et al. (1988) correctly determined the amount of xylem embolism. Therefore, we were able to dismiss the possibility claimed by Canny (1998) of an artifact with this technique. Second, we have shown that cryo-SEM observations of samples frozen after the xylem pressure was released agreed with the hydraulic technique. On the contrary, samples frozen with sap still under negative pressure exhibited an inconsistently high number of air-filled vessels. Therefore, we hypothesized that artifactual cavitations occurred in these vessels if the samples are frozen when the sap is under high negative pressure. We have successfully tested this hypothesis with water-filled capillary tubes exposed to high centrifugal forces and established that the threshold negative pressure below which artifacts may occur was as high as approximately −0.1 MPa. The rather low sample cooling rates we measured on samples dipped in LN2 probably favored these cavitations. However, faster cooling techniques may still produce artifacts. From this series of experiments we concluded that vessel contents are frozen intact only when the xylem pressure is first released to atmospheric value. Partially air-filled conduits were thus artifacts of the cryo-SEM technique in our study. Therefore, we believe that the cryo-SEM observations published recently should be reconsidered before they may be used as arguments against the CT theory and before we may achieve a major revision in some central concepts of plant water relations.
The cryo-SEM technique is unique for assessing vessel content in the smallest parts of the xylem pathways such as rootlets or leaf veins. If properly used, the technique may give new insights into the process and regulation of water transport in plants.
MATERIALS AND METHODS
Plant Material
Experiments were conducted during the summer of 1999 on a mature, 10-m-tall walnut (Juglans regia) tree growing in an orchard at the INRA site of Crouël (Clermont-Ferrand, France). Leaves were randomly collected from the basal sun-exposed part of the tree. Maximum vessel length in the petioles (16 cm) was determined by the air injection method of Ewers and Fisher (1989).
Diurnal Time Courses of Xylem Embolism
Diurnal time courses of xylem embolism in the petioles of the walnut tree were followed for 2 consecutive d (Aug. 23 and 24, 1999). The 1st d was partly cloudy and the 2nd d was very sunny. A total of nine sets of measurements were performed from predawn to sunset at regular time intervals. The Ψx was first measured following the procedure of Turner and Long (1980). Two terminal leaflets were enclosed in airtight plastic bags and covered with aluminum foils at least 4 h before measurements. The terminal leaflets were excised and placed, still bagged, in a Scholander-type pressure chamber (Scholander et al., 1964). The balancing pressure was determined to the nearest 0.025 MPa.
A first set of two samples was then frozen intact on the plant by immersing part of the petiole in a bath of LN2 as described by Canny (1997b) Berndt et al. (1999), or Utsumi et al. (1996, 1998, 1999). Watertight collars made of Styrofoam cups as a container for LN2 were fitted to two intact leaves. The collars were placed near the middle of the leaves, between two pairs of leaflets. The collar was made watertight with terozon placed around the petiole segments. The collars were filled with LN2 and the petioles were allowed to freeze for approximately 30 s (the samples were completely frozen after approximately 10 s, see “Results”). A 4-cm-long segment was then severed from the petiole inside the collar and placed in a container with LN2. The samples were eventually stored at −80°C until they were examined in a cryo-SEM (see below).
A second set of two samples was frozen after the xylem pressures were returned to atmospheric pressure. Two intact leaves were immersed in a bath of tap water and cut, under water, near the petiole insertion on the branch. A 4-cm-long petiole segment was then rapidly excised under water near the middle of the leaf and immediately immersed in a bath of LN2 and then stored at −80°C until examination. Overall, the petioles segments remained for approximately 30 s under water before they were frozen. Thirty seconds was supposedly long enough to release the xylem pressure close to the atmospheric value (0 MPa). Although two samples were collected each time, the cryo-SEM observation was usually performed only on one of them to minimize the access to the microscope and the cost of the analysis.
A third set of five leaves was cut under water as previously described and immediately brought to the laboratory for analysis. In the laboratory, the amount of air embolism was determined with the hydraulic technique (see below) on two petiole segments, 3 to 4 cm long, excised under water with a razor blade on each leaf.
On average, the whole sampling procedure lasted for about 15 min (PLC measurements excluded).
