Abstract
Doxorubicin (also called Adriamycin) is effective in treating a wide range of human cancers and currently considered as one of the most important drugs in cancer chemotherapeutics. The clinical use of doxorubicin is, however, associated with dosage-dependent cardiotoxicity and development of heart failure, which diminish the therapeutic index of this widely used anticancer drug. This article first surveys key research findings on doxorubicin redox biology that may impact its cardiotoxicity as well as anticancer activity. It then discusses emerging concepts, especially the topoisomerase IIb–p53–mitochondrion axis that may lead to the development of mechanistically based novel strategies to protect against cardiotoxicity and enhance the effectiveness of doxorubicin therapy.
Keywords: Anticancer drug, Doxorubicin, Mitochondrial electron transport chain, Oxidative stress, Reactive oxygen species, Redox biology, Redox cycling, Topoisomerase IIb
1. OVERVIEW
Doxorubicin, a member of the anthracycline anticancer agents, is among the most commonly used drugs for treating a wide range of human cancers, including leukemias, Wilms tumor, neuroblastoma, soft tissue and bone sarcomas, breast carcinoma, ovarian carcinoma, transitional cell bladder carcinoma, thyroid carcinoma, gastric carcinoma, Hodgkin’s disease, malignant lymphoma, and lung cancer. Doxorubicin was initially isolated in 1967 in the Farmitalia Research Laboratories in Italy from cultures of a mutant Streptomyces peucetius (Streptomyces peucetius caesius) [1]. The chemical structure of doxorubicin is very similar to that of daunomycin, the very first member of anthracyclines that causes serious cardiotoxicity. Preclinical and clinical studies of the anticancer efficacy of doxorubicin as well as its cardiotoxicity began in the late 1960s and early 1970s [1–5], and the drug was first approved in 1974 by the U.S. Food and Drug Administration (FDA) for clinical use in the United States.
Although doxorubicin has been used clinically for treating a wide variety of human cancers for over 4 decades, its mechanisms of action underlying cancer cell killing and induction of cardiotoxicity and heart failure, a major limiting factor of the drug’s clinical use, remain to be further elucidated. In this context, a number of theories have been proposed to explain doxorubicin’s tumor killing activity and cardiotoxicity, which include redox cycling in mitochondria, topoisomerase inhibition, and oxidative stress. This article surveys key findings on doxorubicin redox biology and discusses emerging concepts that may lead to a better understanding of the molecular pathways underlying its cancer chemotherapeutic activity and cardiotoxicity, and thereby, the development of effective strategies to improve the therapeutic index of this widely used anticancer drug.
2. OXIDATIVE STRESS
2.1. The Concept of Oxidative Stress
Utilization of molecular oxygen by aerobic organisms leads to inevitable formation of reactive oxygen species (ROS), that, when left unchecked, causes detrimental effects on normal cellular constituents, including nucleic acids, proteins, and lipids. Antioxidant defenses have been evolved in aerobic organisms including humans to counteract the harmful effects of ROS and maintain redox homeostasis. Hence, under physiological conditions, cellular levels of ROS are tightly controlled and regulated. The term oxidative stress refers to a condition where the formation of ROS significantly overwhelms the detoxification capacity of the antioxidant system, causing ROS accumulation, leading to potentially detrimental consequences. Oxidative stress condition can be caused by either increased ROS formation or decreased activity of antioxidants or both in aerobic organisms. Moderate oxidative stress causes cell dysfunction, whereas overt oxidative stress usually leads to cell death [6]. Oxidative stress contributes to diverse pathophysiological processes, including many aging-related human disorders, as well as drug-mediated pharmacological effects and adverse reactions [7, 8], though tightly regulated production of ROS is a fundamental mechanism of innate immunity and physiological homeostasis [9–11].
