Abstract
Although many volatile organic compounds (VOCs) are regulated to limit air pollution and the consequent health effects, the photooxidation products generally are not. Thus, we examined the mutagenicity in Salmonella TA100 of photochemical atmospheres generated in a steady-state atmospheric simulation chamber by irradiating mixtures of single aromatic VOCs, NOx, and ammonium sulfate seed aerosol in air. The 10 VOCs examined were benzene; toluene; ethylbenzene; o-, m-, and p-xylene; 1,2,4- and 1,3,5-trimethylbenzene; m-cresol; and naphthalene. Salmonella were exposed at the air-agar interface to the generated atmospheres for 1, 2, 4, 8, or 16 h. Dark-control exposures produced non-mutagenic atmospheres, illustrating that the gas-phase precursor VOCs were not mutagenic at the concentrations tested. Under irradiation, all but m-cresol and naphthalene produced mutagenic atmospheres, with potencies ranging from 2.0 (p-xylene) to 10.4 (ethylbenzene) revertants m3 mgC−1 h−1. The mutagenicity was due exclusively to direct-acting late-generation products of the photooxidation reactions. Gas-phase chemical analysis showed that a number of oxidized organic chemical species enhanced during the irradiated exposure experiments correlated (r ≥ 0.81) with the mutagenic potencies of the atmospheres. Molecular formulas assigned to these species indicated that they likely contained peroxy acid, aldehyde, alcohol, and other functionalities.
Keywords: Mutagenicity, Salmonella TA100, Photooxidation products, Aromatic hydrocarbons, Air quality
Graphical abstract
1. Introduction
With the steady growth in global population and the associated rise in fossil fuel consumption and biomass burning, anthropogenic emissions of NOx (NO + NO2) and hydrocarbons (referred to synonymously as volatile organic compounds/carbon or VOCs) have increased over much of the past century. However, in recent decades, concentrations of VOCs and other criteria pollutants, such as ozone, NOx, SO2, and particulate matter (PM) have generally decreased in most developed countries due to technological developments and regulation (IARC, 2016; Parrish and Stockwell, 2015). Likewise, even in rapidly developing countries such as China and India, concentrations of these criteria pollutants have generally stabilized or declined during the past decade, although episodes of extremely poor regional air quality are still frequent and receive global attention (IARC, 2016).
Fossil fuel processing and combustion represent the single largest anthropogenic source of these compounds, and the majority of these sources tend to co-emit both NOx and VOCs (Jaegle et al., 2005; Piccot et al., 1992). The cycling of NOx between NO and NO2 and the oxidation of VOCs govern tropospheric ozone (O3) production and have important impacts on local air quality (Thornton et al., 2002). Aromatic VOCs represent one specific class of VOCs that are emitted almost exclusively from anthropogenic sources (Simon et al., 2010). The atmospheric photooxidation of aromatic VOCs has been shown to produce compounds that contribute to secondary organic aerosol (SOA) by partitioning to the particle phase or through nucleation to form new particles (de Gouw et al., 2008; Kleindienst et al., 1999; Ng et al., 2007). This results in potential visibility, climate, and public health effects by increasing the mass loadings of particulate matter <2.5 µm in diameter (PM2.5) (Odum et al., 1996).
The concentrations of chemical species that can lead to smog formation and air quality events are controlled by the United States Environmental Protection Agency (U.S. EPA) under the Clean Air Act (2015 U.S. Code, Title 42, Chapter 85). NO2 is regulated under the National Ambient Air Quality Standards (NAAQS) (U.S. EPA, 2015b), and many VOCs such as benzene, 1,3-butadiene, formaldehyde, and other higher molecular weight aldehydes are labeled as hazardous air pollutants due to adverse health effects including carcinogenic, respiratory, and neurological effects, among others (IARC, 2016; U.S. EPA, 2015a; Zhou et al., 2015).
Although oxidized VOCs can be components of primary emissions from a variety of sources, most result from secondary reactions of hydrocarbons emitted into the atmosphere, making them late-generation atmospheric reaction products. Assessments of health effects based solely on direct emissions are incomplete if potentially important contributions from such products are neglected, as has been noted by the Clean Air Act, which mandates consideration of atmospheric transformation products.
Previous studies have shown that the gas-phase of atmospheres produced from mixtures of ozone and VOCs (toluene, acetaldehyde, allyl chloride, or propylene) was a direct-acting mutagen in the Salmonella mutagenicity assay (Dumdei et al., 1988; Shepson et al., 1985a; Shepson et al., 1985b; Shepson et al., 1986; Shepson et al., 1987). Peroxyacyl nitrates (PAN) were especially mutagenic photochemical products (DeMarini et al., 2000; Kleindienst et al., 1990), which have highlighted the important contribution that atmospheric chemical transformation products can make to the mutagenicity of ambient air. However, beyond PAN, photochemical product information was largely lacking in these studies, and it was virtually impossible to identify which oxidation products were responsible for the biological responses. Gas-phase oxidation products of polycyclic aromatic hydrocarbons (PAHs) such as naphthalene have also exhibited mutagenicity using the same assay where exposures were performed after the gas-phase constituents were collected and extracted (Arey et al., 1992; Gupta et al., 1996; Sasaki et al., 1995). These effects were largely attributed to nitro-aromatic compounds. In general though, mutagenicity and health effect studies of the gas-phase are lacking in comparison to that of PM (IARC, 2016), and little work has been performed to incorporated these few studies into risk assessment. Thus, a better understanding of which photooxidation pathways account for the mutagenicity of the gas phase would help to improve assessments of the health effects of air pollution.
In the present work, we present a series of experiments that investigated the mutagenicity of atmospheres containing oxidation products from irradiations of aromatic hydrocarbon/NOx mixtures. These products were monitored using advanced instrumentation and methods (chemical ionization mass spectrometry) capable of directly detecting a wide-range of oxidized organic molecules without prior collection, extraction, or derivatization. Ten aromatic hydrocarbons or structurally related compounds were investigated: benzene; toluene; ethylbenzene; o-, m-, and p-xylene; m-cresol; naphthalene; and 1,2,4- and 1,3,5-trimethylbenzene. All but the last two are EPA-listed hazardous air pollutants. Benzene, toluene, ethylbenzene, and the xylenes (often referred to collectively as BTEX) are significant distillation products and commonly present in fuel mixtures. The same is true for 1,2,4-trimethylbenzene; 1,3,5-trimethylbenzene is emitted from fuel combustion processes. Other considerations for selecting these compounds included that m-cresol is an early generation product of a specific toluene photooxidation pathway. Naphthalene is the simplest PAH and emitted from a variety of industrial activities (Jia and Batterman, 2010). Given the sources of these compounds, they are common in urban air, particularly near high-traffic roads and/or industrial areas, often with mixing ratios exceeding one part per billion by volume (ppbv) in polluted air (Jobson et al., 2004).