Loss of Hydraulic Conductance
The degree of xylem embolism was assessed by measuring the loss of hydraulic conductance due to air blockage (Sperry et al., 1988). The technique consists in measuring the hydraulic conductance of excised petiole segments before and after embolism removal. The PLC is then an indirect estimate of the degree of embolism in the petiole segments. A prototype of a new xylem embolus meter (XYL'EM, Institut National de la Recherche Agronomique, Laboratoire de Physiologie Intègrée de l'Arbre Fruitier et Forestier, Clermont-Ferrand, France) was employed to determine the PLC values. The XYL'EM (version 1.0, June 1999) is a portable apparatus that simultaneously measures (a) the water flow (F, gs−1) entering each sample with a high resolution liquid mass flowmeter (accuracy 1.4 10−5 gs−1); (b) the hydrostatic pressure gradient (P, <3 kPa); and (c) the water bath temperature. A software computes the initial sample hydraulic conductance (Kinit, gs−1 Pa−1) as F/P and corrects for the temperature effects on water viscosity. Once Kinit is measured, the samples are perfused with distilled water pressurize to 0.1 MPa and Kmax determined as above. The software then computes the PLC values as PLC = 100 × (1 − Kinit/Kmax).
VCs
A VC is a graph of the degree of xylem embolism versus the xylem water potential that induced the embolism. Embolisms were induced by three independent techniques. The classical technique consists in dehydrating excised branches on a laboratory bench until the desired Ψx values is obtained. Petiole segments are then excised under water and their PLC value is determined as described above. In this paper we reused that data obtained by Tyree et al. (1993) in our laboratory on similar trees.
For the second technique, well-watered branches were enclosed in a large pressure chamber with the basal end protruding and pressurized to the desired pressure with nitrogen until sap exudation ceased (after 15–60 min depending of the applied pressure; Cochard et al., 1992). The pneumatic pressure was then released to atmospheric and four samples were excised under water for PLC determination. It was necessary to wait at least 1 h before measuring Kinit because air bubbles coming out of the samples perturbed the measurements. The VCs obtained in 1999 were compared with the ones obtained by Tyree et al. (1993) with the same technique.
In the last technique we used the centrifugal force to induce embolism (Pockman et al., 1995; Alder et al., 1997). A high speed spinning device was constructed with a drill (335 rad s−1 or 3,200 rpm maximum) and a domestic centrifuge. A belt connected a 13-cm wheel placed on the drill to a 1.3-cm wheel on the axis of the centrifuge to amplify the angular velocity (ω) of the centrifuge up to at least 1,000 rad s−1 (which corresponded to a pressure of approximately −2.1 MPa). The actual velocity was measured with an optical tachometer (Radiospares, Beauvais, France). The rotor was made of a 16-cm-long aluminum bar on which was glued an Eppendorf-type plastic tube at each extremity. These tubes were designed to be filled with water and to receive the cut ends of the petiole segments. A small hole was made on the upper face of each tube. The distance (d) between the two holes was exactly 0.143 m and the pressure in the middle of the sample was then equal to −0.5 × ρ × (d/2)2ω2, ρ being the water density (Alder et al., 1997). Experiments were conducted on walnut petioles placed under tap water for about 12 h to make sure that they were perfectly rehydrated and that any embolism would have dissolved. Sixteen-centimeter-long petiole segments were excised under water and firmly attached on the rotor with their cut ends inserted into the Eppendorf tube previously filled with distilled water. When the rotor was spun, the excess of water in the tubes was evacuated through the holes and the portion of petiole exposed to negative pressures was exactly equal to d. Once the samples were installed on the rotor, the rotational velocity was increased to the desired value and kept constant for 2 min. The degree of xylem embolism in the middle of the samples was then estimated with the two different techniques.
First, we determined the PLC value with the indirect hydraulic technique. The velocity was returned to zero, the sample was immerged in a bath of tap water for 5 min, a 2.5-cm-long segment was excised under water in the middle of the sample, and the PLC was immediately measured as above. A total of 23 petiole segments were used to construct the VC this way.
Second, the degree of embolism was assessed by direct cryo-SEM observation. The samples were frozen with LN2 directly on the rotor. The container for LN2 was made of a 2-cm-wide and 3-cm-high plastic tube firmly attached in the middle of the rotor. The petiole samples were passing through the container and a watertight seal was made with soft foam. A rubber cork with a 5-mm hole in its center placed at the top of the tube maintained the LN2 into the tube while it was spinning. LN2 was poured in the container through the hole in the cork. The samples were frozen in two different ways. They were either frozen at the end of the 2-min spinning period while the selected centrifugal forces were still exerting their negative pressure on the water column in the xylem conduit, or they were frozen 5 min after the angular speed was returned to zero. Because both sample ends were still immerged in water, we assumed that this period was long enough to release the xylem pressure in the middle of the sample to atmospheric pressure. The central part of petiole was cut outside of the tube and stored at −80°C until examination.