2.2. Doxorubicin-Mediated Oxidative Stress
Oxidative stress is a widely recognized mechanism of doxorubicin-induced cardiotoxicity. Development of this oxidative stress concept is driven primarily by two lines of evidence. One is the increased levels of ROS following treatment with doxorubicin in cellular and animal models. Biomarkers of oxidative stress, such as isoprostanes, are also elevated in human cancer patients treated with doxorubicin. The other line of evidence is the demonstrated protection of doxorubicin cardiotoxicity in animal models by either transgenic overexpression of antioxidant enzymes or administration of antioxidant compounds of diverse structures. Doxorubicin may also cause downregulation of antioxidant enzymes particularly at high dosages and upon prolonged exposure although the exact underlying mechanisms remain largely unknown (see Section 4.3).
Increased ROS levels after doxorubicin treatment in both cellular and animal models as well as cancer patients may result from different mechanisms. As noted above, decreased expression of cellular antioxidant defenses may be one of them. Another mechanism may involve the activation of ROS-generating enzymes/proteins, including NOX2 and Rac1 [12, 13]. The third mechanism may be related to disruption of the mitochondrial electron transport chain (METC) by doxorubicin either directly or indirectly via compromising mitochondrial genome, to cause increased electron leakage from the METC and thereby elevated formation of mitochondrial ROS. The last and probable the most widely recognized mechanism is the potential of doxorubicin to undergo redox cycling to produce ROS (see Section 3.1).
2.3. Redox Modulation of Nrf2 Signaling by Doxorubicin
Short term exposure to doxorubicin may cause activation of Nrf2 (nuclear factor E2-related factor 2), leading to transient increases in antioxidant gene expression [14]. This indicates that doxorubicin may act as a pro-oxidative stimulus to transiently activate Nrf2 signaling. In this context, doxorubicin is suggested as a redox active compound that may undergo redox cycling to generate oxidant species. However, recent evidence does not support a redox cycling property for doxorubicin at pharmacologically relevant concentrations (see Section 3.3). Alternatively, doxorubicin may directly bind to the Nrf2 sequestering protein, Keap1, causing its degradation. Regardless of the molecular mechanisms involved, stimulation of Nrf2 signaling by doxorubicin is not unexpected as activation of the Nrf2 pathway is a common adaptive response to oxidants as well as many other stress factors [15–17].
3. REDOX CYCLING
3.1. The Concept of Chemical Redox Cycling
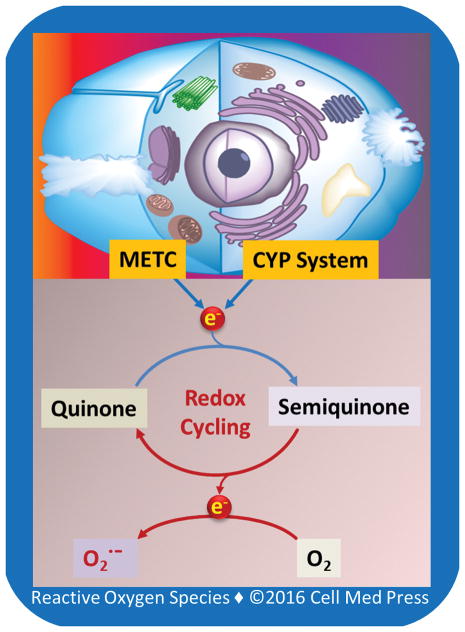
Chemical redox cycling may be defined literally as the reduction and oxidation cycle between two forms of a compound, frequently a quinone molecule [18] (Figure 1). Redox cycling is typically initiated by a univalent reduction of a quinone molecule to form a semiquinone radical species. The cytochrome P450 enzyme system and METC are among the major sources that carry out the one-electron reduction. The semiquinone radical formed can then donate one electron to molecular oxygen, and during this reaction, molecular oxygen is reduced to superoxide anion radical, and the semiquinone radical is oxidized back to the original quinone molecule. Hence, the entire process involving one-electron reduction and oxidation forms a cycle, leading to the persistent production of superoxide anion radical and secondary ROS (e.g., hydrogen peroxide, hydroxyl radical, and peroxynitrite) [18].
FIGURE 1. Schematic illustration of the general concept of quinone redox cycling.