The resulting atmospheres were directed onto Salmonella bacteria at the air-agar interface in separate in vitro exposure chambers to assess the mutagenic activity of the gas-phase photooxidation products. Correlations between the relative abundance of the detected molecular compositions, all of which contained one or more oxygen atoms, and the mutagenic responses provided initial insight into candidate species responsible or related to observed mutagenicity. Lastly, using the precursor VOC that produced the most mutagenic atmosphere, a set of experiments was also performed to examine the relationship between chamber residence time (reaction time) and mutagenicity.
This survey study ultimately examines whether this class of precursors compounds (aromatics) may lead to products with mutagenic effects after atmospheric processing in polluted airsheds. Through correlations, we identify possible late-generation molecular compositions and reaction pathways of interest that could ultimately have an influence on the observed mutagenic effects.
2. Experimental methods
2.1. Steady-state chamber experiments
All of the experiments described here were carried out in the U.S. EPA’s 14.5-m3 fixed-wall atmospheric simulation chamber operated in steady-state mode, which has been described in previous publications (Kleindienst et al., 2006). Two of the chamber walls were equipped with UV-fluorescent light banks that delivered 300 – 400 nm light with a distribution similar to that of tropospheric solar irradiation. For each experiment, the chamber was operated at an average temperature and relative humidity (RH) of 25 °C and 30%, respectively. A typical experiment cycle lasted ~7 days during which the chamber was initialized over days 1 and 2, steady-state measurements and cell exposures occurred during days 3 – 5, and additional measurements and chamber flushing were performed over days 6 and 7. To mitigate any potential losses of chemical species during sampling and exposure, all transfer lines were pre-conditioned to chamber atmospheres for at least 24 h prior to any reported measurements or exposures. The injection, exposure, and instrument sampling flows were such that the volumetric residence time within the chamber was ~4.5 h for each experiment. Residence time is equivalent to the reaction time or the amount of time required for the concentration of an unreactive chemical to decay by 1/e (often referred to as the lifetime).
To begin an experiment, chamber lights remained off with normal instrumental sampling, and the gas-phase precursor compounds (hydrocarbon and NO) were continuously injected into a glass inlet manifold heated to 50 °C where they were mixed and diluted with clean dry air (737 Series Zero Air Generator, Aadco) prior to injection into the chamber. Precursor VOCs were injected at mixing ratios varying from 0.71 – 12.66 parts per million carbon (ppmC) depending on the experiment. In total, 10 VOCs were investigated: benzene; toluene; ethylbenzene; o-, m-, and p-xylene; 1,2,4- and 1,3,5-trimethylbenzene; m-cresol; and naphthalene. All but m-cresol and naphthalene were delivered by bubbling 10 – 100 mL min−1 of low RH clean air through ~50 mL of the neat hydrocarbon in a 250-mL glass bulb. The glass bulb was kept in a temperature-controlled oil bath at 0 – 50 °C depending on the precursor VOC, to ensure a stable evaporation rate. m-Cresol was delivered to the manifold through a septum via a gas-tight syringe connected to a syringe pump, and naphthalene was delivered by passing clean air through the headspace of the glass bulb containing solid naphthalene. Hydrocarbon concentrations were monitored by a gas chromatograph with a flame ionization detector (GC-FID, Hewlett-Packard Model 5890 GC).
NO was delivered from a dilute standard cylinder to the inlet manifold via a mass flow controller such that chamber mixing ratios were ~200 ppbv as measured, along with NO2, by a chemiluminescent NOx analyzer (TECO Model 42C). Chamber O3 was measured with a chemiluminescent ozone monitor (Bendix Model 8002). Ammonium sulfate seed aerosol at 100% RH was injected through a separate chamber port by nebulizing a 10 mg L−1 solution of ammonium sulfate in water (TSI Atomizer Model 9302) such that the seed aerosol mass loading within the chamber was ~3 µg m−3. The inclusion of seed aerosol was necessary in order to provide a substrate for SOA formation and an inorganic component to the chamber aerosol. Also, without the seed, obtaining steady-state in both the gas and particle phases would be more difficult due to the potential for spontaneous SOA nucleation events. The chamber was humidified to 30% RH by vaporizing water into ~10 L min−1 of dry air using a dynamically controlled peristaltic pump. An additional flow (typically ~15 L min−1) was added to the chamber to balance the input and output flows and maintain the desired residence time. After the initialization period when flow inputs were stabilized and before chamber lights were turned on, initial concentrations of the precursor species (precursor VOC and NO) were determined.
Particle-phase organic carbon (OC) concentrations were measured using an automated, semi-continuous organic carbon-elemental carbon analyzer (Model-4 OC-EC, Sunset Laboratories Inc.). The OC-EC instrument was operated with a sampling flow rate of 8 L min−1 with a sample collection time of 30 min, an analysis time of 12.5 min, and a re-equilibration time of 2.5 min for a complete duty cycle of ~ 45 min.
When the loss rate of the various chemical species is equal to the formation rate, then the concentration of said species will remain at a constant value. This condition is referred to as steady-state. After initial measurements were completed, the chamber lights were turned on, and the NO/VOC/seed aerosol mixture was irradiated and allowed to come to steady-state. Any exposures, measurements, or calculations described herein correspond only to the steady-state portion of the experiments. A diagram of the chamber setup is provided in Figure S1. The resulting hydrocarbon-to-NOx ratio of each experiment was selected primarily for practical purposes in order to provide an adequate secondary aerosol yield and an air mixture somewhat representative of a polluted atmosphere that will include particles. With the exception of the benzene precursor experiment and considering the variable reactivity of the precursor hydrocarbons, this ratio was relatively consistent across the experiments.
2.2. Cell exposures and mutagenicity assay
Salmonella mutagenicity plate-incorporation assays were performed as described in Maron and Ames (1983). In brief, 100 µL of overnight culture of strain TA100 minus metabolic activation (S9) was added to molten top agar, and the suspension was poured onto VBME bottom agar in 100-mm plastic Petri dishes, permitting exposure of the cells at the air-agar interface. Strain TA100, which detects base-substitution mutations at GC sites, has been used extensively for smog-chamber studies such as these and been shown to be the strain most sensitive for the detection of mutagenic atmospheric transformation products (DeMarini et al., 2000; Dumdei et al., 1988; Kleindienst et al., 1990; Shepson et al., 1985a; Shepson et al., 1985b; Shepson et al., 1986; Shepson et al., 1987).
When an experiment reached steady-state, a continuous and constant concentration of the chamber atmosphere was delivered into two Modular Incubator Chambers (MIC, Billups-Rothenburg MIC-101Tm), each containing four poured cell plates with lids off. One L min−1 of the chamber atmosphere was drawn through a Tygon tube (1 m length, 9.525 mm inner diameter) and into the MICs. Each MIC contained 4 plates (2 for each exposure time point), cells were exposed for 1, 2, 4, 8, or 16 h, and exposures were performed at least twice, resulting in a total of 4 plates per exposure time point.