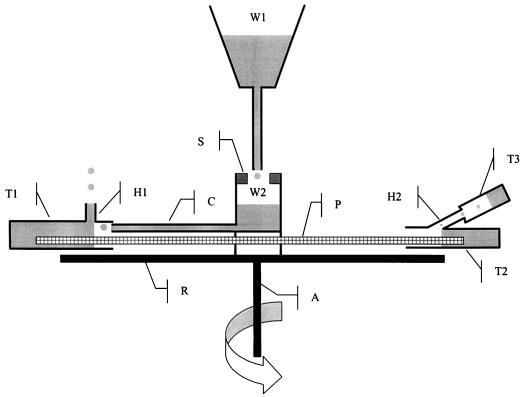
For the third technique for establishing VCs, we used the centrifugal force in a different manner. Our objective was to measure the hydraulic conductance of a petiole segment placed on the rotor of the centrifuge while it was spinning. This was achieved by exposing the segment to a constant positive hydrostatic gradient and by measuring the water flow through the segment. A drawing of the experimental setup is given in Figure 8. The two cut ends of the petiole segment were placed into two water-filled Eppendorf tubes attached to the rotor of the centrifuge. A small hole was drilled on the upper face of each tube. The pressure gradient was created by positioning the hole of the tube 1 (up-stream) 5 mm closer to the axis (dr = r1 + r2 = −5 mm). The P was then equal to 1/2 ρ ω2 (r22 − r12) and the negative centrifugal pressure in the middle of the sample was equal to −1/4 ρ ω2 (r12 + r22) (P. Adler, personal communication). The pressure was positive in the immerged distal ends of the sample and negative elsewhere. To maintain the pressure gradient constant during the centrifugation, water was flowing into tube 1 through a small capillary connected to a water reservoir. The water flow entering tube 1 was much higher than the water flow through the petiole. The excess water was evacuated through the hole, and the level in tube 1 was therefore maintained constant. The water evacuated through hole 2 was collected in a removable vial (T3). At the beginning of each measurement, tubes 1 and 2 were filled with water and the centrifuge spun at low speed for a few seconds. This was necessary to adjust the water level in the tubes. The preweighted vial (T3) was then firmly attached to tube 2 and the sample spun at the desired speed for 30 s. The centrifuge was stopped, the vial removed and weighted, and the whole procedure repeated three times at the same selected velocity. The samples were exposed to increasingly lower negative centrifugal pressures (from about −0.01 MPa to −2.3 MPa). On two occasions the velocity was returned to low values in the middle and at the end of the experiments. A total of six petioles were used to construct the VC.
Figure 8.
Schematic drawing of the experimental set up designed for measuring the hydraulic conductance of a petiole segment exposed to a negative centrifugal pressure. A petiole segment (P) is attached to the rotor (R) of a centrifuge with the cut ends placed into two water-filled tubes (T1 and T2). Water falls from the fixed reservoir (W1) into a reservoir (W2) attached on the centrifuge. A rubber seal (S) maintains water in W2 during the rotation. Water in W2 is forced to T1 through a capillary (C) by the centrifugal force. The excess of water in T1 is evacuated through a small hole (H1) thus maintaining a constant level in T1. Because the distance (r1) between the centrifuge axis (A) and H1 is smaller than the distance (r2) between A and the hole in T2 (H2), a positive hydrostatic pressure gradient is created forcing water from T1 to T2 through the petiole. Water entering T2 is evacuated through H2 into the removable tube (T3) thus maintaining a constant level in T2. The water flow entering T3 equals the water flow through the petiole.
The following sigmoid function was fitted to the experimental data (Cochard et al., 1999):
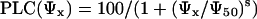 |
1 |
where Ψ50 is the Ψx that induced 50 PLC and s is a slope parameter.