As depicted, the cytochrome P450 (CYP) system and the mitochondrial electron transport chain (METC) are the two major machineries that carry out the initial one-electron reduction of quinone compounds, leading to redox cycling and generation of superoxide anion radical (O2•−). O2• − undergoes dismutation to form hydrogen peroxide either spontaneously or catalyzed by superoxide dismutase (SOD). Hydrogen peroxide then reacts with transition metal ion (e.g., Fe2+, Cu1+), giving rise to hydroxyl radical (not shown).
3.2. Doxorubicin Redox Cycling: the Birth of the Concept
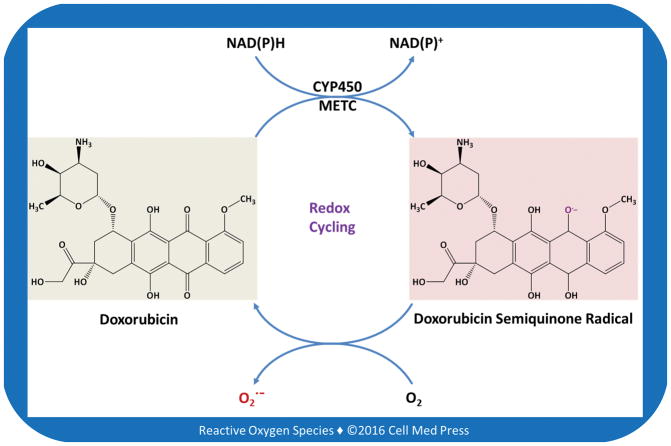
Doxorubicin is a quinone compound, and as such, has been proposed to undergo redox cycling in biological systems [19, 20] (Figure 2). In this regard, both the cytochrome P450 enzyme system and the METC enzyme complexes, especially complex I, have been implicated in mediating the redox cycling of doxorubicin to form ROS. The redox cycling concept of doxorubicin stems from early observations with doxorubicin at non-pharmacologically relevant concentrations in cell-free systems (e.g., submitochondrial particles) [21, 22]. Because of the richness of mitochondria in myocardium in terms of both function and mass of the organelles, METC-mediated redox cycling of doxorubicin has been considered as a major mechanism of doxorubicin-induced cardiotoxicity. Redox cycling of doxorubicin has also been suggested as a mechanism contributing to its cancer cell killing activity. However, as discussed next, there is no direct evidence for doxorubicin to undergo redox cycling to generate ROS at pharmacologically relevant concentrations in target cells and tissues.
FIGURE 2. Schematic illustration of the potential of doxorubicin to undergo redox cycling catalyzed by the cytochrome P450 system (CYP450) and mitochondrial electron transport chain (METC).
As illustrated, doxorubicin contains a p-benzoquinone structure. p-Benzoquinone is actually a weak, if not a non-, redox cycling compound.
3.3. Doxorubicin Redox Cycling: Lack of Evidence under Physiologically Relevant Conditions
With highly sensitive oxygen polarography to measure potassium cyanide-resistant oxygen consumption and using ultra-sensitive bioluminometry to detect ROS formation, we have recently studied the redox cycling properties of doxorubicin at pharmacologically relevant concentrations (0.1–1.0 μM) and a supra-pharmacological concentration (10 μM) in various types of cells, including animal and human primary cardiomyocytes as well as lung cancer cells, neuroblastoma cells, and leukemia cells. We have found no evidence for doxorubicin at the above concentrations to undergo redox cycling to generate ROS. Doxorubicin does not undergo redox cycling either in substrate-driven isolated intact mitochondria under the above conditions. In contrast, with the above methods and cell systems, we have observed dramatic redox cycling of other quinone compounds at concentrations 10–100 times lower (0.01–1.0 μM) than those of doxorubicin. These include beta-lapachone, benzo(a)pyrene-derived quinones, and 9,10-phenathrenequinone. Notably, 9,10-phenanthre-nequinone undergoes redox cycling at concentrations as low as 0.3 nM, and it is the most potent redox cycling compound ever seen with biological systems.