The bacterial cells respond only to gas-phase constituents because unlike mammalian cells they cannot take up aerosols; thus, mutagenic potencies resulted only from gas-phase chemical species even though aerosols were present in the chamber generated atmospheres. After exposure, plates were incubated at 37°C for 72 h, after which colonies (revertants, rev) were counted with an automatic colony counter (ProtoCOL 3, Microbiology International). Controls consisting of 3 spontaneous control plates (no exposure), as well as 2 plates containing a positive control (sodium azide at 3 µg plate−1), were prepared and assessed for each experiment.
Mutagenicity data (rev plate−1) were combined from replicate plates and replicate experiments to construct exposure time-response curves from which linear regressions were calculated resulting in mutagenicity expressed as rev h−1. These were then normalized to the estimated mass concentration of non-precursor gas-phase carbon during the exposure yielding mutagenic potencies with units of rev m3 mgC−1 h−1. A positive mutagenic response was considered a reproducible, dose-related increase in rev plate−1 that approached or exceeded a twofold increase relative to the unexposed spontaneous control and whose slopes were significantly (P < 0.05) greater than zero. Linear regressions (slopes) and r2-values were calculated using Prism (GraphPad, San Diego, CA). Pair-wise comparisons of mutagenic potencies were made by two-tailed Student’s t-tests using Prism.
Using the same methods, dark exposures were also performed with the same initial steady-state chamber conditions to ensure that the precursor VOC and other constituents were non-mutagenic. For the sake of efficiency, precursor VOCs were combined so that multiple VOCs were examined in a single dark experiment. Dark-1 corresponded to the combination of benzene, 1,2,4-trimethylbenzene, and o-xylene; dark-2 to toluene, m-cresol, and m-xylene; dark-3 to ethylbenzene, 1,3,5-trimethylbenzene, and p-xylene; dark-4 to benzene and naphthalene; and dark-5 to 1,2,4-trimethylbenzene and o-xylene.
2.3. CIMS measurements of secondary product levels
In addition to the gas-phase measurements of the precursor compounds described above, secondary products of the photooxidation reactions were also examined using a high-resolution time-of-flight chemical ionization mass spectrometer (CIMS, Aerodyne Research Inc.) with iodide reagent ion chemistry (Lee et al., 2014). The CIMS sampled directly from the atmospheric chamber through perfluorotetrafluoroethylene (PTFE) tubing (10 m length, 3.175 mm inner diameter) at 2 L min−1. Although CIMS data were often collected over the entirety of an experiment, only data taken during the steady-state portion were used in the analysis presented here.
Gas-phase compounds were detected at specific mass-to-charge ratios (m/Q) in the mass spectra corresponding to molecular clusters with the iodide reagent ion. For example, water vapor is detected at m/Q 144.915, which corresponds to the I(H2O)− molecular ion. This measurement technique is most suitable for detection of oxidized and polar species and has low instrumental sensitivity toward nonpolar and weakly polar compounds due to poor clustering with iodide. For this reason, we do not claim to have detected all of the possible products of the photooxidation reactions. Unlike methods used in previous studies (Arey et al., 1992; Gupta et al., 1996; Sasaki et al., 1995), CIMS does not require any collection or extraction before detection, thus minimizing losses of volatile and reactive compounds. Additionally, making use of the high-resolution time-of-flight capabilities allows for detection and molecular composition assignments of products that have not been considered previously (non-targeted or exploratory analysis).
As with other applications of this measurement technique, many of the molecular formulae identified in the mass spectra could represent multiple constitutional isomers contributing to a single m/Q peak. Therefore, we refrained from calibrating to a single potential compound or attaching a mixing ratio to the observed m/Q peak signals. In addition, many of the potential compounds lack authentic standards necessary for calibration.
The sensitivity of the instrument to compounds in the mass spectra is assumed to remain constant between each experiment because the major drivers of compound-specific sensitivity (declustering voltages and RH) were relatively constant across all experiments (Lee et al., 2014; Lopez-Hilfiker et al., 2016). Additionally, all compound signals were normalized to the sum of the I− and I(H2O)− signals to account for any decrease in the total ion current from detector deterioration and/or the radioactive decay of the polonium-210 ionization source between the different experiments.
Molecular compositions whose concentrations were elevated in the irradiated atmospheres relative to the dark atmospheres were selected and correlated with the mutagenic potencies expressed in rev h−1 because this was the most direct measure of the mutagenicity of the generated atmospheres. Correlation coefficients were then calculated as a Pearson r, which is the square root of r2, the coefficient of determination. Compounds responsible for mutagenicity must be electrophilic to interact with DNA, so the CIMS should be especially well-suited toward detection of such compounds considering they are likely polar.
3. Results and discussion
3.1. Concentrations of VOCs and criteria pollutants
The irradiated steady-state mixing ratios for NO, NO2, O3, and the precursor VOC are shown in Table 1. For comparison, we have also included a clean air control experiment with no VOC or NO added to the chamber. Apart from the naphthalene and clean air experiments, the majority of NO was converted to NO2, and substantial levels of O3 were produced. Precursor VOCs were never completely titrated during an experiment except during the m-cresol, but this could have been a result of the lower initial mixing ratio compared to the other experiments. SOA formation was observed across all experiments, and information such as aerosol mass loadings and SOA mass yields for each experiment is provided in the Table S1.
Table 1.
Summary of experiments and results.
| Lights OFF | Lights ON | Mutagenic Potencyb | ||||||
|---|---|---|---|---|---|---|---|---|
| Precursor VOC | VOC (ppmC) | NO (ppbv) | VOC (ppmC) | NO (ppbv) | NO2 (ppbv) | O3 (ppbv) | rev h−1 ± SE | rev m3 mgC−1 h−1 ± SE |
| benzene | 12.66 | 180 | 10.52 | 2 | 44 | 48a | 5.0 ± 0.6 | 5.2 ± 0.7 |
| toluene | 6.05 | 304 | 3.52 | 0 | 116 | 189 | 12.0 ± 0.7 | 10.4 ± 0.6 |
| ethylbenzene | 6.48 | 266 | 4.43 | 1 | 130 | 159 | 10.7 ± 0.8 | 11.4 ± 0.9 |
| o-xylene | 7.58 | 395 | 4.19 | 1 | 219 | 294 | 11.0 ± 0.8 | 7.2 ± 0.6 |
| m-xylene | 5.53 | 293 | 2.30 | 0 | 172 | 256 | 7.4 ± 0.8 | 4.9 ± 0.6 |
| p-xylene | 7.75 | 416 | 4.44 | 0 | 175 | 260 | 3.1 ± 0.6 | 2.0 ± 0.4 |
| 1,2,4-TMB | 7.12 | 392 | 3.99 | 1 | 249 | 309 | 7.1 ± 1.0 | 4.8 ± 0.7 |
| 1,3,5-TMB | 7.78 | 363 | 4.78 | 0 | 272 | 285 | 10.7 ± 1.2 | 7.7 ± 0.9 |
| m-cresol | 0.71 | 87 | 0.07 | 3 | 32 | 48 | 0 | 0 |
| naphthalene | 1.31 | 205 | 0.39 | 66 | 96 | 0 | 0 | 0 |
| clean air | - | 0 | - | 6 | 6 | 0 | 0 | 0 |
We were unable to collect O3 data for the benzene-exposure experiment. In order to place approximate bounds on the O3, the experiment was repeated with similar initial conditions, but additional cell exposures were not performed. The value provided here was from this repeated experiment.