Cryo-SEM Observations
Vessels content in the petioles were observed on a cross-section obtained by cryo-fracturing with a cryo-SEM. The samples stored at −80°C were transported at LN2 temperature to the Laboratory of Electron Microscopy at the INRA-Theix center near Clermont-Ferrand. In the laboratory the samples were manipulated under LN2 until they were transferred to the microscope. A very shallow ring was made in the middle of the sample with a small glass-cutting saw. This was necessary to facilitate the cryo-fraction and to obtain a relatively plane cross-section. The sample was then inserted into a 1-cm-deep hole made in an aluminum bar screwed on the specimen holder. The sample was rapidly transferred to the cryo-preparation chamber of the microscope held at −150°C (model CT 1000, Hexland, UK), and the vacuum was made in the chamber. The cryo-fracture was operated directly in the chamber, and the sample was moved to the sample stage (−150°C) in the column of the SEM (model SEM 505, Philips, Eindhoven, The Netherlands). The samples were observed uncoated at 5 kV. Vessels containing air, even partially, were counted as “embolized.” We found it not reliable to distinguish vessels entirely air-filled to vessels partially filled with air. Indeed, a vessel may appear empty close to the section, but may actually contain sap deeper on. Preliminary observations have shown that counting the embolized vessels on a sample uncoated etched to reveal traces of cell shapes, or coated with gold yielded similar results. Surface etching was achieved by setting the temperature of the stage at −80°C for several minutes. Surface coating with evaporated gold was performed in the cryo-preparation chamber at −150°C for 1 min. The sample was then returned to the column of the microscope and examined at various voltages from 5 to 30 kV. When all the observations were made, the samples were stored in absolute alcohol until the cross-section was observed under a light microscope. A cross-section was obtained by hand with a razor blade and all the xylem vessels larger than approximately 15 μm in diameter were counted to compute a percentage of embolized vessels.
Experiment with Capillaries
The objective of this experiment was to determine whether or not it was possible to freeze intact with LN2 a water column under very negative hydrostatic pressure. Following Briggs (1950) and Lybeck (1959) we spun water-filled glass capillary with a centrifuge, froze them while they were still spinning, and observed if the water column was frozen intact or not. We used two types of capillaries. The first ones were 127 mm long and had a 1.3-mm internal diameter (100-μL glass micro-sampling pipets, Corning, Corning, NY). The second ones were 127 mm long and had an internal diameter of only 0.250 mm (5-μL Pyrex micro-sampling pipets, Corning). The capillary tubes were placed on the rotor of the centrifuge described above where they were maintained by the LN2 container and plastic tapes. The two ends of the capillary were heated and turned backward over a 5-mm length (forming a Z). Doing so, it was possible to hold the water column in the capillary while it was rotating. The capillaries were filled with distilled and partially degassed water filtered to 0.2 μm.
Preliminary experiments demonstrated that it was easy to expose these capillaries to a centrifugal pressure as low as −2 MPa for several minutes without breaking the water column inside the capillary. However, the water column occasionally broke (cavitated) at a lower value, possibly because a particle or a small air bubble was trapped into the tube. Therefore, for each trial we first exposed the capillary to a centrifugal pressure −0.2 MPa lower than the target pressure for several seconds to make sure that the water column would not cavitate inadvertently. The centrifugal pressure was then increased to the target value, maintained constant for several seconds, and LN2 was poured in the LN2 container. A small copper-constantan thermocouple was inserted into the LN2 container close to the capillary and was used to detect precisely when the LN2 was added. The thermocouple was connected to the datalogger (model 21X, Campbell Scientific LTD, Logan, UT) and logged every 0.3 s. The detection of a cavitation event was easy with the large capillary because about 100 μL of water was suddenly expulsed and many small droplets were easily seen on the Plexiglas box placed above the centrifuge for security. The smaller capillary contained only 5 μL of water and a detection of a cavitation event by eye was no longer possible. We detected this event electrically as follow. One end of the capillary was given an “Z” form, the other end having the usual “U” form. The extremity of the Z end was cupped with a small tube that was transpierced at its bottom by two copper wires. Each wire was connected to a copper ring on the axe of the centrifuge thus forming an electrically insulated circuit. With a flexible steel rod in contact with each ring, if was possible to continuously couple this rotating circuit to a current generator. When the tube at the Z end of the capillary was dry, no current could pass through the circuit. When a cavitation event occurred, water was projected in the tube making a current path between the two wires. The current passing in the circuit was monitored every 0.3 s by the datalogger (model 21X, Campbell Scientific LTD). The experiment was repeated on about 10 capillaries of each size.