4. TOPOISOMERASE INHIBITION
4.1. Molecular Biology of DNA Topoisomerases
DNA topoisomerases are ubiquitous and essential enzymes that play critical roles in the fundamental biological processes of DNA replication, transcription, recombination, repair, and chromatin remodeling. They represent a class of enzymes that reduce supercoiling in DNA by breaking and rejoining one or both strands of the DNA molecule. The winding problem of DNA arises due to the intertwined nature of its double-helical structure. During DNA replication and transcription, DNA double-helical structure becomes overwound ahead of a replication fork. If left unabated this tension would build up to a level that would eventually stop the action of the enzymes involved in DNA replication and transcription [23–25]. DNA topoisomerases bind to either single-stranded or double-stranded DNA and cut the phosphate backbone of the DNA. This intermediate break allows the DNA to be untangled or unwound, and, at the end of these processes, the DNA backbone is resealed again. DNA topoisomerases are considered as nature’s molecular tools to resolve the problems of DNA entanglements by enabling topological transformations, thereby regulating the structures of DNA/chromosomes and their associated cellular functions [23–25].
Based on structures and mechanisms, DNA topoisomerases are grouped into type I and II, each of which is further divided into subfamilies A and B. Type I enzymes carry out strand passage through a reversible single-strand break, whereas type II enzymes mediate strand transport through a double-strand DNA gate [23–25]. Table 1 lists the 6 members of mammalian DNA topoisomerases with each encoded by a single gene. Notably, 3 DNA topoisomerases have been found in mammalian mitochondria, including the mitochondrial topoisomerase I, topoisomerase IIIa, and topoisomerase IIb. Topoisomerase IIIa and topoisomerase IIβ have a dual localization and are targeted to both nuclear and mitochondrial compartments. In contrast, mitochondrial topoisomerase I is encoded by a specific nuclear gene and only functions in the mitochondrial compartment.
TABLE 1.
Mammalian DNA topoisomerases
| Type | Member | Location |
|---|---|---|
| IA | Topoisomerase IIIα Topoisomerase IIIβ |
Nucleus and mitochondrion Nucleus |
| IB | Topoisomerase I Mitochondrial topoisomerase I |
Nucleus Mitochondrion |
| IIA | Topoisomerase IIα Topoisomerase IIβ |
Nucleus Nucleus and mitochondrion |
| IIB | None found in mammals | Not applicable |
4.2. Topoisomerase Inhibition in Doxorubicin-Induced Anticancer Activity
Doxorubicin forms complexes with DNA by intercalation between base pairs, and it inhibits DNA topoisomerase II activity by stabilizing the DNA–topoisomerase II complex, preventing the religation portion of the ligation-religation reaction that DNA topoisomerase II catalyzes. The outcomes of the above interactions include DNA strand breaks and inhibition of DNA replication. As cancer cells are rapidly proliferative, DNA topoisomerase inhibition is widely considered as a central mechanism underlying doxorubicin’s cancer cell killing activity. On the other hand, cardiomyocytes are generally quiescent, and as such, DNA topoisomerase inhibition is typically thought to play a minor, if any, role in doxorubicin-induced cardiotoxicity.
4.3. Topoisomerase Inhibition in Doxorubicin-Induced Cardiotoxicity
4.3.1. Topoisomerase IIb as a Novel Molecular Target of Doxorubicin-Induced Cardiotoxicity
In mammalian species, including humans, topoisomerase IIα is found predominantly in proliferating cells and required for DNA replication. As such, it is considered the major molecular target underlying doxorubicin’s tumoricidal activity. On the other hand, topoisomerase IIβ is present in all quiescent cells, including cardiomyocytes, and is recently found to be a major molecular target of doxorubicin cardiotoxicity [26]. Cardiomyocyte-specific deletion of Top2b gene encoding topoisomerase IIβ protects the myocytes from doxorubicin-induced DNA double-strand breaks and transcriptome changes that are responsible for defective mitochondrial biogenesis and increased ROS accumulation [27]. Furthermore, cardiomyocyte-specific deletion of Top2b gene protects mice from the development of doxorubicin-induced progressive heart failure, suggesting that doxorubicin-induced cardiotoxicity is mediated by topoisomerase IIβ in cardiomyocytes [27].