Zero was used to represent the mutagenic potency when the slopes in Figure 1 were not significantly greater than zero.
3.2. Mutagenic potencies
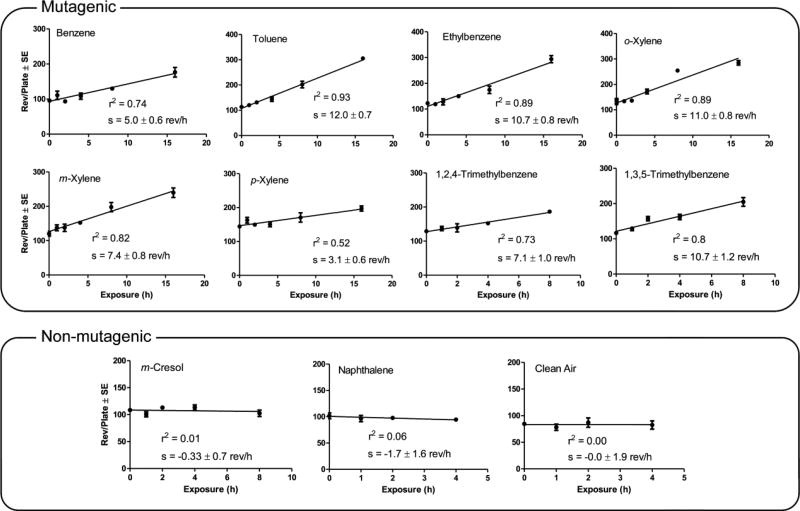
The mutagenicity data (rev plate−1) at the various exposure time points for lights-on (source data in Table S2) and lights-off (source data in Table S3) experiments are plotted in Figure 1, which shows the linear regressions from which the mutagenic potencies (rev h−1) were calculated as the slopes of the regression lines. Positive slopes with P ≤ 0.05 significance from zero indicated mutagenicity; consequently, all of the VOCs except m-cresol and naphthalene produced mutagenic atmospheres; clean air was also not mutagenic.
Fig. 1.
Mutagenicity results from exposures to photooxidation products expressed as revertants plate−1 versus exposure time. Each experiment is identified by the precursor VOC. The “s” is the regression coefficient, which is the mutagenic potency expressed as rev h−1.
None of the dark-cell exposures produced mutagenic atmospheres (Figure S2). Thus, the mutagenicity produced during irradiation had to result from late-generation products from the VOC and NOx photooxidation reactions. Drying of the cells at 8 or more hours of exposure resulted in cytotoxicity for some non-mutagenic atmospheres as indicated by the slightly negative slopes. Thus, positive mutagenic responses at 8 and 16 h for some of the atmospheres in Figure 1 should be considered a lower-bound estimate; however, some dose-responses were linear even up to 16 h, suggesting that either those atmospheres did not dry out the cells or that mutagenicity exceeded cytotoxicity at those exposure times.
Although the number of revertants per hour of exposure was the most direct measurement of the mutagenic potency of each atmosphere, the mutagenicity was also normalized by the estimated mass concentration of non-precursor gas-phase organic species expressed as the mass of gas-phase carbon per cubic meter of chamber air (mgC m−3) (Table S1). This approach helped for a more consistent comparison of the mutagenicity across experiments with varying initial concentrations of precursor hydrocarbons and NO, differing degrees of photooxidation, and different amounts of aerosol formation. These values were calculated as the difference between the total carbon mass of the precursor hydrocarbon consumed and total particulate OC mass formed. The provided errors associated with this metric of mutagenicity were propagated from the standard errors reported for mutagenic potencies in rev h−1.
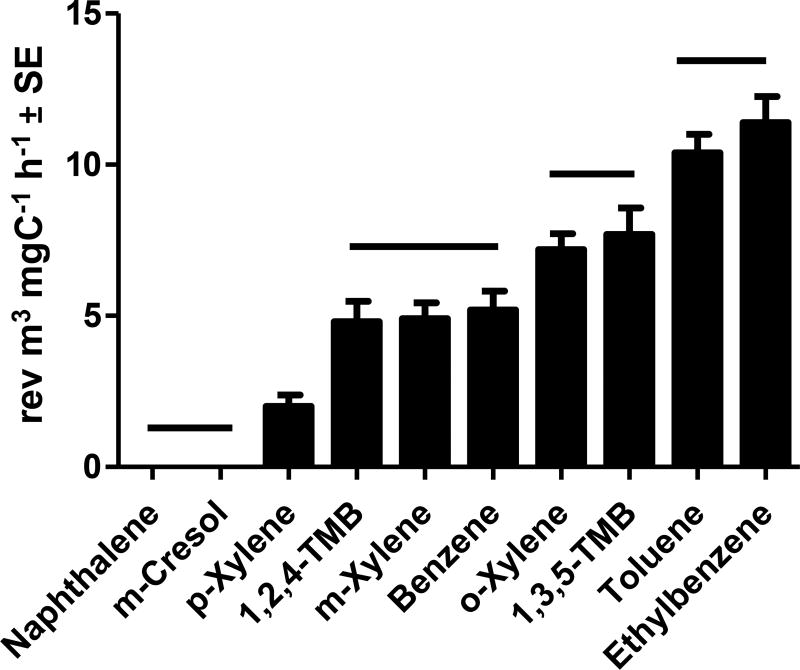
Based on this metric of mutagenic potency, ethylbenzene produced the most mutagenic atmosphere. As shown in Figure 2, when the mutagenic potencies of the atmospheres were expressed in rev m3 mgC−1 h−1, the 10 atmospheres clustered into 5 groups based on P-values (Table S4), resulting in the following ranking of mutagenic potency: ethylbenzene ≈ toluene > o-xylene ≈ 1,3,5-trimethylbenzene > benzene ≈ m-xylene ≈ 1,2,4-trimethylbenzene > p-xylene > m-cresol ≈ naphthalene.
Fig. 2.
Mutagenic potencies in Salmonella TA100-S9 of the atmospheres generated by each of the 10 precursor VOCs examined. Based on comparisons using a two-tailed Student’s t-test, the VOCs under a single horizontal bar produced atmospheres whose mutagenic potencies were not significantly different (P < 0.05).