Cooling Rates
The cooling rates of samples immersed in a bath of LN2 were determined experimentally. A tiny copper-constantan thermocouple was inserted longitudinally approximately one-half of the way in the middle of 3-cm-long petiole segments. The petiole was then immersed in LN2 and the change in temperature recorded every 0.2 s with a datalogger (model 21X, Campbell Scientific LTD). The measurement was repeated on eight different samples with diameter from 2.0 to 3.45 mm. As a control, we also measured the cooling rate of the thermocouple directly immersed in LN2.
ACKNOWLEDGMENTS
We thank Brigitte Martinie for welcoming us in his laboratory, and Maurice Crocombette for his help in the construction of the centrifuge. We are grateful to Pierre Adler (Institut de Physique du Globe de Paris) for the analytical solution of the centrifugal force. We thank Erwin Dreyer, Peter Melcher, John Sperry, and Melvin Tyree for their useful comments on a first draft of our manuscript. The discussions with Martin Canny and Margaret McCully about this work were constructive.
LITERATURE CITED
- Alder NN, Pockman WT, Sperry JS, Nuismer S. Use of centrifugal force in the study of xylem cavitation. J Exp Bot. 1997;48:665–674. [Google Scholar]
- Berndt ML, McCully ME, Canny MJ. Is xylem embolism and refilling involved in the rapid wilting and recovery of plants following root cooling and rewarming? Plant Biol. 1999;1:506–515. [Google Scholar]
- Böhm J. Capillarität und saftsteigen. Ber Dtsch Bot Ges. 1893;11:203–212. [Google Scholar]
- Briggs LI. Limiting negative pressure of water. J Appl Phys. 1950;21:721–722. [Google Scholar]
- Buchard C, McCully ME, Canny MJ. Daily embolism and refilling of root xylem vessels in three dicotyledonous crop plants. Agronomie. 1999;19:97–106. [Google Scholar]
- Canny MJ. A new theory for the ascent of sap-cohesion supported by tissue pressure. Ann Bot. 1995;75:343–357. [Google Scholar]
- Canny MJ. Vessel contents of leaves after excision: a test of Scholander's assumption. Am J Bot. 1997a;84:1217–1222. [PubMed] [Google Scholar]
- Canny MJ. Vessel contents during transpiration: embolisms and refilling. Am J Bot. 1997b;84:1223–1230. [PubMed] [Google Scholar]
- Canny MJ. Applications of the compensating pressure theory of water transport. Am J Bot. 1998a;85:897–909. [PubMed] [Google Scholar]
- Canny MJ. Transporting water in plants. Am Sci. 1998b;86:152–159. [Google Scholar]
- Cochard H, Cruiziat P, Tyree MT. Use of positive pressures to establish vulnerability curves: further support for the air-seeding hypothesis and implications for pressure-volume analysis. Plant Physiol. 1992;100:205–209. doi: 10.1104/pp.100.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Lemoine D, Dreyer E. The effects of acclimation to sunlight on the xylem vulnerability to embolism in Fagus sylvatica L. Plant Cell Environ. 1999;22:101–108. [Google Scholar]
- Comstock JP. Why Canny's theory doesn't hold water. Am J Bot. 1999;86:1077–1081. [PubMed] [Google Scholar]
- Dixon HH, Joly J. On the ascent of sap. Ann Bot. 1894;8:468–470. [Google Scholar]
- Ewers FW, Fisher JB. Techniques for measuring vessel lengths and diameters in stems of woody plants. Am J Bot. 1989;76:645–656. [Google Scholar]
- Holbrook NM, Zwieniecki MA. Embolism repair and xylem tension: do we need a miracle? Plant Physiol. 1999;120:7–10. doi: 10.1104/pp.120.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lybeck BR. Winter freezing in relation to the rise of sap in tall trees. Plant Physiol. 1959;34:482–486. doi: 10.1104/pp.34.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully ME. Root xylem embolisms and refilling: relation to water potentials of soil, roots and leaves, and osmotic potentials of root xylem sap. Plant Physiol. 1999;119:1001–1008. doi: 10.1104/pp.119.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully ME, Huang CX, Ling LEC. Daily embolism and refilling of xylem vessels in the roots of field-grown maize. New Phytol. 1998;138:327–342. doi: 10.1046/j.1469-8137.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- Pate JS, Canny MJ. Quantification of vessel embolisms by direct observation: a comparison of two methods. New Phytol. 1999;141:33–43. [Google Scholar]