4.3.2. The Role of Topoisomerase IIb–p53–Mitochondria Axis in Doxorubicin-Induced Cardiotoxicity
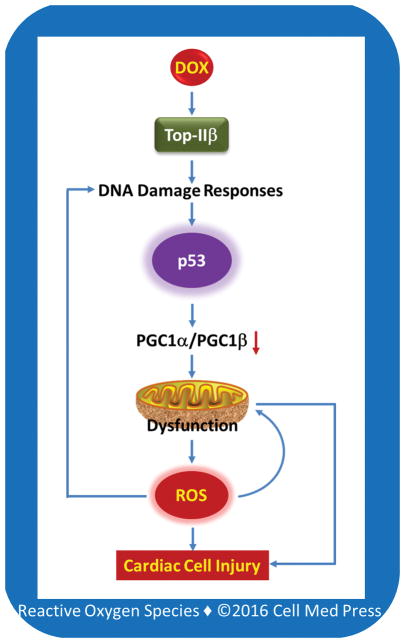
It is suggested that inhibition of topoisomerase IIβ by doxorubicin may trigger stress responses and alter transcriptome, eventually leading to mitochondrial dysfunction and oxidative stress [26–28]. However, the detailed mechanisms by which doxorubicin-mediated inhibition of topoisomerase IIβ causes oxidative stress remain to be elucidated. Since topoisomerase IIβ is present in mitochondria, doxorubicin may compromise the integrity of the mitochondrial genome by inhibiting this enzyme, leading to mitochondrial dysfunction and increased electron leakage and superoxide generation from the METC. However, cardiomyocyte-specific deletion of Top2b gene does not appear to affect mitochondrial function [27], suggesting that a different mechanism might be involved. In this regard, it is proposed that inhibition or poisoning of topoisomerase IIβ by doxorubicin may cause p53 activation via DNA damage responses, and the p53 activation then leads to compromised mitochondrial function through the repression of peroxisome proliferator-activated receptor gamma (PPARγ) co-activator 1α (PGC1α) and PGC1β. Both PGC1α and PGC1β promote mitochondrial biogenesis. This p53-mediated mitochondrial dysfunction leads to increased electron leakage and superoxide formation from the METC, which in turn triggers a cycle of DNA damage, resulting in further p53 activation and mitochondrial dysfunction [29] (Figure 3). Inhibition of p53 protects against doxorubicin cardiotoxicity in mice [30], supporting an important role for p53 signaling in topoisomerase IIb-dependent doxorubicin cardiotoxicity. Regardless of the mechanisms involved, it appears that doxorubicin treatment results in increased formation of mitochondrial superoxide, which causes cardiotoxicity either directly or via the formation of secondary ROS. This notion is in line with the observations that transgenic overexpression of mitochondrial manganese superoxide dismutase (MnSOD) protects against doxorubicin’s cardiotoxicity in mice [31] and that deletion of MnSOD causes dilated cardiomyopathy in mice [32], a characteristic feature of doxorubicin-induced cardiotoxicity [33].
FIGURE 3. Schematic illustration of the potential role of the topoisomerase IIb–p53–mitochondrion axis in doxorubicin cardiotoxicity.
DOX, doxorubicin; Top-IIb, topoisomerase IIb; PGC1α, peroxisome proliferator-activated receptor gamma co-activator 1α; PGC1β, peroxisome proliferator-activated receptor gamma co-activator 1b; ROS, reactive oxygen species. This scheme is based on Ref. [28].