Considering experimental differences (chamber light intensity, initial conditions, extent of reaction, slight differences in exposure times, and other factors), the mutagenic potencies reported here were broadly consistent with those from previous chamber studies that used Salmonella cell assay and generated atmospheres from the photooxidation of a single VOC and NOx. Dumdei et al. (1988) investigated the toluene/NOx system and obtained mutagenic activities of ~10 rev m3 mgC−1 h−1. That study and Shepson et al. (1985b), a predecessor study, were the only prior studies, to our knowledge, that used an aromatic VOC as the precursor compound and direct sampling methods that directly overlapped with the toluene exposure described here. Propylene and acetaldehyde, although not aromatic species, were also investigated previously under similar conditions and produced atmospheres with mutagenic potencies of ~3 and 45–70 rev m3 mgC−1 h−1, respectively (Shepson et al., 1985a; Shepson et al., 1986).
3.3. Correlations between mutagenic potencies and late-generation reaction products
Given that the mutagenicity resulted from some fraction of the oxidized reaction products, the iodide-CIMS was an especially powerful tool for detection of such compounds. In order to link these compounds to the observed mutagenicity, gas-phase molecular compositions identified by the CIMS were examined for their association with the mutagenicity of the generated atmospheres. Compared to dark-exposure experiments, a number of mass spectra peaks corresponding to photooxidation products of the precursor VOCs were enhanced during irradiation. Once a molecular composition was assigned to each of these peaks, the relative levels across all experiments were plotted against the mutagenic potencies and examined for potential correlations.
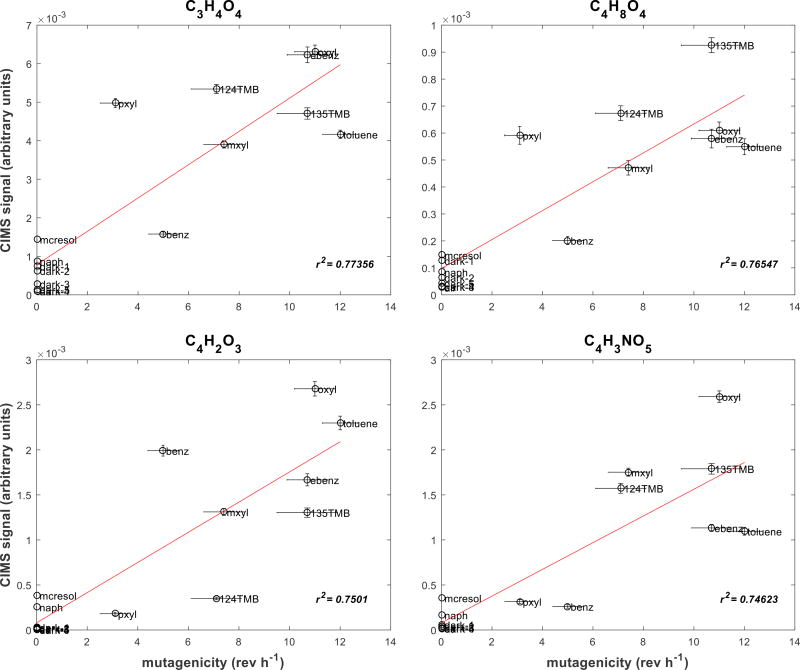
Figure 3 illustrates this approach for four molecular compositions that showed the highest correlations with the mutagenic potencies from Table 1. The vertical error bars correspond to the 2σ variation in the CIMS signals, and the horizontal error bars correspond to the linear fit standard errors from the regressions of the mutagenicity data in Figure 1. The molecular compositions (with iodide removed) are given for each of the panels in Figure 3. Dark exposures were included in the correlation calculation for each plot, which shows positive correlations between the amount of the photooxidation product and the mutagenic potencies of the atmospheres.
Fig. 3.
Correlations between the steady-state levels of four gas-phase molecular compositions (C3H4O4, C4H8O4, C4H2O3, C4H3NO5) measured by CIMS and mutagenicity. Each point represents a single experiment, and each is labeled by precursor VOC abbreviations or as “dark” in the case of the lights-off exposure experiments. Horizontal error bars are the standard errors from the mutagenic potencies. Vertical error bars are the 2σ variation in the CIMS signal averages.
The majority of the enhanced peaks did not show appreciable correlation with observed mutagenicity. However, there were nine molecular compositions, including those shown in Figure 3, that showed good correlations (r ≥ 0.81). These compositions (with iodide removed), along with the respective correlation coefficient values relative to mutagenic potency, are shown in Table 2. Similar to the examples shown in Figure 3, all of the compositions in Table 2 showed significant enhancements with irradiation relative to the dark exposures. The high O:C ratios of the molecular compositions confirmed that these were late-generation photooxidation products.
Table 2.
Correlations between mutagenicity and molecular composition of potential gas-phase photooxidation products.
| Molecular Composition | r | Potential compounds |
|---|---|---|
|
| ||
| C3H4O4 | 0.88 | 2-oxopropaneperoxoic acid, 2-hydroperoxypropanedial |
|
| ||
| C4H8O4 | 0.87 | 2-hydroxybutaneperoxoic acid |
|
| ||
| C4H2O3 | 0.87 | furan-2,5-dione |
|
| ||
| C4H3NO5 | 0.86 | 3-formyl-3-nitro-2-butenoic acid |
|
| ||
| HNO3 | 0.86 | nitric acid |
|
| ||
| C3H4O3 | 0.86 | 2-hydroxypropanedial, 2-oxopropanoic acid, acetic formic anhydride |
|
| ||
| HNO4 | 0.85 | peroxynitric acid |
|
| ||
| C8H8O3 | 0.84 | 2-phenylethaneperoxoic acid, methylbenzenecarboperoxoic acid |
|
| ||
| C4H6O5 | 0.81 | 2-hydroxy-2-methyl-3-oxopropaneperoxoic acid, acetyl 2-hydroperoxyacetate, hydroperoxymethyl 2-oxopropanoate |
| 2-hydroxy-3-oxobutaneperoxoic acid, 4-hydroxy-3-hydroperoxyoxolan-2-one, 2-hydroperoxy-3-hydroxy-butanedial | ||
Using the compositions in Table 2, we tentatively identified potential chemical species resulting from the oxidation of the precursor compounds, considering that other unidentified isomers could also have contributed to the CIMS signals. A subset of the Master Chemical Mechanism (MCM version 3.3.1 – http://mcm.leeds.ac.uk/MCM) was extracted for all of the precursor VOCs and examined for photochemical products that matched the compositions listed in Table 2 (Bloss et al., 2005). Although there was no explicit hydroxyl radical (OH) source in these experiments, such as the photolysis of hydrogen peroxide (Kleindienst et al., 2009), it was nonetheless assumed that non-negligible amounts of OH were present in the chamber. Nitrous acid (HONO) can be formed through heterogeneous chemistry of NO2 and H2O with the chamber aerosol and/or chamber walls. OH could have then been produced from subsequent photolysis of HONO to form OH and NO (see Reactions 1 and 2 below) (Calvert et al., 1994; Finlayson-Pitts et al., 2003; Harris et al., 1982).