- Pockman WT, Sperry JS, Oleary JW. Sustained and significant negative water pressure in xylem. Nature. 1995;378:715–716. [Google Scholar]
- Salleo S, Lo Gullo MA, Depaoli D, Zippo M. Xylem recovery from cavitation-induced embolism in young plants of Laurus nobilis: a possible mechanism. New Phytol. 1996;132:47–56. doi: 10.1111/j.1469-8137.1996.tb04507.x. [DOI] [PubMed] [Google Scholar]
- Scholander PF, Hammel HT, Hemmingsen EA, Bradstreet ED. Hydrostatic pressure and osmotic potential in leaves of mangroves and some other plants. Proc Natl Acad Sci USA. 1964;52:119–125. doi: 10.1073/pnas.52.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane MW, McCully ME. Root xylem embolisms: implications for water flow to the shoot in single-rooted maize plants. Aust J Plant Physiol. 1999;26:107–114. [Google Scholar]
- Sperry JS, Donnelly JR, Tyree MT. A method for measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ. 1988;11:35–40. [Google Scholar]
- Stiller V, Sperry JS. Canny's compensating pressure theory fails a test. Am J Bot. 1999;86:1082–1086. [PubMed] [Google Scholar]
- Turner NC, Long MJ. Errors arising from rapid water loss measurement of leaf water potential by the pressure chamber technique. Aust J Plant Physiol. 1980;7:527–37. [Google Scholar]
- Tyree MT. The cohesion-tension theory of sap ascent: current controversies. J Exp Bot. 1997;48:1753–1765. [Google Scholar]
- Tyree MT, Cochard H, Cruiziat P, Sinclair B, Ameglio T. Drought-induced leaf shedding in walnut: evidence for vulnerability segmentation. Plant Cell Environ. 1993;16:879–882. [Google Scholar]
- Tyree MT, Salleo S, Nardini A, Lo gullo MA, Mosca R. Refilling of embolized vessels in young stems of laurel: Do we need a new paradigm? Plant Physiol. 1999;120:11–21. doi: 10.1104/pp.120.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Sperry JS. Characterization and propagation of acoustic emission signals in woody plants: towards an improved acoustic emission counter. Plant Cell Environ. 1989;12:371–382. [Google Scholar]
- Tyree MT, Yang S. Hydraulic conductivity recovery versus water pressure in xylem of Acer saccharum. Plant Physiol. 1992;100:669–676. doi: 10.1104/pp.100.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi Y, Sano Y, Fujikawa S, Funada R, Ohtani J. Visualization of cavitated vessels in winter and refilled vessels in spring in diffuse-porous trees by cryo-scanning electron microscopy. Plant Physiol. 1998;117:1463–1471. doi: 10.1104/pp.117.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi Y, Sano Y, Funada R, Fujikawa S, Ohtani J. The progression of cavitation in earlywood vessels of Fraxinus mandshurica var. japonica during freezing and thawing. Plant Physiol. 1999;121:897–904. doi: 10.1104/pp.121.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi Y, Sano Y, Ohtani J, Fujikawa S. Seasonal changes in the distribution of water in the outer growth ring of Fraxinus mandshurica var. japonica: a study of cryo-scanning electron microscopy. IAWA J. 1996;17:113–124. [Google Scholar]
- Wei C, Steudle E, Tyree MT. Water ascent in plants: do ongoing controversies have a sound basis? Trends Plant Sci. 1999a;4:372–375. doi: 10.1016/s1360-1385(99)01466-1. [DOI] [PubMed] [Google Scholar]
- Wei C, Tyree MT, Steudle E. Direct measurement of xylem pressure in leaves of intact maize plants: a test of the cohesion-tension theory taking hydraulic architecture into consideration. Plant Physiol. 1999b;121:1191–1205. doi: 10.1104/pp.121.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U, Meinzer FC, Benkert R, Zhu JJ, Schneider H, Goldstein G, Kuchenbrod E, Haase A. Xylem water transport: is the available evidence consistent with the cohesion theory? Plant Cell Environ. 1994;17:1169–1181. [Google Scholar]
- Zwieniecki MA, Holbrook NM. Diurnal variation in xylem hydraulic conductivity in white ash (Fraxinus americana L.), red maple (Acer rubrum L.) and red spruce (Picea rubens Sarg.) Plant Cell Environ. 1998;21:1173–1180. [Google Scholar]