4.3.3. Topoisomerase IIb: Doxorubicin-Mediated Inhibition versus Genetic Deletion
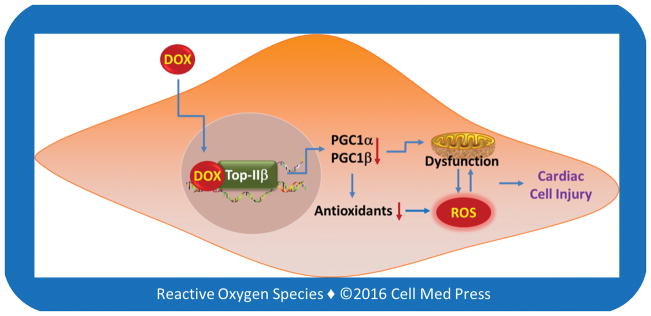
Cardiomyocyte-selective deletion of the gene encoding topoisomerase IIb does not cause mitochondrial dysfunction and cardiomyopathy in mice and, instead, such a genetic deletion renders the mice resistance to doxorubicin-induced cardiotoxicity [27]. On the other hand, inhibition of topoisomerase IIb is considered to be responsible for doxorubicin-induced cardiotoxicity [27]. This seemingly paradoxical notion suggests that interaction of doxorubicin with topoisomerase IIb in cardiomyocytes may alter the enzyme in such a way that converts it to an inciting factor for cardiomyocyte injury. It is suggested that doxorubicin-topoisomerase IIb complex may bind to the promoter region of the genes for PGC1α and PGC1β, leading to the inhibition of their transcription [27]. PGC1α and PGC1β are important players in mitochondrial biogenesis and energetics as well as cellular antioxidant defenses [34], and inhibition of PGC1α and PGC1β signaling causes aging and heart failure [35, 36]. Hence, it is possible that topoisomerase IIb serves as a mediator, rather than an ultimate target, of doxorubicin-induced cardiotoxicity (Figure 4). If this is true, as discussed below, strategies that inhibit doxorubicin’s binding to topoisomerase IIb would be effective in protecting against doxorubicin-induced cardiotoxicity and improving its therapeutic index.
FIGURE 4. Schematic illustration of the potential involvement of the doxorubicin-topoisomerase IIb complex in transcriptional repression of PGC1α and PGC1β, leading to mitochondrial dysfunction, decreased antioxidant expression, and oxidative stress, and ultimately cardiac cell injury.
DOX, doxorubicin; Top-IIb, topoisomerase IIb; PGC1α, peroxisome proliferator-activated receptor gamma co-activator 1α; PGC1β, peroxisome proliferator-activated receptor gamma co-activator 1b; ROS, reactive oxygen species. This scheme is based on Ref. [27].
5. MOLECULAR TARGET-DRIVEN STRATEGIES FOR IMPROVING DOXORUBICIN’S THERAPEUTIC INDEX
Different mechanisms appear to underlie doxorubicin-mediated tumor cell killing and cardiotoxicity, with the former primarily involving topoisomerase IIa inhibition to lead to blockage of DNA replication, and the latter primarily involving interaction with topoisomerase IIb to cause mitochondrial dysfunction and increased ROS formation. The primary involvement of oxidative stress in doxorubicin-mediated cardiotoxicity, but not in its tumor cell killing activity, explains why antioxidant compounds protect against doxorubicin’s cardiotoxicity without interfering with its anticancer activity in various animal models. In animal models, antioxidants of diverse structures also potentiate doxorubicin-induced tumor cell killing. However, so far no antioxidants have been shown to protect against doxorubicin’s cardiotoxicity or augment its anticancer activity in human cancer patients. Currently, dexrazoxane, an iron-chelating compound, is the only agent that shows a limited protection against doxorubicin’s cardiotoxicity in cancer patients, and the cardioprotection likely stems from its ability to compete with doxorubicin for binding to topoisomerase IIb rather than the proposed iron-chelating property or antioxidative activity of the compound [26].