| (1) |
| (2) |
The CIMS detected an increase in signal at m/Q 173.905 after the chamber lights were turned on, corresponding with the I(HONO)− molecular cluster (Figure S3). Thus, it is assumed that most of the formation pathways that led to the chemical species given in Table 2 were through OH-initiated oxidation of the precursor VOC. The majority of the potential species correspond to organic peroxy acids, often with additional carbonyl (aldehydes and ketones) and hydroxy groups. Other potential functional groups include carboxylic acid, dialdehyde, and diketone species, as well as nitrogen-containing species resulting from NO2 addition to the aromatic ring; chemical structures for these species are shown in Figure S4.
Table 2 is not intended to be a definitive list of potential compounds responsible for mutagenicity but rather to illustrate the types of chemical species and reaction channels that are most related to mutagenicity based on the composition and photooxidation mechanisms of the precursor VOCs. The compositions in Table 2 could be used as markers of mutagenic potential in more complex mixtures or perhaps even in ambient air, although additional studies would be needed to confirm this.
3.4. Influence of residence time on mutagenic potency
At the conclusion of the experiments described above, we selected ethylbenzene, because it produced the most mutagenic atmosphere, to explore any association between smog chamber residence time and mutagenic potency. Four experiments were performed with ethylbenzene as the precursor VOC with chamber residence times of 3.5, 5, 7, and 9 h. Atmospheres were generated in the same manner as described above (Table S5).
Table S6 shows the mutagenicity data for these experiments, and the linear regressions used to calculate the mutagenic potencies are shown in Figure S5. Based on mutagenic potencies expressed in rev m3 mgC−1 h−1, there was an inverse association between residence time and mutagenic potency (Figure S6). Although the basis for this observation was not entirely clear, it is likely that at the longer residence times, short-lived reactive species were converted to non-mutagenic products. Alternatively, photooxidation products may have been sufficiently oxidized to partition to the particle phase, resulting in less mutagenic atmospheres at the longer residence times. Indeed, aerosol yields tended to increase at the longer residence times (Table S1).
The ethylbenzene experiments were not designed for the purpose of evaluating the effect of variable initial concentrations on the mutagenic potency of the resulting atmosphere. However, we note that under a standard residence time of 4.5 h, a starting ethylbenzene mixing ratio of 6.48 ppmC with 266 ppbv NO resulted in an atmosphere with a mutagenic potency of 11.4 rev m3 mgC−1 h−1. In contrast, a reaction with a similar residence time (5 h) but a starting concentration of only 3.63 ppmC with 200 ppbv NO resulted in an atmosphere with a mutagenic potency of only 4.1 rev m3 mgC−1 h−1. Although the residence times were not identical, the significantly different results between the two experiments (Figure S7) suggest that reducing the concentration of a non-mutagenic primary VOC and NOx will reduce the mutagenicity of the atmosphere comprised of the photooxidation products of that VOC.
3.5. Assessment of atmospheres produced by m-cresol and naphthalene
Various factors might have accounted for why neither m-cresol nor naphthalene photooxidation products produced mutagenic atmospheres under the experimental conditions examined here. m-Cresol is a first-generation product of toluene photooxidation. Therefore, it was somewhat surprising that the photooxidation of m-cresol itself did not produce a mutagenic atmosphere, whereas toluene did. It is likely that another photooxidation channel of toluene, unrelated to m-cresol formation, was responsible for the toluene-based mutagenicity.
Considering the results of previous studies (Arey et al., 1992; Gupta et al., 1996; Sasaki et al., 1995; Sasaki et al., 1999), it was also unexpected that naphthalene photooxidation resulted in mutagenicity below the level of detection. It is possible that concentrations of OH and/or NO2 in the chamber were insufficient to form the mutagenic nitro-naphthalene or other compounds in appreciable quantities. Assuming our experiments were not limited in steady-state OH concentrations and appropriate quantities of the OH-naphthalene adduct were formed, we can approximate an upper limit to the nitro-naphthalene mixing ratio assuming a yield of 1% from the naphthalene precursor (Nishino et al., 2008). This approach gives a steady-state nitro-naphthalene mixing ratio of ~10 ppbC for the conditions examined here. Given the high concentrations of naphthalene used in our study relative to those of the aforementioned studies, we would have expected sufficient quantities to generate a mutagenic response, but without a reliable estimate of the OH concentrations in the chamber we can only speculate that we were limited in OH.
Another consideration is that both m-cresol and naphthalene had SOA mass yields 2–8 times greater than those of the other precursor compounds (Table S1), and the amount of gas-phase late-generation products may not have been sufficiently concentrated to induce mutagenicity. Similarly, because low initial concentrations of m-cresol and naphthalene were used relative to that of the other VOCs (Table 1), perhaps the concentrations of secondary products were too low to induce detectable mutagenicity. Unfortunately, these two VOCs necessitated a different delivery method compared to that of the other VOCs (see section 2.1. Steady-state chamber experiments above), and we were unable to reach similar initial concentrations.
4. Limitations of this study
When considering the potential compounds in Table 2, correlation between the chemical compositions and mutagenicity does not necessarily mean that those species are mutagenic. Compounds like nitric acid and peroxynitric acid have been included in Table 2 specifically to illustrate this. Neither of these compounds are known to be mutagenic, but each showed good correlation with mutagenicity because the same photooxidation mechanisms that produced these compounds also produced the species responsible for observed mutagenicity. Thus, we cannot state conclusively which species are responsible for the mutagenicity, only that the compositions presented in Table 2 are correlated with the mutagenicity.
Apart from the CIMS-measured levels of late-generation products that trended with mutagenicity, there was no clear explanation for differences in mutagenic potencies between the atmospheres produced from the o-, m-, and p-xylene isomers, where o > m > p (Figure 2). It may have been related to differences in the initial lights-off conditions for each experiment (Table 1) or perhaps differences in the SOA yield (Table S1), but more experiments would be needed to strengthen such arguments. We also note that previous studies have shown differences in the primary yields of dicarbonyl, ring-retaining, and ring-fragmented compounds resulting from the OH-initiated oxidation of m- and p-xylene, which ultimately may have some bearing on the mutagenic potential (Smith et al., 1999; Tuazon et al., 1984). Future experiments are planned to examine the potential for such structural influences on mutagenicity, including isomeric.