The lack of effective drugs for protecting against doxorubicin’s cardiotoxicity and improving its therapeutic index warrants more mechanistic research. Such research is to identify novel molecular targets involved in, and elucidate detailed molecular pathways leading to, doxorubicin-induced cardiotoxicity and progressive heart failure. On the other hand, future research is also needed to develop innovative antioxidant-based modalities to counteract the oxidative stress mechanism of doxorubicin-induced cardiotoxicity. In this context, failure with existing antioxidant compounds in protecting against doxorubicin’s cardiotoxicity does not necessarily invalidate the oxidative stress theory of doxorubicin-induced cardiotoxicity. This is analogous to the notion that failure of certain antibiotics in treating pneumococcal pneumonia does not disapprove the bacterial cause of pneumococcal pneumonia. The lack of efficacy in patients of using existing antioxidant compounds in protecting against doxorubicin cardiotoxicity and in treating oxidative stress-associated disorders as a whole reveals the need to revisit the current thinking on oxidative stress and exogenous antioxidant-based therapy, and to develop innovative concepts to guide effective antioxidant intervention. In this regard, future research may focus on developing antioxidant modalities for targeting specific intracellular compartments of oxidative stress and/or for boosting the endogenous antioxidant defenses in the cellular compartments that are adversely impacted by ROS [37]. Alternatively, efforts may be taken to identify novel agents that enhance doxorubicin-induced tumoricidal activity so that the drug can be used at lower dosages to spare its cardiotoxicity.
Acknowledgments
This work was supported in part by an IIG grant (09A084) from the American Institute for Cancer Research, and a grant (CA192936) from the U.S. National Institutes of Health/National Cancer Institute.
ABBREVIATIONS
- METC
mitochondrial electron transport chain
- MnSOD
manganese superoxide dismutase
- Nrf2
nuclear factor E2-related factor 2
- PGC1α
peroxisome proliferator-activated receptor gamma co-activator 1α
- PGC1β
peroxisome proliferator-activated receptor gamma co-activator 1b
- ROS
reactive oxygen species
References
- 1.Bonadonna G, Monfardini S, De Lena M, Fossati-Bellani F. Clinical evaluation of adriamycin, a new antitumour antibiotic. Br Med J. 1969;3(5669):503–6. doi: 10.1136/bmj.3.5669.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Editorial: Adriamycin and the heart. Lancet. 1974;1(7870):1325. [PubMed] [Google Scholar]
- 3.Smith IE, McElwain TJ. Letter: Adriamycin in acute leukaemia. Lancet. 1974;2(7873):161. doi: 10.1016/s0140-6736(74)91589-x. [DOI] [PubMed] [Google Scholar]
- 4.Cores EP, Holland JF, Wang JJ, Sinks LF. Doxorubicin in disseminated osteosarcoma. JAMA. 1972;221(10):1132–8. doi: 10.1001/jama.221.10.1132. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb JA, Hill CS., Jr Chemotherapy of thyroid cancer with adriamycin. Experience with 30 patients. N Engl J Med. 1974;290(4):193–7. doi: 10.1056/NEJM197401242900404. [DOI] [PubMed] [Google Scholar]
- 6.Li YR, Jia Z, Trush MA. Defining ROS in biology and medicine. Reactive Oxygen Species. 2016;1(1):9–21. doi: 10.20455/ros.2016.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 8.Kasiappan R, Safe SH. ROS-inducing agents for cancer chemotherapy. Reactive Oxygen Species. 2016;1(1):22–37. doi: 10.20455/ros.2016.805. [DOI] [Google Scholar]
- 9.Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol. 2013;13(5):349–61. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312(5782):1882–3. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 11.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475(7354):106–9. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, McLaughlin D, Robinson E, Harvey AP, Hookham MB, Shah AM, et al. Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with doxorubicin chemotherapy. Cancer Res. 2010;70(22):9287–97. doi: 10.1158/0008-5472.CAN-10-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma J, Wang Y, Zheng D, Wei M, Xu H, Peng T. Rac1 signalling mediates doxorubicin-induced cardiotoxicity through both reactive oxygen species-dependent and -independent pathways. Cardiovasc Res. 2013;97(1):77–87. doi: 10.1093/cvr/cvs309. [DOI] [PubMed] [Google Scholar]