There is likely no single factor or molecular composition that explains the observed trends in mutagenicity, which is more likely due to a combination of late-generation reaction products, some of which may not be detectable by the CIMS. More focused studies on the mutagenicity of individual late-generation compounds, rather than the complex mixture of species explored here, would help identify the classes of compounds most responsible. However, many of the potential compounds are not available commercially and are likely unstable, making synthesis of such compounds and subsequent exposures a challenging prospect.
5. Conclusions and implications for control strategies
Other than 1,2,4- and 1,3,5-trimethylbenzene, all of the precursor aromatic VOCs investigated here are classified as hazardous air pollutants by the U.S. EPA, and therefore emissions of these species from industrial activities are controlled under the Clean Air Act (U.S. EPA, 2015a). Nonetheless, of the 8 VOCs that produced mutagenic atmospheres, all did so only under irradiation. Thus, only late-generation reaction products were responsible for the mutagenicity, not the precursor VOCs, raising an interesting point regarding potential control strategies when the photochemistry of certain chemical species is similar to those described here. Non-mutagenic primary compounds may be less likely to be controlled; however, the resulting late-generation products may be more likely to be mutagenic. Therefore, consideration should be given to precursor compounds based not only on their intrinsic health concerns but also on those of their potential late-generation atmospheric photochemical products. It seems that these products, which are typically not monitored or controlled, account for much of the gas-phase direct-acting mutagenicity. Based on these limited studies, reducing the concentrations of primary VOCs would likely result in a corresponding reduction in the concentrations of the products, with a parallel reduction in atmospheric mutagenicity.
Supplementary Material
Highlights.
-
-
Photooxidation of an aromatic VOC, NOx, and aerosol produced mutagenic atmospheres.
-
-
Mutagenicity was due exclusively to gas-phase photooxidation products.
-
-
Levels of certain gas-phase molecular compositions correlated with mutagenicity.
Acknowledgments
We thank Jeff Ross, Jonathan Krug, and William Linak (U.S. EPA) for helpful comments on the paper. The U.S. Environmental Protection Agency through its Office of Research and Development funded and collaborated in the research described here under Contract EP-C-15-008 to Jacobs Technology, Inc. This paper has been subjected to the Agency’s administrative review and approved for publication. The U.S. EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at [insert eventual web address here].
References
- Arey J, Harger WP, Helmig D, Atkinson R. Bioassay-directed fractionation of mutagenic PAH atmospheric photooxidation products and ambient particulate extracts. Mutation Research Letters. 1992;281:67–76. doi: 10.1016/0165-7992(92)90038-j. [DOI] [PubMed] [Google Scholar]
- Bloss C, Wagner V, Jenkin ME, Volkamer R, Bloss WJ, Lee JD, Heard DE, Wirtz K, Martin-Reviejo M, Rea G, Wenger JC, Pilling MJ. Development of a detailed chemical mechanism (MCMv3.1) for the atmospheric oxidation of aromatic hydrocarbons. Atmos. Chem. Phys. 2005;5:641–664. [Google Scholar]
- Calvert JG, Yarwood G, Dunker AM. An evaluation of the mechanism of nitrous acid formation in the urban atmosphere. Research on Chemical Intermediates. 1994;20:463–502. [Google Scholar]
- de Gouw JA, Brock CA, Atlas EL, Bates TS, Fehsenfeld FC, Goldan PD, Holloway JS, Kuster WC, Lerner BM, Matthew BM, Middlebrook AM, Onasch TB, Peltier RE, Quinn PK, Senff CJ, Stohl A, Sullivan AP, Trainer M, Warneke C, Weber RJ, Williams EJ. Sources of particulate matter in the northeastern United States in summer: 1. Direct emissions and secondary formation of organic matter in urban plumes. Journal of Geophysical Research: Atmospheres. 2008:113. [Google Scholar]
- DeMarini DM, Shelton ML, Kohan MJ, Hudgens EE, Kleindienst TE, Ball LM, Walsh D, de Boer JG, Lewis-Bevan L, Rabinowitz JR, Claxton LD, Lewtas J. Mutagenicity in lung of Big Blue® mice and induction of tandem-base substitutions in Salmonella by the air pollutant peroxyacetyl nitrate (PAN): predicted formation of intrastrand cross-links. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2000;457:41–55. doi: 10.1016/s0027-5107(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Dumdei BE, Kenny DV, Shepson PB, Kleindienst TE, Nero CM, Cupitt LT, Claxton LD. MS/MS analysis of the products of toluene photooxidation and measurement of their mutagenic activity. Environmental Science & Technology. 1988;22:1493–1498. doi: 10.1021/es00177a017. [DOI] [PubMed] [Google Scholar]
- Finlayson-Pitts BJ, Wingen LM, Sumner AL, Syomin D, Ramazan KA. The heterogeneous hydrolysis of NO2 in laboratory systems and in outdoor and indoor atmospheres: An integrated mechanism. Physical Chemistry Chemical Physics. 2003;5:223–242. [Google Scholar]
- Gupta P, Harger WP, Arey J. The contribution of nitro- and methylnitronaphthalenes to the vapor-phase mutagenicity of ambient air samples. Atmospheric Environment. 1996;30:3157–3166. [Google Scholar]
- Harris GW, Carter WPL, Winer AM, Pitts JN, Platt U, Perner D. Observations of nitrous acid in the Los Angeles atmosphere and implications for predictions of ozone-precursor relationships. Environmental Science & Technology. 1982;16:414–419. doi: 10.1021/es00101a009. [DOI] [PubMed] [Google Scholar]
- IARC. Outdoor Air Pollution. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2016;109 [PMC free article] [PubMed] [Google Scholar]
- Jaegle L, Steinberger L, Martin RV, Chance K. Global partitioning of NOx sources using satellite observations: Relative roles of fossil fuel combustion, biomass burning and soil emissions. Faraday Discussions. 2005;130:407–423. doi: 10.1039/b502128f. [DOI] [PubMed] [Google Scholar]
- Jia C, Batterman S. A Critical Review of Naphthalene Sources and Exposures Relevant to Indoor and Outdoor Air. International Journal of Environmental Research and Public Health. 2010;7:2903. doi: 10.3390/ijerph7072903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobson BT, Berkowitz CM, Kuster WC, Goldan PD, Williams EJ, Fesenfeld FC, Apel EC, Karl T, Lonneman WA, Riemer D. Hydrocarbon source signatures in Houston, Texas: Influence of the petrochemical industry. Journal of Geophysical Research: Atmospheres. 2004;109 [Google Scholar]