- 14.Nordgren KK, Wallace KB. Keap1 redox-dependent regulation of doxorubicin-induced oxidative stress response in cardiac myoblasts. Toxicol Appl Pharmacol. 2014;274(1):107–16. doi: 10.1016/j.taap.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Kim SB, Pandita RK, Eskiocak U, Ly P, Kaisani A, Kumar R, et al. Targeting of Nrf2 induces DNA damage signaling and protects colonic epithelial cells from ionizing radiation. Proc Natl Acad Sci USA. 2012;109(43):E2949–55. doi: 10.1073/pnas.1207718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keleku-Lukwete N, Suzuki M, Otsuki A, Tsuchida K, Katayama S, Hayashi M, et al. Amelioration of inflammation and tissue damage in sickle cell model mice by Nrf2 activation. Proc Natl Acad Sci USA. 2015 doi: 10.1073/pnas.1509158112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopkins RZ. Nrf2 silencing for neuron maturation. Reactive Oxygen Species. 2016;1(1):53–58. doi: 10.20455/ros.2016.809. [DOI] [Google Scholar]
- 18.Hopkins RZ. Superoxide in biology and medicine: an overview. Reactive Oxygen Species. 2016;1(2):99–109. doi: 10.20455/ros.2016.825. [DOI] [Google Scholar]
- 19.Trush MA, Mimnaugh EG, Gram TE. Activation of pharmacologic agents to radical intermediates: implications for the role of free radicals in drug action and toxicity. Biochem Pharmacol. 1982;31(21):3335–46. doi: 10.1016/0006-2952(82)90609-8. [DOI] [PubMed] [Google Scholar]
- 20.Mimnaugh EG, Trush MA, Ginsburg E, Gram TE. Differential effects of anthracycline drugs on rat heart and liver microsomal reduced nicotinamide adenine dinucleotide phosphate-dependent lipid peroxidation. Cancer Res. 1982;42(9):3574–82. [PubMed] [Google Scholar]
- 21.Davies KJ, Doroshow JH. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem. 1986;261(7):3060–7. [PubMed] [Google Scholar]
- 22.Doroshow JH, Davies KJ. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem. 1986;261(7):3068–74. [PubMed] [Google Scholar]
- 23.Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol. 2002;3(6):430–40. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- 24.Koster DA, Crut A, Shuman S, Bjornsti MA, Dekker NH. Cellular strategies for regulating DNA supercoiling: a single-molecule perspective. Cell. 2010;142(4):519–30. doi: 10.1016/j.cell.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen SH, Chan NL, Hsieh TS. New mechanistic and functional insights into DNA topoisomerases. Annu Rev Biochem. 2013;82:139–70. doi: 10.1146/annurev-biochem-061809-100002. [DOI] [PubMed] [Google Scholar]
- 26.Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014;64(9):938–45. doi: 10.1016/j.jacc.2014.06.1167. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639–42. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 28.Vejpongsa P, Yeh ET. Topoisomerase 2b: a promising molecular target for primary prevention of anthracycline-induced cardiotoxicity. Clin Pharmacol Ther. 2014;95(1):45–52. doi: 10.1038/clpt.2013.201. [DOI] [PubMed] [Google Scholar]
- 29.Sahin E, DePinho RA. Axis of ageing: telomeres, p53 and mitochondria. Nat Rev Mol Cell Biol. 2012;13(6):397–404. doi: 10.1038/nrm3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu W, Soonpaa MH, Chen H, Shen W, Payne RM, Liechty EA, et al. Acute doxorubicin cardiotoxicity is associated with p53-induced inhibition of the mammalian target of rapamycin pathway. Circulation. 2009;119(1):99–106. doi: 10.1161/CIRCULATIONAHA.108.799700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yen HC, Oberley TD, Vichitbandha S, Ho YS, St Clair DK. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest. 1996;98(5):1253–60. doi: 10.1172/JCI118909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11(4):376–81. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 33.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339(13):900–5. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 34.Finkel T. Cell biology: a clean energy programme. Nature. 2006;444(7116):151–2. doi: 10.1038/444151a. [DOI] [PubMed] [Google Scholar]
- 35.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci USA. 2006;103(26):10086–91. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finck BN, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation. 2007;115(19):2540–8. doi: 10.1161/CIRCULATIONAHA.107.670588. [DOI] [PubMed] [Google Scholar]
- 37.Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest. 2014;124(2):617–30. doi: 10.1172/JCI72931. [DOI] [PMC free article] [PubMed] [Google Scholar]