- Kleindienst TE, Edney EO, Lewandowski M, Offenberg JH, Jaoui M. Secondary Organic Carbon and Aerosol Yields from the Irradiations of Isoprene and α-Pinene in the Presence of NOx and SO2. Environmental Science & Technology. 2006;40:3807–3812. doi: 10.1021/es052446r. [DOI] [PubMed] [Google Scholar]
- Kleindienst TE, Lewandowski M, Offenberg JH, Jaoui M, Edney EO. The formation of secondary organic aerosol from the isoprene + OH reaction in the absence of NOx. Atmos. Chem. Phys. 2009;9:6541–6558. [Google Scholar]
- Kleindienst TE, Shepson PB, Smith DF, Hudgens EE, Nero CM, Cupitt LT, Bufalini JJ, Claxton LD, Nestman FR. Comparison of mutagenic activities of several peroxyacyl nitrates. Environmental and Molecular Mutagenesis. 1990;16:70–80. doi: 10.1002/em.2850160204. [DOI] [PubMed] [Google Scholar]
- Kleindienst TE, Smith DF, Li W, Edney EO, Driscoll DJ, Speer RE, Weathers WS. Secondary organic aerosol formation from the oxidation of aromatic hydrocarbons in the presence of dry submicron ammonium sulfate aerosol. Atmospheric Environment. 1999;33:3669–3681. [Google Scholar]
- Lee BH, Lopez-Hilfiker FD, Mohr C, Kurtén T, Worsnop DR, Thornton JA. An Iodide-Adduct High-Resolution Time-of-Flight Chemical-Ionization Mass Spectrometer: Application to Atmospheric Inorganic and Organic Compounds. Environmental Science & Technology. 2014;48:6309–6317. doi: 10.1021/es500362a. [DOI] [PubMed] [Google Scholar]
- Lopez-Hilfiker FD, Iyer S, Mohr C, Lee BH, D'Ambro EL, Kurtén T, Thornton JA. Constraining the sensitivity of iodide adduct chemical ionization mass spectrometry to multifunctional organic molecules using the collision limit and thermodynamic stability of iodide ion adducts. Atmos. Meas. Tech. 2016;9:1505–1512. [Google Scholar]
- Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutation Research/Environmental Mutagenesis and Related Subjects. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Ng NL, Kroll JH, Chan AWH, Chhabra PS, Flagan RC, Seinfeld JH. Secondary organic aerosol formation from m-xylene, toluene, and benzene. Atmos. Chem. Phys. 2007;7:3909–3922. [Google Scholar]
- Nishino N, Atkinson R, Arey J. Formation of Nitro Products from the Gas-Phase OH Radical-Initiated Reactions of Toluene, Naphthalene, and Biphenyl: Effect of NO2 Concentration. Environmental Science & Technology. 2008;42:9203–9209. doi: 10.1021/es802046m. [DOI] [PubMed] [Google Scholar]
- Odum JR, Hoffmann T, Bowman F, Collins D, Flagan RC, Seinfeld JH. Gas/Particle Partitioning and Secondary Organic Aerosol Yields. Environmental Science & Technology. 1996;30:2580–2585. [Google Scholar]
- Parrish D, Stockwell W. Urbanization and air pollution: Then and now. EOS. 2015;96 [Google Scholar]
- Piccot SD, Watson JJ, Jones JW. A global inventory of volatile organic compound emissions from anthropogenic sources. Journal of Geophysical Research: Atmospheres. 1992;97:9897–9912. [Google Scholar]
- Sasaki J, Arey J, Harger WP. Formation of Mutagens from the Photooxidations of 2-4-Ring PAH. Environmental Science & Technology. 1995;29:1324–1335. doi: 10.1021/es00005a027. [DOI] [PubMed] [Google Scholar]
- Sasaki JC, Arey J, Eastmond DA, Parks KK, Phousongphouang PT, Grosovsky AJ. Evidence for oxidative metabolism in the genotoxicity of the atmospheric reaction product 2-nitronaphthalene in human lymphoblastoid cell lines. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 1999;445:113–125. doi: 10.1016/s1383-5718(99)00118-7. [DOI] [PubMed] [Google Scholar]
- Shepson PB, Kleindienst TE, Edney EO, Cupitt LT, Claxton LD. The mutagenic activity of the products of ozone reaction with propylene in the presence and absence of nitrogen dioxide. Environmental Science & Technology. 1985a;19:1094–1098. doi: 10.1021/es00141a013. [DOI] [PubMed] [Google Scholar]
- Shepson PB, Kleindienst TE, Edney EO, Namie GR, Pittman JH, Cupitt LT, Claxton LD. Mutagenic activity of irradiated toluene/NOx/H2O/air mixtures. Environmental Science & Technology. 1985b;19:249–255. doi: 10.1021/es00133a005. [DOI] [PubMed] [Google Scholar]
- Shepson PB, Kleindienst TE, Edney EO, Nero CM, Cupitt LT, Claxton LD. Acetaldehyde: the mutagenic activity of its photooxidation products. Environmental Science & Technology. 1986;20:1008–1013. doi: 10.1021/es00152a007. [DOI] [PubMed] [Google Scholar]
- Shepson PB, Kleindienst TE, Nero CM, Hodges DN, Cupitt LT, Claxton LD. Allyl chloride: the mutagenic activity of its photooxidation products. Environmental Science & Technology. 1987;21:568–573. doi: 10.1021/es00160a007. [DOI] [PubMed] [Google Scholar]
- Simon H, Beck L, Bhave PV, Divita F, Hsu Y, Luecken D, Mobley JD, Pouliot GA, Reff A, Sarwar G, Strum M. The development and uses of EPA’s SPECIATE database. Atmospheric Pollution Research. 2010;1:196–206. [Google Scholar]
- Smith DF, Kleindienst TE, McIver CD. Primary Product Distributions from the Reaction of OH with m-, p-Xylene, 1,2,4- and 1,3,5-Trimethylbenzene. Journal of Atmospheric Chemistry. 1999;34:339–364. [Google Scholar]
- Thornton JA, Wooldridge PJ, Cohen RC, Martinez M, Harder H, Brune WH, Williams EJ, Roberts JM, Fehsenfeld FC, Hall SR, Shetter RE, Wert BP, Fried A. Ozone production rates as a function of NOx abundances and HOx production rates in the Nashville urban plume. Journal of Geophysical Research-Atmospheres. 2002:107. [Google Scholar]
- Tuazon EC, Atkinson R, Macleod H, Biermann HW, Winer AM, Carter WPL, Pitts JN. Yields of glyoxal and methylglyoxal from the NOx air photooxidations of toluene, and m-, and p-xylene. Environmental Science & Technology. 1984;18:981–984. [Google Scholar]
- U.S. EPA. Initial List of Hazardous Air Pollutants with Modifications. 2015a https://www.epa.gov/haps/initial-list-hazardous-air-pollutants-modifications.
- U.S. EPA. NAAQS Table. 2015b https://www.epa.gov/criteria-air-pollutants/naaqs-table.
- Zhou Y, Li C, Huijbregts MAJ, Mumtaz MM. Carcinogenic Air Toxics Exposure and Their Cancer-Related Health Impacts in the United States. PLOS ONE. 2015;10:e0140013. doi: 10.1371/journal.pone.0140013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.